Abstract
On November 27, 2013, President Obama signed into law the Drug Quality Security Act (DQSA). Title II of the DQSA, the Drug Supply Chain Security Act (DSCSA), replaces all existing or future state-wide drug track or trace systems with a new federal drug tracing program that uses pedigrees and product identifiers for verification of the drugs being accepted by the buyer. While the full implementation of the DSCSA is projected to take about ten years from its enactment, both the implementation framework and milestones of the new federal tracing program have been carefully laid out. In this essay, we will explore the current state of the U.S. pharmaceutical supply chain and the imperatives behind the DSCSA. At the crux of this essay is an analysis of the DSCSA implementation plan, its challenges according to feedback from stakeholders, and its potential effectiveness against the entrance of substandard and counterfeit drugs into the U.S. pharmaceutical supply chain.
Keywords: Drug Supply Chain Security Act (DSCSA)
Introduction
Since the enactment of the Prescription Drug Marketing Act (PDMA) of 1987, the federal and state governments have tried to combat counterfeit drugs from entering the United States and the state’s pharmaceutical supply chain. A noteworthy attempt to prevent counterfeit drugs from entering the state drug supply chain was the California E-Pedigree drug tracing program that was passed in 2004 and was to be implemented by the end of 20171. Since all of the states had been using paper format pedigree systems, California E-Pedigree would have been the first electronic pedigree system.
However, California’s e-pedigree provisions came to a halt2. On November 27, 2013, President Obama signed into law the Drug Quality Security Act (DQSA). Title II of the DQSA, the Drug Supply Chain Security Act (DSCSA), removes from all states all existing or future drug track or trace systems, including pedigree systems. The DSCSA replaces those systems with a new federal drug tracing program that uses pedigrees and product identifiers for verification of the drugs being accepted by the buyer. While the full implementation of the DSCSA is projected to take about ten years from its enactment3, both the implementation framework and milestones of the new federal tracing program have been carefully laid out4. In this essay, we will explore the current state of the U.S. pharmaceutical supply chain and the imperatives behind the DSCSA. At the crux of this essay is an analysis of the DSCSA implementation plan, its challenges according to feedback from stakeholders, and its potential effectiveness against the entrance of substandard and counterfeit drugs into the U.S. pharmaceutical supply chain.
I. Why Now? The Current State of the U.S. Pharmaceutical Supply Chain and the Imperatives to Track and Trace Counterfeit5 and Substandard6 Medicines
In 2007, U.S. health officials became alarmed by reports of unexpected allergic-type reactions in patients undergoing dialysis treatment.7 The reactions were linked to a widely used anticoagulant — heparin8 — and specifically to an adulterant that had been introduced during manufacture of the drug in China, which were tested and distributed in the U.S. by Baxter International Inc.9,10 By May 2008, the U.S. Food and Drug Administration (FDA) received 149 reported deaths related to heparin11. The FDA indicates the adulteration of heparin was an economically motivated act12,10— a deliberate breach of the U.S. pharmaceutical supply chain13. While outraging the public, the heparin recall was only one of 203 FDA drug recalls in 200814.
Before diving deeper into the heparin adulteration case, let’s take a look at the current U.S. pharmaceutical supply chain. In the 21st century, drug manufacturing and distribution have become increasingly complex. Prescription and over-the-counter medications come from factories all over the world, moving into the American marketplace through supply chains that can involve numerous processing plants, manufacturers, suppliers, brokers, packagers, and distributors. The number of drug products made at non-U.S. sites doubled between 2001 and 2008, according to FDA estimates.15 An estimated 40 percent of finished drugs used in the United States are made abroad, and 80 percent of the active pharmaceutical ingredients used in U.S. drugs originate from foreign countries.16 The pathway of the pharmaceutical supply chain — from raw materials, to active ingredients, to initially processed products, to finished products, to repackaged products, finally to patient delivery — is inherently complex, and every step is an opportunity for adulteration (see Figure 1, produced by the Pew Health Group17). Moreover, the potential for counterfeit or substandard medicines to enter the system and reach patients has heightened due to globalization, the complexity of pharmaceutical distribution, and the existence of criminal actors taking advantage of supply chain weaknesses.
Figure 1. The pharmaceutical supply chain with examples of vulnerabilties.
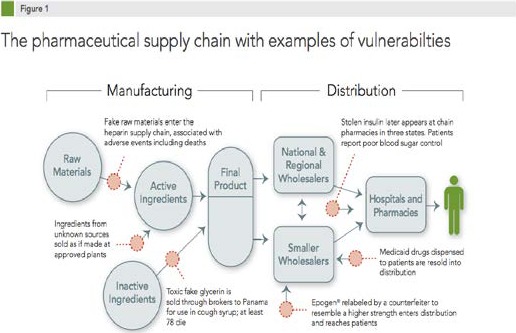
The factors that encourage the proliferation of substandard and counterfeit medicines are different but overlapping. Typically, the neglect of good manufacturing practices, whether accidental or deliberate, drives the distribution of substandard drugs, whereas falsified or counterfeited medicines are rooted from crime and corruption. Both types are able to circulate and remain in the pharmaceutical supply chain because of the unpredictable supply, constant demand for medicines, and the weaknesses in the regulatory system.
There is a significant financial incentive to produce poor-quality or fake prescriptions, whereas the penalties and odds of getting caught are low in comparison. The Federal Food, Drug, and Cosmetic Act (FDCA) penalizes adulteration, misbranding, and counterfeiting at a maximum of $10,000 or three years in prison18. On the other hand, the penalties for trafficking drugs such as heroin and cocaine can have jail sentences up to life and fines in the millions of dollars19. The penalties related to falsified medicines may be too low to deter violations and crime, particularly for pharmaceutical counterfeiting, which is additionally incentivized by high profitability. Based on an estimate by the Department of Commerce, a $1,000 investment in counterfeit prescription drugs could yield up to a $30,000 return, which is 10 times greater than that of the sale of illegal narcotics20.
In the case of the heparin adulteration mentioned previously, a shortage of raw ingredient provided a motivation for deliberate substitutions of cheaper materials. The adulterant, oversulfated chondroitin sulfate (OSCS), entered the supply chain at a time when a widespread swine virus outbreak had greatly diminished Chinese pig herds in 200721. Since crude heparin was (and still is) commonly extracted from pig intestines22, the increase in the price of pigs led to an increase in the price of crude heparin23. OSCS, a synthetic material costing nearly 100 times less to produce than actual heparin24 and mimicking some of heparin’s chemical properties25, was not detected as an adulterant by standard assays or by additional tests used by Baxter26. However, during several retroactive investigations, OSCS was identified in the finished heparin active ingredient made in China by Scientific Protein Laboratories–Changzhou (SPL-CZ) as well as in the crude material provided to SPL-CZ from consolidators.27 This evidence suggests that the OSCS was introduced upstream of SPL-CZ.
However, in the aftermath of the heparin scandal, when Baxter and the FDA sent their own inspectors to evaluate the supply chain, they were repeatedly denied access to investigate upstream workshops and consolidators who are not registered by the FDA.28 Hence, the identities of the perpetrators in the first heparin recall remained unknown, although ample evidence was available to help pinpoint them. More importantly, the case revealed the need for faster regulatory action, improved communication between different branches of FDA, more collaboration with regulatory authorities outside of the US, and greater vigilance within the industry. The FDA failed to take immediate action or issue import alerts to prevent products from these suspicious sites from entering the United States. It was not until five years after the heparin scandal, in 2013, that the FDA finally published a guidance recommending practices for monitoring the quality of crude heparin29, and the U.S. Pharmacopoeia (USP) updated the heparin quality standards meant to boost the safety and quality of the drug.30
Responses from the drug suppliers and regulators could have been more swift and deliberate had there been a track-and-trace system that registers all of the manufacturers, distributors, repackagers, and distributors as a pharmaceutical product moves through the supply chain. Furthermore, a verifiable and secure electronic track-and-trace system that includes every member of the supply chain would enhance the level of responsibility, accountability, and transparency between all supply chain participants and improve the security of the drug supply. This is the system that the Drug Supply Chain Security Act (DSCSA) aims to build31.
II. DSCSA: Implementation Framework and Progress
Enacted in 2013, the DSCSA delineates critical steps to build an electronic, interoperable system to identify and trace most human prescription drugs32 throughout their distribution process in the U.S. by 2023. Drug manufacturers, wholesale drug distributors, repackagers, and dispensers (primarily pharmacies) will be working in cooperation with the FDA to develop the new system. This law requires the FDA to establish standards, issue guidance documents, and develop pilot programs to support effective implementation and compliance.
There are multiple phases to the law, each adding a layer of security around serialization and traceability. The first phase, which started on January 1, 2015 and ends on November 26, 2023, requires supply chain participants share chain-of-ownership data. The second milestone of DSCSA, starting November 2017, requires pharmaceutical manufacturers’ products be marked with a product identifier (GS1 Global Trade Item Number® (GTIN®) or National Drug Code (NDC)), serial number, lot number, and expiration date in both machine-readable and human-readable format. The final phase of DSCSA requires trading partners share chain-of-ownership data in a manner that allows for serialized item traceability back to the product origin (usually the manufacturer). Critical DSCSA implementation dates include:
Lot-level traceability is already in force and has been since 201533;
By November 2017, manufacturers must serialize all pharmaceutical products34;
By November 2023, every stakeholder in the distribution of prescription drugs in America, from manufacturers to dispensers, must have bought into and implemented a system giving electronic traceability of each drug package.35
Although there were a few delays within the 2015 deadlines, supporters of the DSCSA have reasons to believe that its implementation is happening, and that the FDA has been doing a good job of meeting the deadlines and engaging stakeholders in public meetings, workshops, webinars, and online feedbacks. More recently, in October and December of 2016, the FDA issued an updated guidance36 on identifying and notifying suspect drug products in the drug supply chain. In addition, in January 2017, a draft guidance37 on annual reporting to the FDA by prescription drug wholesale distributors and third-party logistics providers as required under the DSCSA. As of April 2017, the FDA has been gathering additional public comments and engaging stakeholders to prepare for upcoming pilot projects38. Hence, there is still a long road ahead for implementation of the DSCSA.
III. Technical challenges for DSCSA Implementation
Catalyzed by the DSCSA, the industry-wide serialization, aggregation, and verification directive is expected to improve traceability of drugs, prevent counterfeit products from entering the supply chain, and ultimately improve patient safety. However, the process of preparing for the full compliance with the law could be time consuming and complex. Many stakeholders have expressed further needs to clarify requirements for each type of member of the supply chain, define the scope for each step of implementation, and to define the scope of responsibilities for each party.
Many stakeholders, ranging from Janssen Pharmaceuticals, Inc. to the American Pharmacists Association (APhA), have urged the FDA to evaluate the costs of implementing a standard serialized data exchange system while considering each player’s different capacity to achieve interoperability and effectively communicate with other stakeholders throughout the supply chain. While larger companies may have the resources to deal with the financial demands of serialization, smaller and medium-sized companies and healthcare institutions may find implementation more challenging or cost-prohibitive. In particular, APhA expresses concerns that “if systems are implemented that are not interoperable with existing pharmacy systems, then trading partners will stop or limit transactions with pharmacies that cannot readily adopt serialized data exchange systems, increasing costs, limiting patient access and potentially driving dispensers out of business. Rural pharmacies that lack or have slow internet connections are especially vulnerable to such exclusion from trading partners”39. On the other hand, companies that do manage to introduce new serialization technology in time for the deadlines may also risk disruption to their product supply if they experience technical issues and downtime as a result of the new process. According to Janssen, “the cost and time to implement serialization and aggregation on an existing packaging line varies widely and is dependent upon several variables, including, age of the line and current technology, number of SKUs packaged on the line, and number of markets served by that line. Time to retrofit a line with serialization requires approximately 50% less time and costs approximately 70% less money than to retrofit a line with both serialization and aggregation… Data gathered to date have revealed some loss of productivity; however, we have been able to recover some of the loss.”40.
Several stakeholders have pointed out vague terms and scopes of responsibilities as described in the law as well as guidance documents published by the FDA. For example, the process of identifying and reporting an illegitimate drug product is confusing for many members of the supply chain. On the dispensing side, APhA’s constituents, most of whom are pharmacists, want more education on the identification and determination of illegitimate products in order to best protect the integrity of the supply chain.41 Hence, APhA encourages the FDA to educate pharmacists on the identification and reporting of illegitimate products. Furthermore, the guidance does not specify what each member of the supply chain is legally required to do when they suspect a drug product is illegitimate. On the distribution side, both the Healthcare Distribution Alliance (HDA)42 and the Pharmaceutical Distribution Security Alliance (PDSA)43 indicate that the FDA’s guidance should be revised to explicitly instruct trading partners to contact the relevant manufacturer to determine whether a product is illegitimate. The FDA should also clarify that the party who makes the illegitimate product determination – which will usually be the manufacturer – is also the party who is responsible for notifying the Agency of the illegitimate product. Without such clarification, multiple members would submit their own notifications for the same illegitimate product, which would result in confusion for the FDA, overload the response system, and delay targeted investigations.
External factors may also hamper effective implementation of DSCSA in the U.S. if neighboring countries, such as Canada and Mexico, do not move in parallel with the U.S. regarding supply chain regulation. The lack of a unified global standard may result in a challenging transition. Studies have shown that most pharmaceutical counterfeits arrive in the U.S. through borders. For example, in response to high prescription drug prices in the U.S., several services have attempted to make prescription drugs available directly from Canadian pharmacies, which often can sell the drugs at a significantly cheaper price. Government controls make prices 20-80% lower than in the United States44. Normally, a patient must have his physician become directly involved in this process in order to import the drugs from Canada. Although it is understandable for a physician to want to help a patient in this manner, there are several potential risks for a physician in the U.S. participating in these programs. In addition, there is a significant risk that the patient may not be getting the drugs prescribed. An estimated one to two million Americans buy prescription drugs from abroad. Although drugs account for only 10% of healthcare costs, the amount has doubled since 1980 and drug prices have tripled45.
With the increased commerce on the Internet comes increased risk for users. While searching for health information online, consumers are offered advice about prescription medications, exposed to drug advertisements, and given links to websites that sell medications. Access, convenience, and privacy are potential benefits of internet pharmacies for the consumer. Internet pharmacies increase access to drugs for those that are disabled or otherwise homebound. Some proponents of internet pharmacies claim that paper prescriptions are often poorly written with illegible handwriting, contain wrong dosages, and may be inappropriate medications. Proponents further claim that e-prescribing can often avoid these errors and save millions of dollars in health care costs. There are also many concerns and risks associated with internet pharmacies, most importantly, those related to using the internet as a means of bypassing the usual regulatory systems. In fact, Bessell and colleagues found that even with tighter standards in many countries, consumers are still at risk for problems when buying nonprescription drugs from internet pharmacies since balanced information about the medications may not be presented.46
IV. The Next Step: Learning from DSCSA-Compliance Pilots
Since the FDA has yet to determined a unifying set of standards for the track-and-trace data exchange format across the supply chain, stakeholders have urged the FDA to encourage the industry stakeholders to conduct pilots, and then select the track-and-trace data exchange models and formats that work across the supply chain. At the early stage, many stakeholders suggest that the new DSCSA-compliant standards would most likely be conforming to GS1 industry standards.
Many groups have already proactively conducted their own pilots that help to inform and prepare themselves for DSCSA requirements. For instance, the National Coalition of Pharmaceutical Distributors (NCPD), which represents specialty and independent pharmaceutical distributors, reports that its member companies “are increasingly adopting technology-intensive methods for processing and forwarding T3 information to and from their supply chain partners”.47 In addition, having collaborated with the GS1 organization on a pilot project, Janssen suggests that the FDA should “continue to encourage and sponsor pilots to test the verification of the product identifier at various points in the supply chain, not just to authenticate returns.”48 According to Janssen, the FDA should learn from experiences from other countries that have implemented track-and-trace system, specifically, Turkey and Argentina.
Currently, the DSCSA-compliance pilot that multiple stakeholders have mentioned repeatedly is the Serialized Returns Pilot (also referred to as “2019 Pilot”) conducted by the Healthcare Distribution Management Association (HDMA). The HDMA 2019 Serialized Returns Pilot aims to investigate different methods for verifying product identifiers, and to identify the most efficient methods for managing the verification process for returns to meet the DSCSA requirements effective on November 27, 2019. The McKesson Corporation, the largest distributor of pharmaceuticals in the U.S., is optimistic that “the 2019 Pilot will provide significant learnings that are broad enough to inform the 2023 Pilot development and DSCSA compliance requirements.”49
In its public comment toward the FDA, however, the HDMA recommends the FDA to further clarify and come to consensus with stakeholders on DSCSA terminologies and responsibilities for all trading partners in order to build pilots that are based upon a common understanding of the statute’s requirements.50 HDMA also advocates for tailoring any FDA-sponsored pilots narrowly (without venturing into testing what the DSCSA does not require) and not seek to impose what the DSCSA does not require.51 The comment from HDMA suggests that the implementation of the DSCSA is still at a vague stage and is desperately in need of further clarification as pilots program are being conducted.
V. How Effective will the DSCSA be in the Fight against Falsified Prescription Drugs?
This discussion will focus on the whether the DSCSA basic design can prevent counterfeit drugs from entering the U.S. pharmaceutical supply chain (USPSC). Using cases of counterfeit drugs entering the USPSC in the past, we examine how the DSCSA will affect those examples of counterfeit drugs entering the USPSC.
The scope of the DSCSA only covers the pharmaceutical supply chain within the U.S. and does not cover the counterfeit active pharmaceutical ingredients (APIs) coming from overseas. However, current FDA rules require that only FDA-approved businesses can make API for U.S. manufacturers52. As was determined in the heparin case in section I, it was at the tertiary level of suppliers where the counterfeit ingredients were introduced into the heparin API. Hence, the heparin contamination crisis, delayed regulatory response, and subsequent dead-end investigations would have been preempted had there been a combination of the existing FDA requirements and a track-and-trace system under the DSCSA.
Here, we consider trading partners as the basic components of the DSCSA supply chain model. The DSCSA defines who trading partners are: manufacturers, wholesale distributors, third party logistics providers, repackagers, and dispensers. All of these trading partners have to be authorized according to the DSCSA53. To be authorized, they must be licensed by the state or by the FDA. In addition, the owner of a distributor must be without a felony conviction related to the USPSC54. The rules of dealing with only authorized trading partners would prevent counterfeit drugs from entering the USPSC. The requirement of only dealing with authorized trading partners and owners without certain convictions makes the USPSC a closed system. However, as we will discuss in the next sub-sections, it does not completely stop a dishonest trading partner from getting an API or drug from an unauthorized third party. We will focus our discussion on three of the major trading partners in the DSCSA supply chain in sequence: manufacturers, wholesale distributors, and dispensers.
a. Manufacturers
In the 1990s, Biochimica Opos SpA, an Italian pharmaceutical manufacturer owned by the French drug company Roussel-Uclaf S.A., falsified records to conceal its use of unauthorized manufacturing plants in Italy, France, and Romania to produce the antibiotic cefaclor. Cefaclor was eventually recalled and Biochimica Opos withdrew its approved marketing applications55. In 2001, the mother company, Roussel-Uclaf, pleaded guilty to felony charges of conspiracy and defrauding the FDA. Roussel-Uclaf was ordered to pay $33 million, $10 million in proceeds and $23 million as a criminal fine, to the U.S. government. Roussel-Uclaf also pleaded guilty on a two-count indictment charge for selling an adulterated drug in the U.S. market in 1995 and 1996 via Biochimica Opos56. The case represents the first time a foreign corporation making a drug product entirely outside the United States received a criminal punishment for defrauding the FDA57.
Ranbaxy Pharmaceuticals Inc. was FDA approved to manufacture and import drugs into the United States. However, the Ranbaxy’s manufacturing plants in India had been selling Guaifenesin LA Tablets 600 mg extended release product in the U.S. without FDA approval in 200258. In 2008, the Department of Justice subpoena motion claimed that Ranbaxy had used API from unapproved FDA sites. On February 25, 2009, the FDA prohibited Ranbaxy’s APIs and finished products from three manufacturing plants from entering the U.S. market. Ranbaxy failed to produce drugs within Good Manufacturing Standards and was found guilty of selling adulterated drugs in the U.S. in 2013, given a fine of $150 million, and had to pay $350 million in claims for a total of $500 million59. On September 16, 2013, an FDA import alert was given for Ranbaxy’s Mohali manufacturing plant which prohibits its drug products from entering the U.S. market60. On January 23, 2014, the FDA prohibited yet another Ranbaxy’s plant in Toansa from manufacturing and distributing APIs for the U.S. market61.
Although the above examples are worst case scenarios, every year the FDA issues warning letters for not following good manufacturing practices and adulteration of API as well as finished pharmaceutical goods in the U.S. and abroad.62 If the manufacturers of API and finished pharmaceutical products only deal with authorized trading partners, many of these severe problems of adulterated drugs will not occur. However, in the case of Ranbaxy, it is the culture of the company that has to change.
b. Wholesale Distributors
Corrupt wholesale distributors have made millions of dollars from buying off the gray market and from secondary suppliers that bought their drugs from non-FDA authorized companies. In 2000 and 2001, Dutchess Business Services, a drug wholesaler licensed in Nevada, and Legend Pharmaceuticals Inc., bought counterfeit Serostim (a drug used to treat excessive weight loss in AIDS patients) and later sold it to McKesson Corp., which then sold it to retail pharmacies63. In addition, Dutchess and Legend were found guilty of doing business with Florida and South Carolina drug wholesale distributor companies that were not authorized to possess the drugs involved in the transactions64. The person that sold the counterfeit drug, Serostim, to the Florida drug wholesale company, Crystal Coast, who sold it to Dutchess, had been operating a drug wholesale business without license in the state of Florida. Although Florida and Nevada have pedigree laws on the buying and selling of drugs in their states, Dutchess and Legend failed to get and properly maintain appropriate pedigree records from their sellers and provide adequate and correct pedigree information to their buyers. The Nevada Board of Pharmacy fined Dutchess $1,000 for each of 399 counts and $250 for each of the remaining 483 counts for a total fine of $519,750 65. Legend was assessed a fine of $250 for each of 125 counts for a total of $31,250 66. Unsurprisingly, Dutchess and Legend lost their wholesale distributor licenses in Nevada.
One of states that seemed to have a problem with wholesale distributors selling diverted and/or counterfeit drugs was Florida. Florida had a pedigree system that was required for secondary wholesalers but it was not enforced 67. On July 21, 2003, nineteen people were indicted for selling counterfeit drugs 68. Eighteen were indicted for a variety of charges from racketeering, conspiracy, and other offenses in regards to prescription drug fraud. A grand jury was convened in 2003 and concluded that the Florida wholesale pharmaceutical industry had become corrupt due to criminal elements 69. Some Florida drug wholesalers who had been given licenses to operate in Florida had one or more felony convictions 70.
Alarmingly, many of those Florida drug wholesalers did not have the proper training or experience to handle, store, or deal in pharmaceuticals71. Moreover, according to the grand jury report, corrupt secondary wholesalers in Florida had done business with millions of dollars of prescription drugs that were later to be found as counterfeits72. Counterfeiters used relabeling, dilution, substitution, and overstating the potency techniques when counterfeiting the drugs.
In 2012, there were two cases that resulted in over a billion dollars in fraud due to drug diversion that covered multiple states where 71 people and three corporations were charged73. Drugs that were dispensed to Medicaid patients were diverted and resold back to corrupt wholesalers, who then sold the drugs to pharmacies. The drug diversion occurred from 2007 until 2011, and although fraudulent drug pedigrees were supplied, it was impossible for the chain and independent pharmacies to trace a pedigree to determine a place of origin 74.
In the cases discussed above, the indicted wholesale distributors were willing to cut corners to gain profits, resulting in injuries or deaths for many patients. In most cases, dealing only with authorized trade partners will prevent the introduction of counterfeit drugs into the USPSC. In the DSCSA, verification is not only with the seller but with the manufacturer or repackager. In a Nevada case of counterfeit drugs, it was not until the manufacturer was contacted before it was determined by the lot number that the drugs were counterfeit75. Furthermore, by contacting the manufacturer, a determination on whether the seller is authorized to sell the drug becomes known. The database of authorized wholesale distributors, which is maintained by the FDA, will help trading partners to determine authorized wholesale distributors. In many of these cases given, the main theme was that none of the incidences were found through inspections but after the fraud was committed and discovered.
c. Dispensers: Pharmacies and Pharmacists
From time to time, counterfeit, diverted, and diluted drugs can and do enter the USPSC via corrupt pharmacies or pharmacists. There is a case of a licensed pharmacist buying counterfeit drugs, Cialis and Viagra, from China for his pharmacy in San Jacinto, Texas76. The special agents of Immigration and Customs Enforcement (ICE) and the FDA Office of Criminal Investigations (OCI) were involved with this purchase by posing as delivery men. The licensed pharmacist was sentenced to two years in federal prison without a chance of parole.
In 2014, a registered pharmacist, who was also an owner of a pharmacy in Des Moines, Iowa, was sentenced to two years in federal prison for diverting hundreds of thousands of narcotic pain pills from the drugstore. The pharmacist had purchased many more hydrocodone pills than the store needed to fill legitimate patient prescriptions and sold the excess pills to drug abusers. To cover up what he was doing, the pharmacist ordered the painkillers from as many as fourteen wholesalers at once77. The Iowa Board of Pharmacy indicated that about 700,000 hydrocodone pills disappeared from the small pharmacy over many years, likely starting from more than a decade earlier, in 200278. The scheme unraveled in 2011, after an admitted drug addict called the Iowa Board of Pharmacy and reporting the drug-diversion scheme79.
In Kansas City, MO for ten years, from 1992 to 2002, a pharmacist and owner of two pharmacies, Robert Courtney, diluted over 98,000 prescriptions written by 400 physicians80. Some of the drugs that were diluted were anticancer drugs Taxol and Gemzar. The rest is unknown as Robert Courtney’s spurious conducts were completed intermittently with no records kept. Lawsuits were filed against the pharmaceutical companies of Eli Lilly and Myers Squibb which settled out of court for $72 million81,82.
The DSCA clearly states that (1) business must be completed between authorized trading partners, (2) transaction records must be exchange between buyer and seller that goes back in history to the manufacturer, and (3) product identifiers are to be verified with the manufacturer or repackager. If the pharmacy or pharmacist follow those rules, the drugs they sell will be authorized and verified successfully throughout the supply chain. However, the DSCA does not cover personal criminal acts, which fall under federal and state laws.
The Effects of Adulterated Drugs on U.S. Economy, Healthcare, and the Population
When drugs imported to the United States are not well regulated and monitored for efficacy and safety, the economy, healthcare system, and the people bear the consequences. Heparin contaminated from China led to a surge of death in 2007 and 2008 due to allergic and hypersensitivity reactions83. The heparin was marketed by Baxter International Inc. who purchased the pharmaceutical ingredients (API) from Changzhou China Scientific Protein Laboratories (SPL-CZ). The FDA approved Baxter to purchase from Changzhou without inspecting the plant. Adulterated drugs posed dangers to the health of the patients and wasted a lot of money, time, and effort to track down the source of the adulteration. Globally, 500 children died from counterfeit cough syrup that was tainted with ethylene glycol84. In addition to this, counterfeit inhalers for the treatment of pediatric cystic fibrosis was found to contain contaminated bacteria that went into the lungs of the children that were treated with the counterfeit medication. The recent increase in opioid abuse also has contributing factors steaming from counterfeit fentanyl. In 2013 and 2014, more than 700 deaths were attributed to fentanyl and fentanyl analogues in the United States. The most recent concern is the cluster of unintentional fentanyl overdoses because of tablets thought to be “Norco” purchased on the street in Northern California 86. Healthcare providers are encouraged to report all cases they feel are the result of counterfeit medications.
On the economy level, the innovation that drives economic growth, research, and U.S. competitiveness in the global marketplace were negatively affected by the growth in adulterated drugs in U.S. Piracy and counterfeit drugs cost U.S. businesses more than $200 billion annually and account for the loss of more than 750,000 jobs85. In addition, these drugs take away enormous amount of money from people’s income and drug companies 84, as well as affecting the environment. The seizure and destruction of seized counterfeit drugs can be a costly process, creating a considerable waste. Counterfeits and piracy drugs could pose a considerable loss to the sale of the original products, pulling its price down and could potentially lead to a loss of market for the genuine drugs. As stated above, the prevalence of adulterated drugs creates a disincentive to innovation. The money to be used for R&D by some of the pharmaceutical companies has been diverted to deter counterfeit drugs.
Conclusion
Although many of the details of the DSCSA are still being developed, the DSCSA basic design and requirements represent a step in the right direction to reduce the probability of counterfeit drugs from entering the U.S. pharmaceutical supply chain. The requirement of obtaining drugs only from FDA authorized trading partners will help to filter out counterfeit drugs from the gray market. Verification of drug products at each node of the supply chain can pinpoint potential counterfeit drugs at their point of entry. Furthermore, the requirement of transaction records as drug products move through the supply chain should make it more difficult for counterfeit drugs to infiltrate the supply chain. While the DSCSA does not completely prevent criminal actions or collusions by deceitful manufacturers, wholesale distributors, pharmacies, and pharmacists, the new regulation does make it harder for their efforts to succeed. Ultimately, the FDA and all participants of the supply chain must stay constantly vigilant to secure the U.S. pharmaceutical supply chain system.
Author Contributions/Acknowledgement
All six authors contributed extensively to the work presented in this paper. L.G., J.O., P.S.L, J.M., W.O. and F.A. all contributed to the planning and gathering research materials for this paper. P.S.L. wrote the initial draft of this paper. J.M. added more materials and expanded to the section about technical challenges for DSCSA implementation. W.O. stated broadly the problems with adulterated drugs on the economy and individuals in the section “The Effects of Adulterated Drugs on U.S. Economy, Healthcare, and the Population.” F.A. helped with the brainstorming session, edited and checked formatting, and added any missing important information. L.G. added information to part V and proofread the paper. J.O. also helped with brainstorming, proofreading the paper, and improving the flow of the paper.
References
- 1.National Association of Boards of Pharmacy . Wholesale Drug Distribution: Protecting the Integrity of the Nation’s Prescription Drug Supply. Mount Prospect, IL: Aug, 2013. [accessed Oct 14;2017 ]. https://nabp.pharmacy/wp-content/uploads/2016/07/Wholesale-Drug-Distribution-08-2013.pdf [Google Scholar]
- 2.California State Board of Pharmacy . “California’s E-Pedigree Law Preempted”. Sacramento, CA: [accessed Oct 14;2017 ]. http://www.pharmacy.ca.gov/laws_regs/e pedigree law preempted.shtml [Google Scholar]
- 3.U.S. Food and Drug Administration, Drug Supply Chain Security Act (DSCSA) Silver Spring; MD: May 1, 2017. [accessed Oct 14;2017 ]. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ updated. [Google Scholar]
- 4.U.S. Food and Drug Administration, Drug Supply Chain Security Act (DSCSA) Silver Spring; MD: Mar 1, 2017. [accessed Oct 14;2017 ]. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ucm382022.htm updated. [Google Scholar]
- 5.U.S. Food and Drug Administration “Counterfeit medicine is fake medicine. It may be contaminated or contain the wrong or no active ingredient. They could have the right active ingredient but at the wrong dose. Counterfeit drugs are illegal and may be harmful to your health.”. [accessed Oct 14;2017 ]. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/
- 6.World Health Organization “Substandard medicines (also called out of specification (OOS) products) are genuine medicines produced by manufacturers authorized by the National Medicines Regulatory Authority (NMRA) which do not meet quality specifications set for them by National standards.”. [accessed May 14;2017 ]. http://www.who.int/medicines/regulation/ssffc/definitions/en/
- 7.U.S. Centers for Disease Control and Prevention Acute Allergic-Type Reactions among Patients Undergoing Hemodialysis—Multiple States, 2007–2008. Morbidity and Mortality Weekly Report. Feb 1, 2008. [accessed Oct 14;2017 ]. pp. 1–2.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm57e201a1.htm 57. (Early Release) [PubMed]
- 8.Blossom David B, Alexander J., Kallen, et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. [accessed May 14;2017 ];N Engl J Med. 2008 Dec 18;359:2674–84. doi: 10.1056/NEJMoa0806450. http://www.nejm.org/doi/fuN/10.1056/NEJMoa0806450#t=article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson Robert L. Chief Executive Officer, Baxter International. Testimony before the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, U.S. House of Representatives. Apr 29, 2008. [accessed Oct 19;2017 ]. https://www.baxter.com/assets/downloads/RLP_testimony.pdf
- 10.Kishimoto Takashi Kei, Viswanathan Karthik, et al. Contaminated Heparin Associated with Adverse Clinical Events and Activation of the Contact System. [accessed Oct 19;2017 ];N Engl J Med. 2008 Jun 5;358:2457–67. doi: 10.1056/NEJMoa0803200. http://www.nejm.org/doi/full/10.1056/NEJMoa0803200#t=article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration Information on Adverse Event Reports and Heparin. Jun 18, 2009. [accessed May 14;2017 ]. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInfor mationforPatientsandProviders/UCM112669 updated.
- 12.FDA’s working definition of “economically motivated adulteration” is the fraudulent, intentional substitution or addition of a substance in a product for the purpose of increasing the apparent value of the product or reducing the cost of its production, i.e., for economic gain. 74 Fed. Reg. 2009 Apr 6;15497 [Google Scholar]
- 13.U.S. Government Accountability Office (October 2010). U.S. Food and Drug Administration: Response to Heparin Contamination Helped Protect Public Health [accessed Oct 19;2017 ]. http://www.gao.gov/assets/320/311879.pdf Controls That Were Needed for Working with External Entities Were Recently Added (Publication No. GAO-11-95)
- 14.Gaffney Alexander. Number of Drug Recalls Surges at FDA, Led by Mid-Level Concerns. Regulatory Affairs Professionals Society. Aug 11, 2014. [accessed Oct 19;2017 ]. http://www.raps.org/Regulatory-Focus/News/2014/08/11/20005/Number-of-Drug-Recalls-Surges-at-FDA-Led-by-Mid-Level-Concerns/
- 15.Autor Deborah M, Director, CDER Office of Compliance. U.S. Food and Drug Administration “Globalization: Challenges and Recent Case Studies”. Presentation at DCAT Week. Mar 18, 2009.
- 16.Hamburg Margaret, Commissioner, U.S. Food and Drug Administration Testimony before the House Committee on Energy and Commerce, Subcommittee on Oversight and Investigations. Apr 13, 2011. [accessed Oct 20;2017 ]. https://www.fda.gov/NewsEvents/Testimony/ucm250710.htm
- 17.Pew Health Group After Heparin: Protecting Consumers from the Risks of Substandard and Counterfeit Drugs. Jul 12, 2011. [accessed Oct 18;2017 ]. http://www.pewtrusts.org/en/research-and-analysis/reports/2011/07/12/after-heparin-protecting-consumers-from-the-risks-of-substandard-and-counterfeit-drugs Figure 1.
- 18. [accessed Oct 20;2017 ]. https://www.law.cornell.edu/uscode/text/21/333 21 USC §333 (a)(2)
- 19.Yeh Brian T., Congressional Research Service “Drug Offenses: Maximum Fines and Terms of Imprisonment for Violation of the Federal Controlled Substances Act and Related Laws.”. Jan 20, 2015. [accessed Oct 20;2017 ]. https://fas.org/sgp/crs/misc/RL30722.pdf
- 20.Ehrenberg R. “Counterfeit crackdown: New scientific tools help tell fake meds from the real thing”. [accessed Oct 20;2017 ];Science News. 2011 179:22–25. http://www.jstor.org/stable/41332416 [Google Scholar]
- 21.Usdin Steve. The Heparin Story. International Journal of Risk & Safety in Medicine. 2009;Vol. 21:99–103. [Google Scholar]
- 22.Tremblay Jean-François. “Making heparin safe”. [accessed Oct 18;2017 ];Chemical and Engineering News. 2016 Oct 10;94(40):30–34. http://cen.acs.org/articles/94/i40/Making-heparin-safe.html [Google Scholar]
- 23.Gardiner Erin, Director, Corporate Communications, Baxter International Inc . After Heparin: Protecting Consumers from the Risks of Substandard and Counterfeit Drugs. Pew Health Group; Feb 19 12, 2010. Jul 19 12, 2011. [accessed Oct 18;2017 ]. Direct communication.http://www.pewtrusts.org/en/research-and-analysis/reports/2011/07/12/after-heparin-protecting-consumers-from-the-risks-of-substandard-and-counterfeit-drugs [Google Scholar]
- 24.Usdin Steve. The Heparin Story. International Journal of Risk & Safety in Medicine. 2009;Vol. 21:99–103. [Google Scholar]
- 25.Tremblay Jean-François. “Making heparin safe”. [accessed Oct 18;2017 ];Chemical and Engineering News. 2016 Oct 10;94(40):30–34. http://cen.acs.org/articles/94/i40/Making-heparin-safe.html [Google Scholar]
- 26.Parkinson Robert L, Chief Executive Officer, Baxter International , editor. Testimony before the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, U.S. House of Representatives. Apr 29, 2008.
- 27.Parkinson Robert L, Chief Executive Officer, Baxter International , editor. Testimony before the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, U.S. House of Representatives. Apr 29, 2008.
- 28.U.S. Government Accountability Office (October 2010). U.S. Food and Drug Administration: Response to Heparin Contamination Helped Protect Public Health [accessed Oct 18;2017 ]. http://www.gao.gov/assets/320/311879.pdf Controls That Were Needed for Working with External Entities Were Recently Added (Publication No. GAO-11-95)
- 29.U.S. Department of Health and Human Services, Food and Drug Administration. et al. Heparin for Drug and Medical Device Use: Monitoring Crude Heparin for Quality. Jun, 2013. [accessed May 18;2017 ]. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegula toryInformation/Guidances/UCM291390.pdf
- 30.U.S. Pharmacopoeia Jul 22, 2013. [accessed May 18;2017 ]. http://www.usp.org/usp-nf/key-issues/heparin Key Issue: Heparin updated.
- 31.Drug Supply Chain Security Act (Title II, Drug Quality and Security Act, 2013) [accessed May 18;2017 ]. https://www.gpo.gov/fdsys/pkg/PLAW-113publ54/pdf/PLAW-113publ54.pdf#page=13
- 32.Exemptions to the DSCSA are listed and discussed in Rodgers, Dirk. “Is Your Drug Exempt From The Federal Drug Supply Chain Security Act?”. Apr 7, 2014. [accessed May 18;2017 ]. https://www.rxtrace.com/2014/04/is-your-drug-exempt-from-the-federal-drug-supply-chain-security-act.html/
- 33.U.S. Food and Drug Administration “Are you ready for the Drug Supply Chain Security Act?”. Mar 03, 2017. [accessed May 18;2017 ]. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ucm427033.htm updated.
- 34.“(C) ELECTRONIC FORMAT. —“(i) IN GENERAL.—Beginning not later than 4 years after the date of enactment of the Drug Supply Chain Security Act , except as provided under clause (ii), a manufacturer shall provide the transaction information, transaction history, and transaction statement required under subparagraph (A)(i) in electronic format”. Drug Supply Chain Security Act (Title II, Drug Quality and Security Act, 2013) [accessed May 20;2017 ]. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ucm376829.htm
- 35.Drug Supply Chain Security Act (Title II, Drug Quality and Security Act, 2013) [accessed May 20;2017 ]. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ucm376829.htm
- 36.U.S. Department of Health and Human Services, Food and Drug Administration. et al. “Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification Guidance for Industry”. Dec 31, 2016. Dec 31, 2018. [accessed May 20;2017 ]. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM400470.pdf Expired.
- 37.U.S. Department of Health and Human Services, Food and Drug Administration. et al. “Annual Reporting by Prescription Drug Wholesale Distributors and Third-Party Logistics Providers: Questions and Answers Guidance for Industry”. Jan, 2017. [accessed May 20;2017 ]. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM535992.pdf
- 38.U.S. Food and Drug Administration “Pharmaceutical Distribution Supply Chain Pilot Projects; Reopening of Comment Period; Request for Information”. Apr 28, 2017. [accessed May 20;2017 ]. https://www.federalregister.gov/documents/2017/04/28/2017-08583/pharmaceutical-distribution-supply-chain-pilot-projects-reopening-of-comment-period-request-for
- 39.American Pharmacists Association (APhA) “Comment RE: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information Docket No. FDA-2016-N-1114”. May 16, 2016. [accessed May 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0008
- 40.Janssen Pharmaceuticals, Inc. “Comment on the Food and Drug Administration (FDA) Notice: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information.”. May 16, 2016. [accessed May 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0006
- 41.American Pharmacists Association (APhA) “Comment RE: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information Docket No. FDA-2016-N-1114”. May 16, 2016. [accessed Oct 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0008
- 42.Healthcare Distribution Alliance (HDA) “Comment on the Food and Drug Administration (FDA) Notice: Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification; Guidance for Industry; Availability.”. Feb 7, 2017. [accessed Oct 19;2017 ]. https://www.regulations.gov/document?D=FDA-2014-D-0609-0024
- 43.Comment from the Pharmaceutical Distribution Security Alliance (PDSA) [accessed Oct 19;2017 ]. https://www.regulations.gov/document?D=FDA-2014-D-0609-0025
- 44.Hopkins Tanne J. Local governments in US buy cheaper prescription drugs from Canada. BMJ: British Medical Journal. 2003;1126;327(7424) doi: 10.1136/bmj.327.7424.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibid.
- 46.Bessell T L, Anderson J N, Silagy C A, Sansom L N, Hiller J E. Surfing, self-medicating and safety: buying non-prescription and complementary medicines via the internet. Qual Saf Health Care. 2003 Apr;12(2):88–92. doi: 10.1136/qhc.12.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Coalition of Pharmaceutical Distributors (NCPD) “Comment on the Food and Drug Administration (FDA) Notice: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information.”. May 16, 2016. [accessed Oct 17;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0009
- 48.Janssen Pharmaceuticals, Inc. “Comment on the Food and Drug Administration (FDA) Notice: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information.”. May 16, 2016. [accessed Oct 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0006
- 49.Comment from McKesson. https://www.regulations.gov/document?D=FDA-2016-N-1114-0007
- 50.Healthcare Distribution Management Association (HDMA) “Comment on the Food and Drug Administration (FDA) Notice: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information.”. May 16, 2016. [accessed Oct 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0004
- 51.Healthcare Distribution Management Association (HDMA) “Comment on the Food and Drug Administration (FDA) Notice: Pharmaceutical Distribution Supply Chain Pilot Projects; Request for Information.”. May 16, 2016. [accessed Oct 20;2017 ]. https://www.regulations.gov/document?D=FDA-2016-N-1114-0004
- 52. [accessed Oct 20;2017 ]. https://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/ucm118003.pdf 21 USC 352(o)
- 53.Drug Supply Chain Security Act (Title II, Drug Quality and Security Act, 2013) [accessed Oct 18;2017 ]. https://www.gpo.gov/fdsys/pkg/PLAW-113publ54/pdf/PLAW-113publ54.pdf#page=13
- 54.Ibid.
- 55.The Pew Charitable Trusts Case Studies: How Unsafe Drugs Can Reach Patients. Feb 26, 2014. [accessed Oct 20;2017 ]. http://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2014/02/26/case-studies-how-unsafe-drugs-can-reach-patients
- 56.Ibid.
- 57.U.S. Food and Drug Administration, Office of Criminal Investigation “Firm Ordered to Pay Criminal Fine of $23,193,600. and Forfeit $10,000,000. To U.S”. The Enforcement Story: Fiscal Year 2001. [accessed Oct 20;2017 ]. https://www.fda.gov/ICECI/EnforcementActions/EnforcementStory/EnforcementStoryArchive/ucm109446.htm#cber
- 58.FDA Warning Letter to Ranbaxy Oct 11, 2012. [accessed Oct 20;2017 ]. p. 71.https://webcache.googleusercontent.com/search?q=cache:QWCDjBQuQbIJ:https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLette rsandNoticeofViolationLetterstoPharmaceuticalCompanies/UCM165 280.pdf+&cd=6&hl=en&ct=clnk&gl=us
- 59.Department of Justice “Generic Drug Manufacturer Ranbaxy Pleads Guilty and Agrees to Pay $500 Million to Resolve False Claims Allegations, cGMP Violations and False Statements to the FDA.”. May 13, 2013. [accessed Oct 20;2017 ]. http://www.justice.gov/opa/pr/2013/May/13-civ-542.html
- 60.U.S. Food and Drug Administration “FDA prohibits manufacture of FDA-regulated drugs from Ranbaxy’s Mohali, India, plant and issues import alert”. Regulatory Action Against Ranbaxy. Sep 16, 2013. [accessed Oct 20;2017 ]. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/enforcementactivitiesbyfda/ucm118411.htm
- 61.U.S. Food and Drug Administration “FDA prohibits Ranbaxy’s Toansa, India facility from producing and distributing drugs for the U.S. market”. Regulatory Action Against Ranbaxy. Jan 23, 2014. [accessed Oct 20;2017 ]. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformati on/enforcementactivitiesbyfda/ucm118411.htm
- 62.FDA Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations. May 18, 2017. [accessed Oct 20;2017 ]. https://www.fda.gov/iceci/enforcementactions/WarningLetters/def ault.htm updated.
- 63.Swafford M. “Las Vegas wholesaler says he unwittingly sold fake AIDS drug.”. Jan 15, 2004. [accessed Oct 20;2017 ]. http://www.lasvegassun.com/news/2004/jan/15/las-vegas-wholesaler-says-he-unwittingly-sold-fake/
- 64.Hardesty J., Supreme Court of Nevada. Dutchess Business Services, Inc.; and Legend Pharmaceuticals, Inc. Appellants v. Nevada State Board of Pharmacy. Sep 11, 2008. [accessed Oct 20;2017 ]. http://caselaw.findlaw.com/nv-supreme-court/1219556.html Respondent. No. 46345. Decided. Retrieved from.
- 65.Hardesty J., Supreme Court of Nevada. Dutchess Business Services, Inc.; and Legend Pharmaceuticals, Inc. Appellants, v. Nevada State Board of Pharmacy. Sep 11, 2008. [accessed Oct 20;2017 ]. http://caselaw.findlaw.com/nv-supreme-court/1219556.html Respondent. No. 46345. Decided. Retrieved from.
- 66.Ibid.
- 67.Stovall S. R. “Drug Pedigrees Are Here, but in What Form?”. [accessed Oct 20;2017 ];Journal of Pharmacy Practice. 2006 Jun 1;19(3):161–164. http://journals.sagepub.com/doi/abs/10.1177/0897190006292947 [Google Scholar]
- 68.The New York Times. [accessed Oct 20;2017 ];“19 Indicted in Florida In Case of Phony Drugs.”. 2003 Jul 23; http://www.nytimes.com/2003/07/22/us/19-indicted-in-florida-in-case-of-phony-drus.html
- 69.Stovall S. R. 2006.
- 70.Hileman B. “Prescription Drug Corruption Rampant Among Florida Wholesalers”. [accessed Oct 20;2017 ];Chemical & Engineering News. 2003 Nov 10;81(45):42–43. http://pubs.acs.org/cen/coverstory/8145/8145drugs1.html Number. [Google Scholar]
- 71.Hileman B. “Prescription Drug Corruption Rampant Among Florida Wholesalers”. [accessed Oct 20;2017 ];Chemical & Engineering News. 2003 Nov 10;81(45):42–43. http://pubs.acs.org/cen/coverstory/8145/8145drugs1.html Number. [Google Scholar]
- 72.Stovall S. R. 2006.
- 73.National Association of Boards of Pharmacy “Wholesale Drug Distribution: Protecting the Integrity of the Nation’s Prescription Drug Supply.”. Aug, 2013. [accessed Oct 20;2017 ]. https://nabp.pharmacy/wp-content/uploads/2016/07/Wholesale-Drug-Distribution-08-2013.pdf
- 74.National Association of Boards of Pharmacy Aug, 2013. See citation 70.
- 75.Swafford M. 2004. See citation 60.
- 76.FDA “FDA Conducts Preliminary Review of Agency’s Diversion and Counterfeit Criminal Case Information.”. Sep, 2011. [accessed Oct 22;2017 ]. http://www.fda.gov/downloads/Drugs/DrugSafety/DrugIntegrityand SupplyChainSecurity/UCM272150.pdf
- 77.Lowery Mark. “Pharmacist accused of diverting 700,000 painkillers”. Drug Topics. Apr 25, 2014. [accessed Oct 22;2017 ]. http://drugtopics.modernmedicine.com/drug-topics/content/tags/bauder-pharmacy/pharmacist-accused-diverting-700000-painkillers?page=full
- 78.Leys Tony. “Ex-owner of Bauder’s Pharmacy sentenced to prison”. The Des Moines Register. Feb 20, 2015. [accessed Oct 22;2017 ]. http://www.desmoinesregister.com/story/news/crime-and-courts/2015/02/20/bauders-pharmacy-mark-graziano-sentencing/23741061/
- 79.Ibid.
- 80.Belluck P. Prosecutors say greed drove pharmacist to dilute drugs. [accessed Oct 22;2017 ];New York Times. 2011 Aug 18; http://www.nytimes.com/2001/08/18/us/prosecutors-say-greed-drove-pharmacist-to-dilute-drugs.html
- 81.Morris M. Appeals court sets back effort to reopen the Robert Courtney drug dilution case. Jul 10, 2014. [accessed Oct 22;2017 ]. http://www.kansascity.com/news/special-reports/kc-true-crime/article705846.html
- 82.Associated Press “Report: Bristol-Myers, Eli Lilly will pay $71 million in diluted drugs settlement.”. Feb 10, 2003. [accessed Oct 22;2017 ]. http://www2.ljworld.com/news/2003/feb/10/report_bristolmyerseli/
- 83.http://www.fda.gov/Cder/drug/infopage/heparin/adverseevents.htmhttps://www.fda.gov/downloads/newsevents/meetingsconferences workshops/ucm163646.pdf The figure is from. It is important to state that the exact number of deaths secondary to contamination is unknown. Adverse event reportedly would have likely increased because of public attention. However, it seems clear that there were excess fatalities.
- 84.Blackstone E. A., Fuhr J. P., Pociask S. The Health and Economic Effects of Counterfeit Drugs. American Health & Drug Benefits. 2014;7(4):216–224. [PMC free article] [PubMed] [Google Scholar]
- 85.Office of the United States Intellectual Property Enforcement Coordinator Intellectual property spotlight. Mar, Apr, 2012. [Accessed Oct 3;2014 ]. www.whitehouse.gov/sites/default/files/omb/
- 86.Fentanyl and a Novel Synthetic Opioid U-47700 Masquerading as Street “Norco” in Central California: A Case ReportArmenian. Patil, et al. Annals of Emergency Medicine. 69(1):87–90. doi: 10.1016/j.annemergmed.2016.06.014. Issue. [DOI] [PubMed] [Google Scholar]


