Abstract
Background:
Broncho-Vaxom® (OM-85 BV) is an extract of infectious respiratory bacteria that is used as an immunostimulant outside of the United States for the prevention and treatment of bronchitis and rhinosinusitis. Prior studies have demonstrated that use of OM-85 BV is associated with reduction in frequency of respiratory infection and decreased duration of antibiotic usage. However, the effects of OM-85 BV on respiratory mucosal innate immunity are unknown.
Methods:
Human sinonasal epithelial cells were grown at an air-liquid interface (ALI).
Ciliary beat frequency (CBF) and nitric oxide (NO) production in response to stimulation with OM-85 BV was measured in vitro. Pharmacologic inhibitors of bitter taste receptor (T2R) signaling were used to determine if this pathway was taste-receptor mediated.
Results:
Apical application of OM-85 BV resulted in an NO-mediated increase in CBF (p<0.05) and increased NO production (p<0.0001) when compared to saline-stimulated control cultures. ALI pre-treatment with taste receptor pathway inhibitors blocked OM-85 BV-induced increases in NO.
Conclusions:
OM-85 BV has ciliostimulatory and immunogenic properties that may be partially responsible for its observed efficacy as a respiratory therapeutic. These responses were NO-dependent and consistent with T2R activation. Further work is necessary to elucidate specific component-receptor signaling relationships.
Keywords: sinusitis, ciliary motility, therapeutics, immunotherapy
Introduction
Chronic rhinosinusitis (CRS) is a common upper respiratory disease affecting nearly 35 million Americans for which treatment involves prolonged glucocorticoid and antibiotic use.1–4 Given the potential for significant health consequences with these treatments, both on the individual and public scale, patients and providers are exploring adjunctive agents to the standard therapies used in the treatment and prevention of sinonasal disease.
Broncho-Vaxom ®, or OM-85 BV, is an over-the-counter oral preparation of lyophilized bacterial lysates of eight common respiratory pathogens (Haemophilus influenzae, Diplococcus pneumoniae, Klebsiella pneumoniae and ozaenae, Staphylococcus aureus, Streptococcus pyogenes and viridans, and Neisseria catarrhalis) and has been used for nearly 30 years in other countries in both adults and children for the treatment and prevention of recurrent respiratory infections. Polyvalent bacterial preparations including OM-85 BV have been studied in several randomized clinical trials (RCT) and have demonstrated a therapeutic benefit in a variety of respiratory and atopic diseases.5–10 In CRS specifically, use of OM-85 BV in children reduced the frequency of rhinosinusitis episodes and days with antibiotic use per month and was shown to provide long-term prophylaxis.11 Use of OM-85BV for treatment in adults with CRS is additionally supported by the European Position Paper on Rhinosinusitis and Nasal Polyps with level 1b evidence.12 Furthermore, OM-85 BV has a good safety profile with only minor gastrointestinal (nausea, vomiting, abdominal pain) and dermatologic reactions (rash and urticarial) reported in clinical trials.9,13
The mechanism of action of immunostimulating agents such as OM-85 BV has been proposed to be through the nonspecific activation of mucosal and general immune responses upon absorption through Peyer’s patches in the gut as well as toll-like receptor-dependent innate immunity. 8,13–18 While previous studies have examined the mechanism of action and therapeutic efficacy of oral administration of OM-85 BV, there have been only a few studies on alternative methods of administration.19–21
Recent research has provided support for the role of taste receptors in the sinonasal epithelium to sense their environment and initiate local immune defenses including mucociliary clearance and antimicrobial product release.22–24 These taste receptors are known to be activated by secreted bacterial products22 and other compounds with biological activity are continuously being discovered.25–28 Given this emerging role of the sinonasal epithelium in the regulation of upper airway immunity, the nasal delivery of topical agents that augment the natural host response is an attractive therapeutic option in the treatment of rhinosinusitis and other local disease. The goal of the present study was thus to evaluate the potential of OM-85 BV as a topical adjunct to conventional treatments through stimulation of immediate mucosal innate defenses, specifically measuring changes in ciliary beat frequency (CBF) and NO production in primary human upper respiratory epithelial cultures treated with OM-85 BV.
Materials and Methods
Reagents and experimental solutions
Phosphate-buffered saline (PBS) and Hank’s Balanced Salt Solution (HBSS) were purchased from Sigma Aldrich. Broncho-Vaxom® Infant Sachets were obtained from Dr. Salomon Waitzel in Mexico City. The contents of the sachet were initially dissolved in PBS to obtain a concentration of 1 mg/ml. The resulting suspension was vortexed for 2 minutes and then centrifuged at room temperature for 5 minutes at 12000 rpm. The supernatant containing the soluble components of OM-85 BV was removed to be used for all experiments.
Human tissue acquisition
Tissue was obtained with informed consent and Institutional Review Board approval. Patients undergoing sinonasal surgery at the Department of Otorhinolaryngology, Division of Rhinology at the University of Pennsylvania and the Philadelphia Veterans Administration Medical Center were recruited and tissue was taken from residual clinical material at surgery. Sinonasal mucosal specimens were transported to the laboratory in saline placed on ice and processed immediately. Patients were excluded if they had antibiotic, corticosteroid, or antibiologic use within one month of surgery, or a history of systemic disease such as Wegener’s or Cystic Fibrosis. Samples from both diseased sinuses and normal epithelium were used in the experiment without discrimination. Our previous work demonstrated that there are not overt differences in ciliary dynamics once cells are grown at an air-liquid interface (ALI) in vitro and terminally differentiated, regardless of disease or nondisease tissue etiology.29
Air-Liquid Interface (ALI) cultures
We have previously described the culture of human nasal epithelial cells at an ALI.22,30 Briefly, human sinonasal epithelial cells were enzymatically dissociated and grown with medium containing Dulbecco’s modified Eagle medium (DMEM)/Ham’s F-12 and bronchial epithelial based medium (BEMB) (Clonetics), in addition to 100 U/ml penicillin and 100 μg/ml streptomycin for 7 days. Following this, cells were trypsinized and placed on porous polyester membranes in transwell cell culture inserts (Transwell-clear, 12-mm diameter, 0.4-μm pores; Corning). These inserts were coated with 100 μl of coating solution (bovine serum albumin 0.1 mg/ml; Sigma-Aldrich), type 1 bovine collagen (30 μg/ml; BD), and fibronectin (10 μg/ml; BD) in LHC basal medium (Invitrogen). After five days, the apical compartment was aspirated dry and the epithelium was allowed to differentiate using a medium of 1:1 DMEM (Invitrogen) and BEBM (Clonetics, Cambrex), with the Clonetics complements [human epidermal growth factor (0.5 ng/ml), epinephrine (5 μg/ml), hydrocortisone (0.5 μg/ml), bovine pituitary extract (0.13 mg/ml), insulin (5 μg/ml), triiodothyronine (6.5 μg/ml), and transferrin (0.5 μg/ml)], supplemented with 100 UI/ml penicillin, 100 g/ml streptomycin, 0.1 nM retinoic acid (Sigma-Aldrich), and 10% fetal bovine serum (Sigma-Aldrich) in the basal compartment. The cultures were exposed to air on the apical surface. For each experimental condition, three to five ALI cultures each from a unique human patient were used. Cultures derived from this same cohort of patients were used across experimental conditions. Individual ALIs were used in a single experiment and discarded.
For experiments regarding the bitter taste receptor T2R38, cultures of different genotypes resulting in functional and non-functional alleles were used. The functional allele of the receptor contains a proline, alanine, and valine (PAV) at positions 49, 262, and 296, while the nonfunctional allele of the receptor contains an alanine, valine, and isoleucine (AVI) at these positions respectively.31
Ciliary beat frequency (CBF) imaging and analysis
Transwell inserts were removed from the basolateral media and placed in a new well with 600μl of HBSS. The apical side of the transwell was copiously washed with PBS to remove any mucus clumps and then 30μl of PBS was added to the surface. Cultures were then allowed to equilibrate for 15 minutes to the ambient 26°C temperature to ensure a steady baseline CBF.32,33 Three conditions were tested: control (PBS), OM-85 BV, and OM-85 BV + L-NG-Nitroarginine methyl ester (L-NAME) (Sigma Aldrich, St. Louis, MO) a specific inhibitor of nitric oxide synthase (NOS). For each condition, three to five ALI cultures from the same cohort of unique human patients were utilized to control for inter-donor variability.
Utilizing the 20× objective on an inverted microscope (Leica Microsystems, Bannockburn, IL), CBF measurements were obtained from individual cultures at several time points. Baseline ciliary beat frequency (CBF) was recorded for 1 minute and percentage change from baseline over 5 minutes after PBS or OM-85 BV addition was determined. Specifically, 30 μl of control or OM-BV 85 was added to the apical surface of the ALI culture at t=0, giving a total apical fluid volume of 60 μl and 1:2 dilution of the soluble components of a 1mg/ml OM-85 BV solution. CBF was measured every 30 seconds after compound addition for 5 minutes. An area scan high-speed monochromatic digital video camera (Model A602f-2; Basler AG, Ahrensburg, Germany) captured images at 100 frames/s with a resolution of 650 × 480 pixels. Images from the camera were individually sampled by an acquisition board (National Instruments, Austin, TX) on a Dell workstation (Model XPS 710; Dell, Inc., Round Rock, TX) running a Windows XP Professional operating system (Microsoft, Redmond, WA). Sisson-Ammons Video Analysis software (SAVA; National Instruments) that is specialized to quantify CBF34 by performing a whole-field analysis of the ciliated apical surface of the cultures was used to analyze images, reporting a CBF that is the arithmetic mean of all of the cilia in the video field. Any videos in which <5% of the imaged field was ciliated were excluded.35 All reported CBF values were normalized to a basal frequency of 100%, with changes in CBF reported as a percentage of this baseline frequency.35,36
Live-cell imaging of reactive NO production in ALI cultures
To track cellular NO production, the fluorescent reactive nitrogen species indicator 4‐amino‐5‐methylamino‐2′,7′‐difluoroflurescein (DAF‐FM; Invitrogen, Carlsbad CA) was imaged as described22 using an IX-81 microscope (×10, 0.3 NA UPlanFLN objective; Olympus, Tokyo, Japan) and the 488‐nm argon laser line of a Fluoview FV1000 laser scanning confocal system. Briefly, new ALI cultures were loaded via the apical side for 60 minutes with DAF-FM-diacetate by incubation in DPBS containing 10 μM DAF-FM-diacetate and 5 μM of the cell‐permeant NO scavenger, 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, (cPTIO) (Cayman Chemical, Ann Arbor, MI). Stock solutions of DAF-FM and cPTIO were made at 1000× in DMSO with working solutions made fresh in PBS daily.
Cultures were washed with PBS to remove any unloaded DAF-FM and cPTIO and incubated for 15 minutes to allow for dye retention. During this time, either 30 μL of PBS (containing 1.8 mM Ca2+) or one of the pharmacologic inhibitors described below were added to the apical side. Cultures were mounted in a custom-made chamber and modified HEPES-buffered HBSS containing 1× MEM amino acids (Invitrogen) was used on the basolateral side to provide a source of arginine (∼0.6 mM) for NO production. After establishing baseline fluorescence for 1 minute, 30 μL of either PBS or OM-85BV was added to the apical side. DAF-FM fluorescence images were acquired at 5‐second intervals. For each condition, three to five ALI cultures from the same cohort of unique human patients were used and 5 distinct areas on each ALI were chosen for analysis. Once an individual ALI was imaged, it was discarded. Microscope and software settings were identical for each experiment.
Pharmacologic Manipulation
To evaluate whether OM-85 BV may function via bitter taste receptor signaling, we utilized several pharmacologic agents to inhibit canonical taste receptor signaling components and effectors, including transient receptor potential cation channel subfamily M member 5 (TRPM5),37 phospholipase C β2 (PLC β2),38 and NO (Figure 5). If OM-85 BV activates taste-receptor signaling pathways, we would expect to see increases in CBF and NO production upon treatment with OM-85 BV. In the presence of these agents, however, we would expect inhibition of any OM-85 BV-mediated changes in CBF or NO production. The NO-synthase inhibitor, L-NG-nitroarginine methyl ester (L-NAME) was used in CBF experiments at a concentration of 50 μM applied for 15 minutes to the apical side only and in DAF-FM experiments at a concentration of 100 μM to both the apical and basolateral sides. Both an active PLC β2 inhibitor, U73122, and its inactive analogue, U73343, were used at 5 μM (Cayman Chemical). The TRPM5 inhibitor,, triphenylphosphine oxide (TPPO; Sigma Aldrich)39 was used at 100 μM. Each of the working solutions was prepared fresh daily in PBS from 1000× DMSO stock solutions.
Figure 5.
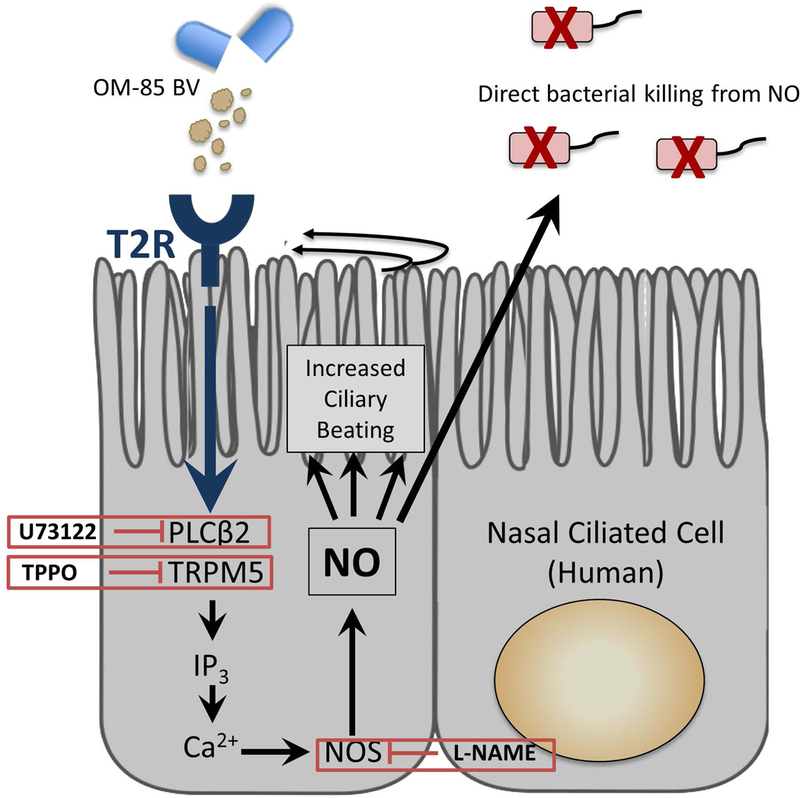
Proposed OM-85BV stimulation of T2R taste receptors on ciliated sinonasal epithelium. Activation of taste-receptor signaling induces innate immune mechanisms including nitric oxide release which is directly bactericidal as well as stimulates ciliary beating. Ca2+= calcium ion; IP3= inositol trisphosphate; L-NAME= L-NG-nitroarginine methyl ester, NO synthase inhibitor; NO= nitric oxide; NOS=nitric oxide synthase; PLCβ2= phospholipase C isoform β2; T2R=bitter taste receptor; TPPO= triphenylphosphine oxide; U73122= active isoform of PLCβ2 blocker
Statistical Analysis
All statistical analyses were performed using GraphPad Prism (student’s t test, ANOVA) as indicated. Unpaired student’s t tests were two-tailed and used for single comparisons. ANOVA with the Dunnett’s post hoc test was used when all values were compared with a control value. P<0.05 was the cutoff for statistical significance. All data are reported as means ± standard errors of mean (SEM).
Results
OM-85 BV Increases CBF in Sinonasal ALIs
We evaluated the soluble fraction of OM-85 BV for effects on ciliary beat frequency in sinonasal epithelium. The experimental solution was prepared in PBS at a concentration of 1mg/ml and the supernatant containing the soluble components was utilized for all experiments. Ciliary beating in ALI cultures was recorded before, during, and after the application of 30 μl of control or OM-85BV supernatant to 30 μl PBS on the apical surface for a 1:2 dilution. Apical application of OM-85BV resulted in a statistically significant increase in CBF up to 115% of baseline CBF compared to saline-stimulated control cultures over a period of 5 minutes following application (p<0.05).
To evaluate whether this immediate increase in ciliary beating might be mediated through nitric oxide (NO) production 22,40–42, we preincubated the cultures with the nitric oxide synthase inhibitor L-NAME. As demonstrated in Figure 1, NOS inhibition partially blocked the OM-85 BV induced increase in ciliary beat frequency, demonstrating a NO-dependent ciliary response to bacterial antigens in the lysate.
Figure 1.
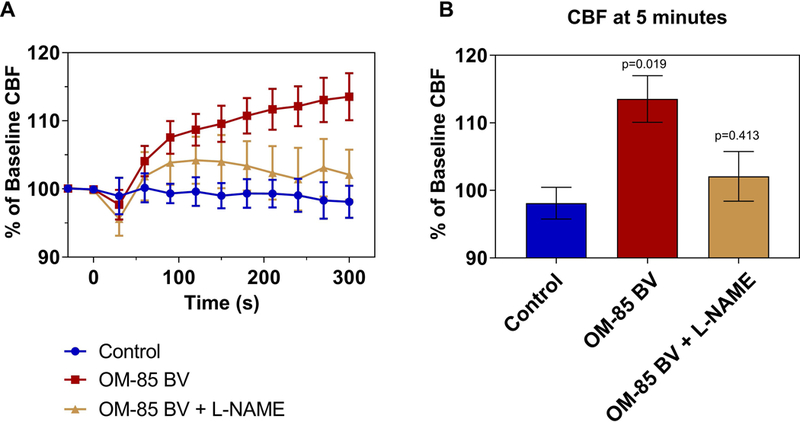
OM-85BV stimulates CBF in a NO-dependent manner. A) CBF changes after addition of 30 μl of OM-85 BV supernatant (red) or PBS (blue) to the apical ciliated surface at t=0 minutes to ALIs or ALIs pre-treated with L-NAME (gold). Percentage change from baseline CBF is shown over 5 minutes with measurements every 30 seconds. B) Graph of change in CBF at 5 minutes after addition of PBS or OM-85 BV. Symbols and bars are means ± standard error of 3–5 cultures each. P-values relative to control. CBF= ciliary beat frequency; PBS= phosphate-buffered saline.
OM-85 BV Stimulates NO Production in ALI Cultures
To confirm that OM-85 BV stimulated nitric oxide production, we measured intracellular production of NO using the fluorescent probe DAF-FM.22,43 DAF-FM reacts with NO-derived reactive nitrogen species to form a fluorescent benzotriazole.44 Changes in NO production were calculated based on changes in fluorescence intensity. NO DAF-FM fluorescence increased in response to the soluble fraction of OM-85 BV almost immediately upon apical application to an average of 176.3 ± 0.4 at 5 minutes (n=5 cultures, p<0.0001) when compared with control (21.5 ±0.6). As expected, this increase was blocked by pre-treatment with L-NAME (28.2 ± 0.5, n= 3 cultures), demonstrating that increase in DAF-FM was in fact due to NO and not a different reactive oxygen or nitrogen species (Figure 2).
Figure 2.
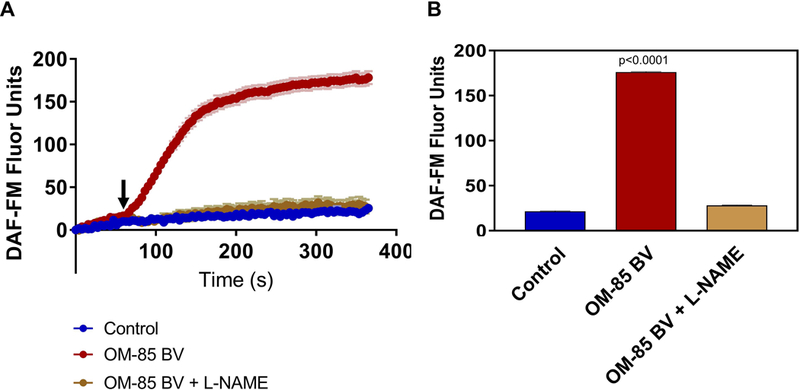
Apical Application of OM-85 BV Stimulates NO Production in Human Sinonasal ALI Cultures. A) Intracellular NO production measured by DAF-FM fluorescence following apical application of the soluble fraction OM-85 BV (red), PBS control (blue). Response is blocked by pre-incubating with the NOS inhibitor L-NAME (gold). B) NO release for each condition as the average DAF-FM fluorescence over last 30 seconds. Symbols and bars are means ± standard error of 3–5 cultures each. P-values relative to PBS control, by 1-way ANOVA with Dunnett’s post-test. L-NAME= L-NG-nitroarginine methyl ester, NO synthase inhibitor; NO= nitric oxide; PBS= phosphate-buffered saline.
Prior work had demonstrated stimulation of nitric oxide synthase via activation of apically expressed bitter taste receptors (T2Rs).22,40–42 Bitter taste receptor signaling pathways depend on signal transduction through transient receptor potential cation channel subfamily M member 5 (TRPM5)37 and phospholipase C β2 (PLC β2).38 To determine whether the OM-85 BV induced changes in intracellular NO resulted from activation of the taste receptor pathway, we blocked these canonical taste signaling components (Figure 3). Cultures were incubated with either the TRPM5 blocker TPPO, the PLC β2 inhibitor U73122, or the inactive analogue U73343 (n=3 cultures each) for 15 minutes prior application of OM-85 BV and subsequent live cell imaging for DAF-FM fluorescence. Incubation with TPPO or the active PLC β2 (U73122) inhibitor blocked OM-85 BV induced intracellular NO generation with changes in DAF-FM fluorescence comparable to control experiments (27.3 ± 0.5 and 23.1 ± 0.6, respectively). Cultures incubated with the inactive analogue (U73343) demonstrated robust NO production in response to OM-85 BV (185.5 ± 0.7). NO release for each condition as the average DAF-FM fluorescence over last 30 seconds is shown in Figure 3B.
Figure 3.
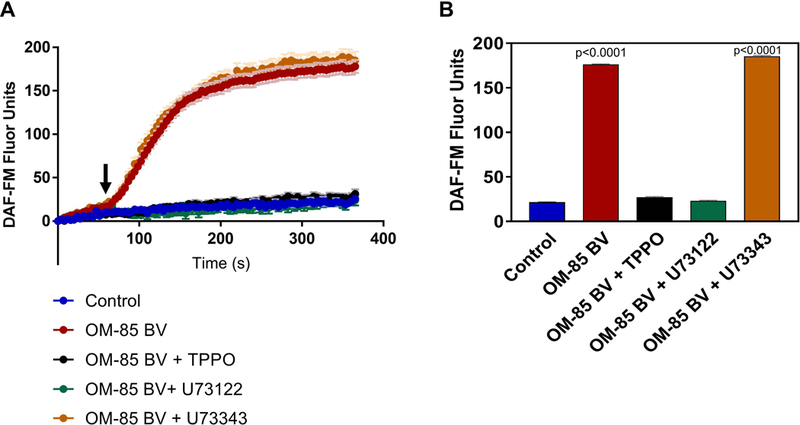
OM-85 BV Stimulates NO Production in ALI Cultures in a Taste Receptor Dependent Manner. A) Intracellular NO responses to apical application of the soluble fraction of OM-85BV(red), PBS control (blue), or OM-85BV in the presence of TPPO (black; a TRPM5 inhibitor), active PLCβ2 blocker U73122 (green) and its inactive analogue U73343 (orange). B) NO release for each condition as the average DAF-FM fluorescence over last 30 seconds. Symbols and bars are means ± standard error of 3–5 cultures each. P-values relative to PBS control, by 1-way ANOVA with Dunnett’s post-test. NO= nitric oxide; PBS= phosphate-buffered saline; PLCβ2= phospholipase C isoform β2; TPPO= triphenylphosphine oxide; U73122= active PLCβ2 blocker; U73433= inactive PLCβ2 blocker.
NO Production in response to OM-85 BV is T2R38 Independent
Prior work has demonstrated that T2R38 is one T2R isoform expressed in ciliated sinonasal epithelial cells and that when activated, triggers nitric oxide production.22 Bitter receptors, and particularly T2R38, are unique among G-protein coupled receptors in the density of their naturally occurring genetic variants.45 Many previous studies have demonstrated that people with the taster form (PAV/PAV) of this receptor report that concentrations of some bitter ligands like phenylthiocarbamide (PTC) are intensely bitter at concentrations which are imperceptible to those with the non-taster form (AVI/AVI).46 As genetic taste variations in T2R38 receptor functionality have also been shown to correlate with NO production and resultant bactericidal activity22, we sought to determine whether components of OM-85 BV may function as T2R38 agonists. We found that all cultures, regardless of whether they were homozygote for the functional allelic form of the receptor (PAV/PAV) or homozygote for the non-functional allele (AVI/AVI), responded with robust increases in NO production when stimulated with OM-85 BV (Figure 4). There was a small but statistically significant difference in fluorescence in PAV/PAV cultures (202.1 ± 0.9, p<0.0001) compared to AVI/AVI cultures (158.5 ± 0.4).
Figure 4.
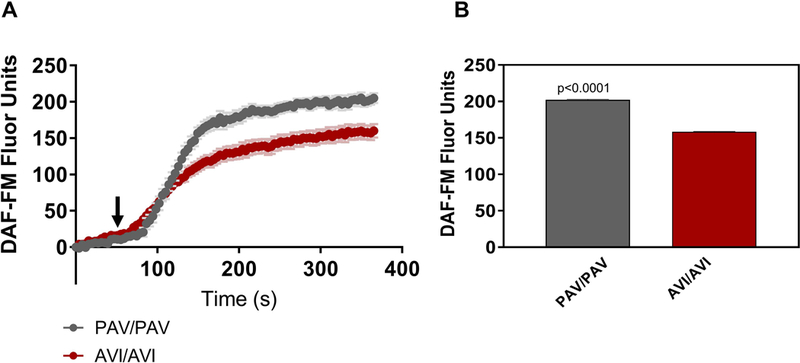
All ALIs, regardless of T2R38 genotype, responded with increased NO production upon stimulation with OM-85BV. Cultures homozygous for the functional form of the T2R38 receptor (PAV/PAV) demonstrated a statistically significant greater increase of approximately 40 DAF-FM units compared to AVI/AVI cultures (n=2–3 cultures per condition). A) NO response to OM-85BV over a period of 5 min B) NO release for each condition as the average DAF-FM fluorescence over last 30 seconds.
Discussion
Adjunct therapies to the traditional topical and systemic therapeutics for sinonasal disease are gaining popularity. This is especially true in light of the significant consequences of glucocorticoid and antibiotic use, including the potential for antibiotic resistance, perturbations to the resident microbiome, and the sequelae of prolonged steroid use. OM-85 BV, sold as Broncho-Vaxom®, is an over-the-counter oral preparation of lyophilized lysates of common respiratory bacteria that has been used for many years in other countries in both children and adults, in a variety of respiratory diseases including COPD, asthma, and rhinosinusitis. Prior studies have demonstrated that use of OM-85BV is cost effective and associated with a reduction in the frequency of respiratory infections and decreased duration of antibiotic usage.5–10 While previous studies have examined the mechanism of action and systemic effects of oral administration of OM-85 BV,8,13–18 alternative methods of bacterial lysate administration have not been extensively studied and there has only been a single study on the immunostimulatory activity of a similar bacterial lysate on the sinonasal epithelium.21 However, the direct application of OM-85 BV in particular has never been evaluated and the aforementioned study only examined the effects after 48 hours of stimulation21, thus assessing the slower innate immune response initiated through TLR activation47 rather than the rapid defensive responses triggered by the novel taste-receptor pathway, which occur on a much more rapid timescale.23,48 Given the known ability of bacteria and their secreted molecules to stimulate an alternative non-TLR immune response through activation of taste receptors in the upper airway,22,23,49 the current study sought to evaluate the immediate innate immune response to OM-85 BV and the potential utility of OM-85 BV as a topical adjunctive therapy in the treatment and prevention of rhinosinusitis.
Utilizing primary human sinonasal epithelial cultures, we tested OM-85 BV for effects on CBF. While application of a compound to the basolateral compartment of an ALI mimics cellular exposure in vivo, the effect of topical therapies is best evaluated by the apical addition of the compound. We found that apical application of the soluble components of OM-85 BV significantly increased CBF to 115% compared to saline-stimulated controls. The magnitude of the effect seen with OM-85BV is similar to what has been reported in studies with other known bitter agonists such as quinine42 and homoserine lactones.22 The observed increase is unlikely to be a response to mechanical stimulation, which can also increase CBF, as the experimental conditions involved a 1:2 dilution to attenuate the effects of acute compound and volume addition to a dry culture. Furthermore, this experimental design approximates in vivo conditions when rhinologic formulations for nasal drug delivery are mixed with an already-present airway surface liquid. This ability to stimulate ciliary beating and mucus clearance may be useful in diseases such as rhinosinusitis where defective mucociliary clearance leads to a vicious cycle of mucus stasis, infection, and airway inflammation.
Prior studies of sinonasal immunity have demonstrated a taste-receptor (T2R) mediated mechanism for increases in CBF and other local immune defenses such as the release of NO and secretion of antimicrobial compounds.22,23 It is known that T2R activation by secreted bacterial products22 as well as other biologically active compounds25–28 results in NO production which is not only directly bactericidal but also increases CBF. To determine whether the topical application of bacterial lysate may work by a similar mechanism of action, ALIs were pre-treated with either a nitric oxide synthase inhibitor or inhibitors of bitter taste-receptor signal transduction. We found that OM-85 BV-induced increases in both CBF and NO production were blocked, demonstrating that the observed effects are in fact NO-dependent as well as TRPM5 and PLCβ2-dependent, implicating T2R activation.
The most well characterized taste receptor in the upper airway innate immunity is T2R38. Genetic variability resulting in a differentially functional receptor has been shown to correlate with both in vitro studies of CBF and NO production as well as clinical severity of disease in CRSwNP.49,50 Prior work has demonstrated the stimulation of multiple T2Rs expressed in ciliated cells all converge on a similar intracellular signaling pathway yielding nitric oxide production.22,41 Utilizing cultures that were homozygote functional and homozygote non-functional for T2R38, that is natural knockouts, we found a statistically significant reduction in NO production of about 40 DAF-FM units between PAV/PAV and AVI/AVI cultures. While this is likely too small to be functionally significant, it suggests that multiple T2Rs are activated by OM-85 BV with a minor contribution from T2R38 as prior studies examining NO production in response to a specific T2R38 agonist (PTC) demonstrated differences of 120–150 DAF-FM units between PAV/PAV and AVI/AVI patient-derived ALI cultures.22 Overall, the effects of OM-85 BV demonstrated in this study were functionally T2R38-independent, suggesting that the components of OM-85 BV stimulate T2R38 as well as other T2R isoforms. Future studies will focus on elucidating the specific bacterial lysate component-receptor signaling relationships.
Overall, this study builds upon prior work on the oral administration of OM-85 BV in the treatment and prevention of respiratory and TH2-mediated disease.6,8,51 We demonstrated that apical application to primary human sinonasal epithelial cells elicits alternative and immediate-onset mechanisms of upper airway immunity through T2R-dependent increases in CBF and NO production (Figure 5), complementing the more sustained and slower response achieved by activation of traditional (TLR, NOD-like receptor) innate immunity pathways. The observed physiologic changes with direct OM-85 BV application to human sinonasal epithelial cells in vitro illustrate its ability to amplify the natural host immune response. This suggests a potential role for purification of the taste receptor stimulatory components and nasal delivery as an adjunctive therapy to current treatments for rhinosinusitis with the potential for quality of life, public health, and economic impacts.
Conclusions
OM-85 BV when applied apically to human sinonasal epithelial cells in vitro, stimulates a robust NO response as well as an increase in ciliary beat frequency. These responses are bitter taste-receptor dependent. This suggests that in contrast to the general immune activation seen with oral administration of OM-85 BV in previous studies, topical application may induce known taste-receptor (T2R) activation of the immune system in direct response to the bacterial antigens contained in the lysate.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health grant R01DC013588 and Veterans Affairs Merit Review CX001617 (awarded to N.A. Cohen).
Footnotes
Disclosure: The authors have no funding, financial relationships, or conflicts of interest to disclose.
Podium Presentation at the American Rhinologic Society at the Combined Otolaryngology Spring Meeting (COSM), National Harbor, MD, April 20, 2018.
References
- 1.Bhattacharyya N Incremental Health Care Utilization and Expenditures for Chronic Rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120(7):423–427. doi: 10.1177/000348941112000701 [DOI] [PubMed] [Google Scholar]
- 2.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x [DOI] [PubMed] [Google Scholar]
- 3.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121(12):2672–2678. doi: 10.1002/lary.21847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya N, Kepnes LJ. Assessment of Trends in Antimicrobial Resistance in Chronic Rhinosinusitis. Ann Otol Rhinol Laryngol. 2008;117(6):448–452. doi: 10.1177/000348940811700608 [DOI] [PubMed] [Google Scholar]
- 5.Yin J, Xu B, Zeng X, Shen K. Broncho-Vaxom in pediatric recurrent respiratory tract infections: A systematic review and meta-analysis. Int Immunopharmacol. 2018;54:198–209. doi: 10.1016/j.intimp.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 6.Koatz AM, Coe NA, Cicerán A, Alter AJ. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung. 2016;194(4):687–697. doi: 10.1007/s00408-016-9880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan L, Jiang X-G, Guo J, Tian Y, Liu C-T. Effects of OM-85 BV in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. J Clin Pharmacol. 2015;55(10):1086–1092. doi: 10.1002/jcph.518 [DOI] [PubMed] [Google Scholar]
- 8.Kearney SC, Dziekiewicz M, Feleszko W. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann Allergy, Asthma Immunol. 2015;114(5):364–369. doi: 10.1016/j.anai.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez-Tarango MD, Berber A. Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest 2001;119(6):1742–1748. [DOI] [PubMed] [Google Scholar]
- 10.Berber AC, Del-Rio-Navarro BE. Use of Broncho-Vaxom in private practice: phase IV trial in 587 children. Clin Ther. 18(6):1068–1079. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Zhou Y, Nie J, et al. Bacterial lysate for the prevention of chronic rhinosinusitis recurrence in children. J Laryngol Otol. 2017;131(06):523–528. doi: 10.1017/S0022215117000524 [DOI] [PubMed] [Google Scholar]
- 12.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi: 10.4193/Rhino50E2 [DOI] [PubMed] [Google Scholar]
- 13.Collet JP, Shapiro S, Ernst P, Renzi P, Ducruet T, Robinson A. Effects of an Immunostimulating Agent on Acute Exacerbations and Hospitalizations in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1997;156(6):1719–1724. doi: 10.1164/ajrccm.156.6.9612096 [DOI] [PubMed] [Google Scholar]
- 14.Luan H, Zhang Q, Wang L, et al. OM85-BV Induced the Productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-Mediated ERK1/2/NF-κB Pathway in RAW264.7 Cells. J Interf Cytokine Res. 2014;34(7):526–536. doi: 10.1089/jir.2013.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bottex C, Cristau B, Corazza JL, Mougin B, Fontanges R Effects of two bacterial extracts, OM-89 and Broncho-Vaxom, on IL-1 release and metabolic activity of a murine macrophage cell-line. Int J Immunother. 1988;4:203–212. [Google Scholar]
- 16.Duchow J, Marchant A, Delville JP, Schandene L GM. Upregulation of adhesion molecules induced by broncho-vaxom on phagocytic cells. Int J Immunopharmacol. 1992;14(5):761–766. [DOI] [PubMed] [Google Scholar]
- 17.Lusuardi M, Capelli A, Carli S, Spada E, Spinazzi A, Donner C. Local airways immune modifications induced by oral bacterial extracts in chronic bronchitis. Chest. 1993;103(6):1783–1791. [DOI] [PubMed] [Google Scholar]
- 18.Emmerich B, Emslander H, Pachmann K, Respiration MH-, 1990 U. Local immunity in patients with chronic bronchitis and the effects of a bacterial extract, Broncho-Vaxom®, on T lymphocytes, macrophages, gamma-interferon and secretory immunoglobulin A in bronchoalveolar lavage fluid and other variables. Respiration. 1990;57(2):90–99. [DOI] [PubMed] [Google Scholar]
- 19.Rial A, Ferrara F, Suárez N, Scavone P, Marqués JM, Chabalgoity JA. Intranasal administration of a polyvalent bacterial lysate induces self-restricted inflammation in the lungs and a Th1/Th17 memory signature. Microbes Infect. 2016;18(12):747–757. doi: 10.1016/j.micinf.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Mora R, Salzano FA, Mora E, Guastini L. Efficacy of a topical suspension of bacterial antigens for the management of chronic suppurative otitis media. Eur Arch Oto-Rhino-Laryngology. 2012;269(6):1593–1597. doi: 10.1007/s00405-011-1816-3 [DOI] [PubMed] [Google Scholar]
- 21.Guaní-Guerra E, Negrete-García MC, Montes-Vizuet R, Asbun-Bojalil J, Terán LM. Human β-defensin-2 induction in nasal mucosa after administration of bacterial lysates. Arch Med Res. 2011;42(3):189–194. doi: 10.1016/j.arcmed.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 22.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–4159. doi: 10.1172/JCI64240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3):1393–1405. doi: 10.1172/JCI72094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107(7):3210–3215. doi: 10.1073/pnas.0911934107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda Y, Ikeda R, Yamazaki T, et al. Activation of human bitter taste receptors by polymethoxylated flavonoids. Biosci Biotechnol Biochem. 2016;80(10):2014–2017. doi: 10.1080/09168451.2016.1184558 [DOI] [PubMed] [Google Scholar]
- 26.Roland WSU, Vincken J-P, Gouka RJ, van Buren L, Gruppen H, Smit G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors hTAS2R14 and hTAS2R39. J Agric Food Chem. 2011;59(21):11764–11771. doi: 10.1021/jf202816u [DOI] [PubMed] [Google Scholar]
- 27.Hariri BM, McMahon DB, Chen B, et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017;292(20):8484–8497. doi: 10.1074/jbc.M116.771949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri BM, McMahon DB, Chen B, et al. Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa. Matsunami H, ed. PLoS One. 2017;12(9):e0185203. doi: 10.1371/journal.pone.0185203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, Antunes MB, Claire SE, et al. Reversal of chronic rhinosinusitis-associated sinonasal ciliary dysfunction. Am J Rhinol. 2007;21(3):346–353. http://www.ncbi.nlm.nih.gov/pubmed/17621822. [DOI] [PubMed] [Google Scholar]
- 30.Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):13–38. [DOI] [PubMed] [Google Scholar]
- 31.Bufe B, Breslin PA, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15(4):322–327. doi: 10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Han D, Song X, Wang H, Wang K, Liu Z. Effects of ephedrine on human nasal ciliary beat frequency. ORL J Oto-Rhino-Laryngology Its Relat Spec. 2008;70(2):91–96. doi: 10.1159/000114531 [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21(10):875–881. doi: 10.1080/08958370802555898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211(Pt 2):103–111. [DOI] [PubMed] [Google Scholar]
- 35.Zhao KQ, Cowan AT, Lee RJ, et al. Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J. 2012;26(8):3178–3187. doi: 10.1096/fj.11-202184 [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Sanderson MJ. The role of cGMP in the regulation of rabbit airway ciliary beat frequency. J Physiol. 2003;551(Pt 3):765–776. doi: 10.1113/jphysiol.2003.041707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27(21):5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Bitter taste transduced by PLC-β 2 -dependent rise in IP 3 and α-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Physiol. 2001;280(4):C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742 [DOI] [PubMed] [Google Scholar]
- 39.Palmer RK, Atwal K, Bakaj I, et al. Triphenylphosphine Oxide Is a Potent and Selective Inhibitor of the Transient Receptor Potential Melastatin-5 Ion Channel. Assay Drug Dev Technol. 2010;8(6):703–713. doi: 10.1089/adt.2010.0334 [DOI] [PubMed] [Google Scholar]
- 40.Carey RM, Workman AD, Yan CH, et al. Sinonasal T2R-mediated nitric oxide production in response to Bacillus cereus. Am J Rhinol Allergy. 2017;31(4):211–215. doi: 10.2500/ajra.2017.31.4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan CH, Hahn S, McMahon D, et al. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. 2017;31(2):85–92. doi: 10.2500/ajra.2017.31.4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Workman AD, Maina IW, Brooks SG, et al. The Role of Quinine-Responsive Taste Receptor Family 2 in Airway Immune Defense and Chronic Rhinosinusitis. Front Immunol. 2018;9:624. doi: 10.3389/fimmu.2018.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol. 1999;516 ( Pt 1):179–190. http://www.ncbi.nlm.nih.gov/pubmed/10066932. Accessed August 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh Y, Ma FH, Hoshi H, et al. Determination and Bioimaging Method for Nitric Oxide in Biological Specimens by Diaminofluorescein Fluorometry. Anal Biochem. 2000;287(2):203–209. doi: 10.1006/abio.2000.4859 [DOI] [PubMed] [Google Scholar]
- 45.Kim U, Wooding S, Ricci D, Jorde LB, Drayna D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 2005;26(3):199–204. doi: 10.1002/humu.20203 [DOI] [PubMed] [Google Scholar]
- 46.Kim UK, Drayna D. Genetics of individual differences in bitter taste perception: lessons from the PTC gene. Clin Genet. 2005;67(4):275–280. doi: 10.1111/j.1399-0004.2004.00361.x [DOI] [PubMed] [Google Scholar]
- 47.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11 http://www.ncbi.nlm.nih.gov/pubmed/11686851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3(6):450–457. doi: 10.1002/alr.21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triantafillou V, Workman AD, Kohanski MA, Cohen NA. Taste Receptor Polymorphisms and Immune Response: A Review of Receptor Genotypic-Phenotypic Variations and their Relevance to Chronic Rhinosinusitis. Front Cell Infect Microbiol. 2018;8:64. doi: 10.3389/FCIMB.2018.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4(1):3–7. doi: 10.1002/alr.21253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Huang R, Yao R, Yang A. The Immunotherapeutic Role of Bacterial Lysates in a Mouse Model of Asthma. Lung. 2017;195(5):563–569. doi: 10.1007/s00408-017-0003-8 [DOI] [PubMed] [Google Scholar]


