Graphical abstract
Keywords: Dental caries, Saliva, Streptococcus mutans
Abstract
Introduction
Dental caries is the most prevalent disease in humans and its incidence is particularly high during childhood. The use of medicinal plants is a common practice in Brazil.
Objective
To evaluate the optimal antimicrobial concentration of Copaifera langsdorffii (copaiba) oil-resin, in the form of dental varnish, against Streptococcus mutans (S. mutans) in children.
Methods
Twenty-four children, caries-free, aged until 6 years old, were selected to participate in this study. The varnish was applied to the occlusal surfaces of all deciduous molars. The antimicrobial activity was analyzed in saliva, whose collection was conducted in two phases: before applying the copaiba varnish and after use to verify the instantaneous effectiveness of Copaifera langsdorffii dental varnish in the reduction of S. mutans. The microbiological analysis was repeated twice, establishing dilutions of 1:10 mL and 1:100 mL.
Results
Comparisons between different times within the same dilution were carried out by repeated measures analysis of variance (ANOVA) associated with Tukey’s multiple comparisons test. Comparisons of conditions prior to and after treatment were performed using the t test for paired samples and it indicated that the 1% formulation promoted a more significant decrease in the number of S. mutans colonies (p = 0,0026).
Conclusion
Copaiba oil-resin, in the form of dental varnish, has antimicrobial activity against S. mutans in all the concentrations studied. Further studies to identify the long-term activity and anticaries effect of this varnish are required to establish its use in caries prevention.
1. Introduction
Dental caries is currently considered a dysbiosis involving interactions between tooth structure, microbial biofilm and sugar exposure, where 35% of cases are not treated worldwide. The early childhood caries (ECC) is characterized by the presence of caries in children up to 6 years of age (Adams et al., 2017, Pitts et al., 2017).
When exposed to a high-sugar diet, diners in the streptococci group metabolize carbohydrates and produce acids that will demineralize the tooth structure. Among the agents that will participate in this process, Streptococcus mutans (S. mutans) is the primary pathogen, whose mechanism of virulence has been the most studied and known when compared to the other species (Pitts et al., 2017, Xiang et al., 2019).
Although it’s not the etiological agent, an increase in the number of S. mutans can be considered a risk factor for the beginning of the lesion, which makes this group of bacteria, which are acidic and acidophilic, associated with the development of the carious lesion (Kaur et al., 2013, Eriksson et al., 2017).
There are physiological mechanisms in the mouth, such as saliva, which aid in remineralization, however when there is a very frequent consumption of sugar in the diet occurs the ecological imbalance of the oral microbiota. Then, the production of acids by acidogenic bacteria, such as S. mutans, is considered an important factor linked in the development of caries (Adams et al., 2017).
S. mutans begins colonization of the mouth early in life and is responsible for mature biofilm formation, resulting in early childhood caries (ECC). Habits such as unrestricted use of baby bottles and feeding (industrialized fruit juices, sweetened teas, fermented milk, milk with fermentable carbohydrates such as starches and sugar) while sleeping are strongly associated with the development of ECC (Ismail et al., 2008, Vasconcelos et al., 2008, Pitts et al., 2017).
In recent years, in the search for new substances with pharmacological and biocompatible potential, the number of studies on the use of natural products in dentistry and alternatives for the management of oral diseases, especially dental caries, has increased (Freires et al., 2015, Lobo et al., 2014).
According to Romero et al., the use of plants for medicinal purposes is a common practice in Brazil and has been passed from one generation to the next either for treatment or prevention of diseases. Medicinal plants have also been studied to evaluate their antimicrobial activity against S. mutans, as described in a study by Lobo et al. (2014).
Copaiba oil is widely used by the brazilian population in the treatment of microbial and inflammatory diseases and is commonly found in public markets, herb markets, and natural product stores. The copaiba tree belongs to the Leguminosae family, Caesalpinioideae subfamily, and Copaifera genus, and is commonly found in Latin America and West Africa. Species of this genus are popularly known as copaiba. This tree is found mainly in the southeast, midwest, and the Amazon regions. Copaifera comprises 72 species of which over 20 are found in Brazil. Chemically, the oil-resin of copaiba is a solution of diterpene acids in an essential oil containing sesquiterpenes, which are categorized into oxygenated and hydrocarbonated components (Paiva et al., 2002, Pieri et al., 2009, Romero et al., 2009).
Several species show demonstrated antimicrobial activity, especially C. multijuga, C. reticulata, C. langsdorffii, C. oblongifolia and C. officinalis (Diefenbach et al., 2017). Copaifera langsdorffii (C. langsdorffii), a species chosen in this study, is native to the Amazon rainforest and common in the Mato Grosso region, where according to a survey it’s the species of greatest importance and use by the population in the region of its georeferencing (Bieski et al., 2015).
Paiva et al. (2002) reported antimicrobial, anti-inflammatory, and wound-healing activities in the diterpenoid fraction of copaiba oil-resin. Further, an in vitro study has investigated the antibacterial activity of a dental cement made with the copaiba oil resin and demonstrated the effectiveness of copaiba oil against S. mutans (Pieri et al., 2009).
Thus, the aim of this study was to evaluate, through microbiological testing, the optimal concentration of the copaiba oil-resin in the varnish form against S. mutans present in the saliva in vivo.
2. Materials and methods
2.1. Extraction and chemical analysis of the oil-resin of copaiba
Samples of copaiba oil-resin, obtained from plant material of Copaifera langsdorffii Desf. (Fabaceae: Caesalpinioideae) deposited in the herbarium of Federal University of Mato Grosso-voucher Silva, R. R. et al. 1749, were received from the Federal University of Mato Grosso and originally obtained from Juruena Valle (Region: Midwest, Latitude: 10°19′05″ S, Longitude: 58°21′32″ W, Height: 300 m). Chemical constituents were identified by specialists at the Department of Chemistry, in the Federal University of Ceara (GC–MS, Shimadzu, model QP 5050, Japan). The main components of the Copaifera langsdorffii oil-resin used in the present study were: β-caryophyllene, α-humulene, cedrene, cadinene and bisabolene. The dental varnish were prepared at the School of Pharmacy of the same university, in random solutions with concentrations of 1%, 5%, 10%, and 20% of the oil-resin.
2.2. Study population
This study was approved by the Ethics Committee of the Federal University of Ceara (approval number 195.096). After signature of informed consent by parents or legal guardians, twenty-four 3–5 year-old children, from both genders, good general health and caries free, with ICDAS II (International Caries Detection and Assentment System) 0 and with high risk of caries, according to the criteria of the AAPD (American Academy of Pediatric Dentistry, 2014) were selected for the study. Children with a history of allergies or allergic diseases, e.g. asthma, urticaria, rhinitis, sinusitis, or intraoral soft tissue lesions were excluded from the study. None of the participants underwent antibiotic treatment up to 3 months prior to study initiation, nor during the course of this clinical trial.
2.3. Treatment application
Children were divided into four groups (6 children/group) with each group corresponding to one of the concentrations mentioned above. Initially, each patient chewed a piece a 3 × 3-cm plastic film (Parafilm®) for 60 s to stimulate the production of saliva and release the bacteria from the dental biofilm. Saliva was collected using a plastic device and stored in sterile microcentrifuge tubes (Eppendorf®), which were stored in polystyrene box containing ice. To minimize the influence of the circadian rhythms on salivary flow, all samples were collected in the same session and conditions by the same operator between 9:00 and 11:00 AM.
Thereafter, the varnish was applied by the same operator to the deciduous molars, with relative insulation, of each patient after Robinson prophylaxis using brushes and pumice. After 10 s, a triple syringe was used to gently dry the varnish. The saliva of each subject was immediately collected and placed into sterile microcentrifuge tubes (Eppendorf®).
2.4. Microbiological analysis
Samples were transported to the laboratory for microbiological analysis in a hermetically sealed case containing ice, and analyzed no longer than 2 h after collection.
Saliva was homogenized on a tube shaker for 30 s. A volume of 0.1 mL of each sample was aseptically drawn and transferred into one sterile test tube containing 0.9 mL of saline. Procedure was repeated twice, establishing dilutions of 1:10 and 1:100. A corresponding volume of ten microliters of each dilution was plated onto Mitis Salivarius-Bacitracin (MSB) agar medium in triplicates. The plates were then incubated at 37 °C, during 48 h, in jars under microaerofilic conditions. Bacterial counts were expressed as colony forming units (CFU)/mL of saliva and followed by phenotypical colony identification, as described elsewhere.
2.5. Statistical analysis
Quantitative variables, number of colony forming units (CFU) and relative reduction of CFU were initially analyzed by the Kolmogorov-Smirnov test to verify the normality of distribution. As such requirement was observed in all cases, then, for the descriptive statistics were calculated the average and standard deviation as well as parametric tests were employed for analytical statistics.
To compare four concentrations (1, 5, 10 and 20%) it was used analysis of variance (ANOVA) test associated with Tukey multiple comparison test to check for differences between the concentrations in pairs. Comparisons between pre- and post-treatment, considering a given concentration were made by t test for paired samples.
In all analyzes, we established the significance level of 0,05 (5%), and considered statistically significant P value less than 0.05. The GraphPad PRISM® software version 5.00 for Windows® (GraphPad Software, San Diego, California, USA, 2007) was used for both the achievement of statistical procedures as for the preparation of graphics.
Results were expressed as reduction in the number of S. mutans colony forming units (CFU) before and after varnish application treatment. Percentage reduction was calculated based on the following formula:
where CFU (before) and CFU (after) correspond to the number of colony-forming units (CFU) obtained before and after treatment, respectively.
3. Results
The patients reported no side effects after the application of different concentrations of varnish.
3.1. Comparison of different concentrations of copaiba
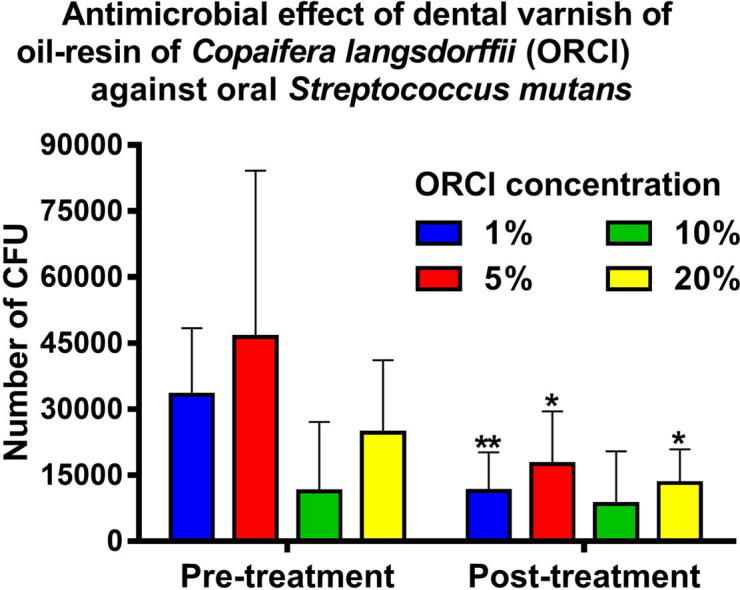
It became clear that the dental varnish copaiba in a concentration of 1% was more effective in reducing bacterial load of S. mutans in the samples analyzed at a dilution of 1:10 (Table 1).
Table 1.
Number of colony forming units (CFU) of Streptococcus mutans in the saliva samples verified in 6 children with 1:10 dilution before and after the treatment with varnish containing the oil resin at concentrations Copaifera langsdorffii 1, 5, 10 and 20%. In each concentration, comparisons between pre- and post-treatment were carried out by using the t test for paired samples.
| Concentration (%) | Pre-treatment |
Post-treatment |
Significance (P value) | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| 1 (n = 6) | 6972,00 | 4159,67 | 2571,83 | 1535,44 | 0,0302* |
| 5 (n = 6) | 5610,83 | 4047,03 | 2160,83 | 1425,83 | 0,0275* |
| 10 (n = 6) | 3479,60 | 4231,43 | 2426,40 | 3751,68 | 0,0928 |
| 20 (n = 6) | 6094,17 | 4448,37 | 3621,83 | 4295,63 | 0,0726 |
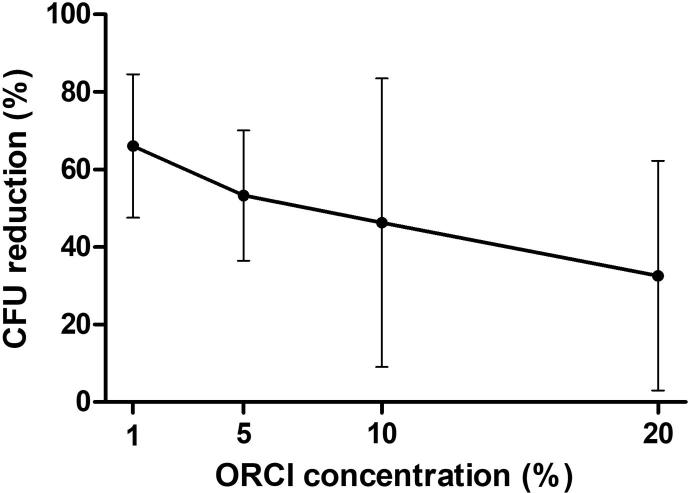
In Fig. 1 it can be verified that at a dilution of 1:10 saliva, the antimicrobial dental varnish activity of copaiba oil-resin decreases with the increase in its concentration. The data are the average and standard deviation of measurements made in the saliva samples of 6 children. Comparisons between treatments were performed by using analysis of variance (ANOVA) test associated with Tukey multiple comparison test to check for differences between the concentrations two by two. No statistically significant differences were found between concentrations (ANOVA: F = 0,3694, P = 0,7760).
Fig. 1.
Effect of different concentrations of the oil resin of Copaifera langsdorffii (ORCI) applied in the form of varnish, in the relative reduction of the number of colony forming units (CFU) of Streptococcus mutans, expressed in percentage terms, measured in saliva samples, dilution 1:10.
In Table 2 by comparing the results of the pre- and post-treatment using a saliva sample at a dilution of 1:100, it is found that the concentration of 1% copaiba oil resin in the dental varnish is truly more effective in reducing the microbial load, in which the statistical difference presents a significant value (P = 0,0026) (see Table 3).
Table 2.
Number of colony forming units (CFU) of Streptococcus mutans observed in the saliva samples of 6 children with dilution of 1:100 before and after the treatment with varnish containing the oil resin of Copaifera langsdorffii on concentrations 1, 5, 10 and 20%. In each concentration, comparisons between pre- and post-treatment were carried out by using the t test for paired samples.
| Concentration (%) | Pre-treatment |
Post-treatment |
Significance (P value) | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| 1 (n = 6) | 33275,00 | 15146,79 | 11443,33 | 8760,58 | 0,0026** |
| 5 (n = 6) | 46388,33 | 37815,41 | 17496,67 | 11998,57 | 0,0430* |
| 10 (n = 6) | 11398,00 | 15693,41 | 8464,00 | 11950,68 | 0,1899 |
| 20 (n = 6) | 24608,33 | 16497,90 | 13218,33 | 7675,31 | 0,0474* |
Table 3.
Relative reduction in the number of colony forming units (CFU) of Streptococcus mutans, expressed in percentage terms, measured in saliva samples from 6 children, with dilution of 1:10 or 1:100, treated with varnish containing the Copaifera langsdorffii oil resin at concentrations of 1, 5, 10 and 20%. In each concentration, comparisons between two dilutions were performed by using the t-test for paired samples.
| Concentration (%) | Dilution 1:10 |
Dilution 1:100 |
Significance (P value) | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| 1 (n = 6) | 57,05 | 27,98 | 66,07 | 18,51 | 0,1924 |
| 5 (n = 6) | 56,10 | 11,01 | 53,30 | 16,86 | 0,6210 |
| 10 (n = 6) | 47,81 | 40,36 | 46,31 | 37,24 | 0,8934 |
| 20 (n = 6) | 41,62 | 32,59 | 32,57 | 29,64 | 0,4323 |
In examining Fig. 2, it is observed, in a similar way, a reduction of antimicrobial activity of dental varnish behavior with increasing concentration of copaiba oil resin incorporated into the formulation. The data correspond to the average and standard deviation of measurements made in the saliva samples of 6 children. Comparisons between treatments were performed by using analysis of variance (ANOVA) test associated with Tukey multiple comparison test to check for differences between the concentrations two by two. No statistically significant differences were found between concentrations (ANOVA: F = 1,698, P = 0,2012).
Fig. 2.
Effect of different concentrations of the oil resin of Copaifera langsdorffii (ORCI) applied in the form of varnish, the relative reduction in the number of colony forming units (CFU) of Streptococcus mutans, expressed in percentage terms, measured in saliva samples, dilution 1:100.
When comparing the average reduction of UFC of S. mutans and standard deviations in the statistical analysis, it is observed that the dilution of saliva of 1:100 presents itself as better analysis option, since it has a higher statistical uniformity evidenced by the lower standard deviation.
4. Discussion
Resistance to synthetic antimicrobials and search for substances with pharmacological properties with lower adverse effects made increase the interest of the pharmaceutical industry for natural products. The development of dental materials with natural products is still quite limited, practically summarizing to topical formulations and without many clinical trials published in the last decades. However, several studies have documented the pharmacological activity of natural products against the dental biofilm, especially the cariogenic one (Marsh et al., 2000, Freires and Rosalen, 2016). In dental materials, phytomedicine has been used as antiinflammatory, antibiotic, analgesic, sedative agents in various types of formulations like toothpaste, mouthwash, endodontic solutions, among others.
In regards of dental caries, there are several risk indicators, those related to the disease directly, such as the exposure to sugar and consequent alteration of the microflora, as well as the modulating factors, such as socioeconomic aspects. Saliva is a biological material, being an excellent biomarker for systemic and oral diseases, especially in relation to dental caries, where approximately 108 microorganisms can be counted in 1 mL of saliva. Research associates counts of S. mutans in saliva to the experience of dental caries (Marsh et al., 2000, Kaur et al., 2013, AAPD, 2014).
Dental varnishes are pharmaceutical formulations for use in dentistry and generally have good acceptance by pediatric patients (Vasconcelos et al., 2008). They are composed of polymer matrices, excipients and the active principle. In the case of the present varnish the chosen matrix was insoluble, in this case ethylcellulose, used to modulate the release of the active principle and thus the substantivity was higher (De Luca et al., 2017). This type of formulation adheres to dental fissures and scars, gradually releasing the active principle, thus becoming a long-term therapeutic agent suitable for antimicrobial formulation (Franca et al., 2014).
The main function of varnishes is the prevention of dental caries because their properties contribute to the disruption of the dental biofilm, thereby decreasing the cariogenic microorganisms on the surface of teeth (Pessan et al., 2008).
We selected the varnish formulation rather than the mouthwash or gel formulations because children under 6 years of age do not have an adequate ability to eject saliva and also because varnishes promote a slow release of active ingredients. In addition to its easy clinical application, varnishes have good adhesive properties to teeth surfaces in children and show no contraindication for the age group studied (Pessan et al., 2008, De Luca et al., 2017).
There are several reasons for the development of new products with antimicrobial properties, since new microbial resistance, toxicity and high costs of dental materials are being marketed. The main antimicrobial agent in dentistry is chlorhexidine, which when used for a prolonged period causes tooth staining, mucosal irritation, alteration of the taste and loses its pharmacological capacity, occurring the recolonization of bacteria as S. mutans (Vale et al., 2014). Current studies demonstrate weak evidence of the use of chlorhexidine for prevention of dental caries and reduction of S. mutans in children and adolescents (Walsh et al., 2015, Flamee et al., 2015).
Investments in patents are increasingly widespread, as the whole functioning of the capitalist system is related to innovation and scientific and technological advancement (Lima, 2006, Franca et al., 2014, Freires and Rosalen, 2016). This study is the first to use copaiba varnish, with patent number BR1020160212628 deposited on the National Institute of Industrial Property (INPI-Brazil).
S. mutans is closely related to the cariogenic biofilm due to the ability to synthesize glucans and fructan from sucrose using various glucosyltransferases (Gtfs) and a fructosyltransferase. Glucans provide specific binding sites for the colonization of S. mutans on the dental surface. Therefore S. mutans is considered a group of highly acidogenic and acid tolerant bacteria, virulence properties highly related to the capacity of dental demineralization and essential for the formation of bacterial biofilm (Klein et al., 2008). The effectiveness of copaiba oil against bacteria present in the oral environment is documented in the literature, not only in relation to Streptococcus sp., But also against Enterococcus sp., Haemophilus sp., Aerococcus sp., Bacillus sp., Lactococcus sp., among others (Klein et al., 2008, Pieri et al., 2016).
A study by Pieri et al (2016) has evaluated the inhibitory activity of copaiba oil-resin against the cariogenic microorganism S. mutans through a minimal inhibitory concentration test using the serial dilution technique in broth, and has reported inhibition of bacterial growth at all concentrations tested. Similarly, a study by Vasconcelos et al.(2008) reported the use of a formulation containing copaiba oleo resin, zinc oxide, and calcium hydroxide (cement). This cement showed antibacterial activity against S. mutans and S. Sanguinis, even in very small amounts at all dilutions analyzed.
Of all the copaiba oil-resin concentrations in dental varnish, the 1% showed the best antimicrobial activity, as evidenced by the relative decline of CFU of S. mutans in the samples studied.
The 1% formulation had stronger antimicrobial activity, probably because the copaiba active ingredients retained in the varnish matrix and is released locally. We also observed that higher concentrations of the copaiba ole resin lost the ability to retain its active ingredient, and in these situations, the active ingredient would be released so quickly that the varnish became unpleasant to patients and partially lost its antimicrobial activity. Therefore, during application, the lowest concentrations of the varnish would better retain the active ingredient, which was gradually released during contact with teeth and gums, there by promoting a higher antimicrobial activity.
Copaiba oil-resin has been tested for its antimicrobial activity against dental plaque-forming bacteria, with good in vitro and in vivo results, which gives credibility to the clinical use of the formulation (Paiva et al., 2002, Vasconcelos et al., 2008, Pieri et al., 2009, Romero et al., 2009). Therefore, this study represents an initial step towards future clinical trials, which can generate the development of a new product in the market, since the copaiba has a great economic importance worldwide. In addition, studies can be carried out with the association of fluoride in the varnish, since chemically it is a compatible association and thus the same also have the capacity of remineralization, since to be based only on bacterial profiles is somewhat limited in the case of dental caries.
5. Conclusions
The present study further supports the antimicrobial bactericidal effect of copaiba against S. mutans. The use of copaiba varnish could be a good prevention strategy for children aged between 3 and 5 years old and warrants the performance of additional randomized clinical trials to identify its antimicrobial efficacy and anticaries effect.
6. Authorship
LARV, MFG, JMM, FOC and EMRN contributed in running the laboratory work and writing the manuscript; Prof. Dr. FVF contributed with data analyze. Profs Dr. MAMB and MMFF, performed extraction, isolation and characterization of isolated constituents. And participated on manuscript preparation and also contributed to critical reading of manuscript. Prof Dr SGFF performed the dental varnishes and also contributed to critical reading of manuscript. Prof Dr CSRF, CBMC and PLDL designed the study, manuscript preparation and also contributed to critical reading of manuscript.
Acknowledgement
Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico-Funcap and Coordenação de Nível Pessoal de Nível Superior-Capes.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams S.E., Arnold D., Murphy B., Carroll P., Green A.K., Smith A.M., Marsh P.D., Chen T., Marriott R.E., Brading M.G. Sci. Reports. 2017;7:43344. doi: 10.1038/srep43344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry (AAPD) Guideline on caries-risk assessment and management for infants, children, and adolescents. Clinical Practice Guidelines. 2014;37:132–139. [Google Scholar]
- Bieski I.C.G., Leonti M., Arnason J.T., Ferrier J., Rapinski M., Violante I.M.P., Balogun S.O., Pereira J.F.C.A., Figueiredo R.C.F., Soares-Lopes C.R., Silva D.R., Pacini A., Albuquerque U.P., Martins D.T.O. Ethnobotanical study of medicinal plants by population of Valley of Juruena Region, Legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 2015;173:383–423. doi: 10.1016/j.jep.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Diefenbach A.L., Muniz F.W.F.G., Oballe H.J.R., Rosing C.K. Antimicrobial activity of copaiba oil (Copaifera ssp.) on oral pathogens: systematic review. Phytotherapy Res. 2017;32:586–596. doi: 10.1002/ptr.5992. [DOI] [PubMed] [Google Scholar]
- Eriksson L., Holgerson P.L., Johansson I. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Sci. Reports. 2017;7:5861. doi: 10.1038/s41598-017-06221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamee S., Gizani S., Caroni S.C., Papagiannoulis L., Twetman S. Eur. Arch. Paediatr. Dent. 2015;16:449–454. doi: 10.1007/s40368-015-0192-x. [DOI] [PubMed] [Google Scholar]
- Franca J.R., De Luca M.P.D., Ribeiro T.G., Castilho R.O., Moreira N.A., Santos V.R., Faraco A.A.G. Propolis-based chitosan varnish: drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complem. Alternative Med. 2014;14:478. doi: 10.1186/1472-6882-14-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freires I.A., Denny C., Benso B., Alencar S.M., Rosalen P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules. 2015;20:7329–7358. doi: 10.3390/molecules20047329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freires I.A., Rosalen P.L. How natural product research has contributed to oral care product development? A critical view. Pharmaceut. Res. 2016;33:1311–1317. doi: 10.1007/s11095-016-1905-5. [DOI] [PubMed] [Google Scholar]
- Ismail A.I., Sohn W., Tellez M., Willem J.M., Betz J., Lepkowski J. Risk indicators for dental caries using the International Caries Detection and Assessment System (ICDAS) Community Dent. Oral Epidemiol. 2008;36:55–68. doi: 10.1111/j.1600-0528.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Kaur R., Gilbert S.C., Sheehy E.C., Beighton D. Salivary levels of Bifidobacteria in caries-free and caries-active children. Int. J. Paediatric Dent. 2013;23:32–38. doi: 10.1111/j.1365-263X.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- Klein M.I., Duarte S., Xiao J., Mitra S., Foster T.H., Koo H. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl. Environ. Microbiol. 2008;75:837–841. doi: 10.1128/AEM.01299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima J.A.A. Digressões sobre Propriedade Intelectual como agente de desenvolvimento, Inovação e Estratégia. Fórum HSM de Estratégia. 2006;10:45–55. [Google Scholar]
- Lobo P.L.D., Fonteles C.S.R., Marques L.A.R.V., Fechine F.V., Fonseca S.G.C., Carvalho C.B.M., Moraes M.E.A. Dose – response evaluation of a novel essential oil against Mutans streptococci in vivo. Phytomedicine. 2014;21:1043–1047. doi: 10.1016/j.phymed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- De Luca M.P., Freires I.A., Gala-García A., Santos V.R., Vale M.P., Alencar S.M. The anti-caries activity and toxicity of an experimental propolis-containing varnish. Braz. Oral Res. 2017 doi: 10.1590/1807-3107BOR-2017.vol31.0045. pp. 31: e45. [DOI] [PubMed] [Google Scholar]
- Marsh P.D., Do T., Beighton D., Devine D.A. Infuence of saliva on the oral microbiota. Periodontology. 2000:1–14. doi: 10.1111/prd.12098. [DOI] [PubMed] [Google Scholar]
- Paiva L.A.F., Cunha K.M.A., Santos F.A., Gramosa N.V., Silveira E.R., Rao V.S.N. Investigation on the wound healing activity of oleo-resin from Copaifera langsdorffi in rats. Phytotherapy Res. 2002;16:737–739. doi: 10.1002/ptr.1049. [DOI] [PubMed] [Google Scholar]
- Pessan J.P., Al Ibrahim N.S., Buzalaf M.A.R., Toumba K.J. Slow-release fluoride devices: a literature review. J. Appl. Oral Sci. 2008;16:238–244. doi: 10.1590/S1678-77572008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri F.A., Mussi M.C., Moreira M.A.S. Óleo de copaíba (Copaifera sp.): histórico, extração, aplicações industriais e propriedades medicinais. Revista Brasileira de Plantas Medicinais. 2009;11:465–472. [Google Scholar]
- Pieri F.A., Souza M.C., Vermelho L.L.R. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Veterinary Res. 2016;12:216. doi: 10.1186/s12917-016-0842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Nat. Rev. 2017;3:1–16. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- Romero A.L., Baptistella L.H.B., Imamura P.M. Absolute configuration of some dinorlabdanes from the copaiba oil. J. Brazilian Chem. Soc. 2009;20:1036–1040. [Google Scholar]
- Vasconcelos K.R.F., Veiga-Junior V.F., Rocha W.C., Bandeira M.F.C.L. Avaliação in vitro da atividade antibacteriana de um cimento odontológico à base de óleo-resina de Copaifera multijuga Hayne. Brazilian J. Pharmacog. 2008;18:733–738. [Google Scholar]
- Vale G.C., Cury A.D.B., Arthur R.A., Cury J.A., Tabchoury C.O.M. Recolonization of mutans streptococci after application of chlorhexidine gel. Brazilian Dental J. 2014;25:485–488. doi: 10.1590/0103-6440201300156. [DOI] [PubMed] [Google Scholar]
- Walsh T., Oliveira-Neto J.M., Moore D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database System. Rev. 2015;4:1–65. doi: 10.1002/14651858.CD008457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S.W., Shao J., He J., Wu X.Y., Xu X.H., Zhao W.H. A membrane-targeted peptide inhibiting PtxA of phosphotransferase system blocks Streptococcus mutans. Caries Res. 2019;53:176–193. doi: 10.1159/000489607. [DOI] [PubMed] [Google Scholar]