Abstract
Objectives:
To determine the prevalence and correlates of subclinical myocardial inflammation in rheumatoid arthritis (RA).
Methods
RA patients (n=119) without known cardiovascular disease (CVD) underwent cardiac 18-fluorodeoxyglucose positron emission tomography with computed tomography (FDG PET-CT). Myocardial FDG uptake was assessed visually and quantitatively by standardized uptake values (SUV). Multivariable linear regression was used to assess the associations of patient characteristics with myocardial SUV. A subset of RA patients escalating disease modifying anti-rheumatic drug (DMARD) therapy (n=8) had a second FDG PET-CT scan after 6 months to assess treatment-associated changes in myocardial FDG uptake.
Results
Visually assessed FDG uptake was observed in 46 (37%) of RA patients, and 21 (18%) had abnormal quantitatively assessed myocardial FDG uptake [i.e. SUVmean≥3.10 units (two standard deviations above the SUVmean of a reference non-RA group (n=27)]. Average SUVmean was 31% higher for those with a clinical disease activity index (CDAI) ≥10 vs. those with lower scores (p=0.005) after adjusting for potential confounders. The average adjusted SUVmean was 26% lower among those treated with non-TNF targeted biologics vs. those treated with conventional (non-biologic) DMARDs (p=0.029). In the longitudinal sub-study, myocardial SUVmean decreased from 4.50 to 2.30 units over 6 months, which paralleled the decrease in average CDAI from 23 to 12 units.
Conclusions
Subclinical myocardial inflammation is frequent in RA, is associated with RA disease activity, and may decrease with RA therapy. Future longitudinal studies will be required to assess whether reduction in myocardial inflammation will reduce heart failure risk in RA.
INTRODUCTION
Heart failure, a key contributor to cardiovascular disease (CVD) morbidity and mortality in RA 1,2, is associated on average with fewer symptoms and higher (preserved) ejection fraction but higher mortality rates when compared with the general population 1–4. In the general population, higher levels of circulating pro-inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin-6 (IL-6), are independent predictors of heart failure 5–9. In rodents, infusion of TNF reduced myocardial contractility 10, and cardiac-specific overexpression of a TNF transgene was associated with myocardial inflammation, remodeling, fibrosis, and eventually heart failure 11–13. In RA, circulating TNF and IL-6 levels are orders of magnitude higher than those shown to predict heart failure in the general population 14; however, little is known about inflammatory processes within the RA myocardium itself. Autopsy studies of RA hearts from the mid-twentieth century suggest that myocarditis may occur in 15–20% of patients 15,16. However, contemporary histologic characterization studies of the myocardium in RA patients are few, mostly limited to patients with a known history of ischemic CVD 14.
The conventional gold standard for diagnosing myocarditis is endomyocardial biopsy, however its sensitivity is limited by the heterogeneous distribution of myocarditis 17,18. This, coupled with its invasiveness, expense, and risk of complications, has limited investigations of subclinical myocarditis in patients with RA. Cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) has been used to identify myocardial abnormalities but clinically approved gadolinium-based contrast agents distribute to the extracellular space19 and are not taken up by cells. Thus, myocardial LGE reflects interstitial edema but cannot directly identify inflammatory infiltrates, nor can LGE identify diffuse myocardial involvement, only focal 20–23. CMR T2-weighted imaging (T2WI), a more sensitive method for measuring myocardial edema that is not dependent on gadolinium, may overcome the latter issue but does not solve the former limitation 24.
In recent years, 18-fluorodeoxyglucose-positron emission tomography-computed tomography (FDG-PET-CT) has been shown to have high sensitivity for detecting myocardial inflammation. Inflammatory cells are metabolically active and avidly take up FDG via glucose transporters (GLUTs); moreover, areas of myocardial FDG uptake strongly correlate with numbers of infiltrating macrophages and T cells on histologic assessment 25,26. In the current study, we assessed myocardial FDG uptake among RA patients with no history of known CVD. In a nested sub-study of RA patients with an inadequate response to methotrexate monotherapy, we evaluated the change in myocardial FDG uptake in response to 6 months of step-up therapy. We hypothesized that myocardial inflammation would be present in a proportion of RA patients without clinical heart failure, its presence would be correlated with RA disease activity and circulating inflammatory mediators, and would decrease upon treatment upregulation.
METHODS
RA patients enrolled in the RHeumatoid arthritis studY of THe Myocardium [RHYTHM], which has been described previously 27, were studied. Participants were recruited from the rheumatology clinics of Columbia University Medical Center and by referral from local rheumatologists. Inclusion criteria included age≥18 years and fulfillment of the American College of Rheumatology 2010 classification criteria for RA 28. Exclusion criteria included: 1) any prior self-reported physician diagnosed CV event or procedure, contraindication to pharmacologic stress agents, and active cancer.
The study sample consisted of 119 RA patients from RHYTHM with FDG PET-CT scans technically adequate to evaluate myocardial FDG uptake. Eight of these 119 took part in a nested longitudinal pilot sub-study that required active disease despite methotrexate monotherapy, specifically a Clinical Disease Activity Index (CDAI)≥ 10 units. Therapy was escalated to either a TNF inhibitor (with continued methotrexate) or triple therapy (i.e. sulfasalazine + hydroxychloroquine with continued methotrexate). Originally escalation was assigned randomly; however, due to slow recruitment, the protocol was switched to open label TNF inhibitor (either etanercept or adalimumab), such that two patients received triple therapy and six received a TNF inhibitor. These RA patients underwent a second FDG PET-CT scan after 6 months of treatment to evaluate change in myocardial FDG uptake with treatment. There is no established normative cutoff for myocardial FDG uptake. Accordingly, we assembled a control group made up of volunteers without RA recruited from friends of RHYTHM participants and advertisements. Additional controls without RA or other rheumatic diseases were added from the CUMC nuclear cardiology archive who underwent FDG PET CT to rule out myocarditis or cardiac sarcoidosis, using a similar scanning protocol during the same enrollment period as the RHYTHM study (i.e. 2011 – 2016) but who did not have visible FDG uptake. The study was approved by the Columbia University Medical center, New York Presbyterian Hospital Institutional Review Board. All subjects provided written informed consent prior to enrollment.
Outcome Assessments
The primary outcome was myocardial FDG uptake, assessed by PET-CT scanning. To suppress physiological uptake of FDG by cardiomyocytes, patients were prescribed a high fat no-carbohydrate diet the day before, followed by a 12-hour fast, before the scan 29,30. Dietary adherence was interrogated on the day of the scan. All patients had a blood sugar concentration of <200 mg/dl at the time of imaging. Imaging was performed on a MCT 64 PET/CT scanner (Siemens Medical Solutions USA, INC., Knoxville, TN). A low dose CT transmission scan was obtained for attenuation correction of the PET data. Patients were injected with 10 mCi of 18F-FDG intravenously. A list mode 3D PET scan was acquired for 10 minutes following a 90-minute uptake period post-18F-FDG injection. Non-gated attenuation-corrected images were reconstructed yielding ~3 mm effective resolution. Corridor4DM v. 7.0 software (Invia Medical Imaging Solutions, Ann Arbor, MI) was used to assess myocardial 18FDG uptake. Cardiac axes were manually defined by marking the base on the vertical long axis, apex on the horizontal long axis, and the left ventricular cavity on the sagittal axis. Qualitative and quantitative assessment of FDG uptake was performed in all scans by the same nuclear cardiologist blinded to disease status. FDG uptake was assessed quantitatively as standardized uptake value (SUV), a measure of radiotracer uptake normalized for injected dose and patient weight. The reconstruction produced a set of transverse slices through the heart perpendicular to the long axis of body, which was used to generate sets of sagittal and coronal slices. LV segments were defined by the segmentation nomenclature of the American Heart Association 31. Contours were placed on the myocardial walls and automated SUV calculations for the mean SUV and the max SUV for each of the segments were derived. The mean of the means (SUVmean) and the mean of the max SUVs (SUVmax) were then obtained.
Other Measures
Coronary artery calcium (CAC).
CAC was assessed from the CT scan and quantified using the Agatston method 32. The presence of CAC was defined as an Agatston score of greater than zero.
Echocardiographic Parameters.
Transthoracic two-dimensional and real-time 3D-echocardiography (RT3DE) was performed using a commercially available system (iE 33; Philips, Andover, MA) by a registered cardiac sonographer according to a standardized protocol. LV end-diastolic (EDVI) and end-systolic (ESVI) volume indices, stroke volume (SV), and ejection fraction (LVEF) were measured by RT3DE using a commercially available software (Philips QLAB Advanced Quantification Software, version 8.1) as previously described 33. LV mass was also assessed by RT3DE by tracings of endocardial and epicardial borders. Left atrial volume and LV diastolic function were measured by 2-dimensional echocardiography as previously described 34. Briefly, in apical 4-chamber view, peak early (E) and late velocity (A) of mitral inflow were measured by pulsed-wave Doppler. Peak early diastolic velocity (E′) of the lateral and septal mitral annulus were evaluated by pulsed-wave Tissue-Doppler and averaged. The E/E’ ratio was calculated as an index of LV filling pressure. LV volumes, stroke volume, cardiac output, and LA volume were indexed by body surface area. LV mass was indexed by height2.7. LV diastolic dysfunction was defined as abnormalities in any of the following parameters: E/A ratio (either <0.8 or >2.0), E deceleration time (>240 msec or <140 msec), peak e’ (< 8 cm/sec), or E/e’ (>15).
Clinical Covariates:
Demographic, lifestyle characteristics and medications were assessed by structured interview. Resting blood pressure (BP) was measured 3 times, and the average of the last 2 measurements was used. Hypertension was defined as systolic BP of ≥140 mm Hg, diastolic BP of ≥90 mm Hg, or use of antihypertensive medications. Diabetes was defined as fasting serum glucose level of ≥126 mg/dl or use of antidiabetic medications. Body mass index was calculated by patient weight (in kilograms) divided by height (in square meters). RA disease duration was calculated from date of diagnosis. Forty-four joints were examined for swelling and tenderness. RA disease activity was calculated with the Clinical Disease Activity Index (CDAI) and the Disease Activity Score (DAS28) using C-reactive protein (CRP) level 35–37. Disability was assessed with the Health Assessment Questionnaire (HAQ) 38. Current and past use of steroids and biologic and non-biologic disease-modifying anti-rheumatic drugs (DMARDs) were queried by examiner-administered questionnaires.
Laboratory Covariates:
Fasting sera and plasma were obtained on the morning of the study visit. Lipids were measured by colormetric assay and Hs-CRP by turbidimetric immunoassay (Roche Diagnostics). Rheumatoid factor (RF), anti-cyclic-citrullinated peptide antibody (anti-CCP), and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA) (IBL America, Inova Diagnostics, R&D Systems, respectively). RF and anti-CCP seropositivity were defined by levels ≥ 40 units and ≥ 60 units, respectively. Brain naturetic peptide (BNP) and troponin-I were measured by ARCHITECT chemiluminescent microparticle immunoassays (Abbott Labs, Abbot Park, IL).
Analytical Methods
Summary statistics for continuous and categorical variables were calculated, including means, standard deviations, ranges, counts and percentages. Multivariable linear regression was used to model the associations of RA patient characteristics with the natural log transformed myocardial SUVmean and SUVmax, first in univariate models with each independent variable modelled as the only covariate in the model. Independent variables associated with the myocardial SUV outcome at the p<0.25 level were carried into multivariable models. Extended models were reduced to more parsimonious models using Akaike’s Information Criterion for nested models. We calculated variance inflation factors to ensure that collinear variables were not co-modelled, and none were detected for any of the primary models. Linear regression was also used to model SUVmean and SUVmax levels according to groups defined by visualized myocardial FDG uptake (i.e. none, focal, diffuse, and focal on diffuse). T-tests were used to compare baseline and follow-up natural log transformed SUVmean and SUVmax levels in the RA groups with baseline levels in the non-RA control group while a paired t-test was used to compare baseline and follow-up myocardial SUV levels within the RA group. Throughout, a two-tailed alpha of 0.05 was used, and analyses were performed using Stata version 14 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics of the 119 RA patients are summarized in Table 1. The mean age was 54 years and median disease duration was 6.7 years. The majority (82%) were female and those of Hispanic or non-Hispanic white race/ethnicity made up 80% of the cohort. Ever versus current smoking was reported in 44% and 11%, respectively. The majority (76%) were seropositive for RF and/or CCP and the distribution of DAS28 indicated that most patients were in the low or moderate disease activity range. Remission (DAS28<2.6 units) and high disease activity (DAS28≥5.2 units) were observed in 15% and 10%, respectively (data not shown). The majority (87%) were treated with DMARDs, with 77% treated with non-biologics (the majority of which were MTX) and 38% treated with biologics (the majority of which were TNF inhibitors). One-third were currently taking prednisone and 41% reported taking NSAIDs. More than two-thirds of the patients had any CAC (> 0) on CT. Troponin-I levels were undetectable in all RA patients (data not shown). The median BNP level was in normal range (Table 1).
Table 1.
Participant Characteristics According to RA Status
| Characteristics | RA patients (n=119) | Controls (n=27) |
|---|---|---|
| Demographic | ||
| Mean Age in years (SD); [range] | 54 (13); [21–80] | 50 (12) |
| Female (%) | 97 (82) | 13 (48) |
| Race/Ethnicity | ||
| Non-Hispanic White (%) | 44 (37) | |
| Non-Hispanic Black (%) | 18 (15) | |
| Hispanic (%) | 53 (44) | |
| Other (%) | 4 (3.4) | |
| Cardio-vascular risk factors | ||
| Ever smoker (%) | 52 (44) | 7 (26) |
| Current smoker (%) | 13 (11) | 1 (4) |
| Hypertension (%) | 46 (39) | 10 (37) |
| Diabetes (%) | 15 (13) | 2 (8) |
| Mean BMI (SD) in kg/m2 | 28.5 (5.9) | 33.6 (4.8) |
| Mean total cholesterol (SD), mg/dL | 191 (37) | |
| Mean LDL-C (SD), mg/dL | 110 (32) | |
| Mean HDL-C (SD), mg/dL | 59 (19) | |
| RA characteristics | ||
| Median RA Duration in years (IQR) | 6.7 (2 – 14.1) | |
| RF or anti-CCP (%) | 91 (76) | |
| Median DAS28-CRP in units (IQR) | 3.9 (3.0–4.7) | |
| Mean CDAI in units (SD) | 18.2 (12.1) | |
| Mean HAQ in units (SD) | 1.7 (.8) | |
| Median AM stiffness in min (IQR) | 20 (5 – 50) | |
| Median CRP (mg/L) (IQR) | 2.51 (1.1 – 6.6) | 0.64 (0.45–2.94) |
| Median IL-6 (pg/mL) (IQR) | 2.3 (1.4 – 7.3) | 0.94 (0.80–2.26) |
| Median troponin-I | undetectable | |
| Median BNP (pg/mL) (IQR) | 15.4 (10–26.2) | |
| No DMARD (%) | 15 (13) | |
| Non-biologic DMARDs (%) | 91 (77) | |
| Methotrexate (%) | 77 (67) | |
| Other non-biologic2(%) | 26 (22) | |
| Biologic DMARD (%) | 45 (38) | |
| TNF inhibitors (%) | 35 (29) | |
| Non-TNF biologics (%) | 10 (8) | |
| Abatacept (%) | 8 (7) | |
| Other Non-TNF biologic3(%) | 2 (2) | |
| Prednisone (%) | 39 (33) | 0 (0) |
| NSAIDs (%) | 49 (41) | 0 (0) |
| CAC score | ||
| CAC score null | 78 (65.6) | 9 (69.2) |
| CAC score 0–99 units | 19 (16) | 2 (15.4) |
| CAC score≥100 units | 22 (18) | 2 (15.4) |
| Myocardial Structure | ||
| Mean LVMI, per grams/ht2.7(SD) | 29.9 (5.41) | |
| Mean EDVI, per mL/m2(SD) | 53.8 (11) | |
| Median ESVI, per mL/m2(IQR) | 19.4 (16–23) | |
| Myocardial Function | ||
| Mean Ejection Fraction (per %) (SD) | 62.9 (4.5) | |
| Mean E/E’ (SD) | 8.5 (2.4) | |
| Mean Stroke Volume Index, per mL/m2(SD) | 58.6 (13.9) | |
| Mean Cardiac Index, per L/min/m2(SD) | 2.29 (0.38) | |
| Diastolic Dysfunction (yes v. no) % | 49 (43) | |
Legend: patients may be on more than one non-biologic disease-modifying anti-rheumatic drug (DMARD).
other non-biologic include Sulfasalazine, hydroxychloroquine or leflunomide
other biologic include Rituximab and Tocilizumab. SD: Standard Deviation, IQR: inter quantile range, RF: rheumatoid factor, CCP: cyclic citrullinated protein antibody, DAS: disease activity score, HAQ: health assessment questionnaire, AM: morning, CRP: C-reactive protein, IL-6: interleukin 6, BNP: brain natriuretic peptide, CAC: coronary artery calcium, LVMI: left ventricle mass indexed to height 2.73, EDVI: end diastolic volume index, ESVI: end systolic volume index.
Myocardial FDG Uptake was Associated with Disease Activity and RA Treatment
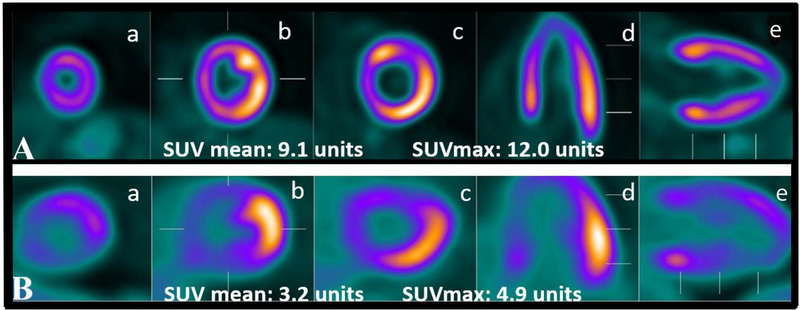
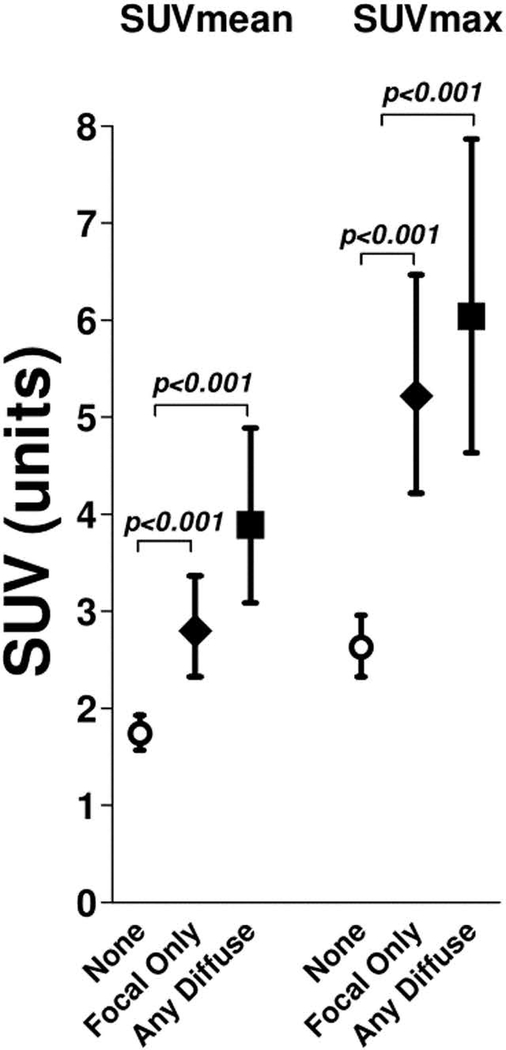
Any visually assessed myocardial FDG uptake was detected in 46 RA patients (39%). Of these, 25 of the 46 (54%) had focal uptake only, and 21 (45%) had any diffuse uptake (n=15 with diffuse and n=6 with focal on diffuse), examples of which are illustrated in Figure 1. Quantified myocardial FDG uptake according to visualized myocardial uptake is depicted in Figure 2. Those with visualized focal only uptake had an average myocardial SUVmean that was 61% higher than those with no visible uptake (2.80 vs. 1.74 units, respectively; p<0.001). Those with any visualized diffuse myocardial FDG uptake had an average myocardial SUVmean that was 124% higher than those with no visible uptake (3.89 vs. 1.74 units, respectively; p<0.001). For SUVmax, compared with those with no visible myocardial FDG uptake, those with focal only uptake had an average myocardial SUVmax that was 98% higher than those with no visible uptake (5.22 vs. 2.63 units, respectively; p<0.001). Those with any diffuse visible myocardial FDG uptake had an average myocardial SUVmax that was 130% higher than those with no visible uptake (6.04 vs. 2.63 units, respectively; p<0.001).
Figure 1: Examples of diffuse and focal myocardial 18F-FDG uptake in two patients with rheumatoid arthritis.
Examples of diffuse (panel A) and focal (panel B) FDG uptake in two different patients with RA. Short axis is represented by a, b and c; horizontal axis in d; and, vertical long axis in e and f.
Figure 2. Quantitative SUVs as a function of categorization by visually assessed (qualitative) SUV.
Means and 95% confidence intervals are depicted for mean and max Standard uptake values (SUV) according to category of visualized myocardial uptake.
We scanned a group of 27 non-RA controls to estimate a cutoff for normal myocardial FDG uptake. Among controls, the mean SUVmean (1.70 units) plus two standard deviations (0.70 units x 2) was 3.10 units. Using this cutoff, 21 (18%) of the RA patients had abnormal (high) myocardial FDG uptake (data not shown).
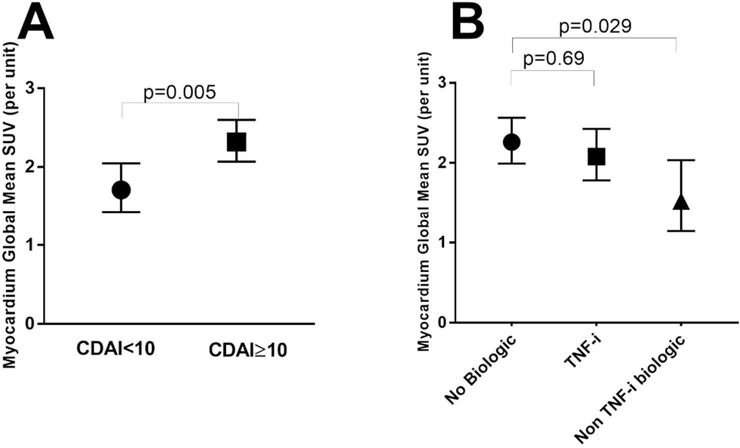
Univariate and multivariable associations of RA participant characteristics with the log transformed myocardial SUVmean are summarized in Table 2. In univariate models, non-Hispanic Black race, higher BMI, higher DAS28, and higher CDAI were each significantly associated with higher log SUVmean, while current use of non-TNF inhibitor biologics was associated with a significantly lower log SUVmean. However, after carrying these into extended and reduced models, only higher disease activity remained significantly associated with higher log SUVmean while non-TNF targeted biologics remained inversely associated with log SUVmean, after adjusting for BMI and CAC level. Specifically, those with a CDAI≥10 had an average adjusted SUVmean that was 31% higher than those with a CDAI<10 (2.30 vs. 1.76 units, respectively; p=0.005: Figure 3A). Those treated with non-TNF targeted biologics had an adjusted SUVmean that was 26% lower compared with those not treated with biologics (1.65 vs. 2.23 units, respectively; p=0.029: Figure 3B). The majority of the non-TNF biologic users (8 out of 10) were receiving abatacept. We did not observe any statistically significant differences in RA characteristics (RF/CCP positivity, CDAI, DAS28, HAQ, CRP, IL-6) in the abatacept users compared to non-abatacept biologic users, or compared to non-biologic users (data not shown). In contrast, the adjusted SUVmean was not significantly different for those treated with TNF inhibitors vs. those not receiving biologics. CDAI≥10 and current use of non-TNF inhibitor biologics were also the only significant correlates of SUVmax after adjusting for BMI (data not shown).
Table 2.
Associations of Participant Characteristics with Log Transformed Myocardial SUVmean
| Univariate Models |
Extended MV Model |
Reduced MV Model |
|||||
|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | ||
| Age, per year | −0.0004 | 0.92 | |||||
| Male vs female | −0.11 | 0.39 | |||||
| Non-Hispanic white | referent | referent | |||||
| Non-Hispanic black | 0.34 | 0.029 | 0.20 | 0.24 | |||
| Hispanic | 0.17 | 0.14 | 0.064 | 0.63 | |||
| Other race | −0.093 | 0.74 | −0.12 | 0.67 | |||
| Ever smoking vs. never | −0.023 | 0.82 | |||||
| Hypertension, yes v. no | 0.0030 | 0.98 | |||||
| BMI, per kg/m2 | 0.018 | 0.042 | 0.014 | 0.10 | 0.015 | 0.068 | |
| Diabetes, yes v. no | −0.17 | 0.27 | |||||
| Total cholesterol, per mg/dL | −0.0017 | 0.22 | −0.0015 | 0.29 | |||
| LDL, per mg/dL | −0.0010 | 0.55 | |||||
| HDL, per mg/dL | −0.0016 | 0.56 | |||||
| log Triglycerides, per mg/dL | −0.11 | 0.36 | |||||
| RA duration (square root), per year | 0.010 | 0.74 | |||||
| RF or anti-CCP, yes v. no | 0.011 | 0.93 | |||||
| DAS28-CRP>3.2 units | 0.22 | 0.039 | |||||
| CDAI >10 units | 0.31 | 0.006 | 0.25 | 0.047 | 0.28 | 0.012 | |
| log CRP, per mg/L | 0.0047 | 0.91 | |||||
| log IL-6, per pg/mL | 0.015 | 0.74 | |||||
| log BNP, per pg/mL | 0.0009 | 0.75 | |||||
| AM stiffness (square root), per min | 0.0052 | 0.71 | |||||
| HAQ, per unit | 0.11 | 0.099 | −0.019 | 0.79 | |||
| Methotrexate, yes v. no | 0.13 | 0.22 | 0.12 | 0.27 | |||
| No biologic | referent | referent | referent | ||||
| TNF inhibitors | −0.080 | 0.48 | −0.029 | 0.80 | −0.023 | 0.83 | |
| Non-TNF biologics | −0.38 | 0.043 | −0.36 | 0.047 | −0.38 | 0.033 | |
| Current prednisone, yes v. no | 0.14 | 0.21 | |||||
| Current NSAID, yes v. no | 0.067 | 0.52 | |||||
| CAC score zero | referent | referent | referent | ||||
| CAC score <100 | 0.018 | 0.90 | 0.041 | 0.77 | 0.037 | 0.78 | |
| CAC score ≥100 | −0.22 | 0.10 | −0.25 | 0.073 | −0.21 | 0.10 | |
| Adjusted R2 | 0.105 | 0.025 | 0.102 | 0.006 | |||
Legend: Beta coefficients represent the average change in the Log Transformed Myocardial SUVmean per 1-unit higher value of the independent continuous variable of interest or for those with versus those without the independent dichotomous variable of interest. RA: rheumatoid arthritis, RF: rheumatoid factor, CCP: cyclic citrullinated protein antibody, DAS: disease activity score, CDAI: clinical disease activity index HAQ: health assessment questionnaire, AM: morning, CRP: C-reactive protein, IL-6: interleukin 6, CAC: coronary artery calcium
Figure 3: Adjusted Mean Global Myocardial FDG Uptake According to CDAI and Current Biologic DMARD Use.
Means and 95% confidence intervals are depicted. Panel A and B are adjusted for DMARD treatment and RA disease group (controlled vs uncontrolled) which were the only significant covariates retained in multivariable modeling. CDAI: Clinical Disease Activity Index, TNF-i: Tumor Necrosis Factor inhibitor. SUV: Standardize Uptake Value
Myocardial FDG Uptake after Escalation of RA Pharmacotherapy
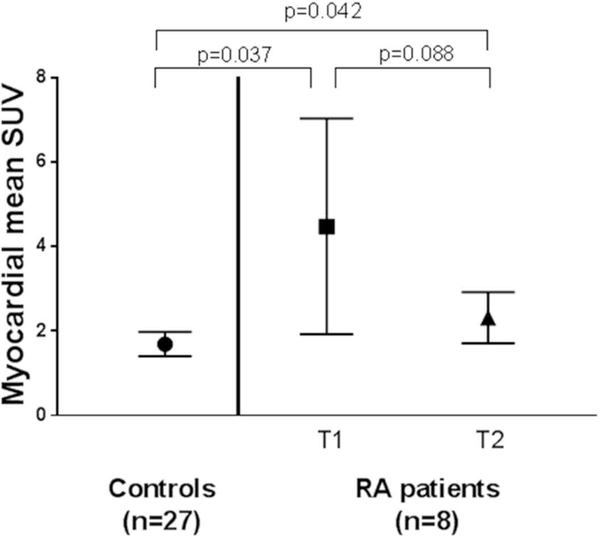
Of the 8 RA patients with longitudinal assessment of myocardial FDG uptake, 6 were escalated to TNF inhibitors and two to triple therapy. At baseline, prior to escalation of therapy, 4 RA patients had visible FDG uptake (two focal and two diffuse) and the average SUVmean was 4.50 units, which was 165% higher than that of a group of non-RA controls (n=27) (p=0.037). Upon rescanning after 6 months of therapy, only one RA patient had visible myocardial FDG uptake, and the average SUVmean in the RA group numerically decreased by almost 50% to 2.3 units, although this change was not statistically significant (p=0.088). The SUVmean at 6 month in the RA group was now only 35% higher than that of the control group, but still significantly higher (p=0.042) (Figure 4). The reduction in myocardial FDG uptake in the RA group paralleled a decrease in mean CDAI score from 23 units at baseline to 12 units at 6 months. A similar, but not statistically significant, trend was observed for SUVmax, which decreased from 7.2 to 3.8 units (p=0.082) after 6 months (data not shown).
Figure 4. Global Myocardial 18F-FDG Uptake Before and After Acceleration of Therapy Compared with non-RA Controls.
Means and 95% confidence intervals are depicted. Controls had only one scan while rheumatoid arthritis patients had a scan before (T1) and after 6-months (T2) of step-up therapy with either TNF-inhibitors or triple therapy with sulfasalazine and hydroxychloroquine on a background of methotrexate.
Association of Myocardial FDG Uptake with LV Structure and Function
We evaluated the association of SUVmean with echocardiographic measures of LV structure (left ventricular mass index [LVMI], end diastolic volume index [EDVI] and end systolic volume index [ESVI]) and of LV function (ejection fraction [EF] and diastolic dysfunction [E/E’ and diastolic dysfunction yes/no]. Except for a high prevalence (43%) of diastolic dysfunction in the RA patients, mean measures of LV structure and function were in normal ranges (Table 1). SUVmean was not associated with any measure of LV structure or function in univariable analyses (Supplementary Table S1). In multivariable analyses of SUVmean with a selected measure of LV structure (LVMI) and of diastolic dsyfunction (E/E’), no association of SUVmean with either was observed (Tables S2 and S4). SUVmax was also not associated with measures of LV structure or function; a representative multivariable analysis of SUVmax with LVMI is shown in Table S3. BNP levels were also not associated with SUVmean (Table 2).
DISCUSSION
To our knowledge, ours is the first study to evaluate and quantify subclinical myocardial inflammation by FDG PET-CT in RA patients, which allowed a detailed assessment of associations of RA characteristics with FDG uptake. It is also the first study, albeit a small pilot study, to longitudinally evaluate the effect of step-up therapy on myocardial inflammation. Cross-sectionally we observed that 37% of RA patients had visible myocardial FDG uptake while abnormal FDG uptake, defined by SUV two standard deviations above the mean of a reference non-RA control group, was prevalent in 18% of RA patients. Additionally, we observed that myocardial FDG uptake was strongly correlated with higher articular disease activity, and was lower in patients using non-TNF targeted biologic agents. In addition, the pilot longitudinal study suggested that myocardial inflammation may improve with treatment with DMARDs.
Our finding of myocarditis in 18–37% of RA patients is largely comparable in magnitude to historical necropsy studies that reported subclinical myocarditis in up to 20% of RA patients 15,16. It is also in accordance with several smaller CMR studies reporting a higher prevalence of LGE in RA patients when compared with non-RA controls 39–41. However, LGE reflects extracellular edema and is therefore less specific than FDG uptake for myocarditis. Moreover, LGE is only useful for identifying focal abnormalities. More recently, T2-weighted imaging (T2WI) has been utilized in CMR studies as a more quantitative, non-gadolinium dependent measure of tissue free water content. In CMR studies in RA patients without clinical CVD, Ntusi et al reported a prevalence of myocardial LGE of 46% versus only 10% by T2WI 39. Similarly in a Japanese cohort of RA patients, Kobayashi et al. observed myocardial LGE in 32%, but T2WI was present in only 12% 41. In non-RA patients with clinically suspected and histopathologically proven myocarditis, T2W1 performed better than standard CMR measures for distinguishing active from inactive myocarditis 24. A future study in RA in which quantitative myocardial FDG uptake and T2W1 CMR studies are performed in the same patients would be useful to determine whether T2W1 correlates with intensity and geographic distribution of FDG uptake.
Importantly, we observed an association of myocardial inflammation with articular disease activity. This observation, which is consistent with several previous CMR studies (39–41) supports the premise that achieving low disease activity or remission of RA activity protects not only the joints but possibly the myocardium as well. However, we did not find an association of myocardial SUV with circulating CRP or IL-6. The known effect of DMARDs on lowering CRP and IL-6 levels may have obscured a relationship with myocardial SUV. It is also possible that systemic inflammation causing myocardial harm may be due to a cytokine(s) not measured in our study. Alternatively, in situ myocardial production of cytokines may play a greater role than systemic inflammation in mediating myocardial inflammation 42–45 but may not be robust enough to contribute to circulating levels of cytokines in this RA cohort without clinical heart failure and without apparent myocardial damage (as suggested by undetectable troponin levels). Other than BMI, we did not observe associations between myocardial FDG uptake and other traditional CVD risk factors. There was also no clear association between myocardial uptake and CAC scores. These findings suggest that myocardial uptake in RA is driven more by features unique to the RA disease state than by CVD risk factors and/or atherosclerosis that may be shared with the general population.
In our cross-sectional analyses, myocardial SUV was lower among patients treated with non-TNF targeted biologics compared with those treated with TNF inhibitors or with non-biologics. The non-TNF targeted biologic group was comprised primarily by patients receiving abatacept (soluble CTLA4-Ig). Although we did not identify any significant differences in RA characteristics between the abatacept users versus the other treatment groups, channeling bias cannot be completely eliminated as an explanation for this finding given the small number of abatacept users. Nonetheless, it is of interest that antibodies that neutralize CTLA4, and are effective as cancer immunotherapy, have been associated with rare but potentially fatal myocarditis 46. Similarly, in experimental models, CTLA-4-deficient mice develop severe myocarditis with lymphocytic infiltration 47,48, while experimental autoimmune myocarditis in rats can be prevented by gene delivery with plasmid encoding CTLA4-Ig49.
Thus, while it is tantalizing to hypothesize that abatacept may act selectively among RA DMARDs in reducing subclinical myocardial inflammation, our small prospective study suggests that this effect may also be observed with escalation of therapy to non-biologics and TNF inhibitors. Taken together, it seems likely that the reduction in myocardial FDG uptake is more dependent on reduction of RA disease activity rather than the specific agent used; however, these interactions require a larger cohort of longitudinally followed patients appropriately powered to explore differences in effect between different treatments and treatment responses. Such studies are currently underway.
We did not observe a relationship of myocardial FDG uptake with measures of LV structure or function or with BNP levels. This may not be surprising since we excluded patients with clinical heart failure, and BNP levels and most of the echocardiographic measures of LV structure and function in this cohort were within normal ranges.
Our study has notable strengths and limitations. Among the strengths, it is the largest study to date to evaluate and quantify the presence of subclinical myocardial inflammation in RA, and the first to utilize FDG PET-CT to do so. It is also the first to evaluate longitudinally, albeit a small pilot study, the effect of step-up therapy on myocardial inflammation. Our cohort includes patients with and without CVD risk factors as well as patients with early and late RA, and is thus a reflection of the typical population of RA patients managed in clinical practice. Among limitations, we assume that FDG uptake corresponds to myocardial inflammation as shown in prior work 25,26, but, for ethical reasons, could not verify this pathologically in this asymptomatic cohort. An additional limitation is that our final multivariable model accounts for only 10% of the variability of SUVmean, inviting the search for other possible contributory variables.
In summary, although larger longitudinal data are needed, the current study supports the hypotheses that myocardial inflammation in RA is related to disease activity, that it may contribute to the increased risk for heart failure in patients with RA compared with controls, and that it may be responsive to step-up therapy.
Supplementary Material
Acknowledgments
The authors would like to thank the RHYTHM study staff (Janine Rose, Rachel Broderick and Thania Perez) for their hard work and dedication, and the participants in the RHYTHM study who graciously agreed to participate in this research. We also thank Drs. Alice Chu, Anna Broder, Teja Kapoor, Edward Dwyer, Jane Kang, Laura Geraldino, Katherine Nickerson, Elizabeth Mayer, Amanda Sammut, Neil Gonter, Alexandru Kimel, and others for generously recommending their patients for this study.
Financial Support:
Research reported in this publication was supported by the National Institute of Arthritis andMusculoskeletal and Skin Diseases under Award Number AR-050026 (JMB), and by theNational Institutes of Health National Center for Translational Science Clinical and Translational Science Award (CTSA) grant UL1 TR000040, of the National Institutes of Health, and by the Rheumatology Research Foundation Award Number RHEUMARF CU12–3892 (JMB). The content is solely the responsibility of the authors and does not necessarily represent the official views of The National Institutes of Health.
REFERENCES
- 1.Nicola PJ, Crowson CS, Maradit-Kremers H, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum 2006;54(1):60–7. doi: 10.1002/art.21560 [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis and rheumatism 2005;52(2):412–20. doi: 10.1002/art.20855 [published Online First: 2005/02/05] [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum 2007;56(11):3583–7. doi: 10.1002/art.22979 [DOI] [PubMed] [Google Scholar]
- 4.Davis JM 3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis and rheumatism 2008;58(9):2603–11. doi: 10.1002/art.23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapadia SR, Yakoob K, Nader S, et al. Elevated circulating levels of serum tumor necrosis factor-alpha in patients with hemodynamically significant pressure and volume overload. J Am Coll Cardiol 2000;36(1):208–12. [published Online First: 2000/07/18] [DOI] [PubMed] [Google Scholar]
- 6.Matsumori A, Yamada T, Suzuki H, et al. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. British heart journal 1994;72(6):561–6. [published Online First: 1994/12/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deswal A, Petersen NJ, Feldman AM, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001;103(16):2055–9. [published Online First: 2001/04/25] [DOI] [PubMed] [Google Scholar]
- 8.Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. The New England journal of medicine 1990;323(4):236–41. doi: 10.1056/nejm199007263230405 [published Online First: 1990/07/26] [DOI] [PubMed] [Google Scholar]
- 9.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000;102(25):3060–7. [published Online First: 2000/01/11] [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber JI, Kim KH, Natterson PD, et al. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. The American journal of physiology 1996;271(4 Pt 2):55. [DOI] [PubMed] [Google Scholar]
- 11.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. The Journal of clinical investigation 1996;98(12):2854–65. doi: 10.1172/JCI119114 [published Online First: 1996/12/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivasubramanian N, Coker ML, Kurrelmeyer KM, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 2001;104(7):826–31. [published Online First: 2001/08/15] [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama T, Nakano M, Bednarczyk JL, et al. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997;95(5):1247–52. [published Online First: 1997/03/04] [DOI] [PubMed] [Google Scholar]
- 14.Grundtman C, Hollan I, Førre OT, et al. Cardiovascular disease in patients with inflammatory rheumatic disease is associated with up-regulation of markers of inflammation in cardiac microvessels and cardiomyocytes. Arthritis and rheumatism 2010;62(3):667–73. doi: 10.1002/art.27264 [DOI] [PubMed] [Google Scholar]
- 15.Cathcart ES, Spodick DH. Rheumatoid heart disease. A study of the incidence and nature of cardiac lesions in rheumatoid arthritis. N Engl J Med 1962;266:959–64. doi: 10.1056/NEJM196205102661901 [published Online First: 1962/05/10] [DOI] [PubMed] [Google Scholar]
- 16.Sokoloff L Cardiac Involvement in Rheumatoid Arthritis and Allied Disorders: Current Concepts. Modern concepts of cardiovascular disease 1964;33:847–50. [published Online First: 1964/04/01] [PubMed] [Google Scholar]
- 17.Bennett MK, Gilotra NA, Harrington C, et al. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circulation Heart failure 2013;6(4):676–84. doi: 10.1161/circheartfailure.112.000087 [published Online First: 2013/06/05] [DOI] [PubMed] [Google Scholar]
- 18.Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. American heart journal 1999;138(2 Pt 1):299–302. [published Online First: 1999/07/30] [DOI] [PubMed] [Google Scholar]
- 19.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 2009;30(6):1259–67. doi: 10.1002/jmri.21969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation 2003;108(16):1945–53. doi: 10.1161/01.CIR.0000095029.57483.60 [published Online First: 2003/10/15] [DOI] [PubMed] [Google Scholar]
- 21.Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation 1995;92(7):1902–10. [published Online First: 1995/10/01] [DOI] [PubMed] [Google Scholar]
- 22.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343(20):1445–53. doi: 10.1056/NEJM200011163432003 [published Online First: 2000/11/18] [DOI] [PubMed] [Google Scholar]
- 23.Lima JA, Judd RM, Bazille A, et al. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation 1995;92(5):1117–25. [published Online First: 1995/09/01] [DOI] [PubMed] [Google Scholar]
- 24.Bohnen S, Radunski UK, Lund GK, et al. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circulation Cardiovascular imaging 2015;8(6) doi: 10.1161/CIRCIMAGING.114.003073 [DOI] [PubMed] [Google Scholar]
- 25.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 2012;59(2):153–63. doi: 10.1016/j.jacc.2011.08.066 [published Online First: 2012/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maya Y, Werner RA, Schutz C, et al. 11C-methionine PET of myocardial inflammation in a rat model of experimental autoimmune myocarditis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2016. doi: 10.2967/jnumed.116.174045 [published Online First: 2016/07/09] [DOI] [PubMed] [Google Scholar]
- 27.Winchester R, Giles JT, Nativ S, et al. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(1):92–102. doi: 10.1002/art.39419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and rheumatism 2010;62(9):2569–81. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 29.Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2017;24(1):86–99. doi: 10.1007/s12350-016-0502-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demeure F, Hanin FX, Bol A, et al. A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: implications for vulnerable coronary plaque imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2014;55(10):1629–35. doi: 10.2967/jnumed.114.138594 [published Online First: 2014/08/02] [DOI] [PubMed] [Google Scholar]
- 31.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105(4):539–42. doi: 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- 32.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 33.Russo C, Jin Z, Homma S, et al. Relationship of Multidirectional Myocardial Strain with Radial Thickening and Ejection Fraction and Impact of Left Ventricular Hypertrophy: A Study in a Community Based Cohort. Echocardiography 2013;30(7):794–802. doi: 10.1111/echo.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology 2011;57(12):1368–74. doi: 10.1016/j.jacc.2010.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue E, Yamanaka H, Hara M, et al. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Annals of the rheumatic diseases 2007;66(3):407–9. doi: 10.1136/ard.2006.054205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevoo MLL, Hof VTMA, Kuper HH, et al. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism 1995;38(1):44–48. doi: 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 37.Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Annals of the Rheumatic Diseases 2009;68(6):954–60. doi: 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis and rheumatism 1980;23(2):137–45. [DOI] [PubMed] [Google Scholar]
- 39.Ntusi NA, Piechnik SK, Francis JM, et al. Diffuse Myocardial Fibrosis and Inflammation in Rheumatoid Arthritis: Insights From CMR T1 Mapping. JACC Cardiovascular imaging 2015;8(5):526–36. doi: 10.1016/j.jcmg.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Giles JT, Hirano M, et al. Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis research & therapy 2010;12(5):R171. doi: 10.1186/ar3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayshi H, Kobayashi Y, Yokoe I, et al. Magnetic Resonance-Detected Myocardial Inflammation and Fibrosis in Rheumatoid Arthritis: Associations of Disease Characteristics and N-terminal pro Brain Natriuretic Peptide Levels. Arthritis care & research 2016. doi: 10.1002/acr.23138 [DOI] [PubMed] [Google Scholar]
- 42.Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circulation research 2016;119(1):159–76. doi: 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- 43.Andersen JK, Oma I, Prayson RA, et al. Inflammatory cell infiltrates in the heart of patients with coronary artery disease with and without inflammatory rheumatic disease: a biopsy study. Arthritis research & therapy 2016;18(1):232. doi: 10.1186/s13075-016-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Totoson P, Maguin-Gate K, Nappey M, et al. Endothelial Dysfunction in Rheumatoid Arthritis: Mechanistic Insights and Correlation with Circulating Markers of Systemic Inflammation. PloS one 2016;11(1):e0146744. doi: 10.1371/journal.pone.0146744 [published Online First: 2016/01/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Totoson P, Maguin-Gate K, Nappey M, et al. Microvascular abnormalities in adjuvant-induced arthritis: relationship to macrovascular endothelial function and markers of endothelial activation. Arthritis & rheumatology (Hoboken, NJ) 2015;67(5):1203–13. doi: 10.1002/art.39065 [published Online First: 2015/02/25] [DOI] [PubMed] [Google Scholar]
- 46.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. The New England journal of medicine 2016;375(18):1749–55. doi: 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3(5):541–47. [DOI] [PubMed] [Google Scholar]
- 48.Love VA, Grabie N, Duramad P, et al. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circulation research 2007;101(3):248–57. doi: 10.1161/CIRCRESAHA.106.147124 [DOI] [PubMed] [Google Scholar]
- 49.Abe S, Hanawa H, Hayashi M, et al. Prevention of experimental autoimmune myocarditis by hydrodynamics-based naked plasmid DNA encoding CTLA4-Ig gene delivery. J Card Fail 2005;11(7):557–64. doi: 10.1016/j.cardfail.2005.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.