Abstract
Calcium carbonate rock dust (RD) is used in mining to reduce the explosivity of aerosolized coal. During the dusting procedures, potential for human exposure occurs, raising health concerns. To improve RD aerosolization, several types of anti-caking surface treatments exist. The aim of the study was to evaluate cytotoxicity of four respirable RD samples: untreated/treated limestone (UL/TL), untreated/treated marble (UM/TM), and crystalline silica (SiO2) as a positive control in A549 and THP-1 transformed human cell lines. Respirable fractions were generated and collected using FSP10 high flow-rate cyclone samplers. THP-1 cells were differentiated with phorbol-12-myristate-13-acetate (20 ng/ml, 48 h). Cells were exposed to seven different concentrations of RD and SiO2 (0–0.2 mg/ml). RD caused a slight decrease in viability at 24 or 72 h post-exposure and were able to induce inflammatory cytokine production in A549 cells, however, with considerably less potency than SiO2. In THP-1 cells at 24 h, there was significant dose-dependent lactate dehydrogenase, inflammatory cytokine and chemokine release. Caspase-1 activity was increased in SiO2- and, on a lesser scale, in TM- exposed cells. To test if the increased toxicity of TM was uptake-related, THP-1 cells were pretreated with Cytochalasin D (CytD) or Bafilomycin A (BafA), followed by exposure to RD or SiO2 for 6 h. CytD blocked the uptake and significantly decreased cytotoxicity of all particles, while BafA prevented caspase-1 activation but not cytotoxic effects of TM. Only TM was able to induce an inflammatory response in THP-1 cells, however it was much less pronounced compared to silica.
Keywords: Rock dust, Marble, Limestone, Silica, Macrophages, Stearate
1. Introduction
During the mining and transport of coal, combustible coal dust can accumulate in the air, thus posing the threat of a large-scale explosion. Despite implementing rigorous safety measures, coal dust explosions are still of great concern, resulting in 65 fatalities and 18 injuries in 2002–2012 in the U.S. alone (CDC/NIOSH, 2012a). Mitigation of explosion hazard in coal mines is achieved by the spreading of fine rock dust (RD), such as pulverized limestone or marble (both having the chemical formulae of CaCO3) to render the coal dust inert. The Office of Mine Safety and Health Research (OMSHR) has investigated the effect of rock dusting on the prevention and suppression of coal dust combustion (Harris et al., 2012; CDC/NIOSH, 2014; 2012b). OMSHR investigators found that 80% total incombustible content of rock dust was needed, which became a federal law in June 2011(“U.S. Code of Federal Regulations. Title 30—Mandatory safety standards. Part 75—underground coal mines. Subpart E. Section 75.403 Maintenance of incombustible content of rock dust.”). Rock dust must meet the following definition stated in 30 CFR 75.2: It shall be comprised of pulverized stone (limestone, dolomite etc.); when particles are wetted and dried will not cohere to form a cake which will not be dispersed into separate particles by a light blast of air; does not contain > 5% combustible matter or > 4% free and combined silica (SiO2) - if such silica concentrations are not available, it must not contain > 5% free and combined silica. Currently, in order to prevent caking in humid conditions and to increase the effectiveness of aerosolization, several types of hydrophobic surface treatments (e.g. fatty acids or silicone) for commercial rock dust have been developed. Experiments conducted with treated rock dust reveled that during the application, respirable particle concentrations in the air may exceed the permissible levels; however, there are administrative controls in place to keep miners minimally exposed to rock dusting during application (Harris, Organiscak, Klima, and Perera, 2017).
A considerable body of literature consisting of studies on coal miners and the coal mining environment supports the notion that exposure to coal mine dust can lead to various respiratory diseases causing disability and premature mortality (Wallace et al., 2011). However, there is little data available to determine whether exposure to calcium carbonate rock dust affects human health. In several epidemiological observations and case reports it was proposed that co-exposure with silica is most likely responsible for the adverse outcomes in susceptible individuals, even though first-hand silica exposures were very low (Angotzi et al., 2005; Bello et al., 2015; Crummy, Carl, Cameron, and Heaney, 2004; Doig, 1955; Yildirim, Akgedik, Akgedik, and Nazaroglu, 2016). We were also unable to find information as to how surface treatments might affect the toxicological profile of the calcium carbonate rock dusts.
In this paper we investigate the comparative cytotoxicity of respirable calcium carbonate rock dust particles with or without specific anti-caking surface treatments, using a surrogate for lung epithelial cells A549, a human lung epithelial adenocarcinoma and widely used cell model for alveolar epithelial function (Hetland et al., 2001; Ovrevik et al., 2006; Vuong et al., 2017). We also used the human promyelocytic cells (THP-1) differentiated with phorbol 12-myristate 13-acetate (PMA), to study the macrophage responses to external stimuli (Chanput, Mes, and Wichers, 2014; Riendeau and Kornfeld, 2003; Tedesco et al., 2018; Van der Meeren, Moureau, Laurent, Laroche, and Angulo, 2016; Voth, Howe, and Heinzen, 2007). The dose ranges selected in this study were relevant to RD concentrations during the dusting procedures in the mines (provided by Pittsburgh Mining Research Division). To better model the in mine scenario, we estimated the number of alveolar epithelial cells and macrophages in the human lungs in determining the dose ranges (See supplementary material).
2. Materials and methods
2.1. Particles
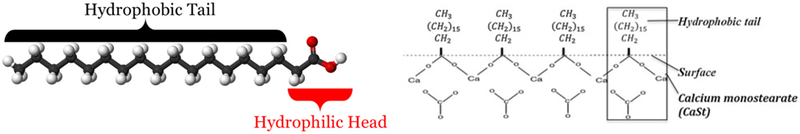
Four types of the rock dust samples were analyzed: untreated limestone (UL, Allegheny Mining Corporation), treated limestone (TL, private enterprise), untreated marble (UM, Micro-White™ 100, Imerys Carbonates), and treated marble (TM, a mix of Kotamite™ and Micro-White™ 100, Imerys Carbonates). Crystalline silica (Min-U-Sil® 5, US Silica Corp.) served as a positive control in this project. The TL silicone-based hydrophobic surface treatment is proprietary and details are unknown. TM samples consist of a blend of 87.5% untreated marble with 12.5% of marble product surface-modified by stearate-based treatment (total 0.125% of stearic acid). During the typical treatment, stearic acid is adsorbed on the surface of CaCO3 particles by covalent bond between the stearic acid “head” group and Ca2+, forming a monolayer of hydrophobic molecules (Cao et al., 2016) (Fig. 1). However, depending of the method used, stearate molecules may or may not form the monolayer or even produce multilayered surface (Shi, Rosa, and Lazzeri, 2010; Jeon et al., 2018). Some of the stearate may also be present in the free state.
Fig. 1.
Stearate-based treatment of Calcium carbonate particulates, partially reprinted from (Cao et al., 2016), with permission from Elsevier.
Respirable fractions were collected from the aerosol generation chamber with FSP10 cyclones (GSA Messgerätebau GmbH, Ratingen, Germany) loaded with polyvinyl chloride filters (PVC, 5 μm pore size, 37 mm, SKC Inc., Eighty Four, PA), washed with a mix of 50% phosphate buffered saline and 50% isopropyl alcohol, centrifuged and dried. For all cell-based experiments, samples were additionally washed three times with saline, pelleted and re-suspended in United States Pharmacopeia (USP) grade sterile Phosphate Buffer Saline (VWR International, Radnor, PA USA) as a stock solution. Dilutions were done in respective clear media with no phenol red, 1% fetal bovine serum (FBS), 1% l-glutamine (HyClone), and 1% penicillin-streptomycin antibiotic mixture (HyClone). Prior to all experiments, endotoxin levels were determined in all samples using LAL Chromogenic Endotoxin Quantitation kit (Pierce, ThermoFisher Scientific, MA USA) according to the manufacturer’s instructions. Endotoxin was assessed in three independent experiments in triplicates at 0.01, 0.05 and 0.2 mg/ml of each particle sample. All RD samples contained < 1% of free silica (Supplementary Table 1) (Soo et al., 2016).
2.2. Dynamic light scattering (DLS) analysis
The average hydrodynamic diameter (Zavg) of respirable rock dust particles was estimated using a Nanotrac 252 (Microtrac, Montgomeryville, PA) and Microtrac particle sizing software, version 10.3.11, with a backscatter angle of 90° and a laser wavelength of 657.0 nm. Stock solutions of rock dust were suspended in ultra-pure USP grade water to achieve 1 mg/ml samples of rock dust. Parameters used included the Refractive indexes (RI) of 1.54 for the samples, and 1.330 for the solvent (water) at the temperature setting of 25 °C. The mean hydrodynamic diameters reported are averages of six measurements obtained using three separate preparations of particles.
2.3. Cell cultures
A549 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin-streptomycin antibiotic mixture (growth medium), at 37 °C in a 5% CO2 humidified environment. THP-1 cells were obtained from the American Type Culture Collection (ATCC®, Manassas, VA) and grown in RPMI 1640 with 10% FBS, 2 mM l-Glutamine and 1% penicillin-streptomycin antibiotic mixture (Invitrogen, Carlsbad, CA). Following the fourth passage, monocyte-like THP-1 were differentiated into macrophages by incubation with 20 ng/ml (32.4 nM concentration) phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO) for 48 h, followed by 24 h incubation in growth medium without PMA. This protocol induces a reproducible phenotype of a macrophage, suitable for the study of inflammatory responses (Lund et al., 2016). Differentiated THP-1 cells were washed, detached using Accutase® enzymatic solution (Innovative Cell Technologies Inc., San Diego, CA) and plated into 96-well plates for further experiments.
2.4. A549 and THP-1 cells’ treatment and toxicological assessment
To assess time- and dose-dependent cytotoxicity, A549 cells (15.6 × 103 per well) were seeded into 96-well plates and treated with 4 different rock dust samples and crystalline silica as a positive control at 0.005, 0.01, 0.025, 0.05, 0.1 or 0.2 mg/ml, equivalent to 0–0.06 mg/cm2 of the cell surface. Cell supernatants were collected 24 and 72 h after incubation for lactate dehydrogenase (LDH) measurements and cells were tested for viability using the Alamar Blue bioassay (Thermo Fisher Scientific, Waltham, MA). Alamar blue reagent was added to cells in 96-well plates (1:10 dilution in clear growth medium) followed by 4h of incubation. Fluorescence was measured using a Synergy H1 hybrid multimode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at Ex530/Em590 nm. Cell viability, proportional to the changes in fluorescence, was normalized to the controls and expressed as percentage viability. Cell culture supernatants were collected for lactate dehydrogenase (LDH) measurements using Liquid LD Reagent Set (Pointe Scientific, Lincoln Park, MI) in 2 independent experiments. Cytokines and chemokines were analyzed in the cell super-natants after 72 h of exposure using a Bio-Rad 27-plex human assay kit (Bio-Rad, Hercules, CA). Two independent experiments were carried out with two replicate measurements for each supernatant.
Differentiated THP-1 were collected after the 24 h resting period, washed with clear exposure medium, seeded into 96-well plates (15.6 × 103 per well) and treated with particles similarly to A549 cells treatment. 24 h after incubation, supernatants were collected for LDH and cytokines measurements, and Alamar Blue bioassay (Thermo Fisher Scientific, MA) in the same manner as with A549 cells. Caspase-1 activity in THP-1 cells, exposed to three concentrations (0.01, 0.05, 0.1 mg/ml for RD and 0.005, 0.02, 0.05 for silica) of particles or clear exposure media, was determined using Caspase-Glo®–1 Inflammasome Assay (Promega Corporation, Madison, WI). We used smaller exposure doses for silica, since it is a known activator of caspase-1 and higher doses were too cytotoxic.
2.5. Transmission electron microscopy (TEM) of particles and cells
Transmission electron micrographs of respirable rock dust particles were obtained on a JEOL TEM 1220 (Peabody, MA) at a working voltage of 80 kV. TEM images were obtained by placing a drop of diluted sample on a formvar-coated copper grid to dry. Several TEM images were analyzed to identify at least 5–10 individual particles per image to estimate approximate dimensions of rock dust particles. For internalization studies, the cells were fixed in 0.5 ml Karnovsky’s fixative (2.5% gluteraldehyde, 2.5% paraformaldehyde in 0.1 M Sodium Cacodylic buffer) and post-fixed in 2% osmium tetroxide for 1 h. The cells were then dehydrated, embedded in epon, thin sectioned, and stained with Reynold’s lead citrate and uranyl acetate. The sections were imaged on a JEOL 1220 transmission electron microscope.
2.6. Uptake and phagolysosome acidification inhibition study
To examine uptake mechanisms involved in treated/untreated RD particles exposures, differentiated THP-1 macrophages were pre-incubated for 30 min at 37 °C in medium containing one of the following inhibitors: 2 μM cytochalasin D (reversible actin polymerization inhibitor) (Sigma-Aldrich, St. Louis, MO), 250 nM Bafylomycin A (inhibitor of vacuolar proton pump) (Sigma-Aldrich, St. Louis, MO) or medium control (0.1% dimethyl sulfoxide, Sigma-Aldrich, St. Louis, MO), used as the vehicle for the inhibitors. After treatment, cells were exposed to 4 types of RD particles (0.1 mg/ml) or silica (0.05 mg/ml) for 6 h, then cell viability, caspase-1 activity, and cytokine production were measured as indicated previously.
2.7. Statistical analysis
Treatment related differences were compared by one-way ANOVA using all pairwise multiple comparison procedures (Holm-Sidak method). All data are presented as means plus the standard error of the mean (SEM). P values of < 0.05 were considered statistically significant.
3. Results
3.1. Particle characterization
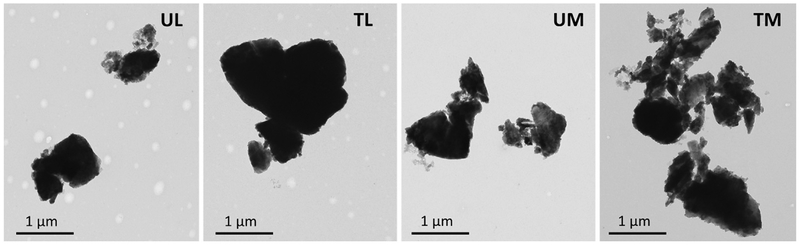
Average hydrodynamic diameters of 863 ± 31 nm, 1209 ± 64 nm, 707 ± 32 nm, 715 ± 21 nm, 1605 ± 107 were found for UM, TM, UL, TL and silica, respectively, using DLS. The Zavg values for each sample were the mean of six measurements. Transmission Electron Microscopy analysis showed that the rock dust particle sizes ranged from 200 nm to 3000 nm (Fig. 2). A detailed airborne rock dust characterization was previously described (Soo et al., 2016). Assessment of rock dust particle dimensions derived from TEM images are in good agreement with the particle sizes estimated by DLS measurements. The level of endotoxin in the highest concentration of washed respirable rock dust samples (1 mg/ml stock) was estimated as 0.078 ± 0.003 EU/ml (7.8 pg/ml). This is a no-observable effect level for endotoxin on cells in culture (Ryan, 2008). The reported endotoxin values for each sample (n = 3) represent the level of endotoxin in the particulate dosing suspensions, but not levels of endotoxin from bulk samples or aerosolized dusts.
Fig. 2.
Representative TEM images of respirable rock dust samples. UL - untreated limestone, TL – treated limestone, UM – untreated marble, TM – treated marble.
3.2. Transmission electron microscopy of the cells
Rock dust and silica particles were clearly seen as electronic dense inclusions in cytoplasm of the A549 cells 72 h after exposure (Supplementary Fig. 1) and THP-1 cells 24 h after exposure (Supplementary Fig. 2).
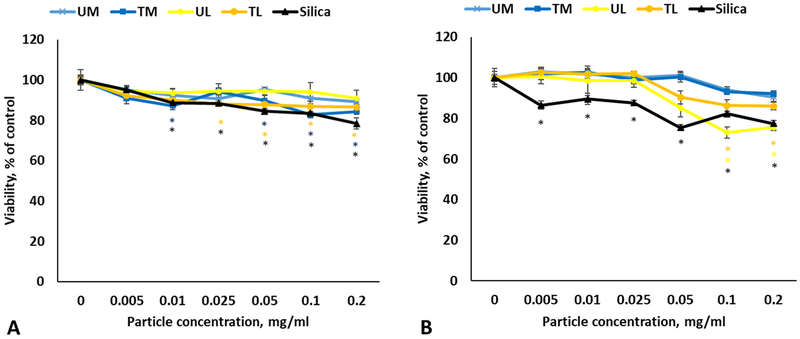
3.3. Cytotoxicity of rock dust and silica in A549 cells
The cytotoxic potential of the respirable fraction of washed RD samples was determined after exposure of A549 cells to various concentrations (0–0.2 mg/ml) of UM, TM, UL, TL and silica for 24 and 72 h. Viability was slightly decreased in TL and TM-treated groups at 24 h (Fig. 3A). There was a small, but statistically significant drop in viability in UL and TL-exposed samples at 72 h (0.05–0.2 mg/ml) compared to control cells (Fig. 3B). Silica exposure caused dose-dependent reduction in viability at both time points (Fig. 3A and B). Cell damage measured as LDH in the cell supernatants after 24 and 72 h exposure was not significantly different between the four rock dusts and control non-exposed cells, while silica treatment caused statistically significant LDH increase only at the concentration of 0.2 mg/ml for both time points (data not shown).
Fig. 3.
Viability of the A549 cells following 24 h (A) and 72 h (B) exposure to various rock dusts and silica. Values are expressed as mean ± SEM. *p < .05 vs. control.
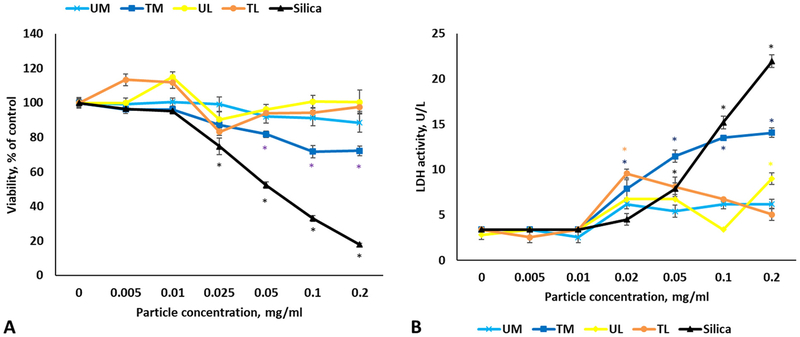
3.4. Cytotoxicity of rock dust and silica in THP-1 cells
24 h after exposure, we observed a significant decrease in viability of the differentiated THP-1 cells treated with TM at 0.05, 0.1 and 0.2 mg/ml doses, while silica caused dose-dependent reduction in viability starting at 0.025 mg/ml (Fig. 4A). Accordingly, LDH activity in supernatants went up for Silica and TM in dose-dependent manner (Fig. 4B).
Fig. 4.
Viability (A) and LDH leakage (B) following exposure to various rock dusts and silica in differentiated THP-1 cells. Values are expressed as mean ± SEM. *p < .05 vs. control.
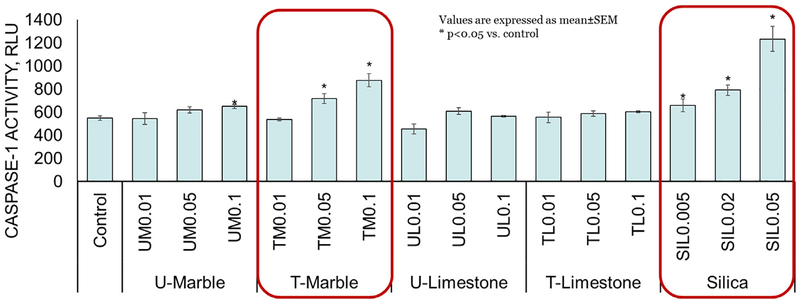
3.5. Caspase-1 activity in THP-1 cells
Macrophages, exposed to all silica concentrations, TM (0.05 and 0.1 mg/ml) and UM (0.1 mg/ml) but not to UL or TL displayed a dose-dependent statistically significant increase in caspase-1 activity (Fig. 5) compared to control cells, which indicates the possible inflammasome involvement.
Fig. 5.
Intracellular caspase-1 activity following 24 h exposure to various concentrations of rock dusts and crystalline silica in THP-1 cells. Values are expressed as mean ± SEM. *p < .05 vs. control.
3.6. Pro-inflammatory cytokine/chemokine secretion in A549 cells following various rock dusts exposures
The release of cytokines in cell supernatants was assessed in the A549 cells exposed to three different concentrations of RD and silica at 72 h post-exposure (Supplementary Table 2). Treated and untreated marble had the most impact on the overall cytokine secretion with statistically significant elevation in the concentrations of IL-6, Il-8, IL-12(p70), TNF-α, IFN-γ and IL-17. Treated marble additionally induced significantly higher IL-10, basic Fibroblast Growth Factor (bFGF) and platelet-derived growth factor (PDGF) secretion compared to other samples.
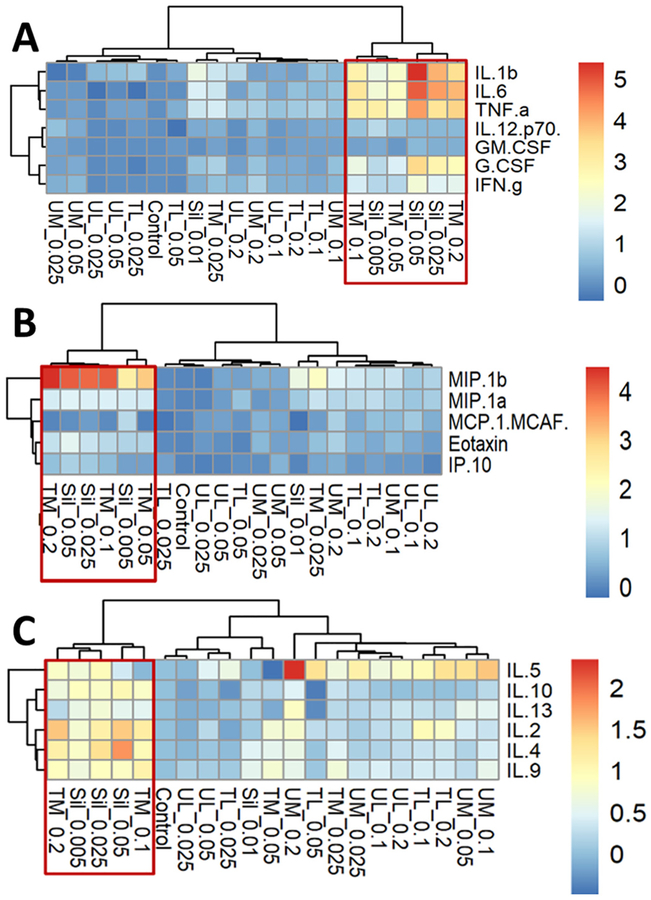
3.7. Hierarchical cluster analysis of cytokine profiles in THP-1 supernatants 24 h post-exposure
The release of cytokines in cell supernatants was assessed in the THP-1 cells exposed to four different concentrations of RD and silica at 24 h post-exposure (Supplementary Table 3). Both TM and silica induced significant dose-dependent increase in the production of proin-flammatory cytokines and together formed a major cluster, separated from other particles (Fig. 6A). Both TM and silica had caused significant increase in the secretion of MIP-1β and eotaxin, again, forming a separate cluster (Fig. 6B), while IL-8 concentration was over the observable range in all samples. Distinct grouping of TM and silica was observed with > 2-fold increase of IL-2, IL-4, and IL-9 concentrations in the cell supernatants. (Fig. 6C).
Fig. 6.
Hierarchical cluster analysis of inflammatory cytokines (A), chemokines (B) and TH2-cytokines (C) in THP-1 supernatants 24 h post-exposure to various rock dusts or crystalline silica.
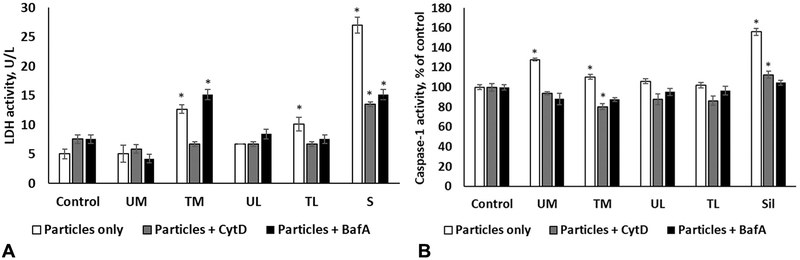
3.8. Uptake-related toxicity study
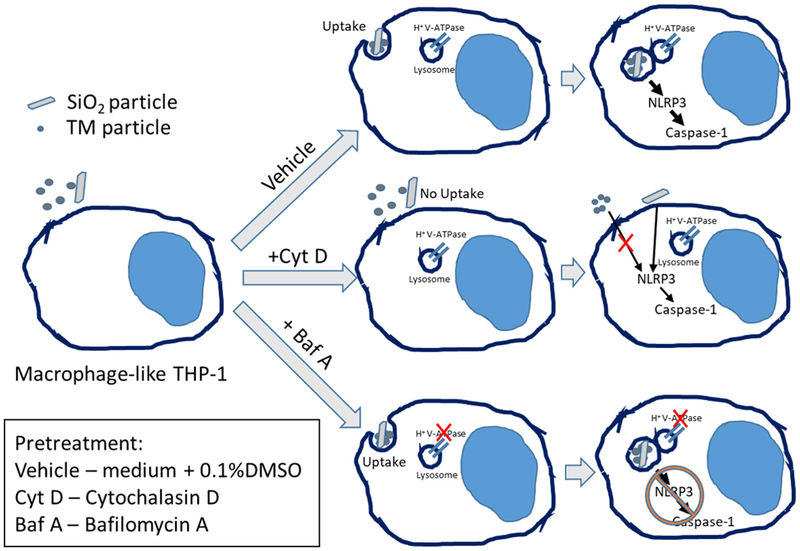
Cytochalasin D (actin polymerization inhibitor) treatment blocked the particles uptake and mitigated or significantly decreased LDH production and caspase-1 activity in treated cells compared to respective controls. Bafilomycin A, an inhibitor of vacuolar H+-ATPase, prevented caspase-1 activation and partially rescued the LDH leakage in SiO2- but not TM-exposed cells (Figs. 7 and 8).
Fig. 7.
LDH leakage in the supernatant (A) and intracellular caspase-1 activity (B) following 6 h of exposure to various rock dusts or silica in THP-1 cells pretreated with Bafilomycin A, Cytochalasin D or exposure medium with 0.1% DMSO. Values are expressed as mean ± SEM. *p < .05 vs. respective control.
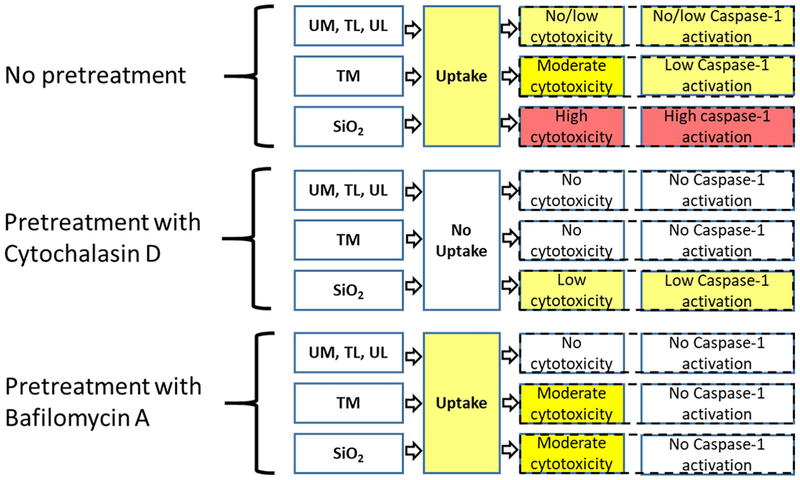
Fig. 8.
The summary of the effects of Cytochalasin D and Bafilomycin A pretreatment on cytotoxicity (LDH leakage) and caspase-1 activation in PMA-differentiated THP-1 macrophages upon exposure to RD or Crystalline Silica.
4. Discussion
Limestone and marble are rocks, mainly comprised of calcium and magnesium carbonate mineral varieties. Since ancient times, they have been used in building and road construction, sculpture, other arts, agriculture, and even the food industry. In mining, the term “rock dust” is applied to a finely crushed (pulverized) stone (e.g. limestone) used to reduce the combustibility of coal dust and prevent an explosion from propagating. It must contain no > 5% combustible matter and no more than a total of 4% free and combined SiO2. The federal register requires that the RD should be effectively applied, but if caking (due to wetting) occurs, the dust loses its ability to disperse and therefore cannot make the coal dust inert or prevent an explosion from propagating. Anti-caking surface treatments exist that increase the dispersibility, but there is no data on the potential health implications of such treatments. Miners’ and other workers’ exposure may occur during the application process, especially downwind from the application. In a recent study that assessed area dust levels at particular locations throughout the mine, treated RD has been shown to produce more respirable particulates during the dusting procedure than non-treated (Harris et al., 2017). Authors also raised concern regarding the re-aerosolization of rock dust covering the floor due to the routine movements of personnel and equipment. Scoop operators using flinger rock dusting machines are also at risk.
Scattered evidence shows little or no cytotoxicity caused by calcium carbonate rock particles, such as limestone, marble, or dolomite (calcium magnesium carbonate). Studies in marble cutters and limestone quarry workers (Doig, 1955; Angotzi et al., 2005; Bwalya, Bratveit, and Moen, 2011; Neghab, Abedini, Soltanzadeh, Iloon Kashkooli, and Ghayoomi, 2012) and several published case reports (Crummy et al., 2004; Bello et al., 2015; Yildirim et al., 2016) discovered increases of respiratory symptom occurrence as well as such adverse health outcomes as COPD, pneumoconiosis, and fibrosis. Co-exposure with silica was proposed to be responsible for adverse outcomes in susceptible individuals, even though the exposure to silica was very low. Previously, several reported studies revealed that the respirable limestone particles were easily taken up by macrophages causing little or no cytotoxicity as compared to silica (Sugiura et al., 1998), and instillation or inhalation of limestone dust in rats or rabbits did not cause any pulmonary fibrosis or tumorigenic outcomes (Mashimo et al., 1998; Oyabu et al., 1998).
Inhaled particles that are deposited in the lung come into contact first with epithelial cells and resident macrophages and induce specific reaction patterns that depend on particle chemical composition, shape, solubility, and surface properties. Reactions range from uneventful clearance to cell damage and full-scale immune activation, accompanied by the release of alarmins and other bioactive compounds, including cytokines and chemokines. With that in mind, our research question was whether the hydrophobic surface treatment of calcium carbonate RDs may influence their bioactivity. In the present study, we assessed cytotoxicity and specific responses to several different types of treated and untreated respirable RDs in comparison to a known fibrogenic and proinflammatory agent, crystalline silica.
Due to the fact that little is known about respirable rock dust levels in personal breathing zones, it is hard to estimate the real workplace exposures. Area sampling performed in a test mine during dusting procedures revealed variability between samples depending on the sampling point and type of the rock dust used. Personal dust monitors can easily become overloaded if located in the rock dust cloud and even at 500 ft. downwind the concentrations of respirable rock dust reached 17 mg/m3. For our calculations, we chose the overall respirable dust standard of 1.5 mg/m3, recently introduced by MSHA (MSHA, 2014). Our mid-range dose (0.01 mg/ml) is equivalent to nine years of human exposure in the case of A549 cells or 1.5 years for THP-1 macrophages. (See supplement material).
Epithelial cell damage and reactivity plays a substantial role in the pathology of lung diseases, including exposure to particulates (Camelo, Dunmore, Sleeman, and Clarke, 2014; Manke, Wang, and Rojanasakul, 2013). A549 cell line models the type II alveolar epithelial cells and is widely used for toxicity, drug metabolism, and transfection studies. A549 cells respond to a number of airborne particulates and pollutants by producing inflammatory mediators (Corsini et al., 2013). There is also a solid number of publications on silica-induced toxicity in A549 cells (Hetland et al., 2001; Vuong et al., 2017; Ovrevik et al., 2006). Previous reports indicated that A549 exhibit phagocytosis of ~1 μm-sized particles, albeit at a much slower rate than macrophages (Kuhn et al., 2014; Rothen-Rutishauser, Muhlfeld, Blank, Musso, and Gehr, 2007), indeed, at 72 h post-exposure we could clearly see the RD and silica inside the cells by TEM. After the RD treatment, dose- and time-dependent cytotoxicity as well as cell damage were only seen at 72 h post exposure with the most effect upon exposure to silica and untreated limestone. Still the viability was relatively high and the cell damage remained low. The extent of cytokine responses, however, has provided more sensitive data. The analysis of inflammatory cytokines/chemokines revealed an overall stronger effect of marble, both treated and untreated, compared to limestone samples. TM induced both greater TH1-type cytokine response, and increased bFGF and PDGF secretion. The former may prompt the localized inflammatory reaction in the lung with edema and recruitment of immune cells, while the latter has been implicated in the remodeling of airways and respiratory compartment (Kelley, 1990; Shimizu et al., 2000). Overall, our results unveiled treatment related differences as well as material dependent changes in biological responses of the surrogate type II alveolar epithelial cells.
Alveolar macrophages (AMs) are the housekeepers of the lung. Aside from participating in local homeostasis, such as the recycling of surfactant components and efferocytosis, they are equipped and armed to deal with particles that get deep into the alveolar region from inorganic particulate matter to saprophytic and pathogenic microflora (Laskin, Malaviya, and Laskin, 2015). Importantly, alveolar macrophages contribute to the overall tolerant immunological milieu, possessing the so-called M2-like phenotype in the resting state (Alber, Howie, Wallace, and Hirani, 2012). Nevertheless, AMs are able to quickly adopt a phenotype (activation) required for playing a major role in a variety of infectious and noninfectious pathologies, including tuberculosis, bacterial and viral pneumonias and pneumoconioses.
Taking into consideration that calcium carbonate is quickly eliminated from the lungs (Oyabu et al., 1998), we did not aim to address long-term toxicity, but rather the acute responses to respirable RD. Thus, we sought to determine the inflammogenic potential of the rock dust compared to silica, using the PMA-differentiated THP-1 cells as a model of macrophages primed toward inflammatory responses (Lund et al., 2016; Sharma, McLeland, Potter, Stern, and Adiseshaiah, 2018). Previously, PMA-differentiated THP-1 cells have been extensively used as a model for alveolar macrophages (Van der Meeren et al., 2016; Kletting et al., 2018; Riendeau and Kornfeld, 2003; Voth et al., 2007).
The ability of silica to induce cell death in macrophages, which contributes to the pathogenesis of silicosis, is well known (Gilberti, Joshi, and Knecht, 2008; Costantini, Gilberti, and Knecht, 2011; Joshi and Knecht, 2013; Hamilton Jr, Thakur, and Holian, 2008). Unsurprisingly, we saw a marked dose-dependent cytotoxicity in the positive control group. Of note, silica-induced inflammation and disease progression is heavily dependent on innate immune components, rather than lymphocyte-mediated responses (Beamer et al., 2010). Studies of silicosis in humans and animal experiments describe the latency period before the onset of the active lung inflammatory reaction (Kawasaki, 2015), which is also separate from the long-term outcomes, such as pulmonary fibrosis. (Rabolli et al., 2011; Re et al., 2014). A recent review hypothesize that this delay and the following exacerbation depends on the concerted action of different immune cell subsets, including inflammatory and so-called alternatively activated, tolerogenic macrophages (Kawasaki, 2015). Among the rock dust samples, only the TM caused a statistically significant decrease in cell viability and increase in extracellular LDH activity in THP-1 cells. Cytokine profiling revealed significant dose-dependent increase in the production of proinflammatory cytokines for both silica and TM, which formed a cluster, separated from control cells, UM and other rock dust particles. With the exception of TM, there was no apparent distinction in cytokine responses between coated and uncoated RD particles, which were clustered together on the concentration basis, lower doses being very close to control samples. These results prompted the question of the possible mechanism responsible for a partial semblance between TM and silica responses. Since we could clearly observe the phagocytosis of all particles and visualize them inside the phagolysosomes, we first wanted to explore the NLRP3 inflammasome pathway, intrinsic in macrophages and implicated in a variety of cell-particle interactions, including asbestos, nanoparticles and crystalline silica exposures (Hornung et al., 2008; Sharma et al., 2018; Cui et al., 2014; Palomaki et al., 2011). Indeed, the activity of caspase-1, an enzyme responsible for the cleavage of IL-1β precursor into the active form and processing of several other cytokines, was also significantly dose-dependently elevated in both TM and silica-exposed cells, as well as in cells exposed to high UM concentration which indicates possible inflammasome involvement (Petrilli, Dostert, Muruve, and Tschopp, 2007).
To test if the particles’ toxicities were uptake-related, we first pre-treated cells with Cytochalasin D, which inhibits the actin polymerization and disrupts phagocytosis in macrophages. As expected, this completely prevented the cytotoxic effect observed in TM-exposed group six hours post exposure. Silica-treated cells still showed slight but statistically significant elevated caspase-1 activity even after Cytochalasin D administration. This could be the result of silica crystals binding to the cell surface, which has been previously shown to be enough stimulus for inflammasome activation in larger particles (Hari et al., 2014). Several studies have reported that crystalline silica exerts its toxicity through the destabilization of lysosomal membranes (Hornung et al., 2008; Jessop, Hamilton Jr., Rhoderick, Fletcher, and Holian, 2017). The specific mechanisms are still debated, although the recent articles pointed out the importance of phagolysosome acid-ification in lysosomal membrane permeabilization and increased NLRP3 inflammasome activity (Jessop et al., 2017; Hornung et al., 2008; Kusaka et al., 2014). To investigate this particular mechanism, we utilized the inhibitor of vacuolar proton pump (vATPase) Bafilomycin A. The pump produces a proton gradient across the lysosomal membrane through the ATP hydrolysis, thus creating the low-pH environment in lysosomes and phagolysosomes, required for the macrophage pathogen and particle clearance function. Bafilomycin A treatment prevented caspase-1 activation, indicating the importance of the phagolysosomes acidification for the activation of inflammasome (Fig. 9). At the same time, Bafilomycin A substantially decreased the LDH leakage in silica-exposed cells, but had no effect on marked LDH release in the supernatant of TM-exposed macrophages, which requires further investigation of TM behavior while inside the phagolysosome.
Fig. 9.
The schematic illustrating the effect of Cytochalasin D and Bafilomycin A on caspase-1 activation in PMA-differentiated THP-1 macrophages upon exposure to treated marble (TM) OR crystalline silica.
Taken together, the data suggests that stearate surface treatment of the innocuous calcite particles increases cytotoxicity (although on a much lesser scale compared to silica) upon the phagocytosis of those particles by macrophages (Fig. 4A). The exact reason for that is currently unclear. American Conference of Governmental Industrial Hygienists (ACGIH®) considers stearates to be of low toxicity, however, ACGIH® opinion was formed based mostly on studies that did not use the inhalation route. One may consider that this low toxicity rating was a factor in the selection of the coating material by the company-producer. It has to be pointed out that the coated marble rock dust product is originally intended to be used as a polymer additive to facilitate the dispersion in organic liquids/solvents, but not for the procedures involving aerosolization.
The existing toxicity data on metallic stearates is scarce. One sub-chronic study in rats given calcium stearate via intratracheal instillation (for 4 to 8 months) revealed pulmonary damage and alveolar emphysema (Tarasenko, Shabalina, and Spiridonova, 1976). Accidental zinc stearate powder aspiration has been implicated in a number of respiratory distress cases in infants (Elder, 1982). TiO2 nanoparticles when coated with stearate exhibited higher cytotoxicity in mouse benign fibrosarcoma cells compared to untreated ones, even facilitating their transformation into aggressive tumor cells (Onuma et al., 2009). Use of nickel ferrite nanoparticles covered with oleic acid also produced increased cytotoxicity observed in neuroblastoma cells (Yin, Too, and Chow, 2005). The surface hydrophobicity itself could impart hazardous properties (Gareth, 2017), however, it is probably not an issue in this case, since TL silicone-based treatment had no observable cytotoxic effects in our study; additionally, other studies observed the beneficial effect of hydrophobic coating on lysosomal damage and overall particulate toxicity (Wang et al., 2012; Hohr et al., 2002). The specific TM biological effects may occur through the formation of the interface between the coating and biomacromolecules and subsequent interaction with lysosomal membrane components (Nel et al., 2009), but without the exact knowledge of particle preparation (due to the proprietary character of the treatment) our speculations are limited. For instance, depending of the method used, stearate molecules may or may not form the monolayer or even produce multilayered surface (Shi et al., 2010; Jeon et al., 2018), with a various degree of (non)polarity, which plays a major role in the cytotoxicities of particulates (Fröhlich, 2012).
Overall, our results support the previously published data showing that respirable calcium carbonate particles themselves probably have little to no effect on epithelial cells or macrophages. Surface treatment of these particles may have no effect either or alter their cytotoxicity profile in an unexpected way. Limitations of our study should be taken into consideration, including the use of transformed cell lines and little information on the specifics of the RD coating. In the context of real-life application, exposure to aerosols with high free silica content still poses more threat than rock dusting. Nevertheless, a precautionary approach is warranted to avoid miners’ exposure downwind of rock dust applications (Harris et al., 2017). Prospective studies can be expanded to include more relevant cell types and in vivo experimental models that would take into account the realistic exposure scenarios. For example, future research should benefit from understanding and evaluating the combined effects of rock dust, silica and/or coal dust.
5. Conclusions
None of the rock dust samples induced significant cytotoxicity in A549 cells following 24 or 72 h of exposure. Rock dust particles were also able to induce some inflammatory cytokine production in A549 cells, however, the response was less pronounced compared to silica. Untreated limestone (UL), untreated marble (UM) and silicone-treated limestone (TL) particles were readily internalized by THP-1 macrophages but caused very little or no toxicity even at high doses. Exposure of THP-1 cells to the blend of untreated (87.5%) and stearate-treated (12.5%) marble (TM) led to significant dose-dependent LDH increase, inflammatory and TH2-type cytokines/chemokines release along with the elevated caspase-1 activity, however the observed effects were substantially less prominent compared to silica at the same mass concentrations. Inhibition of particles uptake by cells using Cytochalasin D prevented the adverse effects of both TM and silica. The use of Bafilomycin A that prevents the acidification of phagolysosomes, reduced the toxic effects of silica treatment, but not TM, perhaps due to different mechanisms of action. Altogether, the obtained results showed that out of all tested rock dust samples only stearate-treated marble caused moderate toxicity in vitro, however still significantly below the effect of silica.
Supplementary Material
Acknowledgements
We would like to thank Sherri Friend for TEM pictures and consultation, Dr. Donald Beezhold and Mackenzie Newman for the helpful review of the manuscript, Dr. Steve Mischler, Dr. Eranda Perera, and Marcia Harris from NIOSH/PMRD Explosions Prevention Group for supplying the rock dust samples and valuable inputs.
Funding
This work was supported by the National Institute for Occupational Safety and Health (NIOSH) 939054L and National Occupational Research Agenda (NORA) 939051G grants.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2018.10.023.
Disclaimer
The content and conclusions of this publication are those of the authors and do not necessarily reflect the views or policies of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors declare no competing financial interest.
References
- Alber A, Howie SE, Wallace WA, Hirani N, 2012. The role of macrophages in healing the wounded lung. Int. J. Exp. Pathol 93, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angotzi G, Bramanti L, Tavarini D, Gragnani M, Cassiodoro L, Moriconi L, Saccardi P, Pinto I, Stacchini N, Bovenzi M, 2005. World at work: Marble quarrying in Tuscany. Occup. Environ. Med 62, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Migliaccio CT, Jessop F, Trapkus M, Yuan D, Holian A, 2010. Innate immune processes are sufficient for driving silicosis in mice. J. Leukoc. Biol 88, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello S, Rinaldi A, Trabucco S, Serafino L, Bonali C, Lapadula G, 2015. Erasmus syndrome in a marble worker. Reumatismo 67, 116–122. [DOI] [PubMed] [Google Scholar]
- Bwalya D, Bratveit M, Moen BE, 2011. Chronic respiratory symptoms among workers at a limestone factory in Zambia. Arch Environ Occup Health 66, 47–50. [DOI] [PubMed] [Google Scholar]
- Camelo A, Dunmore R, Sleeman MA, Clarke DL, 2014. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front. Pharmacol 4, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Zhi, Daly Michael, Clémence Lopez, Geever Luke M., Major Ian, Higginbotham Clement L., Devine Declan M., 2016. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci 378, 320–329. [Google Scholar]
- CDC/NIOSH, 2012a. Mining Feature: Coal Mine Explosion Prevention Accomplishments Accessed 6-26-2018 https://www.cdc.gov/niosh/mining/features/explprevfeature.html. [Google Scholar]
- CDC/NIOSH, 2012b. Publication No. 2012–102, HID 16. Non-conforming Rock Dust U.S. Department of Health and Human Services. [Google Scholar]
- CDC/NIOSH, 2014. Mining Contract: Development of an Anti-Caking Rock Dust. Accessed 6-26-2018 https://www.cdc.gov/niosh/mining//researchprogram/contracts/contract200-2012-52496.html. [Google Scholar]
- Chanput W, Mes JJ, Wichers HJ, 2014. THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharmacol 23, 37–45. [DOI] [PubMed] [Google Scholar]
- Corsini E, Budello S, Marabini L, Galbiati V, Piazzalunga A, Barbieri P, Cozzutto S, Marinovich M, Pitea D, Galli CL, 2013. Comparison of wood smoke PM2.5 obtained from the combustion of FIR and beech pellets on inflammation and DNA damage in A549 and THP-1 human cell lines. Arch. Toxicol 87, 2187–2199. [DOI] [PubMed] [Google Scholar]
- Costantini LM, Gilberti RM, Knecht DA, 2011. The phagocytosis and toxicity of amorphous silica. PLoS One 6, e14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crummy F, Carl I, Cameron CH, Heaney LG, 2004. A possible case of pneumoconiosis in a limestone quarry worker. Occup. Med. (Lond.) 54, 497–499. [DOI] [PubMed] [Google Scholar]
- Cui H, Wu W, Okuhira K, Miyazawa K, Hattori T, Sai K, Naito M, Suzuki K, Nishimura T, Sakamoto Y, Ogata A, Maeno T, Inomata A, Nakae D, Hirose A, Nishimaki-Mogami T, 2014. High-temperature calcined fullerene nanowhiskers as well as long needle-like multi-wall carbon nanotubes have abilities to induce NLRP3-mediated IL-1beta secretion. Biochem. Biophys. Res. Commun 452, 593–599. [DOI] [PubMed] [Google Scholar]
- Doig AT, 1955. Disabling pneumoconiosis from limestone dust. Br. J. Ind. Med 12, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder RL, 1982. Final report of the safety assessment of lithium stearate, aluminum distearate, aluminum stearate, aluminum tristearate, ammonium stearate, calcium stearate, magnesium stearate, potassium stearate, sodium stearate, and zinc stearate. J. Am. Coll. Toxicol 1, 143–177. [Google Scholar]
- Fröhlich Eleonore, 2012. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine 7, 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareth E, 2017. Summary of Evidence – Solvent-Based Hydrophobic Coatings and Risks for Acute Respiratory Toxicity.
- Gilberti RM, Joshi GN, Knecht DA, 2008. The phagocytosis of crystalline silica particles by macrophages. Am. J. Respir. Cell Mol. Biol 39, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF Jr., Thakur SA, Holian A, 2008. Silica binding and toxicity in alveolar macrophages. Free Radic. Biol. Med 44, 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari A, Zhang Y, Tu Z, Detampel P, Stenner M, Ganguly A, Shi Y, 2014. Activation of NLRP3 inflammasome by crystalline structures via cell surface contact. Sci. Rep 4, 7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Marcia L., Sapko MJ, Varley F, Weiss ES, National Institute for Occupational Safety and Health, Office of Mine Safety and Health Research, 2012. Coal dust explosibility meter evaluation and recommendations for application Dept. of Health and Human Services, Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health, Pittsburgh Research Laboratory, Office of Mine Safety and Health Research, Pittsburgh, PA; ; Spokane, WA. [Google Scholar]
- Harris ML, Organiscak J, Klima S, Perera IE, 2017. Respirable dust measured downwind during rock dust application. Min. Eng 69, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland RB, Schwarze PE, Johansen BV, Myran T, Uthus N, Refsnes M, 2001. Silica-induced cytokine release from A549 cells: importance of surface area versus size. Hum Exp Toxicol 20, 46–55. [DOI] [PubMed] [Google Scholar]
- Hohr D, Steinfartz Y, Schins RP, Knaapen AM, Martra G, Fubini B, Borm PJ, 2002. The surface area rather than the surface coating determines the acute in-flammatory response after instillation of fine and ultrafine TiO2 in the rat. Int. J. Hyg. Environ. Health 205, 239–244. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, 2008. Silica crystals and aluminum salts activate the NALP3 in-flammasome through phagosomal destabilization. Nat. Immunol 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Chi, Park Sangwon, Bang Jun-Hwan, Chae Soochun, Song Kyungsun, Lee Seung-Woo, 2018. Nonpolar Surface Modification using Fatty Acids and its effect on Calcite from Mineral Carbonation of Desulfurized Gypsum. Coatings 8, 43. [Google Scholar]
- Jessop F, Hamilton RF Jr., Rhoderick JF, Fletcher P, Holian A, 2017. Phagolysosome acidification is required for silica and engineered nanoparticle-induced lysosome membrane permeabilization and resultant NLRP3 inflammasome activity. Toxicol. Appl. Pharmacol 318, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi GN, Knecht DA, 2013. Silica phagocytosis causes apoptosis and necrosis by different temporal and molecular pathways in alveolar macrophages. Apoptosis 18, 271–285. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, 2015. A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol 27, 363–377. [DOI] [PubMed] [Google Scholar]
- Kelley J, 1990. Cytokines of the lung. Am. Rev. Respir. Dis 141, 765–788. [DOI] [PubMed] [Google Scholar]
- Kletting S, Barthold S, Repnik U, Griffiths G, Loretz B, Schneider-Daum N, de Souza Carvalho-Wodarz C, Lehr CM, 2018. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 35, 211–222. [DOI] [PubMed] [Google Scholar]
- Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B, 2014. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol 5, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K, 2014. Effect of silica particle size on macrophage inflammatory responses. PLoS One 9, e92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Malaviya R, Laskin JD, 2015. Pulmonary macrophages In: Parent RA (Ed.), Comparative Biology of the Normal Lung. Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam; ; Boston. [Google Scholar]
- Lund ME, To J, O’Brien BA, Donnelly S, 2016. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 430, 64–70. [DOI] [PubMed] [Google Scholar]
- Manke A, Wang L, Rojanasakul Y, 2013. Pulmonary toxicity and fibrogenic response of carbon nanotubes. Toxicol. Mech. Methods 23, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo K, Okada M, Aizawa Y, Karube H, Keira T, Watanabe M, Sugiura Y, Kotani M, 1998. Effect of limestone on the lungs of rabbits In: Chiyotani Keizo, Hosoda Yutaka (Eds.), Advances in the Prevention of occupational respiratory diseases: proceedings of the 9th International Conference on Occupational Respiratory Diseases, Kyoto, 13–16 October 1997. Elsevier, New York; ; Amsterdam. [Google Scholar]
- MSHA, 2014. Lowering Miners Exposure to Respirable Coal Mine Dust, Including Continuous Personal Dust Monitors. pp. 24813–24994. [Google Scholar]
- Neghab M, Abedini R, Soltanzadeh A, Iloon Kashkooli A, Ghayoomi SM, 2012. Respiratory disorders associated with heavy inhalation exposure to dolomite dust. Iran Red Crescent Med J 14, 549–557. [PMC free article] [PubMed] [Google Scholar]
- Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M, 2009. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater 8, 543–557. [DOI] [PubMed] [Google Scholar]
- Onuma K, Sato Y, Ogawara S, Shirasawa N, Kobayashi M, Yoshitake J, Yoshimura T, Iigo M, Fujii J, Okada F, 2009. Nano-scaled particles of titanium dioxide convert benign mouse fibrosarcoma cells into aggressive tumor cells. Am. J. Pathol 175, 2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrevik J, Refsnes M, Namork E, Becher R, Sandnes D, Schwarze PE, Lag M, 2006. Mechanisms of silica-induced IL-8 release from A549 cells: initial kinase-activation does not require EGFR activation or particle uptake. Toxicology 227, 105–116. [DOI] [PubMed] [Google Scholar]
- Oyabu T, Kasai T, Yamato H, Ishimatsu S, Morimoto Y, Hashimoto H, Tsuda T, Hori H, Ohgami A, Tanaka i., Baba Y, 1998. Biopersistence and the possibility of fibrosis of limestone in rat Lung In: Chiyotani Keizo, Hosoda Yutaka (Eds.), Advances in the Prevention of occupational respiratory diseases: proceedings of the 9th International Conference on Occupational Respiratory Diseases, Kyoto, 13–16 October 1997. Elsevier, New York; ; Amsterdam. [Google Scholar]
- Palomaki J, Valimaki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H, 2011. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5, 6861–6870. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J, 2007. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol 19, 615–622. [DOI] [PubMed] [Google Scholar]
- Rabolli V, Lo Re S, Uwambayinema F, Yakoub Y, Lison D, Huaux F, 2011. Lung fibrosis induced by crystalline silica particles is uncoupled from lung inflammation in NMRI mice. Toxicol. Lett 203, 127–134. [DOI] [PubMed] [Google Scholar]
- Re SL, Giordano G, Yakoub Y, Devosse R, Uwambayinema F, Couillin I, Ryffel B, Marbaix E, Lison D, Huaux F, 2014. Uncoupling between inflammatory and fibrotic responses to silica: evidence from MyD88 knockout mice. PLoS One 9, e99383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau CJ, Kornfeld H, 2003. THP-1 cell apoptosis in response to Mycobacterial infection. Infect. Immun 71, 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Muhlfeld C, Blank F, Musso C, Gehr P, 2007. Translocation of particles and inflammatory responses after exposure to fine particles and nano-particles in an epithelial airway model. Part Fibre Toxicol 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, 2008. Endotoxin and Cell Culture. Technical Bulletin. [Google Scholar]
- Sharma B, McLeland CB, Potter TM, Stern ST, Adiseshaiah PP, 2018. Assessing NLRP3 Inflammasome Activation by Nanoparticles. Methods Mol. Biol 1682, 135–147. [DOI] [PubMed] [Google Scholar]
- Shi X, Rosa R, Lazzeri A, 2010. On the coating of precipitated calcium carbonate with stearic acid in aqueous medium. Langmuir 26, 8474–8482. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K, 2000. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 279, L503–L510. [DOI] [PubMed] [Google Scholar]
- Soo JC, Lee T, Chisholm WP, Farcas D, Schwegler-Berry D, Harper M, 2016. Treated and untreated rock dust: Quartz content and physical characterization. J. Occup. Environ. Hyg 13, D201–D207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Keira T, Aizawa Y, Okada M, Watanabe M, Harada H, Mashimo K, Kotani M, 1998. Effect of limestone on the alveolar macrophages of hamster In: Chiyotani Keizo, Hosoda Yutaka (Eds.), Advances in the Prevention of occupational respiratory diseases: proceedings of the 9th International Conference on Occupational Respiratory Diseases, Kyoto, 13–16 October 1997. Elsevier, New York; ; Amsterdam. [Google Scholar]
- Tarasenko NY, Shabalina LP, Spiridonova VS, 1976. Comparative toxicity of metal stearates. Int. Arch. Occup. Environ. Health 37, 179–192. [DOI] [PubMed] [Google Scholar]
- Tedesco S, De Majo F, Kim J, Trenti A, Trevisi L, Fadini GP, Bolego C, Zandstra PW, Cignarella A, Vitiello L, 2018. Convenience versus Biological significance: are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages when Studying in Vitro Polarization? Front. Pharmacol 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “U.S. Code of Federal Regulations. Title 30—Mandatory safety standards. Part 75—underground coal mines. Subpart E. Section 75.403 Maintenance of incombustible content of rock dust In. Washington, DC: National Archives and Records Administration; Office of the Federal Register. [Google Scholar]
- Van der Meeren A, Moureau A, Laurent D, Laroche P, Angulo JF, 2016. In vitro assessment of plutonium uptake and release using the human macrophage-like THP-1 cells. Toxicol In Vitro 37, 25–33. [DOI] [PubMed] [Google Scholar]
- Voth DE, Howe D, Heinzen RA, 2007. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun 75, 4263–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong NQ, Goegan P, De Rose F, Breznan D, Thomson EM, O’Brien JS, Karthikeyan S, Williams A, Vincent R, Kumarathasan P, 2017. Responses of A549 human lung epithelial cells to cristobalite and alpha-quartz exposures assessed by toxicoproteomics and gene expression analysis. J Appl Toxicol 37, 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WE, Keane MJ, Harrison JC, Stephens JW, Brower PS, Grayson RL, Attfield MD, 2011. Coal Mine Dust Exposures and Associated Health Outcomes. National Institute for Occupational Safety and Health (NIOSH). [Google Scholar]
- Wang X, Xia T, Duch MC, Ji Z, Zhang H, Li R, Sun B, Lin S, Meng H, Liao YP, Wang M, Song TB, Yang Y, Hersam MC, Nel AE, 2012. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett 12, 3050–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim BB, Akgedik R, Akgedik S, Nazaroglu H, 2016. Pulmonary alveolar proteinosis in a marble worker. Int J Occup Med Environ Health 29, 871–876. [DOI] [PubMed] [Google Scholar]
- Yin H, Too HP, Chow GM, 2005. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials 26, 5818–5826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.