Abstract
Serine/threonine protein kinase C (PKC) is activated by diacylglycerol that is released from membrane lipids by phospholipase C in response to activation of G protein-coupled receptors or receptor tyrosine kinases. PKC isoforms are particularly relevant for proliferation and differentiation of cells including osteoblasts. Osteoblasts/osteocytes produce fibroblast growth factor 23 (FGF23), a hormone regulating renal phosphate and vitamin D handling. PKC activates NFκB, a transcription factor complex controlling FGF23 expression. Here, we analyzed the impact of PKC on FGF23 synthesis. Fgf23 expression was analyzed by qRT-PCR in UMR106 osteoblast-like cells and in IDG-SW3 osteocytes, and FGF23 protein was measured by ELISA. Phorbol ester 12-O-tetradecanoylphorbol-13-acetate (PMA), a PKC activator, up-regulated FGF23 production. In contrast, PKC inhibitors calphostin C, Gö6976, sotrastaurin and ruboxistaurin suppressed FGF23 formation. NFκB inhibitor withaferin A abolished the stimulatory effect of PMA on Fgf23. PKC is a powerful regulator of FGF23 synthesis, an effect which is at least partly mediated by NFκB.
Introduction
Protein kinase C (PKC) isoforms are related serine/threonine kinases probably expressed in all cell types. Classically, PKC activity is induced upon stimulation of various Gq protein-coupled receptors and growth factor receptor tyrosine kinases [1]. Three classes of PKC isoforms can be distinguished: classical PKC (cPKC) isoforms are activated by both, diacylglycerol (DAG) and an increase in the intracellular Ca2+ concentration whereas novel PKC (nPKC) isoforms require only DAG, and atypical PKC (aPKC) isoforms are induced by other mechanism [2]. The classical activation is dependent on phospholipase Cβ or Cγ-mediated breakdown of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) yielding inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 binds the IP3 receptor releasing Ca2+ from the endoplasmic reticulum (ER), while membrane-bound DAG activates PKC [1].
PKC is crucial for most cellular responses including the regulation of gene expression, cell migration, proliferation, differentiation, and apoptosis [3]. Moreover, PKC is implicated in the pathophysiology of frequent disorders such as heart failure, diabetes, Alzheimer and Parkinson disease, as well as inflammatory and immune disorders [3]. PKC is particularly relevant for various malignancies, owing to its tumor and metastasis-promoting properties [3]. Plant-derived phorbol esters are potent carcinogens that are effective through stimulating PKC activity [3].
Inflammation [4,5], renal and cardiovascular disease [6–8] are major triggers of the production of fibroblast growth factor 23 (FGF23), a proteohormone mainly produced in the bone [9] and implicated in the regulation of phosphate reabsorption and 1,25(OH)2D3 (active vitamin D [10]) formation in the kidney [11]. The FGF23-mediated inhibition of renal phosphate transporter NapiIIa and CYP27B1, the key enzyme for the generation of 1,25(OH)2D3, is dependent on Klotho, a transmembrane protein [9]. The induction of left ventricular hypertrophy is, however, solely mediated by FGF23 without the involvement of Klotho [8,12,13], whereas vitamin D partly overcomes the FGF23 effect on cardiac hypertrophy [14]. FGF23 also impacts on neutrophil recruitment [15], erythropoiesis [16,17], or hepatic cytokine secretion [18] in a paracrine manner.
Klotho or FGF23 deficiency results in rapid aging, a very short life span, and multiple age-associated diseases affecting most organs and tissues [11,19]. This dramatic phenotype of Klotho or FGF23 null mice is almost completely rescued by a phosphate- or vitamin D-deficient diet [20,21], pointing to the predominant role of calcification in the pathophysiology of Klotho or FGF23 deficiency [22]. Apart from its endocrine and paracrine effects that are still incompletely understood, FGF23 is a putative disease biomarker [6,23]. The plasma level of FGF23 correlates well with progression of chronic kidney disease (CKD) and is a very sensitive marker that is elevated even before onset of hyperphosphatemia or hyperparathyroidism [24,25]. Furthermore, FGF23 levels are elevated in acute kidney injury [26,27]. Whether and to which extent FGF23 not only indicates disease but actively contributes to disease progression, as shown for the heart, remains unclear.
The identification of molecular regulators of FGF23 production is of high interest and relevance. Known regulators include PTH [28], 1,25(OH)2D3 [29], phosphate [30,31], inflammatory cytokines and factors such as tumor necrosis factor α (TNFα) [32,33], interleukin (IL)-1/6 [4,33–35], NFκB [33,36], transforming growth factor (TGF)β [37], AMP-dependent protein kinase (AMPK) [38] or insulin-dependent PI3 kinase signaling [39].
The present study explored the contribution of PKC signaling to the production of FGF23 in bone cells.
Materials and methods
Cell culture
Cell culture and experiments with UMR106 rat osteoblast-like cells (purchased from ATCC, Manassas, VA, USA) were conducted as described before [39]. Briefly, cells were cultured in DMEM high-glucose medium containing 10% FBS and penicillin-streptomycin at 37°C and 5% CO2.
IDG-SW3 bone cells (purchased from Kerafast, Boston, MA, USA) were cultured as described earlier [40]. Briefly, non-differentiated cells were kept at 33°C in AlphaMEM medium (with L-glutamine and deoxyribonucleosides) containing 10% FBS, penicillin-streptomycin and interferon-gamma (INF-γ; 50 U/ml). For differentiation, cells were plated on collagen-coated dishes at 37°C in medium with 50 μg/ml ascorbic acid and 4 mM β-glycerophosphate but without INF-γ. All reagents were from ThermoFisher unless indicated.
IDG-SW3 osteocytes were used after 35 days of differentiation, and UMR106 cells were pretreated with 100 nM 1,25(OH)2D3 (Tocris, Wiesbaden-Nordenstadt, Germany) for 24h before the experiment. Cells were then incubated with activator phorbol ester 12-O-tetradecanoylphorbol-13-acetate (PMA; Sigma, Schnelldorf, Germany; 0.1 μM; 6 h) with or without 1 μM PKC inhibitors calphostin C (Tocris), Gö6976 (Tocris), sotrastaurin (Selleckchem, München, Germany), ruboxistaurin (Selleckchem), or NFκB inhibitor withaferin A (Tocris; 0.5 μM), or with vehicle only for another 24 h.
Expression analysis
Total RNA was extracted using peqGOLD TriFast reagent (VWR, Dresden, Germany). Complementary DNA (cDNA) was synthesized from 1.2 μg RNA using random primers and the GoScript™ Reverse Transcription System (Promega, Mannheim, Germany) at 25°C for 5 min, 42°C for 1 h, and 70°C for 15 min.
The expression profile of PKC isoforms in UMR106 cells was studied by RT-PCR using the GoTaq Green Master Mix (Promega) and the primers listed in Table 1. For PCR, 2 μl synthesized cDNA were used. Settings were: 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 60°C for 30s, 72°C for 45s. PCR products were loaded on a 1.5% agarose-gel and visualized by Midori Green (Biozym, Hessisch Oldendorf, Germany), and a 100 bp DNA ladder (Jena Bioscience, Jena, Germany) was used as a size marker.
Table 1. Primer sequences used for expression analysis.
| name | sequence (5'-3') | product |
|---|---|---|
| Pkcα_forward | CTGAACCCTCAGTGGAATGAGT | 326 bp |
| Pkcα_reverse | GGCTGCTTCCTGTCTTCTGAA | |
| Pkcβ_forward | AACGGCTTGTCAGATCCCTA | 250 bp |
| Pkcβ_reverse | CCACTCCGGCTTTCTGTAGT | |
| Pkcγ_forward | AGTCCCACGGACTCCAAGAG | 397 bp |
| Pkcγ_reverse | CGGCATAGAATGCTGCGTG | |
| Pkcδ_forward | TGTGAAGACTGCGGCATGAA | 265 bp |
| Pkcδ_reverse | AGGTGAAGTTCTCAAGGCGG | |
| Pkcε_forward | CGAGGACGACTTGTTTGAATCC | 389 bp |
| Pkcε_reverse | CAGTTTCTCAGGGCATCAGGTC | |
| Pkcζ_forward | GTGGACCCCACGACAACTT | 207 bp |
| Pkcζ_reverse | GATGCTTGGGAAAACGTGGA | |
| Pkcη_forward | CCATGAAGATGCCACAGGGATC | 249 bp |
| Pkcη_reverse | TCATCGATCGGAGTTAAAACAGG | |
| Pkcθ_forward | TGCCGACAATGTAATGCAGC | 219 bp |
| Pkcθ_reverse | ACACTTGAGACCTTGCCTCG | |
| Pkcι_forward | CTCCTGATCCACGTGTTCCC | 321 bp |
| Pkcι_reverse | GGATGACTGGTCCATTGGCA |
qRT-PCR
Relative expression levels of Fgf23 and other relevant genes were determined by qRT-PCR using 2 μl synthesized cDNA (for primers see Table 2), and GoTaq qPCR Master Mix (Promega) on a Rotor-Gene Q (Qiagen, Hilden, Germany). PCR conditions were 95°C for 3 min, followed by 35 cycles of 95°C for 10 s, 58°C for 30 s and 72°C for 45 s. After normalization to Tbp (TATA box-binding protein) expression, relative quantification of gene expression was carried out based on the double-delta Ct (threshold cycle) method.
Table 2. Primer sequences used for qRT-PCR.
| name | sequence (5'-3') |
|---|---|
| Tbp_forward | ACTCCTGCCACACCAGCC |
| Tbp_reverse | GGTCAAGTTTACAGCCAAGATTCA |
| Fgf23_forward | TGGCCATGTAGACGGAACAC |
| Fgf23_reverse | GGCCCCTATTATCACTACGGAG |
| Il6_forward | CAGAGTCATTCAGAGCAATAC |
| Il6_reverse | CTTTCAAGATGAGTTGGATGG |
| Tnfα_forward | CTCACACTCAGATCATCTTC |
| Tnfα_reverse | GAGAACCTGGGAGTAGATAAG |
| Nfatc1_forward | GAAGACTGTCTCCACCACCA |
| Nfatc1_reverse | CCGATGTCTGTCTCCCCTTT |
| Nfatc3_forward | TGATGGCCTTGGATCTCAGT |
| Nfatc3_reverse | CCCTCGGCTACCTTCAGTTT |
| Nfatc4_forward | GAAAGAGATGGCTGGCATGG |
| Nfatc4_reverse | CACCTCAATCCTCAGCTCCA |
| Hif1α_forward | GAAAGGATTACTGAGTTGATGG |
| Hif1α_reverse | CAGACATATCCACCTCTTTTTG |
| Phex_forward | ATGGCTGGATAAGCAATAAC |
| Phex_reverse | GCTTTTTCAATCGCTTTCTC |
| Dmp1_forward | ACTGTTATCCTCCTTACGTTC |
| Dmp1_reverse | GGTCTATACTGGCTTCTGTC |
FGF23 ELISA (C-Term)
UMR106 osteoblast-like cell culture supernatant was collected and frozen at -80°C. The medium was concentrated using Sartorius Vivaspin 6 Centrifugal Concentrators (Sartoritus, Göttingen, Germany). The concentration of C-terminal FGF23 was determined by an ELISA (Immutopics, San Clemente, CA, USA) according to the manufacturer’s instructions.
Statistics
Arithmetic means ± SEM were calculated, and n represents the number of independent experiments. Comparisons of two groups were made by unpaired Student’s t test, and for more than two groups, comparisons were calculated via one-way ANOVA, followed by Tukey’s or Dunnett’s multiple comparison tests, using GraphPad Prism. Differences were considered significant if p < 0.05.
Results
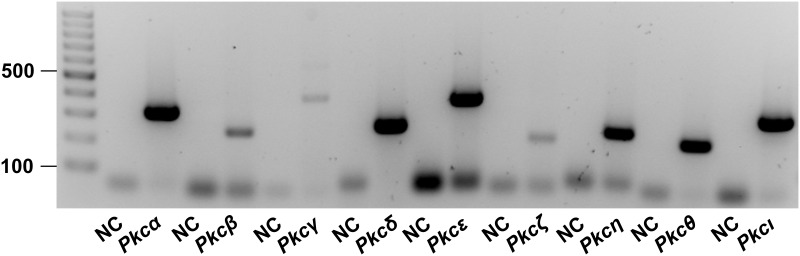
The relevance of PKC activity for the synthesis of FGF23 was studied in UMR106 osteoblast-like cells and IDG-SW3 osteocytes. First, the expression of Pkc isoforms was explored by RT-PCR. As demonstrated in Fig 1, mRNA specific for Pkcα, Pkcδ, Pkcε, Pkcη, Pkcθ, and Pkcι could readily be detected. The bands indicating the abundance of Pkcβ, Pkcγ, Pkcζ mRNA in UMR106 cells were weaker albeit detectable.
Fig 1. Expression of Pkc isoforms in UMR106 osteoblast-like cells.
Original agarose gel photo showing Pkcα, -β, -γ, -δ, -ε, -ζ, -η, -θ or -ι specific cDNA in UMR106 cells. NC: non-template control.
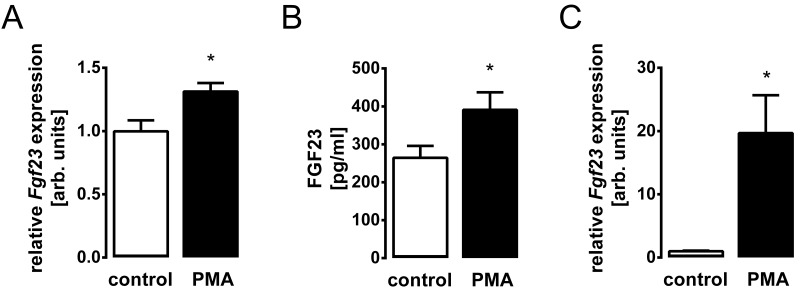
Phorbol ester 12-O-tetradecanoylphorbol-13-acetate (PMA) is a potent activator of PKC [3]. We treated UMR106 cells with and without PMA and determined Fgf23 transcripts by qRT-PCR. PMA treatment significantly up-regulated the abundance of Fgf23 mRNA (Fig 2A). As a next step, we explored whether PMA-stimulated Fgf23 gene expression translates into enhanced FGF23 production. To this end, we determined FGF23 protein in the supernatant of UMR106 cells. As shown in Fig 2B, PMA indeed stimulated FGF23 synthesis. Similar to osteoblasts, PKC activation with PMA enhanced Fgf23 gene expression in IDG-SW3 osteocytes (Fig 2C). These results suggest that PKC activity drives Fgf23 gene expression in osteoblasts and osteocytes.
Fig 2. PKC activator PMA induces FGF23 production in UMR106 osteoblast-like cells and in IDG-SW3 osteocytes.
Arithmetic means ± SEM (n = 6) of relative Fgf23 mRNA abundance normalized to Tbp in UMR106 osteoblast-like cells (A) or IDG-SW3 osteocytes (C), and FGF23 concentration in the cell culture supernatant of UMR106 cells (B) incubated without (white bars) or with (black bars) 0.1 μM PKC activator PMA. * p < 0.05 indicates significant difference. arb., arbitrary.
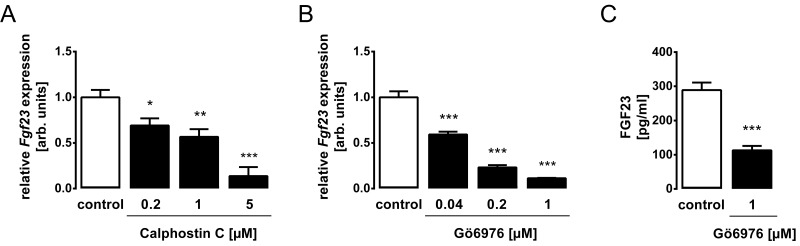
Our next series of experiments tested whether inhibition of PKC interferes with FGF23 expression. To this end, UMR106 cells were exposed to PKC inhibitors. As demonstrated in Fig 3, PKC inhibitor calphostin C (Fig 3A) and also PKCα/β inhibitor Gö6976 (Fig 3B) significantly and dose-dependently down-regulated Fgf23 gene expression in UMR106 cells. PKCα/β inhibitor Gö6976 also lowered the FGF23 protein concentration in the cell culture supernatant (Fig 3C). Thus, PKC is a stimulator of FGF23 expression.
Fig 3. PKC inhibitors Calphostin C and Gö6976 decrease FGF23 expression levels in UMR106 osteoblast cells.
UMR106 cells were treated without and with PKC inhibitors Calphostin C (A) or Gö6976 (B, C) at the indicated concentrations. Arithmetic means ± SEM (n = 6) of the relative Fgf23 mRNA abundance in UMR106 cells (A, B). Gene expression was normalized to Tbp as housekeeping gene. Arithmetic means ± SEM (n = 6) of FGF23 protein concentration in the cell culture supernatant (C). *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant difference. arb., arbitrary.
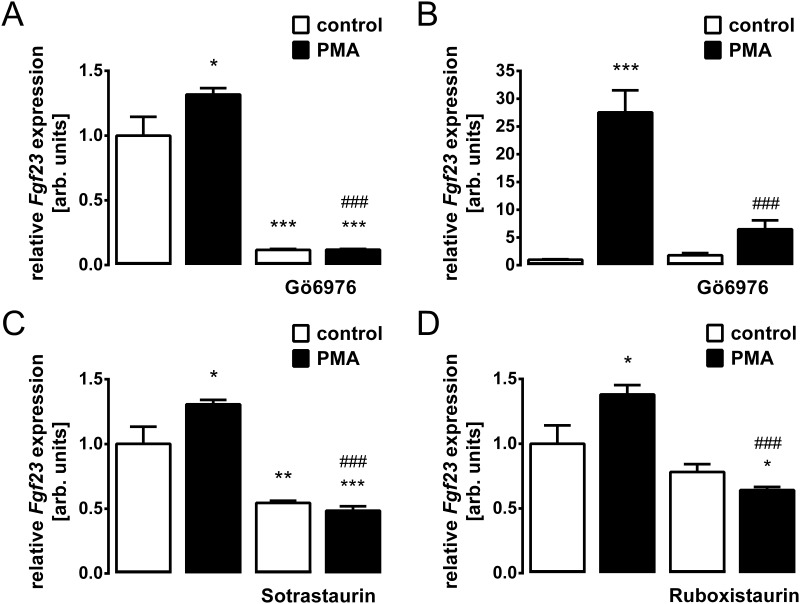
We investigated whether PMA-stimulated Fgf23 gene expression is indeed dependent on PKC activity using UMR106 and IDG-SW3 cells. As demonstrated in Fig 4, the PMA effect on Fgf23 gene expression was completely abrogated by PKC inhibitor Gö6976 in UMR106 osteoblast-like cells (Fig 4A) and in IDG-SWR3 osteocytes (Fig 4B), and also by PKC inhibitors sotrastaurin (Fig 4C) and ruboxistaurin (Fig 4D) in UMR106 cells.
Fig 4. PKC inhibition abrogates the PMA-induced increase in Fgf23 gene expression in UMR106 osteoblast-like cells and in IDG-SW3 osteocytes.
Relative Fgf23 transcript levels in UMR106 cells (A,C,D) or in IDG-SW3 cells (B) incubated without or with PMA (0.1 μM, A-D) in the absence and presence of PKCα/β inhibitor Gö6976 (1 μM, A,B), pan PKC inhibitor Sotrastaurin (1 μM, C) or PKCβ inhibitor Ruboxistaurin (1 μM, D). Gene expression was normalized to Tbp as a housekeeping gene, and the values are expressed as arithmetic means ± SEM (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant difference from vehicle (first bar). ###p < 0.001 indicates significant difference from the absence of PKC inhibitor (second bar vs. fourth bar). arb., arbitrary.
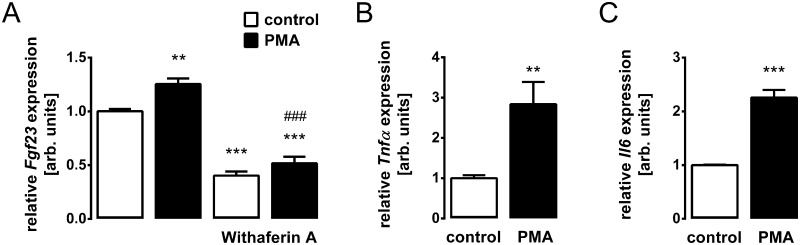
Finally, we sought to identify the mechanism of PKC-dependent FGF23 regulation. Since PKC is an activator of NFκB [41], a known regulator of FGF23, we treated UMR106 cells with and without NFκB inhibitor withaferin A in the absence and presence of PMA and determined Fgf23 gene expression. As demonstrated in Fig 5A, the PMA effect was indeed abrogated by withaferin A. Hence, PKC was, at least in part, effective through NFκB activity. The pro-inflammatory cytokines TNFα [32,33] and IL-6 [33,35] are also regulators of FGF23 synthesis. We therefore checked whether PKC activity impacts on their expression. According to Fig 5B and 5C PKC activation resulted in enhanced gene expression of Tnfα and Il-6 in UMR106 cells.
Fig 5. The PKC effect on Fgf23 gene expression is dependent on inflammation.
Relative Fgf23 transcript levels in UMR106 cells incubated without or with PMA (0.1 μM) in the absence and presence of NFκB inhibitor Withaferin A (0.5 μM) (A). Relative Tnfα (B) and Il6 (C) transcript levels in UMR106 cells incubated without or with PMA (0.1 μM). Gene expression was normalized to Tbp as a housekeeping gene, and the values are expressed as arithmetic means ± SEM (n = 6). **p < 0.01, and ***p < 0.001 indicate significant difference from vehicle (first bar). ###p < 0.001 indicates significant difference from the absence of withaferin A (second bar vs. fourth bar). arb., arbitrary.
Further transcription factors involved in FGF23 production include NFAT [42,43] and HIF-1α [44–46]. Therefore, we tested whether PKC induces the expression of these transcription factors. As a result, incubation of UMR106 cells with 0.1 μM PMA did not significantly affect Nfatc1 mRNA (control: 1.0 ± 0.02; PMA: 1.03 ± 0.02; n = 6), Nfatc4 mRNA (control: 1.0 ± 0.03; PMA: 1.02 ± 0.06; n = 6) or HIF-1α mRNA (control: 1.0 ± 0.02; PMA: 1.0 ± 0.05; n = 6). The abundance of Nfat3 mRNA was significantly down-regulated upon incubation with PMA (control: 1.0 ± 0.02; PMA: 0.79 ± 0.03; p < 0.001; n = 6).
The peptidase PHEX (phosphate regulating gene with homologies to endopeptidases on the X chromosome) and matrix protein DMP1 (dentin matrix protein-1) are further important regulators of FGF23 [47,48]. PKC activation did not affect Phex mRNA (control: 1.0 ± 0.02; PMA: 1.14 ± 0.04; n = 6) but significantly up-regulated Dmp1 mRNA (control: 1.0 ± 0.02; PMA: 255.76 ± 12.82; p < 0.001; n = 6).
Discussion
Our study discloses PKC as a novel regulator of FGF23 production. We provide experimental evidence that activation of PKC induces and inhibition of PKC suppresses Fgf23 gene expression: Four different PKC inhibitors were similarly capable of completely blocking PMA-induced Fgf23 expression. Moreover, altered Fgf23 gene expression translated into protein secretion as demonstrated by FGF23 protein measurements in the cell culture supernatant. These results unequivocally demonstrate the powerful role of PKC in regulating FGF23 production.
FGF23 is mainly produced by osteoblasts/osteocytes in the bone [9]. The differentiation of osteoblasts is driven by transcription factor MSX2 [49]. PKC enhances the proliferation of osteoblasts [50]. In contrast, PKC inhibits the differentiation of osteoblasts by targeting MSX2 [51] whereas FGF23 induces MSX2. [52]. Transcriptional activity of Runx2, also implicated in osteoblast differentiation, is enhanced by both PKC [53] and FGF23 [54].
We could show that four different pharmacological PKC inhibitors potently suppressed Fgf23 gene expression. Enhanced PKC activity contributes to the pathophysiology of several disorders, and pharmacological PKC inhibition has therefore been suggested as a therapeutic approach in multiple diseases including cancer, sequelae of diabetes, cardiovascular diseases, or inflammatory disorders such as psoriasis [55]. Some of these diseases, particularly renal and cardiovascular diseases, are associated with elevated FGF23 plasma levels [6], and FGF23 not only indicates disease, but actively contributes at least to left heart hypertrophy [12,13]. It therefore appears to be possible that the therapeutic benefit of PKC inhibition in some of these diseases is at least in part also due its FGF23-lowering properties.
Two different cell lines representing both osteoblasts and mature osteocytes were used in our study to decipher the effect of PKC on FGF23: PKC activation with PMA enhanced and PKC inhibition with different PKC inhibitors suppressed Fgf23 gene expression in both UMR106 osteoblast-like cells and IDG-SW3 osteocytes. This result suggests PKC-dependent regulation of FGF23 formation as a universal mechanism for the control of this hormone.
In an attempt to identify the underlying mechanism, we found that PKC-mediated up-regulation of Fgf23 expression is sensitive to NFκB inhibition. NFκB, as a p65/p50 dimer, is inactivated by binding inhibitory κB (IκB). The inhibitory κB kinase (IKK) phosphorylated IκB, leading to its ubiquitination and degradation, paralleled by the release and activation of NFκB [56]. Therefore, the activation of the IKK complex plays a crucial role in the induction of NFκB, and PKC activates NFκB through this kinase [41]. Clearly, inflammation is a major trigger of FGF23 production [4,36]. In detail, NFκB up-regulates Ca2+ release-activated Ca2+ (CRAC) channel Orai1/STIM1 accomplishing store-operated Ca2+ entry (SOCE) and inducing the transcription of the Fgf23 gene [36]. According to our experiment with NFκB inhibitor withaferin A, the PKC effect on FGF23 was, at least partly, dependent on NFκB pointing to the decisive role of this pro-inflammatory transcription factor complex in the regulation of FGF23. Moreover, PKC activation induced the expression of pro-inflammatory cytokines TNFα and IL-6 which are themselves stimulators of FGF23 production [33]. Therefore, the enhanced formation of these cytokines may contribute to the PKC effect on FGF23. NFAT isoforms and HIF1α, further regulators of FGF23, were not affected by PKC activity in UMR106 cells.
We observed a PKC-mediated up-regulation of FGF23 regulator DMP1 in UMR106 cells which would be expected to lower FGF23 levels. Possibly, the PKC effect on FGF23 involving NFκB overcomes the expected down-regulation of FGF23 due to PKC-mediated DMP1 induction. Alternatively, enhanced PKC-stimulated FGF23 formation is limited by counter regulatory DMP1 induction. Clearly, further studies are warranted to address this question. In addition, since our study was carried out in vitro only, its in vivo relevance will also have to be verified separately.
In conclusion, our study identified PKC as a novel regulator of FGF23 production in both osteoblast and osteocytes, being at least partially effective via NFκB signaling. Therefore, the therapeutic modulation of PKC activity in chronic disease may impact on the plasma FGF23 concentration.
Acknowledgments
The authors acknowledge the technical assistance of S. Ross and F. Reipsch.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The study was supported by the Deutsche Forschungsgemeinschaft [Fo 695/2-1].
References
- 1.STEINBERG SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008; 88: 1341–1378. 10.1152/physrev.00034.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet. Nat Rev Cancer. 2007; 7: 554–562. 10.1038/nrc2168 [DOI] [PubMed] [Google Scholar]
- 3.Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol. 2018; 48: 36–52. 10.1016/j.semcancer.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 4.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016; 89: 135–146. 10.1038/ki.2015.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutiérrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS ONE. 2015; 10: e0122885 10.1371/journal.pone.0122885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnedl C, Fahrleitner-Pammer A, Pietschmann P, Amrein K. FGF23 in Acute and Chronic Illness. Dis Markers. 2015; 2015: 358086 10.1155/2015/358086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spichtig D, Zhang H, Mohebbi N, Pavik I, Petzold K, Stange G, et al. Renal expression of FGF23 and peripheral resistance to elevated FGF23 in rodent models of polycystic kidney disease. Kidney Int. 2014; 85: 1340–1350. 10.1038/ki.2013.526 [DOI] [PubMed] [Google Scholar]
- 8.Itoh N, Ohta H. Pathophysiological roles of FGF signaling in the heart. Front Physiol. 2013; 4: 247 10.3389/fphys.2013.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuro-O M, Moe OW. FGF23-αKlotho as a paradigm for a kidney-bone network. Bone. 2017; 100: 4–18. 10.1016/j.bone.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 10.Tsuprykov O, Chen X, Hocher C-F, Skoblo R, Lianghong Y, Hocher B. Why should we measure free 25(OH) vitamin D. J Steroid Biochem Mol Biol. 2018; 180: 87–104. 10.1016/j.jsbmb.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113: 561–568. 10.1172/JCI19081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121: 4393–4408. 10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faul C. Cardiac actions of fibroblast growth factor 23. Bone. 2017; 100: 69–79. 10.1016/j.bone.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leifheit-Nestler M, Grabner A, Hermann L, Richter B, Schmitz K, Fischer D-C, et al. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant. 2017; 32: 1493–1503. 10.1093/ndt/gfw454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossaint J, Oehmichen J, van Aken H, Reuter S, Pavenstädt HJ, Meersch M, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016; 126: 962–974. 10.1172/JCI83470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agoro R, Montagna A, Goetz R, Aligbe O, Singh G, Coe LM, et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018; 32: 3752–3764. 10.1096/fj.201700667R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flamme I, Ellinghaus P, Urrego D, Krüger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS ONE. 2017; 12: e0186979 10.1371/journal.pone.0186979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016; 90: 985–996. 10.1016/j.kint.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390: 45–51. 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 20.Kuro-O M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013; 9: 650–660. 10.1038/nrneph.2013.111 [DOI] [PubMed] [Google Scholar]
- 21.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007; 18: 2116–2124. 10.1681/ASN.2006121385 [DOI] [PubMed] [Google Scholar]
- 22.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, Feger M, et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest. 2013; 123: 812–822. 10.1172/JCI64093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuro-O M. Klotho and endocrine fibroblast growth factors: marker of chronic kidney disease progression and cardiovascular complications. Nephrol Dial Transplant. 2018. 10.1093/ndt/gfy126 [DOI] [PubMed] [Google Scholar]
- 24.Graciolli FG, Neves KR, Barreto F, Barreto DV, Dos Reis LM, Canziani ME, et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017; 91: 1436–1446. 10.1016/j.kint.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 25.Pulskens WP, Verkaik M, Sheedfar F, van Loon EP, van de Sluis B, Vervloet MG, et al. Deregulated Renal Calcium and Phosphate Transport during Experimental Kidney Failure. PLoS ONE. 2015; 10: e0142510 10.1371/journal.pone.0142510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egli-Spichtig D, Zhang MYH, Perwad F. Fibroblast Growth Factor 23 Expression Is Increased in Multiple Organs in Mice With Folic Acid-Induced Acute Kidney Injury. Front Physiol. 2018; 9: 1494 10.3389/fphys.2018.01494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christov M. Fibroblast growth factor 23 in acute kidney injury. Curr Opin Nephrol Hypertens. 2014; 23: 340–345. 10.1097/01.mnh.0000447021.51722.2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010; 299: F882–9. 10.1152/ajprenal.00360.2010 [DOI] [PubMed] [Google Scholar]
- 29.Masuyama R, Stockmans I, Torrekens S, van Looveren R, Maes C, Carmeliet P, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006; 116: 3150–3159. 10.1172/JCI29463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari SL, Bonjour J-P, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005; 90: 1519–1524. 10.1210/jc.2004-1039 [DOI] [PubMed] [Google Scholar]
- 31.Rodelo-Haad C, Rodríguez-Ortiz ME, Martin-Malo A, Pendon-Ruiz de Mier MV, Agüera ML, Muñoz-Castañeda JR, et al. Phosphate control in reducing FGF23 levels in hemodialysis patients. PLoS ONE. 2018; 13: e0201537 10.1371/journal.pone.0201537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glosse P, Fajol A, Hirche F, Feger M, Voelkl J, Lang F, et al. A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFα upregulation. Nutr Diabetes. 2018; 8: 36 10.1038/s41387-018-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015; 399: 208–218. 10.1016/j.mce.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki M, Kawai M, Miyagawa K, Ohata Y, Tachikawa K, Kinoshita S, et al. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J Bone Miner Metab. 2015; 33: 342–354. 10.1007/s00774-014-0598-2 [DOI] [PubMed] [Google Scholar]
- 35.Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018; 94: 315–325. 10.1016/j.kint.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Yan J, Umbach AT, Fakhri H, Fajol A, Schmidt S, et al. NFκB-sensitive Orai1 expression in the regulation of FGF23 release. J Mol Med. 2016; 94: 557–566. 10.1007/s00109-015-1370-3 [DOI] [PubMed] [Google Scholar]
- 37.Feger M, Hase P, Zhang B, Hirche F, Glosse P, Lang F, et al. The production of fibroblast growth factor 23 is controlled by TGF-β2. Sci Rep. 2017; 7: 4982 10.1038/s41598-017-05226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glosse P, Feger M, Mutig K, Chen H, Hirche F, Hasan AA, et al. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int. 2018; 94: 491–501. 10.1016/j.kint.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 39.Bär L, Feger M, Fajol A, Klotz L-O, Zeng S, Lang F, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci U S A. 2018; 115: 5804–5809. 10.1073/pnas.1800160115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011; 26: 2634–2646. 10.1002/jbmr.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moscat J, Diaz-Meco MT, Rennert P. NF-kappaB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003; 4: 31–36. 10.1038/sj.embor.embor704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bär L, Großmann C, Gekle M, Föller M. Calcineurin inhibitors regulate fibroblast growth factor 23 (FGF23) synthesis. Naunyn Schmiedebergs Arch Pharmacol. 2017; 390: 1117–1123. 10.1007/s00210-017-1411-2 [DOI] [PubMed] [Google Scholar]
- 43.Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018; 94: 60–71. 10.1016/j.kint.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 44.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011; 108: E1146–55. 10.1073/pnas.1110905108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014; 29: 361–369. 10.1002/jbmr.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, et al. The hypoxia-inducible factor-1α activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016; 4: 16011 10.1038/boneres.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowe PSN. Regulation of Bone—Renal Mineral and Energy Metabolism: The PHEX, FGF23, DMP1, MEPE ASARM Pathway. Crit Rev Eukar Gene Expr. 2012; 22: 61–86. 10.1615/CritRevEukarGeneExpr.v22.i1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011; 25: 2551–2562. 10.1096/fj.10-177816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004; 279: 34015–34022. 10.1074/jbc.M403621200 [DOI] [PubMed] [Google Scholar]
- 50.Galea GL, Meakin LB, Williams CM, Hulin-Curtis SL, Lanyon LE, Poole AW, et al. Protein kinase Cα (PKCα) regulates bone architecture and osteoblast activity. J Biol Chem. 2014; 289: 25509–25522. 10.1074/jbc.M114.580365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong HM, Jin Y-H, Choi YH, Yum J, Choi J-K, Yeo C-Y, et al. PKC signaling inhibits osteogenic differentiation through the regulation of Msx2 function. Biochim Biophys Acta. 2012; 1823: 1225–1232. 10.1016/j.bbamcr.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 52.Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, et al. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014; 85: 1103–1111. 10.1038/ki.2013.332 [DOI] [PubMed] [Google Scholar]
- 53.Kim H-J, Kim J-H, Bae S-C, Choi J-Y, Ryoo H-M. The protein kinase C pathway plays a central role in the fibroblast growth factor-stimulated expression and transactivation activity of Runx2. J Biol Chem. 2003; 278: 319–326. 10.1074/jbc.M203750200 [DOI] [PubMed] [Google Scholar]
- 54.Orfanidou T, Iliopoulos D, Malizos KN, Tsezou A. Involvement of SOX-9 and FGF-23 in RUNX-2 regulation in osteoarthritic chondrocytes. J Cell Mol Med. 2010; 13: 3186–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target. Nat Rev Drug Discov. 2012; 11: 937–957. 10.1038/nrd3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8: 49–62. 10.1038/nrm2083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.