Abstract
Changes in cognition due to age have been associated with falls and reduced standing postural control. Sensory integration is one component of postural control that may be influenced by certain aspects of cognitive functioning. This study investigated associations between measures of cognitive function and sensory integration capabilities for healthy young and older adults. Dynamic posturography was performed using the Equitest Sensory Organization Test (SOT) protocol to evaluate sensory integration during standing using sway-referencing of the platform and/or visual scene to alter somatosensory and visual inputs. The Equilibrium Score was used as a measure of sway. Cognitive testing examined aspects of cognitive function that have been associated with falls in older adults. A correlational analysis investigated associations between the cognitive measures and postural sway during the altered sensory conditions of the SOT. For older subjects only, slower decision processing speed was associated with increased sway during SOT conditions whenever somatosensation was altered. Reduced perceptual inhibition was associated with increased sway whenever somatosensation was intact, and particularly when vision was altered in the presence of somatosensation. Visuospatial construct ability was associated with sway only when the eyes were closed during altered somatosensation. Task switching was associated with sway only when vision and somatosensation were intact. With increased age, deficits in decision speed and inhibition appear associated with the sensory integration crucial for balance maintenance. Associations are modulated by the availability of somatosensation and vision. These associations define situations and individual differences in aspects of cognition that may relate to situational loss of balance in older adults.
Keywords: Sensory Integration, Cognition, Posture, Balance, Aging
Introduction
Sensory integration for postural control refers to the ability to centrally combine sensory information from three main sources (vestibular, visual and somatosensory) to estimate body position and motion (Horak and Macpherson 1996). Dynamic posturography is a test that probes the sensory integration capabilities of standing balance by systematically altering sensory information and measuring the postural response (Nashner et al. 1982). Sensory integration capability is impacted by aging, with postural sway in older adults being typically greater than those in young adults when there is an alteration in sensory inputs requiring sensory reweighting (Cham et al. 2007; Cohen et al. 1996; Horak et al. 1989; Woollacott et al. 1986).
Measures of cognition have been related to balance and falls in older adults. Numerous studies have implicated tests of executive functions (Herman et al. 2010; Holtzer et al. 2007; Mirelman et al. 2012; Taylor et al. 2017). Executive functioning refers to a broad set of processes that control behavior and decision making in the absence of automatic or well-learned control. Such processes include inhibition, working memory and selective attention (Diamond 2013). Measures tapping these different aspects of executive function have been associated with falls and imbalance in older adults; such as trail making tests, Stroop, go-no-go, task-switching and digit symbol substitution. (See (Kearney et al. 2013) for a recent review.) Other measures conceptually relevant to sensory integration, but not thought of as executive function, have also been implicated in falls including reaction time and tests of visual-spatial abilities (Anstey et al. 2006; Taylor et al. 2014; Taylor et al. 2017). The decline in basic processing speed with age is well established as evident in basic perceptual motor tasks as well as impacting more complex tasks (Salthouse 1996) Visuospatial ability refers to spatial memory, navigation, mental rotation, and mental representation of three-dimensional space and also is known to decline with age (Bigelow and Agrawal 2015; Klencklen et al. 2012). We know little about the relationships between the decline in these aspects of cognition and postural control under the conditions that challenge sensory integration, e.g. challenges of the SOT protocol.
Our past research has been focused on understanding how sensory integration for balance may be subject to processing capacity limitations evident when multiple tasks compete for the control of action (Humphreys et al. 2013). Using dual-task paradigms, we have shown that concurrent processing of simple speeded decision processing tasks compete with sensorimotor integration for balance in sensory conflict situations for older, but not younger, adults (Fuhrman et al. 2015; Redfern et al. 2001). Similarly, variants of our simple processing tasks requiring inhibition of well-learned associations were also specifically associated with the ability of older adults to maintain balance under conditions in which sensory conflict challenged sensorimotor integration for balance (Redfern et al. 2001; Redfern et al. 2009). To date, little is known about the possible impact of other aspects of cognition related to aging on balance in the presence of different challenges to sensory integration.
The purpose of this study was to examine the associations between aspects of cognition that are potentially related to sensory integration capabilities in young and older adults. Our hypothesis was that aspects of cognition known to be associated with falling in older adults would also be associated with sensory integration capabilities when challenged by alterations in vision and somatosensation. Specifically, we hypothesized that reduced processing speed for simple decisions, interference control and visuospatial ability would be associated with reduced sensory integration capability in older adults.
Methods
Subjects:
Thirty-four healthy community dwelling older adults (mean age 76.0± 4.0, 13M) and forty four healthy young adults (mean age 23.5 ± 2.9, 12M) were included in this study. All participants were screened for normal sensory, musculoskeletal and cognitive health. Inclusionary criteria were normal sensory and motor function, including no history of stroke, heart failure, peripheral neuropathy, inner ear disorder, macular degeneration or glaucoma. Subjects also were screened for use of medications that may affect performance, including sedatives, “anti-dizziness” medications, or other psychoactive medications. Vestibular function was assessed with oculomotor, positional, and rotational testing using video-oculography. (Furman and Cass 1996) Ankle joint proprioceptive sensation was evaluated using a Rydel Seiffer graduated tuning fork with an exclusion cutoff of 4. (Martina et al. 1998) Plantar foot cutaneous pressure threshold was determined using Semmes – Weinstein monofilaments, with an exclusionary cutoff of 4.3 (Holewski et al. 1988). Further exclusionary criteria were abnormal binocular visual acuity (with corrective lenses) of worse than 20/40 and hearing loss. Older subjects were also required to have a Mini Mental State Examination (MMSE) score of greater than 23 to be included in the study. The MMSE scores of the included subjects were older (μ = 28.9, s.d.=1.2, range=26–30) and young (μ = 29.6, s.d.=0.6, range=28–30). All subjects had a minimum of a high school education (12 yrs). Education levels for the older subjects (μ = 14.9 yrs, s.d.=2.7, range=12–22) and young (μ = 15.8 yrs, s.d.=2,2, range=12–24) were not significantly different (F(1,79)=2.3; p=.13). Informed consent was obtained prior to any participation. The Institutional Review Board of the University of Pittsburgh approved this study.
Dynamic Posturography:
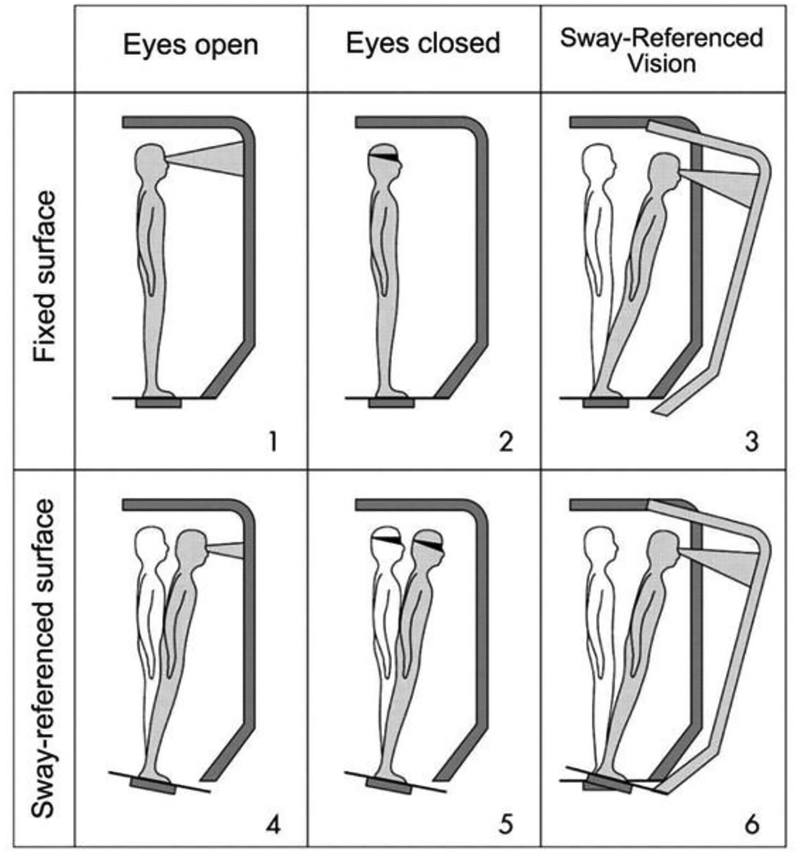
Clinical dynamic posturography was performed on the subject’s first visit using the Sensory Organization Test (SOT) protocol on the Neurocom Smart Equitest (Natus Balance & Mobility, Inc.) The six conditions tested include alterations to visual and somatosensory information during standing (Black 2001; Nashner et al. 1982). (Figure 1) The somatosensory conditions include a fixed and sway-referenced platform. The visual conditions include a fixed visual scene, eyes closed, and a sway-referenced visual scene. Sway-referencing is accomplished through rotation in direct proportion to the individual’s sway in the anteroposterior (AP) direction. (Nashner et al. 1982) Sway-referencing uses a low-pass filter of recorded center of pressure (COP) to estimate the center of gravity in the antero-posterior direction in an attempt to keep the ankle angle constant. This rotation of the support surface reduces the reliability of somatosensory information from the feet and ankles for balance. Sway referencing the visual scene uses the same principle to provide erroneously stable vision even though the person is swaying.
Figure 1:
Conditions performed in the Equitest Sensory Organization Test. Combinations of visual conditions (Eyes Open, Eyes Closed and Sway-referenced) and somatosensory conditions (Fixed surface, Sway-references surface) are used, resulting in six tests. (Source: Neurocom, http://resourcesonbalance.com/program/role/cdp/protocols)
Cognitive Testing:
Cognitive testing was performed on the subject’s second visit. Cognitive tests included three batteries: Perceptual and Motor Inhibition Test (MAPIT), CNS-Vital Signs, and the Repeatable Battery for Assessment of Neuropsychological Status (RBANS). The MAPIT protocol, based upon the work of Nassauer and Halperin (Nassauer and Halperin 2003), is a computer-based test that uses reaction times to visual stimuli on a screen. (Redfern et al. 2009) Stimuli are arrows pointing either to the right or left. Responses required are key presses from either the right or left index finger. Two types of reaction time (RT) tasks are used: one that creates a perceptual conflict and one that creates a motor conflict. (see (Jennings et al. 2011; Mendelson et al. 2010; Redfern et al. 2009) for methodological details) Briefly, the perceptual task uses an arrow pointing to the right or left either positioned on the right side or left side of the screen. The subject is asked to respond by pushing the button on the side toward which the arrow points and ignoring the spatial location of the arrow. Two conditions (congruous and incongruous) are intermixed within a block of trials for the perceptual inhibition test. The congruous condition has the spatial location of the arrow and the direction of the arrow being the same. The incongruous condition has a conflict between the direction of the arrow and the spatial location of the arrow. Forty trials in each condition are presented randomly intermixed within each block. The motor task consisted of two conditions as well. The first condition has an arrow appearing in the center of the computer screen pointing in the right or left direction. Subjects are asked to press the button on the side toward which the arrow pointed. The second condition has the participant press the button on the side “opposite” to the direction of the arrow. These trials are blocked by congruency. The median RT for each subject for each condition were calculated. The CNS-Vital Signs battery (CNS Vital Signs LLC, Morrisville, NC) is a commercially available computerized series of neurocognitive tests that assess a broad spectrum of cognitive functions. The battery has been used with a large variety of subjects over a wide range of ages, as well as with different clinical groups. The test–retest reliability of the measures has been established and compared to other neuropsychological tests (Gualtieri and Johnson 2006). (Detailed descriptions of each test is provided at CNS Vital Signs, LLC website (http:www.cnsvitalsigns.com).) The RBANS assesses attention, language, immediate memory, delayed memory, and visuospatial construction. (Randolph et al. 1998)
Data Analysis
Equilibrium (EQ) Scores:
The Equilibrium (EQ) scores provided by the Smart Equitest device were used as the measures of postural response to each condition. The EQ score is computed according to Equation 1, where 12.5° represents the maximum normal postural sway in the anterior-posterior direction; θ represents the participant’s s calculated maximum anterior-posterior center of gravity displacements. (NeuroCom International 2004) A score of 100 represents perfect stability; a score of 0 indicates a loss of balance.
| (1) |
Inhibitory Measures:
The median RTs for each of the MAPIT conditions within subject were computed. The right and left hand median RTs were averaged together within each condition, resulting in a congruous RT and incongruous RT for the perceptual conditions and for the motor conditions. The motor congruous RT is the baseline RT used in computing the inhibition scores. The perceptual inhibition measure was calculated as the incongruous RTs minus the baseline RT. The motor inhibition score was calculated as the difference between the motor incongruous RT and the baseline RT. A previous study showed that the reliability (Cronbach’s alpha) of these scores was r=.70 or greater. (Jennings et al. 2011) The greater the inhibition score, the greater the slowing associated with incongruous displays. Thus, the interpretation is that greater inhibition scores reflect increased difficulty in inhibition.
Preliminary analyses of the cognitive measures were performed to determine the most appropriate variables to address our hypotheses regarding decision speed, interference control, and visuospatial abilities, and to reduce the number of statistical comparisons between neuropsychological tests and balance outcomes. Our goal was to refine the cognitive measures used to relate to the three aspects of cognition deemed relevant. Refinement was necessary given the number of measures collected, the overlap between large batteries, i.e. the RBANS and CNS Vital Signs, and the lack of seeming relevance of some of the measures, e.g. verbal comprehension. A mix of conceptual and statistical procedures focused only on the neuropsychological measures prior to relating these to balance indices. Bivariate correlations among tests were examined and these were supplemented by targeted principal component analyses focused on measures seemingly related to decision speed, interference control, or visuospatial ability (Statistica, Dell, Inc. Tulsa, OK). Retained scores were then submitted to a final preliminary analysis to further reduce redundant information. The retained set of measures best assessed dimensions of decision processing speed (tapping, choice RT), inhibition (perceptual inhibition and motor inhibition), associative inhibition (task shifting), and visuospatial ability (visuospatial construct and visual memory). We also retained two cognitive measures (digit symbol substitution and Stroop interference) that have been shown specifically to relate to falls in older adults in the literature.
Statistical analyses included mixed design repeated measures ANOVAs to compare the age groups on their EQ scores and on the retained cognitive measures. Associations between EQ scores and the different cognitive measures were evaluated using a correlation analysis within each age group. A significance level of α<.01 was set for the analysis due to the multiple comparisons being examined in correlating these neuropsychological scores with EQ scores. In addition, a Bonferroni-Holm correction for multiple comparisons was also conducted and reported. Exploratory correlations were also computed to ensure that excluded tests, e.g. language skill, were unrelated to the EQ scores. Whenever more than one cognitive test was related to the balance score for a particular SOT condition, then a partial correlation analysis was performed. This was done to see if the covariance shared by the predicting tests was related to the balance score relative to each test independently.
Results
EQ and Cognitive Measures:
The EQ scores varied across the SOT conditions (F(5,385)=375, p<.0001) and between the two Age groups (F(1,77)=6.4, p=.01). (Table 1) Specific contrasts showed Age differences within SOT conditions for SOT1, SOT2, SOT3 and SOT6. (p<.05). Table 2 presents the cognitive measures for young and older subjects. All variables were significantly different between the age groups at p<.0001, except for visuospatial construction which was significantly different at p=.02 (F(1,77)=6.2).
Table 1:
Equilibrium Scores for each Sensory Organization Test (SOT) condition (described in Figure 1) for young and older adults. (error=1 s.d.) *=significant differences between age groups (p<.05)
| SOT Condition | Age Group | |
|---|---|---|
| Young | Older | |
| SOT 1* | 94(2) | 91(3) |
| SOT 2* | 91(3) | 89(3) |
| SOT 3* | 91(4) | 88(4) |
| SOT 4* | 77(11) | 75(4) |
| SOT 5 | 62(11) | 60(11) |
| SOT 6* | 68(11) | 62(10) |
| Composite | 77(7) | 72(8) |
Table 2:
Cognitive test scores (mean (s.d.)) for young and older subjects. All differences between young and older were significant (p<.02).
| Cognitive Measure | Young | Older |
|---|---|---|
| Perceptual Inhibition (ms) | 77(30) | 128(65) |
| Motor Inhibition (ms) | 35(26) | 118(122) |
| Motor Speed (taps) | 56(10) | 47(7) |
| Visual Memory (# correct) | 50(4) | 44(7) |
| Stroop RT (ms) | 608(95) | 808(122) |
| Task Switching (# correct) | 58(8) | 44(11) |
| Digit Symbol Substitution (ms) | 119(22) | 161(42) |
| Choice Reaction Time (ms) | 302(28) | 410(62) |
| Visuospatial Construct (standard score) | 104(15) | 97(13) |
EQ Scores – Cognition Correlations:
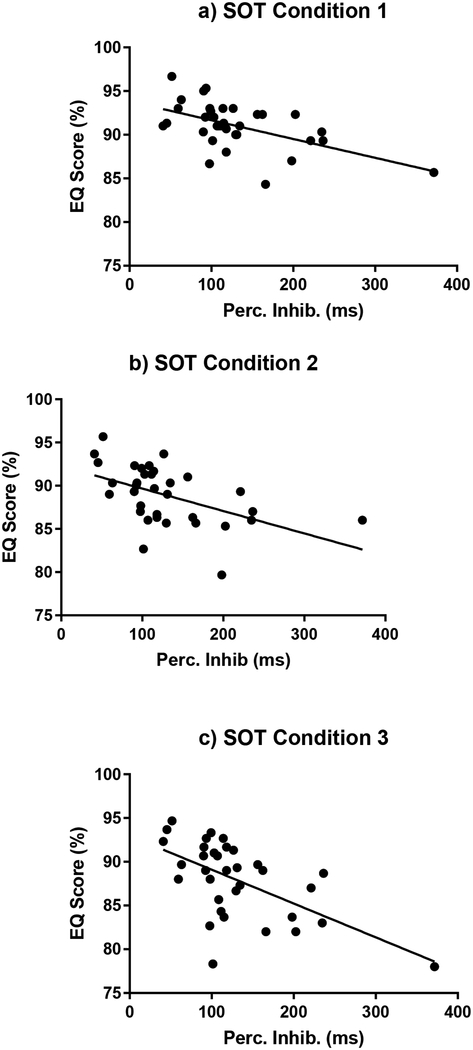
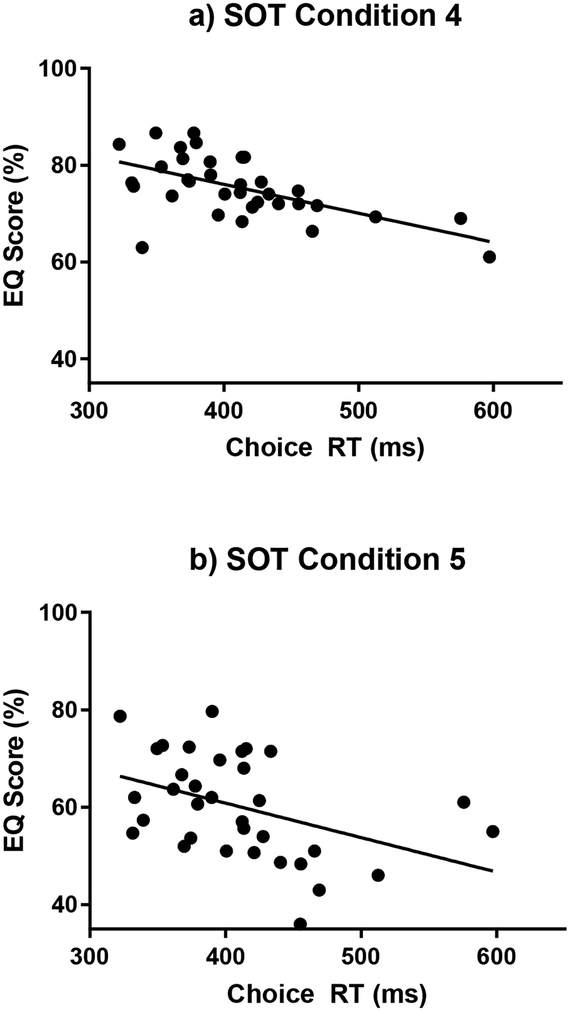
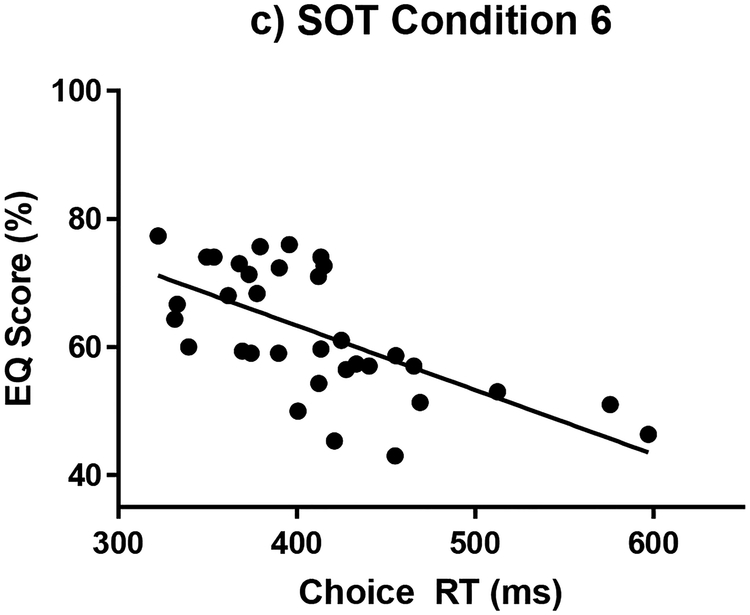
There were no significant correlations between the EQ scores and the cognitive variables for the young subjects. Therefore, all subsequent correlative analyses are focused on the older adults. There were significant correlations for the older subjects between the EQ scores and four cognitive measures within the SOT conditions. (Table 3) Perceptual inhibition was negatively associated with EQ scores for the conditions with a fixed platform (SOT 1, SOT 2, SOT 3), with the strongest correlation during the SOT 3 condition. (Figure 2) Increasing perceptual inhibition score (i.e. worsening inhibitory performance) was associated with decreasing EQ scores (i.e. poorer balance). Choice RT was negatively correlated with EQ score for the conditions with a sway referenced platform (SOT 4, SOT 5, SOT 6). (Figure 3) Slower choice RTs were associated with decreasing EQ scores. Visuospatial construction was correlated with EQ score on condition SOT 5 where the platform is sway-referenced and eyes are closed. Poorer visuospatial performance was associated with decreasing EQ score. Task Switching was correlated with EQ score for SOT 1, with poorer performance associated with decreased EQ score. The EQ Composite Score was correlated with choice RT and perceptual inhibition. When the Bonferroni-Holm correction for multiple comparisons criteria was applied, only the perceptual inhibition correlations with EQ scores for SOT 1, 2 and 3 and the choice RT correlations for SOT 4 and 5 were found to be statistically significant.
Table 3:
Significant (α=.01) correlations in bold (r, p-value) between the equilibrium scores and cognitive measures during the SOT conditions for older subjects. For non-significant correlations, * included when p<.05.
| Task Switching | Visuospatial Construct | Choice RT | Perceptual Interference | |
|---|---|---|---|---|
| SOT 1 |
r=.47 P=.006 |
r=.11 | r=.36* |
r=−.54 p=.001 |
| SOT 2 | r=.33 | r=.22 | r=−.23 |
r=−.51 p=.002 |
| SOT 3 | r=.14 | r=.31 | .40* |
r=−.59 p=.0002 |
| SOT 4 | r=.29 | r=.05 |
r=−.58 p=.0003 |
r=.05 |
| SOT 5 | r=.12 |
r=.47 p=.005 |
r=−.42 p=.01 |
r=−.21 |
| SOT 6 | r=.26 | r=.34 |
r=−.63 p<.0001 |
r=−.39* |
| EQ Composite | r=.05 | r=.33 |
r=−.56 p=.0005 |
r=−.54 p=.001 |
Figure 2:
Perceptual Inhibition measure versus EQ scores for older adults during the fixed platform conditions with: a. Eyes open (SOT 1), b. Eyes closed (SOT 2), and c. sway referenced vision (SOT 3)
Figure 3:
Choice Reaction Time measure versus EQ scores for older adults during the sway referenced platform conditions with: a. Eyes open (SOT 4), b. Eyes closed (SOT 5), and c. sway referenced vision (SOT 6)
Partial correlation analyses were conducted for two SOT conditions where two cognitive tests were related to the balance score. For SOT 1, task switching and perceptual inhibition were both correlated with the EQ score. The partial correlation for EQ score and perceptual inhibition was significant when task switching was partialed out (rpartial=−0.44, p=0.01). The partial correlation for EQ score and task switching when perceptual inhibition was partialed out was rpartial=0.33, p=.06. For SOT 5, EQ Score and visuospatial construction were significantly correlated when choice RT was partialed out (rpartial=0.41, p=.02). EQ score and choice RT were correlated when visuospatial construction was partialed out (rpartial=0.−35, p=.05). The partial correlations calculated for the SOT Composite scores were not significant: Choice RT (rpartial=−0.35, p=.05) and Perceptual Inhibition (rpartial=−0.29, p=.11). Overall, whenever more than one cognitive measure related to a balance measure, both measures tended to independently contribute to the prediction.
Discussion
In older adults we found that cognitive measures related to decision processing speed, interference control (perceptual motor and task interference) and visuospatial abilities were correlated with postural sway. It is interesting, but not surprising, that relationships were observed with measures related to the two primary realms of cognition associated with aging, processing speed and executive function. Our results focus on specific processes within these realms rather than being relevant to whether one type of cognition is more impacted by age than the other form of cognition ( e.g., Albinet et al. 2012). Different processes were related to sway depending upon the sensory integration required. Specifically, poorer perceptual inhibition was associated with greater sway (decreased EQ score) for conditions where the platform was fixed (SOT 1, 2 and 3); task level inhibition (task switching) was similarly related, but only for SOT1 in which both vision and somatosensation were present. Slower choice RT was associated with greater sway when the platform was sway referenced (SOT 4, 5, and 6). Visuospatial construction was associated with sway when the eyes were closed on a sway referenced platform (SOT 5).
The measure of perceptual inhibition in older adults was correlated with sway when somatosensory input was correct; a relationship that was even more marked when visual conflict was present (SOT 3). This result suggests that inhibition may be involved in integrating visual information when somtosensation is the primary available sensory input. Given that older adults become more visually dependent for balance with age (Borger et al. 1999; Lord et al. 1991), the ability of integrating visual information even when somatosensation is available for balance is of increasing importance. Interestingly, balance with both somatosensation and vision available (SOT 1) was related to task level inhibition, which refers to the inhibition of the prior stimulus-response mappings of an immediately preceding task (task switching). The partial correlation analysis suggested that there are contributions of both perceptual and task inhibition, with perceptual inhibition being more significant. Conceivably, the integration of intact vision and somatosensory information requires coordinating sensory and motor aspects of perceptual-motor associations to maintain balance.
The perceptual inhibition results reported here appear to contradict an earlier study that showed perceptual inhibition was involved when both somatosensory and visual sensory conflict occur (Redfern et al. 2009). However, the measure used in this previous study differed from the one used here. In the previous study (Redfern et al. 2009), perceptual inhibition was defined as the difference between the perceptual incongruent RTs and the perceptual congruent RTs. This measure confounds speeding due to congruency with the perceptual inhibition related to incongruency. Aging’s influences on cognitive processing speed and executive inhibition are not mutually exclusive, and so controlling for speed is important in examining the influence of inhibition (Albinet et al. 2012). In the current study, perceptual inhibition is calculated without this confound using the difference between the perceptual incongruent RTs and the baseline RTs in an attempt to reduce any impact of decision processing speed on the measures of inhibition. Thus, the current measure explored the association between perceptual inhibition and sway with reduced confound with decision processing speed.
The relationship between the choice RT measure and postural sway during sway-referenced platform conditions suggests that decision processing speed plays an important role in sensory integration when somatosensory is not reliable. Interestingly, motor speed was not correlated with sway so the motor component does not appear to be related; rather, decision processing is more strongly associated with postural control under these somatosensory conflict conditions. The finding is consistent with reports that decreased decision processing speed is associated with falls, both in healthy older adults and those with mild cognitive impairment (Anstey et al. 2009; Holtzer et al. 2007; Mirelman et al. 2012; Pijnappels et al. 2010; Taylor et al. 2014; Woolley et al. 1997) and with balance (Saverino et al. 2016). The cognitive processing speed may be involved in maintaining sensory reweighting when somatosensory input is erroneous. One caution relative to this interpretation is that maintaining balance on a sway referenced platform is generally more difficult that on a stable floor. So, an alternative explanation is that balance task difficulty could be associated with processing speed and not necessarily the sensory integration mechanism specifically.
Overall, the results suggest that sensory reweighting for balance control requires higher cognitive processing, and that processing is altered by aging. Decision processing speed impacts the up-weighting of visual inputs and/or the down-weighting of incorrect somatosensory inputs. However, when visual information is erroneous and must be inhibited, such as during the SOT 3 condition, perceptual inhibition is necessary. The relationship between poorer visuospatial construction scores and increase sway during SOT 5 condition suggests that visuospatial abilities may be required when vision and somatosensation are removed. These visuospatial abilities contribute independently from the decision speed ability which also is related to SOT 5 performance. One interpretation is that visuospatial ability is involved when balance is primarily reliant on vestibular inputs. If so, visuospatial abilities are involved in interpreting vestibular motion sensory inputs for spatial awareness and postural control.
Some cognitive measures were not correlated with sway scores. For example, the Stroop Interference measure was not correlated with sway under any conditions. This is somewhat surprising, as the Stroop measure of inhibition has been associated with falls (Saverino et al. 2016), gait variability (Hausdorff et al. 2005), and balance (Liu-Ambrose et al. 2007). A possible explanation is that the Stroop measure is less spatially challenging as it uses incongruity in words and colors, as opposed to spatial locations used in the perceptual inhibition test. So, while both measure inhibitory function, perceptual inhibition may be more sensitive at subclinical levels. Other cognitive measures did not meet the criterion of α<.01 used in this analysis, and so no conclusion could be made. For example, visual memory was close to significance during both SOT 3 and SOT 6 (i.e. when visual sway-referencing occurred) (p=.02). Thus, there may be a relationship between visual memory and inhibition of erroneous visual information, but the statistical power of this experiment limited any conclusions to be made.
No significant relationships between cognitive measures and balance were observed for the young subjects. One explanation is that there are sufficient cognitive resources in these domains for sensory integration in younger adults. The significant difference between young and older subjects on the measures is consistent with this explanation. Hence, our hypothesis is that there is a reduction of cognitive capacity in decision processing speed and inhibition that influences the sensory integration system in older adults.
The current observations are limited to some degree by the relatively small sample size. Hence, one future direction is to focus on specific cognitive dimensions found in this study (perceptual and task inhibition, decision processing speed, and visuospatial ability) in a larger cohort of older adults. Such a study should expand the measures for each of the three aspects, but most particularly of visuospatial ability. A second future direction is to focus on older individuals with either reduced cognitive function or with balance disorders. Such work might show that additional or different processes are important when either cognition or balance is impaired. Assessing specific relationships with falls occurring in different situations would also be of great value.
In summary, our results suggest that different aspects of cognition are engaged in the sensory integration process for balance control for older adults depending upon the sensory environment. Decision processing speed is influential when somatosensation is unreliable. Perceptual inhibition appears to be engaged when somatosensation is reliable and visual information needs to be resolved, particularly when that visual input is absent or erroneous. Interestingly, visuospatial construction appears to be involved when vision is absent and somatosensation is unreliable, suggesting the ability to mentally manipulate spatial information is important when vestibular information becomes the primary reliable input. The results highlight the influence of cognitive functioning in balance control and a potential pathway for the impact of aging on postural control.
Acknowledgements:
Susan Strelinski and Anita Lieb are much appreciated for helping to conduct this research.
Funding source: This work was funded through the National Institutes of Health (R01 AG14116), and the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827).
Footnotes
Conflict of interest statement: The authors have no financial or personal relationships with other people or organizations that could inappropriately influence or bias their work.
References
- Albinet CT, Boucard G, Bouquet CA, Audiffren M (2012) Processing speed and executive functions in cognitive aging: how to disentangle their mutual relationship? Brain Cogn 79:1–11 doi: 10.1016/j.bandc.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Luszcz MA (2006) An 8-year prospective study of the relationship between cognitive performance and falling in very old adults Journal of the American Geriatrics Society 54:1169–1176 doi: 10.1111/j.1532-5415.2006.00813.x [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR (2009) Different cognitive profiles for single compared with recurrent fallers without dementia Neuropsychology 23:500–508 doi: 10.1037/a0015389 [DOI] [PubMed] [Google Scholar]
- Bigelow RT, Agrawal Y (2015) Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory Journal of vestibular research : equilibrium & orientation 25:73–89 doi: 10.3233/VES-150544 [DOI] [PubMed] [Google Scholar]
- Black FO (2001) What can posturography tell us about vestibular function? Ann N Y Acad Sci 942:446–464 [DOI] [PubMed] [Google Scholar]
- Borger LL, Whitney SL, Redfern MS, Furman JM (1999) The influence of dynamic visual environments on postural sway in the elderly Journal of vestibular research : equilibrium & orientation 9:197–205 [PubMed] [Google Scholar]
- Cham R, Perera S, Studenski SA, Bohnen NI (2007) Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults Gait & posture 26:516–525 doi: 10.1016/j.gaitpost.2006.11.204 [DOI] [PubMed] [Google Scholar]
- Cohen H, Heaton LG, Congdon SL, Jenkins HA (1996) Changes in sensory organization test scores with age Age Ageing 25:39–44 [DOI] [PubMed] [Google Scholar]
- Diamond A (2013) Executive functions Annu Rev Psychol 64:135–168 doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman SI, Redfern MS, Jennings JR, Furman JM (2015) Interference between postural control and spatial vs. non-spatial auditory reaction time tasks in older adults Journal of vestibular research : equilibrium & orientation 25:47–55 doi: 10.3233/ves-150546 [DOI] [PubMed] [Google Scholar]
- Furman JM, Cass SP (1996) Laboratory testing In: Baloh RW, Halmagyi M (eds) Electronystagmography and rotational testing. Disorders of the vestibular system. Oxford Press, New York, NY, [Google Scholar]
- Gualtieri CT, Johnson LG (2006) Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs Arch Clin Neuropsychol 21:623–643 doi: 10.1016/j.acn.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N (2005) Walking is more like catching than tapping: gait in the elderly as a complex cognitive task Experimental brain research 164:541–548 doi: 10.1007/s00221-005-2280-3 [DOI] [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM (2010) Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling J Gerontol A Biol Sci Med Sci 65:1086–1092 doi: 10.1093/gerona/glq077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holewski JJ, Stess RM, Graf PM, Grunfeld C (1988) Aesthesiometry: quantification of cutaneous pressure sensation in diabetic peripheral neuropathy J Rehabil Res Dev 25:1–10 [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J (2007) The relationship between specific cognitive functions and falls in aging Neuropsychology 21:540–548 doi: 10.1037/0894-4105.21.5.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM (1996) Postural orientation and equilibrium In: Rowell L, Shepard NP (eds) Handbook of physiology. Oxford University Press, New York, pp 255–292 [Google Scholar]
- Horak FB, Shupert CL, Mirka A (1989) Components of postural dyscontrol in the elderly: a review Neurobiol Aging 10:727–738 [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Kumar S, Yoon EY, Wulff M, Roberts KL, Riddoch MJ (2013) Attending to the possibilities of action Philos Trans R Soc Lond B Biol Sci 368:20130059 doi: 10.1098/rstb.2013.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Mendelson DN, Redfern MS, Nebes RD (2011) Detecting age differences in resistance to perceptual and motor interference Exp Aging Res 37:179–197 doi: 10.1080/0361073x.2011.554512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T (2013) The relationship between executive function and falls and gait abnormalities in older adults: a systematic review Dement Geriatr Cogn Disord 36:20–35 doi: 10.1159/000350031 [DOI] [PubMed] [Google Scholar]
- Klencklen G, Despres O, Dufour A (2012) What do we know about aging and spatial cognition? Reviews and perspectives Ageing Res Rev 11:123–135 doi: 10.1016/j.arr.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Liu-Ambrose T, Pang MY, Eng JJ (2007) Executive function is independently associated with performances of balance and mobility in community-dwelling older adults after mild stroke: implications for falls prevention Cerebrovasc Dis 23:203–210 doi: 10.1159/000097642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW (1991) Visual acuity and contrast sensitivity in relation to falls in an elderly population Age Ageing 20:175–181 [DOI] [PubMed] [Google Scholar]
- Martina IS, van Koningsveld R, Schmitz PI, van der Meche FG, van Doorn PA (1998) Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. European Inflammatory Neuropathy Cause and Treatment (INCAT) group Journal of neurology, neurosurgery, and psychiatry 65:743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson DN, Redfern MS, Nebes RD, Richard Jennings J (2010) Inhibitory processes relate differently to balance/reaction time dual tasks in young and older adults Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 17:1–18 doi: 10.1080/13825580902914040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A et al. (2012) Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition Plos One 7:e40297 doi: 10.1371/journal.pone.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Black FO, Wall C 3rd (1982) Adaptation to altered support and visual conditions during stance: patients with vestibular deficits J Neurosci 2:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassauer KW, Halperin JM (2003) Dissociation of perceptual and motor inhibition processes through the use of novel computerized conflict tasks J Int Neuropsychol Soc 9:25–30 [DOI] [PubMed] [Google Scholar]
- NeuroCom International IC (2004) Instructions for use: EquiTest system operator’s manual. Version 8.2. 2018
- Pijnappels M, Delbaere K, Sturnieks DL, Lord SR (2010) The association between choice stepping reaction time and falls in older adults--a path analysis model Age Ageing 39:99–104 doi: 10.1093/ageing/afp200 [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN (1998) The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity J Clin Exp Neuropsychol 20:310–319 doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM (2001) Attention influences sensory integration for postural control in older adults Gait & posture 14:211–216 [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Mendelson D, Nebes RD (2009) Perceptual inhibition is associated with sensory integration in standing postural control among older adults J Gerontol B Psychol Sci Soc Sci 64:569–576 doi: 10.1093/geronb/gbp060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (1996) The processing-speed theory of adult age differences in cognition Psychol Rev 103:403–428 [DOI] [PubMed] [Google Scholar]
- Saverino A, Waller D, Rantell K, Parry R, Moriarty A, Playford ED (2016) The Role of Cognitive Factors in Predicting Balance and Fall Risk in a Neuro-Rehabilitation Setting Plos One 11:e0153469 doi: 10.1371/journal.pone.0153469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME, Delbaere K, Lord SR, Mikolaizak AS, Brodaty H, Close JC (2014) Neuropsychological, physical, and functional mobility measures associated with falls in cognitively impaired older adults J Gerontol A Biol Sci Med Sci 69:987–995 doi: 10.1093/gerona/glt166 [DOI] [PubMed] [Google Scholar]
- Taylor ME, Lord SR, Delbaere K, Kurrle SE, Mikolaizak AS, Close JC (2017) Reaction Time and Postural Sway Modify the Effect of Executive Function on Risk of Falls in Older People with Mild to Moderate Cognitive Impairment The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 25:397–406 doi: 10.1016/j.jagp.2016.10.010 [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A, Nashner LM (1986) Aging and posture control: changes in sensory organization and muscular coordination Int J Aging Hum Dev 23:97–114 doi: 10.2190/VXN3-N3RT-54JB-X16X [DOI] [PubMed] [Google Scholar]
- Woolley SM, Czaja SJ, Drury CG (1997) An assessment of falls in elderly men and women J Gerontol A Biol Sci Med Sci 52:M80–87 [DOI] [PubMed] [Google Scholar]