Abstract
Ashwagandha (Withania somnifera) is a herb commonly used in Ayurvedic medicine to promote youthful vigor, enhance muscle strength and endurance, and improve overall health. In this 16-week, randomized, double-blind, placebo-controlled, crossover study, its effects on fatigue, vigor, and steroid hormones in aging men were investigated. Overweight men aged 40–70 years, with mild fatigue, were given a placebo or an ashwagandha extract (Shoden beads, delivering 21 mg of withanolide glycosides a day) for 8 weeks. Outcome measures included the Profile of Mood States, Short Form (POMS-SF), Aging Males’ Symptoms (AMS) questionnaire, and salivary levels of DHEA-S, testosterone, cortisol, and estradiol. Fifty-seven participants were enrolled, with 50 people completing the first 8-week period of the trial and 43 completing all 16 weeks. Improvements in fatigue, vigor, and sexual and psychological well-being were reported over time, with no statistically significant between-group differences. Ashwagandha intake was associated with an 18% greater increase in DHEA-S (p = .005) and 14.7% greater increase in testosterone (p = .010) compared to the placebo. There were no significant between-group differences in cortisol and estradiol. In conclusion, the intake of a standardized ashwagandha extract (Shoden beads) for 8 weeks was associated with increased levels of DHEA-S and testosterone, although no significant between-group differences were found in cortisol, estradiol, fatigue, vigor, or sexual well-being. Further studies with larger sample sizes are required to substantiate the current findings.
Keywords: ashwagandha, Withania somnifera, DHEA, testosterone, hormones, fatigue, energy, herbal
Fatigue is a common complaint among young and older adults, presenting in approximately 20%–40% of individuals (Lerdal, Wahl, Rustoen, Hanestad, & Moum, 2005). It is one of the most common symptoms encountered in patients receiving primary care medical treatment, reported by 14%–40% of patients (Cathebras, Robbins, Kirmayer, & Hayton, 1992; Hickie et al., 1996). While fatigue is a central symptom associated with several medical and psychiatric conditions, idiopathic fatigue also has a substantial impact on an individual’s self-care (Rhodes, Watson, & Hanson, 1988) and overall quality of life (Yoo et al., 2018). Fatigue is also a strong predictor of future morbidity and mortality (Appels & Mulder, 1988; Avlund, Schultz-Larsen, & Davidsen, 1998). Higher fatigue levels in daily activities is also an early indicator of aging as it is associated with several poor health outcomes (Avlund, 2010).
The bulk of evidence suggests that rates of self-reported fatigue increase with age (Loge, Ekeberg, & Kaasa, 1998; Van Mens-Verhulst & Bensing, 1998). In a study of over 2,000 men between the ages of 18 and 92 years, an incremental increase in physical, mental, and general fatigue was associated with increasing age (Beute, Wiltink, Schwarz, Weidner, & Brahler, 2002). Given the association between fatigue, disease, and quality of life, strategies to slow this decline are important.
Along with increasing fatigue, reductions in steroid hormones commonly occur as men age. It is estimated that males experience a decline in testosterone levels at the rate of 1%–2% per annum after the age of 40 years (Feldman et al., 2002; Stanworth & Jones, 2008). Dehydroepiandrosterone sulfate (DHEA-S) concentrations also decrease by an average of 1%–4% per year between the ages of 40 and 80 years (Tannenbaum, Barrett-Connor, Laughlin, & Platt, 2004; Walther, Philipp, Lozza, & Ehlert, 2016). Testosterone and DHEA have several important roles in the body as they influence sexual health, lean body mass, mental health, cognition, bone density, cardiovascular function, and metabolic activity, just to name a few (Kelly & Jones, 2013; Rutkowski, Sowa, Rutkowska-Talipska, Kuryliszyn-Moskal, & Rutkowski, 2014). In men, testosterone can be converted by the enzyme aromatase into estradiol. Although estradiol is a hormone often associated with women, it also tends to decline as men age (Orwoll et al., 2006). It has several important roles in males, including having a critical role in male sexual function, adiposity levels, neurological activity, cardiovascular health, and immunity (Cooke, Nanjappa, Ko, Prins, & Hess, 2017; Schulster, Bernie, & Ramasamy, 2016).
Low serum testosterone levels in men are strongly associated with increased morbidity (Maggi, Schulman, Quinton, Langham, & Uhl-Hochgraeber, 2007) and commonly occur in men with major depressive disorder (Joshi et al., 2010), cardiovascular disease (Corona et al., 2011), obesity (Di Vincenzo, Busetto, Vettor, & Rossato, 2018; A. M. Traish & Zitzmann, 2015), and type 2 diabetes (Yao, Wang, An, Zhang, & Ding, 2018). Lower testosterone concentrations are also adversely associated with a reduced quality of life (Khera, 2016). It has been reported that DHEA levels predict longevity in men (Enomoto et al., 2008), and higher concentrations are associated with improvements in mood and reductions in fatigue (Saad, Hoesl, Oettel, Fauteck, & Rommler, 2005). Given the influence of steroid hormones such as testosterone and DHEA on mental and physical well-being, health-related quality of life, and disease morbidity and mortality, treatments options to slow their progressive decline as men age may be prudent. Chronic and acute stress are factors that can influence concentrations of steroid hormones. Although research on the directional impact of stress on DHEA concentrations is inconsistent, there is strong evidence confirming that high stress, as indicated by elevated cortisol, is regularly associated with lower testosterone concentrations (Collomp et al., 2016). This indicates that strategies to reduce stress, or cortisol concentrations, may be an effective way to optimize steroid hormone concentrations in men. This is supported by findings of increased DHEA-S and testosterone following participation in a stress reduction program in men (Antoni, 2003).
Adaptogens such as ashwagandha, Rhodiola rosea, Siberian ginseng, and Bacopa monnieri are defined as nontoxic substances, often of plant origin, that increase the body’s ability to resist the damaging effects of stress and promote or restore normal physiological functioning. Adaptogens exhibit neuroprotective, anti-fatigue, antidepressant, anxiolytic, nootropic, and central nervous system–stimulating activity (Panossian & Wikman, 2010). Many adaptogenic herbs demonstrated an anti-fatigue effect, particularly during times of chronic or acute stress (Panossian & Wikman, 2009). Ashwagandha (also known as Withania somnifera, Indian ginseng, or winter cherry) is an adaptogenic herb that is commonly used in Ayurvedic medicine to promote “youthful vigor,” enhance muscle strength and endurance, and improve overall health. It is also believed to offer health-restorative properties by counteracting chronic fatigue, weakness, impotence, sterility, nervous exhaustion, senility, and premature aging (Kulkarni & Dhir, 2008). The mental and physical effects of ashwagandha have been investigated in several research studies, with identified positive effects in reducing stress and anxiety (Pratte, Nanavati, Young, & Morley, 2014) and increasing memory and cognition (Choudhary, Bhattacharyya, & Bose, 2017). In a recent meta-analysis comprising four clinical trials, it was concluded that ashwagandha supplementation was associated with significant increases in sperm concentration, semen volume, and sperm motility in oligospermic males. Increases in serum testosterone and luteinizing hormone levels were also identified (Durg, Shivaram, & Bavage, 2018). It has been demonstrated in several animal studies that ashwagandha can influence the hypothalamic–pituitary–gonadal hormonal axis and increase testosterone concentrations (Azgomi et al., 2018; Sengupta et al., 2018). In a study on chronically stressed adults, ashwagandha supplementation for 60 days was associated with reductions in anxiety (as measured by the Hamilton Anxiety Scale) and increases in serum DHEA-S (Auddy, Hazra, Mitra, Abedon, & Ghosal, 2008).
The aims of this study were to identify the effects of ashwagandha supplementation over an 8-week period in overweight men aged 40–70 years with mild-to-moderate, self-reported fatigue. Given the positive association of steroid hormones such as testosterone and DHEA-S on general well-being and quality of life, the influence of ashwagandha on these hormones was also investigated. Since cortisol is consistently associated with stress and can have an adverse effect on energy and androgenic hormones, the effects of ashwagandha on this stress-related hormone was also examined. To help clarify the impact of ashwagandha supplementation on hormonal pathways and enzymatic activity in men (specifically aromatase), estradiol levels were also measured.
Materials and Methods
Study Design
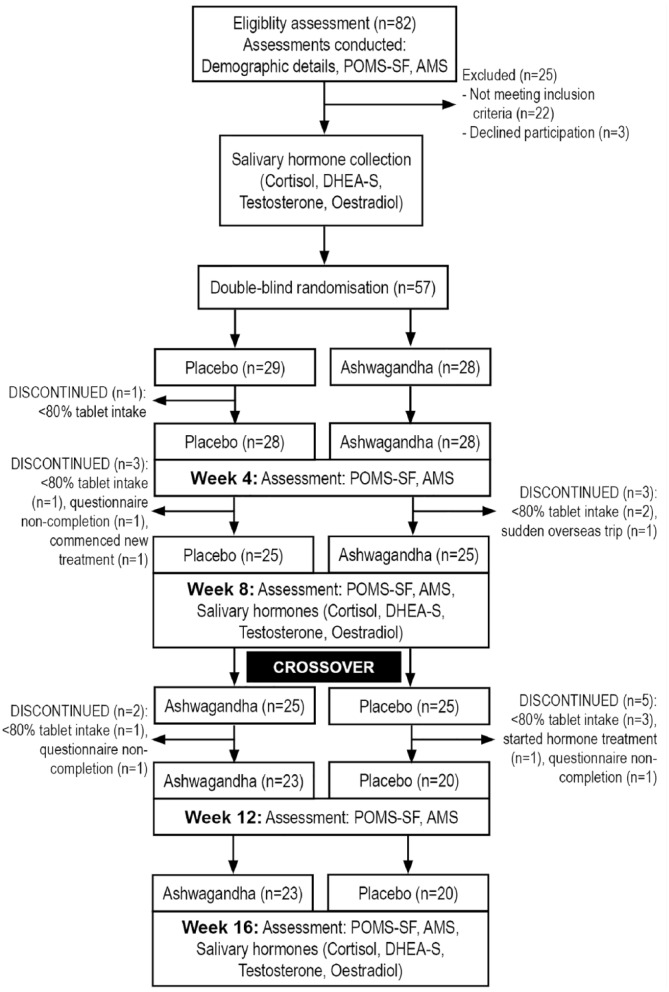
This was a 16-week, randomized, double-blind, placebo-controlled, crossover trial evaluating the effect of an ashwagandha extract (Shoden beads) on salivary hormones and symptoms of fatigue and vitality in healthy, overweight men. No washout period was included in this crossover trial as the aim in the second period of the trial was to investigate the durability of changes after discontinuation of the active treatment. The trial protocol was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee at Murdoch University, Western Australia, and was prospectively registered with the Australian New Zealand Clinical Trials Registry (Trial ID. ACTRN12617000971336; date of registration 05/07/2017). Participants were recruited through social media advertisements between December 2017 and February 2018, across Western Australia, and they gave their written informed consent for inclusion before they participated in the study. Details of the study design are outlined in Figure 1.
Figure 1.
Systematic illustration of study design.
Participants were randomly and equally allocated into two groups (placebo followed by ashwagandha or ashwagandha followed by placebo) using a randomization calculator (http://www.randomization.com). The randomization structure comprised seven randomly permuted blocks, containing eight participants per block. Participant identification number was allocated according to the order of participant enrolment in the study. All tablets were packed in identical containers labeled by intervention code numbers. Intervention codes were held by the sponsor and a university investigator not directly involved in study recruitment and data collection. Participants and study investigators were not informed of treatment group allocation until all questionnaire data was collected.
As this was a pilot study, the sample size was a convenience sample. Past studies have demonstrated that ashwagandha has an effect size of 0.8–1.2 on symptoms of stress and anxiety in stressed adults (Auddy et al., 2008; Chandrasekhar, Kapoor, & Anishetty, 2012). Assuming a power of 80%, a type I error rate (alpha) of 5%, and a 10% dropout rate, the total number of participants to find an effect was calculated as 57.
Participants
Participants were informed about the study and, if agreeable, were assessed by the principal investigator for eligibility based on the following inclusion/exclusion criteria:
Inclusion criteria
Healthy males aged between 40 and 70 years reporting mild-to-moderate symptoms of fatigue or reduced vitality were eligible to participate (as measured by a Profile of Mood States [POMS] Fatigue-Inertia score above the 50th percentile, or POMs total score or Vigor-Activity score below the 50th percentile). Participants were also required to be nonsmokers, be medication-free for at least 3 months, and have a body mass index (BMI) between 25 and 35. Participants were not planning to participate in any weight-loss program or significant lifestyle-related changes during the study.
Exclusion criteria
Participants were ineligible for participation in the study if they had a diagnosable mental health disorder (e.g., depression, anxiety-related disorder, eating disorder, psychosis/schizophrenia) or medical illness including diabetes, autoimmune diseases, cardiovascular disease, hypertension, chronic fatigue syndrome, or asthma. Those suffering from an infection or illness over the past month (including the common cold), those who reported drinking greater than 14 standard servings of alcoholic drinks per week, or those who reported a current or history of illicit drug abuse were also ineligible to participate. Additionally, current intake of any herbal preparations or a known hypersensitivity to ashwagandha, herbal supplements, or other herbs and spices also resulted in ineligibility for study participation.
Eligibility was initially assessed via the completion of an online questionnaire that screened for current medication use, energy/stamina levels, height and weight, physical and mental health, illnesses over the past month, alcohol consumption, and nicotine intake. If deemed as likely eligible, volunteers then participated in a phone interview with the primary investigator. The phone interview comprised a structured series of questions examining the eligibility criteria already specified.
Interventions
Tablets containing either an ashwagandha extract (Shoden beads; Arjuna Natural Ltd., Aluva, Kerala, India), delivering 10.5 mg of withanolide glycosides, or a placebo (roasted rice powder) were used for the intervention and placebo periods, respectively. Shoden beads uses a patented Bioactive Ingredient Protection System (BIPS) technology comprising an ashwagandha extract from roots and leaves standardized to 35% withanolide glycosides. Both intervention and placebo were made into tablets, each weighing 300 mg, and were produced in a good manufacturing practice (GMP)–certified facility. Participants were instructed to take two tablets once daily, 2 hours away from food (preferably after dinner). The total daily intake of withanolide glycoside during the treatment condition was 21 mg (two tablets). Tablets were identical in appearance, shape, color, and packaging, comprising round maroon-colored tablets.
Medication compliance was measured by participant pill count at Weeks 4, 8, 12, and 16. Efficacy of participant treatment blinding was examined by asking participants to predict group allocation (placebo, ashwagandha, or uncertain) at the completion of each phase of the study (Weeks 8 and 16).
Outcome Measures
Outcome Measure 1: symptomatic changes
Aging Males’ Symptoms (AMS) self-report measure
The AMS is a 17-item, self-report questionnaire measuring psychological, somatic, and sexual symptoms. Items are rated on a 5-point Likert scale from 1 (none) to 5 (extremely severe). The AMS is a commonly used and reliable/valid measure of aging symptoms in men and their impact on quality of life (Daig et al., 2003). The AMS was completed at baseline, Week 4, Week 8, Week 12, and Week 16 after the commencement of tablet intake.
Profile of Mood States, Short Form (POMS-SF); Fatigue-Inertia and Vigor-Activity subscale scores
The POMS-SF is a 35-item, self-report questionnaire with subscales including Anger-Hostility, Confusion-Bewilderment, Depression-Dejection, Fatigue-Inertia, Tension-Anxiety, Vigor-Activity, and Friendliness. Items are rated on a 5-point Likert scale from 0 (not at all) to 4 (extremely). The POMS-SF is a commonly used and reliable/valid questionnaire (Heuchert & McNair, 2012) with the Fatigue-Inertia subscale demonstrating good validity in a patient population (Fink et al., 2010). The POMS-SF fatigue-inertia and Vigor-Activity subscale standardized scores were used to examine symptomatic change over time.
Outcome Measure 2: hormonal changes
Salivary testosterone, cortisol, DHEA-S, and estradiol
Participants were required to collect a morning, fasting saliva sample at baseline, Week 8 (end of Phase 1), and Week 16 (end of Phase 2). Participants were instructed to fill the collection tube with saliva (approximately 5 ml) between 6 a.m. and 8 a.m. or within 30 min of rising. They were asked to collect saliva samples before eating, drinking liquids, or brushing teeth. Salivary hormones were tested in duplicate using a fully automated enzyme-linked immunosorbent assay (ELISA) platform.
Statistical Analysis
An independent samples t-test was used to compare demographic variables across the two groups. To examine self-reported symptomatic changes, AMS total score, and standardized subscale scores for the POMS-SF Fatigue-Inertia and Vigor-Activity subscales were analyzed using a 2 × 2 crossover, two-sample t-test (Senn, 2002). There were no significant outliers in data as assessed by the visual inspection of Q-Q plots. Hormonal changes (i.e., salivary cortisol, DHEA-S, testosterone, and estradiol) were analyzed (placebo-ashwagandha and ashwagandha-placebo) for treatment effects using a 2 × 2 crossover, two-sample t-test. Grubbs’ single-outlier test and Rosner’s ESD many-outliers test for response variables were examined for Week 8 and Week 16 (Period 1 and Period 2) and outliers were eliminated. Shapiro–Wilk test was used to test for normality. Carryover effects and period effects were also calculated with no significant effects being observed.
Data from participants were included in analyses if questionnaire data were received on at least two time points across the two treatment phases (intention to treat, with last observation carried forward for missing values). For all the tests, statistical significance was set at p < .05 (two-tailed). All data were analyzed using SPSS (version 24; IBM, Armonk, NY) and NCSS 12.
Results
Demographic Details and Baseline Data
Eighty-two people were screened for participation in the study and 57 met inclusion criteria and were enrolled to participate. Forty-three (75%) participants complied with all necessary treatment requirements (i.e., consumed >80% of capsules, completed self-report inventories on at least two time points across the two treatment phases, and collected salivary samples) over the 16-week trial. Six participants (11%) dropped out of the placebo–ashwagandha condition, and 8 (14%) dropped out of the ashwagandha–placebo condition. There were no significant differences between the dropout rates across treatment groups. Reasons for withdrawal included inconsistent tablet intake (n = 8, 14%), failure to complete questionnaires/collect saliva samples (n = 3, 5%), commencement of new medical treatment (n = 2, 4%), and unexpected overseas trip (n = 1, 2%). No participant withdrew from the study due to self-reported adverse effects from tablet intake.
Demographic characteristics are presented in Table 1 and indicate that the study population was homogeneous, with no statistically significant differences between the groups on baseline demographic characteristics.
Table 1.
Participant Baseline Demographic Characteristics.
| Placebo to ashwagandha (mean and SE) |
Ashwagandha to placebo (mean and SE) |
p value | |
|---|---|---|---|
| Sample size (n) | 29 | 28 | Not applicable |
| Age | 51.66 (1.19) | 50.07 (1.26) | .363a |
| BMI | 27.93 (0.65) | 26.72 (0.55) | .164a |
| Shift workers/remote travel occupations | 6 (21%) | 6 (21%) | .945b |
| Outcome measures | |||
| AMS total score | 38.17 (1.85) | 36.18 (1.46) | .403a |
| POMS Fatigue-Inertia score | 55.83 (1.75) | 52.29 (1.36) | .117a |
| POMS Vigor-Activity score | 41.28 (1.78) | 42.11 (1.81) | .745a |
| Cortisol (nmol/L) | 30.64 (2.87) | 25.54 (1.79) | .137a |
| DHEA-S (nmol/L) | 8.57 (0.79) | 8.96 (1.07) | .772a |
| Testosterone (pmol/L) | 346.56 (27.20) | 354.22 (24.18) | .834a |
| Estradiol (pmol/L) | 35.44 (3.71) | 29.37 (3.55) | .242a |
Note. AMS = Aging Males’ Symptoms; POMS = Profile of Mood States; SE = standard error.
Independent samples t-test. bPearson’s chi-square.
Outcome Measure 1: symptomatic changes
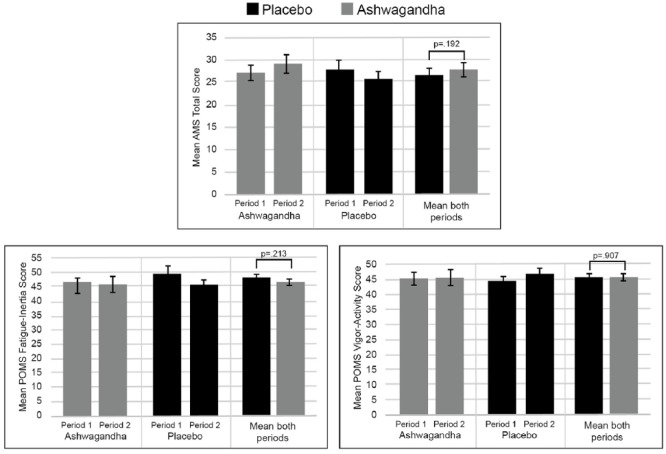
Mean scores in the AMS total score, POMS Fatigue-Inertia subscale score, and POMS Vigor-Activity subscale score during the crossover period for the two treatment groups are detailed in Table 2 and Figure 2. There were nonsignificant between-group differences in AMS total score (T41 = 1.33, p = .192), POMS Fatigue-Inertia subscale score (T41 = −1.26, p = .213), and POMS Vigor-Activity subscale score (T41 = −0.12, p = .907). A within-group, paired-samples t-test for Period 1 of the study demonstrated that there were significant improvements in most symptom scores from baseline to Week 8, in both the placebo (AMS, p = .001; POMS Fatigue-Inertia, p = .001; POMS Vigor-Activity, p = .005) and ashwagandha (AMS, p = .002; POMS Fatigue-Inertia, p = .348; POMS Vigor-Activity, p = .017) conditions.
Table 2.
Symptom Scores After Each Crossover Period.
| Placebo | Ashwagandha | Placebo mean (both periods) | Ashwagandha mean (both periods) | Mean differencea | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Period | ||||||||
| 1 | 2 | 1 | 2 | ||||||
| AMS total score | n | 24 | 19 | 19 | 24 | ||||
| Mean (SE) | 27.79 (1.92) | 25.79 (1.85) | 29.11 (2.34) | 27.21 (1.63) | 26.7 (1.36) | 28.1 (1.39) | 1.37 (1.03) | .192 | |
| POMS Fatigue-Inertia score | n | 23 | 20 | 20 | 23 | ||||
| Mean (SE) | 49.78 (2.26) | 45.6 (1.37) | 46.20 (1.96) | 46.13 (2.34) | 47.72 (1.37) | 46.17 (1.56) | –1.55 (1.23) | .213 | |
| POMS Vigor-Activity score | n | 23 | 20 | 20 | 23 | ||||
| Mean (SE) | 44.13 (2.32) | 46.65 (1.91) | 45.10 (2.20) | 45.39 (2.49) | 45.39 (1.53) | 45.25 (1.68) | –0.15 (1.24) | .907 | |
Note. AMS = Aging Males’ Symptoms; POMS = Profile of Mood States; SE = standard error.
Treatment effect: mean score during ashwagandha period minus mean score during the placebo period.
Figure 2.
Mean symptom scores after each crossover period.
Outcome Measure 2: hormonal changes
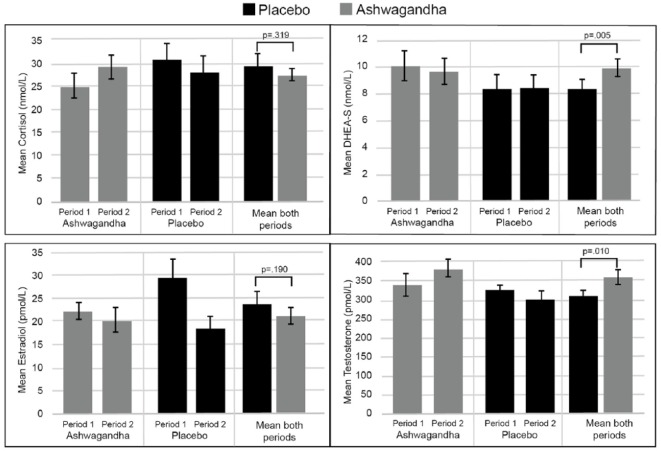
Mean salivary hormone levels during each crossover period are detailed in Table 3 and Figure 3. The 2 × 2 crossover, two-sample t-test confirmed significantly higher levels of DHEA-S (T37 = 2.97, p = .005) and testosterone (T36 = 2.74, p = .010) during ashwagandha intake, compared to placebo intake (18.0% and 14.7%, respectively). Nonsignificant decreases in cortisol (T38 = −1.01, p = .319) and estradiol (T38 = −1.34, p = .189) were found during ashwagandha intake, compared to placebo intake (7.8% and 11.6% lower, respectively).
Table 3.
Hormonal Scores After Each Crossover Period.
| Placebo | Ashwagandha | Placebo mean (both periods) | Ashwagandha mean (both periods) | Mean differencea | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Period | ||||||||
| 1 | 2 | 1 | 2 | ||||||
| Cortisol (nmol/L) | n | 21 | 19 | 19 | 21 | ||||
| Mean (SE) | 30.95 (3.19) | 27.9 (3.78) | 25.06 (2.72) | 29.2 (2.66) | 29.42 (2.46) | 27.13 (1.90) | –2.29 (2.27) | .319 | |
| DHEA-S (nmol/L) | n | 21 | 19 | 19 | 21 | ||||
| Mean (SE) | 8.3 (0.98) | 8.24 (1.12) | 9.98 (1.18) | 9.54 (1.00) | 8.27 (0.74) | 9.76 (0.77) | 1.49 (0.50) | .005 | |
| Testosterone (pmol/L) | n | 21 | 19 | 19 | 21 | ||||
| Mean (SE) | 324.57 (18.44) | 295.41 (25.25) | 332.77 (35.59) | 378.38 (27.22) | 309.99 (15.29) | 355.57 (22.02) | 45.58 (16.64) | .01 | |
| Estradiol (pmol/L) | n | 21 | 19 | 19 | 21 | ||||
| Mean (SE) | 29.52 (4.37) | 18.38 (2.91) | 22.21 (2.28) | 20.14 (3.06) | 23.95 (2.68) | 21.18 (1.94) | –2.78 (2.08) | .19 | |
Note. SE = standard error.
Treatment effect: mean score during ashwagandha period minus mean score during placebo period.
Figure 3.
Mean hormonal scores after each crossover period.
To examine the durability of increases in DHEA-S and testosterone from ashwagandha supplementation, mean levels at Period 1 (ashwagandha) can be compared to Period 2 (placebo). As demonstrated in Table 3, mean DHEA-S levels dropped from 9.98 nmol/L to 8.24 nmol/L and mean testosterone levels dropped from 332.77 pmol/L to 295.41 pmol/L. Although the sample size available was small (n = 19), the results of a paired-samples t-test confirmed that the reduction in DHEA-S was statistically significant (T17 = 2.28, p = .035), and there was a tendency to suggest testosterone levels were not sustained (T16 = 1.34, p = .198). This indicates that the effects of ashwagandha supplementation on DHEA-S and testosterone were not sustained 8 weeks later.
Adverse Events and Treatment Compliance
At Weeks 4, 8, 12, and 16, participants were asked to list any adverse effects, symptoms, or illnesses experienced during the study period (whether they believed it was associated with tablet intake or not). Ashwagandha was well tolerated with no significant differences in reported adverse events between placebo and active drug treatment groups. Compliance with tablet intake was also high, as 86% of participants consumed greater than 80% of allocated tablets (as measured by self-reported tablet number at Weeks 4, 8, 12, and 16).
Efficacy of Participant Blinding
To evaluate the efficacy of condition concealment over the study, participants were asked at the completion of each phase of the study to predict condition allocation (i.e., placebo, ashwagandha, or uncertain). Efficacy of group concealment was high as only 35% of participants correctly guessed treatment allocation, 30% of participants were uncertain of treatment allocation, and the remaining 35% incorrectly guessed group allocation.
Discussion
In this 16-week, randomized, double-blind, crossover study, the 8-week intake of an ashwagandha extract (Shoden beads, delivering 21 mg of withanolide glycosides) was associated with significant changes in salivary DHEA-S and testosterone in healthy, overweight males aged between 40 and 70 years reporting mild-to-moderate symptoms of fatigue or reduced vitality. No statistically significant change in salivary cortisol or estradiol was observed. Improvements in fatigue, vigor, and sexual and psychological well-being were observed after both ashwagandha and placebo supplementation with no statistically significant group differences. Ashwagandha supplementation was well tolerated with no reported adverse events or participant withdrawal associated with its intake.
The mood-lifting and antianxiety effects of ashwagandha have been investigated in several studies, confirming its positive antianxiety effects in stressed, healthy adults (Auddy et al., 2008; Chandrasekhar et al., 2012); stressed, overweight adults (Choudhary, Bhattacharyya, & Joshi, 2017); and adults with a diagnosed anxiety disorder (Andrade, Aswath, Chaturvedi, Srinivasa, & Raguram, 2000; Khyati & Anup, 2013). Anti-stress and antidepressant effects of ashwagandha have also been observed in animal studies comprised of unpredictable foot shock, elevated plus-maze test, and the forced swim test (Bhattacharya, Bhattacharya, Sairam, & Ghosal, 2000; Bhattacharya & Muruganandam, 2003). Although an area of lesser focus, ashwagandha supplementation also has demonstrated positive effects on energy and fatigue as evidenced by greater exercise-induced muscle recovery in adults undergoing resistance training (Wankhede, Langade, Joshi, Sinha, & Bhattacharyya, 2015) and breast cancer patients undergoing chemotherapy (Biswal, Sulaiman, Ismail, Zakaria, & Musa, 2013).
In the current study, significant improvements over time in levels of vigor and emotional or sexual well-being from ashwagandha supplementation were identified; however, this was not significantly different to the placebo. This suggests that in the population examined (i.e., healthy, overweight males aged 40–70 years reporting mild-to-moderate symptoms of fatigue or reduced vitality), ashwagandha had no advantage over a placebo in alleviating fatigue, increasing vigor, or enhancing sexual vitality. There are several potential explanations for these findings. Average pretreatment, self-reported levels of fatigue, vigor, and sexual well-being were of only mild intensity. This increases the likelihood of floor effects, and as only moderate improvements are likely, larger sample sizes would be required to identify statistically significant differences. Nonsignificant between-group differences may also be associated with the method of participant recruitment. Volunteers were recruited via social media, and this likely included individuals motivated to improve their general well-being. While participants were encouraged to not engage in any dietary, exercise, or lifestyle changes during the study, it is possible that these motivated individuals may have implemented changes that had positive effects on their energy, vigor, and general well-being (e.g., changes in work, relationships, diet, and physical activity). Unfortunately, monitoring of changes in these areas over the study duration was not undertaken. In addition, a large portion of participants (approximately 20%) were engaged in shift work and/or were employed in occupations requiring travel to remote, mining communities. This may moderate the therapeutic efficacy of ashwagandha due to ongoing sleep-related disturbances and stresses associated with living away from home. Research confirms that shift work is associated with a greater need for recovery and a higher risk of disability (Gommans, Jansen, Stynen, de Grip, & Kant, 2015). This hypothesis was supported in the current study, as a post hoc analysis of trial data revealed an average 4.8% improvement in fatigue during the first phase of the study in shift/mine workers compared to a larger improvement of 12.6% in the remaining participants. Undertaking statistical analyses by excluding shift workers was considered inappropriate due to the small sample and increased potential for type I errors. The nonsignificant findings may also result from the high observed placebo effect (e.g., AMS total score reduction of 25% in Period 1 of the study). This is significantly higher than effects observed in most previously published studies on ashwagandha. This suggests greater treatment expectations in the recruited population of Australian participants. This may be culturally influenced, as previous studies have mostly been conducted on Indian populations.
In the current study, an examination of hormonal changes over time demonstrated that ashwagandha supplementation over an 8-week period was associated with 15% higher levels of salivary testosterone and 18% higher levels of DHEA-S compared to placebo. However, there were no statistically significant differences in levels of salivary cortisol or estradiol compared to the placebo. The effects of ashwagandha in increasing levels of testosterone have been demonstrated in men undergoing resistance training (Wankhede et al., 2015), oligospermic males (Ambiye et al., 2013), and infertile men (Gupta et al., 2013). Increases in DHEA-S from ashwagandha supplementation were also identified in chronically stressed males (Auddy et al., 2008). In animal studies, ashwagandha has been consistently demonstrated to have a lowering effect on corticosterone concentrations (Baitharu et al., 2013; Bhatnagar, Sharma, & Salvi, 2009; Bhattacharya & Muruganandam, 2003). Animal studies investigating its effects on sex hormones are limited, although there are preliminary supportive findings (Abdel-Magied, Abdel-Rahman, & Harraz, 2001; Rahmati et al., 2016).
Findings from the current study are consistent with previous findings, as significantly higher levels of testosterone and DHEA-S were identified from ashwagandha supplementation compared to the placebo. It seems that these increases are not sustained over time, as DHEA-S, and to a lesser extent testosterone, was lower after 8 weeks of discontinued ashwagandha supplementation. This suggests that ongoing ashwagandha intake is required to sustain changes, or longer intake is required for more enduring changes. The effects of DHEA-S and testosterone in aging males have important health implications as lower levels are associated with increased morbidity, reduced longevity, and lower quality of life (Enomoto et al., 2008; Khera, 2016; Maggi et al., 2007; Nagaya, Kondo, & Okinaka, 2012).
Although ashwagandha was associated with increases in DHEA-S and testosterone, no statistically significant effects on morning salivary cortisol levels (a nonsignificant 7.8% lower level compared to placebo) or estradiol concentrations (a nonsignificant 11.6% lower level compared to placebo) were identified. The effect of ashwagandha on estradiol has not been previously investigated, although there are several studies confirming its cortisol-lowering effects in adults. The current findings, therefore, contrast with the results from studies confirming ashwagandha’s cortisol-reducing influence in chronically stressed adults (Auddy et al., 2008), adults with an anxiety disorder (Chandrasekhar et al., 2012), and overweight, stressed adults (Choudhary, Bhattacharyya, & Joshi, 2017). These findings suggest that ashwagandha was ineffective at lowering cortisol levels in the examined male population. However, in contrast to previous studies, salivary cortisol was measured rather than serum. Moreover, as mentioned previously, a significant portion of the recruited population (approximately 20%) were shift and mine workers. There is research confirming a strong influence of shift work on diurnal cortisol concentrations (Li et al., 2018; Ulhôa, Marqueze, Burgos, & Moreno, 2015) and there is some evidence, albeit inconsistent, suggesting a lowering effect on testosterone concentrations (Deng, Haney, Kohn, Pastuszak, & Lipshultz, 2018). Recruiting shift workers may have therefore moderated the potential effects of ashwagandha on cortisol and other steroid hormones. In addition, contrary to previous investigations on ashwagandha, a stressed or anxious population, where higher cortisol levels are commonly observed, was not specifically recruited. Rather, a population with self-reported fatigue was recruited. There is evidence to suggest lower cortisol concentrations in people with symptoms of burnout (Lennartsson, Sjors, Wahrborg, Ljung, & Jonsdottir, 2015) and chronic fatigue syndrome (Nijhof et al., 2014). Consequently, if the recruited sample presented with premorbid low cortisol levels, further reductions would unlikely occur. This hypothesis requires investigation in future studies.
Understanding the mechanisms associated with ashwagandha’s influence on DHEA and testosterone production requires further investigation although there are several plausible mechanisms. DHEA is produced in the zona reticularis of the adrenal cortex and is influenced by activity of the hypothalamus–pituitary–adrenal (HPA) axis, particularly circulating levels of adrenocorticotropic hormone (ACTH; Klinge, Clark, & Prough, 2018). As already discussed, in several studies ashwagandha lowered cortisol concentrations, suggesting a dampening effect on HPA axis activity. Potentially via ashwagandha’s influence on HPA axis activity, DHEA and testosterone concentrations may be elevated. However, this mechanism was not confirmed in the current study as ashwagandha had no appreciable effect on cortisol concentrations. DHEA and testosterone are also produced by Leydig cells in the testes, which are influenced by gonadotropin-releasing hormone (GnRH). It has been demonstrated through in vitro and animal studies that ashwagandha upregulates the activity of GnRH (Al-Qarawi et al., 2000; Kataria, Gupta, Lakhman, & Kaur, 2015). Therefore, through its effect on GnRH activity, increases in DHEA and testosterone concentrations from ashwagandha intake may occur. A bidirectional relationship between inflammation, oxidative stress, and androgen hormones such as testosterone has also been regularly observed in animal and human studies (Mohamad et al., 2018; Tostes, Carneiro, Carvalho, & Reckelhoff, 2016; A. Traish, Bolanos, Nair, Saad, & Morgentaler, 2018; Wang, Chen, Ye, Zirkin, & Chen, 2017). Ashwagandha has demonstrated antioxidant and anti-inflammatory activity, thereby potentially contributing to its influence on DHEA and testosterone (Ganguly, Kumar, Ahmad, & Rastogi, 2018; Mishra, Singh, & Dagenais, 2000). Finally, it is important to note that although there was no statistically significant change in estradiol concentrations from ashwagandha supplementation, there was a trend signifying reduced levels. This contrasts with the increased levels of estradiol prohormones, DHEA-S and testosterone. Elevated estradiol levels would, therefore, be expected. As this did not occur, it is hypothesized that ashwagandha may be an inhibitor of aromatase, an enzyme required for the conversion of testosterone into estradiol. The aromatase-inhibiting effect of a plant from the same Solanaceae family, Withania coagulans, was identified in one study (Haq et al., 2013). W. coagulans, like ashwagandha, contains high concentrations of withanolides. This very speculative observation requires investigation in future studies.
Study Limitations and Directions for Future Research
There are several limitations associated with this study that may have impacted on the findings and require consideration in future research. The sample size recruited was small, making it difficult to develop definitive conclusions. Future studies should, therefore, involve larger samples. Participants were recruited via social media, which may have biased the findings. It is expected that such individuals comprise a motivated cohort of volunteers. Consequently, in addition to participating in a study and taking supplements, these individuals may have made dietary, occupational, lifestyle, and/or social changes that contributed to the fatigue-lowering and vigor-enhancing changes observed in both treatment conditions. While participants were encouraged to maintain regular lifestyle habits during the study, this was not adequately monitored. It may be beneficial in future studies to monitor such changes (e.g., diet, exercise, weight, life stressors) through questionnaires and other monitoring instruments. It is also important to consider the potential impact of participant expectations. This is particularly important when there are strong advertising campaigns promoting the virtues of a product or drug. In the sample of Australian participants recruited in this study, most reported limited knowledge about ashwagandha and its benefits. However, this will require consideration in countries such as India and increasingly in the United States, where knowledge about ashwagandha by health-conscious individuals is increasing.
To increase compliance and reduce the demands on participants, hormonal concentrations were evaluated via fasting salivary collections rather than in serum. It has been confirmed in several studies that salivary measurements of testosterone, DHEA-S, cortisol, and estradiol correlate well with serum levels (Arregger, Contreras, Tumilasci, Aquilano, & Cardoso, 2007; Francavilla et al., 2018; Lood et al., 2018). However, their accuracy as an outcome measure can be compromised by their rapid diurnal variability (Wood, 2009). Although participants were instructed to collect saliva samples within 15 min of waking, and in a fasting state, sample collection times could not be adequately monitored as they were undertaken in participants’ homes. Inconsistency in such collections will have a negative impact on the accuracy and reliability of measurements. In future studies, to enhance the robustness of findings, it would be important to control for or accurately monitor such variables. Measuring hormonal concentrations in both saliva and serum may also be advantageous. Measurement methods and standardization must also be considered due to increasing concerns around the accuracy and variability associated with testosterone measurements (Vesper & Botelho, 2010).
As previously noted, approximately 20% of volunteers were either shift or mine workers. Given the limited sample size, subgroup analyses could not be adequately undertaken to examine the effect of shift work on measured outcomes. However, there is research confirming the adverse effects of shift work on general well-being, morbidity, and hormonal concentrations such as cortisol and testosterone. It will be important in future studies to control for the recruitment of shift workers and/or to specifically examine the effects of ashwagandha in this group of individuals. Given their variable sleep and daily routines, an examination into the impact of variable dosing and timing may also be helpful.
Increases in steroid hormones such as testosterone and DHEA-S in men are commonly considered to have positive health-enhancing effects. However, this may not always be the case. For example, there is evidence to suggest that testosterone replacement therapy may be associated with adverse effects in men with high cardiovascular risk, preexisting prostate disease, and sleep apnea (Bhasin et al., 2010; Grossmann, 2011; Snyder et al., 2016). While these risks are specifically associated with testosterone replacement therapy (and continue to be debated particularly in relation to cardiovascular risk), the safety of ashwagandha in such populations requires further consideration and evaluation. However, serious adverse effects seem unlikely with ashwagandha, as a moderate 15% increase in testosterone level, which remained within normal endogenous concentrations, was observed in this study. Previous studies have also confirmed that ashwagandha is not associated with withdrawal effects and has not been associated with abuse (Durg et al., 2018; Pratte et al., 2014). In the current study, ashwagandha intake was not associated with any significant adverse events and was well tolerated by participants. This strong safety profile is supported by previously conducted studies where systematic reviews confirmed that ashwagandha intake was not associated with significant adverse reactions in stressed and anxious adults (Pratte et al., 2014) or infertile males (Durg et al., 2018). However, most studies have been of short duration, so safety and efficacy over longer term administration are required.
Finally, in this study, we used a patented ashwagandha extract, Shoden beads, standardized to withanolide glycosides. This extract is manufactured in a GMP-certified facility (Arjuna Natural Ltd.). The quality of herbal extracts and the manufacturing practices used can vary significantly, likely impacting on therapeutic potency. Therefore, generalizing the findings from this study to alternative ashwagandha extracts should be done cautiously.
In conclusion, the findings from this study demonstrated that 8 weeks of supplementation with an ashwagandha extract (Shoden beads, 600 mg daily delivering 21-mg withanolide glycoside) was associated with significant improvements in salivary DHEA-S and testosterone, but not cortisol and estradiol, in healthy males aged between 40 and 70 years. Furthermore, supplementation had no significant effect on symptoms of fatigue, vigor, or sexual or psychological well-being. The robustness and generalizability of the findings are moderated by the small sample size and the high number of shift and mine workers recruited. Future investigations utilizing larger sample sizes, varying treatment doses and duration, and divergent study populations, including both males and females, are necessary to further understand the potential therapeutic and adaptogenic effects of ashwagandha.
Acknowledgments
The authors gratefully acknowledge Arjuna Natural Ltd. for funding the project and supplying Shoden® for use in this study.
Footnotes
Author Contributions: AL, PD, and SS contributed to the study design, data collection, writing, statistical analyses, and data interpretation of the clinical trial of the present research. All the authors read and approved the final draft of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Arjuna Natural Ltd. was not involved in the design of the research, analysis of data, or in the writing of the report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Arjuna Natural Ltd.
ORCID iD: Adrian L. Lopresti  https://orcid.org/0000-0002-6409-7839
https://orcid.org/0000-0002-6409-7839
References
- Abdel-Magied E. M., Abdel-Rahman H. A., Harraz F. M. (2001). The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. Journal of Ethnopharmacology, 75(1), 1–4. [DOI] [PubMed] [Google Scholar]
- Al-Qarawi A. A., Abdel-Rahman H. A., El-Badry A. A., Harraz F., Razig N. A., Abdel-Magied E. M. (2000). The effect of extracts of Cynomorium coccineum and Withania somnifera on gonadotrophins and ovarian follicles of immature Wistar rats. Phytotherapy Research, 14(4), 288–290. [DOI] [PubMed] [Google Scholar]
- Ambiye V. R., Langade D., Dongre S., Aptikar P., Kulkarni M., Dongre A. (2013). Clinical evaluation of the spermatogenic activity of the root extract of ashwagandha (Withania somnifera) in oligospermic males: A pilot study. Evidence-Based Complementary and Alternative Medicine, 2013, 1–6. doi: 10.1155/2013/571420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C., Aswath A., Chaturvedi S. K., Srinivasa M., Raguram R. (2000). A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian Journal of Psychiatry, 42(3), 295–301. [PMC free article] [PubMed] [Google Scholar]
- Antoni M. H. (2003). Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: Empirical support for a psychoneuroimmunological model. Stress, 6(3), 173–188. doi: 10.1080/1025389031000156727 [DOI] [PubMed] [Google Scholar]
- Appels A., Mulder P. (1988). Excess fatigue as a precursor of myocardial infarction. European Heart Journal, 9(7), 758–764. [DOI] [PubMed] [Google Scholar]
- Arregger A. L., Contreras L. N., Tumilasci O. R., Aquilano D. R., Cardoso E. M. (2007). Salivary testosterone: A reliable approach to the diagnosis of male hypogonadism. Clinical Endocrinology, 67(5), 656–662. doi: 10.1111/j.1365-2265.2007.02937.x [DOI] [PubMed] [Google Scholar]
- Auddy B., Hazra J., Mitra A., Abedon B., Ghosal S. (2008). A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: A double-blind, randomized, placebo-controlled study. Journal of the American Nutraceutical Association, 11, 50–56. [Google Scholar]
- Avlund K. (2010). Fatigue in older adults: An early indicator of the aging process? Aging Clinical and Experimental Research, 22(2), 100–115. [DOI] [PubMed] [Google Scholar]
- Avlund K., Schultz-Larsen K., Davidsen M. (1998). Tiredness in daily activities at age 70 as a predictor of mortality during the next 10 years. Journal of Clinical Epidemiology, 51(4), 323–333. [DOI] [PubMed] [Google Scholar]
- Azgomi R. N. D., Zomorrodi A., Nazemyieh H., Fazljou S. M. B., Bazargani H. S., Nejatbakhsh F., … AsrBadr Y. A. (2018). Effects of Withania somnifera on reproductive system: A systematic review of the available evidence. BioMed Research International, Article ID 4076430, 17 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitharu I., Jain V., Deep S. N., Hota K. B., Hota S. K., Prasad D., Ilavazhagan G. (2013). Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. Journal of Ethnopharmacology, 145(2), 431–441. doi: 10.1016/j.jep.2012.10.063 [DOI] [PubMed] [Google Scholar]
- Beute M. E., Wiltink J., Schwarz R., Weidner W., Brahler E. (2002). Complaints of the ageing male based on a representative community study. European Urology, 41(1), 85–92, discussion 92–83. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Cunningham G. R., Hayes F. J., Matsumoto A. M., Snyder P. J., Swerdloff R. S., Montori V. M. (2010). Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 95(6), 2536–2559. doi: 10.1210/jc.2009-2354 [DOI] [PubMed] [Google Scholar]
- Bhatnagar M., Sharma D., Salvi M. (2009). Neuroprotective effects of Withania somnifera Dunal: A possible mechanism. Neurochemical Research, 34(11), 1975–1983. doi: 10.1007/s11064-009-9987-7 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Bhattacharya A., Sairam K., Ghosal S. (2000). Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: An experimental study. Phytomedicine, 7(6), 463–469. doi: 10.1016/S0944-7113(00)80030-6 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Muruganandam A. V. (2003). Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacology Biochemistry and Behavior, 75(3), 547–555. [DOI] [PubMed] [Google Scholar]
- Biswal B. M., Sulaiman S. A., Ismail H. C., Zakaria H., Musa K. I. (2013). Effect of Withania somnifera (ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integrative Cancer Therapies, 12(4), 312–322. doi: 10.1177/1534735412464551 [DOI] [PubMed] [Google Scholar]
- Cathebras P. J., Robbins J. M., Kirmayer L. J., Hayton B. C. (1992). Fatigue in primary care: Prevalence, psychiatric comorbidity, illness behavior, and outcome. Journal of General Internal Medicine, 7(3), 276–286. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar K., Kapoor J., Anishetty S. (2012). A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian Journal of Psychological Medicine, 34(3), 255–262. doi: 10.4103/0253-7176.106022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D., Bhattacharyya S., Bose S. (2017). Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. Journal of Dietary Supplements, 14(6), 599–612. doi: 10.1080/19390211.2017.1284970 [DOI] [PubMed] [Google Scholar]
- Choudhary D., Bhattacharyya S., Joshi K. (2017). Body weight management in adults under chronic stress through treatment with ashwagandha root extract: A double-blind, randomized, placebo-controlled trial. Evidence-Based Complementary and Alternative Medicine, 22(1), 96–106. doi: 10.1177/2156587216641830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collomp K., Baillot A., Forget H., Coquerel A., Rieth N., Vibarel-Rebot N. (2016). Altered diurnal pattern of steroid hormones in relation to various behaviors, external factors and pathologies: A review. Physiology & Behavior, 164(Pt A), 68–85. doi: 10.1016/j.physbeh.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Cooke P. S., Nanjappa M. K., Ko C., Prins G. S., Hess R. A. (2017). Estrogens in male physiology. Physiological Reviews, 97(3), 995–1043. doi: 10.1152/physrev.00018.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G., Rastrelli G., Monami M., Guay A., Buvat J., Sforza A., … Maggi M. (2011). Hypogonadism as a risk factor for cardiovascular mortality in men: A meta-analytic study. European Journal of Endocrinology, 165(5), 687–701. doi: 10.1530/EJE-11-0447 [DOI] [PubMed] [Google Scholar]
- Daig I., Heinemann L. A. J., Kim S., Leungwattanakij S., Badia X., Myon E., … Thai D. M. (2003). The Aging Males’ Symptoms (AMS) scale: Review of its methodological characteristics. Health and Quality of Life Outcomes, 1, 77. doi: 10.1186/1477-7525-1-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng N., Haney N. M., Kohn T. P., Pastuszak A. W., Lipshultz L. I. (2018). The effect of shift work on urogenital disease: A systematic review. Current Urology Reports, 19(8), 57. doi: 10.1007/s11934-018-0815-y [DOI] [PubMed] [Google Scholar]
- Di Vincenzo A., Busetto L., Vettor R., Rossato M. (2018). Obesity, male reproductive function and bariatric surgery. Frontiers in Endocrinology, 9, 769. doi: 10.3389/fendo.2018.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durg S., Shivaram S. B., Bavage S. (2018). Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine, 50, 247–256. doi: 10.1016/j.phymed.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Enomoto M., Adachi H., Fukami A., Furuki K., Satoh A., Otsuka M., … Imaizumi T. (2008). Serum dehydroepiandrosterone sulfate levels predict longevity in men: 27-year follow-up study in a community-based cohort (Tanushimaru Study). Journal of the American Geriatrics Society, 56(6), 994–998. doi: 10.1111/j.1532-5415.2008.01692.x [DOI] [PubMed] [Google Scholar]
- Feldman H. A., Longcope C., Derby C. A., Johannes C. B., Araujo A. B., Coviello A. D., … McKinlay J. B. (2002). Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts Male Aging Study. The Journal of Clinical Endocrinology & Metabolism, 87(2), 589–598. doi: 10.1210/jcem.87.2.8201 [DOI] [PubMed] [Google Scholar]
- Fink A. M., Eckhardt A. L., Fennessy M. M., Jones J., Kruse D., VanderZwan K. J., … Zerwic J. J. (2010). Psychometric properties of three instruments to measure fatigue with myocardial infarction. Western Journal of Nursing Research, 32(7), 967–983. doi: 10.1177/0193945910371320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla V. C., Vitale F., Ciaccio M., Bongiovanni T., Marotta C., Caldarella R., … Mazzucco W. (2018). Use of saliva in alternative to serum sampling to monitor biomarkers modifications in professional soccer players. Frontiers in Physiology, 9, 1828. doi: 10.3389/fphys.2018.01828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly B., Kumar N., Ahmad A. H., Rastogi S. K. (2018). Influence of phytochemical composition on in vitro antioxidant and reducing activities of Indian ginseng [Withania somnifera (L.) Dunal] root extracts. Journal of Ginseng Research, 42(4), 463–469. doi: 10.1016/j.jgr.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommans F., Jansen N., Stynen D., de Grip A., Kant I. (2015). The ageing shift worker: A prospective cohort study on need for recovery, disability, and retirement intentions. Scandinavian Journal of Work, Environment & Health, 41(4), 356–367. doi: 10.5271/sjweh.3497 [DOI] [PubMed] [Google Scholar]
- Grossmann M. (2011). Low testosterone in men with type 2 diabetes: Significance and treatment. The Journal of Clinical Endocrinology & Metabolism, 96(8), 2341–2353. doi: 10.1210/jc.2011-0118 [DOI] [PubMed] [Google Scholar]
- Gupta A., Mahdi A. A., Shukla K. K., Ahmad M. K., Bansal N., Sankhwar P., Sankhwar S. N. (2013). Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. Journal of Ethnopharmacology, 149(1), 208–214. doi: 10.1016/j.jep.2013.06.024 [DOI] [PubMed] [Google Scholar]
- Haq I., Mirza B., Kondratyuk T. P., Park E. J., Burns B. E., Marler L. E., Pezzuto J. M. (2013). Preliminary evaluation for cancer chemopreventive and cytotoxic potential of naturally growing ethnobotanically selected plants of Pakistan. Pharmaceutical Biology, 51(3), 316–328. doi: 10.3109/13880209.2012.728612 [DOI] [PubMed] [Google Scholar]
- Heuchert J. P., McNair D. M. (2012). POMS-2 manual. North Tonawanda, NY: MHS. [Google Scholar]
- Hickie I. B., Hooker A. W., Hadzi-Pavlovic D., Bennett B. K., Wilson A. J., Lloyd A. R. (1996). Fatigue in selected primary care settings: Sociodemographic and psychiatric correlates. The Medical Journal of Australia, 164(10), 585–588. [DOI] [PubMed] [Google Scholar]
- Joshi D., van Schoor N. M., de Ronde W., Schaap L. A., Comijs H. C., Beekman A. T., Lips P. (2010). Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clinical Endocrinology, 72(2), 232–240. doi: 10.1111/j.1365-2265.2009.03641.x [DOI] [PubMed] [Google Scholar]
- Kataria H., Gupta M., Lakhman S., Kaur G. (2015). Withania somnifera aqueous extract facilitates the expression and release of GnRH: In vitro and in vivo study. Neurochemistry International, 89, 111–119. doi: 10.1016/j.neuint.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Kelly D. M., Jones T. H. (2013). Testosterone: A metabolic hormone in health and disease. Journal of Endocrinology, 217(3), R25–R45. doi: 10.1530/JOE-12-0455 [DOI] [PubMed] [Google Scholar]
- Khera M. (2016). Male hormones and men’s quality of life. Current Opinion in Urology, 26(2), 152–157. doi: 10.1097/MOU.0000000000000256 [DOI] [PubMed] [Google Scholar]
- Khyati S., Anup B. (2013). A randomized double blind placebo controlled study of ashwagandha on generalized anxiety disorder. International Ayurvedic Medical Journal, 1, 1–7. [Google Scholar]
- Klinge C. M., Clark B. J., Prough R. A. (2018). Dehydroepiandrosterone research: Past, current, and future. Vitamins and Hormones, 108, 1–28. doi: 10.1016/bs.vh.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Kulkarni S. K., Dhir A. (2008). Withania somnifera: An Indian ginseng. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 32(5), 1093–1105. doi: 10.1016/j.pnpbp.2007.09.011 [DOI] [PubMed] [Google Scholar]
- Lennartsson A.-K., Sjors A., Wahrborg P., Ljung T., Jonsdottir I. H. (2015). Burnout and hypocortisolism – a matter of severity? A study on ACTH and cortisol responses to acute psychosocial stress. Frontiers in Psychiatry, 6, 8. doi: 10.3389/fpsyt.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerdal A., Wahl A., Rustoen T., Hanestad B. R., Moum T. (2005). Fatigue in the general population: A translation and test of the psychometric properties of the Norwegian version of the Fatigue Severity Scale. Scandinavian Journal of Public Health, 33(2), 123–130. doi: 10.1080/14034940410028406 [DOI] [PubMed] [Google Scholar]
- Li J., Bidlingmaier M., Petru R., Pedrosa Gil F., Loerbroks A., Angerer P. (2018). Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. Journal of Occupational Medicine and Toxicology, 13, 23. doi: 10.1186/s12995-018-0204-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loge J. H., Ekeberg O., Kaasa S. (1998). Fatigue in the general Norwegian population: Normative data and associations. Journal of Psychosomatic Research, 45(1), 53–65. [DOI] [PubMed] [Google Scholar]
- Lood Y., Aardal-Eriksson E., Webe C., Ahlner J., Ekman B., Wahlberg J. (2018). Relationship between testosterone in serum, saliva and urine during treatment with intramuscular testosterone undecanoate in gender dysphoria and male hypogonadism. Andrology, 6(1), 86–93. doi: 10.1111/andr.12435 [DOI] [PubMed] [Google Scholar]
- Maggi M., Schulman C., Quinton R., Langham S., Uhl-Hochgraeber K. (2007). The burden of testosterone deficiency syndrome in adult men: Economic and quality-of-life impact. The Journal of Sexual Medicine, 4(4 Pt 1), 1056–1069. doi: 10.1111/j.1743-6109.2007.00531.x [DOI] [PubMed] [Google Scholar]
- Mishra L. C., Singh B. B., Dagenais S. (2000). Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Alternative Medicine Review, 5(4), 334–346. [PubMed] [Google Scholar]
- Mohamad N. V., Wong S. K., Wan Hasan W. N., Jolly J. J., Nur-Farhana M. F., Ima-Nirwana S., Chin K. Y. (2018). The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male, 1–12. doi: 10.1080/13685538.2018.1482487 [DOI] [PubMed] [Google Scholar]
- Nagaya T., Kondo Y., Okinaka T. (2012). Serum dehydroepiandrosterone-sulfate reflects age better than health status, and may increase with cigarette smoking and alcohol drinking in middle-aged men. Aging Clinical and Experimental Research, 24(2), 134–138. [DOI] [PubMed] [Google Scholar]
- Nijhof S. L., Rutten J. M., Uiterwaal C. S., Bleijenberg G., Kimpen J. L., Putte E. M. (2014). The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology, 42, 199–206. doi: 10.1016/j.psyneuen.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Lambert L. C., Marshall L. M., Phipps K., Blank J., Barrett-Connor E., … Cummings S. (2006). Testosterone and estradiol among older men. The Journal of Clinical Endocrinology & Metabolism, 91(4), 1336–1344. doi: 10.1210/jc.2005-1830 [DOI] [PubMed] [Google Scholar]
- Panossian A., Wikman G. (2009). Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Current Clinical Pharmacology, 4(3), 198–219. [DOI] [PubMed] [Google Scholar]
- Panossian A., Wikman G. (2010). Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals (Basel), 3(1), 188–224. doi: 10.3390/ph3010188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratte M. A., Nanavati K. B., Young V., Morley C. P. (2014). An alternative treatment for anxiety: A systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). Journal of Alternative and Complementary Medicine, 20(12), 901–908. doi: 10.1089/acm.2014.0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati B., Ghosian Moghaddam M. H., Khalili M., Enayati E., Maleki M., Rezaeei S. (2016). Effect of Withania somnifera (L.) Dunal on sex hormone and gonadotropin levels in addicted male rats. International Journal of Fertility and Sterility, 10(2), 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes V. A., Watson P. M., Hanson B. M. (1988). Patients’ descriptions of the influence of tiredness and weakness on self-care abilities. Cancer Nursing, 11(3), 186–194. [PubMed] [Google Scholar]
- Rutkowski K., Sowa P., Rutkowska-Talipska J., Kuryliszyn-Moskal A., Rutkowski R. (2014). Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs, 74(11), 1195–1207. doi: 10.1007/s40265-014-0259-8 [DOI] [PubMed] [Google Scholar]
- Saad F., Hoesl C. E., Oettel M., Fauteck J. D., Rommler A. (2005). Dehydroepiandrosterone treatment in the aging male – What should the urologist know? European Urology, 48(5), 724–733, discussion 733. doi: 10.1016/j.eururo.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Schulster M., Bernie A. M., Ramasamy R. (2016). The role of estradiol in male reproductive function. Asian Journal of Andrology, 18(3), 435–440. doi: 10.4103/1008-682X.173932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P., Agarwal A., Pogrebetskaya M., Roychoudhury S., Durairajanayagam D., Henkel R. (2018). Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reproductive BioMedicine, 36(3), 311–326. doi: 10.1016/j.rbmo.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Senn S. (2002). Cross-over trials in clinical research (2nd ed.). West Sussex, England: John Wiley & Sons, Ltd. [Google Scholar]
- Snyder P. J., Bhasin S., Cunningham G. R., Matsumoto A. M., Stephens-Shields A. J., Cauley J. A., … Ellenberg S. S. (2016). Effects of testosterone treatment in older men. The New England Journal of Medicine, 374(7), 611–624. doi: 10.1056/NEJMoa1506119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanworth R. D., Jones T. H. (2008). Testosterone for the aging male; current evidence and recommended practice. Clinical Interventions in Aging, 3(1), 25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C., Barrett-Connor E., Laughlin G. A., Platt R. W. (2004). A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: The Rancho Bernardo Study. European Journal of Endocrinology, 151(6), 717–725. [DOI] [PubMed] [Google Scholar]
- Tostes R. C., Carneiro F. S., Carvalho M. H., Reckelhoff J. F. (2016). Reactive oxygen species: Players in the cardiovascular effects of testosterone. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 310(1), R1–R14. doi: 10.1152/ajpregu.00392.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A., Bolanos J., Nair S., Saad F., Morgentaler A. (2018). Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. Journal of Clinical Medicine, 7(12), 549. doi: 10.3390/jcm7120549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A. M., Zitzmann M. (2015). The complex and multifactorial relationship between testosterone deficiency (TD), obesity and vascular disease. Reviews in Endocrine and Metabolic Disorders, 16(3), 249–268. doi: 10.1007/s11154-015-9323-2 [DOI] [PubMed] [Google Scholar]
- Ulhôa M. A., Marqueze E. C., Burgos L. G., Moreno C. R. (2015). Shift work and endocrine disorders. International Journal of Endocrinology, 2015, 1–11. doi: 10.1155/2015/826249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mens-Verhulst J., Bensing J. (1998). Distinguishing between chronic and nonchronic fatigue, the role of gender and age. Social Science & Medicine, 47(5), 621–634. [DOI] [PubMed] [Google Scholar]
- Vesper H. W., Botelho J. C. (2010). Standardization of testosterone measurements in humans. The Journal of Steroid Biochemistry and Molecular Biology, 121(3–5), 513–519. doi: 10.1016/j.jsbmb.2010.03.032 [DOI] [PubMed] [Google Scholar]
- Walther A., Philipp M., Lozza N., Ehlert U. (2016). The rate of change in declining steroid hormones: A new parameter of healthy aging in men? Oncotarget, 7(38), 60844–60857. doi: 10.18632/oncotarget.11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen F., Ye L., Zirkin B., Chen H. (2017). Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction, 154(4), R111–R122. doi: 10.1530/REP-17-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankhede S., Langade D., Joshi K., Sinha S. R., Bhattacharyya S. (2015). Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. Journal of the International Society of Sports Nutrition, 12, 43. doi: 10.1186/s12970-015-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. (2009). Salivary steroid assays – research or routine? Annals of Clinical Biochemistry, 46(Pt 3), 183–196. doi: 10.1258/acb.2008.008208 [DOI] [PubMed] [Google Scholar]
- Yao Q. M., Wang B., An X. F., Zhang J. A., Ding L. (2018). Testosterone level and risk of type 2 diabetes in men: A systematic review and meta-analysis. Endocrine Connections, 7(1), 220–231. doi: 10.1530/EC-17-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo E. H., Choi E. S., Cho S. H., Do J. H., Lee S. J., Kim J. H. (2018). Comparison of fatigue severity and quality of life between unexplained fatigue patients and explained fatigue patients. Korean Journal of Family Medicine, 39(3), 180–184. doi: 10.4082/kjfm.2018.39.3.180 [DOI] [PMC free article] [PubMed] [Google Scholar]