Abstract
Objective
To develop a nanoparticle platform that can expand both CD4+ and CD8+ T regulatory cells (Tregs) in vivo for the suppression of autoimmune responses in systemic lupus erythematosus (SLE).
Methods
Poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs) encapsulating IL-2 and TGF-β were coated with anti-CD2/CD4 antibodies and administered to mice with lupus-like disease induced by the transfer of DBA/2 T cells into (C57BL/6 x DBA/2)F1 (BDF1) mice. Peripheral frequency of Tregs was monitored ex vivo by flow cytometry. Disease progression was assessed by measuring serum anti-dsDNA antibodies by ELISA. Kidney disease was evaluated as proteinuria and by renal histopathology.
Results
Anti-CD2/4 antibody-coated, but not non-coated NPs encapsulating IL-2 and TGF-β, induced CD4+ and CD8+ Foxp3+ Tregs in vitro. In vivo studies in non-lupus mice determined the optimal dosing regimen of NPs for expansion of CD4+ and CD8+ Tregs that was then tested in BDF1 lupus mice. The administration of anti-CD2/4 antibody-coated NPs encapsulating IL-2 and TGF-β resulted in the expansion of CD4+ and CD8+ Tregs, a marked suppression of anti-DNA antibody production, and reduced renal disease.
Conclusion
This study shows for the first time that T cell-targeted PLGA NPs encapsulating IL-2 and TGF-β can expand both CD4+ and CD8+ Tregs in vivo and suppress murine lupus. This approach that enables the expansion of Tregs in vivo and inhibits pathogenic immune responses in SLE could represent a potential new therapeutic modality in autoimmune conditions characterized by impaired Tregs function associated with IL-2 deficiency.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a disorder of immune regulation where genetic and environmental factors contribute to the disruption of immune homeostasis. In SLE, normally quiescent self-reactive T and B cells become activated and are no longer held in check by the mechanisms of peripheral tolerance, including the suppression by T regulatory cells (Tregs) - which are specialized cells that in SLE have an impaired function (1).
Since the levels of IL-2 and TGF-β that are required for the induction, expansion and function of Tregs are compromised in SLE (1) and because IL-2 and TGF-β help induce both CD4+ and CD8+ Tregs (in normal and disease settings), it has been proposed to manipulate these cytokines for possible restoration of the Tregs deficits (2). For example, low dose IL-2 therapy has been used to correct defects in Tregs and to improve clinical disease in SLE patients (3–4), supporting the concept that the functional impairment of the Tregs in SLE that is attributable to cytokine deficiency can be corrected by selective cytokine modulation.
We recently reported that nanoparticles (NPs) composed of the biocompatible FDA-approved biodegradable polymer, poly(lactic-co-glycolic) acid (PLGA) coated with anti-CD4 Ab (for the targeting of CD4+ T cells) that encapsulate IL-2 and TGF-β can induce ex vivo functional, stable CD4+CD25+Foxp3+ Tregs (5). Here, we extend those findings by exploring the therapeutic effects of targeted delivery of IL-2 and TGF-β to T cells for the induction of Tregs in vivo. In addition to targeting CD4+ T cells by coating NPs with anti-CD4 Ab, we co-coated NPs with anti-CD2 Ab to also target CD8+ T cells (2) and induce CD8+ Tregs ex vivo (because of the protective effects of CD8+ Tregs in SLE) (6–7).
Specifically, the effects of the NPs encapsulating IL-2 and TGF-β were investigated in a murine lupus model where donor CD4+ T cells from DBA/2 mice injected into (C57Bl/6 x DBA/2)F1 (BDF1) mice recognize the host major histocompatibility complex (MHC) antigens, become activated, and drive B cell hyperactivity (8–9). In this model, the outcome of the cell transfer is lymphoid hyperplasia, polyclonal B cell activation and anti-dsDNA antibody production within 2 weeks and immune complex glomerulonephritis within 4–6 weeks (8–9). Of note, an advantage of this model is that a single transfer of DBA/2 CD4+ and CD8+ Tregs generated ex vivo with IL-2 and TGF-β is sufficient to block B cell activation, autoantibody production and immune complex nephritis (10), allowing the testing of the therapeutic potential of targeted delivery of IL-2 and TGF-β to T cells for the induction of Tregs in vivo. Our results indicate that NPs encapsulating IL-2 and TGF-β induced both CD4+ and CD8+ Tregs in vivo, with subsequent reduced lupus disease manifestations.
MATERIALS AND METHODS
Nanoparticle preparation and characterization
Cytokine-encapsulating PLGA NPs were prepared according to a previously reported water/oil/water double emulsion protocol (5). Briefly, 60 mg PLGA (50:50, Durect Corp.) were dissolved in 3 ml of chloroform in a glass test tube. A primary emulsion was generated by adding 200 μl of an aqueous solution containing 2.5 μg carrier free TGF-β and 1.25 μg IL-2 (Peprotech). Addition was carried out dropwise while continuously vortexing the chloroform polymer solution. The resulting primary emulsion was sonicated using an Ultrasonic Processor GEX600 model probe at 38% amplitude for a 10” pulse, and added dropwise to a continuously vortexed glass test tube containing 4 ml of 4.7% PVA and 0.625 mg/ml avidin-palmitate conjugate, as previously described (11). The resulting double emulsion was sonicated with three 10” pulses with 20” breaks in an ice bath in between before transfer to a beaker containing 200 ml of 0.25% PVA. Particles were allowed to harden by stirring at room temperature for 3 hours. Hardened NPs were washed three times by cycles of pelleting at 18,000 r.c.f. and resuspension in MilliQ water. Washed NPs were flash-frozen in liquid nitrogen and lyophilized for multiple days to enable long-term storage. NPs were stored at −20° C until use and prepared from lyophilized stocks for each experiment. For cell targeting, NPs were freshly prepared in PBS at the target concentration and reacted with the biotinylated targeting antibody at a concentration ratio of 2 μg Ab/mg NPs 10 minutes prior to use. NPs size was quantified using dynamic light scattering (DLS) with Malvern Nanozetasizer. Cytokine encapsulation and release were measured by BD OptEIA™ ELISA kits, either after disrupting particles in DMSO or by supernatant analysis of release study aliquots. For the release assay, a 1 wt/v% solution of Pluronic F127 in PBS was used as release buffer, to help stabilize released cytokine and prevent binding to the tube surface and loss of capture/detection Ab binding ability.
Mice
C57Bl/6, DBA/2 and BALB/c mice (including DO11.10, H2d) were purchased from The Jackson Laboratory (Bar Harbor, ME). Female C57Bl/6 mice and male DBA/2 mice were bred for the generation of (C57Bl/6 x DBA/2)F1 (BDF1) mice. At the age of eight weeks, BDF1 mice were induced to develop disease by the transfer of parent DBA/2 cells according to standard protocols (10). BDF1 mice were then given i.p. injection of vehicle as control or PLGA NPs encapsulating IL-2 and TGF-β - coated or not (control) with anti-CD2 and anti-CD4 Ab (BD Biosciences). Mice were monitored bi-weekly to measure the frequency of circulating Tregs by flow cytometry. Serum sampling was obtained via retro-orbital bleeding. Proteinuria was measured using Albustix strips (Siemens). Mice were maintained in specific pathogen-free (SPF) facilities at the University of California Los Angeles. Experiments were approved by the Institutional Animal Research Committee.
In vitro assays
For T cell proliferation, splenocytes were incubated at 37°C at a concentration of 2 × 105 cells/well in 96-well plates (Corning) in complete RPMI medium (100 U/ml penicillin, 100 μg/ml streptomycin, 10% heat-inactivated FCS) for 72 hours in the absence (control) or in the presence of plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml) Ab (BD Biosciences). In some experiments, OVA323–339 peptide (Thermo Fisher Scientific) was added, with or without NPs, with paired control without peptide. 3H-thymidine was added during the last 16 hours before cells harvesting on a Tomtec Harvester 96. Stimulation index was calculated as mean c.p.m. of antigen-stimulated wells/mean c.p.m. of wells with medium only.
ELISA
ELISA measurement of anti-dsDNA antibodies was performed using kits from Alpha Diagnostics International, according to the manufacturer’s instructions. Optical densities (O.D.) were measured at 450 nm.
Flow cytometry
PBMC or splenocytes were isolated according to standard procedures and single cell suspensions were used for phenotypic analyses using combinations of fluorochrome-conjugated antibodies. After Fc blocking, fluorochrome-conjugated anti-mouse Ab (BD Biosciences) to CD4, CD8, CD25, CD19, CD11b, CD11c, Gr-1 or isotype control antibodies were used for staining prior to acquisition on an FACSCalibur flow cytometer (BD Biosciences) and subsequent analysis using FlowJo software (Tree Star). For intracellular staining of Foxp3, cells were first stained for the expression of cell-surface markers before fixing and permeabilization and Foxp3 staining using the eBioscience Foxp3 Staining Kit, according to the manufacturer’s instructions. The gating strategy used for the different immune cell populations is shown in Supplementary Figure 1.
Histology
Kidney sections (4-μm-thick) were stained with hematoxylin and eosin (H&E) according to standard procedures (12). For the assessments of pathologic changes as glomerular activity score (GAS) and tubulointerstitial activity score (TIAS), sections were scored in a blinded manner, using a scale of 0–3, where 0 = no lesions, 1 = lesions in <30% of glomeruli, 2 = lesions in 30–60% of glomeruli, and 3 = lesions in >60% of glomeruli. The glomerular activity score includes glomerular proliferation, karyorrhexis, fibrinoid necrosis, inflammatory cells, cellular crescents, and hyaline deposits. The tubulointerstitial activity score includes interstitial inflammation, tubular cell necrosis and/or flattening, and epithelial cells or macrophages in the tubular lumen. The raw scores were averaged to obtain a mean score for each feature, and the mean scores were summed to obtain an average score from which a composite kidney biopsy score was obtained (13). For indirect immunofluorescence (IIF) studies, sections were fixed in cold acetone for 5 minutes, washed, and blocked with 2% bovine serum albumin (BSA) for 1 hour before staining with rabbit anti-mouse IgG (Fisher Scientific).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 5.0 software. Parametric testing was done using the unpaired t test; nonparametric testing was used when data were not normally distributed. Significance level was set at a P value <0.05.
RESULTS
Preparation of nanoparticles.
The prepared cytokine-encapsulating NPs were characterized through examination of physical properties, encapsulation metrics, and release kinetics (Figure 1) as previously described (5, 14). By dynamic light scattering, NPs were found to have an average hydrodynamic diameter of 245.3 ± 2.2 nm with a low polydispersity index (0.06 ± 0.01), indicative of a uniform NP population with a relatively tight size distribution (Figure 1B). Cytokine encapsulation was measured by ELISA after disrupting the NPs using DMSO. Standard curves were generated using cytokine standards but all wells were supplemented to contain 5 v/v% DMSO and the appropriate concentration of empty NPs. Using this method, NPs were found to contain 7.4 ± 0.4 ng TGF-β and 1.9 ± 0.1 ng IL-2 per mg NP. For TGF-β, the percent encapsulation efficiency was 17.8 ± 1.1; for IL-2 was 9.1 ± 0.4. The release assay was performed using 1 mg/ml aliquots of particles in release buffer. At each time point, aliquots were spun down in a microcentrifuge and supernatant was isolated from the particle pellet. The pellet was then resuspended in fresh release buffer until the next time point. Supernatant samples were frozen until the end of the study, at which point ELISA analysis was performed. As seen in Figure 1C, release of TGF-β and IL-2 from the NP system exhibits burst release during the first 24 hours, followed by a slower, more sustained release profile over the course of the tested 14-day period.
Figure 1. Schematic representation of PLGA NPs encapsulating IL-2 and TGF-β.
A. Depiction of the PLGA NP system showing co-encapsulation of IL-2 and TGF-β and the surface coating of targeting anti-CD2 and anti-CD4 antibodies. B. IL-2/TGF-β NP size distribution. Physical characterization of NPs size was performed by using dynamic light scattering. NPs had a negative surface charge as measured by zeta potential of −15. 1 ± 0.5 mV in MilliQ water. C. Loading and release of the two encapsulated cytokines quantified by ELISA.
Establishment of the conditions for the induction of CD4+ and CD8+ Tregs
To induce simultaneously CD4+ and CD8+ Tregs, we used PLGA NPs encapsulating IL-2 and TGF-β in amounts that had been used previously (5). Scalar doses of NPs coated with anti-CD4/CD2 Ab (Figure 1A) were added in culture to mouse purified CD3+ cells for the delivery to T cells, in a paracrine fashion, of IL-2 and TGF-β that induce Tregs in vitro. Since anti-CD3/CD28 Ab stimulation with 50 μg/ml NPs promoted a significant increase in the frequency of both CD4+ and CD8+ Tregs (Figure 2), we proceeded to study the conditions for generation of Tregs in vivo.
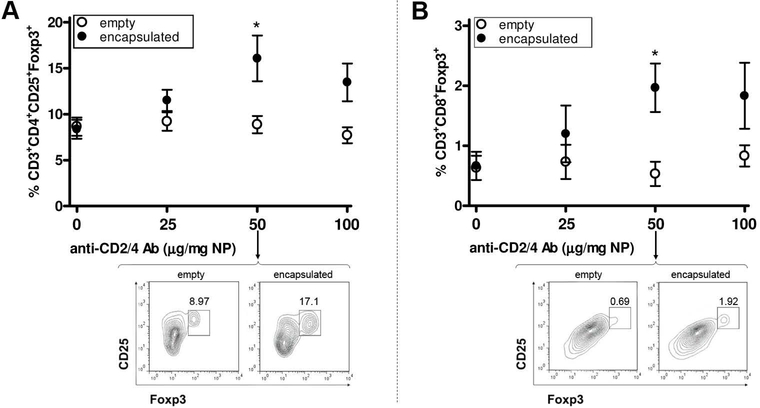
Figure 2. Establishment of the conditions for in vitro induction of CD4 and CD8 Tregs with anti-CD2/CD4 Ab-coated PLGA NPs encapsulated with IL-2 and TGF-β.
Sorted CD3+ T cells (negatively selected with magnetic beads) from 12 weeks-old BALB/c mouse splenocytes were cultured in the presence of anti-CD3/28 Ab and scalar doses of NPs. After three days, flow cytometry analysis compared the numbers of CD4+ Tregs (A) and CD8+ Tregs (B) in cultures with NPs encapsulated with IL-2/TGF-β (closed circles) vs. cultures with empty NPs (empty circles). Significant differences were observed at a dose of 50 μg/mg NP (*P<0.05), for which the figure includes representative flow cytometry plots on pregated CD4 (A) and CD8 (B) cells. Representative of four experiments, each with three mice per group.
Establishment of in vivo conditions for the induction of CD4+ and CD8+ Tregs in non-lupus mice
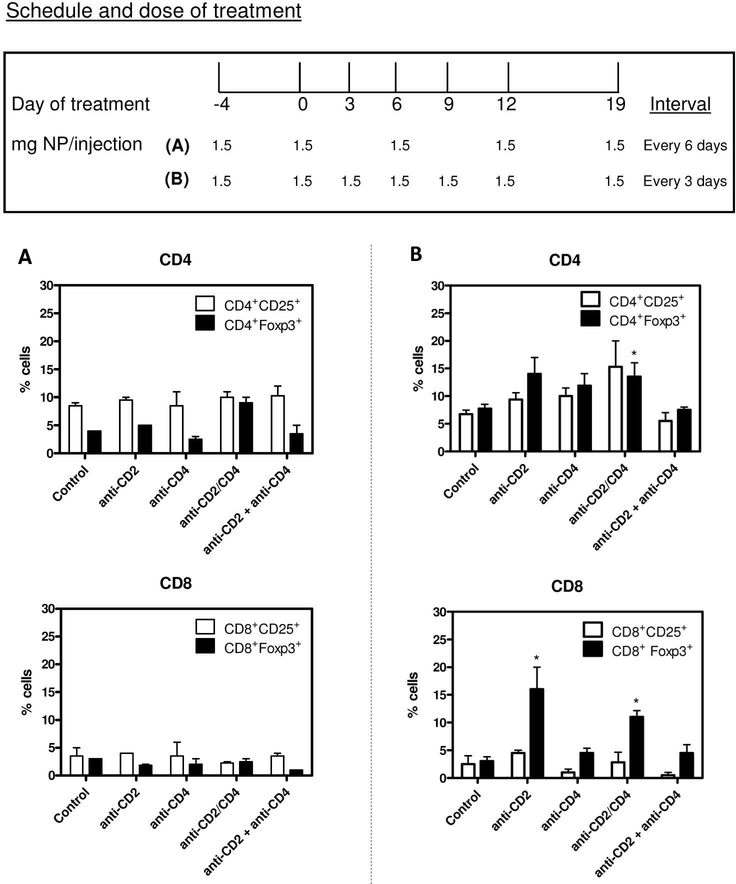
Treatment with anti-CD2/4 Ab-coated NPs was compared with that of NPs coated with only anti-CD2 or anti-CD4 Ab, keeping constant the total amount of NPs (all encapsulating IL-2 and TGF-β) (Figure 3). After a loading dose, 1.5 mg NPs were injected into BALB/c mice every 3 days or 6 days for the first 12 days. One week later, both groups of mice received another 1.5 mg NPs. Analysis of Tregs at day 21 among the circulating PBMCs revealed that only those animals that had received NPs every 3 days had significant increases in Tregs (Figure 3B and Supplementary Figure 2). Importantly, the coating antibodies needed to be attached to the same NPs (co-coated), since coating of anti-CD2 and anti-CD4 Ab independently on NPs was not effective in expanding Tregs. Anti-CD2 Ab coating expanded CD8+Foxp3+ cells (Figure 3B), and the percentage of CD8+Foxp3+ cells induced by anti-CD2 Ab-coated NPs was higher than that from anti-CD2/4 Ab-coated NPs (likely due to lower per-NP coating of anti-CD2 Ab in the co-coated system and increased competitive binding to CD4+ T cells). In view of the superiority of the anti-CD2/4 Ab-coated NPs in inducing Tregs as compared to NPs coated with each single antibody, we performed the subsequent experiments in lupus mice using anti-CD2/4 Ab-coated NPs encapsulating IL-2 and TGF-β. This treatment for the expansion of Tregs did not affect the T cell responsiveness to antigenic stimulation (Supplementary Figure 3), suggesting that the binding of NPs to CD2 or CD4 co-receptors did not impede activation through the T cell receptor.
Figure 3. Protocol of PLGA NP administration for the identification of an optimal dose for the expansion of Tregs in vivo.
Comparison of anti-CD2/4 Ab-coated NPs with NPs coated with anti-CD2 and anti-CD4 Ab alone, or in combination. A. Percentages of CD4+ Tregs (top) and CD8+ Tregs (bottom) in mice treated according to protocol (A) indicated in the figure quadrant. B. Percentages of CD4+ Tregs (top) and CD8+ Tregs (bottom) in mice treated according to protocol (B) indicated in the figure quadrant. Representative of two experiments with four mice per group; *P<0.05 by Mann Whitney U test comparisons with control.
In vivo studies in BDF1 lupus mice
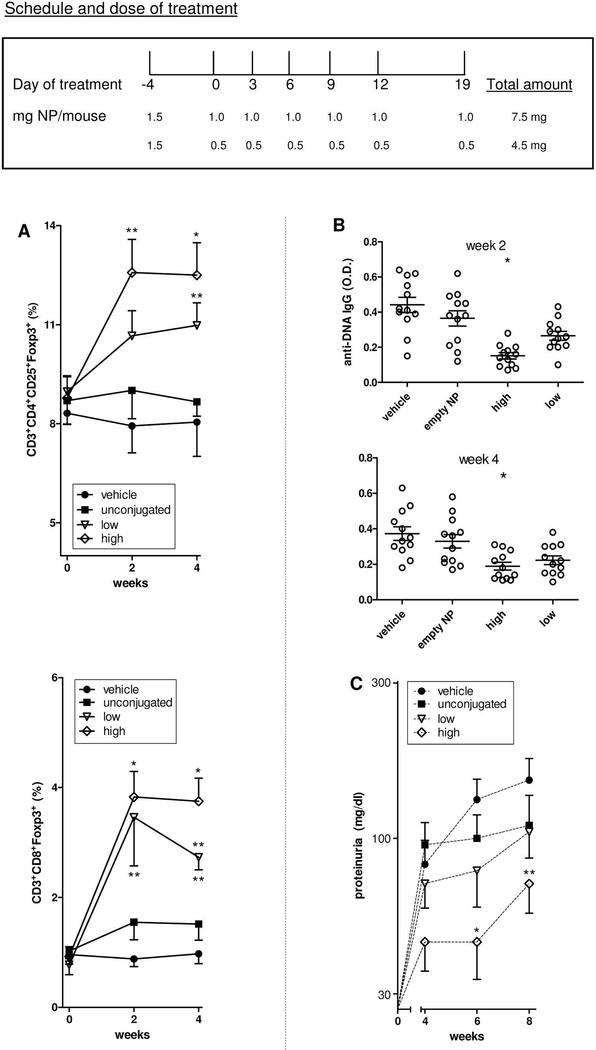
With the protocol of Figure 4 in BDF1 lupus mice, treatment with anti-CD2/4 Ab-coated NPs encapsulating IL-2 and TGF-β resulted in increased numbers of circulating CD4+ and CD8+ Tregs (Figure 4A and Supplementary Figure 4), and afforded protection from lupus disease manifestations when high doses of NPs were used (Figure 4B-C).
Figure 4. Expansion of Tregs after treatment of BDF1 mice with T cell-targeted PLGA NPs encapsulated with IL-2/TGF-β associates with reduced lupus manifestations.
A. Percentages of peripheral CD4+ and CD8+ Tregs at the indicated time points after treatment (x axis). *P<0.05; **P<0.005 in the comparison between unconjugated NPs vs. NPs coated with anti-CD4/CD2 Ab. B. ELISA measurements of anti-dsDNA antibodies from sera collected at the indicated time points. *P<0.05 vs unconjugated NPs. C. Proteinuria at the indicated time points in the mice shown in panel A. *P<0.05 vs unconjugated NPs. Each group included 12 mice.
In BDF1 mice, disease onset after transfer of DBA/2 cells is rapid, with anti-DNA autoantibodies appearing by 2 weeks and proteinuria due to immune complex glomerulonephritis by 6 weeks after transfer (8–10). Figure 4 shows that a total dose of 7.5 mg NPs was superior to that of 4.5 mg in inducing tolerogenic effects. This higher dose was associated with an increase of about two-fold for CD4+ Tregs and about four-fold for CD8+ Tregs but not with changes in the frequency of other immune cell populations (Supplementary Figure 5), and with a significant reduction in the production of anti-dsDNA autoantibodies (Figure 4B) and proteinuria (Figure 4C).
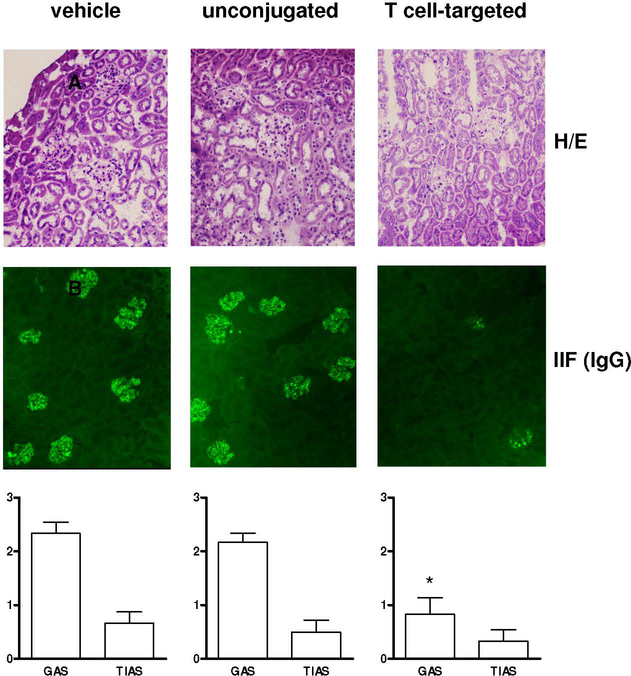
We had previously reported that PLGA NPs needed to be targeted to induce and expand CD4+ Tregs (5). Here, NPs also needed to be targeted for the expansion of CD4+ and CD8+ Foxp3-expressing Tregs and associated with protection of the mice from developing anti-DNA autoantibodies and proteinuria. Indeed, untargeted NPs containing IL-2 and TGF-β administered at equivalent doses had none of these effects (Figure 4). Of note, the decreased proteinuria in mice treated with T cell-targeted NPs encapsulating IL-2 and TGF-β was reflected by histopathological kidney changes that indicated preserved glomeruli and reduced IgG precipitation (Figure 5). Conversely, control mice (including those treated with untargeted NPs) displayed glomerular hypercellularity and proliferative changes characteristic of lupus nephritis and IgG precipitation that associated with worse renal disease scores (Figure 5).
Figure 5. Renal histopathology of BDF1 lupus mice after treatment with NPs encapsulated with IL-2/TGF-β and coated with anti-CD4/CD2 Ab.
Histology of 4-μm kidney sections stained with hematoxylin/eosin (top) and indirect immunofluorescence for IgG (middle). Representative of six mice per group. Magnification: 20x. C. Cumulative glomerular activity score (GAS) and tubulointerstitial activity score (TIAS) in kidneys from mice treated with vehicle, unconjugated of T-cell targeted NPs (n=6 per group) (bottom). Readings were done six weeks after start of treatment. Values are mean ±SD; *P<0.001 vs vehicle group for both GAS and TIAS.
DISCUSSION
We describe for the first time a biodegradable formulation of NPs that can expand both CD4+ and CD8+ Tregs in vivo sufficiently to suppress lupus manifestations in mice. The coating with anti-CD2/CD4 Ab enabled NPs to bind both CD4+ and CD8+ T cells for the expansion of both cell types in vivo, in non-lupus mice and in BDF1 lupus mice, with resulting reduction of anti-dsDNA autoantibodies and immune-complex glomerulonephritis in the latter.
Several tolerogenic strategies enhance the ability of lupus Tregs to suppress production of pathogenic autoantibodies, including anti-DNA. These include an induction and expansion of Tregs or the administration of tolerogenic peptides that induce both CD4+ and CD8+ Tregs (10, 15–19). Regarding the latter, the immunotherapeutic potential of CD8+ Tregs in SLE has not been examined thoroughly, although it is known that improved function of CD8+ Tregs in human SLE is associated with disease remission (20–21). We had shown that IL-2 and TGF-β could induce CD8+ cells to become Tregs (22), with a protective activity in humanized mice (23). Notably, when we used both CD4+ and CD8+ Tregs induced ex vivo with IL-2 and TGF-β to suppress lupus-like disease in BDF1 mice, the therapeutic effects were much stronger than when the mice were treated with CD4+ Tregs alone, suggesting an important role of CD8+ Tregs in suppressing lupus autoimmunity (10). Here anti-CD2 Ab targeted CD8+ cells in vivo and induced Foxp3+ Tregs, as known to happen with this stimulus together with CD3 stimulation (24) and in line with the notion that anti-CD2 Ab and the CD2-specific fusion protein alefacept have immunosuppressive effects in autoimmune patients (25–26). Mechanistically, the observed synergy of anti-CD2 and anti-CD4 Ab could underscore two potential non-mutually-exclusive possibilities: 1) antibody administration to target cells with nanoscale reagents could afford multivalency (i.e., multiple copies of antibodies binding the targets would increase avidity, and thus pharmacological effects); 2) targeted proximal release of IL-2 and TGF-β would promote local expansion of Tregs. In this context, the encapsulant released from NPs is known to be most effective within nanoscale distances from the target cell. We previously modeled mathematically the “flattening” of the cell interface as it interacts with the particle, identifying a significantly enhanced magnitude of cytokine accumulation at the cell-particle interface (27–29). This phenomenon of “paracrine effect post-release” suggests that targeting, and therefore ligation, via anti-CD2 and anti-CD4 Ab could bring particles and T cells within nanoscale ligand-receptor distances, increasing the local concentration of cytokines available to cells with great efficacy (5). This phenomenon occurs in systems of artificial antigen presentation, where IL-2 encapsulated in NPs has an equivalent T cell stimulatory effect to soluble IL-2 at 1000-fold higher concentration (27–29). NPs also create a local acidic microenvironment that can convert endogenous latent TGF-β to its active form, and this could synergize with IL-2 in extending Tregs expansion - even after the TGF-β stores in the NPs are depleted. All together, these features suggest an advantage in the use of nanoparticulate delivery systems to afford cytokine delivery at local levels in minute doses, mitigating high dose-related toxicity while retaining high bioactivity.
Presently, the treatment of SLE (and other autoimmune diseases) includes agents that target proinflammatory cytokines, effector cells, or signaling pathways (30). Although those agents can block disease progression, they rarely induce remission because they also target the compensatory regulatory pathways that are required to stop disease. Other attempts to treat SLE have tried to “reset” the immune system to cause remission. For example, lymphoid cell depletion followed by autologous stem cell transplantation resulted in extended disease remission in SLE but this strategy - while valid as proof-of-concept - associated with post-operative patient mortality (31).
Multiple approaches that use ex vivo-expanded CD4+ Tregs in autoimmune diseases are under investigation, especially in type 1 diabetes (32), but carry problems such as the need of autologous Tregs that remain functionally stable in vivo, in addition to requiring technically cumbersome procedures to prepare Tregs in large numbers. Given these considerations, the use of NPs can represent a new therapeutic possibility for the targeting of T cells (or antigen-presenting cells [APCs]) in the induction of immune tolerance. PLGA NPs encapsulating tolerogenic peptides or peptides and rapamycin that targeted APCs prevented/reversed disease in animal models of autoimmune diabetes and multiple sclerosis through TGF-β-dependent induction of Tregs (33–34). Moreover, NP-mediated delivery of immunosuppressive drugs of Ca2+/calmodulin-dependent protein kinase IV (CaMK4) inhibitor ameliorated murine SLE (35–37).
Additionally, a recent study showed that iron oxide NPs coated with MHC-peptides could convert IFN-γ-producing Th1 cells into IL-10-producing Tr1 cells, affording therapeutic effects in mouse models of autoimmune disease (38). Although CD4+ Tregs induced DCs to become tolerogenic and protect secondary hosts (39), that system had limitations. It did not permit encapsulation/sustained release of multiple therapeutic agents, and different MHC-specific peptides would be needed to match the MHC diversity encountered in human autoimmune diseases. Also, extended iron oxide accumulation is toxic. Conversely, biodegradable PLGA NPs - unlike the iron oxide NPs that act on differentiated Th1 cells - acts upstream at the level of T cells that differentiate to become suppressor cells.
Before concluding, we acknowledge that our study raises questions to address in future experiments. For example, we expanded both CD4+ and CD8+ Tregs but do not know the precise relative contribution of each subset on the timing of the response, and possible reciprocal cell interactions in the suppression of lupus manifestations. Another unexplored aspect is that anti-CD2 Ab can also bind NK cells (2), and the contribution of this interaction might be important (since CD8/NK cell interactions induce NK cells to produce TGF-β) (2, 22). In this sense, NPs loaded with IL-2 and a TGF-β inhibitor greatly impacted cancer cells and tumor microenvironment by primarily affecting NK cells (40). We will also need to address whether the induced Tregs could transfer suppressive activity to conventional T cells through “infectious tolerance” (41), and whether the combination of TGF-β and IL-2 protected T cells from apoptosis, e.g. via a modulation of the anti-apoptotic protein Bcl-xL (42). Notwithstanding these considerations, our findings suggest that NPs that carry cytokines that are deficient in SLE (43) could represent a novel strategy to restore tolerogenic responses in the disease, and that targeting CD2 and CD4 simultaneously might facilitate synergistic therapeutic effects.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the NIH grants AI109677 (to ALC) and GM007205 (to SB).
Footnotes
Disclosure: D.A.H. is Chief Executive Officer at General Nanotherapeutics, LLC.
Study conception and design
D. A. Horwitz, T. M. Fahmy, A. La Cava.
Acquisition of data
S. Bickerton, A. La Cava.
Analysis and interpretation of data
D. A. Horwitz, S. Bickerton, M. Koss, T. M. Fahmy, A. La Cava.
REFERENCES
- 1.Ferretti C, La Cava A. Overview of the pathogenesis of systemic lupus erythematosus In: Tsokos GC, editor. Systemic lupus erythematosus. Basic, applied and clinical aspects. Cambridge: Academic Press; 2016. P. 55–62. [Google Scholar]
- 2.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-β: contrasting effects of anti-CD2 and anti-CD3. J Immunol 1998;160:2248–54. [PubMed] [Google Scholar]
- 3.von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, Enghard P, Sawitzki B, Hiepe F, Radbruch A, Burmester GR, Riemekasten G, Humrich JY. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 2016;75:1407–15. [DOI] [PubMed] [Google Scholar]
- 4.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X,Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med 2016;22:991–3. [DOI] [PubMed] [Google Scholar]
- 5.McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials 2015;59:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinesh RK, Skaggs BJ, La Cava A, Hahn BH, Singh RP. CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun Rev 2010;9:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGF-β-secreting CD8+ T cell suppressors. J Immunol 2005;175:7728–37. [DOI] [PubMed] [Google Scholar]
- 8.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today 1988;9:207–13. [DOI] [PubMed] [Google Scholar]
- 9.Rus VA, Svetic A, Nguyen P, Gausen WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease: regulatory role of donor CD8+ T cells. J Immunol 1995;155:2396–406. [PubMed] [Google Scholar]
- 10.Zheng SG, Wang JH, Koss MN, Quismorio F, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-β suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol 2004;172:1531–9. [DOI] [PubMed] [Google Scholar]
- 11.Fahmy TM, Samstein RM, Harness CC, Saltzman MW. Surface modification of biodregradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials 2005;26:5727–36. [DOI] [PubMed] [Google Scholar]
- 12.Lourenço EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci USA 2016;113:10637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrera F, Hahn BH, Rizzi M, Anderson M, Fitzgerald J, Millo E, Indiveri F, Shi FD, Filaci G, La Cava A. Protection against renal disease in (NZB x NZW)F1 lupus-prone mice after somatic B cell gene vaccination with anti-DNA immunoglobulin consensus peptide. Arthritis Rheum 2007;56:1945–53. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T cell lineage outcomes with targeted nanoparticle mediated cytokine delivery. Mol Pharm 2011;8:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black x New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol 2004;173:3542–8. [DOI] [PubMed] [Google Scholar]
- 16.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol 2007;178:7649–57. [DOI] [PubMed] [Google Scholar]
- 17.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol 2005;174:3247–55. [DOI] [PubMed] [Google Scholar]
- 18.Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J Immunol 2008;181:3243–51. [DOI] [PubMed] [Google Scholar]
- 19.Scalapino KJ, Daikh DI. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS One 2009;24:e6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol 2012;189:2118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-β-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol 2009;183:6346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirokawa M, Horwitz DA. The role of transforming growth factor β in the generation of suppression: an interaction between CD8+T and NK cells. J. Exp Med 1994;180:1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz DA, Pan S, Ou JN, Wang J, Chen M, Gray JD, Zheng SG. Therapeutic polyclonal human CD8+CD25+FoxP3+TNFR2+PD-L1+ regulatory cells induced ex vivo. Clin Immunol 2013;149:450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol 2005;174:6993–7005. [DOI] [PubMed] [Google Scholar]
- 25.Hafler DA, Ritz J, Schlossman SF, Weiner HL. Anti-CD4 and anti-CD2 monoclonal antibody infusions in subjects with multiple sclerosis. Immunosuppressive effects and human anti-mouse responses. J Immunol 1988;141:131–8. [PubMed] [Google Scholar]
- 26.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Shippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 2015;125:3285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labowsky M, Lowenthal J, Fahmy TM. An in silico analysis of nanoparticle/cell diffusive transfer: application to nano-artificial antigen-presenting cell:T-cell interaction. Nanomedicine 2015;11:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labowsky M, Fahmy TM. Diffusive transfer between two intensely interacting cells with limited surface kinetics. Chem Eng Sci 2012;74:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J Biol Chem 2011;286:34883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong M, La Cava A. Lupus, the current therapeutic approaches. Drugs Today (Barc) 2011;47:289–302. [DOI] [PubMed] [Google Scholar]
- 31.Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, Verda L, Krosnjar N, Quigley K, Yaung K, Villa Bs M, Takahashi M, Jovanovic B, Oyama Y. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006;295:527–35. [DOI] [PubMed] [Google Scholar]
- 32.Gitelman SE, Bluestone JA. Regulatory T cell therapy for type 1 diabetes: may the force be with you. J Autoimmun 2016;71:78–87. [DOI] [PubMed] [Google Scholar]
- 33.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD and Miller SD. A biodegradable nanoparticle platform for the induction of antigen-specific tolerance for treatment of autoimmune disease. ACS Nano 2014;25:2148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA 2015;112:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, Fahmy TM. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J Clin Invest 2013;123:1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otomo K, Koga T, Mizui M, Yoshida N, Kriegel C, Bickerton S, Fahmy TM, Tsokos GC. Cutting edge: nanogel-based delivery of an iinhibitor of CaMK4 to CD4+ T cells suppresses experimental autoimmune encephalomyelitis and lupus-like disease in mice. J Immunol 2015;195:5533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda K, Otomo K, Yoshida N, Abu-Asab MS, Ichinose K, Nishino T, Kono M, Ferretti A, Bhargava R, Maruyama S, Bickerton S, Fahmy TM, Tsokos MG, Tsokos GC. CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. J Clin Invest. 2018;128:3445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016;530:434–40. [DOI] [PubMed] [Google Scholar]
- 39.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, Liu Z, Zheng SG. Polyclonal CD4+Foxp3+ Treg cells induce TGF-β-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol 2012;4:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 2012;11:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β-dependent manner. J Exp Med 2008;205:1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28- regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci USA 2007;104:17459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest 2015;125:2220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.