Abstract
Plant phenology (e.g. timing of spring green-up, flowering) is among the most sensitive indicator of ecological response to ongoing climate variability and change. While previous studies have documented changes in the timing of spring green-up and flowering across different parts of the world, empirical evidence regarding how such ongoing ecological changes impact allergic disease burden at population level is lacking. Because earlier spring green-up may increase season length for tree pollen, we hypothesized that early onset of spring (negative anomaly in start of season (SOS)) will be associated with increased hay fever burden. To test this, we first calculated a median cardinal date for SOS for each county within the contiguous US for the years 2001–2013 using phenology data from the National Aeronautics and Space Administration’s Moderate Resolution Imaging Spectroradiometer (MODIS). We categorized yearly deviations in SOS for each county from their respective long-term averages as: very early (>3 wks early), early (1–3 wks early), average (within 1 wk), late (1–3 wks late) and very late (> 3 wks late). We linked these data to 2002–2013 National Health Interview Survey data, and investigated the association between changes in SOS and hay fever prevalence using logistic regression. We observed that adults living in counties with a very early onset of SOS had a 14% higher odds of hay fever compared to the reference group, i.e. those living in counties where onset of spring was within the normal range (Odds Ratios (OR): 1.14. 95% Confidence Interval (CI): 1.03–1.27). Likewise, adults living in counties with very late onset of SOS had a 18% higher odds hay fever compared to the reference group (OR: 1.18, CI: 1.05–1.32). Our data provides the first-ever national scale assessment of the impact of changing plant phenology–linked to ongoing climate variability and change–on hay fever prevalence. Our findings are likely tied to changes in pollen dynamics, i.e early onset of spring increases the duration of exposure to tree pollen, while very late onset of spring increases the propensity of exposure because of simultaneous blooming.
Introduction
Allergic rhinitis, commonly referred to as hay fever, affects 25 million Americans, and costs the US economy an estimated $11.2 billion/year [1;2]. It is a chronic condition characterized by itching, clear rhinorrhea, and sneezing [3–7]. Besides economic costs, hay fever also reduces quality of life and leads to missed work and school days[2;3;7;8]. The common triggers of hay fever, among others, include seasonal exposures to aeroallergens such as mold and pollen[3–6;8].
In North America, the dominant sources of pollen include trees in the spring, grass in the summer and weeds in the fall[9]. Timing of flowering of these plants is a primary determinant of pollen season length—an important determinant of exposure to allergenic pollen[10;11]. The timing of flowering is sensitive to temperature, particularly for plants that flower in early spring, such as trees.[12–15] Consequently, this important phenological event, along with the start of spring season (SOS), as well as growing season length (GSL) has undergone noticeable changes over the past several decades in response to changing climate.[12–22] For example, studies based on in situ observations have shown that the duration of the ragweed pollen season over North America has been increasing in recent decades, with the most pronounced changes observed in northern latitudes; consistent with higher rates of warming[23]. Furthermore, ragweed plants grown at an urban location showed earlier onset as well as significantly higher rates of pollen production compared to a rural location where the temperature and the CO2 concentration were 3.6°F and 30% lower, respectively[24]. These studies, among others, provide indirect evidence of how climate change is impacting the exposure dynamics of allergenic pollen, resulting in more severe and frequent exacerbations of allergic diseases[25]. This mechanism involving temperature, flowering phenology and pollen dynamics has also been suggested as one of the possible explanations for the observed association between exposure to extreme heat events—projected to grow in frequency, duration, and intensity in response to changing climate[26–28]—and increased risk of allergic diseases. [29;30] However, no study has directly linked the changes in plant phenology with allergic diseases or provided quantitative assessment of this association on a national scale.
Despite mounting interest in the links between climate change and adverse health outcomes[31], quantitative assessments of this association are sparse. This is due, in part, to insufficient exposure metrics that can reliably quantify, then link “exposure” to climate-induced environmental change to human health outcomes over a long temporal scale. However, in the context of hay fever, satellite based measures of floral phenology lends itself as a robust exposure metric that enables climate-induced changes to be directly linked with allergic diseases for several reasons: 1) previous studies have shown plant phenology to be a sensitive indicator of changing climate[16]; 2) floral phenology is directly related to pollen dynamics, including pollen load and pollen season length[11;16–18]; and 3) phenology data collected with identical methodology is currently available on a global scale with uniform temporal and spatial resolution.
In this study, we quantified and evaluated the association between alteration in plant phenology that have been tied with ongoing climate change and hay fever burden in the contiguous United States. We further investigated how this association varied across age, race, sex, SES status, and geographic areas.
Materials and methods
Exposure metric
We downloaded phenology data for the contiguous United States from the United States Geological Survey, (USGS) phenology network (http://phenology.cr.usgs.gov) at a 250 m spatial resolution. These data were derived based on the changes in weekly time series of Normalized Difference Vegetation Index (NDVI) from the National Aeronautics and Space Administration Moderate Resolution Imaging Spectroradiometer (MODIS) data as described previously[32–34]. In brief, a delayed moving average (DMA) is predicted based on previous pixel level observations, and compared to the smoothed NDVI data values. A point of departure (cardinal date) is identified when the smoothed NDVI value becomes larger than the predicted DMA value, and referred to as the start of the growing season (SOS) for that particular pixel[32–34]. We aggregated this gridded SOS data to county level by taking a median of all the pixels within a county. Because there is considerable variability in SOS within the contiguous US, we calculated a median cardinal date for onset of greening for each county using the available MODIS phenology data (2001–2013). We then calculated how yearly SOS in each county deviated from their respective long term (13 years: 2001–2013) averages and categorized these observed deviations into 5 groups: very early (>3 wks early), early (1–3 wks early), normal (within 1 wk), late (1–3 wks late) and very late (> 3 wks late). We refer to this county specific yearly deviation in SOS as alteration in plant phenology, and use it as an exposure metric in our analysis.
Health outcome data
We obtained the health outcome data from the National Health Interview Survey (NHIS) for years 2002 through 2013. The NHIS is a cross-sectional survey of a nationally representative sample of the civilian non-institutionalized US population, which has been conducted continuously since 1957, although the survey instrument has changed over time[35]. During the study period, NHIS sampled roughly 40,000 households per year, with some households having multiple families. For each family, a sample adult was selected for a detailed interview, with a response rate ranging from 60 to 80%[36].
We identified hay fever using responses to the question: “During the past 12 months, have you been told by a doctor or other health professional that you had hay fever?” For demographic characteristics, we included age (18–34, 35–49, 50–64, 65+ years), race/ethnicity (Hispanic, non-Hispanic black, non-Hispanic white, all other races and ethnicities), sex (female, male), education level (less than high school/GED, high school/GED, some college, Bachelor’s degree, Graduate degree), and family income relative to the federal poverty threshold (less than 100%, 100% to less than 200%, 200% to less than 400%, 400% or above the poverty threshold)[37;38]. We used the NHIS multiply-imputed income data to assign poverty status level to records with missing values (percent missing ranged from 4.50% to 10.01% over 1997–2013) using NCHS-recommended methods[39]. We also included a geographical covariate to categorize each county into urban, suburban and rural categories.
Statistical analysis
We merged the exposure metric (alteration in phenology) with the adult NHIS respondents (2002–2013) by county and survey year. Since the outcome measure is 12 month period prevalence, we used the interview month to lag exposure window to the previous 12 months and extracted the relevant exposure metric (SOS) from that temporal window. We used logistic regression to examine the association between alteration in plant phenology and self-reported hay fever after adjusting for age, race, sex and education, using SUDAAN to account for the complex clustered sample design of the NHIS.[40] Since some of the respondents (18%) lived in counties that had SOS outside the spring season (March through May), we conducted sensitivity analysis that included only those respondents who lived in the counties where the onset of greening occurred during spring season.
Results
During 2002 through 2013, 8.1% of the respondents (N = 26,565) reported having hay fever (Table 1). Overall, slightly more female respondents reported having hay fever in the previous 12 months. Those who reported having hay fever tended to be more non-Hispanic whites, more educated, and insured. The vast majority of the study population (82%) lived in counties where the SOS (onset of greening) was detected in the spring season. Likewise, the majority of the study population (59%) lived in counties where the onset of greening occurred within 1 week of the long-term average. The distribution of respondents across the exposure variable (deviation in SOS) by survey year is depicted in S1 Table. Yearly variability in SOS across the Contiguous United States is publically available through USGS portal at https://phenology.cr.usgs.gov/get_data_250w.php.
Table 1. Demographic characteristics of the NHIS Respondents: 2002–2013.
| Demographic Characteristics | Hay Fever Status | ||||
|---|---|---|---|---|---|
| NO | YES | ||||
| N | Weighted % | N | Weighted % | ||
| Sex | |||||
| Male | 134524 | 48.7 | 10215 | 42.4 | |
| Female | 167159 | 51.3 | 16350 | 57.6 | |
| Age | |||||
| 18–34 | 88560 | 31.7 | 4895 | 20.2 | |
| 35–49 | 81896 | 27.9 | 8962 | 35.2 | |
| 50–64 | 70301 | 23.6 | 8013 | 30.1 | |
| > = 65 | 60926 | 16.9 | 4695 | 14.5 | |
| Race/Ethnicity | |||||
| Non-Hispanic White | 188344 | 70.3 | 18686 | 77.7 | |
| Non-Hispanic Black | 46566 | 11.9 | 3348 | 9.1 | |
| Hispanic | 50636 | 12.8 | 3191 | 8.6 | |
| Other | 16137 | 5 | 1340 | 4.7 | |
| Education | |||||
| <High School | 139008 | 44.4 | 9343 | 33.8 | |
| High School | 87631 | 29.6 | 8436 | 31.7 | |
| College | 49178 | 17.1 | 5441 | 21.4 | |
| > College | 25866 | 8.9 | 3345 | 13 | |
| Poverty | |||||
| Poverty Ratio <1 | 50792 | 12.8 | 3660 | 10.1 | |
| Poverty Ratio 1 to 2 | 63325 | 18.8 | 4675 | 15.1 | |
| Poverty Ratio 2 to 4 | 90579 | 30.8 | 7507 | 28.8 | |
| Poverty Ratio >4 | 96987 | 37.7 | 10723 | 46 | |
| Insurance Status | |||||
| Un-Covered | 53421 | 17 | 3213 | 11.3 | |
| Covered | 248262 | 83 | 23352 | 88.7 | |
| Timing of onset of greenness | |||||
| Spring | 242974 | 82.3 | 21283 | 81.6 | |
| Summer | 18966 | 6 | 1578 | 5.6 | |
| Fall | 13203 | 3.8 | 1316 | 4.5 | |
| Winter | 26540 | 7.9 | 2388 | 8.4 | |
| Changes in Plant Phenology | |||||
| > 3 wks early | 15844 | 4.7 | 1531 | 5.1 | |
| >1–3 wks early | 54392 | 18 | 4562 | 17.2 | |
| 1 wk early—1 wk late | 174140 | 59.5 | 15141 | 59.1 | |
| >1–3 wks later | 35366 | 11.2 | 3335 | 11.8 | |
| > 3 wks later | 21941 | 6.6 | 1996 | 6.9 | |
Non-Hispanic white adults had a 42% higher odds of reporting hay fever in the previous 12 months (OR 1.42, 95% CI 1.34–1.50) compared to Hispanic adults (Table 2). Likewise, individuals with a college education were more likely to report having hay fever in the previous 12 months compared to those who had less than a high school education (OR 1.71, 95% CI: 1.62–1.8). Individuals residing in counties where SOS arrived very early (> 3 weeks earlier than the long term (13-year) average) had a13% increased odds of hay fever (OR 1.13, 95% CI: 1.05–1.22) compared to those who lived in areas where the SOS arrival was within normal range (+/- 1 week of long term average). Similarly, individuals living in counties where SOS arrived very late (> 3 weeks later than the long term average) had a 14% increased odds of hay fever (OR 1.14, 95% CI: 1.06–1.21). When we restricted the analysis to the counties with springtime SOS (82% of study population), the effects were slightly stronger for very late SOS (OR 1.14, 95% CI: 1.06–1.21 vs OR 1.18, 95% CI 1.05–1.32 for full model and restricted model, respectively).
Table 2. Odds Ratios (OR) and 95% Confidence Intervals (CI) for hay fever prevalence among NHIS respondents (2002–2013).
| OR | 9%% CI | OR1 | 95% CI | ||
|---|---|---|---|---|---|
| Changes in Plant Pnenology | |||||
| > 3wk Early | 1.13 | 1.05–1.22 | 1.14 | 1.03–1.27 | |
| 1–3 wk Early | 0.96 | 0.92–1.00 | 0.95 | 0.9–1.00 | |
| 1 wk early to 1 wk late | 1.00 | REF | 1.00 | REF | |
| 1–3 wk Late | 1.05 | 0.99–1.11 | 1.05 | 0.99–1.12 | |
| >3 wk Late | 1.14 | 1.06–1.21 | 1.18 | 1.05–1.32 | |
| Age Groups | |||||
| 18–34 | 1.00 | REF | 1.00 | REF | |
| 35–49 | 1.91 | 1.82–2 | 1.86 | 1.76–1.96 | |
| 50–64 | 1.90 | 1.81–1.99 | 1.85 | 1.75–1.95 | |
| > = 65 | 1.32 | 1.25–1.39 | 1.25 | 1.17–1.32 | |
| Race/Ethnicity | |||||
| Non-Hispanic White | 1.42 | 1.34–1.5 | 1.39 | 1.29–1.5 | |
| Non-H Black | 1.05 | 0.98–1.13 | 1.04 | 0.95–1.14 | |
| Hispanic | 1.00 | REF | 1.00 | REF | |
| Other | 1.14 | 1.04–1.26 | 1.11 | 0.99–1.25 | |
| Sex | |||||
| Male | 1.00 | REF | 1.00 | REF | |
| Female | 1.30 | 1.26–1.34 | 1.29 | 1.25–1.34 | |
| Education | |||||
| <High School | 1.00 | REF | 1.00 | REF | |
| High School | 1.37 | 1.32–1.42 | 1.38 | 1.32–1.43 | |
| College | 1.53 | 1.46–1.6 | 1.54 | 1.46–1.62 | |
| > College | 1.71 | 1.62–1.8 | 1.72 | 1.62–1.83 | |
1Springtime Interview only
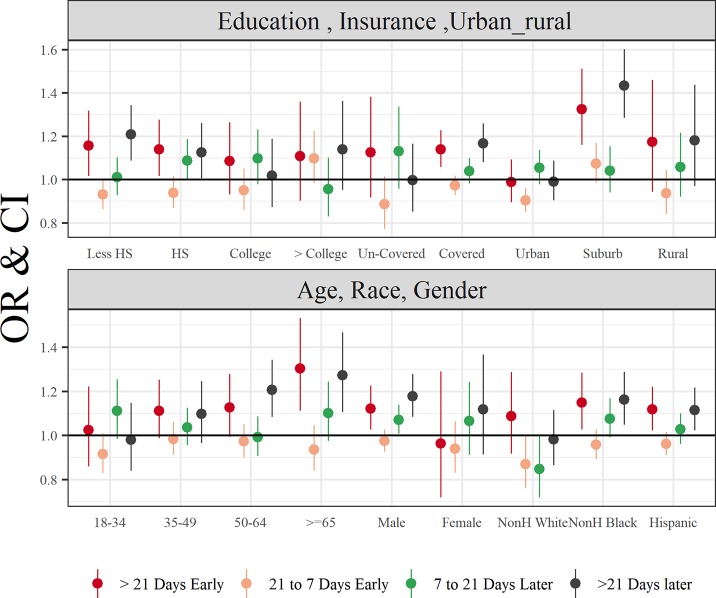
We stratified the analysis by demographic characteristics and urban/rural status (Fig 1). This analysis revealed a consistent U-shaped pattern of the exposure-response relationship, i.e. very early SOS as well as very late SOS were associated with an increased odds of hay fever prevalence. The relationship was most pronounced among those with less education (high school or lower), with insurance coverage, older age groups, and among those living in suburban areas. While both non-Hispanic black and Hispanic adults had increased odds of hay fever associated with both earlier and late onset of SOS, such associations were not evident among non-Hispanic white adults (Fig 1).
Fig 1. Odds Ratios and 95% CIs for changes in plant phenology and prevalence of hay fever, stratified by demographic characteristics, urban/rural status, and insurance coverage.
Discussion
Previous studies have shown that temporal changes in plant phenology are among the most sensitive indicators of ecological response to climate change.[12–16;21;41] Here we show that such alteration in plant phenology is associated with increased prevalence of hay fever among U.S. adults. This is, to our knowledge, the first national scale assessment linking climate driven alteration in plant phenology with prevalence of hay fever in the contiguous United States.
Our findings are based on health outcomes that were derived from a nationally representative sample of the US population, and exposure metrics that were derived using satellite based observations. Our data show that very early SOS is associated with higher prevalence of hay fever. Early SOS is likely associated with earlier onset of flowering among species that bloom in the spring, such as trees[10;11;18;21]. It is plausible that earlier tree flowering may lead to longer tree pollen season, which is the dominant source of allergenic pollen during spring [42;43]. In addition, we found that very late SOS was also associated with an increased prevalence of hay fever in the contiguous US. While unexpected, this particular finding is likely related to the propensity of pollen exposure–i.e. during late SOS, most trees bloom simultaneously. It is likely that simultaneous bloom, or anthesis may result in very sharp increases in pollen concentrations, albeit for a short duration. This U-shaped exposure-response function observed suggests that there may exist a suitable window of exposure for minimal risk. Our data also suggest that changes in this particular window–both early or late—contribute to an overall increase in hay fever burden. Given over 10% of Americans are sensitized to tree pollen[44] while over 8% of US adults suffer from hay fever[36], even a modest changes in the timing of tree flowering can have a substantial impact on hay fever morbidity.
This is of particular concern among people who suffer from allergic diseases given the increasing number of studies that have shown that climate change is directly contributing to alteration in plant phenology[45]. Recent data from Europe has shown that ongoing warming has been advancing flowering by 5.2 days/decade[46], while others have reported SOS in the US is advancing by as much as 0.3 days/year.[47] The extent to which the warming climate may alter plant phenology depends upon several factors including fulfillment of the chilling requirement.[11;48–50]. If wintertime temperature is well below the chilling requirement, then the decreased winter chilling related to climatic warming does not impact the thermal time required for budburst and anthesis [11;48;49]. In such cases, warming temperature lead to earlier budburst. However, if wintertime temperature is not cold enough, vernalization may not occur, in which case plants will require larger thermal time to reactivate growth. This may result in minimal change in the timing of budburst and flowering. [11;48;49] Overall, chilling or vernalization will also need to be taken into account to improve the empirical links between pollen phenology and human health.
Overall the current study used a large nationally representative sample of non-institutionalized populations (N = 328,248) covering over a decade of survey data (2002–2013). The data were collected using identical protocols, minimizing temporal bias in outcome assessment. Our exposure metric was derived using satellite observations that incorporated the same consistent method. By using deviation in SOS from the long-term average, we controlled for the inherent variability in the timing of SOS that exists across a large geographic area. Thus, our results are associated with true alteration in plant phenology, rather than simple geographical contrasts that exist by virtue of latitude.
While the current effort represents a robust analysis, there are additional caveats that should also be considered. For example, NHIS is a multipurpose survey, and does not include more in-depth information such as date of onset of the symptoms or quantification of clinical indicators of allergen sensitization, nor does it contain information regarding allergen exposure. Furthermore, the outcome measure “hay fever” is a lay term that is often used to describe seasonal allergic rhinitis[5]. It is unclear the extent to which this term is misunderstood, and how this may vary across different ethnic groups or if the understanding has changed over time. We also did not investigate how hay fever is related to other outcomes including asthma and eczema in this study population. In addition, we used ordinal variable to categorize the SOS deviation into five groups. While the variable maintains a clear ordering of the categories, the exact distance between the categories may not be the same. Thus comparison of risk estimates across the exposure strata should be done judiciously. Likewise, we did not account for the temperature or moisture content that are related to chilling requirement and plant growth.
Conclusion
Our results provide the first ever national-scale assessment of the association between climate change related alteration in plant phenology and hay fever among nationally representative sample of US adults. As such, they confirm a clear link between floral phenology and allergenic disease that is perturbed by climate change.
Supporting information
(DOCX)
Acknowledgments
The authors would like to thank Drs. Jennifer Parker and Lara Akinbami at NCHS for their generous support throughout the study including study design and statistical analysis.
Data Availability
The satellite data used in this manuscript are third-party data publicly available through NASA and USGS websites described in the method section and the references. The National Health Interview Survey (NHIS) data is available directly through the National Center for Health Statistics (https://www.cdc.gov/nchs/index.htm). But users need to apply for permission to access the geocodes of respondents. Users who want to access the geocodes of respondents (restricted data file) have to get approval from NCHS before accessing the location information. The authors did not have special access privileges that other users would not.
Funding Statement
This work was supported by the National Institute of Environmental Health Sciences (NIEHS 1R21ES021422-01A1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention. Allergies and Hay Fever. 2016. 10-12-2016.
- 2.Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc 2010. September;31(5):375–80. 10.2500/aap.2010.31.3329 [DOI] [PubMed] [Google Scholar]
- 3.van CP, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy 2000. February;55(2):116–34. [DOI] [PubMed] [Google Scholar]
- 4.Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg 2015. February;152(1 Suppl):S1–43. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008. August;122(2 Suppl):S1–84. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein JA. Allergic and mixed rhinitis: Epidemiology and natural history. Allergy Asthma Proc 2010. September;31(5):365–9. 10.2500/aap.2010.31.3380 [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008. April;63 Suppl 86:8–160. [DOI] [PubMed] [Google Scholar]
- 8.Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res 2011. July;3(3):148–56. 10.4168/aair.2011.3.3.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziska LH, Beggs PJ. Anthropogenic climate change and allergen exposure: The role of plant biology. J Allergy Clin Immunol 2012. January;129(1):27–32. 10.1016/j.jaci.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 10.Emberlin J, Smith M, Close R, dams-Groom B. Changes in the pollen seasons of the early flowering trees Alnus spp. and Corylus spp. in Worcester, United Kingdom, 1996–2005. International Journal of Biometeorology 2007;51(3):181–91. 10.1007/s00484-006-0059-2 [DOI] [PubMed] [Google Scholar]
- 11.Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, Rantio-Lehtimaki A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. International Journal of Biometeorology 2002;46(4):159–70. 10.1007/s00484-002-0139-x [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Mozo H., Mestre A, Galan C. Phenological trends in southern Spain: A response to climate change. Agricultural and Forest Meteorology 2010;150:575–80. [Google Scholar]
- 13.Garcia-Mozo H, Oteros J, Galan C. Phenological changes in olive (Ola europaea L.) reproductive cycle in southern Spain due to climate change. Ann Agric Environ Med 2015;22(3):421–8. 10.5604/12321966.1167706 [DOI] [PubMed] [Google Scholar]
- 14.Galan C, Garcia-Mozo H, Vazquez L, Ruiz L, de la Guardia CD, Trigo MM. Heat requirement for the onset of the Olea europaea L. pollen season in several sites in Andalusia and the effect of the expected future climate change. Int J Biometeorol 2005. January;49(3):184–8. 10.1007/s00484-004-0223-5 [DOI] [PubMed] [Google Scholar]
- 15.Ahas R, Aasa A. The effects of climate change on the phenology of selected Estonian plant, bird and fish populations. Int J Biometeorol 2006. September;51(1):17–26. 10.1007/s00484-006-0041-z [DOI] [PubMed] [Google Scholar]
- 16.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, et al. Ecological responses to recent climate change. Nature 2002;416(6879). [DOI] [PubMed] [Google Scholar]
- 17.Penuelas J, Rutishauser T, Filella I. Phenology feedbacks on climate change. Science 2009. May 15;324(5929):887–8. 10.1126/science.1173004 [DOI] [PubMed] [Google Scholar]
- 18.Menzel A. Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol 2000. August;44(2):76–81. [DOI] [PubMed] [Google Scholar]
- 19.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science 2002. May 31;296(5573):1689–91. 10.1126/science.1071617 [DOI] [PubMed] [Google Scholar]
- 20.Fu Y.H., Piao S, Op de Beeck M, Cong N, Zhao H, Zhang Y, et al. Recent spring phenology shifts in western Central Europe based on multiscale observations. Global Ecology and Biogeography 201;23:1255–63. [Google Scholar]
- 21.Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, et al. European phenological response to climate change matches the warming pattern. Global Change Biology 2006;12:1969–76. [Google Scholar]
- 22.Fu YH, Zhao H, Piao S, Peaucelle M, Peng S, Zhou G, et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015. October 1;526(7571):104–7. 10.1038/nature15402 [DOI] [PubMed] [Google Scholar]
- 23.Ziska L, Knowlton K, Rogers C, Dalan D, Tierney N, Elder MA, et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proceedings of the National Academy of Sciences of the United States of America 2011;108(10):4248–51. 10.1073/pnas.1014107108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: Common ragweed, urbanization, and public health. Journal of Allergy and Clinical Immunology 2003;111(2):290–5. [DOI] [PubMed] [Google Scholar]
- 25.Portier CJ, Thigpen TK, Carter SR, Dilworth CH, Grambsch AE, Gohlke J, et al. A Human Health Perspective on Climate Change: A Report Outlining the Research Needs on the Human Health Effects of Climate Change. Environmental Health Perspectives and National Institute of Enviornmental Health Sciences; 2010. [Google Scholar]
- 26.Climate Change 2014- Mitigation of Climate Change. Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, Uk, and New York, USA: Cambridge University Press; 2014. [Google Scholar]
- 27.Managing the risks of extreme events and disasters to advance climate change adaptation: Special report to the Intergovernmental Panel on Climate Change Cambirdge, UK; New York, USA: Cambridge Uniersity Press; 2012. [DOI] [PubMed] [Google Scholar]
- 28.Luber G, Knowlton K, Balbus J, Frumkin H, Hayden M, Hess J, et al. Climate Change Impacts in the United States In: Melillo JM, Richmond T, Yohe G, editors. The Third National Climate Assessment.Washington DC: US Global Change Research Program; 2014. p. 220–56. [Google Scholar]
- 29.Soneja S, Jiang C, Fisher J, Upperman CR, Mitchell C, Sapkota A. Exposure to extreme heat and precipitation events associated with increased risk of hospitalization for asthma in Maryland, U.S.A. Environ Health 2016;15:57 10.1186/s12940-016-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upperman CR, Parker JD, Akinbami LJ, Jiang C, He X, Murtugudde R, et al. Exposure to Extreme Heat Events Is Associated with Increased Hay Fever Prevalence among Nationally Representative Sample of US Adults: 1997–2013. J Allergy Clin Immunol Pract 2017. March;5(2):435–41. 10.1016/j.jaip.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein L, Bosch P, Canziani O, Chen A, Christ R, Davidson O, et al. Intergovernmental Panel on Climate Change (IPCC): Synthesis Report Geneva, Switzerland: IPCC; 2007. [Google Scholar]
- 32.U.S.Geographical Survey (USGS). Methods for Deriving Metrics. http://phenology/cr/usgs/gov/methods_deriving/php 2016
- 33.Swets DL, Reed BC, Rowland JR, Marko SE. A weighted least-square approach to temporal NDVI smoothing. Proceedings of the 1999 ASPRS Annual Conference, from Image to Information, Portland Oregeon, May 17–21. American Society for Photogrammetry and Remote Sensing; 1999.
- 34.Jenkerson C, Maiersperger T, Schmidt G. eMODIS: A user-friendly data source. Reston, VA: U.s. Geological Survey (USGS); 2010. Report No.: Open-File Report 2010–1055.
- 35.Centers for Disease Control and Prevention. About the National Health Interview Survey. 2016. 8-15-2016. Ref Type: Online Source
- 36.Upperman CR, Parker JD, Akinbami LJ, Jiang C, He X, Murtugudde R, et al. Exposure to Extreme Heat Events Is Associated with Increased Hay Fever Prevalence among Nationally Representative Sample of US Adults: 1997–2013. J Allergy Clin Immunol Pract 2016. November 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief 2013. May;(121):1–8. [PubMed] [Google Scholar]
- 38.U.S.Census Bureau. US Census Bureau Poverty main page. 2016. 8-15-2016.
- 39.Ingram DD, Franco SJ. NCHS urban-rural classification scheme for counties. Vital Health Stat 2 2012. January;(154):1–65. [PubMed] [Google Scholar]
- 40.RTI International. SUDAAN Available from http://sudaansupport.rti.org. 2018.
- 41.Donnelly A, Yu R. The rise of phenology with climate change: an evaluation of IJB publications. Int J Biometeorol 2017. September;61(Suppl 1):29–50. 10.1007/s00484-017-1371-8 [DOI] [PubMed] [Google Scholar]
- 42.Ugolotti M, Pasquarella C, Vitali P, Smith M, Albertini R. Characteristics and trends of selected pollen seasons recorded in Parma (Northern Italy) from 1994 to 2011. Aerobiologia 2015;31:341–52. [Google Scholar]
- 43.Lind T, Ekebom A, Alm KK, Ostensson P, Bellander T, Lohmus M. Pollen Season Trends (1973–2013) in Stockholm Area, Sweden. PLoS One 2016;11(11):e0166887 10.1371/journal.pone.0166887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salo PM, Arbes SJ Jr., Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol 2014. August;134(2):350–9. 10.1016/j.jaci.2013.12.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziska L. Impacts of Climate Change on Allergen Seasonality In: Beggs PJ, editor. Impacts of Climate Change on Allergens and Allergic Diseases.Cambridge: Cambridge University Press; 2016. p. 74–91. [Google Scholar]
- 46.Bock A, Sparks TH, Estrella N, Jee N, Casebow A, Schunk C, et al. Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Glob Chang Biol 2014. November;20(11):3508–19. 10.1111/gcb.12579 [DOI] [PubMed] [Google Scholar]
- 47.Li X, Zhou Y, Asrar GR, Meng L. Characterizing spatiotemporal dynamics in phenology of urban ecosystems based on Landsat data. Sci Total Environ 2017. December 15;605–606:721–34. 10.1016/j.scitotenv.2017.06.245 [DOI] [PubMed] [Google Scholar]
- 48.Cannell MG, Smith RI. Climatic warming, spring budburst and frost damage on trees. Journal of Applied Ecology 1986;23:177–91. [Google Scholar]
- 49.Murray MB, Cannell MG, Smith RI. Date of budburst of fifteen tree species in Britain following climatic warming. Journal of Applied Ecology 1989;26:693–700. [Google Scholar]
- 50.Cook BI, Wolkovich EM, Parmesan C. Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci U S A 2012. June 5;109(23):9000–5. 10.1073/pnas.1118364109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The satellite data used in this manuscript are third-party data publicly available through NASA and USGS websites described in the method section and the references. The National Health Interview Survey (NHIS) data is available directly through the National Center for Health Statistics (https://www.cdc.gov/nchs/index.htm). But users need to apply for permission to access the geocodes of respondents. Users who want to access the geocodes of respondents (restricted data file) have to get approval from NCHS before accessing the location information. The authors did not have special access privileges that other users would not.