Abstract
Children of women treated with antidepressants during pregnancy are more likely to develop neurodevelopmental problems than are unexposed children. Associations between prenatal antidepressant exposure and neurodevelopmental problems could reflect a causal effect or could be partially or fully explained by other factors that differ between exposed and unexposed offspring, including having mothers with conditions requiring antidepressant treatment (e.g., depression), environmental risk factors, and/or genetic risk factors shared across disorders.
This translational review aims to provide a brief overview of findings from rodent experiments and critically evaluate observational studies in humans to assess the extent to which associations between prenatal antidepressant exposure and neurodevelopmental problems are due to causal mechanisms versus other influences. We focus our review on two important neurodevelopmental outcomes – autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD).
In general, rodent studies have reported adverse effects of perinatal antidepressant exposure on neurodevelopment. Between-species differences raise questions about the generalizability of these findings to humans. Indeed, converging evidence from studies using multiple designs and approaches suggest that observed associations between prenatal antidepressant exposure and neurodevelopmental problems in humans are largely due to confounding factors.
We also provide specific recommendations for future research. Animal research should explicitly evaluate the impact of timing of exposure and dosage of medications, as well as better map outcome measures in rodents to human neurodevelopmental problems. Observational studies should investigate specific confounding factors, specific antidepressant drugs and classes, the potential impact of timing of exposure, and a wider range of other potential offspring outcomes.
The findings summarized in this review may help women and their doctors make informed decisions about antidepressant use during pregnancy by providing reassurance that use of these medications during pregnancy is unlikely to substantially increase the risk of ASD and ADHD.
Keywords: antidepressants, pregnancy, prenatal antidepressant exposure, neurodevelopmental problems, autism spectrum disorder, attention-deficit/hyperactivity disorder, causal inference
Introduction
Antidepressant use, particularly use of selective serotonin reuptake inhibitors (SSRIs), among pregnant women is common and increasing (Andrade et al., 2016; Jimenez-Solem et al., 2013). For example, in a sample of 1.9 million insured pregnant women in the U.S., approximately 2% filled a prescription for an SSRI during pregnancy in 2001, whereas 12% filled a prescription for an SSRI in 2013 (Andrade et al., 2016). Concerns that prenatal antidepressant exposure may be harmful to fetal development have led some medical professionals to recommend that pregnant women avoid using antidepressants (e.g., Campagne, 2007). These recommendations are informed by a history of research on teratology – the study of agents that may alter fetal development and, thereby, increase the risk of proximal adverse effects (e.g., death, reduced fetal growth, and birth defects) or neurological impairments that appear later in childhood (Alwan & Chambers, 2015; Georgieff, Tran, & Carlson, 2018). Perhaps the most salient example of a teratogen is thalidomide -- a medication that was widely prescribed to treat nausea during pregnancy until it was found to cause severe birth defects (Alwan & Chambers, 2015). Though the teratogenic effects of thalidomide are well established, the potential effects of many other medications (e.g., antidepressants and mood stabilizers/antiepileptic medications) are less clear (Galbally, Crabb, & Snellen, 2018; Rubin, 2018). Animal and human research has attempted to answer the questions of whether prenatal antidepressant exposure increases the risk of adverse outcomes in offspring. Although considering the potential consequences of prenatal antidepressant exposure on a wide range of offspring outcomes is important for clinical decision-making, this review will focus on offspring neurodevelopment in particular.
Rodent studies have shown that perinatal antidepressant exposure causes abnormalities in brain structure and function, as well as adverse behavioral outcomes (Ko, Lee, & Li, 2014; Lee, 2009; Rodriguez-Porcel et al., 2011; Schaefer et al., 2013; Simpson et al., 2011; Sprowles et al., 2016; Weaver, Paul, Lin, & Simpson, 2010; Zimmerberg & Germeyan, 2015). Additionally, observational studies with humans have shown that antidepressants cross the placenta (Heikkinen, Ekblad, & Laine, 2001, 2002; Loebstein, Lalkin, & Koren, 1997) and can be found in amniotic fluid (Fokina et al., 2016; Hostetter, Ritchie, & Stowe, 2000; Loughhead, Fisher, et al., 2006) and cord blood (Fokina et al., 2016; Hendrick et al., 2003; Loughhead, Stowe, et al., 2006; Rampono, Proud, Hackett, Kristensen, & Ilett, 2004; Rampono et al., 2009; Sit et al., 2011). Observational studies have also documented associations between prenatal antidepressant exposure and adverse offspring outcomes, including neurodevelopmental problems, such as autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD; Morales, Slattery, Evans, & Kurz, 2018).
However, it is important to consider that these findings may not reflect a causal effect of prenatal antidepressant exposure. While randomization in rodent experiments helps ensure that there are no systematic differences between the exposed and unexposed, it is unclear if effects in rodents apply to humans given between-species differences related to pregnancy and drug metabolism rates (Bourke, Stowe, & Owens, 2014). Additionally, associations between prenatal antidepressant exposure and neurodevelopmental problems documented in observational studies may be due to systematic differences between exposed and unexposed offspring. Unlike randomized control trials where the exposed and unexposed group could theoretically be swapped without alternating results, exposed and unexposed individuals in observational studies are not guaranteed to be exchangeable with respect to their pre-exposure risk of the outcome. If some of the determinants of exposure also influence the risk of outcome, the exposure and outcome will appear associated. This means that in addition to a potential causal influence of prenatal antidepressant exposure, other factors that differ between exposed and unexposed offspring could contribute to observed associations. Factors other than the exposure of interest that contribute to an observed association are referred to as confounding factors. It is important to note that multiple plausible influences, including causal mechanisms and/or confounding, could simultaneously contribute to the associations between prenatal antidepressant exposure and neurodevelopmental problems.
Figure 1 presents a simplified model to illustrate some of the main processes through which prenatal antidepressant exposure could be associated with neurodevelopmental problems. As depicted by the solid arrows labeled a and b in Figure 1, prenatal antidepressant exposure could cause an increased risk of neurodevelopmental problems through mechanisms of action. For example, the developmental hyperserotonemia (DHS) model of autism postulates that high levels of maternal blood serotonin could enter the developing brain of the fetus and cause a loss of serotonin receptors through a negative feedback mechanism. In support of the DHS model, research has documented that individuals with ASD have elevated blood levels of serotonin (Hranilovic et al., 2007; Naffah-Mazzacoratti et al., 1993) and decreased activity, synthesis, and binding potential of serotonin in several brain areas (Chugani et al., 1999; Chugani et al., 1997; Hadjikhani, 2010; Murphy et al., 2006; Yang, Tan, & Du, 2014).
Figure 1.
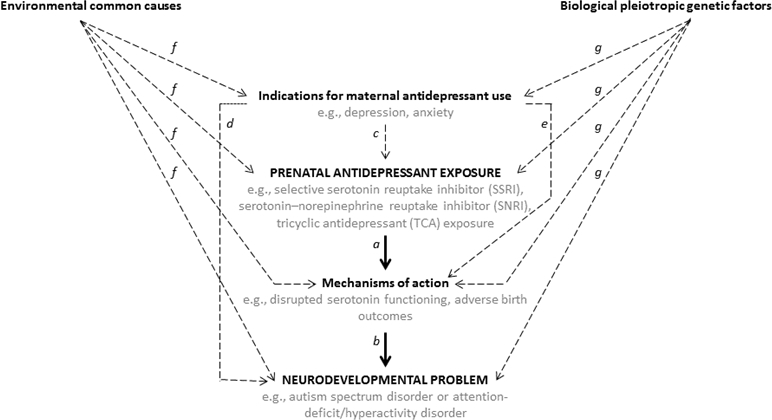
Hypothesized influences on neurodevelopmental outcomes among offspring exposed to antidepressants during pregnancy. Solid lines a and b represent a plausible causal pathway from prenatal antidepressant exposure to neurodevelopmental problems through mechanisms of action. Dashed lines represent plausible confounding from indications for maternal antidepressant use (paths c, d, and e), environmental common causes (path f), and/or biological pleiotropic genetic factors (path g).
Another possible causal pathway is through adverse birth outcomes, such as reduced fetal growth and shorter gestation. Numerous studies, including studies that used advanced methods to account for potential confounding factors, have found associations between prenatal antidepressant exposure and adverse birth outcomes (e.g., Huang, Coleman, Bridge, Yonkers, & Katon, 2014; Sujan et al., 2017; Viktorin et al., 2016) and associations between adverse birth outcomes and neurodevelopmental problems, including ASD and ADHD (e.g., Class, Rickert, Larsson, Lichtenstein, & D’Onofrio, 2014; D’Onofrio et al., 2013; Murray et al., 2016; Pettersson et al., 2015).
Aside from causal mechanisms, there are several non-causal processes that could partially or fully explain observed associations between prenatal antidepressant exposure and neurodevelopmental problems. In order to introduce this possibility, we review several such processes below.
As illustrated in Figure 1 by the dotted arrow c, maternal indications for antidepressant use are determinants of prenatal antidepressant exposure. Although antidepressants can be prescribed to treat a variety of conditions, such as insomnia, pain, and migraines, their primary use is for treatment of depressive and anxiety disorders (Wong et al., 2016), which can be influenced by stressful life events (e.g., Kendler, Karkowski, & Prescott, 1999; Pine, Cohen, Johnson, & Brook, 2002). Moreover, several observational studies have shown that stress during pregnancy, as well as depression, anxiety, and other maternal psychiatric conditions, are associated with increased risk of offspring ASD (Class, Abel, et al., 2014; Rai et al., 2013) and ADHD (Class, Abel, et al., 2014; Grizenko, Fortier, Gaudreau-Simard, Jolicoeur, & Joober, 2015; Martini, Knappe, Beesdo-Baum, Lieb, & Wittchen, 2010; O’Connor, Heron, Golding, Beveridge, & Glover, 2002; O’Connor, Heron, Golding, & Glover, 2003; Van den Bergh & Marcoen, 2004). That is, as shown by the dotted arrow labeled d in Figure 1, maternal underlying conditions could influence offspring neurodevelopment. For example, maternal depressive symptoms may lead to poorer parenting practices (e.g., Lovejoy, Graczyk, O’Hare, & Neuman, 2000), which in turn are associated with offspring neurodevelopmental problems (e.g., Ellis & Nigg, 2009). Alternatively, as shown by the dotted arrow labeled e in Figure 1, maternal conditions like depression could also influence mechanisms, such as adverse birth outcomes (Bonari et al., 2004). Thus, there are multiple plausible pathways through which the maternal condition for which antidepressants are used during pregnancy could contribute to an increased likelihood for the exposed offspring to develop ASD or ADHD.
As shown by the dotted arrows labeled f in Figure 1, other environmental factors that could influence indications for maternal antidepressant use may, through some mechanism of action, also influence the risk of neurodevelopmental problems. There are several plausible candidates for such environmental common causes of prenatal antidepressant exposure and neurodevelopmental outcomes. For example, low socioeconomic status predicts the development of depression (Lorant et al., 2003) and is associated with reduced availability of healthy foods (Chai, Fan, & Wen, 2018), and poorer nutrition has been linked with cognitive (Ampaabeng & Tan, 2013), behavioral (Galler et al., 2012), and attention (Galler et al., 2012) problems. Maternal conditions that could contribute to an adverse intrauterine environment, such as preexisting health problems (e.g., high body mass index, diabetes, and chronic hypertension), are also associated with antidepressant use during pregnancy, and, thus, may confound associations (Ericson, Kallen, & Wiholm, 1999; Reis & Kallen, 2010).
Additionally, prenatal antidepressant exposure and neurodevelopmental problems may share genetic influences. The phenomenon that the same genetic factors can influence multiple phenotypes and, thus, confound associations between phenotypes is known as biological pleiotropy (Solovieff, Cotsapas, Lee, Purcell, & Smoller, 2013). The potential influence of biological pleiotropy is depicted by the dotted arrows labeled g on Figure 1, which show that genetic factors could influence indications for maternal antidepressant use during pregnancy, as well as the liability for neurodevelopmental problems in offspring. Research has provided support for the influence of biological pleiotropy. Specifically, twin studies (Cole, Ball, Martin, Scourfield, & McGuffin, 2009; Scherff et al., 2014) and genome-wide association studies (Lee et al., 2013) have suggested that genetic liability is shared across internalizing problems – such as depression and anxiety disorders – and neurodevelopmental disorders – such as ASD and ADHD.
In sum, observed associations between prenatal antidepressant exposure and neurodevelopmental problems could be due to several influences, including a causal effect of the exposure, indications for maternal antidepressant use, environmental common causes, and/or biological pleiotropy. Importantly, these influences are not necessarily mutually exclusive. For example, an observed association could be partly due to a causal effect of prenatal antidepressant exposure and partly due to maternal depression, indicating that the true increase in risk attributable to antidepressant exposure is nonzero but less than might be expected based on the overall association.
Aim of the Review
The primary aim of this translational review is to briefly summarize findings from experimental studies in rodents and critically evaluate evidence from observational studies in humans, with the goal of assessing the extent to which the observed associations between prenatal antidepressant exposure and neurodevelopmental problems are due to causal mechanisms versus other alternative influences. The outcomes we focus on in observational studies are ASD and ADHD. We focus on these outcomes for several reasons. First, they are two of the most common neurodevelopmental problems with approximately one in 68 children having ASD (Christensen et al., 2016) and one in 19 children having ADHD (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015), and they frequently co-occur (Leitner, 2014). Second, they are associated with significant impairment. Third, they are the most studied neurodevelopmental outcomes associated with prenatal antidepressant exposure. We conclude the paper by providing recommendations for future research directions and discussing implications for clinical practice.
Literature search method
We used Web of Science and Google Scholar to conduct our literature review. We also reviewed work cited in relevant identified publications. We aimed to identify a representative sample of studies examining the effects of perinatal antidepressant exposure on brain and behavioral outcomes in rodents. In contrast, we aimed to identify all observational studies and meta-analyses on prenatal antidepressant exposure and risk for ASD and ADHD in humans published prior to June 1st, 2018. We identified 4 individual studies examining both ASD and ADHD, 13 individual studies examining ASD but not ADHD, and 5 individual studies examining ADHD but not ASD (Tables S1 and S2). We excluded one descriptive study on ASD that reported a crude association and the prevalence of prenatal antidepressant exposure among ASD cases and controls but did not use any method to account for confounding (Eriksson, Westerlund, Anderlid, Gillberg, & Fernell, 2012).
We identified 1 meta-analysis on ASD and ADHD, 8 meta-analyses on ASD but not ADHD, and 2 meta-analysis on ADHD but not ASD (Table 2). Although no single meta-analyses included all individual studies, the meta-analysis conducted by Morales et al. (2018) was the most comprehensive both in terms of number of studies and different designs included.
Table 2.
Meta-analyses of observational studies
| Study | Exposure | Outcome | Included studies | Tested for confounding by unmeasured factors? | Pooled Association | |
|---|---|---|---|---|---|---|
| n | Specific studies | |||||
| Andalib et al. (2017) | DP SSRI | ASD | 7 | 2,6,9,11,12,17,19 | No | aOR=1.8,95% CI:1.6–2.1 |
| Brown, Hussain-Shamsy, et al. (2017) | DP SSRI | ASD | 4 | 5,6,11,17 | No | aOR=1.4,95% CI:1.0–2.0 |
| DP SSRI | 3 | 5,6,17 | No (MP adjusted) | aOR=1.4,95% CI:0.9–2.2 | ||
| DP SSRI | 2 | 2,12 | No (MP adjusted) | aOR=1.5,95% CI:0.9–2.7 | ||
| 1T SSRI | 4 | 5,6,11,17 | No | aOR=1.7,95% CI:1.1–2.6 | ||
| 1T SSRI | 3 | 5,6,17 | No (MP adjusted) | aOR=1.8,95% CI:1.1–3.1 | ||
| Jiang et al. (2017) | BP AD | ADHD | 3 | 15,16,20 | Yes | aHR=1.8,95% CI:1.5–2.2 |
| BP SSRI | 3 | 15,16,20 | Yes | aHR=1.6,95% CI:1.3–1.9 | ||
| DP AD | 6 | 1,10,13,15,16,20 | No | aHR=1.3,95% CI:1.1–1.6 | ||
| DP AD | 2 | 15,16 | Yes (Compared to BP AD exposure) | aHR=0.9,95% CI:0.7–1.1 | ||
| DP AD | 2 | 10, 15 | No (Compred to MP exposure) | aHR=1.0,95% CI:0.8–1.2 | ||
| DP AD | 3 | 13,16,20 | Yes (Sibling comapriosn) | aHR=0.9,95% CI:0.7–1.1 | ||
| DP SSRI | 5 | 1,13,15,16,20 | No | aHR=1.5,95% CI:1.4–1.6 | ||
| DP non-SSRI | 3 | 1,13,16 | No | aHR=1.5,95% CI:1.2–1.8 | ||
| 1T AD | 4 | 1,13,16,20 | No | aHR=1.4,95% CI:1.3–1.5 | ||
| 2T/3T AD | 3 | 1,13,16, | No | aHR=1.4,95% CI:1.2–1.6 | ||
| Kaplan et al. (2017) | DP SSRI | ASD | 3 | 7,12,15 | No | aHR=1.6,95% CI:1.2–2.3 |
| DP SSRI | 4 | 7,12,15,19 | No | aHR=1.7,95% CI:1.3–2.1 | ||
| Kaplan et al. (2016) | BP SSRI | ASD | 3 | 5,6,9 | Yes (Timing of exposure) | aOR=1.8,95% CI:1.5–2.3 |
| DP SSRI | 5 | 5,6,9,11,17 | No | aOR=1.7,95% CI:1.2–2.2 | ||
| DP non-SSRI AD | 3 | 5,6,17 | No | aOR=2.1,95% CI:1.2–3.5 | ||
| 1T SSRI | 4 | 5,6,9,11 | No | aOR=1.9,95% CI:1.3–2.8 | ||
| 2T SSRI | 4 | 5,6,9,11 | No | aOR=1.7,95% CI:1.2–2.6 | ||
| 3T SSRI | 4 | 5,6,9,11 | No | aOR=1.6,95% CI:0.8–3.2 | ||
| 3T SSRI | 3 | 5,6,9 | No | aOR=2.5,95% CI:1.7–3.6 | ||
| 3T SSRI | 3 | 5,6,11 | No | aOR=1.1,95% CI:0.7–1.9 | ||
| Kobayashi et al. (2016) | DP SSRI | ASD | 7 | 5,6,7,11,12,16,17 | No | aOR=1.5,95% CI:1.2–1.8 |
| DP SSRI | 7 | 5,6,7,11,16,17,19 | No | aOR=1.6,95% CI:1.3–1.9 | ||
| DP SSRI | 5 | 5,6,11,16,17 | No | aOR=1.4,95% CI:1.1–1.7 | ||
| DP SSRI | 2 | 7,12 | No | aOR=1.7,95% CI:0.8–3.6 | ||
| DP SSRI | 2 | 7,19 | No | aOR=1.9,95% CI:1.2–3.0 | ||
| DP SSRI | 3 | 7,11,12 | No (Compared to MP exposure) | aOR=1.0,95% CI:0.6–1.6 | ||
| DP SSRI | 3 | 7,11,19 | No (Compared to MP exposure) | aOR=1.2,95% CI:0.7–2.1 | ||
| DP SSRI | 2 | 6,17,19 | Yes (Other ADs as active comparator) | cOR=1.1,95% CI:0.7–2.0 | ||
| Man et al. (2015) | DP SSRI | ASD | 4 | 6,9,11,17 | No | aOR=1.8,95% CI:1.5–2.2 |
| Man et al. (2018) | BP AD | ADHD | 5 | 5,8,15,16,20 | Yes (Timing of exposure) | aRR=1.6,95% CI:1.3–2.0 |
| DP AD | 7 | 1,5,8,13,15,16,20 | No | aRR=1.4,95% CI:1.2–1.6 | ||
| DP AD | 3 | 13,16,20 | Yes (Sibling comparison) | aRR=0.9,95% CI:0.8–1.2 | ||
| 1T AD | 6 | 1,4,8,13,16,20 | No | aRR=1.3,95% CI:1.0–1.6 | ||
| 1T AD | 4 | 1,13,16,20 | No | aRR=1.3,95% CI:1.0–1.6 | ||
| 1T AD | 2 | 4,8 | No | aRR=1.1,95% CI:0.5–2.6 | ||
| 2T AD | 5 | 1,4,8,13,16 | No | aRR=1.4,95% CI:1.2–1.7 | ||
| 2T AD | 2 | 1,13,16 | No | aRR=1.4,95% CI:1.2–1.6 | ||
| 2T AD | 2 | 4,8 | No | aRR=1.8,95% CI:0.7–5.1 | ||
| 3T AD | 5 | 1,4,8,13 | No | aRR=1.1,95% CI:0.7–1.5 | ||
| 3T AD | 2 | 1,13 | No | aRR=1.3,95% CI:1.1–1.6 | ||
| 3T AD | 2 | 4,8 | No | aRR=0.6,95% CI:0.3–1.1 | ||
| Mezzacappa et al. (2017) | BP AD | ASD | 4 | 4,5,6,9 | Yes (Timing of exposure) | aOR=2.0,95% CI:1.7–2.3 |
| BP AD | 4 | 4,5,6,9 | Yes (Timing of exposure; MP adjsuted) | aOR=1.8,95% CI:1.5–2.1 | ||
| DP AD | 6 | 4,5,6,9,11,17 | No | aOR=1.8,95% CI:1.5–2.0 | ||
| DP AD | 5 | 4,5,6,9,17 | No (MP adjusted) | aOR=1.5,95% CI:1.1–2.1 | ||
| DP AD | 2 | 2,12 | No (MP adjusted) | aHR=1.3,95% CI:0.9–1.7 | ||
| 1T AD | 6 | 4,5,6,9,11,17 | No | aOR=2.1,95% CI:1.7–2.6 | ||
| 1T AD | 5 | 4,5,6,9,17 | No (MP adjusted) | aOR=1.7,95% CI:1.3–2.5 | ||
| 1T AD | 2 | 2,12 | No (MP adjusted) | aHR=1.1,95% CI:0.7–1.8 | ||
| 2T AD | 5 | 4,5,6,9,11 | No | aOR=2.0,95% CI:1.6–2.6 | ||
| 2T AD | 4 | 4,5,6,9 | No (MP adjusted) | aOR=1.7,95%CI:1.1–2.5 | ||
| 3T AD | 5 | 4,5,6,9,11 | No | aOR=2.0,95% CI:1.7–2.3 | ||
| 3T AD | 4 | 4,5,6,9 | No (MP adjusted) | aOR=1.5, 95% CI:0.8–2.9 | ||
| Morales et al. (2018) | BP AD | ASD | 7 | 2,3,4,5,6,12,20 | Yes (Timing of exposure) | aRR=1.5,95% CI:1.3–1.7 |
| DP AD | 10 | 2,3,4,5,6,7,11,12,15,17,20 | No | aRR=1.5,95% CI:1.3–1.8 | ||
| DP AD | 6 | 2,3,11,12,15,21 | No (MP adjusted) | aRR=1.2,95% CI:0.9–1.5 | ||
| DP AD | 3 | 3,19,20 | Yes (Sibling comparison) | aRR=1.0,95% CI:0.7–1.4 | ||
| Paternal DP AD | 2 | 19,20 | Yes (Paternal comparison) | aRR=1.3,95% CI:1.1–1.5 | ||
| 1T AD | 7 | 2,4,5,6,11,12,20 | No | aRR=1.4,95% CI:1.1–1.8 | ||
| 2T AD | 5 | 4,5,6,9,11 | No | aRR=1.6,95% CI:1.2–2.2 | ||
| 3T AD | 5 | 4,5,6,9,11 | No | aRR=1.5,95% CI:0.9–2.5 | ||
| BP AD | ADHD | 5 | 4,5,8,16,20 | Yes (Timing of exposure) | aRR=1.4,95% CI:1.1–1.7 | |
| DP AD | 7 | 4,5,8,13,15,16,20 | No | aRR=1.4,95% CI:1.1–1.7 | ||
| DP AD | 3 | 13,16,20 | Yes (Sibling comparison) | aRR=0.9,95% CI:0.7–1.1 | ||
| 1T AD | 6 | 4,5,8,13,16,20 | No | aRR=1.4,95% CI:1.0–1.8 | ||
| 2T AD | 5 | 4,5,8,13,16 | No | aRR=1.3,95% CI:0.9–1.7 | ||
| 3T AD | 5 | 4,5,8,13,16 | No | aRR=0.9,95% CI:0.4–1.9 | ||
| Zhou et al. (2018) | BP AD | ASD | 2 | Not reported | Yes (Timing of exposure) | aRR=1.3, 95% CI:0.9–1.7 |
| BP AD | 4 | Not reported | Yes (Timing of exposure) | aOR=1.7, 95% CI:1.4–2.0 | ||
| BP SSRI | 2 | Not reported | Yes (Timing of exposure) | aOR=1.8, 95% CI:1.4–2.3 | ||
| DP AD | 7 | 2,3,12,15,18,20.21 | No | aRR=1.1, 95% CI:0.9–1.4 | ||
| DP AD | 6 | 4,5,6,9,11,17 | No | aOR=1.5, 95% CI:1.2–2.0 | ||
| DP AD | 5 | 3,15,18,19,20 | Yes (Four were sibling comparisons) | aRR=1.0, 95% CI:0.8–1.2 | ||
| DP AD | 4 | 4,5,9,17 | No (MP adjsuted) | aOR=1.4, 95% CI:1.0–2.0 | ||
| DP SSRI | 3 | Not reported | No | aRR=1.2, 95% CI:0.8–1.8 | ||
| DP SSRI | 4 | Not reported | No | aOR=1.8, 95% CI:1.5–2.2 | ||
| 1T AD | 5 | Not reported | No | aRR=1.0, 95% CI:0.8–1.2 | ||
| 1T AD | 5 | Not reported | No | aOR=1.7, 95% CI:1.2–2.4 | ||
| 1T SSRI | 3 | Not reported | No | aRR=1.0, 95% CI:0.8–1.3 | ||
| 1T SSRI | 2 | Not reported | No | aOR=2.1, 95% CI:1.6–2.8 | ||
| 2T AD | 5 | Not reported | No | aOR=1.6, 95% CI:1.2–2.2 | ||
| 2T SSRI | 3 | Not reported | No | aOR=1.9, 95% CI:1.4–2.7 | ||
| 3T AD | 5 | Not reported | No | aOR=1.5, 95% CI:0.9–2.4 | ||
| 3T SSRI | 3 | Not reported | No | aOR=2.3, 95% CI:1.6–3.3 | ||
| 2T/3T AD | 4 | Not reported | No | aRR=1.4, 95% CI:1.0–1.9 | ||
| 2T/3T SSRI | 3 | Not reported | No | aRR=1.4, 95% CI:0.8–2.4 | ||
BP=before pregnancy. DP=during pregnancy. 1T=1st trimester. 2T=2nd trimester. 3T=3rd trimester. AD=antidepressant. SSRI=selective serotonin reuptake inhibitor. ASD=autism spectrum disorder. ADHD=attention-deficit/hyperactivity disorder. MP=maternal psychopathology. aOR=adjusted odds ratio. aHR=adjusted hazard ratio. aRR=adjusted relative risk. cOR=crude odds ratio. The following studies were included in the meta-analyses: [1]Boukhris, Sheehy, and Berard (2017), [2]Boukhris et al. (2016), [3]Brown, Ray, et al. (2017), [4]Castro et al. (2016), [5]Clements et al. (2015), [6]Croen et al. (2011), [7]El Marroun et al. (2014), [8]Figueroa (2010), [9]Gidaya et al. (2014), [10]Grzeskowiak et al. (2016), [11]Harrington, Lee, Crum, Zimmerman, and Hertz-Picciotto (2014), [12]Hviid, Melbye, and Pasternak (2013), [13]Laugesen et al. (2013), [14]Liu et al. (2017), [15]Malm et al. (2016), [16]Man et al. (2017), [17]Rai et al. (2013), [18]Rai et al. (2017), [19]Sorensen et al. (2013), [20]Sujan et al. (2017), [21]Viktorin, Uher, Reichenberg, Levine, and Sandin (2017).
Rodent Studies
As controlled experiments, rodent studies have two major strengths. First, they allow very tight control of exposure, effectively avoiding bias from exposure misclassification (i.e., measurement error). Second, randomization of exposure eliminates any bias from confounding by ensuring that there are no systematic differences between the exposed and unexposed rodents.
Here we provide a succinct overview of findings from rodent experiments because other in-depth reviews on effects of perinatal antidepressant exposure in rodents have been published recently (e.g., Zucker, 2017). We review studies on intrauterine antidepressant exposure, as well as studies of early postnatal antidepressant exposure. We include early postnatal studies because rodent models of risk factors occurring late in pregnancy often are conducted on neonates due to rodents having shorter gestational periods than humans (Bourke et al., 2014).
Findings
We review rodent studies that explored several domains associated with neurodevelopment to illustrate the findings from animal studies. Table 1 summarizes methods and findings from a sample of rodent studies that explored multiple outcomes related to neurodevelopmental problems.
Table 1.
Rodent studies
| Exposure | ||||
|---|---|---|---|---|
| Study | Animal | Timing | Medication | Findings |
| Christensen et al. (2000) | CD-1 mice | GD −14 to PD 0 | 30 mg/kd/day paroxetinea | Failed to find an effect on learning and memory. |
| Ko et al. (2014) | Wistar rats | PD 0 to 4 | 20 mg/kg 2xd fluoxetinea | Reduced social behavior. |
| Lee (2009) | Wistar rats | PD 0 to 6 | 10 mg/kg fluoxetinea | Functional and structural changes to the somatosensory system. |
| McAllister et al. (2012) | C57BL/6 mice | GD 15 to PD 12 | 30 mg/kd/day fluoxetinea | Failed to find effects on spatial memory and memory retention. |
| Rodriguez-Porcel et al. (2011) | Long Evans rats | PD 8 to 21 | 10mg/kg 2xd citaloprama, 5 mg/kg 2xd fluoxetinea, or 15mg/kg 2xd buproprion | Reduced social behavior. |
| Schaefer et al. (2013) | Sprague–Dawley CD rats | PD 11 to 20 | 5 mg/kg 2xd or 7.5 mg/kg 2xd citaloprama | Caused learning deficits. |
| Simpson et al. (2011) | Rats | PD 8 to 21 | 20 mg/kg citaloprama | Caused altered myelination of corpus callosum axons. Reduced social behavior. |
| Sprowles et al. (2016) | Sprague-Dawley rats | GD 6 to 21 and PD 1 to 20 | 10 mg/kg 2xd citaloprama | Caused learning deficits. |
| Vorhees et al. (1994) | Sprague–Dawley CD rats | GD 7 to PD 20 | 1 mg/kg/d, 5 mg/kg/d, or 12 mg/kg/d fluoxetinea | Failed to find an effect on spatial memory. |
| Weaver et al. (2010) | Long Evans rats | PD 8 to 21 | 5 mg/kg, 10 mg/kg, or 20 mg/kg citaloprama | Reduced hippocampal serotonin transporter fiber density. |
| Zimmerberg and Germeyan (2015) | Norway rats | PD 2 to 7 | 10mg/kg/d fluoxetinea | Reduced social behavior. |
PD=postnatal day. GD=gestational day.
Selective serotonin reuptake inhibitor.
Significant findings.
Several rodent studies have shown that perinatal antidepressant exposure causes abnormalities in brain structure and function, as well as behavioral outcomes.
Abnormalities in brain structure and function.
Rodent studies have shown that perinatal exposure to SSRIs can alter serotonin circuitry (e.g., reduce serotonin transporter fiber density; Weaver et al., 2010). Rodent studies have also shown that SSRI exposure can cause structural and functional changes in brain areas that have been implicated in ASD (Khan et al., 2015) and ADHD (Seidman, Valera, & Makris, 2005) in humans, such as the somatosensory cortex (Lee, 2009) and the corpus callosum (Simpson et al., 2011).
Behavioral outcomes.
Several studies have also demonstrated effects of perinatal antidepressant exposure on behaviors related to neurodevelopmental problems. For example, perinatal antidepressant exposure reduced social behavior (Ko et al., 2014; Rodriguez-Porcel et al., 2011; Simpson et al., 2011; Zimmerberg & Germeyan, 2015) and caused learning deficits (Schaefer et al., 2013; Sprowles et al., 2016) in rats. As shown in Table 1, these studies have used a variety of strains of rats and mice, exposure time periods, medication dosages, and types of antidepressants, though medications are primarily from the SSRI class.
Null findings.
Though several rodent studies have reported effects of perinatal antidepressant exposure on neurodevelopment, a few rodent studies have failed to find such effects. For example, some published studies have reported that perinatal SSRI exposure did not have a statistically significant effect on some indices of learning and memory (Christensen, Rayburn, & Gonzalez, 2000; McAllister, Kiryanova, & Dyck, 2012; Vorhees et al., 1994). It is difficult to determine what accounts for the discrepant findings because there is substantial between-study variability that could contribute to the different findings. For example, differences in handling stress experienced, the type of rodent studied (e.g., rats versus mice), timing of exposure, medication dosage, specific antidepressant used, or the specific behavioral measure assessed could have contributed to the discrepant findings.
Limitations
The primary limitation of rodent studies is that the applicability of findings to humans is unclear. There are three key between-species differences that could impact generalizability. First, rodent gestation is considerably shorter than human gestation. Therefore, neonate rodents are used to model exposure late in human pregnancy. As such, rodent studies modeling the end of the human pregnancy actually examine postnatal – rather than intrauterine – exposure and, therefore, may not generalize to human pregnancies (Bourke et al., 2014). Second, as shown by the variability in dosages used in rodent studies (Table 1), between-species differences in drug metabolism rates make it difficult to estimate the appropriate doses to correspond with doses used in people (Bourke et al., 2014; Shoulson, Stark, & Garland, 2014). Third, there are major between-species differences in brain size, cortical complexity, and behavior. As such, outcome measures used to model neurodevelopmental problems in rodents may not fully correspond to neurodevelopmental problems in humans (Nestler & Hyman, 2010).
There are two other limitation of rodent studies that are also limitations of observational studies. First, studies have focused on examining consequences of prenatal exposure to SSRIs. Therefore, the consequences of exposure to other types of antidepressants are unclear. Second, publication bias could affect the overall conclusions the field draws. The overall evidence may be biased towards suggesting an effect of perinatal antidepressant exposure due to preferential submission and acceptance of statistically significant findings. Additionally, given that findings with larger effect sizes are more likely to be statistically significant than findings with smaller effect sizes, preferential submission and acceptance of statistically significant findings may lead to an overestimation of effect sizes. For reviews on publication bias see Maxwell (2004) and Mlinarić, Horvat, and Šupak Smolčić (2017).
Summary
In sum, most rodent studies have found effects of perinatal antidepressant exposure on neuronal, brain, and behavioral indices related to neurodevelopmental problems (Zucker, 2017). However, between-species differences raise questions about the generalizability of these findings to humans. Given this uncertainty, the field should be wary about making strong conclusions based on results from rodent studies alone.
Observational Studies
Ethical concerns have prevented the use of randomized control trials of antidepressant use during pregnancy in humans. Instead, researchers have relied on observational data and methodological approaches to try to account, in varying degree, for the influence of confounding factors that could bias the associations between antidepressant use during pregnancy and offspring neurodevelopmental problems.
Types of designs
Several different types of observational designs have been used to study antidepressant use during pregnancy and offspring neurodevelopment. Some of these designs are better able to test the role of potential confounding factors than others. However, all methods have specific limitations and require assumptions when interpreting the results. We briefly review the strengths and limitations of these designs below.
Designs that target measured potential confounders.
The majority of observational studies have used methods to account for characteristics that have been measured by the researchers. These methods include using measured characteristics as covariates in regression models, using propensity scores (Austin, 2011; Wood, Lapane, van Gelder, Rai, & Nordeng, 2018), matching cases and controls on a set of measured characteristics (Song & Chung, 2010), and restricting the comparison group to offspring of women with (measured) psychiatric disorders who did not use antidepressants during pregnancy. These approaches help rule out the possible confounding role of the measured characteristics. In particular, the practice of restricting comparison groups to offspring of mothers with diagnosed psychiatric conditions helps account for confounding by indication. It is important to note, though, this method does not alone account for differences in severity of indication between medicated and unmedicated mothers. Importantly, any method that only adjusts for measured characteristics is unlikely to adequately account for confounding because researchers realistically cannot measure every salient plausible confounding factor (Academy of Medical Sciences Working Group, 2007). Furthermore, error in the measurement of the characteristics may lead to far less confounding control than intended (Westfall & Yarkoni, 2016).
Designs that target unmeasured potential confounding.
To more rigorously assess the consequences of prenatal antidepressant exposure, some researchers have used measured covariates in combination with advanced observational methods with design features that either account for or evaluate the potential role of unmeasured confounding factors. Although such designs are typically able to capture more background factors than possible with measured characteristics alone, the net ability to capture confounding factors will depend on the degree to which these are among the targeted unmeasured factors and measured characteristics combined. We briefly review several methods that target unmeasured confounding below.
Paternal antidepressant use as a negative control.
The negative control design compares what happens to the association with offspring neurodevelopmental problems if the exposure to maternal antidepressant use during pregnancy is replaced with exposure to paternal use of antidepressants during the pregnancy period. By definition, an ideal negative control is influenced by all of the same confounding factors as the exposure of interest but has no causal effect on the outcome. Given that paternal antidepressant use during the pregnancy period is unlikely to result in fetal exposure, any observed association between paternal use and an offspring outcome suggests that the observed association with maternal use is influenced by confounding to some extent. Because confounding factors and a causal influence of antidepressant exposure could simultaneously contribute to an association, a paternal association would have to be similar in magnitude to the maternal association in order to completely rule out a causal effect of intrauterine antidepressant exposure. In other words, the paternal use association helps indicate the degree of confounding in the maternal use association.
Importantly, the negative control assumption that maternal and paternal antidepressant use are influenced by the same confounding factors may be violated for several reasons. For example, paternal depression may be less likely to lead to antidepressant use than maternal depression because men may be less likely to seek treatment for mental health problems, including depression, than women (Galdas, Cheater, & Marshall, 2005). Alternatively, maternal antidepressant use during pregnancy may be associated with more severe depression than paternal use because mothers may be more inclined to discontinue antidepressant use during pregnancy because of concerns about fetal exposure. Another limitation of this design is that it assumes equal measurement error of antidepressant use across mothers and fathers. For more information on negative control analyses and the use of paternal comparisons as negative controls see Lipsitch, Tchetgen Tchetgen, and Cohen (2010); Richmond, Al-Amin, Smith, and Relton (2014); and Smith (2008).
Maternal use of another psychotropic medication as an active comparator.
The active comparator design compares individuals exposed to a medication under study (antidepressants) to individuals exposed to a theoretically safe alternative medication with a similar indication for use. Based on the assumption that the active comparator medication is not harmful, a null association with antidepressant exposure compared to exposure to the active comparator medication would suggest that antidepressant exposure does not have an adverse effect. The active comparator design accounts for all unmeasured confounding factors that are common to the use of both types of medication, such as common indications. However, interpretation of the results rests on the assumption that the other medication does not influence the offspring outcome through different etiological mechanisms. For more information about active comparator designs see Lund, Richardson, and Stürmer (2015) and Ray and Griffin (1989).
Timing of exposure comparison.
The timing of exposure design compares the offspring outcome following maternal antidepressant use before pregnancy only, to the offspring outcome following maternal antidepressant use during pregnancy. This design tests for confounding by all factors shared by women on antidepressant treatment around the time of pregnancy, such as having a condition for which treatment is indicated. The finding that risk of the outcome does not differ across the exposure time-periods would be inconsistent with a causal effect because use before but not during pregnancy is unlikely to result in fetal exposure.
There are two key limitations of the design. First, it assumes that preconception antidepressant exposure does not affect embryonic or fetal development. Second, the design also does not account for unmeasured confounding factors that differ between the mothers who use antidepressants during pregnancy and those who only use antidepressants before pregnancy. For example, women who discontinue their antidepressant use before pregnancy are likely to have less severe depression than women who continue their treatment during pregnancy. To illustrate the limitations of timing of exposure comparisons, we conducted analyses using a Swedish population dataset (n=708,450 offspring born between 2006 and 2012; see Sujan et al., 2017 for more details about this sample) and found that women who filled prescriptions for antidepressants before and during pregnancy (i.e., continuation of use during pregnancy) were more likely to have several risk factors for offspring psychopathology, including older age at childbearing (i.e., 35 years or older; odds ratio [OR]=1.2, 95% confidence interval [CI]:1.1–1.3), history of hospitalization for severe psychiatric illness (OR=1.6, 95% CI:1.5–1.8), use of other psychotropic medications during pregnancy (OR=2.1, 95% CI:1.9– 2.3), and a lifetime history of suicide attempts (OR=1.2, 95% CI:1.1– 1.3) than women who filled prescriptions for antidepressants before but not during pregnancy (i.e., discontinuation of use). The results suggest that associations seen in timing of exposure comparisons may be influenced by differences between the comparison groups, including severity of the underlying condition and other potentially confounding factors.
For more information about timing of exposure comparisons see Smith (2008).
Sibling comparison.
Siblings share approximately 50% of their segregating genes and often have similar early environments. The sibling-comparison design makes use of these similarities. A contrast of siblings that are discordant for prenatal antidepressant exposure will account for all unmeasured genetic and environmental factors that make siblings similar, including all maternal characteristics and other factors that remain stable across pregnancies. Similar risk for an outcome among differentially exposed siblings would suggest that there is no causal effect of the exposure. Given that genetic confounding is likely to exist (Solovieff et al., 2013), this design is particularly helpful in drawing causal inferences because it helps test for genetic and family-level confounding.
Although sibling comparisons provide a rigorous test of confounding by familial factors, the design by itself cannot account for unmeasured confounding by factors that vary across a woman’s pregnancies. However, researchers can and should use measured covariates to account for factors that vary across pregnancies in sibling-comparison studies. Sibling-comparison designs also assume no carry-over effects from the exposed siblings to the unexposed siblings (e.g., antidepressant use during one pregnancy affecting subsequent pregnancies). Additionally, it is possible that an observed attenuation of associations in sibling-comparison studies may be due to random measurement error or mediators shared by the siblings. Sibling comparisons also require large samples to obtain an adequate number of discordant siblings.
For more information about sibling comparisons see D’Onofrio, Lahey, Turkheimer, and Lichtenstein (2013); Frisell, Oberg, Kuja-Halkola, and Sjolander (2012); Lahey and D’Onofrio (2010); Sjölander and Zetterqvist (2017); Wood et al. (2018).
Findings of Observational Studies
For this review, we focus on observational studies that examined associations between prenatal antidepressant exposure and ASD and ADHD. We first review meta-analyses (Table 2). We also review individual studies that adjusted for measured characteristics only (Table S1), as well as individual studies that used methods to test the role of unmeasured confounding (Table S2). Though we focus on studies on ASD and ADHD, studies have also examined associations with other important neurodevelopmental outcomes, such as speech and language development (Brown et al., 2016; Nulman et al., 2002; Skurtveit, Selmer, Roth, Hernandez-Diaz, & Handal, 2014), IQ scores (Nulman et al., 2015; Nulman et al., 2002), and intellectual disability (Viktorin, Uher, Kolevzon, et al., 2017). Recent reviews have also discussed a range of neurodevelopmental outcomes, including cognitive, motor, and language development (e.g., Rotem-Kohavi & Oberlander, 2017).
Analyses that only target measured potential confounders.
Meta-analyses have pooled estimates from studies that relied on the use of measured characteristics only to account for confounding. Specifically, 9 meta-analyses have examined associations with ASD and 3 meta-analyses have examined associations with ADHD. These meta-analyses include overlapping studies (Table 2). Although none of the meta-analyses included all published individual studies, the most comprehensive reported a pooled aRR of 1.5 (95% CI: 1.3–1.8) from 10 ASD studies and a pooled aRR of 1.4 (95% CI: 1.1–1.7) from 7 ADHD studies (Morales et al., 2018). The meta-analyses on ASD have reported pooled adjusted point estimates (i.e., odds, hazard, or relative risk ratios) ranging from approximately 1.0 to 2.5 (Table 2; Andalib et al., 2017; Brown, Hussain-Shamsy, Lunsky, Dennis, & Vigod, 2017; Kaplan, Keskin-Arslan, Acar, & Sozmen, 2016, 2017; Kobayashi, Matsuyama, Takeuchi, & Ito, 2016; Man et al., 2015; Mezzacappa et al., 2017; Morales et al., 2018; Zhou, Li, Ou, & Li, 2018).The meta-analyses on ADHD have generally reported pooled point estimates (i.e., odds or relative risk ratios) of about 1.5 (Table 2; Jiang, Peng, Zhang, & Ruan, 2017; Man et al., 2018; Morales et al., 2018).
It is important to note that the findings and conclusions across individual studies (Table S1) are inconsistent. First, variability in effect sizes appear to be due in part to heterogeneity in the measured statistical adjustments. A recent review and meta-analysis of prenatal antidepressant exposure and ASD and ADHD documented that there is enormous variability in the measured characteristics included in studies. (See Tables 2 and 3 in Morales et al., 2018 for a review of specfic measured characterstics adjusted for in studies.) For example, most studies have adjusted for year of birth. However, few studies have adjusted for paternal characteristics and severity of maternal depression (Morales et al., 2018). Statistical adjustments for appropriate measured characteristics (i.e., confounding factors) will results in reduced effect sizes. Meta-analyses on prenatal antidepressant exposure and neurodevelopmental problems have demonstrated that adjusted associations (e.g., aRR=1.5, 95% CI:1.3–1.8 for ASD and aRR=1.4, 95% CI:1.1–1.7 for ADHD) are attenuated compared to crude associations (e.g., RR=1.9, 95% CI:1.6–2.2 for ASD association and RR=2.0, 95% CI:1.6–2.6 for ADHD association; Morales et al., 2018). Notably, recent studies that adjusted for more covariates have reported weaker associations (Morales et al., 2018).
Second, studies assessing associations with exposure in specific trimesters have shown mixed results, making it unclear whether there are sensitive periods of exposure during pregnancy. For example, though some research has suggested only first-trimester exposure is associated with ASD (e.g., Croen, Grether, Yoshida, Odouli, & Hendrick, 2011), other research has suggested only exposure later in pregnancy is associated with ASD (e.g., Boukhris, Sheehy, Mottron, & Berard, 2016). Similarly, some research has shown similar associations with ADHD across trimester (Man et al., 2017), whereas other research has found stronger associations with ADHD for first-trimester exposure (Clements et al., 2015). As a result, there is ongoing debate about timing effects. For example, several researchers have written commentaries questioning the result showing only an association for exposure later in pregnancy reported in the Boukhris et al. (2016) paper, citing several arguments, such as a greater vulnerability during the first-trimester due to the immaturity of the blood-brain barrier (e.g., Fombonne, 2016; Kaplan, Keskin-Arslan, & Acar, 2016).
Third, some studies found similar effect sizes but differed in statistical significance, and some discrepancies in the conclusion drawn were based on reliance on formal statistical significance tests. The differences in statistical significance were most likely due to differences in sample size and statistical power. For example, two studies reported elevated aHRs of similar magnitude for an association between second and/or third-trimester antidepressant exposure and ASD. However, one study conducted on a sample of 145,456 offspring reported a statistically significant result (aHR=1.9, 95% CI:1.2–3.0; Boukhris et al., 2016), whereas the other study reported a statistically non-significant finding in a sample of 35,906 offspring (after inverse probability of treatment weighting based on high-dimensional propensity scores aHR=1.6, 95% CI:1.0–2.7; Brown, Ray, et al., 2017). The study that found a statistically significant result concluded that second and/or third-trimester antidepressant exposure increases the risk of ASD, whereas the study that found a statistically non-significant result concluded that confounding factors may be responsible for the association.
Limitations.
A critical limitation of these studies is their reliance of measured characteristics to account for confounding. A number of recent reviews have emphasized that solely adjusting for measured characteristics is unlikely to adequately capture all confounding influences (Morales et al., 2018; Pourhoseingholi, Baghestani, & Vahedi, 2012).
There are also several limitations to analyses that only target measured potential confounders that are shared by all by all observational studies. First, findings could be biased by misclassification of the exposure (e.g., medication non-compliance) or outcomes. Second, including causal mechanisms in analyses would attenuate associations and could, thereby, lead researchers to inaccurately conclude associations are influenced by confounding. For example, some studies adjusted for birth outcomes (e.g., gestational age and birth weight; Malm et al., 2016; Sorensen et al., 2013). If an adverse birth outcome fully mediated a (true) effect of antidepressant exposure on an offspring outcome, inclusion of the mediator would result in a null association between prenatal antidepressant exposure and the outcome. Third, findings may lack generalizability. For example, findings may not generalize from a population sample of women with less severe depression to a clinic sample of women with more severe depression and vice versa. Fourth, some studies, particularly those examining exposure by trimester, have lacked adequate statistical power. This has resulted in imprecise estimates and, consequently, limited the interpretability of the results. Fifth, most studies to date have examined SSRIs as a class. Thus, our knowledge about the potential consequences of exposure to specific medications and other antidepressant classes (e.g., serotonin-norepinephrine reuptake inhibitors) is lacking. Sixth, as discussed in the section on limitations of rodent studies, publication bias for statistically significant associations could influence the overall evidence from observational studies towards an overestimation of effect sizes. Most meta-analyses were not able to systematically evaluate the impact of publication bias due to an insufficient number of studies. However, a recent meta-analysis on prenatal antidepressant exposure and ASD found asymmetry in funnel plots for both case-control and cohort studies, indicating a potential for publication bias (Zhou et al., 2018).
Summary.
Many observational studies have documented associations between prenatal antidepressant exposure and ASD and ADHD, and, in general, adjustment for measured characteristics partially attenuated these associations. However, there are inconsistencies across findings, and the threat of unmeasured confounding ultimately precludes the field from being able to draw strong conclusions from these studies.
Analyses that target unmeasured potential confounding.
We review findings from meta-analyses (Table 2) and individual studies (Table S2) that use different design features to help evaluate the role of unmeasured confounding.
Paternal antidepressant use as a negative control.
A meta-analysis of two studies (i.e., Sorensen et al., 2013; Sujan et al., 2017) reported a pooled aRR of 1.3 (95% CI: 1.1–1.5) for the association between paternal antidepressant use during the pregnancy period and ASD (Table 2; Morales et al., 2018). One of the included studies reported an elevated and statistically significant association (eTable 2; Sujan et al., 2017 [aHR=1.3, 95% CI: 1.1–1.6]), though the other did not (Table S2; Sorensen et al., 2013 [aHR=1.1, 95% CI: 0.9–1.3]). Results from two other paternal comparison studies not included in the meta-analysis also showed weak, non-statistically significant associations, with effect sizes close to the null for the association between paternal antidepressant treatment during pregnancy and ASD (Table S2; Liu et al., 2017 [aHR=1.1, 95% CI: 0.8–1.4]; Rai et al., 2017 [aHR=1.1, 95% CI: 0.7–1.9]). Only one study assessed the association between paternal antidepressant treatment during pregnancy and offspring risk of ADHD. This study found an aHR of 1.7 (95% CI:1.4–2.2; Table S2; Sujan et al., 2017). Given that paternal use during pregnancy is unlikely to result in fetal exposure, any observed associations with paternal use suggests that observed associations with maternal use during pregnancy are influenced by familial confounding. Therefore, these results, particularly the results from the meta-analysis, suggest that observed associations with maternal antidepressant use during pregnancy are at least in part due to familial confounding.
Maternal use of another psychotropic medication as an active comparator.
A meta-analysis that pooled unadjusted associations of three studies (i.e., Croen et al., 2011; Rai et al., 2013; Sorensen et al., 2013) found that compared to exposure to non-SSRI antidepressants, exposure to SSRIs during pregnancy was not associated with increased risk of ASD (OR=1.1, 95% CI: 0.7–2.0; Kobayashi et al., 2016). This finding suggests that confounding by indication rather than prenatal SSRI exposure may be responsible for observed associations between maternal SSRI use during pregnancy and increased risk for ASD in offspring.
One study also evaluated the risk of antidepressant exposure for ADHD by using maternal antipsychotic treatment during pregnancy as an active comparator (Table S2). The study found that, compared to prenatal exposure to antipsychotic medications, prenatal exposure to antidepressants was not statistically significantly associated with an increased risk of ADHD (aHR=1.3, 95% CI: 0.7–2.2; Man et al., 2017), suggesting that factors related to maternal use of psychotropic medications during pregnancy may confound associations between prenatal antidepressant exposure and ADHD. However, the conclusions that can be drawn from this study are limited given that the point estimate was elevated and the CI was wide. It is also important to note that the active comparator approach rests on the assumption that the active comparator medication is not harmful. However, based on research to date, the effects of antipsychotics on fetal development are not clear (Abel, 2011). For example, prenatal antipsychotic exposure has been linked to gestational diabetes (Bodén, Lundgren, Brandt, Reutfors, & Kieler, 2012), and gestational diabetes has been linked to offspring neurodevelopmental problems (Nomura et al., 2012). Therefore, a similar risk compared to exposure to antipsychotic medications may not be indicative of non-causal effect of antidepressant exposure because antipsychotic medication exposure could influence offspring neurodevelopmental problems through different mechanisms of action.
Timing of exposure comparisons.
Four meta-analyses, which included overlapping studies, found statistically significant associations between antidepressant use before pregnancy and ASD (Table 2; Kaplan, Keskin-Arslan, Acar, et al., 2016; Mezzacappa et al., 2017; Morales et al., 2018; Zhou et al., 2018), and three meta-analyses also including overlapping studies found statistically significant associations between antidepressant use before pregnancy and ADHD (Table 2; Jiang et al., 2017; Man et al., 2018; Morales et al., 2018). (eTable 2 includes results from individual studies.) These associations were of similar magnitude as associations reported for use during pregnancy. These studies suggest that factors related to maternal antidepressant use around the time of pregnancy may contribute to the observed associations between prenatal antidepressant exposure and neurodevelopmental problems.
While several studies reported associations with use before pregnancy, two studies found that before-pregnancy antidepressant use was not associated with ASD (Table S2; Boukhris et al., 2016; Liu et al., 2017), and two studies found that before-pregnancy use was not associated with ADHD (Table S2; Clements et al., 2015; Figueroa, 2010). It is important to note that three of these four studies (Boukhris et al., 2016; Clements et al., 2015; Figueroa, 2010) did not directly compare use during pregnancy to use before pregnancy, and in all four studies the CIs for the association with use before pregnancy overlapped with the CIs for the association with use during pregnancy. Therefore, these studies were limited in their ability to assess whether antidepressant exposure during pregnancy had an effect over and above indications for antidepressant use around the time of pregnancy. Moreover, meta-analyses suggest that there is an association between maternal antidepressant use before pregnancy and ASD and ADHD, indicating that the studies that failed to find an association had atypical results, perhaps due to inadequate statistical power.
Sibling comparisons.
Four sibling-comparison studies have examined associations between prenatal antidepressant exposure and ASD (Table S2; Brown, Ray, et al., 2017; Rai et al., 2017; Sorensen et al., 2013; Sujan et al., 2017). A meta-analysis of three of these studies (i.e., Brown, Ray, et al., 2017; Sorensen et al., 2013; Sujan et al., 2017) reported a pooled aRR of 0.9 (95% CI: 0.8–1.2; Table 2; Morales et al., 2018). Two of the individual studies also reported aHRs of approximately one. Specifically, Sorensen et al. (2013) reported an aHR of 1.1 (95% CI: 0.5–2.3) in a sample of 668,468 offspring, and Sujan et al. (2017) an aHR of 0.8 (95% CI: 0.6–1.1) in a sample of 1,580,629 offspring. These results suggest that confounding by familial factors could account for the association between prenatal antidepressant exposure and ASD.
However, two of the individual studies that examined associations with ASD reported elevated point estimates. Specifically, Brown, Ray, et al. (2017) found an aHR of 1.6 (95% CI:0.7–3.7) in a sample of 35,906 offspring, and Rai et al. (2017) found an aOR of 1.4 (95% CI:0.8–2.2) in a sample of 254,610 offspring. It is important to note that CIs in these studies were wide and overlapped with one, which limits the conclusions that can be drawn.
Three individual sibling-comparison studies have examined associations between prenatal antidepressant exposure and ADHD (Table S2; Laugesen, Olsen, Telén Andersen, Frøslev, & Toft Sørensen, 2013; Man et al., 2017; Sujan et al., 2017). Three meta-analyses have been conducted and they each included all three of these sibling-comparison studies (Table 2; Jiang et al., 2017; Man et al., 2018; Morales et al., 2018). All three meta-analyses and all three individual studies found adjusted point estimates at or below 1.0 for associations with ADHD. Together, the sibling-comparison studies suggest that genetic and/or environmental confounding factors that make sibling similar may be responsible for observed associations between prenatal antidepressant exposure and ASD and ADHD.
Limitations.
There are a number of limitations to analyses that target unmeasured potential confounders. First, no observational method, even those that target unmeasured confounding, is likely to completely eliminate bias from confounding. Importantly, studies that targeted unmeasured confounding may not have accounted for confounding by severity of the indication. For example, offspring born to mothers who use antidepressants during pregnancy may have been exposed to more severe depression than offspring in the comparison groups. Specifically, offspring born to mothers who used antidepressants during pregnancy may have been exposed to more severe depression than offspring born to (a) fathers who use antidepressants during pregnancy (paternal comparisons), (b) mothers who use another psychotropic medication during pregnancy (active comparator design), (c) mothers who only use antidepressants before pregnancy only (timing of exposure comparisons), and (e) mothers who used antidepressants during the focal pregnancy but not during another pregnancy (sibling comparisons).
Second, because the majority of studies used large datasets in order to have adequate statistical power, they were unable to obtain in-depth assessments of antidepressants use, and therefore, were subject to exposure misclassification. For example, many large-scale studies relied on records of prescriptions filled at pharmacies, and researchers could not confirm the actual use of the medications from these records. To address this limitations future smaller scale studies could obtain maternal blood samples to confirm the use of antidepressants.
Third, researchers have not used methods that target unmeasured confounding when evaluating the potential impact of timing of exposure (Table S2).
Fourth, research using designs that target unmeasured confounding to estimate associations with specific antidepressant classes and medications is limited (Table S2).
Summary.
Several studies have used methods that help account for unmeasured confounding in combination with measured covariates to study antidepressant use during pregnancy and offspring neurodevelopmental problems. Converging evidence from these studies does not provide support for a large causal influence of maternal antidepressant use during pregnancy on offspring ASD and ADHD. Although these studies cannot rule out a small effect, they suggest the observed population-wide associations are largely due to confounding factors.
Overall Summary of Research
Both rodent experiments and observational studies in humans have been used to study the potential effects of maternal antidepressant use during pregnancy on offspring neurodevelopment. Rodent studies have reported adverse effects of antidepressant exposure. However, between-species differences may impact the translation of these findings to humans. Therefore, researchers and clinicians should be wary about making strong conclusions based on findings from rodent studies alone. Additionally, converging evidence from observational studies suggest that maternal antidepressant use during pregnancy either does not have any influence on offspring risk of ASD and ADHD or the influence on these outcomes is small and may not be clinically significant. In other words, research to date does not provide strong support for a causal pathway from prenatal antidepressant exposure to increased risk of neurodevelopmental problems through mechanism of actions (Figure 1 arrows a and b). Rather, there is growing evidence to suggest that observed population-wide associations between prenatal antidepressant exposure and of ASD and ADHD are largely due to other factors that differ between exposed and unexposed offspring. However, based on the research to date it is not clear which non-causal pathways contribute to the observed associations. Specifically, we do not know the extent to which indications for maternal antidepressant use (Figure 1, arrows c, d, and e), environmental common causes (Figure 1, arrows f), and biological pleiotropic genetic factors (Figure 1, arrows g) contribute to the observed associations. Given concerns surrounding drug safety during pregnancy it is important to note that the body of research to date indicates that prenatal antidepressants exposure is unlikely to have strong adverse effects for ASD and ADHD. In other words, research to date suggests that antidepressants use during pregnancy is relatively safe, particularly for risk of long-term neurodevelopmental outcomes.
Future Research Directions
There are number of outstanding questions that need to be addressed in order to provide a clearer picture of the implications of maternal antidepressant use during pregnancy. First, research needs to reconcile findings from rodent studies, which generally suggest that antidepressants may cause harm, and human studies, which do not provide support for a large causal effect. To help reconcile these discrepancies, the modeling of antidepressant exposure in non-human animal models needs to be improved, particularly with regard to timing, dose, and mapping the measures used to human neurodevelopmental problems. Several reviews have been published describing possible methods to improve non-human animal models of prenatal exposures and neurodevelopmental disorders. For example, researchers have suggested developing a non-primate animal model whose physiology is more relevant to human parturition (e.g., the guinea pig; Mitchell & Taggart, 2009) and identifying and using specific behaviors in the mouse repertoire that are sufficiently relevant to each category of the diagnostic symptoms of neurodevelopmental disorders as defined by the Diagnostic and Statistical Manual of Mental Disorders (Crawley, 2012).
Second, future research needs to evaluate the potential implications of publication bias. One meta-analysis evaluated publication bias for observational studies on prenatal antidepressant exposure and ASD (Zhou et al., 2018). However, the impact of publication bias in rodent studies and observational studies on ADHD has not been evaluated.
Third, future studies should continue to explore what specific factors other than prenatal antidepressant exposure may be responsible for the increased occurrence of ASD and ADHD among offspring born to women who use antidepressants during pregnancy. The finding that observed associations between prenatal antidepressant exposure and ASD and ADHD are largely due to confounding factors underscores the importance of fully elucidating what specific factors are responsible for the associations. Identifying these specific factors would inform our understanding of the causes of neurodevelopmental problems and help with prevention efforts. Both small-scale and large-scale observation studies are needed to identify specific confounding factors. Small-scale studies will allow researchers to obtain fine grained measurements of plausible confounding factors (Yonkers, Forray, & Smith, 2017). It will also be important for researcher to conduct large-scale studies with valid measurements as these studies will allow researchers to obtain precise estimates, which is important particularly when studying rare exposures and outcomes.
Fourth, given that studies to date have focused on examining associations with exposure to any antidepressant or SSRIs specifically, future studies of specific medications and other classes are needed to evaluate if these medications have different effects on the developing fetus. This is an important area for future research because antidepressant medications have different mechanisms of action and target different neurotransmitters. For example, SSRIs increase serotonin levels in the brain by binding to serotonin transporters and blocking reuptake of serotonin, serotonin-norepinephrine reuptake inhibitors boost serotonin and norepinephrine throughout the brain by combining robust serotonin transporter inhibition with various degree of norepinephrine transporter inhibition. Tricyclic antidepressants increase norepinephrine and sometimes serotonin by blocking the reuptake pumps for norepinephrine or both norepinephrine and serotonin (Stahl, 2013). Given that most studies have focused on prenatal SSRI exposure, the potential consequences of increased fetal exposure to norepinephrine are less clear.
Fifth, more studies examining the role of timing of exposure are needed to assess if there are particularly sensitive periods of exposure during pregnancy. Studies examining associations with exposure during specific trimesters have shown mixed results. Some researchers have hypothesized that exposure early in pregnancy may be more harmful due to the immaturity of the blood-brain barrier (e.g., Fombonne, 2016; Kaplan, Keskin-Arslan, & Acar, 2016). However, the second and third trimesters are sensitive periods for fetal growth and brain development (Rice & Barone, 2000; Zeanah, Gunnar, McCall, Kreppner, & Fox, 2011), and reduced fetal growth could be a mechanism for an effect of antidepressant exposure on neurodevelopmental problems (Huang et al., 2014; Pettersson et al., 2015). It is difficult to use observational data to study timing effects because most women who use antidepressants early in pregnancy also use antidepressants later in pregnancy. For example, in a sample of 708,450 Swedish offspring born between 2006 and 2012, less than one percent (0.11%) of offspring were born to mothers who were only dispensed an antidepressant in the second and/or third trimester of pregnancy (Sujan et al., 2017). Therefore, in order to evaluate timing effects in observational studies, researchers will need to obtain particularly large samples so that enough offspring are exposed during specific periods of pregnancy. To do so, researchers may need to use population-based samples with extended follow-up. However, these types of datasets may not be available for a number of years. It may also be possible to use randomized clinical trials to study timing effects by recruiting pregnant women who have already made the decision to use their antidepressant medication during pregnancy and randomly assigning the women to use their medications during different pregnancy periods. In fact, some researchers have recently called for use of randomized clinical trials to study the effects of medication use, and antidepressant use specifically, during pregnancy given the limitations of observational studies and the additional information that could be gained from randomized clinical trials (Howland, 2013; Illamola et al., 2018).
Sixth, although this review focused on ASD and ADHD, future studies will also need to study other important offspring outcomes, including birth outcomes (e.g., preterm birth, fetal growth, and congenital malformations) and other neurodevelopmental outcomes (e.g., intellectual disabilities, learning disorders, and tics) so that pregnant women and their doctors can have more complete information when making decisions about the risk and benefits of using antidepressants during pregnancy.
Implications for Clinical Practice
While it is important that pregnant women and their doctors consider a wide variety of potential consequences for offspring development, including adverse birth outcomes (e.g., preterm birth and congenital malformation) and neurodevelopmental problems, the research to date should provide women considering antidepressant use during pregnancy reassurance because it suggests intrauterine antidepressant exposure does not substantially increase the risk for two concerning neurodevelopmental problems -- ASD and ADHD.
In addition to being exposed to antidepressants during pregnancy, children born to women who use antidepressants during pregnancy are exposed to several other risk factors, including having a mother with an indication for antidepressant treatment. These other risk factors appear to be largely responsible for the increased occurrence of ASD and ADHD among children prenatally exposed to antidepressants. Therefore, it is critical that services are provided to women with depressive and anxiety disorders and their children in order to reduce the risk of offspring neurodevelopmental problems. In order to do this, the first step is identifying pregnant women who may need treatment. Recently, the US Preventive Services Task Force issued the recommendation that medical practitioners should routinely screen all adults, including pregnant women, for depression with validated self-report screening measures, such as the Patient Health Questionnaire, the Hospital Anxiety and Depression Scale, and the Edinburgh Postnatal Depression Scale (Siu et al., 2016). Additionally, recent advances in computer adaptive testing may also help expedite the screening of psychiatric problems (Gibbons, Weiss, Frank, & Kupfer, 2016), including depression (Gibbons, Weiss, Pilkonis, & et al., 2012) and anxiety (Gibbons et al., 2014).
Experts have recommended a stepped approach to treatment of depression during pregnancy (Stewart, 2011). Specifically, women with mild depression with a recent onset (i.e., two weeks or less) should be monitored and encouraged to exercise and seek social support. Women with mild depression that does not improve within two weeks of diagnosis and women with moderate–to–severe depression should seek/be offered evidence-based treatment. Research has suggested that evidence-based psychotherapies, such as cognitive therapy, and antidepressants are roughly equally effective at treating depression (DeRubeis, Hollon, Amsterdam, & et al., 2005).
The American Psychiatric Association and the American College of Obstetricians and Gynecologists have published recommendations about decision-making regarding antidepressant treatment during pregnancy (Yonkers et al., 2009). In their report, they suggest that antidepressants may be a superior treatment option to psychotherapy for some women. Women with a history of severe suicide attempts or severe depression who have previously experienced symptom reduction with antidepressant treatment may respond to antidepressants better than psychotherapy. They also suggest it may be advisable for some women who have previously relapsed when discontinuing antidepressant treatment to continue antidepressants use during pregnancy. Additionally, women who have tried psychotherapy but have not achieved adequate symptom reduction may also need antidepressant treatment during pregnancy. They also point out that some women may have a preference for antidepressant treatment over psychotherapy. In sum, their report indicates that women and their doctors should work together and consider severity of current symptoms, previous mental health history, and patient treatment preferences when making decisions about antidepressant use during pregnancy.
Conclusion
There are many important offspring outcomes that need to be considered in clinical decision-making, and this review only focused on two specific neurodevelopmental problems – ASD and ADHD. Yet, the findings summarized in this review may help women and their doctors make decisions about antidepressant use during pregnancy by providing reassurance that use of these medications during pregnancy is unlikely to substantially increase the risk ASD and ADHD.
Supplementary Material
Key points.
Associations between prenatal antidepressant exposure and neurodevelopmental problems could reflect a causal effect of antidepressant exposure or be influenced by other factors that differ between exposed and unexposed offspring.
Between-species differences raise questions about the generalizability of findings from rodent studies, which suggest an effect of perinatal antidepressant exposure on neurodevelopment.
Although observational studies cannot rule out a small effect of prenatal antidepressant exposure on neurodevelopment in humans, growing evidence – especially from studies assessing unmeasured confounding – suggests that observed associations are largely explained by confounding factors.
Future research needs to investigate discrepancies between rodent and human studies, specific confounding factors, specific antidepressant drugs and classes, potential sensitive periods of exposure, and a wider range of potential offspring outcomes.
Acknowledgements
Research reported in this publication was supported by a National Science Foundation Graduate Research Fellowship [1342962], the National Institute of Mental Health of the National Institutes of Health [T32MH103213], the National Institute on Drug Abuse of the National Institutes of Health [K99DA040727], Indiana Clinical and Translational Sciences Institute: Pediatric Project Development Team, the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM) framework [340-2013-5867], the Swedish Research Council for Health, Working Life and Welfare (FORTE) [50623213], and the Swedish Research Council [2014-38313831]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Main findings from analyses that adjusted for measured characteristics only.
Table S2. Main findings from analyses that used designs to adjust for unmeasured confounding.
Conflict of interest statement: No conflicts declared.
References
- Abel K (2011). Review: teratogenicity of first- and second-generation antipsychotics in pregnancy is unclear. Evidence-based mental health, 14(1), 31–31. doi: 10.1136/ebmh.14.1.31 [DOI] [PubMed] [Google Scholar]
- Academy of Medical Sciences Working Group. (2007). Identifying the environmental causes of disease: How should we decide what to believe and when to take action? London: Academy of Medical Sciences. [Google Scholar]
- Alwan S, & Chambers CD (2015). Identifying Human Teratogens: An Update. Journal of Pediatric Genetics, 4(2), 39–41. doi: 10.1055/s-0035-1556745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampaabeng SK, & Tan CM (2013). The long-term cognitive consequences of early childhood malnutrition: The case of famine in Ghana. Journal of Health Economics, 32(6), 1013–1027. doi: 10.1016/j.jhealeco.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Hoilund-Carlsen PF, Vafaee MS, & Michel TM (2017). Maternal SSRI exposure increases the risk of autistic offspring: A meta-analysis and systematic review. European Psychiatry, 45, 161–166. doi: 10.1016/j.eurpsy.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Andrade SE, Reichman ME, Mott K, Pitts M, Kieswetter C, Dinatale M,… Toh S (2016). Use of selective serotonin reuptake inhibitors (SSRIs) in women delivering liveborn infants and other women of child-bearing age within the US Food and Drug Administration’s Mini-Sentinel program. Archives of Womens Mental Health, 19(6), 969–977. doi: 10.1007/s00737-016-0637-1 [DOI] [PubMed] [Google Scholar]
- Austin PC (2011). An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behavioral Research, 46(3), 399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodén R, Lundgren M, Brandt L, Reutfors J, & Kieler H (2012). Antipsychotics during pregnancy: Relation to fetal and maternal metabolic effects. Archives of General Psychiatry, 69(7), 715–721. doi: 10.1001/archgenpsychiatry.2011.1870 [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, & Koren G (2004). Perinatal risks of untreated depression during pregnancy. Can J Psychiatry, 49(11), 726–735. doi: 10.1177/070674370404901103 [DOI] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, & Berard A (2017). Antidepressant Use in Pregnancy and the Risk of Attention Deficit with or without Hyperactivity Disorder in Children. Paediatric and Perinatal Epidemiology, 31(4), 363–373. doi: 10.1111/ppe.12378 [DOI] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, Mottron L, & Berard A (2016). Antidepressant Use During Pregnancy and the Risk of Autism Spectrum Disorder in Children. JAMA Pediatr, 170(2), 117–124. doi: 10.1001/jamapediatrics.2015.3356 [DOI] [PubMed] [Google Scholar]
- Bourke CH, Stowe ZN, & Owens MJ (2014). Prenatal antidepressant exposure: clinical and preclinical findings. Pharmacol Rev, 66(2), 435–465. doi: 10.1124/pr.111.005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Gyllenberg D, Malm H, McKeague IW, Hinkka-Yli-Salomaki S, Artama M, … Sourander A (2016). Association of Selective Serotonin Reuptake Inhibitor Exposure During Pregnancy With Speech, Scholastic, and Motor Disorders in Offspring. JAMA Psychiatry, 73(11), 1163–1170. doi: 10.1001/jamapsychiatry.2016.2594 [DOI] [PubMed] [Google Scholar]
- Brown HK, Hussain-Shamsy N, Lunsky Y, Dennis CE, & Vigod SN (2017). The Association Between Antenatal Exposure to Selective Serotonin Reuptake Inhibitors and Autism: A Systematic Review and Meta-Analysis. J Clin Psychiatry, 78(1), e48–e58. doi: 10.4088/JCP.15r10194 [DOI] [PubMed] [Google Scholar]
- Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, & Vigod SN (2017). Association Between Serotonergic Antidepressant Use During Pregnancy and Autism Spectrum Disorder in Children. JAMA, 317(15), 1544–1552. doi: 10.1001/jama.2017.3415 [DOI] [PubMed] [Google Scholar]
- Campagne DM (2007). Fact: antidepressants and anxiolytics are not safe during pregnancy. Eur J Obstet Gynecol Reprod Biol, 135(2), 145–148. doi: 10.1016/j.ejogrb.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, … Perlis RH (2016). Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry, 6, e708. doi: 10.1038/tp.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai WW, Fan JX, & Wen M (2018). Association of Individual and Neighborhood Factors with Home Food Availability: Evidence from the National Health and Nutrition Examination Survey. Journal of the Academy of Nutrition and Dietetics, 118(5), 815–823. doi: 10.1016/j.jand.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KVN, Bilde D, Charles J, Constantino JN, … Yeargin-Allsopp M (2016). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen HD, Rayburn WF, & Gonzalez CL (2000). Chronic prenatal exposure to paroxetine (Paxil) and cognitive development of mice offspring. Neurotoxicology and Teratology, 22(5), 733–739. doi: 10.1016/S0892-0362(00)00099-4 [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, & Chugani HT (1999). Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol, 45(3), 287–295. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, … Chugani HT (1997). Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol, 42(4), 666–669. doi: 10.1002/ana.410420420 [DOI] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, … D’Onofrio BM (2014). Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med, 44(1), 71–84. doi: 10.1017/S0033291713000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Rickert ME, Larsson H, Lichtenstein P, & D’Onofrio BM (2014). Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry, 205(5), 355–361. doi: 10.1192/bjp.bp.113.143693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, … Perlis RH (2015). Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry, 20(6), 727–734. doi: 10.1038/mp.2014.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Ball HA, Martin NC, Scourfield J, & McGuffin P (2009). Genetic overlap between measures of hyperactivity/inattention and mood in children and adolescents. J Am Acad Child Adolesc Psychiatry, 48(11), 1094–1101. doi: 10.1097/CHI.0b013e3181b7666e [DOI] [PubMed] [Google Scholar]
- Crawley JN (2012). Translational animal models of autism and neurodevelopmental disorders. Dialogues in Clinical Neuroscience, 14(3), 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, & Hendrick V (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry, 68(11), 1104–1112. doi: 10.1001/archgenpsychiatry.2011.73 [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, & Lichtenstein P (2013). Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry, 70(11), 1231–1240. doi: 10.1001/jamapsychiatry.2013.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, & Lichtenstein P (2013). The critical need for family-based, quasi-experimental research in integrating genetic and social science research. American Journal of Public Health, 103, S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, & et al. (2005). Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry, 62(4), 409–416. doi: 10.1001/archpsyc.62.4.409 [DOI] [PubMed] [Google Scholar]
- El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernandez G, Schoemaker NK, … Tiemeier H (2014). Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry, 205(2), 95–102. doi: 10.1192/bjp.bp.113.127746 [DOI] [PubMed] [Google Scholar]
- Ellis B, & Nigg J (2009). Parenting practices and attention-deficit/hyperactivity disorder: new findings suggest partial specificity of effects. J Am Acad Child Adolesc Psychiatry, 48(2), 146–154. doi: 10.1097/CHI.0b013e31819176d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson A, Kallen B, & Wiholm BE (1999). Delivery outcome after the use of antidepressants in early pregnancy. European Journal of Clinical Pharmacology, 55(7), 503–508. doi: DOI10.1007/s002280050664 [DOI] [PubMed] [Google Scholar]
- Eriksson MA, Westerlund J, Anderlid BM, Gillberg C, & Fernell E (2012). First-degree relatives of young children with autism spectrum disorders: some gender aspects. Res Dev Disabil, 33(5), 1642–1648. doi: 10.1016/j.ridd.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Figueroa R (2010). Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. Journal of Developmental and Behavioral Pediatrics, 31(8), 641–648. [DOI] [PubMed] [Google Scholar]
- Fokina VM, West H, Oncken C, Clark SM, Ahmed MS, Hankins GD, & Nanovskaya TN (2016). Bupropion therapy during pregnancy: the drug and its major metabolites in umbilical cord plasma and amniotic fluid. Am J Obstet Gynecol, 215(4), 497 e491–497. doi: 10.1016/j.ajog.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2016). Prenatal antidepressant use and risk of autism spectrum disorders in the children. JAMA Pediatrics, 170(7), 711–712. doi: 10.1001/jamapediatrics.2016.0745 [DOI] [PubMed] [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R, & Sjolander A (2012). Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology, 23(5), 713–720. [DOI] [PubMed] [Google Scholar]
- Galbally M, Crabb C, & Snellen M (2018). Designing research that can untangle the effects in pregnancy of pharmacological treatments for mental disorders. The Lancet Psychiatry, 5(8), 608–610. doi: 10.1016/S2215-0366(18)30214-1 [DOI] [PubMed] [Google Scholar]
- Galdas PM, Cheater F, & Marshall P (2005). Men and health help-seeking behaviour: literature review. Journal of Advanced Nursing, 49(6), 616–623. doi: 10.1111/j.1365-2648.2004.03331.x [DOI] [PubMed] [Google Scholar]
- Galler JR, Bryce CP, Waber DP, Hock RS, Harrison R, Eaglesfield GD, & Fitzmaurice G (2012). Infant malnutrition predicts conduct problems in adolescents. Nutr Neurosci, 15(4), 186–192. doi: 10.1179/1476830512y.0000000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK, Tran PV, & Carlson ES (2018). Atypical fetal development: Fetal alcohol syndrome, nutritional deprivation, teratogens, and risk for neurodevelopmental disorders and psychopathology. [Article]. Development and Psychopathology, 30(3), 1063–1086. doi: 10.1017/s0954579418000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Weiss DJ, Frank E, & Kupfer D (2016). Computerized Adaptive Diagnosis and Testing of Mental Health Disorders In Cannon TD & Widiger T (Eds.), Annual Review of Clinical Psychology, Vol 12 (Vol. 12, pp. 83–104). Palo Alto: Annual Reviews. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Weiss DJ, Pilkonis PA, & et al. (2012). Development of a computerized adaptive test for depression. Archives of General Psychiatry, 69(11), 1104–1112. doi: 10.1001/archgenpsychiatry.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Weiss DJ, Pilkonis PA, Frank E, Moore T, Kim JB, & Kupfer DJ (2014). Development of the CAT-ANX: A Computerized Adaptive Test for Anxiety. American Journal of Psychiatry, 171(2), 187–194. doi: 10.1176/appi.ajp.2013.13020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, & Newschaffer CJ (2014). In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord, 44(10), 2558–2567. doi: 10.1007/s10803-014-2128-4 [DOI] [PubMed] [Google Scholar]
- Grizenko N, Fortier ME, Gaudreau-Simard M, Jolicoeur C, & Joober R (2015). The effect of maternal stress during pregnancy on IQ and ADHD symptomatology. J Can Acad Child Adolesc Psychiatry, 24(2), 92–99. [PMC free article] [PubMed] [Google Scholar]
- Grzeskowiak LE, Morrison JL, Henriksen TB, Bech BH, Obel C, Olsen J, & Pedersen LH (2016). Prenatal antidepressant exposure and child behavioural outcomes at 7 years of age: a study within the Danish National Birth Cohort. Bjog, 123(12), 1919–1928. doi: 10.1111/1471-0528.13611 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N (2010). Serotonin, pregnancy and increased autism prevalence: is there a link? Med Hypotheses, 74(5), 880–883. doi: 10.1016/j.mehy.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, & Hertz-Picciotto I (2014). Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics, 133(5), e1241–1248. doi: 10.1542/peds.2013-3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Ekblad U, & Laine K (2001). Transplacental transfer of amitriptyline and nortriptyline in isolated perfused human placenta. Psychopharmacology (Berl), 153(4), 450–454. doi: 10.1007/s002130000597 [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Ekblad U, & Laine K (2002). Transplacental transfer of citalopram, fluoxetine and their primary demethylated metabolites in isolated perfused human placenta. Bjog-an International Journal of Obstetrics and Gynaecology, 109(9), 1003–1008. doi: Doi10.1016/S1470-0328(02)01467-2 [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, & Haynes D (2003). Placental passage of antidepressant medications. Am J Psychiatry, 160(5), 993–996. doi: 10.1176/appi.ajp.160.5.993 [DOI] [PubMed] [Google Scholar]
- Hostetter A, Ritchie JC, & Stowe ZN (2000). Amniotic fluid and umbilical cord blood concentrations of antidepressants in three women. Biol Psychiatry, 48(10), 1032–1034. doi: 10.1016/S0006-3223(00)00958-6 [DOI] [PubMed] [Google Scholar]
- Howland RH (2013). Antidepressant medication and pregnancy: time for randomized controlled trials. Journal of psychosocial nursing and mental health services, 51(2), 11–14. doi: 10.3928/02793695-20130109-01 [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Bujas-Petkovic Z, Vragovic R, Vuk T, Hock K, & Jernej B (2007). Hyperserotonemia in adults with autistic disorder. J Autism Dev Disord, 37(10), 1934–1940. doi: 10.1007/s10803-006-0324-6 [DOI] [PubMed] [Google Scholar]
- Huang H, Coleman S, Bridge JA, Yonkers K, & Katon W (2014). A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen Hosp Psychiatry, 36(1), 13–18. doi: 10.1016/j.genhosppsych.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid A, Melbye M, & Pasternak B (2013). Use of Selective Serotonin Reuptake Inhibitors during Pregnancy and Risk of Autism. New England Journal of Medicine, 369(25), 2406–2415. doi: 10.1056/Nejmoa1301449 [DOI] [PubMed] [Google Scholar]
- Illamola SM, Bucci-Rechtweg C, Costantine MM, Tsilou E, Sherwin CM, & Zajicek A (2018). Inclusion of pregnant and breastfeeding women in research - efforts and initiatives. Br J Clin Pharmacol, 84(2), 215–222. doi: 10.1111/bcp.13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Peng CT, Zhang X, & Ruan B (2017). Antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in the children: a meta-analysis of cohort studies. Bjog. doi: 10.1111/1471-0528.15059 [DOI] [PubMed] [Google Scholar]
- Jimenez-Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp-Pedersen C, & Poulsen HE (2013). Prevalence of antidepressant use during pregnancy in Denmark, a nation-wide cohort study. PLOS One, 8(4). doi: 10.1371/journal.pone.0063034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan Y, Keskin-Arslan E, & Acar S (2016). Prenatal antidepressant use and risk of autism spectrum disorders in children. JAMA Pediatrics, 170(7), 712. doi: 10.1001/jamapediatrics.2016.0727 [DOI] [PubMed] [Google Scholar]
- Kaplan YC, Keskin-Arslan E, Acar S, & Sozmen K (2016). Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: A systematic review and meta-analysis. Reprod Toxicol, 66, 31–43. doi: 10.1016/j.reprotox.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Kaplan YC, Keskin-Arslan E, Acar S, & Sozmen K (2017). Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. British journal of clinical pharmacology, 83(12), 2798–2806. doi: 10.1111/bcp.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, & Prescott CA (1999). Causal Relationship Between Stressful Life Events and the Onset of Major Depression. American Journal of Psychiatry, 156(6), 837–841. doi: 10.1176/ajp.156.6.837 [DOI] [PubMed] [Google Scholar]
- Khan S, Michmizos K, Tommerdahl M, Ganesan S, Kitzbichler MG, Zetino M, … Kenet T (2015). Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain, 138(Pt 5), 1394–1409. doi: 10.1093/brain/awv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee LJ, & Li Y (2014). Long-term consequences of neonatal fluoxetine exposure in adult rats. Dev Neurobiol, 74(10), 1038–1051. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuyama T, Takeuchi M, & Ito S (2016). Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: A systematic review and meta-analysis. Reproductive Toxicology, 65, 170–178. doi: 10.1016/j.reprotox.2016.07.016 [DOI] [PubMed] [Google Scholar]
- Lahey BB, & D’Onofrio BM (2010). All in the Family: Comparing Siblings to Test Causal Hypotheses Regarding Environmental Influences on Behavior. Current Directions in Psychological Science, 19(5), 319–323. doi: 10.1177/0963721410383977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, & Toft Sørensen H (2013). In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open, 3(9). doi: 10.1136/bmjopen-2013-003507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ (2009). Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotox Res, 15(3), 212–223. doi: 10.1007/s12640-009-9022-4 [DOI] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, … Genetic IIBD (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics, 45(9), 984–+. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner Y (2014). The Co-Occurrence of Autism and Attention Deficit Hyperactivity Disorder in Children – What Do We Know? Frontiers in Human Neuroscience, 8(268). doi: 10.3389/fnhum.2014.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Tchetgen Tchetgen E, & Cohen T (2010). Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology, 21(3), 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Agerbo E, Ingstrup KG, Musliner K, Meltzer-Brody S, Bergink V, & Munk-Olsen T (2017). Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ, 358. doi: 10.1136/bmj.j3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebstein R, Lalkin A, & Koren G (1997). Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet, 33(5), 328–343. doi: 10.2165/00003088-199733050-00002 [DOI] [PubMed] [Google Scholar]
- Lorant V, Deliege D, Eaton W, Robert A, Philippot P, & Ansseau M (2003). Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol, 157(2), 98–112. doi: 10.1093/aje/kwf182 [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, & Stowe ZN (2006). Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiatry, 163(1), 145–147. doi: 10.1176/appi.ajp.163.1.145 [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, DeVane CL, & Owens MJ (2006). Placental passage of tricyclic antidepressants. Biol Psychiatry, 59(3), 287–290. doi: 10.1016/j.biopsych.2005.06.040 [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, & Neuman G (2000). Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev, 20(5), 561–592. [DOI] [PubMed] [Google Scholar]
- Lund JL, Richardson DB, & Stürmer T (2015). The active comparator, new user study design in pharmacoepidemiology: Historical foundations and contemporary application. Current Epidemiology Reports, 2(4), 221–228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomaki S, McKeague IW, … Sourander A (2016). Gestational Exposure to Selective Serotonin Reuptake Inhibitors and Offspring Psychiatric Disorders: A National Register-Based Study. J Am Acad Child Adolesc Psychiatry, 55(5), 359–366. doi: 10.1016/j.jaac.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, & Wong IC (2015). Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev, 49, 82–89. doi: 10.1016/j.neubiorev.2014.11.020 [DOI] [PubMed] [Google Scholar]
- Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, … Wong ICK (2018). Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: A systematic review and meta-analysis. Neurosci Biobehav Rev, 86, 1–11. doi: 10.1016/j.neubiorev.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, … Wong ICK (2017). Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. Bmj-British Medical Journal, 357. doi: 10.1136/bmj.j2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, & Wittchen HU (2010). Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev, 86(5), 305–310. doi: 10.1016/j.earlhumdev.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Maxwell SE (2004). The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol Methods, 9(2), 147–163. doi: 10.1037/1082-989x.9.2.147 [DOI] [PubMed] [Google Scholar]
- McAllister BB, Kiryanova V, & Dyck RH (2012). Behavioural outcomes of perinatal maternal fluoxetine treatment. Neuroscience, 226, 356–366. doi: 10.1016/j.neuroscience.2012.09.024 [DOI] [PubMed] [Google Scholar]
- Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, … Gressier F (2017). Risk for Autism Spectrum Disorders According to Period of Prenatal Antidepressant Exposure: A Systematic Review and Meta-analysis. JAMA Pediatr, 171(6), 555–563. doi: 10.1001/jamapediatrics.2017.0124 [DOI] [PubMed] [Google Scholar]
- Mitchell BF, & Taggart MJ (2009). Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol, 297(3), R525–R545. doi: 10.1152/ajpregu.00153.2009 [DOI] [PubMed] [Google Scholar]
- Mlinarić A, Horvat M, & Šupak Smolčić V (2017). Dealing with the positive publication bias: Why you should really publish your negative results. Biochemia Medica, 27(3), 030201. doi: 10.11613/BM.2017.030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DR, Slattery J, Evans S, & Kurz X (2018). Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Medicine, 16(1), 6. doi: 10.1186/s12916-017-0993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, Daly E, Schmitz N, Toal F, Murphy K, Curran S, … Travis M (2006). Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger’s syndrome: an in vivo SPECT study. Am J Psychiatry, 163(5), 934–936. doi: 10.1176/ajp.2006.163.5.934 [DOI] [PubMed] [Google Scholar]
- Murray E, Pearson R, Fernandes M, Santos IS, Barros FC, Victora CG, … Matijasevich A (2016). Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? Evidence from a comparison between high-income and middle-income cohorts. J Epidemiol Community Health, 70(7), 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffah-Mazzacoratti MG, Rosenberg R, Fernandes MJ, Draque CM, Silvestrini W, Calderazzo L, & Cavalheiro EA (1993). Serum serotonin levels of normal and autistic children. Braz J Med Biol Res, 26(3), 309–317. [PubMed] [Google Scholar]
- Nestler EJ, & Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat Neurosci, 13(10), 1161–1169. doi: 10.1038/nn.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, & Halperin JM (2012). Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med, 166(4), 337–343. doi: 10.1001/archpediatrics.2011.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Koren G, Rovet J, Barrera M, Streiner DL, & Feldman BM (2015). Neurodevelopment of children prenatally exposed to selective reuptake inhibitor antidepressants: Toronto sibling study. J Clin Psychiatry, 76(7), e842–847. doi: 10.4088/JCP.14m09240 [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, & Koren G (2002). Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry, 159(11), 1889–1895. doi: 10.1176/appi.ajp.159.11.1889 [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, & Glover V (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years - Report from the Avon Longitudinal Study of Parents and Children. British Journal of Psychiatry, 180, 502–508. doi: DOI10.1192/bjp.180.6.502 [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, & Glover V (2003). Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry, 44(7), 1025–1036. doi: 10.1111/1469-7610.00187 [DOI] [PubMed] [Google Scholar]
- Pettersson E, Sjölander A, Almqvist C, Anckarsäter H, D’Onofrio BM, Lichtenstein P, & Larsson H (2015). Birth weight as an independent predictor of ADHD symptoms: a within‐twin pair analysis. J Child Psychol Psychiatry, 56(4), 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Johnson JG, & Brook JS (2002). Adolescent life events as predictors of adult depression. J Affect Disord, 68(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, & Rohde LA (2015). Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry, 56(3), 345–365. doi: 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- Pourhoseingholi MA, Baghestani AR, & Vahedi M (2012). How to control confounding effects by statistical analysis. Gastroenterology and Hepatology From Bed to Bench, 5(2), 79–83. [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, & Magnusson C (2013). Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ, 346, f2059. doi: 10.1136/bmj.f2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Newschaffer C, Lewis G, & Magnusson C (2017). Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ, 358. doi: 10.1136/bmj.j2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Proud S, Hackett LP, Kristensen JH, & Ilett KF (2004). A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmacol, 7(3), 329–334. doi: 10.1017/S1461145704004286 [DOI] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, … Forman T (2009). Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry, 42(3), 95–100. doi: 10.1055/s-0028-1103296 [DOI] [PubMed] [Google Scholar]
- Ray WA, & Griffin MR (1989). Use of medicaid data for pharmacoepidmiology. American Journal of Epidemiology, 129(4), 837–849. doi: 10.1093/oxfordjournals.aje.a115198 [DOI] [PubMed] [Google Scholar]
- Reis M, & Kallen B (2010). Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological Medicine, 40(10), 1723–1733. doi: 10.1017/s0033291709992194 [DOI] [PubMed] [Google Scholar]
- Rice D, & Barone S (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives, 108(3), 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, Al-Amin A, Smith GD, & Relton CL (2014). Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Hum Dev, 90(11), 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Porcel F, Green D, Khatri N, Harris SS, May WL, Lin RC, & Paul IA (2011). Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat Rec (Hoboken), 294(10), 1726–1735. doi: 10.1002/ar.21402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem-Kohavi N, & Oberlander TF (2017). Variations in Neurodevelopmental Outcomes in Children with Prenatal SSRI Antidepressant Exposure. Birth Defects Research, 109(12), 909–923. doi: 10.1002/bdr2.1076 [DOI] [PubMed] [Google Scholar]
- Rubin R (2018). Addressing barriers to inclusion of pregnant women in clinical trials. JAMA. doi: 10.1001/jama.2018.9989 [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Grace CE, Braun AA, Amos-Kroohs RM, Graham DL, Skelton MR, … Vorhees CV (2013). Cognitive impairments from developmental exposure to serotonergic drugs: citalopram and MDMA. Int J Neuropsychopharmacol, 16(6), 1383–1394. doi: 10.1017/S1461145712001447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherff A, Taylor M, Eley TC, Happe F, Charman T, & Ronald A (2014). What causes internalising traits and autistic traits to co-occur in adolescence? A community-based twin study. J Abnorm Child Psychol, 42(4), 601–610. doi: 10.1007/s10802-013-9796-y [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, & Makris N (2005). Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11), 1263–1272. doi: 10.1016/j.biopsych.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Shoulson RL, Stark RL, & Garland M (2014). Pharmacokinetics of fluoxetine in pregnant baboons (Papio spp.). J Am Assoc Lab Anim Sci, 53(6), 708–716. [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, … Lin RC (2011). Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A, 108(45), 18465–18470. doi: 10.1073/pnas.1109353108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit D, Perel JM, Wisniewski SR, Helsel JC, Luther JF, & Wisner KL (2011). Mother-infant antidepressant concentrations, maternal depression, and perinatal events. J Clin Psychiatry, 72(7), 994–1001. doi: 10.4088/JCP.10m06461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, … Pignone MP (2016). Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA, 315(4), 380–387. doi: 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- Sjölander A, & Zetterqvist J (2017). Confounders, Mediators, or Colliders: What Types of Shared Covariates Does a Sibling Comparison Design Control For? Epidemiology, 28(4), 540–547. doi: 10.1097/ede.0000000000000649 [DOI] [PubMed] [Google Scholar]
- Skurtveit S, Selmer R, Roth C, Hernandez-Diaz S, & Handal M (2014). Prenatal exposure to antidepressants and language competence at age three: results from a large population-based pregnancy cohort in Norway. BJOG, 121(13), 1621–1631. doi: 10.1111/1471-0528.12821 [DOI] [PubMed] [Google Scholar]
- Smith GD (2008). Assessing Intrauterine Influences on Offspring Health Outcomes: Can Epidemiological Studies Yield Robust Findings? Basic & Clinical Pharmacology & Toxicology, 102(2), 245–256. doi: 10.1111/j.1742-7843.2007.00191.x [DOI] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, & Smoller JW (2013). Pleiotropy in complex traits: challenges and strategies. Nature reviews. Genetics, 14(7), 483–495. doi: 10.1038/nrg3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, & Chung KC (2010). Observational Studies: Cohort and Case-Control Studies. Plastic and reconstructive surgery, 126(6), 2234–2242. doi: 10.1097/PRS.0b013e3181f44abc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen MJ, Gronborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, & Pedersen LH (2013). Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol, 5, 449–459. doi: 10.2147/CLEP.S53009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowles JL, Hufgard JR, Gutierrez A, Bailey RA, Jablonski SA, Williams MT, & Vorhees CV (2016). Perinatal exposure to the selective serotonin reuptake inhibitor citalopram alters spatial learning and memory, anxiety, depression, and startle in Sprague-Dawley rats. Int J Dev Neurosci, 54, 39–52. doi: 10.1016/j.ijdevneu.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Stahl SM (2013). Antidepressants Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications (4 ed). Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Stewart DE (2011). Clinical practice. Depression during pregnancy. N Engl J Med, 365(17), 1605–1611. doi: 10.1056/NEJMcp1102730 [DOI] [PubMed] [Google Scholar]
- Sujan AC, Rickert ME, Oberg AS, Quinn PD, Hernandez-Diaz S, Almqvist C, … D’Onofrio BM (2017). Associations of Maternal Antidepressant Use During the First Trimester of Pregnancy With Preterm Birth, Small for Gestational Age, Autism Spectrum Disorder, and Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA, 317(15), 1553–1562. doi: 10.1001/jama.2017.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, & Marcoen A (2004). High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev, 75(4), 1085–1097. [DOI] [PubMed] [Google Scholar]
- Viktorin A, Lichtenstein P, Lundholm C, Almqvist C, D’Onofrio BM, Larsson H, … Magnusson PKE (2016). Selective serotonin re-uptake inhibitor use during pregnancy: association with offspring birth size and gestational age. International Journal of Epidemiology, 45(1), 170–177. doi: 10.1093/ije/dyv351 [DOI] [PubMed] [Google Scholar]
- Viktorin A, Uher R, Kolevzon A, Reichenberg A, Levine SZ, & Sandin S (2017). Association of antidepressant medication use during pregnancy with intellectual disability in offspring. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2017.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorin A, Uher R, Reichenberg A, Levine SZ, & Sandin S (2017). Autism risk following antidepressant medication during pregnancy. Psychological Medicine, 1–10. doi: 10.1017/s0033291717001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Fisher JE, Moran MS, & Buelke-Sam J (1994). A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fundam Appl Toxicol, 23(2), 194–205. [DOI] [PubMed] [Google Scholar]
- Weaver KJ, Paul IA, Lin RC, & Simpson KL (2010). Neonatal exposure to citalopram selectively alters the expression of the serotonin transporter in the hippocampus: dose-dependent effects. Anat Rec (Hoboken), 293(11), 1920–1932. doi: 10.1002/ar.21245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall J, & Yarkoni T (2016). Statistically Controlling for Confounding Constructs Is Harder than You Think. PLoS ONE, 11(3), e0152719. doi: 10.1371/journal.pone.0152719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Motulsky A, Eguale T, Buckeridge DL, Abrahamowicz M, & Tamblyn R (2016). Treatment Indications for Antidepressants Prescribed in Primary Care in Quebec, Canada, 2006–2015. JAMA, 315(20), 2230–2232. doi: 10.1001/jama.2016.3445 [DOI] [PubMed] [Google Scholar]
- Wood ME, Lapane KL, van Gelder M, Rai D, & Nordeng HME (2018). Making fair comparisons in pregnancy medication safety studies: An overview of advanced methods for confounding control. Pharmacoepidemiol Drug Saf, 27(2), 140–147. doi: 10.1002/pds.4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CJ, Tan HP, & Du YJ (2014). The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience, 267, 1–10. doi: 10.1016/j.neuroscience.2014.02.021 [DOI] [PubMed] [Google Scholar]
- Yonkers K, Forray A, & Smith MV (2017). Maternal antidepressant use and pregnancy outcomes. JAMA, 318(7), 665–666. doi: 10.1001/jama.2017.9182 [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, … Lockwood C (2009). The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry, 31(5), 403–413. doi: 10.1016/j.genhosppsych.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Gunnar MR, McCall RB, Kreppner JM, & Fox NA (2011). Sensitive periods. Monographs of the society for research in child development, 76(4), 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X-H, Li Y-J, Ou J-J, & Li Y-M (2018). Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Molecular Autism, 9(1), 21. doi: 10.1186/s13229-018-0207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, & Germeyan SC (2015). Effects of neonatal fluoxetine exposure on behavior across development in rats selectively bred for an infantile affective trait. Dev Psychobiol, 57(2), 141–152. doi: 10.1002/dev.21264 [DOI] [PubMed] [Google Scholar]
- Zucker I (2017). Risk mitigation for children exposed to drugs during gestation: A critical role for animal preclinical behavioral testing. Neuroscience & Biobehavioral Reviews, 77, 107–121. doi: 10.1016/j.neubiorev.2017.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


