Abstract
Testicular macrophages (tMΦ) are the most abundant immune cells residing in the testis, an immune-privileged organ. TMΦ are known to exhibit different functions, such as protecting spermatozoa from auto-immune attack by producing immunosuppressive cytokines and trophic roles in supporting spermatogenesis and male sex hormone production. They also contribute to fetal testicular development. Recently, we characterized two distinct tMΦ populations based on their morphology, localization, cell surface markers, and gene expression profiling. Here, we focus and describe in detail the phenotypical distinction of these two tMΦ populations by fluorescence-activated cell sorting (FACS) using multicolor panel antibodies combining with high-resolution immunofluorescence (IF) imaging. These two techniques enable to classify two tMΦ populations: interstitial tMΦ and peritubular tMΦ.
Keywords: Macrophage, Testis, FACS, IF, Spermatogenesis, Immune-privilege organ
Background
The testis is the male reproductive organ where spermatogenesis and testosterone production occurs. tMΦ might be considered as “guardians of fertility” by their immuno-suppressive function to assure the immune-privilege status of the testis and their trophic roles in spermatogenesis and male hormone production, but the distinct functions of these macrophage populations are only starting to be elucidated (Mossadegh-Keller and Sieweke, 2018). Tissue-resident macrophages exhibit tissue-specific functions and gene expression patterns depending of their organs of residency ( Gentek et al., 2014 ; Lavin et al., 2014 ) but also share common core tissue-resident macrophage markers as F4/80, CD11b, CD64, M-CSFR and for some tissues CX3CR1 (Gordon, 2002; Gautier et al., 2012 ; Yona et al., 2013 ; Gentek et al., 2014 ). Recently, two testicular macrophages (tMΦ) populations have been characterized using IF imaging and FACS ( DeFalco et al., 2015 ; Mossadegh- Keller et al., 2017 ). Interstitial tMΦ can be identified by microscopy by their rounded morphology and localization in the interstitial space of the testis in close contact with testosterone-producing Leydig cells (Smith and Walker, 2014; DeFalco et al., 2015 ; Mossadegh- Keller et al., 2017 ). In contrast, the peritubular tMΦ exhibit an elongated morphology and surround the seminiferous tubules housing spermatogonial stem cells (SSC) ( DeFalco et al., 2015 ; Mossadegh- Keller et al., 2017 ). Confirming previous observations ( DeFalco et al., 2015 ), we observed that both tMΦ were positive for CX3CR1 and F4/80 by IF. Interstitial tMΦ can be distinguished by the strong expression of M-CSFR from peritubular tMΦ that selectively express high levels of MHCII (Figure 2) (Mossadegh- Keller et al., 2017 ). We further analyzed in depth the interstitial and peritubular tMΦ populations by FACS. We established an extended antibody panel going beyond previous protocols ( DeFalco et al., 2015 ), excluding monocytes and dendritic cells and including CD64 as a key tissue-resident macrophage ( Gautier et al., 2012 ; Mossadegh- Keller et al., 2017 ). Within the tMΦ CD45+Ly6C-CD11cloF4/80+CD11b+ fraction, we defined interstitial tMΦ as M-CSFR+CD64hiMHCII- cells and peritubular tMΦ as M-CSFRloCD64loMHCII+ cells (Figure 3).
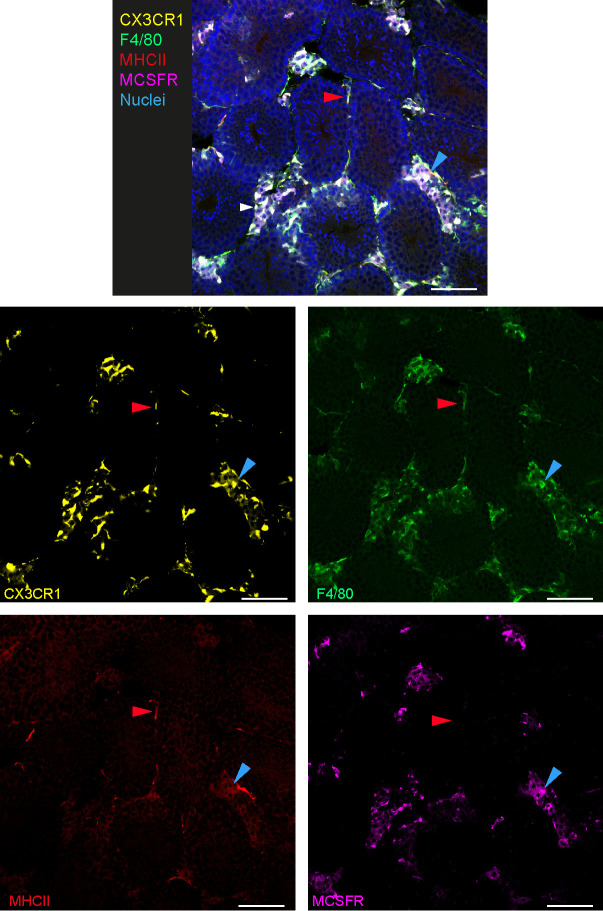
Figure 2. Phenotypic characterization of interstitial and peritubular macrophages by IF.
IF imaging of CX3CR1GFP/+ adult mouse testis is revealing morphology, localization, F4/80, MHCII and M-CSFR staining of interstitial and peritubular testicular macrophages. One example of interstitial macrophages is indicated with a blue arrow showing a cell with round morphology and expressing CX3CR1, F4/80 and M-CSFR. One example of peritubular macrophages is indicated with a red arrow showing a cell with elongated morphology and expressing CX3CR1, F4/80 and MHCII. scale bars = 40 μm.
Figure 3. Phenotypic characterization of interstitial and peritubular macrophages by FACS.
Gating strategy describing in CD45+Ly6C-CD11c-F4/80+CD11b+ adult mouse testis fraction, the distinction of interstitial M-CSFR+CD64+MHCII- and peritubular M-CSFRloCD64loMHCII+ population, respectively represented by blue and red gates.
Here, we describe IF and FACS protocols that will be instrumental to define and isolate these two tMΦ populations for further phenotypic and functional characterization.
Materials and Reagents
Pipette tips
Microscope slides (SuperFrost Plus, VWR, catalog number: 631-0108)
Cover glass (VWR, catalog number: 470820)
Hydrophobic pen, Mini PAP Pen (Life Technologies, catalog number: 008877)
Simport Scientific Disposable Base Molds (Fisher Scientific, catalog number: 11670990)
Eppendorf tube 1.5 ml (Sigma-Aldrich, catalog number: Z606340-1000EA)
Sterilin tube (Thermo Fisher Scientific, catalog number: 129A)
5 ml Falcon Polystyrene Round-Bottom Tube (Fisher Scientific, Corning, catalog number: 352008)
Filter 50 μm Filcon, Sterile, Syringe-type (Becton, catalog number: 340601)
Wild-type C57BL/6J mice from Janvier labs
CX3CR1GFP/+ mice ( Jung et al., 2000 )
Ice
DPBS 1x (gibco, life Technologies, catalog number: 14190-094)
HBSS 1x (gibco, life Technologies, catalog number: 14025-050)
BSA (Sigma-Aldrich, catalog number: A2153)
Fetal Calf Serum (Biosera, catalog number: FB-1001/500)
Saponin (Sigma-Aldrich, catalog number: 47036)
Antigenfix (Diapath, catalog number: P0014)
Optimum Cutting Temperature (O.C.T.) Tissue-Tek (VWR, catalog number: 256008-930)
Isopentane (VWR, catalog number: 24872-323)
-
Antibodies:
Anti-CD16/32 (clone 2.4G2, BD Biosciences, catalog number: 553142, working dilution 1/200)
Anti-CD45-BV421 (clone 30F11, BD Biosciences, catalog number: 560501, working dilution 1/200)
Anti-F4/80-BV785 (clone BM8, BioLegend, catalog number: 123141, working dilution 1/200)
Anti-CD11b-BV605 (clone M1/70, BD Biosciences, catalog number: 563015, working dilution 1/800)
Anti-CD64-PerCP/Cy5.5 (clone X54-5/7.1, BioLegend, catalog number: 139308, working dilution 1/300)
Anti-CD11c-BV711 (clone N418, BioLegend, catalog number: 117349, working dilution 1/300)
Anti-CD115-PE (clone AFS98, eBiosciences, catalog number: 112-1152-82, working dilution 1/200)
Anti-Ly6C-APC/Cy7 (clone HK1.4, BioLegend, catalog number: 128026, working dilution 1/300)
Anti-I-A/I-E (MHCII)-PE/Cy7 (clone M5/114.15.2, BioLegend, catalog number: 107630, working dilution 1/300)
Anti-F4/80-Alexa Fluor647 (clone BM8, BioLegend, catalog number: 123122, working dilution 1/200)
Anti-MCII-efluor450 (clone M5/114.15.2, eBiosciences, catalog number: 48-5321-82, working dilution 1/500)
Anti-M-CSFR (clone C-20, Santa Cruz, catalog number: sc-692, working dilution 1/800)
Anti-rabbit-Alexa Fluor 594 (Jackson Immunoresearch, catalog number: 711-585-152, working dilution 1/500)
Prolong Gold antifade reagent (Life Technologies, catalog number: P36930)
Zombie Violet fixable live/dead staining (BioLegend, catalog number: BLE423113, working dilution 1/1,000)
30% sucrose (Sigma-Aldrich, catalog number: S9378) (see Recipes)
Blocking Buffer for IF (see Recipes)
Wash Buffer for IF (see Recipes)
Prolong containing Sytox blue dye (Life Technologies, catalog number: S34857) (see Recipes)
-
Enzymatic digestion mix (see Recipes)
Collagenase II (Worthington Biochemicals/Serlabo Technologies, catalog number: LS004174, 125 U/mg dry weight)
Dnase I (Roche, catalog number: 10104159001)
Working enzymatic digestion (500 µl/testis sample)
Equipment
Pipettes
Microscope slide holder/box (Heathrow Scientific, catalog number: 15994G)
Vertical plastic staining rack
Dissection scissors (Harvard Apparatus, catalog number: 72-8422)
Milligramme balance (Mettler Toledo, catalog number: 30029085)
SnapFrost80 (Alphelys)
Cryostat (Leica, model: CM3050S)
Confocal microscope (ZEISS, model: LSM 780)
40x/1.4 oil differential interference contrast objective (Plan-Apochromat)
FACS LSRII instrument (BD)
Thermomixer comfort (Eppendorf, catalog number: 5382000015)
Rocking agitator (VWR, catalog number: 444-0756)
Centrifuge
Vortexer
4 °C refrigerator
-80 °C freezer
Software
FlowJo (Version 10.0.8)
ImageJ (Version 1.49s, National Institutes of Health)
Adobe Illustrator CS6 (Version 16.0.3)
Procedure
-
Preparing tissue testis sections for IF (Figure 1)
Euthanize mice by cervical dislocation.
Excise the whole testis organ and place it directly in a Sterilin tube with 1 ml cold Antigenfix. Incubate on a rocking agitator for 3 h at 4 °C.
Wash the fixed testis in cold PBS 1x at 4 °C for 20 min.
Incubate the testis organ in 5 ml of 30% sucrose solution overnight at 4 °C, until the testis sinks completely to the bottom of the tube.
Transfer the testis into disposable base molds in a coronal plane and place immediately into cold (-80 °C) isopentane inside the SnapFrost80. After 2 min, remove the frozen molds and store at -80 °C.
Section the mouse testis at 20 µm-thick with the cryostat on SuperFrost slides and store at -20 °C.
-
Immunofluorescence (Figure 2)
Place slides in a microscope slide holder/box, delimit the testis section with a hydrophobic pen and add 100 µl PBS 1x per section for 10 min at room temperature (RT).
Incubate in blocking buffer for 1 h at RT.
Wash 3 times with the wash buffer solution for 5 min at RT by moving the slide rack up and down between each wash.
Place wet tissues into the slide holder/box in order to make a humid chamber for the staining.
-
Incubate with antibodies (100 µl per section) in PBS/0.05% saponin overnight at 4 °C at the following dilutions:
Anti-F4/80-Alexa Fluor647 dilution 1/200
Anti-MCII-efluor450 dilution 1/500
Anti-M-CSFR dilution 1/800
After three washes using the wash buffer for 5 min each at RT, incubate for 1 h at RT with anti-rabbit-Alexa Fluor 594, dilution 1/500, for anti-MCSFR antibody detection.
Wash slides three times with PBS/0.05% saponin for 5 min each at RT by moving the slide rack up and down several times. Place slides on a vertical plastic staining rack, protected from the light, in order to dry the slides.
Mount slides in Prolong containing Sytox blue dye (see Recipes).
-
Fluorescence-Activated Cell Sorting (FACS) (Figure 3)
Euthanize mice by cervical dislocation.
Collect the whole testis organ and place it directly in a 1.5 ml Eppendorf tube in cold PBS containing 1 mg/ml Collagenase II/0.15 mg/ml Dnase I. Mechanically dissociate the testis by mincing with scissors directly inside the tube.
Place the minced tissue in a thermomixer at 37 °C for 40 min by shaking for enzymatic digestion.
Transfer the digested testis through a 50 μm filter placed on top of a 5 ml Falcon tube and filter using 1 ml PBS.
Centrifuge the digested tissue in a refrigerated centrifuge for 5 min at 300 × g at 4 °C.
Resuspend the pellet of cells in 200 µl of cold PBS and incubate with blocking anti-CD16/32 antibody, dilution 1/200, leaving it on ice for 15 min.
Wash in 1 ml cold PBS and centrifuge for 5 min at 300 × g at 4 °C.
-
Incubate single-cell suspensions in cold PBS and stain for expression of surface antigens for 20 min on ice. The following antibodies are used in the same mix preparation:
Anti-F4/80-BV785 dilution 1/200
Anti-CD11b-BV605 dilution 1/800
Anti-CD64-PerCP/Cy5.5 dilution 1/300
Anti-CD11c-BV711 dilution 1/300
Anti-M-CSFR-PE dilution 1/200
Anti-Ly6C-APC/Cy7 dilution 1/300
Anti-I-A/I-E (MHCII)-PE/Cy7 dilution 1/300
Anti-CD45-BV421 dilution 1/200
Zombie Violet fixable live/dead cell dye as a viability marker dilution 1/1,000
Wash in 1 ml cold PBS and centrifuge for 5 min at 300 × g at 4 °C. Take up single cell suspension in 800 µl of PBS and pass the solution through a 50 µm filter. The sample is ready for the analysis on FACS.
Figure 1. Coronal testis plane preparation.
The testis is placed into disposable base molds with OCT in a coronal plane.
Data analysis
Confocal microscopy acquisitions were performed on a confocal microscope (LSM780; ZEISS) at room temperature, and slides were imaged with a 40x/1.4 oil differential interference contrast objective (Plan-Apochromat). Different lasers were used (405 nm, 488 nm, 56 nm, and 633 nm) to excite the fluorophores (Sytox blue, eFluor 450, Alexa Fluor 488, Alexa Fluor 594, and Alexa Fluor 647). Fluorescence was recorded in individual channels acquired in a sequential mode to avoid cross-talk using a highly sensitive 32-channel gallium arsenide phosphide detector. The pinhole was set to 1 airy unit. Image processing was done with ImageJ (National Institutes of Health). Only a median filter was applied to the images to remove salt and pepper noise.
FACS data were acquired on an LSR II instrument (BD) using violet laser 405 nm, blue laser 488 nm, green laser 561 nm, and red laser 633 nm. FACS data were analyzed using FlowJo software (V10.0.8). The FACS gating strategy to distinguish Interstitial CD64hiMHCII- tMΦ and peritubular CD64loMHCII+ tMΦ of adult mouse testis and a representative FACS profile is shown in Figure 2. This can be used to quantify the proportion of these two tMΦ populations and the details regarding the analysis can be found in the original article (Mossadegh- Keller et al., 2017 ; e.g., Figures 2A and 2B).
Recipes
-
30% sucrose
30 g sucrose
Bring the volume to 100 ml with PBS
Store at 4 °C
-
Blocking Buffer for IF
2 g BSA
1 ml FCS
0.1 g saponin
Bring the volume to 100 ml with PBS
Store at 4 °C
-
Wash Buffer for IF
0.05 g saponin
Bring the volume to 100 ml with PBS
Store at 4 °C
-
Prolong containing Sytox blue dye
1 ml of Prolong without DAPI
Add 3 µl of Sytox blue dye
Vortex 3 min
Add 1 ml Prolong without DAPI
Store at -20 °C
-
Enzymatic digestion mix
-
Collagenase 50 mg/ml stock solution
Resuspend 1 g of collagenase in 20 ml of HBSS
Make aliquots of 1 ml
Store at -20 °C
-
Dnase I 10 mg/ml stock solution
Resuspend 100 mg of DNase in 10 ml of HBSS
Make aliquots of 500 µl
Store at -20 °C
-
Working enzymatic digestion (500 µl/testis sample)
10 µl of collagenase stock solution
7.5 µl of Dnase I stock solution, bring the volume to 500 µl with PBS
-
Acknowledgments
We thanks the Centre d’Immunologie de Marseille-Luminy (CIML) flow cytometry and mouse house facilities for support. We acknowledge the PICSL imaging facility of the CIML (ImagImm), member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04).
This work was supported by institutional grants from Institut National de la. Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Aix-Marseille University to the CIML and grants to M.H. Sieweke from the Agence Nationale de la Recherche (ANR-11-BSV3-0026), Fondation pour la Recherche Médicale (DEQ. 20110421320) a INSERM-Helmholtz cooperation grant and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement number 695093 MacAge). M.H. Sieweke has been supported as a BIH-Einstein visiting fellow at the MDC and an Alexander von Humboldt Professor at CRTD/TU Dresden.
Competing interests
The authors declare no competing financial interests.
Ethics
In vivo procedures were performed under specific pathogen-free conditions following protocols approved by the Ethics Committee of Marseille in accordance with institutional, national, and European regulations (approval nos. APAFIS 3292-2015 1221 09359224 and APAFIS 10545-2017 0710 08253541).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. DeFalco T., Potter S. J., Williams A. V., Waller B., Kan M. J. and Capel B.(2015). Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep 12(7): 1107-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gautier E. L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K. G., Gordonov S., Mazloom A. R., A. Ma'ayan, Chua W. J., Hansen T. H., Turley S. J., Merad M., Randolph G. J. and Immunological Genome C.(2012). Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13(11): 1118-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gentek R., Molawi K. and Sieweke M. H.(2014). Tissue macrophage identity and self-renewal. Immunol Rev 262(1): 56-73. [DOI] [PubMed] [Google Scholar]
- 4. Gordon S.(2002). Pattern recognition receptors: doubling up for the innate immune response. Cell 111(7): 927-930. [DOI] [PubMed] [Google Scholar]
- 5. Jung S., Aliberti J., Graemmel P., Sunshine M. J., Kreutzberg G. W., Sher A. and Littman D. R.(2000). Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20(11): 4106-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S. and Amit I.(2014). Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159(6): 1312-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mossadegh-Keller N. and Sieweke M. H.(2018). Testicular macrophages: Guardians of fertility. Cell Immunol 330: 120-125. [DOI] [PubMed] [Google Scholar]
- 8. Mossadegh-Keller N., Gentek R., Gimenez G., Bigot S., Mailfert S. and Sieweke M. H.(2017). Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med 214(10): 2829-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith L. B. and Walker W. H.(2014). The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30: 2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E. and Jung S.(2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38(1): 79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]