Abstract
Morphological characterization and multi-locus DNA sequence analysis of fungal isolates obtained from 32 clinical cases of equine fungal keratitis (FK) was performed to identify species and determine associations with antifungal susceptibility, response to therapy and clinical outcome. Two species of Aspergillus (A. flavus and A. fumigatus) and three species of Fusarium (F. falciforme, F. keratoplasticum, and F. proliferatum) were the most common fungi isolated and identified from FK horses. Most (91%) equine FK Fusarium nested within the Fusarium solani species complex (FSSC) with nine genetically diverse strains/lineages, while 83% of equine FK Aspergillus nested within the A. flavus clade with three genetically diverse lineages. Fungal species and evolutionary lineage were not associated with clinical outcome. However, species of equine FK Fusarium were more likely (p = 0.045) to be associated with stromal keratitis. Species of Aspergillus were more susceptible to voriconazole and terbinafine than species of Fusarium, while species of Fusarium were more susceptible to thiabendazole than species of Aspergillus. At the species level, A. fumigatus and A. flavus were more susceptible to voriconazole and terbinafine than F. falciforme. Natamycin susceptibility was higher for F. falciforme and A. fumigatus compared to A. flavus. Furthermore, F. falciforme was more susceptible to thiabendazole than A. flavus and A. fumigatus. These observed associations of antifungal sensitivity to natamycin, terbinafine, and thiabendazole demonstrate the importance of fungal identification to the species rather than genus level. The results of this study suggest that treatment of equine FK with antifungal agents requires accurate fungal species identification.
Introduction
Fungal keratitis (FK) is a severe, progressive, inflammatory ocular disease resulting from invasive growth of fungi into the cornea. Fungal keratitis is challenging to manage and can lead to blindness or loss of the affected eye.[1] The incidence of human FK has increased in the past several decades.[2, 3] In subtropical areas, fungal infections are reported to cause up to 35% of all documented keratitis cases in humans, especially in China and India.[1, 3, 4] Fungal keratitis is less common in the US where it is predominantly observed in south Florida and Texas.[2, 5] Nearly half of the causative organisms in FK are filamentous fungi, predominantly species of Aspergillus and Fusarium, of approximately equal frequency, followed in incidence by species of Candida, a dimorphic yeast.[1, 3–5] Fusarium spp. and Aspergillus spp. accounted for 31% and 25% of filamentous FK isolates from South India; [4] 28% Fusarium spp. and 22% Aspergillus spp. from East India; [3] and 48% Fusarium spp. and 19% Aspergillus spp. from Northeast China. [1] In these studies, the fungal species associated with FK were not identified.
Filamentous fungi and yeasts are part of the normal ocular surface microbiome, are soil saprobes and plant pathogens, and thought to be opportunistic when invading the cornea in FK.[1] Predisposing factors for developing FK in humans include advanced age, trauma (≤ 89% of cases) especially with vegetative foreign bodies, workers in rural or agricultural areas, immunosuppression, and past antibiotic, antifungal, or steroid use.[3, 4, 6] Mechanisms of fungal invasion and virulence have been extensively studied, including the requirement for transition from yeast to hyphal forms with Candida, expression of specialized proteins, such as adhesins and invasins on the cell surface, and development of biofilms.[7] Many of these virulence mechanisms represent areas of scientific investigation for developing new antifungal compounds or methods to prevent fungal invasion.[8, 9]
Identification of fungi as a possible causative organism of keratitis has traditionally been evaluated using direct cytological smears and the gold standard of culture and morphological-based identification.[2] Cultures reliably differentiate Aspergillus, Candida, and Fusarium, but due to the large degree of morphological variability at various developmental stages of growth, this traditional mycological classification approach does not provide consistent or discriminatory resolution to the species or genotype (lineage) level for identifying pathogenic fungal species known to infect the cornea.[2] Fungal molecular phylogenetic studies further define evolutionary lineages of fungi (i.e., a group of organisms that consists of all descendants of a common ancestor) that are animal and human pathogens beyond culture and routine identification techniques.[10–13] The ocular pathogens classified as Fusarium, for example, do not represent a single species but rather are members of a diverse species complex consisting of at least 18 phylogenetically distinct species.[10, 13] Species may exhibit differences in disease aggressiveness (e.g., corneal invasion and virulence) and susceptibility to antifungal medications, which if identified, could dramatically improve FK management since corneal ulcers are currently treated empirically routinely without susceptibility data.[6] Precise genotypic identification of FK etiological agents may also improve understanding of the environmental reservoir of each fungal species and epidemiology.[12] Molecular phylogenetic analysis and placement of fungal organisms causing FK is critical for diagnosis, therapy, particularly when correlated with disease outcome and prognostic aspects.[2]
Fungal keratitis is the most common cause of blindness in horses of the Southeastern USA and is a widespread disease in horses from all states east of the Rocky Mountains.[14–18] Similar to human keratitis, the most common causative organisms of FK in horses are the filamentous fungi, Aspergillus and Fusarium.[19] Clinically, FK in horses is also similar to human FK with characteristic diagnostic criteria of a raised corneal ulcer with a feathery border, satellite lesions, and secondary uveitis with hypopyon.[6, 16, 18] Once FK develops, current treatment is the same for all cases, regardless of fungal species, and greater than 50% of horses with FK do not respond to medical therapy and either require surgical repair or enucleation.[16] The similarity between human and equine FK suggests that there is high value in studying this naturally-occurring model of FK using molecular phylogenetic studies to predict aggressiveness and virulence of specific FK causative organisms and to select effective antifungal therapies.
The purpose of this study was to better understand the pathogenesis and treatment of FK by associating antifungal susceptibility and multi-locus sequence-based fungal identification with clinical outcome of a naturally occurring model of FK in horses.
Methods
Animals, disease assessment, and sample collection
Horses that were presented with FK to the ophthalmology service at North Carolina State University or Auburn University, confirmed through hyphae identified on wet mount cytological analysis with light microscopy, had culture samples collected from the clinically infected eye prior to initiating antifungal therapy. Following informed consent, samples were collected (using a sterile rayon swab or handle end of a sterile surgical blade) directly from the FK lesion. Samples were immediately plated using C-shaped streaks on Sabouraud dextrose agar (SDA) and trypticase soy agar with 5% sheep blood (CBA) and maintained at 25°C and 37°C for growth and microbiological identification. Signalment (age, breed and sex) and historical treatment and health information were also collected from each patient. Horses were treated with standard of care topical, subconjunctival, and/or systemic antifungal medications.[20] If medical therapy (MT) did not resolve the FK, then a surgical therapy (ST) such as a superficial keratectomy, keratectomy, conjunctival graft, or penetrating keratotomy was considered.[21, 22] Advanced disease, severe discomfort, or perforation of the eye usually resulted in enucleation (E).
Fungal culture and identification
Inoculated SDA and CBA plates from the clinic were incubated and evaluated per standard operating procedures of the North Carolina State University Microbiology & Molecular Diagnostics Laboratory. Plates were incubated for up to 21 days, and evaluated biweekly for evidence of fungal growth. Initial fungal identification was performed based on examination of colony morphology and microscopic characteristics including shape and size of conidia, filamentous hyphae, chlamydospores, and conidiogenous cells following staining with lactophenol cotton blue.[1]
DNA extraction, amplification and multi-locus sequencing
All fungi were sub-cultured onto Potato Dextrose Agar (PDA) to ensure cultures were pure and grown at 30°C for seven days in the dark. Mycelia of Fusarium spp. were harvested by straining through cheesecloth, lyophilized for 3 days, and stored at -80°C until DNA extraction. For cultures with characteristics of Aspergillus, conidia were harvested from the plates of PDA by flushing with 0.05% Triton X-100 and transferring the conidial suspension into a 2 mL Eppendorf tube. Tubes were stored at -20°C until DNA extraction. DNA was extracted using MOBIO UltraClean Kit protocol for Aspergillus and DNeasy Plant Mini Kit for Fusarium, following manufacturer's recommendations.
Multi-locus sequence typing (MLST) was performed with species-specific oligonucleotide primers (S1 Table) to identify species and evolutionary lineages. Initially, DNA for all isolates were amplified and sequenced with fungal-specific nuclear ribosomal internal transcribed spacer (ITS1) and the nuclear large-subunit rRNA (LR3) primers [23] to tentatively identify each fungus to genus/species level. Isolates of Aspergillus flavus were further genotyped using six loci: two aflatoxin cluster regions (aflM/alfN and aflW/aflX) and four non-cluster regions (amdS, trpC, mfs, and MAT) that provide resolution of specific A. flavus evolutionary lineages (IA, IB and IC) [24] and subspecies (A. oryzae).[25, 26] The MAT1-1and MAT1-2 mating type genes in A. flavus were determined using oligonucleotide primers and methods described previously.[27] Isolates putatively identified as members of the Fusarium solani species complex were further genotyped using a portion of the DNA-directed RNA polymerase subunit 1 (RPB1) gene and two segments of the RPB2 gene that were previously reported to provide resolution of Fusarium strains recovered from equine FK infected eyes.[13] See S1 Table for sequences of PCR primers used for multi-locus typing of A. flavus and F. solani. All samples were sequenced with forward primers with the exception of ITS1-LR3, which were sequenced with both forward and reverse primers (underlined in S1 Table). PCR master mix corresponding to each genus was made using Apex 2.0X Taq RED Master Mix, primers, and water. Each reaction contained 24 μL of master mix and 2 μL of DNA (1–3 ng/μL). All reactions were run in an Eppendorf Mastercycler ep Gradient S Thermocycler (Eppendorf, Hamburg, Germany) using cycling conditions presented in S2 Table. Amplified DNA products were subjected to electrophoresis in a 1.5% agarose gel with ethidium bromide to verify product size. Amplified PCR products were submitted for cleanup and Sanger sequencing at the North Carolina State University Genomic Sciences Laboratory.
Phylogenetic placement and species identification
Sequences were examined in Sequencher version 5.4.6 (Gene Codes Corporation, Ann Arbor, MI). Ends were trimmed using default parameters to create unaligned FASTA sequence files for each locus. The Tree-Based Alignment Selector (T-BAS) toolkit v. 2.1 was used to integrate phylogenetic and taxonomic information, DNA sequence alignments, and clinical metadata, and to perform BLAST and phylogenetic placement of query FASTA sequences in the context of a predetermined reference tree.[28] BLASTn similarity searches of ITS sequences against the UNITE fungal database (Release 7, http://unite.ut.ee/index.php) [29] provided preliminary identification at the genus/species level. This was further corroborated with two-locus (ITS and LSU) likelihood-based placement on the published fungal [30] and Pezizomycotina [28] reference trees using the Evolutionary Placement Algorithm (EPA) in RAxML version 8 [31] accessible through the RESTful services at CIPRES.[32] Published reference trees, voucher information and multiple sequence alignments for Aspergillus section Flavi [25] and the Fusarium solani species complex [13] were imported into T-BAS v2.1 for reference-guided alignment and placement. This involves aligning query sequences for each locus to the homologous reference sequence alignment using MAFFT [33] and then running EPA on the newly extended multiple sequence alignments. A likelihood weight greater than 0.96 was used for identifying the nearest matching reference species, evolutionary lineage or MLST. Likelihood weights less than 0.5 indicate a weak match to the reference taxa and this could result in multiple equally probable or incorrect placements. In this case, MLSTs were determined directly for query isolates by collapsing multi-locus sequence alignments using SNAP Map [34] in the Mobyle SNAP Workbench. [35, 36]
Assessment of antifungal minimum inhibitory concentration (MIC)
In vitro fungal susceptibility to voriconazole (VRC), natamycin (NAT), fluconazole (FLC), thiabendazole (THB), and terbinafine (TRB) were assayed in 96-well microplates using a modified protocol of the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (M38-A2 protocol) for filamentous fungi.[37] Moxifloxacin (MXF) was included as an antibacterial control. Antimicrobial compounds represented analytical grade formulations obtained from Sigma-Aldrich (St. Louis, MO) and were diluted with DMSO as a carrier agent. Agents were added to wells in 1 μl aliquots; the final concentration of the DMSO carrier was 0.5%. Each antimicrobial was tested in a 5x dilution series (0.01, 0.05, 0.25, 1.25, 6.25, 31, 70, 156 μg/ml) with 70 μg/ml inserted between 31 and 156 μg/ml and also in a 2x dilution series (0.063, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 μg/ml) to refine the MIC determine within the middle part of the 5x dilution range. All isolates were evaluated in duplicate. Control wells included untreated wells and wells treated only with the DMSO carrier. None of the DMSO control wells showed inhibition of fungal growth. To avoid edge effects on treated wells, all edge wells were untreated. The volume of 50% Potato Dextrose Broth (PDB) in each well was 200 μl.
Isolates were cultured on PDA (Difco) and incubated at 30°C for seven days. Plates were flooded with 50% PDB (Difco) and filtered through cheesecloth to prepare conidial suspensions. Conidia concentrations were calculated using a hemocytometer and adjusted based on fungal genus (Aspergillus adjusted to 200,000 conidia/mL; Fusarium adjusted to 40,000 conidia/mL). Fifty μl of inoculum was delivered per well resulting in 10,000 conidia per well for Aspergillus and 2000 conidia per well for Fusarium. Microplates were incubated in the dark at 30°C for 72 hours. Minimum inhibitory concentrations (MICs), defined as the lowest concentration of an antifungal agent that substantially inhibits fungal growth, [37] were determined visually for each isolate using a magnified reading mirror.
Data and statistical analysis
Associations among isolate, species, evolutionary lineage, mating type, signalment, disease type, and outcome (response to medical therapy, surgical therapy or enucleation) were evaluated using Wilcoxon signed rank and Fischer Exact tests. Associations between MIC values (using the lower MIC value of a range) and isolate, species, evolutionary lineage, mating type, signalment, disease type or outcome were determine using ANOVA, student t test, and Tukey’s post hoc analysis for multiple comparisons. Differences were considered significant at p ≤ 0.05 and all probabilities and results were calculated using computerized statistical software (JMP Pro, v. 13.2; SAS Inc., Cary, NC, USA). Additional statistical analyses of the MIC values were conducted as follows. First, median MIC values for each unique isolate–antifungal combination were calculated so that each isolate would be given equal weight. Then using Minitab 18, State College, PA, USA) ANOVA analyses were conducted. If the effect of interest (e.g. Isolate), was significant at the p ≤ 0.05 level, then Tukey mean separation was conducted with α = 0.05.
Ethics statement
Animal use in this study adhered to the Association for Research in Vision and Ophthalmology Statement for use of animals in ophthalmic and vision research. Additionally, this study was approved and monitored by the North Carolina State University Institutional Care and Use Committee (IACUC) (Protocol approval # #12-013-O) and NC State Veterinary Hospital Board. The use of animals in research at NC State University is governed by institutional policy and at least two US federal statutes, including The Animal Welfare Act (Public Law 89–544, 1966, as amended [P.L. 91–579, P.L. 94–279, and P.L. 99–198]) and The Health Research Extension Act (P.L. 99–158, 1985, “Animals in Research”).
Results
Association between fungi isolated and clinical outcome
Data and samples from 32 horses with fungal keratitis (FK) were evaluated. There were 15 breeds of horses affected with FK in this study, the most common of which mirrored the clinical population and included eight Quarter horses, six Thoroughbred, three Holsteiners, and three Tennessee walking horses. All horses were from the Southeastern US, with ~81% (26/32) from North Carolina. There were 22 males and 10 females, with a mean age of 14.0 years with a range of 0.6 to 36 years of age. The disease affected 18 right eyes and 14 left eyes with 13 eyes diagnosed with superficial FK (Fig 1) and 19 eyes presented with stromal FK (Fig 2). Outcome of the 32 eyes included eight (25%) that healed with medical therapy (MT), 12 (37.5%) that healed with surgical therapy (ST), and 12 (37.5%) that were either enucleated or the horse was euthanized because of severe FK (E). There was no significant association with outcome when evaluating horse breed, sex, age, eye affected, or type of corneal lesion (Tables 1 and 2).
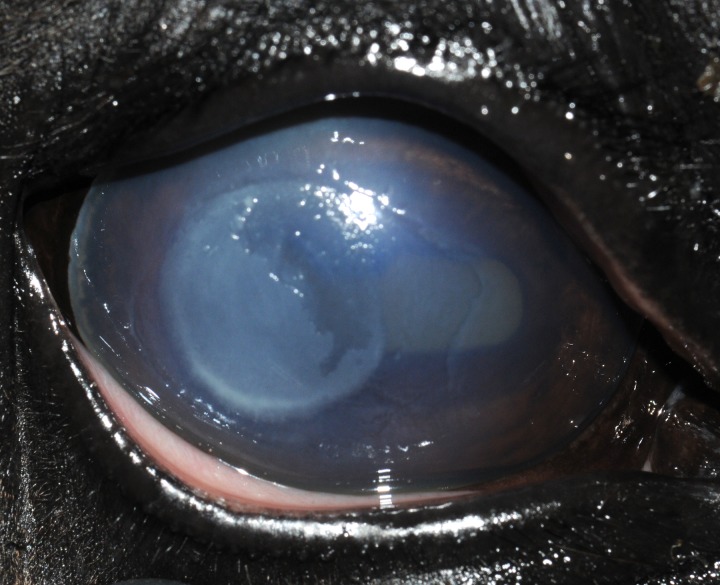
Fig 1. Superficial keratitis in a 24-year-old Thoroughbred horse (Horse #16) where Aspergillus fumigatus was isolated.
This horse’s keratitis eventually healed following surgical keratectomy.
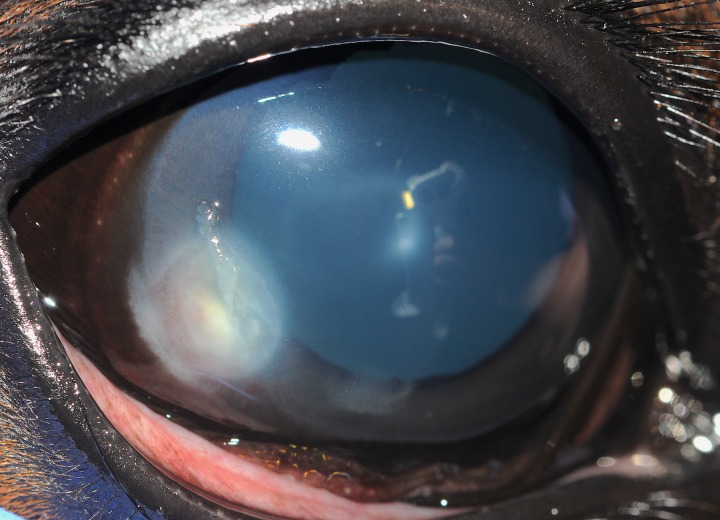
Fig 2. Stromal keratitis in a 6-month-old Holsteiner horse (Horse #29) where Fusarium falciforme was isolated.
This horse’s keratitis healed with medical therapy consisting of topical voriconazole and natamycin.
Table 1. Signalment, type of corneal disease, outcome, bacteriological result, and fungal species metadata for equine fungal keratitis patients.
| Patient # | Breed | Sex | Age at diagnosis (years) | City/State of Origin | Type of corneal disease | Fungal Species* | MLST** (Lineage) | Mating Type | Bacteriology Result | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Thoroughbred | MC | 20 | Cary, NC | Ulcerative–superficial | Aspergillus flavus | AF1 (IB) | MAT1-1 | No growth | Healed with surgery |
| 2 | Paint Horse | MC | 13 | Eastover, SC | Ulcerative–stromal | Aspergillus flavus | AF1 (IB) | MAT1-1 | No growth | Enucleation |
| 3 | Saddlebred | MC | 15 | Winston-Salem, NC | Ulcerative–stromal | Aspergillus flavus | AF2 (IB) | MAT1-2 | No growth | Enucleation |
| 4 | Quarter Horse | MC | 17 | Raleigh, NC | Ulcerative—superficial | Aspergillus flavus | AF2 (IB) | MAT1-2 | No growth | Enucleation |
| 5 | Pony | MC | 22 | Apex, NC | Ulcerative—stromal | Aspergillus flavus | AF2 (IB) | MAT1-2 | No growth | Healed with medical therapy |
| 6 | Walking Horse | MC | 17 | Southern Pines, NC | Ulcerative—stromal | Aspergillus flavus | AF3 (IB) | MAT1-2 | No growth | Healed with surgery |
| 7 | Fox Trotter | MC | 10 | Mount Olive, NC | Ulcerative—superficial | Aspergillus flavus | AF1 (IB) | MAT1-1 | No growth | Healed with surgery |
| 8 | Thoroughbred | F | 21 | Roanoke, VA | Ulcerative—stromal | Aspergillus flavus | AF4 (IC) | MAT1-2 | No growth | Healed with surgery |
| 9 | Walking Horse | MC | 11 | Marshville, NC | Ulcerative—stromal | Aspergillus flavus | AF5 (IC) | MAT1-2 | No growth | Healed with surgery |
| 10 | Holsteiner | MC | 7 | Aberdeen, NC | Ulcerative—stromal | Aspergillus flavus | AF6 (IC) | MAT1-1 | Staphylococcus sp. | Healed with surgery |
| 11 | Morgan | MC | 10 | Mooresville, NC | Ulcerative—superficial | Aspergillus flavus | AF7 (IC) | MAT1-1 | Kocuria rosea | Enucleation |
| 12 | Quarter Horse | MC | 22 | Aberdeen, NC | Ulcerative—superficial | Aspergillus flavus | AF8 (IC) | MAT1-2 | No growth | Enucleation |
| 13 | Quarter Horse | MC | 14 | Wake Forest, NC | Ulcerative—superficial | Aspergillus flavus | AF9 (IC) | MAT1-1 | No growth | Healed with surgery |
| 14 | Thoroughbred | MC | 2 | Ocala, FL | Ulcerative—stromal | Aspergillus flavus | AF8 (IC) | MAT1-2 | No growth | Enucleation |
| 15 | Quarter Horse | F | 12 | Birmingham, AL | Ulcerative—superficial | Aspergillus flavus | AF10 (IA) | MAT1-1 | Streptococcus equisimilis | Healed with medical therapy |
| 16 | Thoroughbred | F | 20 | Southern Pines, NC | Ulcerative—superficial | Aspergillus fumigatus | n.d. | n.d. | No growth | Healed with surgery |
| 17 | Arabian | F | 15 | Hillsborough, NC | Ulcerative—stromal | Aspergillus fumigatus | n.d. | n.d. | No growth | Healed with surgery |
| 18 | Saddlebred | F | 12 | Colfax, NC | Ulcerative—superficial | Aspergillus fumigatus | n.d. | MAT1-2 | No growth | Euthanasia |
| 19 | Thoroughbred | F | 15 | Oriental, NC | Ulcerative—stromal | Fusarium falciforme | FF1 (4dddd) | n.d. | No growth | Healed with surgery |
| 20 | Quarter Horse | MC | 37 | Ashboro, NC | Ulcerative—stromal | Fusarium falciforme | FF2 (4dddd, 4gggg) | n.d. | Bacillus spp. | Enucleation |
| 21 | Holsteiner | M | 0.6 | Midland, NC | Ulcerative—stromal | Fusarium falciforme | FF3 (4eee) | n.d. | No growth | Healed with medical therapy |
| 22 | Walking Horse | MC | 17 | Southern Pines, NC | Ulcerative—stromal | Fusarium falciforme | FF3 (4eee) | n.d. | No growth | Healed with surgery |
| 23 | Dutch Warmblood | F | 15 | Williamsburg, VA | Ulcerative—stromal | Fusarium falciforme | FF4 (4eeee, 4uuu) | n.d. | Streptococcus zooepidemicus | Healed with medical therapy |
| 24 | Selle Francais | MC | 16 | Davidson, NC | Ulcerative—stromal | Fusarium falciforme | FF5 (4hhhh) | n.d. | No growth | Enucleation |
| 25 | Quarter Horse | MC | 11 | Advance, NC | Ulcerative—stromal | Fusarium falciforme | FF6 (4hhhh, 4ffff) | n.d. | No growth | Healed with medical therapy |
| 26 | Warmblood | MC | 10 | Hillsborough, NC | Ulcerative—superficial | Fusarium falciforme | FF7 (4hhhh, 4ffff) | n.d. | No growth | Enucleation |
| 27 | Warmblood | F | 14 | Reidsville, NC | Ulcerative—stromal | Fusarium falciforme | FF8 (4hhhh, 4ffff) | n.d. | No growth | Enucleation |
| 28 | Percheron | MC | 22 | Sedley, VA | Ulcerative—stromal | Fusarium keratoplasticum | FK1 (2u) | n.d. | No growth | Enucleation |
| 29 | Holsteiner | M | 7 | Midland, NC | Ulcerative—stromal | Fusarium proliferatum | FP1 | n.d. | No growth | Healed with medical therapy |
| 30 | Quarter Horse | F | 5 | Warsaw, NC | Ulcerative—superficial | Mucor sp. | n.d. | n.d. | Staphylococcus aureus | Healed with medical therapy |
| 31 | Quarter Horse | F | 11 | Summerton, SC | Ulcerative—stromal | Byssochlamys sp. | n.d. | n.d. | No growth | Healed with medical therapy |
| 32 | Thoroughbred | MC | 11 | Wilmington, NC | Ulcerative—superficial | Exserohilum sp. | n.d. | n.d. | Bacillus spp. | Healed with surgery |
n.d. = Not Determined
*Classification to species level was based on multi-locus phylogenetic placement.
**Multi-locus sequence type (MLST) designations are labeled with the first two uppercase letters for the species (AF = A. flavus; FF = F. falciforme; FK = Fusarium keratoplasticum; and FP = Fusarium proliferatum) followed by a number for the unique haplotype within each species. In parentheses are lineage or species haplotype designations derived from reference trees used for phylogenetic placements. In A. flavus, lineage membership (IA, IB, or IC) is from Moore et al 2017 (25). In Fusarium, species haplotypes are shown instead of lineage and are from O’Donnell et al. 2016 (13), where species are designated with Arabic numerals (2 = F. keratoplasticum; and 4 = F. falciforme) followed by lowercase letters to represent unique haplotypes within each species (e.g. 4dddd and 4gggg represent different multi-locus haplotypes).
Table 2. Summary table—Genetic lineage haplotypes, species haplotypes and clinical outcomes in fungal keratitis.
| Fungal identification | Lineage haplotypes | Clinical type (n) | Outcome (n)* |
|---|---|---|---|
| Aspergillus spp. | A. flavus lineage IB | ||
| AF1 | Superficial (2); Stromal (1) | HS (2) E (1) |
|
| AF2 | Superficial (1); Stromal (2) | HM (1) E (2) |
|
| AF3 | Stromal (1) | HS (1) | |
| A. flavus lineage IC | |||
| AF4 | Stromal (1) | HS (1) | |
| AF5 | Stromal (1) | HS (1) | |
| AF6 | Stromal (1) | HS (1) | |
| AF7 | Superficial (1) | E (1) | |
| AF8 | Superficial (1); Stromal (1) | E (2) |
|
| AF9 | Superficial (1) | HS (1) | |
| A. flavus lineage IA | |||
| AF10 | Superficial (1) | HS (1) | |
| A. fumigatus | Superficial (2); Stromal (1) | E (1)a HS (2) | |
| Total | Superficial (9); Stromal (9) | HM (1) HS (10) E (6) E (1)a | |
| Fusarium spp. | Species haplotypes: | ||
| F. falciforme FF1 | Stromal (1) | HS (1) | |
| F. falciforme FF2 | Stromal (1) | E (1) | |
| F. falciforme FF3 | Stromal (2) | HM (1) HS (1) | |
| F. falciforme FF4 | Stromal (1) | HM (1) | |
| F. falciforme FF5 | Stromal (1) | E (1) | |
| F. falciforme FF6 | Stromal (1) | HM (1) | |
| F. falciforme FF7 | Superficial (1) | E (1) | |
| F. falciforme FF8 | Stromal (1) | E (1) | |
| F. proliferatum FP1 | Stromal (1) | HM (1) | |
| F. keratoplasticum FK1 | Stromal (1) | E (1) | |
| Total | Superficial (1); Stromal (10)1 | HM (4) HS (2) E (5) | |
| Other | Mucor circinelloides | Superficial (1) | HM (1) |
| Byssochlamys sp. | Stromal (1) | HM (1) | |
| Exserohilum sp. | Superficial (1) | HS (2) | |
| Total | Superficial (2); Stromal (1) | HM (2) HS (1) E (0) | |
| Total Isolates | Superficial (12); Stromal (20) | HM (7) HS (13) E (11) E (1)a |
*HM–healed with medical treatment only
a Euthanasia instead of enucleation
HS- healed with surgical intervention. E–enucleated
1Fusarium sp. fungal keratitis significantly more likely to be associated with stromal keratitis (Fishers Exact test, p = 0.045)
On routine fungal culture, characteristic microconidia (oval and 1–2 cells) and macroconidia (curved (falcate) and >2 cells) and chlamydospores typical of Fusarium and oval chains of conidia attached to phialides and metulae arising for vesicle typical of Aspergillus were observed for 90.6% (29/32) of the cultures. The most common fungi isolated based on morphological and DNA analysis in horses with FK were species of Aspergillus (18 of 32 [50%]) and Fusarium (11 of 32 [34%]). In addition, three other fungal species (Byssochlamys, Mucor, and Exserohilum) were identified and associated with equine FK (Table 1). Bacterial outcomes were reported for six horses with FK (18.8%) and consisted of species of Bacillus (1), Staphylococcus (2), Streptococcus (2) and Kocuria rosea (1) (Table 1). There were no statistical associations among fungal species, type of corneal lesion, presence of bacterial co-infection, or patient outcome (Tables 1 and 2). However, species of Fusarium sampled and cultured from FK horses were significantly (p = 0.045; Fisher’s Exact test) more likely to be associated with stromal keratitis.
Horse eyes infected with Fusarium were significantly (Chi-Square p = 0.04) more likely to heal with medical therapy than eyes infected with Aspergillus. But the enucleation level was essentially the same whether the eye was infected with Aspergillus or Fusarium (p = 0.88) because of improved healing with surgery for eyes infected with Aspergillus.
To delimit species in Fusarium, sequences were examined using multi-locus EPA placement on the reference tree published by O'Donnell et al. [13] Fusarium multi-locus haplotypes were based on collapsing of concatenated RPB1 and RPB2 sequence alignments. In our naming convention, multi-locus haplotypes are labeled with the first two uppercase letters for the species (e.g., FF = F. falciforme) followed by a number for the unique haplotype within each species. Of the Fusarium species isolated from equine FK, 10/11 (91%) samples belonged to the Fusarium solani species complex (FSSC) (i.e., nine isolates of Fusarium falciforme and one isolate of Fusarium keratoplasticum). An additional isolate of Fusarium proliferatum belonging to the Fusarium fujikuroi species complex (FFSC) was also sampled from equine FK. FSSC haplotypes were labeled FF1–8, FP1 and FK 1 (Tables 1 and 2). Fusarium species, haplotypes, isolates, or presence of bacterial co-infection was not significantly associated with lesion type or FK outcome.
To determine lineage membership for species identified as Aspergillus flavus, sequences were examined using multi-locus EPA placement on the Aspergillus section Flavi reference tree. [25] Aspergillus lineage haplotypes were based on aflM/alfN, aflW/aflX, amdS, trpC, mfs, and MAT. Of the species of Aspergillus isolated from equine FK, 15 were classified as Aspergillus flavus, seven of which were lineage IB, seven belonging to lineage IC and one from lineage IA. Three isolates were classified as Aspergillus fumigatus (Tables 1 and 2). Aspergillus flavus lineage haplotypes were labeled AF1–10 (Tables 1 and 2). Aspergillus species, evolutionary lineage and haplotypes, or presence of co-infection was not significantly associated with lesion type or outcome of FK (Tables 1 and 2).
Three other fungi isolated from equine FK included species of Mucor, Byssochlamys, and Exserohilum. Mucor and Exserohilum spp. both had bacterial co-infections, however, all three patients healed, two with medical treatment only, and one with surgical treatment (Table 1). Overall, these outcome results were more favorable than FK with Aspergillus spp. (2 HM; 9 HS; 7 E) or Fusarium spp. (4 HM; 2 HS; 5 E) (Tables 1 and 2).
Association between in vitro antifungal susceptibility and fungal taxonomy
In vitro antifungal susceptibility of VRC, NAT, FLC, THB, TRB, and MXF (as a negative control) was evaluated for isolates of Aspergillus and Fusarium from equine FK (Table 3; Fig 3). None of the fungal isolates were susceptible to MXF even at concentrations as high as 156 μg/ml. All Fusarium isolates and most Aspergillus isolates grew in the presence of FLC at concentrations as high as 156 μg /ml. There were no significant association in mean MIC values for FLC and MXF among isolates, species, evolutionary lineages, degree of corneal invasion, or disease outcome (Table 3).
Table 3. Minimal inhibitory concentrations (MIC) (μg/mL) of isolates from equine fungal keratitis.
| Patient # | Type of corneal disease | Fungal Species | MLST* (Lineage) | Voriconazole MIC | Natamycin MIC | Fluconazole MIC | Thiabendazole MIC | Terbinafine MIC | Moxifloxacin MIC | Antifungal (s) used | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ulcerative—superficial | Aspergillus flavus | AF1 (IB) | 0.5 | n.d. | >156 | n.d. | n.d. | >156 | Voriconazole, natamycin | Healed with surgery |
| 2 | Ulcerative—stromal | Aspergillus flavus | AF1 (IB) | 0.5–1 | 16 | ≥156 | 6.25 | 0.05 | >156 | n.a. | Enucleation |
| 3 | Ulcerative—stromal | Aspergillus flavus | AF2 (IB) | 0.5–1 | 32–70 | ≥156 | 6.25 | 0.05 | >156 | Voriconazole, fluconazole | Enucleation |
| 4 | Ulcerative—superficial | Aspergillus flavus | AF2 (IB) | 0.25–1 | 16–70 | ≥156 | 6.25 | 0.05 | n.d. | Voriconazole, amphotericin B | Enucleation |
| 5 | Ulcerative—stromal | Aspergillus flavus | AF2 (IB) | 0.5–1 | 16 | >156 | 6.25 | 0.05–0.25 | n.d. | Voriconazole, fluconazole | Healed with medical therapy |
| 6 | Ulcerative—stromal | Aspergillus flavus | AF3 (IB) | 0.5–1 | 16–70 | ≥156 | 6.25 | 0.05–1 | >156 | n.a. | Healed with surgery |
| 7 | Ulcerative—superficial | Aspergillus flavus | AF1 (IB) | 0.5–1 | ≥32 | >156 | 6.25 | 0.05–0.0625 | n.d. | Voriconazole | Healed with surgery |
| 8 | Ulcerative—stromal | Aspergillus flavus | AF4 (IC) | 0.05–0.26 | 16–70 | ≥156 | 4–6.25 | 0.05–0.25 | >156 | Voriconazole | Healed with surgery |
| 9 | Ulcerative—stromal | Aspergillus flavus | AF5 (IC) | 1.25–4 | 70 | >156 | 6.25–16 | 0.05–0.125 | >156 | n.a. | Healed with surgery |
| 10 | Ulcerative—stromal | Aspergillus flavus | AF6 (IC) | 0.25–0.5 | 4–6.25 | >156 | 16 | 1 | n.d. | n.a. | Healed with surgery |
| 11 | Ulcerative—superficial | Aspergillus flavus | AF7 (IC) | 1 | 32–70 | >156 | 6.25 | 0.125–0.25 | n.d. | Voriconazole | Enucleation |
| 12 | Ulcerative—superficial | Aspergillus flavus | AF8 (IC) | 1–1.25 | 70 | >156 | 6.25 | 0.0625 | n.d. | None | Enucleation |
| 13 | Ulcerative—superficial | Aspergillus flavus | AF9 (IC) | 1 | 32 | >156 | 6.25 | 0.05 | n.d. | Voriconazole | Healed with surgery |
| 14 | Ulcerative—stromal | Aspergillus flavus | AF8 (IC) | 0.5–1 | 32–70 | ≥156 | 6.25 | 0.05 | n.d. | Miconazole, Voriconazole, Amphotericin B | Enucleation |
| 15 | Ulcerative—superficial | Aspergillus flavus | AF10 (IA) | 0.5–1 | 16–70 | ≥156 | 6.25 | 0.05–1 | >156 | Voriconazole, natamycin | Healed with medical therapy |
| 16 | Ulcerative—superficial | Aspergillus fumigatus | n.d. | 0.25–0.5 | 4–6.25 | >156 | 16 | 1 | >156 | Voriconazole | Healed with surgery |
| 17 | Ulcerative—stromal | Aspergillus fumigatus | n.d. | 0.25–0.5 | 4–6.25 | >156 | 16 | 1–1.25 | >156 | Voriconazole, natamycin | Healed with surgery |
| 18 | Ulcerative—superficial | Aspergillus fumigatus | n.d. | 0.25–1.25 | 4 | >156 | 16 | 1.25 | >156 | None | Euthanasia |
| 19 | Ulcerative—stromal | Fusarium falciforme | FF1 (4dddd) | 2–6.25 | 4–32 | >156 | 1.25 | 6.25–16 | >156 | n.a. | Healed with surgery |
| 20 | Ulcerative—stromal | Fusarium falciforme | FF2 (4dddd, 4gggg) | 2 | 4–8 | >156 | 1 | 6.25–16 | >156 | Voriconazole | Enucleation |
| 21 | Ulcerative—stromal | Fusarium falciforme | FF3 (4eee) | 2–4 | 4–32 | >156 | 4–6.25 | 6.25 | >156 | Voriconazole | Healed with medical therapy |
| 22 | Ulcerative—stromal | Fusarium falciforme | FF3 (4eee) | 2–4 | 4–32 | >156 | 4 | 6.25 | >156 | n.a. | Healed with surgery |
| 23 | Ulcerative—stromal | Fusarium falciforme | FF4 (4eeee, 4uuu) | 4 | 4–70 | >156 | 1.25 | 6.25–16 | >156 | Voriconazole | Healed with medical therapy |
| 24 | Ulcerative—stromal | Fusarium falciforme | FF5 (4hhhh) | 1–4 | 4–32 | >156 | 0.25–1.25 | 6.25–8 | >156 | Voriconazole | Enucleation |
| 25 | Ulcerative—stromal | Fusarium falciforme | FF6 (4hhhh, 4ffff) | 6.25 | 4 | >156 | 4 | 16 | n.d. | Voriconazole, fluconazole | Healed with medical therapy |
| 26 | Ulcerative—superficial | Fusarium falciforme | FF7 (4hhhh, 4ffff) | 1–4 | 0.125–32 | >156 | 0.25–2 | 6.25–8 | >156 | Voriconazole, fluconazole | Enucleation |
| 27 | Ulcerative—stromal | Fusarium falciforme | FF8 (4hhhh, 4ffff) | 1–6.25 | 1.25–32 | >156 | 1–4 | 6.25 | >156 | Voriconazole, fluconazole | Enucleation |
| 28 | Ulcerative—stromal | Fusarium keratoplasticum | FK1 (2u) | 4–6.25 | 6.25–32 | >156 | 1.25–6.25 | 16 | >156 | n.a. | Enucleation |
| 29 | Ulcerative—stromal | Fusarium proliferatum | FP1 | 1.25–4 | 1.25–4 | >156 | 8 | 1.25 | >156 | Voriconazole | Healed with medical therapy |
| 30 | Ulcerative—superficial | Mucor sp. | n.d. | >156 | 6.25 | >156 | n.d. | n.d. | >156 | Voriconazole | Healed with medical therapy |
| 31 | Ulcerative—stromal | Byssochlamys sp. | n.d. | 4 | n.d. | >156 | n.d. | n.d. | >156 | Voriconazole, fluconazole | Healed with medical therapy |
| 32 | Ulcerative—superficial | Exserohilum sp. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | Voriconazole | Healed with surgery |
n.d. = Not Determined. n.a. = Not Available
*See Table 1 for a description of MLST (Lineage
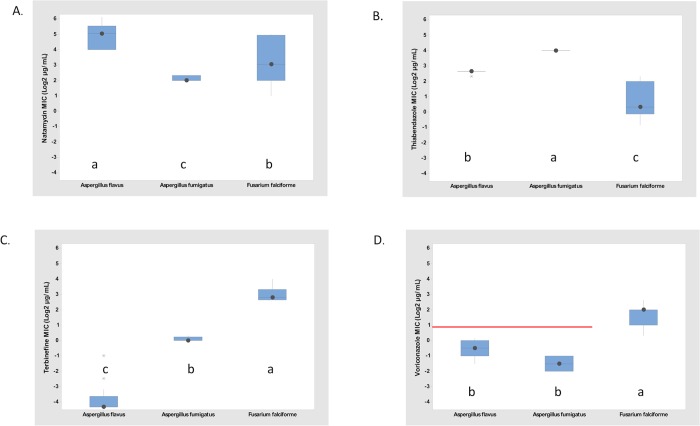
Fig 3. Fungal species boxplots of isolates sampled from equine fungal keratitis.
A. Natamycin. B. Thiabendazole. C. Terbinafine. and D. Voriconazole. Minimal inhibitory concentration (MIC) values converted to log base 2 in parallel to the 2x dose steps used. ANOVA 2-factor Analysis: P values were all < 0.001 for fungus and antifungal main effects and for fungus x antifungal interaction. Mean separation of the fungus x antifungal interaction: Tukey mean with α = 0.05. Different letters indicate significant differences, CLSI susceptibility working breakpoint for voriconazole for Aspergillus is ≤1 μg/mL (red line). No breakpoints are available for natamycin, thiabendazole and terbinafine. Number of isolates: Aspergillus flavus: n = 13; Aspergillus fumigatus: n = 5; and Fusarium falciforme: n = 10.
Minimal inhibitory concentration values for VRC ranged from 0.25 μg/ml (five isolates of Aspergillus flavus) to 6.25 μg/ml for four Fusarium isolates. An isolate of Mucor sp. had an MIC of >156 μg/ml for VRC. For NAT, MIC ranged from 0.125 μg/ml (one isolate of Fusarium falciforme [FF7]) to 32 μg/ml for five isolates of A. flavus. Minimal inhibitory concentration values for THB ranged from 0.25 μg/ml against two isolates of Fusarium falciforme to 16 μg/ml for an A. flavus and three A. fumigatus isolates. For TRB, MIC values ranged from 0.05 μg/ml from 11 isolates of Aspergillus spp. to 16 μg/ml for a F. falciforme and F. keratoplasticum isolate. (Table 3).
At the fungal genus level, there were highly significant differences in sensitivity of Aspergillus and Fusarium isolates for three compounds, VRC, THB, and TRB (p<0.001). Aspergillus was more sensitive to VRC and TRB than Fusarium; whereas Fusarium was more sensitive to THB than Aspergillus (Table 3, Fig 3). For NAT, the strong species effect within Aspergillus resulted in one Aspergillus species being more sensitive and one species being less sensitive than the Fusarium isolates which were intermediate in sensitivity between the two species of Aspergillus. Therefore, patterns of sensitivity to NAT have to be considered at the species level not at the genus level.
At the fungal species level, there were significant (p<0.001) differences among species for four antifungal agents, VRC, THB, NAT, and TRB (Figs 3 and 4). Three species had multiple isolates and thus could be tested for MIC species differences: Aspergillus flavus, A. fumigatus, and Fusarium falciforme. A. flavus (mean MIC of 33.5 +/- SD 16.1 μg/ml) was less susceptible than F. falciforme (mean MIC of 14.4 +/- SD 12.4) and A. fumigatus (mean MIC of 4.4 +/- SD 0.5) to NAT. Both species of Aspergillus (with mean MIC of 0.10 +/- SD 0.13 μg/ml for A. flavus and 1.1 +/- SD 0.11 μg/ml for A. fumigatus) were more susceptible than F. falciforme (mean MIC of 8.5 +/- SD 3.1) to TRB. For VRC the two species of Aspergillus had mean MIC values of 0.7 +/- SD 0.3 μg/ml for A. flavus and 0.4 +/- SD 0.1 μg/ml for A. fumigatus and exhibited greater susceptibility than F. falciforme with a mean MIC of 3.4 +/- SD 1.5 μg/ml. In contrast, Fusarium falciforme (mean MIC of 2.1 +/- SD 1.6) was more susceptible to THB than both species of Aspergillus (mean MIC of 6.2 +/- SD 0.3 μg/ml for A. flavus and 16.0 +/- SD 0.0 μg/ml for A. fumigatus) (Figs 3 and 4).
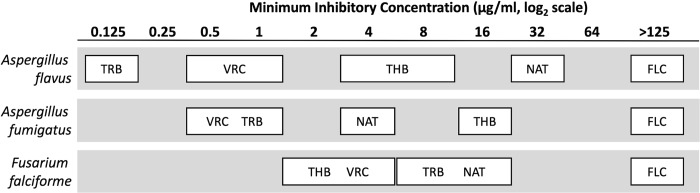
Fig 4. Minimal inhibitory concentration (MIC) comparisons among isolates from equine fungal keratitis.
Antifungal agents within a box do not have significantly different MIC values, while antifungal agents in different boxes have significantly different MIC values. ANOVA, 2-factor. P < 0.001 for fungus x antifungal agent interaction. Mean separation: Tukey with α = 0.05. FLC = fluconazole, NAT = Natamycin, TRB = Terbinafine, THB = Thiabendazole, and VRC = Voriconazole.
There were significant differences in susceptibility between the two species of Aspergillus for NAT, THB, and TRB. A. fumigatus was more susceptible to NAT than A. flavus (mean MIC of 4.4 +/- SD 0.5 μg/ml for A. fumigatus and 33.5 +/- SD 16.1 μg/ml for A. flavus) whereas A. flavus exhibited higher susceptibility to TBH and to TRB than A. fumigatus (TBH: mean MIC of 6.2 +/- SD 0.3 μg/ml for A. flavus and 16.0 +/- SD 0.0 μg/ml for A. fumigatus; TRB: mean MIC of 0.1 +/- SD 0.1 μg/ml for A. flavus and 1.1 +/- SD 0.1 μg/ml for A. fumigatus). These statistically significant species differences in antifungal agent susceptibility within Aspergillus demonstrate the importance of accurate identification of the causal fungal pathogen to the species level. In the case of Fusarium, it was not possible to evaluate interspecies differences because there was only one isolate of F. keratoplasticum and F. proliferatum. However, the pattern of TRB and THB MIC values between the single isolate of F. proliferatum and the 9 isolates of F. falciforme suggest that there may be interspecies susceptibility differences within Fusarium. The median TRB MIC value for F. proliferatum at 1.25 μg/ml was lower than the TRB MIC values for all 9 isolates of F. falciforme. The median THB MIC for F. proliferatum at 8 μg/ml was higher than the THB MIC values for all 9 isolates of F. falciforme.
There were no statistically significant differences in antifungal agent susceptibility between IB and IC lineages within A. flavus and among different lineages of F. falciforme in antifungal agent susceptibility to TRB, TBH and VRC. Lineage group FF6-7-8 of F. falciforme was more susceptible than lineages FF1, 2, 4, and 5 to NAT. Neither of these lineage groups of F. falciforme differed in susceptibility to the two isolates belonging to lineage FF3 which were classified as intermediate.
The antifungal used for treatment in these equine FK cases included most commonly topical voriconazole (n = 23/32), topical natamycin (n = 3/32), oral fluconazole (n = 7/23), and subconjunctival amphotericin B (n = 2/32) (Table 2). The selection and route of these antifungals was based on formulation availability and clinician preferences, and not on susceptibility testing. There was no correlation between in vitro sensitivity testing, antifungal used, and FK outcome (Table 3).
Discussion
FK is a common and aggressive disease in horses. In this study, 25% of equine FK cases were resolved with medical therapy and over 37% of the patients had loss of the eye due to infection. To better understand the pathogenesis and treatment of this disease, we used multi-locus DNA sequence analysis to accurately determine fungal species and evolutionary lineages and to examine associations with in vitro antifungal agent susceptibility, and outcome of equine FK. Analogous to human patients, misidentification of causative agents of filamentous FK and use of inadequate therapy may lead to blindness. Therefore, species-level identification of putative pathogen and antifungal agent susceptibility of the causal fungi is important for successful FK therapy.[38]
In this study of 32 cases of FK in horses, filamentous fungi predominated: 56% of FK cases were associated with Aspergillus spp., 34% with Fusarium spp., and 3% were Mucor sp., Byssochlamys sp., or Exserohilum sp. Our results are consistent with previous reports using standard mycological culture techniques in horses where the occurrence and isolation of species of Aspergillus predominate in equine FK, with species of Fusarium sampled and isolated in a lower frequency than Aspergillus.[39–41] Associated fungal species in human FK vary, but similar to horses, filamentous fungi predominate. In most studies of human FK investigations, a slightly higher percentage of species of Fusarium (approximately 28–48%) is observed compared to species of Aspergillus (19–25%).[1,3,4] However, in a study from China, FK in humans were more commonly associated with A. fumigatus (65%)[1], while another study from south Florida demonstrated A. flavus (42%) as the most common fungal associate in human cases of FK,[6] suggesting a regional geographic difference in pathogenic fungal species in FK.
In both equine and human Fusarium FK, fungi most commonly isolated belong to the F. solani species complex (FSSC) (i.e., Fusarium falciforme, Fusarium keratoplasticum and Fusarium sp. FSSC 12). Gajjar et al.[6], Homa et al. [42] and Oechsler et al. [2] also found that FK Fusarium sampled from human eyes nested most commonly into the FSSC. For example, Gajjar et al. [6] used a single locus (ITS1 and 4 regions) for phylogenic analysis and placement and reported that all identified isolates of Fusarium placed into the FSSC. Homa et al. [42] conducted a two-locus (β-tubulin and elongation factor 1-α) and Oechsler et al. [2] a single locus (ITS) phylogenetic analyses of Fusarium collected from human eyes in India and South Florida, respectively, also demonstrated that 75–76% of Fusarium causing FK belonged to the FSSC. O’Donnell [13] described species of Fusarium isolated from a variety of veterinary sources and found that the most commonly sampled veterinary Fusaria were isolated from eyes of horses (31% of those reported). Furthermore, they deployed a three-locus phylogenetic analysis (TEF1, RPB2, and ITS) of 17 isolates of Fusarium sampled from 17 equine eyes, most of which were from the southeastern US. Similar to our results, O’Donnell reported 14 of 17 (82%) isolates sampled from an equine FK source belonged the FSSC and represented 12 genetically diverse strains/lineages.[13] In our study, 91% of equine Fusarium FK nested within the FSSC and represented nine genetically diverse strains/lineages. Only MLSTs from horse numbers 21, 22 and 29 had cumulative likelihood weights > 0.96 and are considered reliable placements within the FSSC; F. falciforme haplotype FF3 for patient 21 and 22 matched F. falciforme haplotype 4eee from equine eye (NRRL 54964); F. proliferatum FP1 for patient 29 matched rhinoceros horn (NRRL 54994) and equine eye (NRRL 62546); all other strains showed weak placements and hit multiple F. falciforme haplotypes as nearest siblings. It is common for members of the FSSC that share the same multi-locus haplotypes to cause infections in humans, animals and plants.[43] This is true also in F. falciforme which was reported as an emerging pathogen on lima bean in Brazil [44] and shares a most recent common ancestor with F. falciforme haplotypes in this study based on phylogenetic placement of a portion of the RPB2 gene (data not shown). Updating the FSSC reference tree with these strains would increase phylogenetic and host diversity of F. falciforme, and improve resolution and reliability of future placements.
In our study, 15/18 (83%) of equine Aspergillus FK nested within the A. flavus clade, and included three genetically diverse lineages, IA, IB and IC. Only one A. flavus isolate belonged to IA and the other 14 strains were equally split between IB and IC, which is consistent with the frequency of IB and IC isolated from soil in agricultural fields.[25, 45] Interestingly, 10/14 (71%) of the A. flavus strains had A. oryzae as their nearest common ancestor in both lineages IB (7/7) and IC (3/7), supporting a close relationship between wild and domesticated A. flavus strains.[26, 46] Putative clonal lineages within IB (AF1) and IC (AF8) were associated with both superficial and stromal keratitis infections in different horses and states, suggesting that strains with close affinities to A. flavus/A. oryzae harbor characteristics (e.g. metabolites) that serve as effective conduits for equine FK disease. Three additional isolates of Aspergillus were identified as A. fumigatus (17%). Further differentiation of these strains is possible using mating types [47] and microsatellite markers [48] but we have limited information on evolutionary lineages in A. fumigatus from multi-locus DNA sequence data. There are fewer studies specifically evaluating the genetic diversity of Aspergillus in human FK.[49] However, in one study in India [49], fungi identified through multi-locus sequence analysis (ITS1-5.8S-ITS2, calmodulin, and β-tubulin) were similar to what we found in horses where 75% of human FK aspergillosis were identified as A. flavus and 12% were A. fumigatus.
Although A. flavus/A. oryzae and F. falciforme were recovered predominantly from equine FK infected eyes, species, haplotypes, isolates, or evolutionary lineage of Aspergillus or Fusarium were not significantly associated with lesion type or FK outcome in horse eyes in this study. This suggests that FK disease severity or virulence are complex phenotypes determined by multiple factors that are not closely linked to multi-locus markers examined in this study. Additional factors such as initiating injury (the type and nature of injury is typically unknown in horses), delay of owners of horses to seek treatment, and variable treatment prior to examination may determine severity of infection and outcome in equine FK. However, in this study we demonstrated that Fusarium species sampled and cultured from FK horses were significantly more likely to be associated with stromal keratitis compared to Aspergillus.
Although there was no statistical association among antifungal agent susceptibility and disease severity or outcome, significant differences in susceptibility was observed at the fungal genus, species, and evolutionary lineage levels. Most notably, at the fungal genus level, Aspergillus was more susceptible to VRC and TRB than Fusarium; whereas Fusarium was more susceptible to THB than Aspergillus. At the species level, A. flavus was statistically less sensitive to NAT than F. falciforme and A. fumigatus. Both species of Aspergillus were more susceptible to TRB than F. falciforme and the two species of Aspergillus were more susceptible to VRC than F. falciforme. In contrast, Fusarium falciforme was more susceptible to THB than were both Aspergillus species. There were no statistically significant differences in antifungal agent susceptibility between IB and IC lineages within Aspergillus flavus. However, within different lineages of Fusarium falciforme, FF6-7-8 was more susceptible to NAT than FF1, 2, 4, and 5. These statistically significant species differences in antifungal agent susceptibilities within Aspergillus demonstrate the importance of accurate identification of the potential fungal pathogen to the species level.
However, we did not find a correlation between in vitro sensitivity testing, antifungal used clinically, and FK outcome in these horses (Table 3). This may suggest that the clinical relevance of in vitro fungal testing is low and that additional methods are needed for better translate these results to clinical fungal keratitis. This subject is being currently investigated by our laboratories. Factors other than drug susceptibility may influence outcome in these clinical patients, such as variability of disease severity and host response to injury (e.g., host immune response and healing rates). Therefore, larger case numbers, MLST identification, and susceptibility testing are needed.
Further study of these equine FK isolates against other common antifungal agents is needed, including itraconazole, amphotericin B, clotrimazole, ketaconazole, and econazole. One study of Aspergillus from human FK demonstrated that A. flavus was susceptible to econazole, clotrimazole and ketoconazole while A. fumigatus was susceptible to amphotericin B, natamycin, voriconazole, and itraconazole.[47] In another study, amphotericin B and natamycin where shown to be effective against species of Fusarium, while species of Aspergillus were sensitive to amphotericin B and itraconazole.[6] Homa et al. [42] reported that terbinafine, natamycin, and amphotericin B followed by voriconazole were the most effective antifungal drugs for the majority of Fusarium isolates from human FK. As a whole, the results from these published studies support our data, but suggest that amphotericin B and possibly itraconazole are two antifungals that should be evaluated against isolates of Aspergillus and Fusarium from equine FK. O’Donnell et al. [11] showed that human FK isolates of the FSSC phylogeny complex (19 isolates representing 18 species) were insensitive to 10 antifungal agents tested in vitro. In contrast, we found that FSSC complex composed of F. falciforme was susceptible to natamycin and thiabendazole, but less susceptible to voriconazole and terbinafine. MIC values for Aspergillus spp. obtained in this equine FK study match those reported for human FK; as examples, for A. flavus 0.7 and 33.5 versus 1 and 32 μg/ml [50] for voriconazole and natamycin respectively; for A. fumigatus 0.4 and 4.4 versus 0.5 and 4 μg/ml [50] for voriconazole and natamycin respectively. In the case of Fusarium spp., there are both similarities and differences between MIC values in this equine FK study with those obtained from human FK studies in part due to the high variability among human FK studies.[51, 52]
Although fungal species and evolutionary lineage were not associated with clinical outcome in this study, associations regarding antifungal agent susceptibility demonstrated the importance of identifying the potential fungal pathogen to the species and lineage levels and not just to the generic level. These results also suggest that antifungal agent treatment of equine keratitis should be tailored to the infecting fungi and that accurate fungal species identification is critical to determine response to therapeutic agents and for developing effective treatment recommendations. Therefore, it is recommended to perform MLST typing routinely in FK to help choose appropriate antifungal therapy based on likely susceptibility and with a large sample size, ultimately, predict outcome.
Supporting information
The primers used for Sanger sequencing are underlined.
(DOCX)
(DOCX)
Acknowledgments
The authors thank Kerry O’Donnell, Jacklyn H Salmon, Allison Blanchard, and Erin Barr. We thank Dr. Richard McMullen and staff at Auburn University College of Veterinary Medicine and the clinical staff and technicians at North Carolina State University College of Veterinary Medicine for sample collection, patient care, and management of samples.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
NC State University College of Agricultural and Life Sciences and the College of Veterinary Medicine provided seed funding. This work was partially supported by the Agriculture and Food Research Initiative Competitive Grants Program grant no. 2013-68004-20359 from the USDA National Institute of Food and Agriculture (NIFA) to IC. The development of T-BAS is supported by the National Science Foundation (NSF) Genealogy of Life (GoLife) program to IC (DEB-1541418). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.He D, Hao J, Zhang B, Yang Y, Song W, Zhang Y, et al. Pathogenic spectrum of fungal keratitis and specific identification of Fusarium solani. Investig Ophthalmol Vis Sci 2011;52: 2804–2808. [DOI] [PubMed] [Google Scholar]
- 2.Oechsler RA, Feilmeier MR, Miller D, Shi W, Hofling-Lima AL, Alfonso EC. Fusarium keratitis: Genotyping, in vitro susceptibility and clinical outcomes. Cornea 2013;32: 667–673. 10.1097/ICO.0b013e318277ac74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy P, Das S, Singh NP, Saha R, Kajla G, Snehaa K, et al. Changing trends in fungal and bacterial profile of infectious keratitis at a tertiary care hospital: A six-year study. Clin Epidemiol Glob Heal 2017;5: 40–45. [Google Scholar]
- 4.Mascarenhas J, Lalitha P, Prajna NV, Srinivasan M, Das M, D’Silva SS, et al. Acanthamoeba, fungal, and bacterial keratitis: A comparison of risk factors and clinical features. Am J Ophthalmol 2014;157: 56–62. 10.1016/j.ajo.2013.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, et al. Trends in Fungal keratitis in the United States, 2001 to 2007. Ophthalmology 2010;117: 2263–2267. 10.1016/j.ophtha.2010.03.048 [DOI] [PubMed] [Google Scholar]
- 6.Gajjar DU, Pal AK, Ghodadra BK, Vasavada AR. Microscopic evaluation, molecular identification, antifungal susceptibility, and clinical outcomes in Fusarium, Aspergillus and, dematiaceous keratitis. Biomed Res Int 2013: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013;4: 119–128. 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Nadales E, Almeida Nogueira MF, Baldin C, Castanheira S, El Ghalid M, Grund E, et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Genet Biol 2014;70: 42–67. 10.1016/j.fgb.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua X, Yuan X, Wilhelmus KR. A fungal pH-responsive signaling pathway regulating Aspergillus adaptation and invasion into the cornea. Investig Ophthalmol Vis Sci 2010;51: 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol 2006;44: 2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, et al. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol 2008;46: 2477–2490. 10.1128/JCM.02371-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell K, Sarver BAJ, Brandt M, Chang DC, Noble-Wang J, Park BJ, et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J Clin Microbiol 2007;45: 2235–2248. 10.1128/JCM.00533-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donnell K, Sutton DA, Wiederhold N, Robert VARG , Crous PW , Geiser DM. Veterinary fusarioses within the United States. J Clin Microbiol 2016;54: 2813–2819. 10.1128/JCM.01607-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeiss C, Neaderland M, Yang FC, Terwilliger G, Compton S. Fungal polymerase chain reaction testing in equine ulcerative keratitis. Vet Ophthalmol 2013;16: 341–351. 10.1111/vop.12004 [DOI] [PubMed] [Google Scholar]
- 15.Andrew SE, Nguyen A, Jones GL, Brooks DE. Seasonal effects on the aerobic bacterial and fungal conjunctival flora of normal thoroughbred brood mares in Florida. Vet Ophthalmol 2003;6: 45–50. [DOI] [PubMed] [Google Scholar]
- 16.Andrew SE, Brooks DE, Smith PJ, Gelat KN, Chmielewski NT, et al. Equine ulcerative keratolmycosis: visual outcome and ocular survival in 39 cases (1987–1996). Equine Vet J 1998;30: 109–116. [DOI] [PubMed] [Google Scholar]
- 17.Pearce JW, Giuliano EA, Moore CP. In vitro susceptibility patterns of Aspergillus and Fusarium species isolated from equine ulcerative keratomycosis cases in the midwestern and southern United States with inclusion of the new antifungal agent voriconazole. Vet Ophthalmol 2009;12: 318–324. 10.1111/j.1463-5224.2009.00721.x [DOI] [PubMed] [Google Scholar]
- 18.Betbeze CM, Wu CC, Krohne SG, Stiles J. In vitro fungistatic and fungicidal activities of silver sulfadiazine and natamycin on pathogenic fungi isolated from horses with keratomysis. Am J Vet Res 2006;67: 1788–1793. 10.2460/ajvr.67.10.1788 [DOI] [PubMed] [Google Scholar]
- 19.Sherman AB, Clode AB, Gilger BC. Impact of fungal species cultured on outcome in horses with fungal keratitis. Vet Ophthalmol 2017;20: 140–146. 10.1111/vop.12381 [DOI] [PubMed] [Google Scholar]
- 20.Ledbetter EC. Antifungal therapy in equine ocular mycotic infections. Vet Clin North Am Equine Pract 2017;33: 583–605. 10.1016/j.cveq.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 21.Utter ME, Davidson EJ, Wotman KL. Clinical features and outcomes of severe ulcerative keratitis with medical and surgical management in 41 horses (2000–2006). Equine Vet Educ 2010;21: 321–327. [Google Scholar]
- 22.Denis HM. Equine corneal surgery and transplantation. Vet Clin North Am Equine Pract 2004;20: 361–380. 10.1016/j.cveq.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 23.Vilgalys R, Gonzalez D. Organization of ribosomal DNA in the basidiomycete Thanatephorus praticola. Curr Genet 1990;18: 277–280. [DOI] [PubMed] [Google Scholar]
- 24.Geiser DM, Dorner JW, Horn BW, Taylor JW. The Phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet Biol 2000;31: 169–179. 10.1006/fgbi.2000.1215 [DOI] [PubMed] [Google Scholar]
- 25.Moore GG, Olarte RA, Horn BW, Elliott JL, Singh R, O’Neal CJ, et al. Global population structure and adaptive evolution of aflatoxin-producing fungi. Ecol Evol 2017;7: 9179–9191. 10.1002/ece3.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtzman CP, Smiley MJ, Robnett CJ, Wicklow DT. DNA Relatedness among wild and eomesticated species in the Aspergillus flavus group. Mycologia 1986;78: 955. [Google Scholar]
- 27.Ramirez-Prado J. H., Moore G. G., Horn B. W. and Carbone I. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet Biol 2008;45: 1292–1299 10.1016/j.fgb.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 28.Carbone I, White JB, Miadlikowska J, Arnold AE, Miller MA, Kauff F, et al. T-BAS: Tree-Based Alignment Selector toolkit for phylogenetic-based placement, alignment downloads and metadata visualization: an example with the Pezizomycotina tree of life. Bioinformatics 2017;33: 1160–1168. 10.1093/bioinformatics/btw808 [DOI] [PubMed] [Google Scholar]
- 29.Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 2013;22: 5271–5277. 10.1111/mec.12481 [DOI] [PubMed] [Google Scholar]
- 30.James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006;443: 818–822. 10.1038/nature05110 [DOI] [PubMed] [Google Scholar]
- 31.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014;30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MA, Schwartz T, Pickett BE, He S, Klem EB, Scheuermann RH, et al. A RESTful API for Access to Phylogenetic Tools via the CIPRES Science Gateway. Evol Bioinforma 2015;11: EBO.S21501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh K, Toh H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010;26: 1899–1900. 10.1093/bioinformatics/btq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aylor DL, Price EW, Carbone I. SNAP: Combine and map modules for multilocus population genetic analysis. Bioinformatics 2006;22: 1399–1401. 10.1093/bioinformatics/btl136 [DOI] [PubMed] [Google Scholar]
- 35.Price EW, Carbone I. SNAP: workbench management tool for evolutionary population genetic analysis. Bioinformatics 2005. 21: 402–404. 10.1093/bioinformatics/bti003 [DOI] [PubMed] [Google Scholar]
- 36.Monacell JT, Carbone I. Mobyle SNAP Workbench: A web-based analysis portal for population genetics and evolutionary genomics. Bioinformatics 2014. 30: 1488–90 10.1093/bioinformatics/btu055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rex JH, Alexander BD, Andes D, Arthington-Skaggs B, Brown SD, Chaturveli V, et al. M38-A2 Reference Method for Broth Dilution Antifungal susceptibility testing of filamentous fungi. Clinical and Laboratory Standard Institute; 2008;28:29. [Google Scholar]
- 38.Kredics L, Narendran V, Shobana CS, Vágvölgyi C, Manikandan P. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses 2015;58: 243–260. 10.1111/myc.12306 [DOI] [PubMed] [Google Scholar]
- 39.Reed Z, Thomasy SM, Good KL, Maggs DJ, Magdesian KG, Pusterla N, et al. Equine keratomycoses in California from 1987 to 2010 (47 cases). Equine Vet J 2013;45: 361–366. 10.1111/j.2042-3306.2012.00623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voelter-Ratson K, Pot SA, Florin M, Spiess BM. Equine keratomycosis in Switzerland: A retrospective evaluation of 35 horses (January 2000-August 2011). Equine Vet J 2013;45: 608–612. 10.1111/evj.12042 [DOI] [PubMed] [Google Scholar]
- 41.Wada S, Hobo S, Ode H, Niwa H, Moriyama H. Equine keratomycosis in Japan. Vet Ophthalmol 2013;16: 1–9. [DOI] [PubMed] [Google Scholar]
- 42.Homa M, Shobana CS, Singh YRB, Manikandan P, Selvam KP, Kredics L, et al. Fusarium keratitis in South India: Causative agents, their antifungal susceptibilities and a rapid identification method for the fusarium solani species complex. Mycoses 2013;56: 501–511. 10.1111/myc.12062 [DOI] [PubMed] [Google Scholar]
- 43.Zhang N, Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM. Members of the Fusarium solani Species Complex That Cause Infections in Both Humans and Plants Are Common in the Environment. Journal of Clinical Microbiology 2006;44(6):2186 10.1128/JCM.00120-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa ES, Melo MP, Mota JM, Sousa EMJ, Beserra JEA, Matos KS. First Report of Fusarium falciforme (FSSC 3 + 4) Causing Root Rot in Lima Bean (Phaseolus lunatus L.) in Brazil. Plant Disease 2017; 101:11, 1954–1954 10.1094/PDIS-02-16-0144-RE [DOI] [PubMed] [Google Scholar]
- 45.Olarte RA, Horne B, JW D. Effect of sexual recombination on population diversity in aflatoxin production of Apergillus flavus and evidence for cryptic heterokaryosis. Mol Ecol 2012;21: 1453–1476. 10.1111/j.1365-294X.2011.05398.x [DOI] [PubMed] [Google Scholar]
- 46.Wicklow DT, McAlpin CE, Peterson SW. Common genotypes (RFLP) within a diverse collection of yellow-green aspergilli used to produce traditional Oriental fermented foods. Mycoscience 2002;43: 289–297. [Google Scholar]
- 47.Paoletti M, Rydholm C, Schwier E. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol 2005;15: 1242–1248. 10.1016/j.cub.2005.05.045 [DOI] [PubMed] [Google Scholar]
- 48.Ashu E, Hagen F, Chowdhary A, Meis J, Xu J. Global population genetic analysis of Aspergillus fumigatus. mSphere 2017;2: e00019–17. 10.1128/mSphere.00019-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manikandan P, Varga J, Kocsubé S, Anita R, Revathi R, Németh TM, et al. Epidemiology of Aspergillus keratitis at a tertiary care eye hospital in South India and antifungal susceptibilities of the causative agents. Mycoses 2013;56: 26–33. [DOI] [PubMed] [Google Scholar]
- 50.Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea 2012;31(6): 662–667. 10.1097/ICO.0b013e31823f8ae0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walther G, Stasch S, Kaerger K, Hamprecht A, Roth M, Cornely OA, et al. Fusarium Keratitis in Germany. J Clin Microbiol. 2017;55(10):2983–95. 10.1128/JCM.00649-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tupaki-Sreepurna A, Al-Hatmi AMS, Kindo AJ, Sundaram M, de Hoog GS. Multidrug-resistant Fusarium in keratitis: a clinico-mycological study of keratitis infections in Chennai, India. Mycoses. 2017. April;60(4):230–3. 10.1111/myc.12578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The primers used for Sanger sequencing are underlined.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.