Abstract
Background
Angiogenesis and vascular remodeling are complementary, innate responses to ischemic cardiovascular events, including peripheral artery disease (PAD) and myocardial infarction, which restore tissue blood supply and oxygenation; the endothelium plays a critical function in these intrinsic protective processes. C-type natriuretic peptide (CNP) is a fundamental endothelial signaling species that coordinates vascular homeostasis. Herein, we sought to delineate a central role for CNP in angiogenesis and vascular remodeling in response to ischemia.
Methods
The in vitro angiogenic capacity of CNP was examined in pulmonary microvascular endothelial cells (PMEC) and aortic rings isolated from wild type (WT), endothelium-specific CNP knockout (ecCNP-/-), and global natriuretic peptide receptor (NPR)-B-/- and NPR-C-/- animals, and in human umbilical vein endothelial cells (HUVEC). These studies were complemented by in vivo investigation of neovascularization and vascular remodeling following ischemia or vessel injury, and CNP/NPR-C expression & localization in tissue from patients with PAD.
Results
Clinical vascular ischemia is associated with reduced levels of CNP and its cognate NPR-C. Moreover, genetic or pharmacological inhibition of CNP and NPR-C, but not NPR-B, reduces the angiogenic potential of PMEC, HUVEC and isolated vessels ex vivo. Angiogenesis and remodeling are impaired in vivo in ecCNP-/- and NPR-C-/-, but not NPR-B-/-, mice; the detrimental phenotype caused by genetic deletion of endothelial CNP, but not NPR-C, can be rescued by pharmacological administration of CNP. The pro-angiogenic effect of CNP/NPR-C is dependent on activation of Gi, ERK1/2 and PI3Kγ/Akt at a molecular level.
Conclusions
These data define a central (patho)physiological role for CNP in angiogenesis and vascular remodeling in response to ischemia and provide the rationale for pharmacological activation of NPR-C as an innovative approach to treating PAD and ischemic cardiovascular disorders.
Keywords: Natriuretic peptide, natriuretic peptide receptor, endothelium, angiogenesis, vascular remodeling, arteriogenesis, ischemia, peripheral artery disease
Introduction
The morbidity and mortality associated with peripheral arterial disease (PAD) is an expanding unmet medical need as a consequence of the ageing population and growing prevalence of metabolic disorders1, 2. PAD affects more than 200 million individuals worldwide and typically manifests as intermittent claudication but often progresses to, or even presents as, critical limb ischemia (CLI) characterized by pain, ulceration and gangrene; many patients face amputation and early death1, 2. Pathology is triggered by atherosclerotic vascular occlusions and is underpinned by an insufficient angiogenic (hypoxia-triggered de novo blood vessel formation) and arteriogenic (shear stress-induced remodeling of the collateral network) response; compensatory and complementary mechanisms invoked to reinstate blood supply to the ischemic tissue3. A central pathway coordinating both processes is driven by vascular endothelial growth factor (VEGF)-A4, which pre-clinical studies indicated was a potential treatment for PAD5. However, large-scale clinical trials using VEGF-A to promote angiogenesis and/or arteriogenesis in PAD and CLI patients have proven disappointing6–8. Therefore, delineation of the mechanisms and signaling pathways that underlie these intrinsic, restorative pathways are critical if the therapeutic potential of a pro-angiogenic strategy is to be harnessed.
The endothelium is pivotal in triggering angiogenesis, vascular remodeling and minimizing functional deficit following ischemia9. Endothelium-derived C-type natriuretic peptide (CNP) plays a fundamental role in regulating vascular homeostasis10–12; CNP controls local blood flow in the resistance vasculature and systemic blood pressure, reduces the reactivity of leukocytes and platelets, and prevents the development of atherogenesis and aneurysm. Several facets of CNP biology suggest it may play a central role in angiogenesis. For instance, two of the primary stimuli for CNP release from endothelial cells are shear stress13 and TGFβ14, both of which are well-validated triggers for angiogenesis & arteriogenesis. In addition, HIF-1α, a fundamental driver of the ischemic angiogenic response, is a potent enhancer of natriuretic peptide expression, particularly in cardiomyocytes15. Our previous studies revealed that pulmonary microvascular endothelial cells from endothelium-specific CNP-/- (ecCNP-/-) and global NPR-C-/- mice proliferate significantly more slowly compared to wild type (WT) cells16, implying that CNP has a physiological role in regulating growth. The peptide also slows neointimal hyperplasia and promotes re-endothelialisation in vein grafts17, in damaged carotid arteries18, and following balloon angioplasty19, 20. CNP also maintains capillary density after myocardial infarction21 and hind-limb ischemia22. Whilst these observations intimate that pharmacological administration of CNP may promote arteriogenesis & angiogenesis, previous work has reported that CNP blocks VEGF signaling to attenuate angiogenesis in vitro23, and reduces sponge implant neovascularisation in vivo24, demonstrating the function of CNP in this setting remains unclear and requires further investigation. Moreover, there is no evidence demonstrating that endothelium-derived CNP either directly stimulates angiogenesis, or triggers endothelial cell processes critical for angiogenesis. Therefore, given recent findings showing an important role for CNP in vascular homeostasis10–12, we sought to investigate whether endogenous CNP is a key regulator of angiogenesis and vascular remodeling.
Methods
Ethical permission
All murine studies conformed to the UK Animals (Scientific Procedures) Act of 1986 and had approval from the local Animal Welfare and Ethical Review Body (AWERB) within Bart’s and The London School of Medicine. The human tissue studies were permitted under Local Research Ethics Committee decision 16/WA/0198 (IRAS project ID: 193340) with informed patient consent. The authors declare that all supporting data are available within the article (and its online Supplemental files).
Endothelial 3D-tube formation assay
Primary murine pulmonary endothelial cells (PMECs) were isolated as we have described previously16. PMEC were re-suspended in diluted ECGM (1:3 in DMEM/F12) and plated on 15 mg/mL reduced-growth factor extracellular matrix (Cultrex, Trevigen, USA) at a density of 1.75 × 105 cells/cm2. Tubule formation was determined by measuring branch number and length using NIH Image J software, 2, 4 and 6 h after treatment with CNP (1 nM to 1 μM, GenScript, USA) or VEGF (30 ng/mL, Pre-protech, USA).
Tubule formation in HUVEC was measured following treatment with CNP (1 nM) or ANP (10 nM) in the absence or presence of selective NPR-C antagonist M372049 (10 μM), the selective PI3Kγ inhibitor AS605240 (100 nM; Sigma), or the selective PKG blocker KT5283 (2 μM; Sigma) over a 16 h period. A cohort of cells was transfected using lipofectamine 2000 (Thermofisher Scientific) with either non-specific siRNA (Mission Control, Sigma Aldrich), NPR-B- or NPR-C- specific siRNA (Sigma Aldrich) and were treated with CNP or the selective NPR-C agonist, cANF4–23 (2 nM). Silencing of NPR-B and NPR-C was confirmed at the mRNA and protein level using RT-PCR and immunoblot (Supplemental Figure 1A-C).
Cell migration assay
Endothelial cells were plated in gelatin-coated wells of a 96 well plate (Corning® Biocoat, UK) at a density of 1.75 × 105 cells/cm2 and left to reach confluence. A scratch was performed using a 10 μL sterile pipette and images were taken at regular intervals over a 24 h period to monitor scratch closure. Cell populations were treated with CNP (1 nM to 1 μM) or VEGF (30 ng/mL).
Aortic sprouting assay
Mice were killed by cervical dislocation and thoracic aortas were removed, trimmed of all extraneous tissue and flushed with media via the lumen to remove all blood. Rings of ~0.5 mm were cut and embedded 1 mg/mL collagen matrix (type 1 rat tail, Millipore, Germany) and incubated for 1 h at 37°C. Opti-MEM + Glutamax media (Opti-MEM, Gibco, UK) containing 2.5% bovine serum (Gibco, UK), 50 U/mL penicillin and 0.5 mg/mL streptomycin (Sigma-Aldrich, UK) and either CNP (1 nM to 1 μM) or VEGF (30 ng/mL) or was added to the wells. Interventions were refreshed every 2 days and images of sprouting aortae were obtained after 7 days.
Matrigel plug neovascularization
Mice were injected subcutaneously with 15 mg/mL reduced-growth factor extracellular matrix (Cultrex, Trevigen, USA) containing 30 ng/mL VEGF (Pre-protech, USA) and 50 U Heparin (Pfizer, UK), which formed a solid ‘plug’ at body temperature. Plugs were extracted after 14 days and homogenized in 0.5 mL of cell lysis buffer and centrifuged at 6000 x g at 4 °C for 60 min. Hemoglobin was detected at 400 nm wavelength, using a colorimetric assay (Sigma-Aldrich, UK). Histological analysis of fixed and paraffin embedded matrigel plugs was performed using hematoxylin and eosin (H&E) and isolectin B4 staining.
Hindlimb ischemia (HLI)
Mice were anaesthetized with 1.5-2% isoflurane vaporized in oxygen, placed on a heated blanket to maintain body temperature and a small incision (~10 mm) was made in the hindlimb skin directly over the femoral vasculature. All mice received pre-operative analgesia (buprenorphine, 0.1mg/kg, Vetergesic, Alstoe Animal health). A portion of the femoral artery was exposed via a 2 cm incision and two ligations were performed using eight nylon sutures, first distal to the origin of the profunda femoris artery and second proximal to the saphenous artery. The femoral artery was then excised between the ligation sites and the skin was closed with non-continuous absorbable suture. Non-invasive laser Doppler imaging (Moor LDI2, Moor Instruments Ltd, UK) was used to assess hindlimb blood flow at baseline and immediately after undergoing HLI, with subsequent imaging at day 3, 7, 14, 21 and 28 post HLI. The rate of reperfusion in the hindlimb was calculated as a ratio of blood flow in the ipsilateral ischemic versus non-ischemic in the contralateral limb. In some experiments, mice were implanted with osmotic mini-pumps (1002; Alzet) containing CNP (0.2mg/kg/day) at day 0 to explore if any phenotype produced by gene KO could be reversed by pharmacological application of the peptide. At the end of the study (Day 28), mice were briefly anaesthetized with isoflurane and blood was collected via cardiac puncture. Mice were culled by cervical dislocation and the gastrocnemius muscle from each leg was harvested for post analysis. In some experiments, the gastrocnemius muscle from each leg was harvested at day 3 and day 7 post HLI from WT mice, treated with an RNA stabilizer (RNAlater, Sigma, UK) and stored at -20°C before RNA isolation.
Carotid injury
Mice were anaesthetized using 1.5-2 % isoflurane in oxygen. Left internal carotid arteries were exposed and isolated from surrounding nerves and tissue. A 0.35 mm wire catheter was introduced via a small incision and inserted and extracted repeatedly, 3 times, over ~15 mm length of vessel, to remove the endothelial cell layer. Vessels were then and skin sutured using sterile 6-0 silk and mice allowed to recover. Mice were given buprenorphine (0.1 mg/kg) at the beginning of surgery for management of post-surgical pain. After 14 days, mice were anaesthetized with sodium pentobarbital (100 mg/mL). Blood was cleared using phosphate buffered saline, and mice were then perfusion fixed with 4% paraformaldehyde at 100 mmHg. Injured left and control right carotid arteries were dissected out and embedded in paraffin. 4 μm sections every 500 μm were cut and stained with H&E and α-smooth muscle actin (1:5000) for histological analysis. Vessel dimensions were measured using Image J analysis after calibration with a micrometer on H&E stained sections and intimal-media thickness ratio was calculated.
Cutaneous wound healing
The rate of skin wound closure following injury was determined using a cutaneous wound healing model. Mice were anesthetized with 1.5-2 % isoflurane and the dorsal skin was shaved and cleaned with alcohol. Per mouse, one bilateral full-thickness skin wound were created, using a sterile 3 mm biopsy punch on the dorsorostral back skin without injuring the underlying muscle. Wounds were digitally photographed at 0, 4, and 7 days after injury using a digital camera (Sony Europe Limited, Weybridge, UK).
Protein and mRNA quantification and immunohistochemistry
These were conducted according to standard protocols. Further information can be found in the Supplemental Information and Supplemental Table 1.
Data analysis
Results are expressed as mean ± s.e.mean, and the n value denotes the number of animals or independent experiments per group. Statistical analyses were performed (GraphPad Prism version 6; GraphPad, La Jolla, CA, USA) using Student’s t-test, one-way or two-way ANOVA with Bonferroni post hoc tests as appropriate.
Results
Expression of CNP and NPR-C are downregulated in human CLI patients
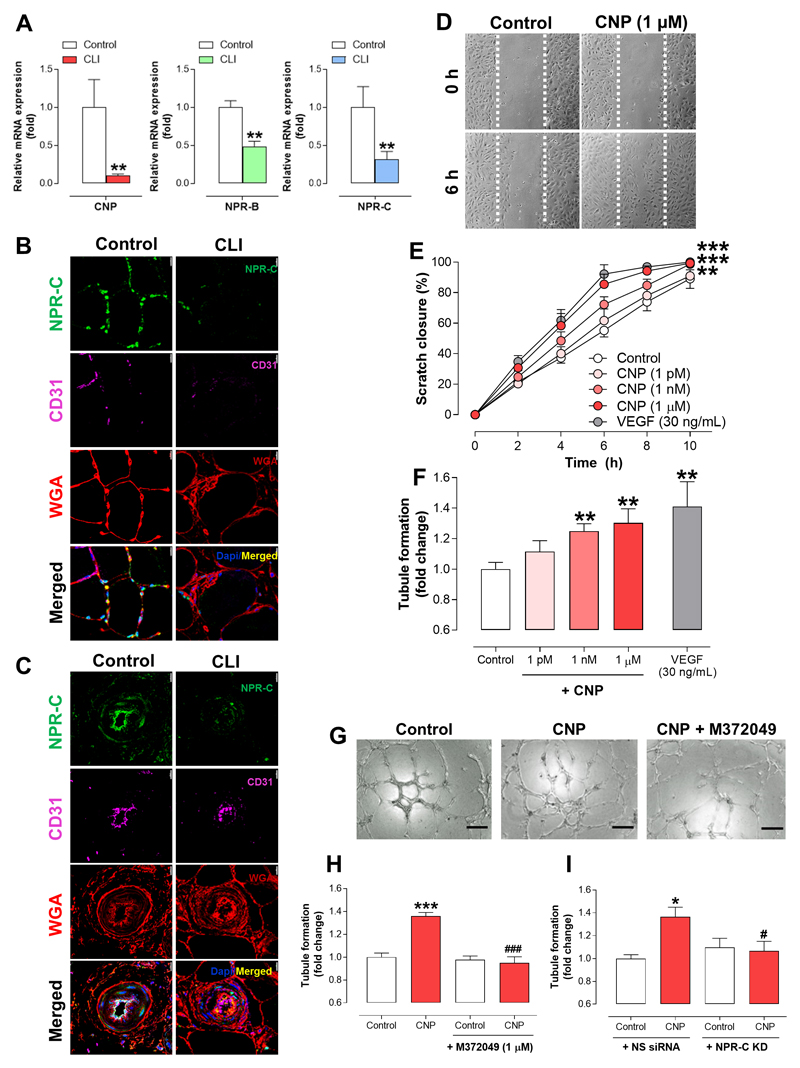
Expression of VEGF-A and VEGF receptor 2 (VEGFR2) mRNA was significantly reduced whereas hypoxia-inducible factor 1α (HIF-1α), apoptosis regulator Bcl-2 associated X protein (Bax), vascular cell adhesion protein-1 (VCAM-1), interleukin-6 (IL-6) and C-C Motif Chemokine Ligand 2 (CCL2) mRNA were all elevated in gastrocnemius muscle biopsies from CLI patients in comparison to healthy controls, commensurate with a compromised angiogenic, re-modelling environment25, 26 (Supplemental Figure 2A). Evidence that CNP/NPR-C signalling might be critical to a pro-angiogenic, pro-arteriogenic host defense response to ischemia was provided by a parallel reduction in both CNP (mRNA) and NPR-C (mRNA & protein) levels in CLI tissue (NPR-B mRNA expression was also reduced; Figure 1A & Supplemental Figure 3A). Furthermore, NPR-C protein expression, which co-localized with the cell membrane (WGA-positive) and vascular endothelium (CD31-positive) was markedly reduced around both the myofibers (Figure 1B) and blood vessels (Figure 1C) in gastrocnemius samples of CLI patients compared to healthy controls.
Figure 1. C-type natrireutic peptide promotes angiogenesis in human umbilical vein endothelial cells via natriuretic peptide receptor-C.
(A) C-type natriuretic peptide (CNP), natriuretic peptide receptor (NPR)-B and NPR-C mRNA expression is markedly reduced in gastrocnemius muscle biopsies of critical limb ischemia (CLI) patients compared to healthy subjects (Control; n=6-7), NPR-C protein levels are significantly reduced in gastrocnemius (B) myofibers and (C) blood vessels of CLI patients compared to healthy controls (cell membrane marker wheat germ agglutinin, WGA; endothelium specific marker, CD31; scale bars, 15 μm; n=6-7), (D) Representative images and (E) quantitative analysis showing that CNP (1 pM – 1 μM) accelerates human umbilical endothelial cell (HUVEC) migration (i.e. scratch closure) with a time-course similar to vascular endothelial growth factor (VEGF; 30 ng/mL) (n=6); (F) CNP (1 pM – 1 μM) promotes tubule formation in HUVECs with a maximal magnitude commensurate with VEGF (30 ng/mL) (n=4), (G & H) the NPR-C antagonist, M372049 (10 μM) and (I) NPR-C knockdown attenuates CNP (1 nM)-induced tubule formation (scale bars, 100 μm; n=6). Data are presented as mean ± SEM. Statistical analyses by (A) Student’s t-test, (E) two-way ANOVA or (F, H & I) one-way ANOVA with Bonferoni post hoc test. *P<0.05, **P<0.01, ***P<0.001 versus healthy/control or #P<0.05, ###P<0.001 versus CNP alone.
CNP promotes angiogenesis in human and murine endothelial cells via NPR-C
Accordingly, exogenous CNP increased the rate of migration of HUVECs in a concentration-dependent manner with the highest concentration producing a response with a similar time-course and magnitude to the archetypal pro-angiogenic mediator, VEGF (Figure 1D-E). Pharmacological administration of CNP also promoted tubule formation in HUVEC with a maximal effect commensurate with that produced by VEGF (Figure 1F). Combination of CNP and VEGF result in a modest additive effect on tubule forming activity across genotypes (Supplemental Figure 4A), suggesting the two mediators are not inter-dependent. In order to substantiate the thesis that NPR-C activation underpins the pro-angiogenic action of CNP, genetic and pharmacological deletion of NPR-C was employed in HUVEC to demonstrate an anti-angiogenic phenotype. Indeed, in the presence of both the selective NPR-C antagonist M237204927 (Figure 1G-H) and siRNA knockdown of NPR-C (Figure 1I & Supplemental Figure 1A-B), the tubule formation driven by CNP was abrogated. Furthermore, siRNA knockdown of NPR-B did not alter the pro-angiogenic capacity of CNP (Supplemental Figure 1C-D). The selective NPR-C agonist27, cANF4–23, also produced an essentially identical increase in tubule formation that was sensitive to blockade by M372049 (Supplemental Figure 4B). These data establish the pro-angiogenic capacity of CNP-NPR-C signaling in human endothelial cells and provide direct evidence that such a pathway is downregulated in human PAD/CLI. Interestingly, increases in tubule formation driven by ANP were not sensitive to blockade by M372049 (Supplemental Figure 5), intimating that this member of the natriuretic peptide family is unable to trigger angiogenic behavior in endothelial cells via NPR-C; this finding fits well with previous work identifying NPR-A/cGMP/PKGI as the key pathway underpinning ANP/BNP-driven revascularization following ischemia22, 28.
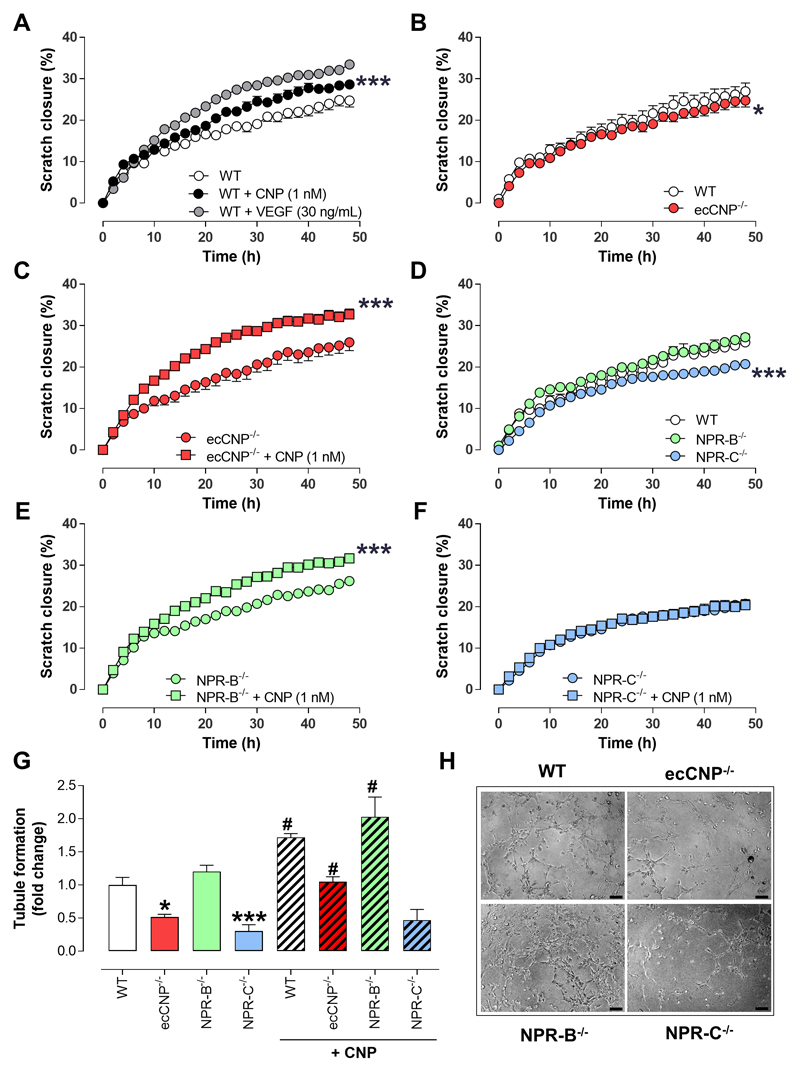
To reiterate the importance of endothelium-derived CNP in angiogenic signalling, murine PMECs were isolated from ecCNP-/- animals, in which CNP is conditionally deleted in endothelial cells, and from WT littermates and used to investigate in vitro migration and tubule formation. Consistent with the HUVEC data, CNP promoted migration in a scratch closure assay in WT murine PMECs in a concentration-dependent manner and with a similar maximal activity to VEGF (Figure 2A & Supplemental Figure 6). The inherent migratory capacity of PMECs was reduced subtly in cells from ecCNP-/- mice compared to WT littermates (Figure 2B). However, exogenous CNP was able to increase the rate of scratch closure in cells from both genotypes to a similar extent to that observed in HUVEC (Figure 2A,C). Of note, the relative increase in migration in PMECs from ecCNP-/- mice was greater than that in WT cells, suggesting a sensitization of the underlying signaling pathway. PMECs from mice with global deletion of NPR-B or NPR-C were utilized to confirm the NPR subtype underlying the actions of CNP on endothelial migration and tube formation. PMECs from NPR-B-/- mice had an intrinsic migratory capacity equivalent to WT (Figure 2D), whereas PMECs from NPR-C-/- animals exhibited a significantly slower scratch closure (Figure 2D). Whilst pharmacological addition of CNP promoted endothelial migration in NPR-B-/- cells (Figure 2E), it had no significant effect in NPR-C-/- cells (Figure 2F). This receptor-specific activity was recapitulated in assays of endothelial tubule formation, in which ecCNP-/- and NPR-C-/- cells displayed significantly impaired intrinsic tubule-forming activity compared to cells from WT and NPR-B-/- mice (Figure 2G-H). Likewise, the ability of exogenous CNP to stimulate tubule development was only apparent in WT, ecCNP-/- and NPR-B-/- cells, but was abrogated in cells from NPR-C-/- mice (Figure 2G-H). These findings corroborate a fundamental role for endothelial CNP-NPR-C signaling in stimulating cellular functions essential for angiogenesis.
Figure 2. C-type natriuretic peptide increases scratch closure and tubule formation in murine pulmonary microvascular endothelial cells via natriuretic peptide receptor-C.
(A) Migration (i.e. scratch closure) of murine pulmonary microvascular endothelial cells (PMEC) from wild type (WT) mice is facilitated by C-type natriuretic peptide (CNP; 1 nM) in a similar manner to vascular endothelial growth factor (VEGF; 30 ng/mL; n=6), (B) PMEC migration is impaired in cells from endothelium-specific CNP knockout (ecCNP-/-) mice compare to WT littermates (n=6), but (C) can be reversed by pharmacological addition of CNP (1 nM; n=6), (D) PMEC migration is arrested in cells from global natriuretic peptide receptor-C knockout (NPR-C-/-) but not global NPR-B-/- animals (n=6), (E & F) CNP (1 nM) augments PMEC migration in cells from NPR-B-/-, but not NPR-C-/- mice (n=6), (G & H) Intrinsic tubule forming capacity in PMEC is impaired in cells from ecCNP-/- and NPR-C-/-, but not NPR-B-/-, mice whereas CNP (1 nM) promotes tubule formation in WT, ecCNP-/- and NPR-B-/- PMEC but is unable to do so in NPR-C-/- cells (scale bars, 100 μm; n=12-15). Data are presented as mean ± SEM. Statistical analyses by (A-F) two-way ANOVA or (G) one-way ANOVA with Bonferroni post hoc test. *P<0.05, ***P<0.001 versus WT or absence of CNP (A-F), *P<0.05, ***P<0.001 versus WT or #P<0.05 versus corresponding genotype in the absence of CNP (G).
De novo sprouting ex vivo and neovascularization in vivo is diminished in vessels lacking either endothelial CNP or NPR-C
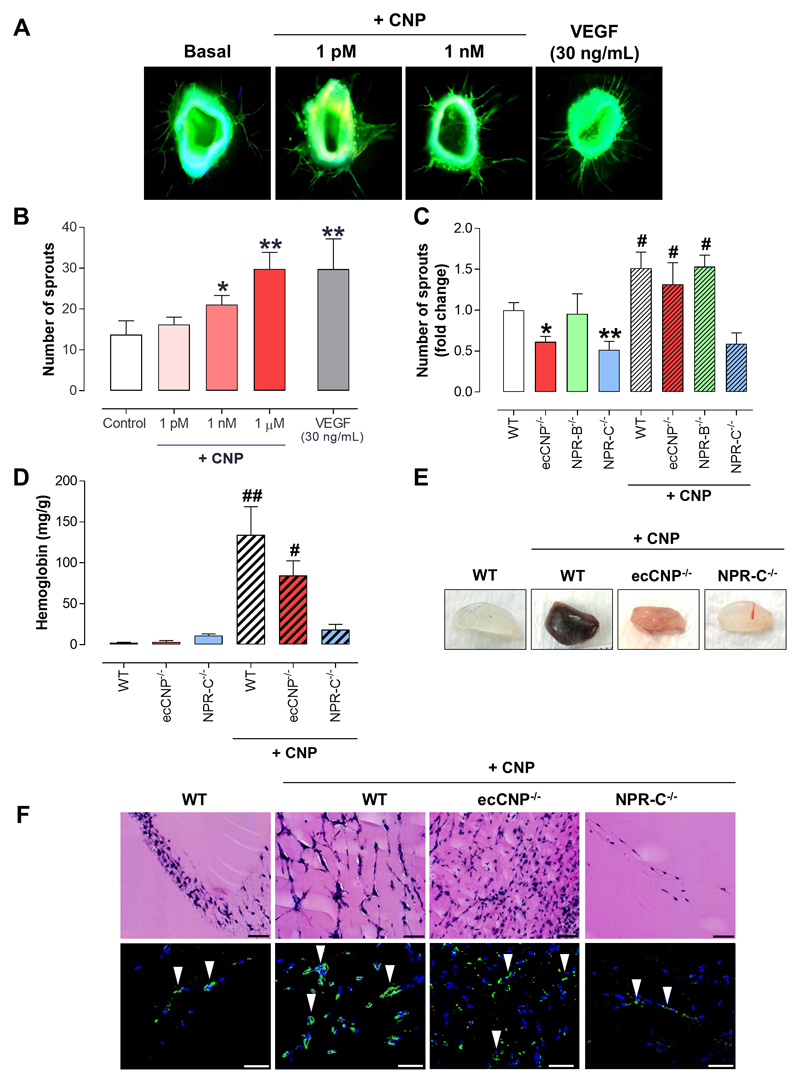
A more complex ex vivo model of angiogenic sprouting was subsequently employed to verify this novel angiogenic role for endothelium-derived CNP. Aortic sprouting in vessel segments from WT animals was potentiated in a concentration-dependent manner by treatment with CNP with a time-course and response magnitude equivalent to those of VEGF (Figure 3A-B). Furthermore, spontaneous sprouting was overtly impaired in aortic rings from ecCNP-/- and NPR-C-/- mice in comparison to WT animals (Figure 3C). In sharp contrast, in vessel segments from NPR-B-/- mice, de novo sprouting was similar to that in WT aortic rings (Figure 3C). The requirement of NPR-C activation for the pro-angiogenic action of CNP in this assay was demonstrated by the failure of exogenous CNP to promote aortic sprouting in NPR-C-/- aortic rings, as compared with vessels from ecCNP-/- and NPR-B-/- mice (Figure 3C). These data dovetail well with the rudimentary in vitro models (above) establishing that CNP-NPR-C signaling is critical to the angiogenic potential of the endothelium.
Figure 3. Aortic sprouting and matrigel plug neovascularization is diminished in vessels lacking either endothelial C-type natriuretic peptide or natriuretic pepide receptor-C.
(A-C) C-type natriuretic peptide (CNP; 1 pM – 1 μM) promotes de novo vessel sprouting ex vivo with a maximal effect equivalent to that produced by vascular endothelial growth factor (VEGF; 30 ng/mL; n=10), whereas inherent vessel sprouting capacity is diminished in aortic rings from endothelium-specific CNP knockout (ecCNP-/-) and global natriuretic peptide receptor-C knockout (NPR-C-/-), but not global NPR-B-/-, mice and the pro-angiogenic action of CNP (1 nM) is maintained in wild type (WT), ecCNP-/- and NPR-B-/- vessels but absent in NPR-C-/- aortae (n=6), (D) CNP (1 nM) facilitates neovascularisation of matrigel plugs in vivo in WT and ecCNP-/- mice but is unable to recapitulate this pro-angiogenic activity in NPR-C-/- animals (n=12), as interrogated by (E) hemoglobin content, (F) Hematoxylin and eosin (H&E; upper panel) and immunofluorescence (lower panel) staining of transverse sections of the excised matrigel plugs (DAPI nuclear stain, blue; endothelium marker isolectin B4, green; arrows denote overt endothelial staining; scale bars, 50 μm). Data are presented as mean ± SEM. Statistical analyses by one-way ANOVA with Bonferroni post hoc test. *P<0.05, **P<0.01 versus Control or WT and #P<0.05, ##P<0.01 versus corresponding genotype in the absence of CNP.
Next, we investigated the influence of CNP on neovascularization in vivo using an assay in which subcutaneously implanted matrigel plugs exhibit a significant increase in de novo vessel formation and hemoglobin content (as an index of mature, perfused vasculature; Figure 3D-F). In accord with our data from aortic ring assays, CNP caused a striking stimulation of neovascularization in matrigel plugs implanted in WT, ecCNP-/- and NPR-B-/- mice but had no significant effect on angiogenesis in NPR-C-/- mice (Figure 3D-F & Supplemental Figure 7). These findings taken together with our data from cell-based and aortic ring assays provide strong evidence that CNP potently promotes angiogenesis specifically via the NPR-C subtype.
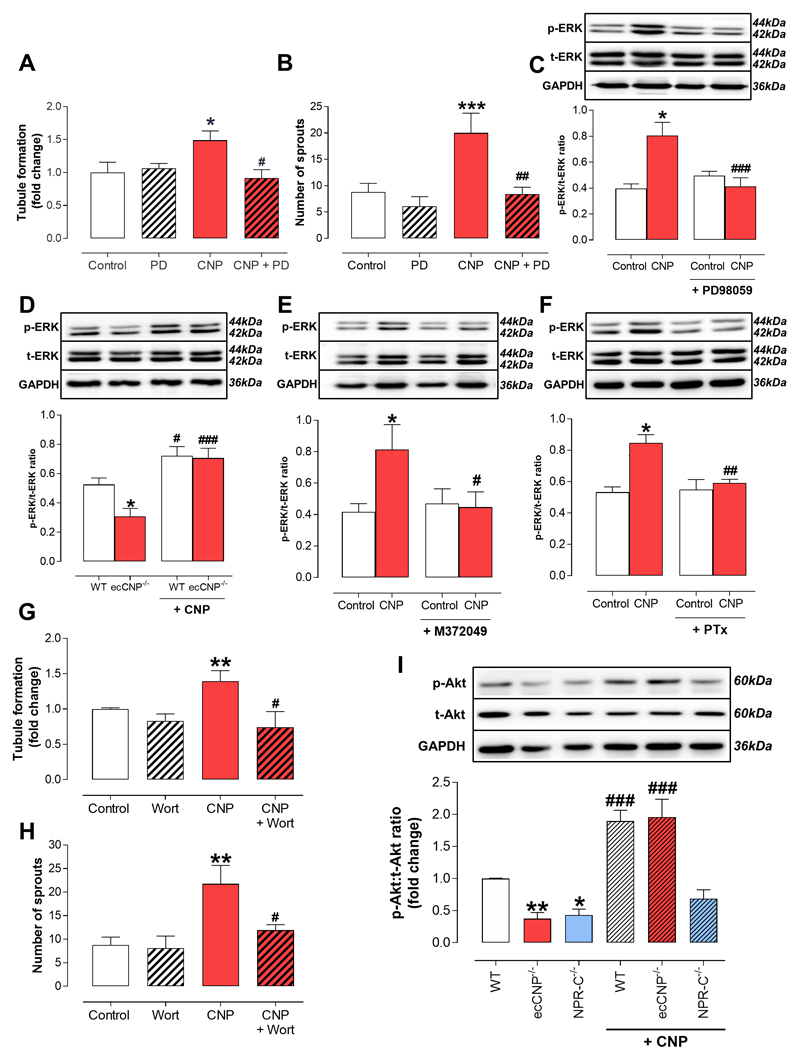
CNP governs angiogenesis via NPR-C-dependent activation of ERK1/2 and Akt/Protein kinase B
We have previously reported that NPR-C-dependent ERK1/2 phosphorylation is key to augmenting CNP-driven endothelial cell proliferation16. In accord, we investigated if a similar transduction pathway is responsible for the angiogenic actions of CNP. The ability of exogenous CNP to trigger tubule formation in PMECs and aortae from WT animals was significantly attenuated by the selective ERK1/2 inhibitor, PD98059 (Figure 4A-B). The functional blockade by PD98059 was paralleled by a reduced ERK1/2 phosphorylation in response to CNP (Figure 4C). Endothelium-derived CNP was also required for basal ERK1/2 activation since PMEC from ecCNP-/- mice exhibited a significantly lower basal ERK1/2 phosphorylation compared to WT controls (Figure 4D), an effect that was fully rescued by addition of exogenous CNP, which stimulated ERK1/2 activation to a similar extent in WT and ecCNP-/- cells (Figure 4D). The role of NPR-C in conveying CNP angiogenic signaling was investigated using the selective NPR-C antagonist, M372049. Treatment with M372049 completely inhibited CNP-induced ERK1/2 activation in WT PMEC (Figure 4E). The mechanism linking NPR-C activation with ERK1/2 phosphorylation was examined with the use of the Gi-blocker pertussis toxin (PTx). As shown in Figure 4F, PTx strongly inhibited the ability of CNP to trigger ERK1/2 phosphorylation. We next investigated the role of the phosphoinositide 3-kinase (PI3K)/Akt (protein kinase B, PKB) pathway, which is essential to the migratory capacity of endothelial cells29. Here, the ability of CNP to promote endothelial tubule formation and aortic sprouting was blocked in the presence of the selective PI3K inhibitor wortmannin (Figure 4G-H). In addition, studies with the selective inhibitor AS605240 revealed that, specifically, PI3Kγ activation is a prerequisite for CNP-driven tubule formation and aortic sprouting (Supplemental Figure 8A-B). Basal Akt/PKB phosphorylation was also significantly reduced in endothelial cells from ecCNP-/- and NPR-C-/- mice, but increased by incubation with CNP (Figure 4I). Finally, to rule out a role for NPR-B/cGMP signalling in the pro-angiogenic actions of CNP, tubule formation and aortic sprouting were investigated in the presence of the PKG inhibitor KT5823; in this setting, CNP-facilitated responses were unchanged (Supplemental Figure 8C-D). In concert, these observations establish that CNP-NPR-C-Gi coupling activates ERK1/2 and PI3Kγ/Akt/PKB signaling to promote angiogenesis, similar to the downstream pathways activated by VEGF and other well-established pro-angiogenic mediators3. These data are also consistent with previous reports linking NPR-C activation with Gi-signaling in the vasculature30.
Figure 4. C-type natriuretic peptide triggers extracellular signal-regulated kinase 1/2 and Akt/Protein kinase B phosphorylation via natriurertic peptide receptor-C-dependent Gi activation.
(A) Tubule formation (n=6), (B) aortic sprouting (n=12) and (C) extracellular signal-regulated kinase (ERK)1/2 phosphorylation (n=6) in response to C-type natriuretic peptide (CNP; 1 nM) are inhibited by the selective ERK1/2 inhibitor PD90859 (10 μM), (D) basal (Control) ERK1/2 phosphorylation in pulmonary microvascular endothelial cells (PMEC) from endothelium-specific CNP knockout (ecCNP-/-) mice is lower than wild type (WT) littermates but can be similarly increased by CNP (1 nM) in both genotypes (n=10), (E & F) CNP (1 nM)-driven ERK1/2 phosphorylation is blocked by the selective natriuretic pepide receptor (NPR)-C antagonist M372049 (10 μM; n=7) and the selective Gi inhibitor pertussis toxin (PTx, 100 ng/mL; n=7), (G) Tubule formation and (H) aortic sprouting in response to CNP (1 nM) are blocked by the Akt/protein kinase B inhibitor wortmannin (100 nM) (n=12), (I) basal (Control) Akt/protein kinase B phosphorylation is lower in PMECs from ecCNP-/- and global NPR-C knockout (NPR-C-/-) mice compared to wild type (WT) littermates and CNP (1 nM) promotes Akt phosphorylation in WT and ecCNP-/-, but not NPR-C-/-, animals (n=5). Data are presented as mean ± SEM. Statistical analyses by one-way ANOVA with Bonferroni post hoc test. ***P<0.05 versus Control or #P<0.05, ##P<0.01 versus CNP alone (A-C, E-H), *P<0.05, **P<0.01 versus WT and #P<0.05, ###P<0.001 versus corresponding genotype in the absence of CNP (D & I).
Restoration of blood flow following hindlimb ischemia is impaired by loss of endothelium-derived CNP but can be reversed by targeting NPR-C pharmacologically
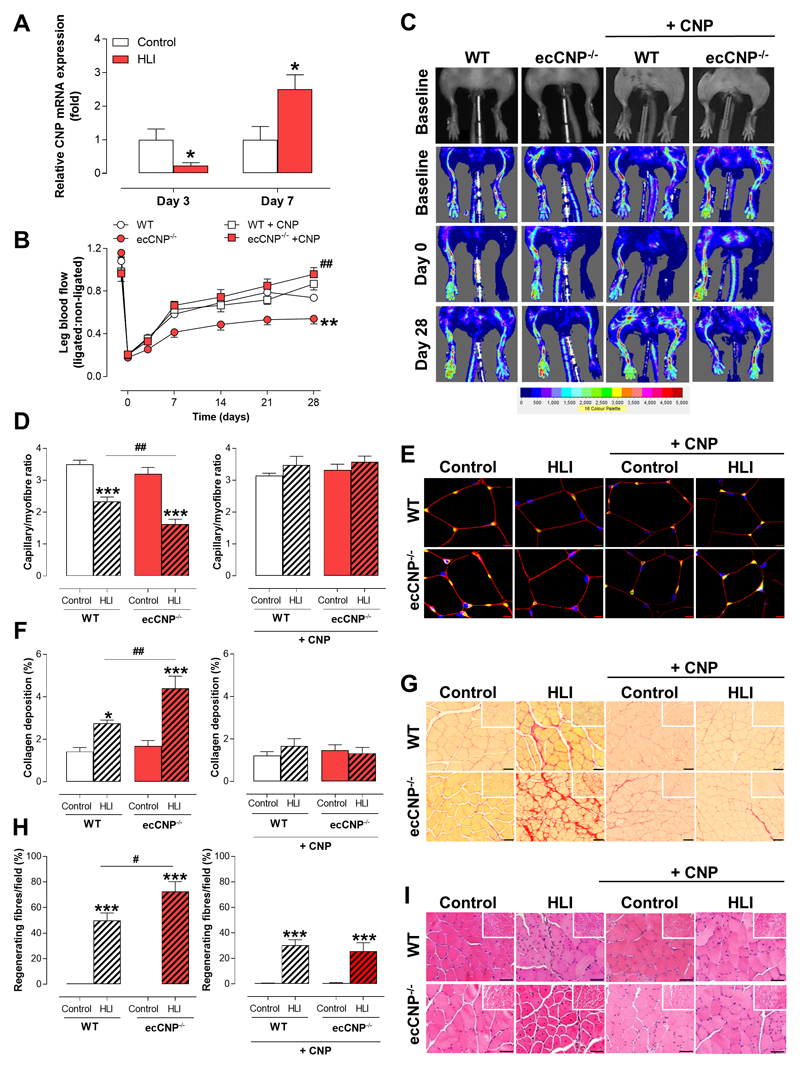
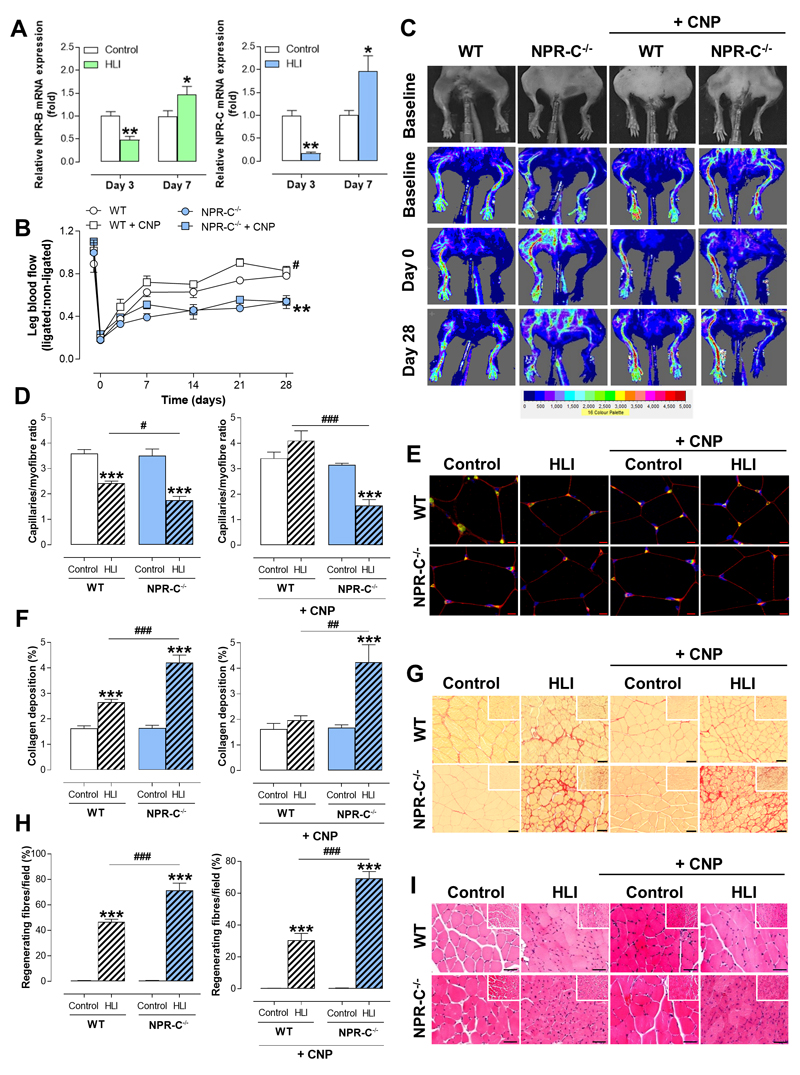
We utilized a well-established in vivo model of CLI to verify that endothelium-derived CNP, via NPR-C activation, is a prerequisite for optimal engagement of restorative angiogenic and arteriogenic pathways following pathological ischemia in vivo. Following unilateral ligation and excision of the femoral artery (hindlimb ischemia, HLI), mice undergo a pronounced remodeling to restore an adequate blood supply that supports essentially complete recovery (i.e. perfusion, motor function) over 28 days. Consistent with the findings in human tissue, the expression of both CNP and VEGF-A mRNA were reduced, with a marked increase in HIF-1α, Bax, VCAM-1, IL-6 and CCL2 in the ischemic gastrocnemius muscle at day 3 following HLI compared to the non-ischemic tissue in WT mice, with a subsequent increase in expression at day 7 (Figure 5A & Supplemental Figure 2B-C). ecCNP-/- mice exhibited a significantly impaired recovery following HLI in terms of perfusion (Figure 5B-C & Supplemental Figure 9A), blood vessel density (i.e. capillary/myofiber ratio; Figure 5D-E), fibrosis (i.e. collagen deposition; Figure 5F-G), and regeneration of muscle fibers (Figure 5H-I). This adverse phenotype in ecCNP-/- animals could be rescued by pharmacological administration of CNP (Figure 5B-I). Indeed, exogenous CNP improved indices of injury in WT mice implying that therapeutic targeting of the CNP-NPR-C pathway is likely to be of benefit above and beyond any endogenous signaling (paralleling observations in PMEC where CNP-stimulated migration in ecCNP-/- was greater than that in WT). NPR-C mRNA and protein expression followed an identical temporal profile to CNP and VEGF-A, with an initial reduction (Day 3) followed by a significant increase (Day 7; Figure 6A & Supplemental Figures 2B-C & 3B), suggesting the expression of CNP-NPR-C signalling is coordinated with the angiogenic response. However, NPR-C-/- mice presented with an overly more injurious phenotype after being subjected to HLI (Figure 6B-I). Significantly, in sharp contrast to ecCNP-/- animals, pharmacological administration of CNP did not rescue phenotype in NPR-C-/- mice (Figure 6B-I & Supplemental Figure 9B). These data substantiate a pro-angiogenic, pro-remodeling function for endothelium-derived CNP in vivo and verify that activation of NPR-C underpins this process. Moreover, strategies aimed at pharmacologically activating NPR-C are likely to be of benefit in CLI patients.
Figure 5. Restoration of blood flow following hindlimb ischemia is impaired in endothelium-specific C-type natriuretic peptide (CNP) knockout mice but can be reversed by pharmacological administration of CNP.
(A) The mRNA expression of C-type natriuretic peptide (CNP) is reduced in ischemic gastrocnemius muscles (HLI) from wild type (WT) mice, compared to non-ischemic controls (Control), with a significant increase at day 7 following HLI (n=5-7), (B & C) Restoration of leg blood flow is impaired in endohelium-specific CNP knockout (ecCNP-/-) mice compared to WT littermates but can be phenotypically-rescued by administration of CNP (0.2mg/kg/day; n=6-12), (D & E) Reduction in capillary density (representative images showing immunostaining with DAPI nuclear stain, blue, endothelium marker isolectin B4, green, cellular membrane marker wheat germ agglutinin, red; scale bars, 50 μm), (F & G) increased fibrotic burden (representative images of collagen-specific Picrosirius staining; scale bars, 50 μm), and (H & I) impaired regeneration of muscle fibers (representative images of haematoxylin & eosin [H&E] staining; scale bars, 50 μm) are all exacerbated in ecCNP-/- mice compared to WT littermates but this phenotypic deficit is significantly reversed by addition of CNP (0.2mg/kg/day; n=6-12). Data are presented as mean ± SEM. Statistical analyses by (A & B) two-way ANOVA or (D, F & H) one-way ANOVA with Bonferroni post hoc test. *P<0.05, **P<0.01. ***P<0.001 versus WT or #P<0.05, ##P<0.01, P<0.001 versus ecCNP-/- + CNP alone (A & B), *P<0.01, ***P<0.001 versus corresponding control genotype or ##P<0.01 versus WT HLI (C-E).
Figure 6. Restoration of blood flow following hindlimb ischemia is impaired in global natriuretic peptide receptor-C knockout mice but cannot be rescued by pharmacological administration of C-type natriuretic peptide.
(A) The mRNA expression of natriuretic peptide receptor (NPR)-C is reduced in ischemic gastrocnemius muscles (HLI) from wild type (WT) mice, compared to non-ischemic controls (Control), with a significant increased at day 7 following HLI (n=5-7), (B & C) Restoration of leg blood flow is impaired in global natriuretic peptide receptor-C knockout (NPR-C-/-) mice compared to WT littermates but is not amenable to phenotypic rescue by administration of C-type natriuretic peptide (CNP; 0.2mg/kg/day; n=6-12), (D & E) Reduction in capillary density (representative images showing immunostaining with DAPI nuclear stain, blue, endothelium marker isolectin B4, green, cellular membrane wheat germ agglutinin, red; scale bars, 50 μm), (F & G) increased fibrotic burden (representative images of collagen-specific Picrosirius staining; scale bars, 50 μm), and (H & I) impaired regeneration of muscle fibers (representative images of haematoxylin & eosin [H&E] staining; scale bars, 50 μm) are all exacerbated in NPR-C-/- mice compared to WT littermates and this phenotypic deficit cannot be reversed by addition of CNP (0.2mg/kg/day; n=6-12). Data are presented as mean ± SEM. Statistical analyses by (A & B) two-way ANOVA or (D, F & H) one-way ANOVA with Bonferroni post hoc test. *P<0.05, **P<0.01. ***P<0.001 versus WT or #P<0.05, ##P<0.01, ###P<0.001 versus ecCNP-/- + CNP alone (A & B), *P<0.01, ***P<0.001 versus corresponding control genotype or ##P<0.01 versus WT HLI (C-E).
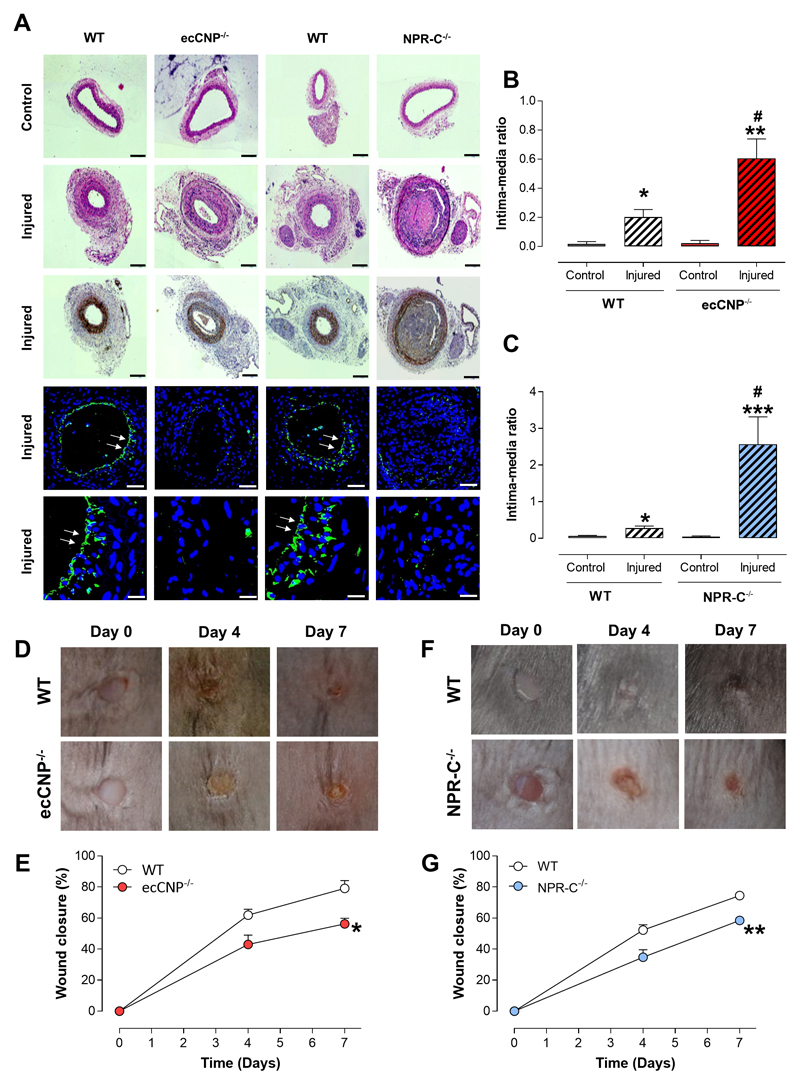
Reponses to vessel injury and wound healing in vivo are impaired in ecCNP-/- and NPR-C-/- mice
In order to demonstrate a more widespread role for endothelial CNP in the host defense response to injury, we utilized two further independent, etiologically-distinct pre-clinical models. First, we examined the remodeling in wire-injured murine carotid arteries (akin to percutaneous coronary intervention, PCI). In this setting, WT mice exhibited a typical, marked intimal hyperplasia following injury that was significantly exacerbated in ecCNP-/- and NPR-C-/- animals (Figure 7A-C). Indeed, the intimal thickening was particularly pronounced in NPR-C-/- mice, with almost complete occlusion of the vessel following injury (Figure 7C). Second, we investigated cutaneous wound healing following a biopsy on the dorsal skin. Here, wound closure was essentially complete in WT animals after 7 days (Figure 7D-G). However, this reparative process was markedly delayed in both ecCNP-/- (Figure 7D-E) and NPR-C-/- mice (Figure 7F-G). These observations support the conclusion that CNP-NPR-C signaling has an important role in the tissue repair responses to injury, and warrants further attention from both a (patho)physiological and therapeutic perspective.
Figure 7. Reponses to vessel injury and wound healing in vivo are impaired in endothelium-specific C-type natriuretic peptide knockout and global natriuretic peptide receptor-C knockout mice.
(A-C) Neointimal hyperplasia (intima:media ratio) in response to wire injury in the carotid artery is exacerbated in endothelium-specific C-type natriuretic peptide knockout (ecCNP-/-) and global natriuretic peptide receptor-C knockout (NPR-C-/-) mice compared to wild type (WT) littermates (n=6-7). Representative images showing hematoxylin & eosin (H&E) staining, immunostaining with alpha smooth muscle actin (brown; panels 1-3, scale bar, 100μm) and immunofluorescence with DAPI nuclear stain (blue) and endothelium marker isolectin B4 (green; arrows denote overt endothelial staining; panels 4 & 5, scale bars, 50μm and 20μm, respectively). Wound healing (% closure) is impaired in (D & E) ecCNP-/- and (F & G) NPR-C-/- mice compared to WT littermates (n=9-13). Data are presented as mean ± SEM. Statistical analyses by (B & C) one-way ANOVA with Bonferroni post hoc test or (D, F & H) two-way ANOVA. *P<0.05, ***P<0.001 versus corresponding genotype uninjured and #P<0.05 versus WT injured (A) or *P<0.05, **P<0.01 versus WT (B).
Discussion
Ischemic cardiovascular disease, typified by PAD and CLI, MI and stroke, represents a major cause of global morbidity and mortality31. Restoration of adequate blood supply to hypoxic tissue, via complementary angiogenic and arteriogenic mechanisms, in response to ischemia is decisive for both host repair and therapeutic intervention. Previous attempts to harness these endogenous corrective pathways to improve outcome in PAD have focused primarily on promoting the actions of VEGF, yet these strategies have proven largely ineffective clinically, whether cell-, gene- or small-molecule- based6–8. Herein, we establish a previously undefined role for endothelium-derived CNP in angiogenesis and vascular remodeling. This critical function is dependent on activation of NPR-C and, via Gi-coupling, stimulation of the classical ERK1/2 and Akt/protein kinase B pathways that are harnessed by many factors promoting endothelial proliferation, migration and survival, including VEGF3, 32. Clinical evidence utilizing gastrocnemius muscle biopsies from CLI patients confirmed that expression of CNP and NPR-C is down-regulated, matching that observed at the earliest timepoints following HLI in the experimental model; yet mice are able to up-regulate CNP and NPR-C expression to coincide with the angiogenic and arteriogenic response to ischemia. Diminution of CNP/NPR-C signalling in CLI may therefore underpin the inability to promote compensatory angiogenesis and vascular remodeling to maintain appropriate blood supply. However, targeting this pathway pharmacologically facilitates angiogenesis and vascular remodeling, implying therapeutic approaches triggering NPR-C activation are likely to be of benefit in PAD/CLI and other ischemic disorders including MI.
Endothelium-derived CNP plays a fundamental role in regulating vascular function local blood flow, systemic blood pressure and the reactivity of circulating leukocytes and platelets10–12. Previous work has also suggested that this peptide contributes to the maintenance of blood vessel integrity and response to injury17–22. The signaling pathway(s) underpinning these vasoprotective functions of CNP remain unresolved. In terms of endothelial and vascular smooth muscle cell hyperplasia both cognate receptors, NPR-B and NPR-C, have been implicated33. However, in the present study, cells and tissues isolated from NPR-B-/- mice behaved in an identical fashion to WT in complementary in vitro and in vivo models of angiogenesis, whether in terms of intrinsic activity or in response to exogenous CNP; the pro-angiogenic effects of CNP in vitro were also insensitive to PKG inhibition. These findings demonstrate that NPR-B-triggered cGMP formation is unlikely to underlie the restorative capacity of CNP. Rather, genetic deletion of NPR-C produced an identical anti-angiogenic, anti-arteriogenic phenotype, both in vitro and in vivo, akin to that observed in mice lacking endothelium-derived CNP. This NPR-C-dependency adds to the growing number of physiological signaling roles of this G-protein coupled receptor traditionally thought solely to clear natriuretic peptides from the circulation34. Evidence now supports the conclusion that NPR-C is a cellular transduction trigger in the cardiovascular system, regulating diverse processes including cell growth16, oxidative stress35 and SA node conduction36. Moreover, Gi-coupling appears to mediate the majority of these actions30. Herein, we show that the pro-angiogenic effects of CNP, driven through NPR-C activation, are also Gi-dependent and involve signaling via both ERK1/2 and PI3Kγ/Akt/Protein kinase B; pathways well-established to synchronize angiogenesis & arteriogenesis3, 37 (Supplemental Figure 10). These observations fit well with the profile of CNP and NPR-C expression in response to HLI (herein and 38), first falling and then increasing in a temporal manner that aligns with the restoration of blood flow and influence of VEGF, and thereby provide new mechanistic insight into the vasoprotective role of this receptor. Intriguingly, the loss of this protective phenotype observed in vitro, and to some extent in vivo, appeared to be more severe in global NPR-C-/- cells and tissues in comparison to those from ecCNP-/- mice. This differential activity may stem, in part, from an incomplete deletion of CNP from endothelial cells in the transgenic strain employed (~80%10) but may also originate from loss of constitutive receptor activity, a characteristic of GPCRs, in NPR-C-/- animals. Whether NPR-A/cGMP/PKGI signalling in response to ANP and/or BNP, which is established to promote revascularization following ischemia 22, 28, might offset the loss of CNP/NPR-C signalling remains to be determined. However, the anti-angiogenic consequence of NPR-C deletion implies that increased activity of ANP/BNP (acting via NPR-A/cGMP/PKGI) resulting from elimination of the clearance capacity of NPR-C is not sufficient to compensate overtly. Cursory investigation herein suggests that ANP is also unable to exert a similar pro-angiogenic effect via NPR-C.
Additionally, we demonstrate clear therapeutic potential for pharmacologically activating NPR-C in PAD and other ischemic cardiovascular disorders. Administration of CNP (i.e. an NPR-C agonist) is able to significantly improve revascularization in vivo following ischemia, and the multi-faceted nature of this protective influence was highlighted by improvement in several indices of disease severity including improved leg and paw perfusion, increased vessel density, reduced fibrosis and accelerated myofibre regeneration. In the setting of PAD/CLI, the anti-leukocyte and anti-platelet actions of endothelial-derived CNP (via NPR-C)10 will proffer an additional level of protection from prospective atherosclerotic occlusion and represent additional therapeutic value. One possible caveat is the diminished NPR-C expression observed in amputated limbs from CLI patients, which might limit efficacy of CNP/NPR-C-based treatments. However, at initial stages of PAD (e.g. onset of claudication) the components of the signalling pathway are likely to be expressed at a higher level, advocating early intervention. Moreover, the human CLI tissue was derived from extensively necrotic areas and it is conceivable that in the border region between healthy and diseased limb NPR-C expression is higher and therefore more tractable in terms of therapy (comparable to day 7 following HLI). Indeed, herein CNP delivery in the murine model was initiated at the nadir of NPR-C expression which matches that in CLI tissue. Regardless, pharmacological administration of CNP is incredibly effective at restoring blood flow, providing proof-of-concept that this strategy might be effective in CLI patients.
Interestingly, there appears to be a reciprocal relationship between CNP and VEGF in the regulation of angiogenesis. VEGF inhibits CNP secretion from endothelial cells39 whilst CNP (and other natriuretic peptides) have been reported to inhibit VEGF production40. Whether this represents temporal and/or spatial feedback communication between the parallel pathways remains to be explored, but a linking mechanism may involve TGFβ1 signaling, which is key to tissue repair following ischemia, since both CNP and VEGF have TGFβ1-responsive promoters41. A further common mechanism of regulation is likely provided by HIF-1α, which is known to be a key inducer of VEGF transcription (in addition to its downstream signaling components)42, and which also upregulates natriuretic peptide expression15. Indeed, the Nppc (CNP) promoter region contains several hypoxia response elements (HREs). Regardless, CNP-NPR-C signaling appears to represent a novel, innate angiogenic pathway independent of and equal magnitude to that proffered by VEGF (at least in assays described herein).
The Gi-linked reparative action of CNP-NPR-C closely resembles the pro-angiogenic processes regulated by growth factors such as sphingosine-1-phosphate and insulin like growth factor43, 44, both of which signal via single transmembrane domain Gi-coupled receptors. Thus, the present study suggests NPR-C, which similarly belongs to this ‘atypical’ type 1 membrane GPCR family, can also be considered to function as a growth factor receptor in the context of angiogenesis (in contrast to more acute, functional changes in vascular tone and blood pressure10). Furthermore, we provide compelling evidence that CNP-NPR-C signaling is a widespread mechanism invoked to expedite tissue repair after injury. In two etiologically-distinct experimental models, wire-induced carotid damage and wound healing, recovery from insult was overtly impaired in animals endothelial CNP or global NPR-C null mutants. These more complex systems illustrate the significant contribution of CNP-NPR-C signaling to tissue repair and implicate additional roles above and beyond the processes of angiogenesis and arteriogenesis (the central focus of this study). Indeed, a previous report showed that pharmacological addition of CNP promotes angiogenesis in the skin of mice (albeit accompanied by slowed wound healing)45 and that CNP expression is up-regulated in human coronary atherosclerotic lesions46 and in the neointima following percutaneous coronary intervention47. These latter vasoprotective facets of CNP biology which drive vessel repair highlight the opposing effects of CNP on endothelial (stimulatory) and vascular smooth muscle/myofibroblast (inhibitory) proliferation. In turn, this may result from altered expression patterns of NPR-B and NPR-C on these cells; NPR-B is highly expressed in all cell types whereas NPR-C expression is higher on endothelial cells compared to vascular smooth muscle48. This dual activity may also underpin the more severe phenotype of global NPR-C-/-, versus ecCNP-/-, mice in these particular injurious experimental models.
In sum, endothelium-derived CNP plays a pivotal role in angiogenesis and vascular remodeling following ischemia via specific activation of NPR-C, Gi-stimulation, and triggering of ERK1/2 and PI3kγ/Akt/protein kinase B phosphorylation (Supplemental Figure 10). Furthermore, pharmacological targeting of NPR-C promotes vascular repair after injury and offers a novel avenue for the therapy of PAD and other ischemic cardiovascular disorders.
Supplementary Material
Clinical Perspective.
What is new?
Angiogenesis and arteriogenesis are harmonized responses to ischemia (e.g. peripheral arterial disease and myocardial infarction) which restore tissue perfusion.
This study defines a central (patho)physiological role for endothelium-derived C-type natriuretic peptide (CNP), via activation of cognate natriuretic peptide receptor (NPR)-C, in angiogenesis and vascular remodeling.
Moreover, the work demonstrates the therapeutic utility of pharmacologically targeting NPR-C to restore deficits in these processes following ischemia and injury.
What are the clinical implications?
Previous approaches aimed at facilitating angiogenesis in peripheral arterial disease have proven ineffective in clinical trials, the only currently effective management is surgery.
The novel signaling system we have identified offers promise to radically improve and resolve the poor perfusion in PAD patients, reducing the need for invasive intervention and amputation.
Targeting the CNP/NPR-C pathway may therefore offer a tangible pharmacological approach to improve PAD and other ischemic cardiovascular disorders.
Funding Sources
This work was supported by British Heart Foundation grants PG/14/14/30690 & PG/17/74/33111.
Footnotes
Disclosures
AJH is a scientific advisory board member for Palatin Technologies Inc. and is a named inventor on a patent describing NPR-C agonists.
References
- 1.Berger JS, Hiatt WR. Medical therapy in peripheral artery disease. Circulation. 2012;126:491–500. doi: 10.1161/CIRCULATIONAHA.111.033886. [DOI] [PubMed] [Google Scholar]
- 2.Hess CN, Norgren L, Ansel GM, Capell WH, Fletcher JP, Fowkes FGR, Gottsater A, Hitos K, Jaff MR, Nordanstig J, Hiatt WR. A Structured Review of Antithrombotic Therapy in Peripheral Artery Disease With a Focus on Revascularization: A TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) Initiative. Circulation. 2017;135:2534–2555. doi: 10.1161/CIRCULATIONAHA.117.024469. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 5.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 7.Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, Annex BH, Hiatt WR. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–1773. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 8.Gorenoi V, Brehm MU, Koch A, Hagen A. Growth factors for angiogenesis in peripheral arterial disease. Cochrane Database Syst Rev. 2017;6:CD011741. doi: 10.1002/14651858.CD011741.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009;7(Suppl 1):35–37. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 10.Moyes AJ, Khambata RS, Villar I, Bubb KJ, Baliga RS, Lumsden NG, Xiao F, Gane PJ, Rebstock AS, Worthington RJ, Simone MI, et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. doi: 10.1172/JCI74281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao K, Kuwahara K, Nishikimi T, Nakagawa Y, Kinoshita H, Minami T, Kuwabara Y, Yamada C, Yamada Y, Tokudome T, Nagai-Okatani C, et al. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension. 2017;69:286–296. doi: 10.1161/HYPERTENSIONAHA.116.08219. [DOI] [PubMed] [Google Scholar]
- 12.Spiranec K, Chen W, Werner F, Nikolaev VO, Naruke T, Koch F, Werner A, Eder-Negrin P, Dieguez-Hurtado R, Adams RH, Baba HA, et al. Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure. Circulation. 2018;138:494–508. doi: 10.1161/CIRCULATIONAHA.117.033383. [DOI] [PubMed] [Google Scholar]
- 13.Okahara K, Kambayashi J, Ohnishi T, Fujiwara Y, Kawasaki T, Monden M. Shear stress induces expression of CNP gene in human endothelial cells. FEBS Lett. 1995;373:108–110. doi: 10.1016/0014-5793(95)01027-c. [DOI] [PubMed] [Google Scholar]
- 14.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of "vascular natriuretic peptide system". J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun YS, Hyun JY, Kwak YG, Kim IS, Kim CH, Choi E, Kim MS, Park JW. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1. Biochem J. 2003;370:149–157. doi: 10.1042/BJ20021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khambata RS, Panayiotou CM, Hobbs AJ. Natriuretic peptide receptor-3 underpins the disparate regulation of endothelial and vascular smooth muscle cell proliferation by C-type natriuretic peptide. Br J Pharmacol. 2011;164:584–597. doi: 10.1111/j.1476-5381.2011.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno N, Itoh H, Ikeda T, Ueyama K, Yamahara K, Doi K, Yamashita J, Inoue M, Masatsugu K, Sawada N, Fukunaga Y, et al. Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation. 2002;105:1623–1626. doi: 10.1161/01.cir.0000014985.50017.6e. [DOI] [PubMed] [Google Scholar]
- 18.Furuya M, Aisaka K, Miyazaki T, Honbou N, Kawashima K, Ohno T, Tanaka S, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem Biophys Res Commun. 1993;193:248–253. doi: 10.1006/bbrc.1993.1616. [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Haruno A, Morisaki N, Furuya M, Kangawa K, Takeshita A, Saito Y. Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation. 1997;96:2272–2279. doi: 10.1161/01.cir.96.7.2272. [DOI] [PubMed] [Google Scholar]
- 20.Doi K, Ikeda T, Itoh H, Ueyama K, Hosoda K, Ogawa Y, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, et al. C-type natriuretic peptide induces redifferentiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler Thromb Vasc Biol. 2001;21:930–936. doi: 10.1161/01.atv.21.6.930. [DOI] [PubMed] [Google Scholar]
- 21.Del Ry S, Cabiati M, Martino A, Cavallini C, Caselli C, Aquaro GD, Battolla B, Prescimone T, Giannessi D, Mattii L, Lionetti V. High concentration of C-type natriuretic peptide promotes VEGF-dependent vasculogenesis in the remodeled region of infarcted swine heart with preserved left ventricular ejection fraction. Int J Cardiol. 2013;168:2426–2434. doi: 10.1016/j.ijcard.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Yamahara K, Itoh H, Chun TH, Ogawa Y, Yamashita J, Sawada N, Fukunaga Y, Sone M, Yurugi-Kobayashi T, Miyashita K, Tsujimoto H, et al. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc Natl Acad Sci U S A. 2003;100:3404–3409. doi: 10.1073/pnas.0538059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedram A, Razandi M, Hu RM, Levin ER. Vasoactive Peptides Modulate Vascular Endothelial Cell Growth Factor Production and Endothelial Cell Proliferation and Invasion. Journal of Biological Chemistry. 1997;272:17097–17103. doi: 10.1074/jbc.272.27.17097. [DOI] [PubMed] [Google Scholar]
- 24.Almeida SA, Cardoso CC, Orellano LA, Reis AM, Barcelos LS, Andrade SP. Natriuretic peptide clearance receptor ligand (C-ANP4-23) attenuates angiogenesis in a murine sponge implant model. Clin Exp Pharmacol Physiol. 2014;41:691–697. doi: 10.1111/1440-1681.12251. [DOI] [PubMed] [Google Scholar]
- 25.van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM, Lindeman JH, Delis-van Diemen PM, van Hinsbergh VW, van Bockel JH, Quax PH. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler Thromb Vasc Biol. 2007;27:1426–1432. doi: 10.1161/ATVBAHA.107.139642. [DOI] [PubMed] [Google Scholar]
- 26.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 27.Veale CA, Alford VC, Aharony D, Banville DL, Bialecki RA, Brown FJ, Damewood JR, Jr, Dantzman CL, Edwards PD, Jacobs RT, Mauger RC, et al. The discovery of non-basic atrial natriuretic peptide clearance receptor antagonists. Part 1. Bioorg Med Chem Lett. 2000;10:1949–1952. doi: 10.1016/s0960-894x(00)00387-5. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn M, Volker K, Schwarz K, Carbajo-Lozoya J, Flogel U, Jacoby C, Stypmann J, van EM, Gambaryan S, Hartmann M, Werner M, et al. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–2030. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 30.Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- 31.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW, Bell K, Caporusso J, Durand-Zaleski I, Komori K, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 33.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Sarkar O, Brochu M, Anand-Srivastava MB. Natriuretic peptide receptor-C attenuates hypertension in spontaneously hypertensive rats: role of nitroxidative stress and Gi proteins. Hypertension. 2014;63:846–855. doi: 10.1161/HYPERTENSIONAHA.113.01772. [DOI] [PubMed] [Google Scholar]
- 36.Egom EE, Vella K, Hua R, Jansen HJ, Moghtadaei M, Polina I, Bogachev O, Hurnik R, Mackasey M, Rafferty S, Ray G, et al. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. J Physiol. 2015;593:1127–1146. doi: 10.1113/jphysiol.2014.283135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Pressly ED, Abendschein DR, Hawker CJ, Woodard GE, Woodard PK, Welch MJ. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med. 2011;52:1956–1963. doi: 10.2967/jnumed.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doi K, Itoh H, Komatsu Y, Igaki T, Chun TH, Takaya K, Yamashita J, Inoue M, Yoshimasa T, Nakao K. Vascular endothelial growth factor suppresses C-type natriuretic peptide secretion. Hypertension. 1996;27:811–815. doi: 10.1161/01.hyp.27.3.811. [DOI] [PubMed] [Google Scholar]
- 40.Pedram A, Razandi M, Levin ER. Natriuretic peptides suppress vascular endothelial cell growth factor signaling to angiogenesis. Endocrinology. 2001;142:1578–1586. doi: 10.1210/endo.142.4.8099. [DOI] [PubMed] [Google Scholar]
- 41.Ohta S, Takeuchi M, Deguchi M, Tsuji T, Gahara Y, Nagata K. A novel transcriptional factor with Ser/Thr kinase activity involved in the transforming growth factor (TGF)-beta signalling pathway. Biochem J. 2000;350(Pt 2):395–404. [PMC free article] [PubMed] [Google Scholar]
- 42.Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Licht T, Tsirulnikov L, Reuveni H, Yarnitzky T, Ben-Sasson SA. Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3) Blood. 2003;102:2099–2107. doi: 10.1182/blood-2002-12-3634. [DOI] [PubMed] [Google Scholar]
- 44.Cho YL, Hur SM, Kim JY, Kim JH, Lee DK, Choe J, Won MH, Ha KS, Jeoung D, Han S, Ryoo S, et al. Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J Biol Chem. 2015;290:467–477. doi: 10.1074/jbc.M114.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuehnl A, Pelisek J, Ring A, Spindler N, Hatz R, Jauch KW, Eckstein HH, Langer S. C-type natriuretic peptide slows down wound healing but promotes angiogenesis in SKH1-hr hairless mice. Int Wound J. 2013;10:425–430. doi: 10.1111/j.1742-481X.2012.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naruko T, Ueda M, van der Wal AC, van der Loos CM, Itoh H, Nakao K, Becker AE. C-type natriuretic peptide in human coronary atherosclerotic lesions. Circulation. 1996;94:3103–3108. doi: 10.1161/01.cir.94.12.3103. [DOI] [PubMed] [Google Scholar]
- 47.Naruko T, Itoh A, Haze K, Ehara S, Fukushima H, Sugama Y, Shirai N, Ikura Y, Ohsawa M, Ueda M. C-Type natriuretic peptide and natriuretic peptide receptors are expressed by smooth muscle cells in the neointima after percutaneous coronary intervention. Atherosclerosis. 2005;181:241–250. doi: 10.1016/j.atherosclerosis.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Pelisek J, Kuehnl A, Rolland PH, Mekkaoui C, Fuchs A, Walker GF, Ogris M, Wagner E, Nikol S. Functional analysis of genomic DNA, cDNA, and nucleotide sequence of the mature C-type natriuretic peptide gene in vascular cells. Arterioscler Thromb Vasc Biol. 2004;24:1646–1651. doi: 10.1161/01.ATV.0000137387.78515.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.