Abstract
The aim of the current study was to evaluate and compare the toxicities of different types of mosquito repellents i.e. coils, mats and liquid vapors in animal models. Different types of mosquito repellents including liquid vaporizers, coils and mats have been extensively used by the people to get protection from the mosquitoes and diseases associated with them. The active constituents of these repellents include; allethrins, pyrethrins, paraffin and various other derivatives, are well known for their toxicities. Exposure of albino mice to these repellents for 3 h per day over a period of 20 days produced significant toxicological effects on vital body organs including; liver, lungs, kidneys, brain and heart. The order of toxicity of different repellents on nervous and hepatic tissues was found to be: Coil > Liquid > Mat while in renal and cardiac tissues, the coil was again found to be the most toxic one, mat with medium toxicity whereas liquid as least toxic (Coil > Mat > liquid). Lungs tissues are almost equally affected by all the repellants. On the basis of current findings, it has been concluded that exposure to various types of mosquito repellents can be deleterious to health and can cause various health related issues by producing pathological changes in the vital organs.
Keywords: Allethrin, Mosquito repellents, Subchronic toxicity, Vital organs, Mice
1. Introduction
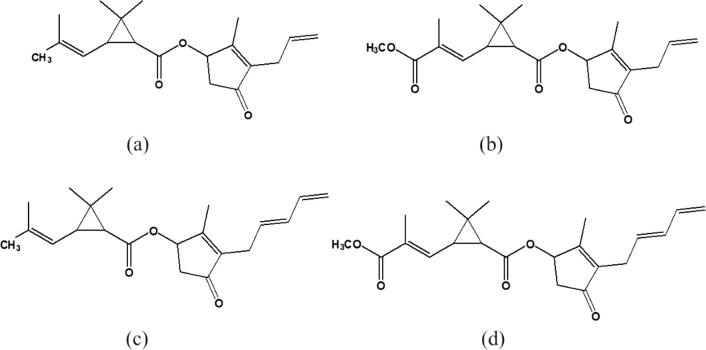
In various countries, mosquitoes are known as the main vector of transmitting various diseases such as filariasis, malaria, dengue fever, nile virus and yellow fever, etc. In order to control mosquito population especially in residential areas, people adopt various ways including use of mosquito repellants in different forms. Most commonly used residential repellents are available in the form of sprays, coils, mats and vaporizers (Soderlund and Bloomquist, 1989). Mosquito coils and mats are preferred to use as repellent in most of the populations because of their economical price. Major active ingredients of these coils/mats are pyrethrins/pyrethroids which account for 3-4% of coil mass (John and John, 2015). The insecticidal potential of pyrethrins was discovered a century before when scientists isolated this chemical from Chrysanthemum plants, for the use of insecticidal purposes around the year 1800 in Asia. Many species of the plant Chrysanthemum (family Asteraceae) such as C. cinerariaefolium and C. coccineum contain pyrethrins in their flowers. Pesticides as synthetic (Pyrethroids) or in natural form (pyrethrins), are used as insecticides worldwide and are the most shared content of mosquito coil, mats, and liquidators (Macan et al., 2006). Allethrins are another group of related synthetic pyrethroids, found in the flowers part of Chrysanthemum and used as insecticides. Chemical structures of allethrin and pyrethrins are shown in Fig. 1.
Fig. 1.
Chemical structures of chief constituents present in the mosquito repellants (a) Allethrin I, (b) Allethrin II, (c) Pyrethrin I and (d) Pyrethrin II.
These coils also contain small particles (<1 μm) metal fumes, vapors and free radicals, which upon reaching to alveolar regions of the lungs causes irritation in upper respiratory tract (Chang and Lin, 1998). The other constituents of mosquito coils are dyes and binders organic fillers; ignition of these compounds produces large amount of sub-micrometer particles which upon reaching to the lower respiratory tract can cause carcinogenesis (John and John, 2015). It is found that gas phase of these coil smoke contains carbonyl compounds including acetaldehyde and formaldehyde which can produce strong irritant effect in the upper respiratory system (Koo and Ho, 1994, Chang and Lin, 1998). The main target site of pyrethroids is sodium channel, they remain open for long time, results in increasing the influx of Na+ ions and hence, causes the hyper excitation of the nervous system that finally results in the insect paralysis (Casida, 1980, Soderlund and Bloomquist, 1989, Chang and Lin, 1998, Macan et al., 2006). In various rodent models, pyrethroids decreases the antioxidant levels and produces oxidative stress (El-Demerdash, 2011, Mossa et al., 2013). Synthetic pyrethroids e.g. allethrin can cross the blood brain barrier (BBB) and hence, can delay the maturity of BBB and also produces some biochemical changes associated with the health risk especially in the early years of life (Gupta et al., 1999). Some researchers found that the long term exposure to coil can produce wheezing and asthma in youngsters along with that it produces metaplasia in the tracheal epithelium of rodents (Liu and Sun, 1988, Liu and Wong, 1987). Long term use of these coils can also alter the biochemical parameters of blood (Idowu et al., 2013, Narendra et al., 2007). Some studies have reported that use of pyrethrin based coils can considerably increase the leukocytes count, especially basophils and lymphocytes (Garba et al., 2007). Some cellular, tissues and organ injuries are also associated with the use of this toxic substance (Moya-Quiles et al., 1995). Mosquito coil smoke can cause chromosomal changes in bone marrow and pulmonary alveolar macrophages (Das et al., 1994, Moorthy and Murthy, 1994). Recently, a study has shown that mosquito coil is associated with the increased reactive oxygen species (ROS) production as well as over expression of the gene p53 (Madhubabu and Yenugu, 2012). DNA damage, mediated by oxidative stress causes an up-regulation of p53 which is considered to be an early stage of mitochondrial mediated cellullar apoptosis (Madhubabu and Yenugu, 2012).
Despite of multiple scientific reports showing the toxicities of different individual mosquito preparations, still there is no comparative study has been conducted which can show the order of toxicities of coils, mats and liquid repellents on vital organs in rodents.
2. Materials and Methods
2.1. Chemicals and reagents
Commonly used mosquito repellents including coils, mats and liquid were purchased from the open market of Gujranwala, Pakistan. Biochemical parameters in serum were analyzed by using commercially available kits.
2.2. Animals
The experiments were carried out with Swiss albino mice weighing 30–35 g obtained from the Lab ACU (Animal Care Unit), College of Pharmacy, Prince Sattam Bin Abdulaziz University (PSAU), Al-Kharj, Saudi Arabia. Research on experimental animals was conducted in accordance with the internationally accepted principles for laboratory animal use and care. Mice were kept in polypropylene cages (22.5 × 37.5 cm) and were maintained under standard housing conditions (room temperature, 22 ± 4 °C and humidity, 60–65%) with a 12-h light and dark cycle. They were allowed free access to standard pellet diet and water, ad libitium.
2.3. Experimental design
For the evaluation of the subchronic toxicity of the coils, mats and liquids on the experimental animals by direct inhalation treatment, the mice were randomly divided into four groups each consisted of six animals (n = 6).
Group I (Normal control): Animals were kept in the cages of similar ventilation without any type of repellent for the period of 20 days.
Group II (Coil smokes): Animals have been exposed to coil smokes for 3 h per day over a period of 20 days via whole body inhalation.
Group III (Mat vapors): Animals have been exposed to mat vapors for 3 h per day over a period of 20 days via whole body inhalation.
Group IV (Liquid vapors): Animals has been exposed to liquid vapors for 3 h per day over a period of 20 days via whole body inhalation.
After 24 h of the last exposure to mosquito repellants, all mice were assessed for physical appearance and motor activity followed by withdrawal of blood from retro orbital plexus under light ether anesthesia for the biochemical estimations in serum. After blood collection all the mice were sacrificed by cervical dislocation and vital organs (Liver, Lungs, Kidney, Brain and Heart) were isolated for histopatholgical studies.
2.4. Physical appearance and motor activity
An overall assessment of the health and welfare of a research mouse includes an evaluation of the animal in its home cage. Observing the mouse in its home cage provided information about the animal’s overall appearance and activity level, the interaction with the environment, including nest building, and its behavior with respect to its cage mates.
2.5. Biochemical estimation in serum
For the biochemical assessment, the blood samples were collected from the retro-orbital plexus under light ether anesthesia. Serum was separated by the centrifugation (3000 rpm × 10 min, 4EC) process, and transferred to pre-labeled eppendorf tubes for assessment of various biochemical parameters. The estimation of ALT and AST was determined by colorimetric method, as described previously by Reitman and Frankel (1957). Blood urea nitrogen (BUN) level was estimated by enzymatic colorimetric method (Fawcett and Scott, 1960). Serum total protein was measured by Biuret method, originally described by Josephson and Gyllensward (1957). Total cholesterol was estimated by enzymatic-(hydrolysis and oxidation) colorimetric reaction (CHOD-PAP-method), described originally by Allain et al. (1974). The levels of TG and LDL were determined by enzymatic colorimetric reaction (GPA-PAP Method) as reported earlier (Bucolo and David, 1973, Okada et al., 1998).
2.6. Histopathological studies
The lungs, brain, kidneys, heart, and liver were isolated and preserved in 10% formalin solution (Giri et al., 2004). The tissues were cleared with xylene and impregnated with paraffin wax and cut in to thin sections (∼5 µ) followed by stained with haematoxylin and eosin. These were mounted on slide for light microscopic examination.
2.7. Statistical analysis
Statistical analysis of the results was done with one-way ANOVA followed by Dunnett's test using GraphPad Prism 7 Software (GraphPad Software Inc., San Diego, USA). The data were expressed as the mean ± SD (n = 6). The significance was set at *P < 0.05 compared to control group.
3. Results
3.1. Physical appearance and motor activity
Some animals exposed to the fumes of mosquito coil experienced a change in the fur condition; it became pale and rough as compare to the other test and control groups.
No significant variation in motor activity has been observed in the test groups as compare to the control group. Some of the animals exposed to coil showed some symptoms of lethargy.
3.2. Biochemical alterations of blood parameters
Toxicological effects of coil smokes, mat and liquid vapors on different biochemical parameters of blood are presented in Table 1. The blood profiles, representing liver function, show significant changes in coil smoke inhaled group (Group I) in comparison with the control. Data indicates that the activity of two key hepatic enzymes i.e. ALT and AST were increased significantly (at P < 0.05) by 58 and 59%, respectively in mice exposed to coil smokes. In the same group, the activity of BUN and total protein was decreased by 17 and 13% respectively. In the lipid profile test, levels of cholesterol, LDL and TG were observed higher in the treated mice with resultant increase of 30, 35 and 17% as compared to control, respectively.
Table 1.
Effect of mosquito repellants (coil, mat and liquid smokes) on biochemical parameters.
| Parameters | Control | Direct inhalation treatment |
||
|---|---|---|---|---|
| Coils | Mats | Liquids | ||
| ALT (U/L) | 10.50 ± 1.34 | 16.63 ± 2.46* | 12.23 ± 0.95 | 14.36 ± 2.28* |
| AST (U/L) | 39.10 ± 3.43 | 62.46 ± 5.6* | 48.83 ± 4.38* | 56.40 ± 4.20* |
| Cholesterol (mg/dl) | 98.31 ± 5.13 | 127.2 ± 8.85* | 104.2 ± 5.67 | 115.4 ± 5.03* |
| TG (mg/dl) | 85.08 ± 2.83 | 99.23 ± 5.89* | 92.23 ± 5.20 | 95.82 ± 4.45* |
| LDL (mg/dl) | 65.06 ± 3.65 | 87.5 ± 6.68* | 68.45 ± 7.80 | 74.25 ± 4.8* |
| BUN (mg/dl) | 9.42 ± 0.72 | 7.85 ± 0.45* | 8.72 ± 0.73 | 8.42 ± 0.68 |
| Total protein (g/dl) | 5.50 ± 0.44 | 4.76 ± 0.21* | 5.15 ± 0.25 | 5.32 ± 0.25 |
Values are expressed as Mean ± SD (n = 6), significance was set at *P < 0.05 with respect to the control group.
The blood profiles, representing liver function in mats vapor inhaled group (Group II) indicates that the activity of ALT and AST were increased significantly (at P < 0.05) by 16% and 24%, respectively. On the other hand, the activity of BUN and total protein was decreased by 7% and 6% respectively whereas, increased levels of cholesterol, LDL and triglyceride were observed in the treated mice with mat vapour by 6%, 5% and 8% than the control, respectively (Table 1).
In the liquid inhaled group (Group III), levels of ALT and AST increased by 37% and 44%, respectively when compared with negative control (Table 1). The activity of BUN and total protein was decreased by 10% and 11% respectively while levels of cholesterol, LDL and triglyceride were observed increase by 17, 14 and 13%, respectively than the control.
Taken together, the biochemical parameters representing hepatotoxicity (ALT and AST) were found raised in the studied repellents in order of Coil > Liquid > Mat (Table 1).
3.3. Histopathological studies
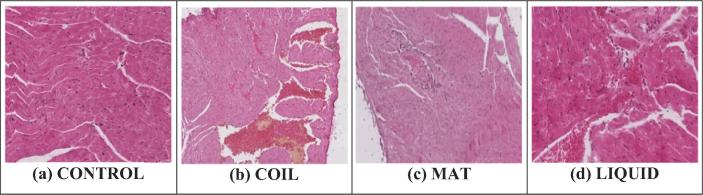
Photomicrograph of heart sections of normal control group showed clear integrity of myocardial cell membrane with no infiltration and edema whereas toxic control group mice exposed by different mosquito repellants showed significant degeneration, atrophy and hyperemia. In comparison to all mosquito repellants, mosquito coil produced highest toxicity in heart tissues (Fig. 2 & Table 2).
Fig. 2.
Histopathological photomicrograph of cardiac tissues showing microscopically changes on exposure with different mosquito repellants (H and E × 400).
Table 2.
Effect of mosquito repellants (coil, mat and liquid smokes) on histopathological studies of heart tissue.
| Groups | Degeneration | Atrophy | Hyperemia |
|---|---|---|---|
| Control | |||
| Liquid | ++ | ++ | + |
| Mat | ++ | ++ | ++ |
| Coil | +++ | + | ++++ |
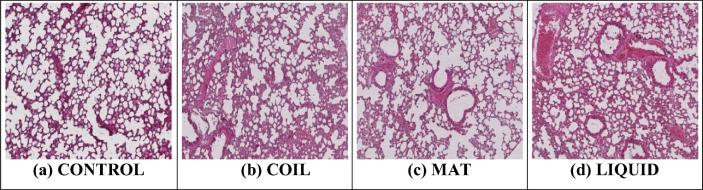
Lung sections of normal control group mice showed normal photomicrograph whereas toxic control mice with exposure of different mosquito repellents showed significant hyperemia, necrosis, and occlusion by hyaline material and infiltration of connective tissue by inflammatory cells. Mosquito mat produced more toxic effects in lung tissue as compared with other repellents (Fig. 3 & Table 3).
Fig. 3.
Histopathological photomicrograph of lung tissues showing microscopically changes on exposure with different mosquito repellants (H and E × 400).
Table 3.
Effect of mosquito repellants (coil, mat and liquid smokes) on histopathological studies of lung tissue.
| Groups | Hyperemia | Occlusion by hyaline | Infiltration of connective tissue by Inflammatory cells | Necrosis |
|---|---|---|---|---|
| Control | − | − | − | − |
| Liquid | +++ | ++ | +++ | − |
| Mat | +++ | +++ | ++ | ++ |
| Coil | +++ | ++ | +++ | ++ |
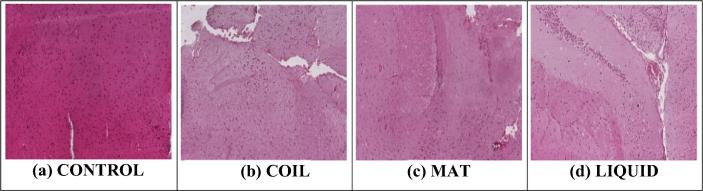
Nervous tissues of control group show a normal configuration with a compact structure, whereas the exposure of mosquito repellents has shown remarkable toxic effect on these tissues. The animals exposed to these repellents show a significant degeneration of nervous tissues, decrease in number of neurons along with decreased uptake of eosin material. The order of toxicity of mosquito repellents on nervous tissues was found to be: Coil > Liquid > Mat (Fig. 4 & Table 4).
Fig. 4.
Histopathological photomicrograph of brain tissues showing microscopically changes on exposure with different mosquito repellants (H and E × 400).
Table 4.
Effect of mosquito repellants (coil, mat and liquid smokes) on histopathological studies of brain tissue.
| Groups | Degeneration | Number of neuron decreased | Lack of eosin uptake |
|---|---|---|---|
| Control | − | − | − |
| Liquid | ++++ | ++ | ++++ |
| Mat | +++ | + | +++ |
| Coil | ++++ | +++ | ++++ |
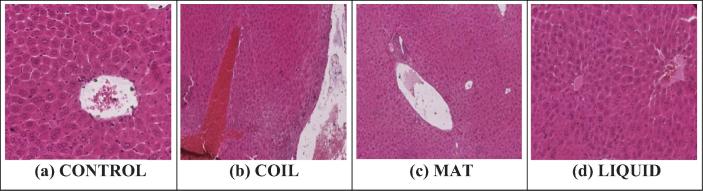
Hepatic tissues of mice exposed to different types of mosquito repellents showed various degrees of histological changes as compare to the control group. Microscopic structure of control liver showed a compact and regular structure with organized arrangement of parenchymal cells and central veins. Whereas, the tissues of animals exposed to mosquito repellents showed various degrees of tissue injuries including; hyperemia, occlusion, necrosis and aggregation of inflammatory cells at the site of tissue injury. Among different types of mosquito repellents mosquito coil produced more destruction of hepatic cells as compare to the others. The order of hepatotoxicity of different repellents was found to be: Coil > Liquid > Mat (Fig. 5 & Table 5).
Fig. 5.
Histopathological photomicrograph of liver tissues showing microscopically changes on exposure with different mosquito repellants (H and E × 400).
Table 5.
Effect of mosquito repellants (coil, mat and liquid smokes) on histopathological studies of liver tissue.
| Groups | Occlusion by hyaline | Hyperemia | Necrosis | Inflammatory cells |
|---|---|---|---|---|
| Control | − | − | − | − |
| Liquid | +++ | + | +++ | − |
| Mat | ++ | ++ | ++ | |
| Coil | +++ | ++++ | +++ | +/− |
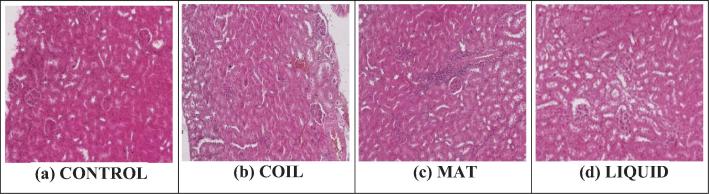
The histological structures of the control group showed a normal arrangement of renal pelvis and glomeruli. Animals exposed to various mosquito repellents over a period of 20 days show alteration in the histological features of renal cells as compare to the control group. The histological changings observed in the mosquito repellent exposed group include; degeneration of the tissues in renal pelvis, occlusion of renal tubules by hyaline and necrosis is in the glomeruli. The order of toxicity of these different repellents on renal cells was; Coil > Mat > liquid (Fig. 6 & Table 6).
Fig. 6.
Histopathological photomicrograph of kidney tissues showing microscopically changes on exposure with different mosquito repellants (H and E × 400).
Table 6.
Effect of mosquito repellants (coil, mat and liquid smokes) on histopathological studies of kidney tissue.
| Groups | Occlusion of tubules by hyaline | Degeneration | Necrosis | Hyperemia | Inflammatory cell aggregation |
|---|---|---|---|---|---|
| Control | |||||
| Liquid | + | ++ | + | ||
| Mat | ++ | + | +++ | ||
| Coil | ++ | +++ | ++ | ||
4. Discussion
Repeated exposure of Swiss albino mice to the various mosquito repellents produced significant changes in their physical appearance followed by biochemical and histological changes. Swiss albino mice exposed to mosquito repellants smoke for duration of 3 h a day, for 20 days presented with smoke-induced histopathological lesions, including degeneration, atrophy and hyperemia in cardiac tissues, hyperemia, necrosis, and infiltration of connective tissue by inflammatory cells in lung tissue, degeneration of nervous tissues, various degrees of hepatic and renal injury.
The toxicological effects of smoke from three locally purchased mosquito repellants on mice revealed significantly (P < 0.05) increase in the levels of AST, ALT, CHO, LDL and TG, whereas, BUN and total protein levels were significantly (P < 0.05) decreased in the serum. The increased levels of enzymes in serum indicated that the enzymes were released from the damaged tissues into the bloodstream. Thus, the study indicated that mosquito coil fumes do initiate gradual damage to the host. The increase in AST and ALT may indicate damage to the liver (Idowu et al., 2013). The findings also suggest that there may be health consequences in human in case of long duration exposure to all mosquito repellants fumes. These pathological effects must be confirmed and compared with more detailed studies in humans which will be immensely helpful when regulating their long-term and indoor usage for different parasites control programmes.
Volatile organic components and free radicals in the smoke and vapors of mosquito repellents are major contributors of tissue injury and DNA damage. They have a potential to cause carcinogenesis, necrosis, inflammation and apoptosis. Most of the mosquito repellents contain pyrethrins and allethrins in their composition as an insecticide (John and John, 2015). Although pyrethrum-based insecticides have relatively low-risk toxicity in mammals due to fast metabolism and almost no significant accumulation, but they can cause serious adverse health effects due to chronic exposure.
These repellents are mostly used in the night especially in Asian countries and the overnight combustion or release of vapors can cause general and systemic toxicities (Cheng et al., 1995). Overnight burning of mosquito coil releases carbon particles, aldehyde and heavy metals. Inhalation of these components produces cellular injury of lungs tissues along with the destruction of mucus membrane (Liu and Wong, 1987).
Existing knowledge is not adequate to support safe residential use of mosquito coils containing uncharacterized combustion products, and it is unfortunate that the environmental data and experimental studies have not been more prominent in risk management. Extensive research is indicated in reference to standard size of the room, ventilation, and number of people sleeping in the room pertaining to safety and efficacy of mosquito repellants use. The guidelines for safe use of mosquito coil with standard defined regulations need to be ear marked and popularized by educating the general masses. The companies, manufacturing these mosquito coils, mats and vapors should specify the ingredients used with scientific references regarding its safe use and duration of use of such coils.
Thus, educating masses for safe use, practices, and standardization of the different mosquito repellants products will help in minimizing the exposure risk and health ill effects.
5. Conclusion
Exposure of albino mice to different commercially available forms of mosquito repellents produced subchronic toxicological effects on the physical appearance and behavior, biochemical parameters and histopathology of the vital organs in these animals. The order of toxicity of studied repellents on nervous and hepatic tissues was found to be: Coil > Liquid > Mat while in renal and cardiac tissues, the coil was again found to be the most toxic one, mat with medium toxicity and liquid as least toxic (Coil > Mat > liquid) whereas lungs tissues were almost equally affected by all the repellants. On the basis of current findings, it has been concluded that exposure to various types of mosquito repellents can be deleterious to health and might cause various health related issues by producing pathological changes in the vital organs.
Acknowledgments
Acknowledgments
The authors are thankful to Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia for providing necessary facilities to carry out this research.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Allain C.C., Poon L.S. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- Casida J.E. Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. 1980;34:189–202. doi: 10.1289/ehp.8034189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.Y., Lin J.M. Aliphatic aldehydes and allethrin in mosquito coil smoke. Chemosphere. 1998;36:617–624. doi: 10.1016/s0045-6535(97)00357-3. [DOI] [PubMed] [Google Scholar]
- Cheng H., Yang W. Large-scale spraying of bednets to control mosquito vectors and malaria in Sichuan, China. Bull. World Health Organ. 1995;73(3):321–328. [PMC free article] [PubMed] [Google Scholar]
- Das R.K., Sahu K. Induction of chromosome aberrations and micronuclei in pulmonary alveolar macrophages of rats following inhalation of mosquito coil smoke. Mutat. Res. 1994;320:285–292. doi: 10.1016/0165-1218(94)90081-7. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F.M. Oxidative stress and hepatotoxicity induced by synthetic pyrethroids-organophosphate insecticides mixture in rat. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2011;29:145–158. doi: 10.1080/10590501.2011.577679. [DOI] [PubMed] [Google Scholar]
- Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba S.H., Shehu M.M. Toxicological effects of inhaled mosquito coil smoke on the rat spleen: a haematological and histological study. J. Med. Sci. 2007;7(1):94–99. [Google Scholar]
- Giri S.N., Al-Bayati M.A. Amelioration of doxorubicin-induced cardiac and renal toxicity by pirfenidone in rats. Cancer Chemother. Pharmacol. 2004;53:141–150. doi: 10.1007/s00280-003-0703-z. [DOI] [PubMed] [Google Scholar]
- Gupta, A., Nigam, D., et al., 1999. Effect of pyrethroid‐based liquid mosquito repellent inhalation on the blood–brain barrier function and oxidative damage in selected organs of developing rats. In: J. Applied Toxicol: An International Forum Devoted to Research and Methods Emphasizing Direct Clinical, Industrial and Environmental Applications 1999, Chichester, UK: John Wiley & Sons, Ltd., vol. 19, No. 1, pp. 67–72. [DOI] [PubMed]
- Idowu E.T., Aimufua O.J. Toxicological effects of prolonged and intense use of mosquito coil emission in rats and its implications on malaria control. Rev. Biol. Trop. 2013;61:1463–1473. [PubMed] [Google Scholar]
- John N.A., John J. Prolonged use of mosquito coil, mats, and liquidators: a review of its health implications. Int. J. Clin. Exp. Physiol. 2015;2:209–213. [Google Scholar]
- Josephson B., Gyllensward C. The development of the protein fractions and of cholesterol concentration in the serum of normal infants and children. Scand. J. Clin. Lab Invest. 1957;9:29–38. doi: 10.3109/00365515709088110. [DOI] [PubMed] [Google Scholar]
- Koo L.C., Ho J.H. Mosquito coil smoke and respiratory health among Hong Kong Chinese: results of three epidemiological studies. Indoor Built. Environ. 1994;3:304–310. [Google Scholar]
- Liu W.K., Sun S.E. Ultrastructural changes of tracheal epithelium and alveolar macrophages of rats exposed to mosquito-coil smoke. Toxicol. Lett. 1988;41:145–157. doi: 10.1016/0378-4274(88)90088-4. [DOI] [PubMed] [Google Scholar]
- Liu W.K., Wong M.H. Toxic effects of mosquito coil (a mosquito repellent) smoke on rats. Toxicol. Lett. 1987;39:223–239. doi: 10.1016/0378-4274(87)90237-2. [DOI] [PubMed] [Google Scholar]
- Macan J., Varnai V.M. Health effects of pyrethrins and pyrethroids. Arh. Hig. Rada. Toksikol. 2006;57:237–243. [PubMed] [Google Scholar]
- Madhubabu G., Yenugu S. Effect of continuous inhalation of allethrin-based mosquito coil smoke in the male reproductive tract of rats. Inhal. Toxicol. 2012;24(3):143–152. doi: 10.3109/08958378.2011.649189. [DOI] [PubMed] [Google Scholar]
- Moorthy M.V., Murthy P.B. Analysis of sister chromatid exchange, micronucleus and chromosomal aberration frequencies in rodents exposed to mosquito coil smoke by inhalation route. Toxicol. Lett. 1994;70:357–362. doi: 10.1016/0378-4274(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Mossa A.T., Refaie A.A. Amelioration of Prallethrininduced oxidative stress and hepatotoxicity in rat by the administration of Origanummajorana essential oil. BioMed. Res. Int. 2013:1–11. doi: 10.1155/2013/859085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Quiles M.R., Munoz-Delgado E. Effect of the pyrethroid insecticideallethrin on membrane fluidity. Biochem. Mol. Biol. Int. 1995;36:1299–1308. [PubMed] [Google Scholar]
- Narendra M., Bhatracharyulu N.C. Prallethrin induced biochemical changes in erythrocyte membrane and red cell osmotic haemolysis in human volunteers. Chemosphere. 2007;67:1065–1071. doi: 10.1016/j.chemosphere.2006.11.064. [DOI] [PubMed] [Google Scholar]
- Okada M., Matsui H. Low-density lipoprotein cholesterol can be chemically measured: a new superior method. J. Lab Clin. Med. 1998;132:195–201. doi: 10.1016/s0022-2143(98)90168-8. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Soderlund D.M., Bloomquist J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989;34(1):77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]