Abstract
Myelin disruptions are frequently reported in human immunodeficiency virus (HIV)-infected individuals and can occur in the CNS very early in the disease process. Immature oligodendrocytes (OLs) are quite sensitive to toxic increases in [Ca2+]i caused by exposure to HIV-1 Tat (transactivator of transcription, a protein essential for HIV replication and gene expression), but sensitivity to Tat-induced [Ca2+]i is reduced in mature OLs. Tat exposure also increased the activity of Ca2+/calmodulin-dependent kinase IIβ (CaMKIIβ), the major isoform of CaMKII expressed by OLs, in both immature and mature OLs. Since CaMKIIβ is reported to interact with glycogen synthase kinase 3β (GSK3β), and GSK3β activity is implicated in OL apoptosis as well as HIV neuropathology, we hypothesized that disparate effects of Tat on OL viability with maturity might be due to an altered balance of CaMKIIβ-GSK3β activities. Tat expression in vivo led to increased CaMKIIβ and GSK3β activity in multiple brain regions in transgenic mice. In vitro, immature murine OLs expressed higher levels of GSK3β, but much lower levels of CaMKIIβ, than did mature OLs. Exogenous Tat upregulated GSK3β activity in immature, but not mature, OLs. Tat-induced death of immature OLs was rescued by the GSK3β inhibitors valproic acid or SB415286, supporting involvement of GSK3β signaling. Pharmacologically inhibiting CaMKIIβ increased GSK3β activity in Tat-treated OLs, and genetically knocking down CaMKIIβ promoted death in mature OL cultures treated with Tat. Together, these results suggest that the effects of Tat on OL viability are dependent on CaMKIIβ-GSK3β interactions, and that increasing CaMKIIβ activity is a potential approach for limiting OL/myelin injury with HIV infection.
Keywords: neuroAIDS, cell death, myelination, calcium, hippocampus, striatum, cortex
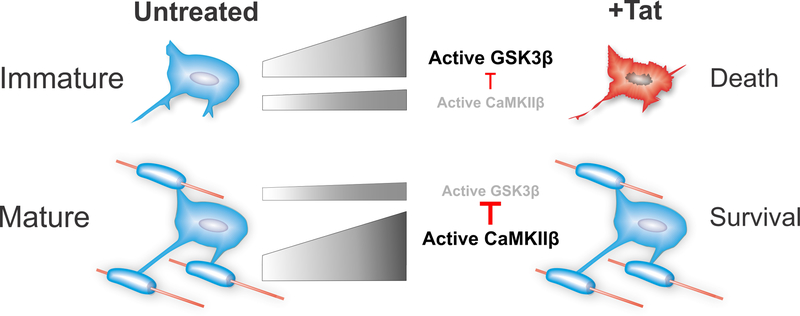
SUMMARIZING SCHEMATIC
The HIV-1 protein Tat (transactivator of transcription) is documented to cause significant neurotoxicity. We have reported that immature oligodendrocytes (OLs) are also quite vulnerable to toxic effects of HIV-1 Tat, through Ca2+-mediated mechanisms. More mature OLs experience similarly elevated [Ca2+]i but display significantly less cytotoxicity. Based on findings presented here, we propose that this differential toxicity may reflect changes in the relative levels of GSK3β versus CaMKIIβ as OLs mature. HIV-1 Tat-dependent increases in [Ca2+]i activate both GSK3β and CaMKIIβ. In mature OLs, large amounts of cytoplasmic CaMKIIβ are activated to inhibit GSK3β, protecting OLs from death. Conversely, lower levels of CaMKIIβ in immature OLs are insufficient to protect against the high levels of activated GSK3β. This relative overactivation of GSK3β triggers signaling events leading to immature OL death. The findings support the concept that increasing CaMKIIβ levels/activity may mitigate [Ca2+]i-induced toxicity in multiple injury/disease situations. Grey bars represent the relative activity levels in OLs at different levels of maturity and activition.
INTRODUCTION
Infiltration of human immunodeficiency virus (HIV) into the central nervous system (CNS) occurs at an early, asymptomatic stage of the disease process. Despite effective combined antiretroviral therapy (cART), ~50% of HIV+ individuals still suffer a variety of mild neuronal deficits, collectively called HIV-associated neurocognitive disorders (HAND) (Sacktor et al. 2001). White matter tracts are commonly affected in HAND patients (Gorry et al. 2003). In the white matter of post-mortem HIV tissues, oligodendrocytes (OLs) exhibit up-regulated p53 and BAX (Jayadev et al. 2007), indicating activation of cell death signaling. OLs are unlikely to be directly infected by HIV given their lack of CD4 expression (Sattentau et al. 1986) and the typically low viral load in the CNS, especially following cART (Yilmaz et al. 2006). Although HIV virions have been observed in OLs by electron microscopy (Gyorkey et al. 1987), and HIV has been proposed to infect OLs using galactosylceramide on the cell surface as alternative receptors (Albright et al. 1996), those reports remain quite controversial. Thus, OL damage is more likely to be caused by glial inflammatory responses or toxicity from secreted viral proteins. Although infection- or virotoxin-induced inflammation is thought to be the main cause of HAND pathogenesis, the extent to which adjunctive anti-inflammatory drug therapy improves neurocognitive scores in HIV+ individuals maintained on cART is debated, suggesting that additional injury mechanisms may be operative (Sacktor et al. 2011, Tan & McArthur 2012, McArthur et al. 2010).
The HIV-1 transactivator of transcription (Tat) is a highly conserved protein, which is essential to HIV replication and gene expression (Dayton et al. 1986, Fisher et al. 1986). Expression of Tat starts early during HIV infection and continues through the viral lifespan, even during cART-mediated suppression of viral replication (Bagashev & Sawaya 2013, Heaton et al. 2010, Johnson & Nath 2016). Significant quantities of Tat are released from infected cells, and extracellular or exogenous Tat can be actively internalized by (Ensoli et al. 1993, Frankel & Pabo 1988), or palmitoylated within the cell membrane (Chopard et al. 2018) of different bystander cells types, and can modulate host gene expression and cellular functions, including cell survival and development. Previously, we reported that Tat activates OL surface ionotropic glutamate receptors (iGluRs), resulting in elevated intracellular calcium ([Ca2+]i) and Ca2+/Calmodulin dependent kinase IIβ (CaMKIIβ) activity. Different cell death responses in immature and mature OLs (Zou et al. 2015) suggested activation of divergent downstream signaling pathways. We have been intrigued by the potential relationship between CaMKIIβ and glycogen synthase kinase 3β (GSK3β), one of the two isoforms of GSK3 that was originally discovered as a serine/threonine kinase vital for glucose metabolism. Inhibiting GSK3β activity in vivo can promote OL precursor cell proliferation, OL survival, and subsequent myelination (Azim & Butt 2011), indicating its involvement in OL development and function. Song et al. (2010) showed that inhibitory phosphorylation of GSK3, especially GSK3β at its N-terminal serine residue by CaMKII, promotes survival of cerebellar granule neurons. Interestingly, the major isoform of CaMKII in cerebellar granule neurons, CaMKIIβ, is also the predominant CaMKII in OLs (Burgin et al. 1990, Waggener et al. 2013), and CaMKIIβ is critical for OL maturation under physiological conditions (Waggener et al. 2013).
Since the activities of GSK3β and CaMKIIβ can both be regulated by increased [Ca2+]i (Hartigan & Johnson 1999, Hudmon & Schulman 2002b), we hypothesized that a difference in the balance of GSK3β and CaMKIIβ levels in immature and mature OLs may contribute to divergent responses following Tat-driven [Ca2+]i increases. Here we report that Tat expression in vivo in the adult brain led to increased activity of both CaMKIIβ and GSK3β in multiple regions. Tat also increased GSK3β activity in immature OLs in vitro; this increase in GSK3β activity was accompanied by a decrease in survival, which was rescued by the GSK3β inhibitors, valproic acid (VPA) or SB415286. Conversely, mature OLs expressed relatively low basal levels of GSK3β compared to immature OLs, and Tat did not increase their GSK3β activity or alter their survival. Tat activated OL CaMKIIβ at both developmental stages, although mature OLs had significantly higher basal levels of CaMKIIβ. Inhibiting CaMKIIβ pharmacologically increased GSK3β activity and genetically knocking down CaMKIIβ made mature OL cultures vulnerable to Tat. Thus it appears that mature OLs are protected against Tat toxicity by a combination of high basal levels of CaMKIIβ, low basal levels of GSK3β activity, and failure of Tat to increase GSK3β activity.
Together, these results suggest that Tat effects on OL viability reflect a balance of CaMKIIβ-GSK3β signaling, and infer that therapeutic approaches to alter activity in these pathways to favor CaMKIIβ might protect against OL/myelin deficits in HIV+ individuals, perhaps delaying the appearance of HAND.
MATERIALS AND METHODS
Experiments involving animals were carried out strictly in compliance with procedures reviewed and approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (Approval AM10158). All mice were housed 3–5 per cage with littermates in the Department of Animal Resources vivarium, in a temperature and humidity-controlled room on a 12 h light-dark cycle (lights off 18:00), with ad libitum access to food and chow. Anti-nociceptive medication was not required by any animal in these studies. The studies were not pre-registered. For experiments presented in Fig. 2 animals were sorted into groups based on their genotype and could not be randomized. In all other studies, animals were arbitrarily selected from among litters of like animals. Analyses in Fig. 2 were performed in a blinded manner using eartag identification numbers.
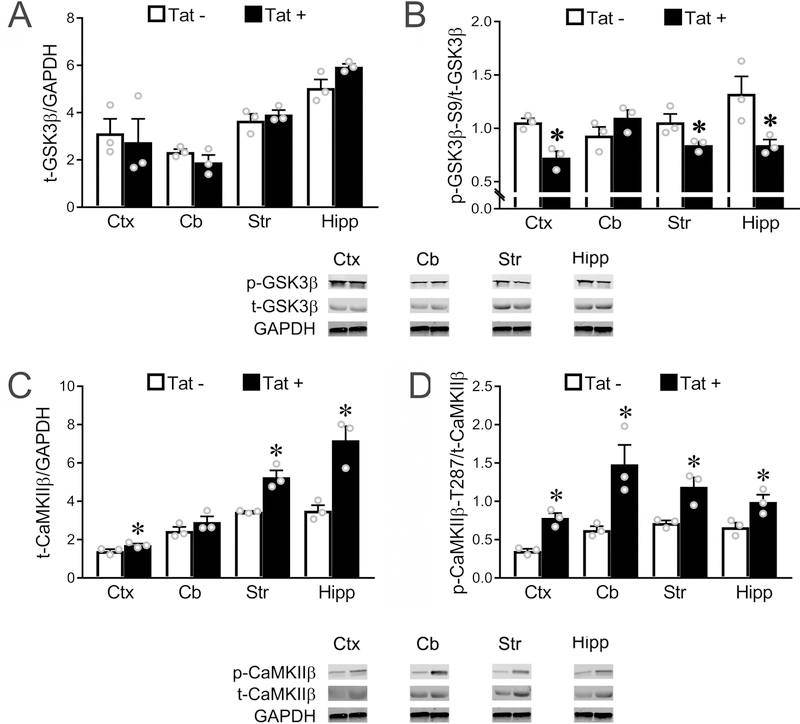
Figure 2. In vivo expression of Tat activates GSK3β and CaMKIIβ in multiple brain regions.
(A) In vivo expression of Tat has no effect on total GSK3β level in any of the four brain regions we examined. (B) In vivo Tat expression leads to upregulated activity of GSK3β (decreased ratio of p-GSK3β-S9/t-GSK3β) in cortex, striatum and hippocampus, but not cerebellum (p = 0.077). (C-D) Western blots show that Tat expression in vivo leads to an increased total CaMKIIβ level in cortex, striatum, and hippocampus (C, cerebellum: p = 0.09), and upregulated CaMKIIβ activity (increased p-CaMKIIβ-T287/t-CaMKIIβ ratio) in all four regions examined (* p < 0.05 vs. corresponding control). In A-D, Ctx: cortex; Cb: cerebellum; Str: striatum; Hipp: hippocampus. Data in A-D are based on the results from N=3 individual Tat+ and Tat− mice, each from a different litter.
Transgenic Mice
Transgenic mice carrying a doxycycline (DOX)-inducible, human glial fibrillary acidic protein-driven Tat expression vector were developed by Dr. Avindra Nath, bred in our facility and utilized as previously described in multiple publications from our lab and others (Zou et al. 2015). The original C3H x C57Bl/6J line has been repeatedly backcrossed to C57Bl/6J. Three-month old male mice (4 Tat− and 4 Tat+; 25–30 g; genotyped by PCR and identified by eartag) were given ad libitum access to DOX-containing chow (6 g/kg) for 10 d to induce Tat expression, then humanely euthanized by exposure to isofluorane followed by cervical dislocation. Frontal cortex, cerebellum, striatum, and hippocampus were dissected on ice and stored in −80°C before proteins were extracted for Western blotting. Camk2bfl/fl mice carrying a targeted and loxP-modified Camk2b allele were produced in Dr. Babette Fuss’s lab. The mice were generated from strain Camk2btm1a(EUCOMM)Hgmu obtained from the European Mouse Mutant Archive (RRID:IMSR_EM:05782). Re-derivation of embryos and initial breeding was done by the Transgenic Mouse Facility at VCU. The Cre-mediated excision of the loxP-flanked exon 2 of the Camk2b gene was achieved by adding cell-permeable Cre recombinase (50 nM, #EG-1025, Excellgen, Rockville, MD) to culture media. Both transgenic lines are available upon reasonable request.
Oligodendroglial cultures
Mixed glial cultures from postnatal day 0–1 CD-1 (RRID:IMSR_CRL:22, Charles River Laboratory, Wilmington, MA) or Camk2bfl/fl mice were prepared as described previously (Zou et al. 2011). Brains were dissected after decapitation, minced, and mixed with 2.5 mg/ml trypsin (#T9935, Sigma, St. Louis, MO) and 0.015 mg/ml DNase (#D5025, Sigma) in Dulbecco’s Modified Eagle’s Medium (DMEM) (#11995–065, Life Technologies, Carlsbad, CA). Dissociated tissues were incubated for 30 min (37°C, 5% CO2), triturated, and re-suspended in DMEM supplemented with fetal bovine serum (10%, # SH3007003HI, Thermo Scientific HyClone, Logan, UT), glucose (6 g/L, #G7021, Sigma), sodium bicarbonate (#25080–094, 3.7 g/L, Life Technologies), and penicillin/streptomycin (#15070063, Life Technologies). Cells were filtered sequentially through 100 μm (#542000) and 40 μm (#542040) EASYstrainer sieves (Greiner Bio-One, Monroe, NC) and plated in poly-L-lysine-coated (1 mg/ml, #P2636, Sigma) flasks (T25, Corning Inc., Corning, NY) at a density of 2 brains/flask. Eight days later, flasks were rotated at 100 rpm for 20 min to dislodge loosely-attached microglia. The medium was then replaced with DMEM containing 5% fetal bovine serum, and flasks were stroked against the table 5–10 times to release O2A/glial progenitor cells. The resulting cell suspension was panned on a plastic, uncoated culture dish for 2 h to allow adherent glia to attach. Progenitors in the suspension were spun down and plated on poly-L-lysine-coated surfaces in DMEM supplemented with CNTF (10 ng/ml, #450–13, Peprotech, Rocky Hill, NJ), NAC (5 μg/ml, #A9165, Sigma), and triiodothyronine (15 nM, #T6397, Sigma). Culture medium was changed every other day.
Viral protein and drug treatments
OLs were treated with HIV-1 Tat1–86 (1–100 nM, clade B, #1002–2, ImmunoDX LLC, Woburn, MA), VPA (0.01–1 mM, #P4543, Sigma), SB415286 (0.01–0.05 mM, #S3567, Sigma), KN-92 (10 μM, #4130/1, Tocris Bioscience, Bristol, UK), KN-93 (10 μM, #5215/1, Tocris), MK801 (20 μM, #0924/10, Tocris) and CNQX (20 μM, #1045/1, Tocris), at 2 or 7 d after enrichment. Cultures at 2 d after enrichment largely contain immature OLs that are O4+ and have multiple, thin processes but have not yet formed myelin-like membranes. Cultures at 7 d after enrichment largely contain more mature OLs that are also O4+, with myelin-like membranes produced from along the lengths of their processes. Inhibitors were applied 30 min prior to Tat, for either 24 or 96 h. Concentrations of drugs were chosen as previously reported (Salter & Fern 2005, Masvekar et al. 2015, Waggener et al. 2013). For all the experiments, vehicle refers to the diluent that is used to dissolve the drug. In experiments where both DMSO and ultrapure water were used to dissolve different treatments/drugs (Fig. 5), DMSO was used as vehicle. Responses to the Tat protein are relevant in patients receiving anti-retroviral therapy, since Tat is produced by and released from HIV-infected cells even when HIV replication is suppressed by cART (Cysique et al. 2018, Johnson & Nath 2016).
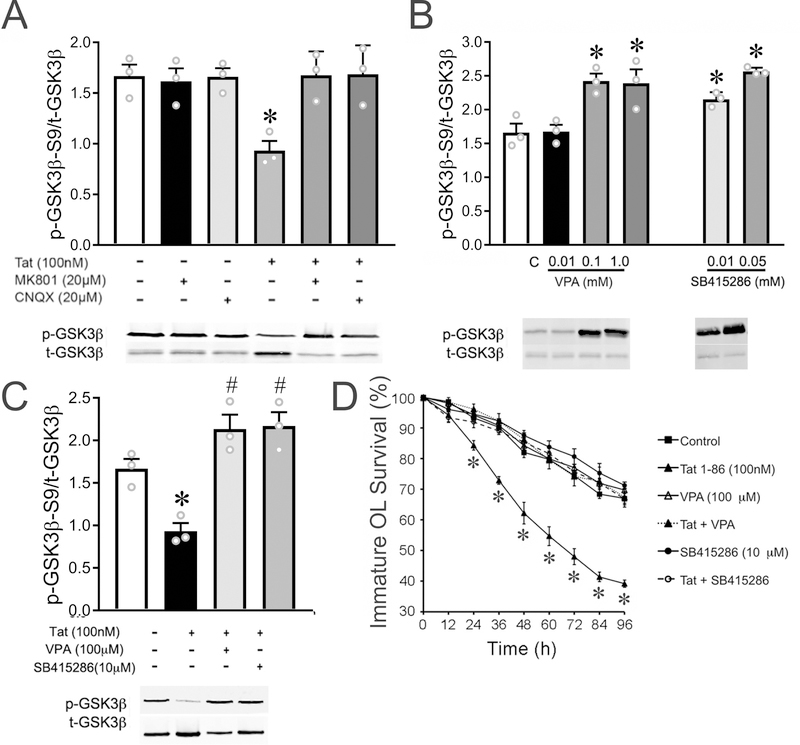
Figure 5. Inhibiting GSK3β activation rescued immature OLs from Tat-induced death in vitro.
(A) Both MK801 and CNQX fully reversed the upregulation of GSK3β activity induced by Tat. (B-C) Both VPA (0.1–10 mM) and SB415286 (0.01–0.05 mM) downregulated basal GSK3β activity in immature OLs (B), and reversed the elevation of GSK3β activity caused by 24 h Tat (C). (D) Both VPA (100 μM) and SB415286 (10 μM) rescued immature OL death induced by 96 h Tat, although neither VPA nor SB415286 by themselves affected immature OL viability. (*, #: p < 0.05 vs. non-treated control.) Experiments in A–C represent results from N=3 independent cultures prepared from mice of different litters. For each of N=4 independent experiments in D, ≥ 50 OLs were tracked for every treatment during the course of the entire 96 h time period and survival effects were analyzed by a repeated measures two-way ANOVA.
Time-lapse analysis
Time-lapse analysis was performed as previously published (Zou et al. 2011)(Masvekar et al. 2015). In brief, OLs were cultured in 12-well plates and treated with Tat and GSK3β inhibitors. Plates were maintained in the environmental chamber of the Zeiss Axio Observer Z1 system (37°C, 5% CO2; Carl Zeiss Microscopy, LLC, Thornwood, NY). For each treatment group, ≥ 50 OLs at the same stage of morphological development were chosen in sequential grids starting at the center of the plate. OLs were initially identified in digital images based on their distinctive morphology, and immunostaining for O4 was conducted at the end of experiments to positively identify OLs or OL cellular debris. Time-lapse images of all selected cells were made at hourly intervals for 96 h using a computer-controlled stage encoder and Zeiss Axiovision 4.8 software (RRID:SCR_002677; Carl Zeiss). Cells were maintained in an environmental chamber throughout the experiment. At the experimental endpoint we assessed the pre-selected OLs for their viability in the digital images. Cell death was determined by careful assessment of rigorous morphological criteria, including but not restricted to: (1) loss of phase brightness; (2) degenerating processes; and (3) involution or fragmentation of cell soma. OL death was confirmed in selected experiments using a LIVE/DEAD Viability/Cytotoxicity Kit (#L3324; Life Technologies). Data are presented as mean percentage of survival ± standard error of the mean (SEM) from at least 4 individual experiments using cultures established from different litters. Blinding to treatment group was not possible.
Fluorescent viability assay
Survival/death of OLs was confirmed using a LIVE/DEAD Viability/Cytotoxicity Kit (#L3324; Life Technologies). Working solutions were prepared by adding ethidium homodimer-1 (EthD-1) and calcein-AM to sterile, tissue culture–grade D-PBS to a final concentration of 4 μM and 2 μM, respectively. After 96 h time-lapse imaging, 24-well plates were quickly rinsed once with sterile D-PBS and immersed in working solution for 30 min at room temperature. Images were taken using a Zeiss Axio Observer Z1 microscope with Zen 2012 software (RRID:SCR_013672; Carl Zeiss). Living or dead OLs were defined by green (calcein-AM, Em ~515 nm) or red (EthD-1, Em ~617 nm) fluorescence, respectively, and the number of green/red fluorescent pixels was assessed using ImageJ software (RRID:SCR_003070; National Institutes of Health). Each N represents a separate culture from a different litter (biological replicates); 20 images from a pre-determined region in the center of duplicate coverslips (technical replicates) were imaged, analyzed, and averaged per N.
Western blot
Proteins from cultured cells were extracted using RIPA buffer (#R0278,Sigma) with protease and phosphatase inhibitors (#78443, Thermo Scientific). Tissues from transgenic mice were homogenized with 3 strokes (15 s/stroke) in a 2 ml ceramic bead tube (#13113–50, MO BIO Laboratories, Carlsbad, CA) using a Precellys 24 Homogenizer (#P000669-PR240-A; Bertin Technologies, Rockville, MD) before protein was extracted as per cultured cells. All protein concentrations were measured using a BCA assay (#23227, Thermo Scientific). Lysates were mixed 1:1 with 2x Laemmli buffer (#1610747 BioRad, Hercules, CA), and equal amounts of total protein (5–10 μg) from each sample were loaded on Criterion Precast gels (#3450032, 4–20%, BioRad) and separated under constant voltage (130 V) for 1.5 h. Proteins were transferred onto polyvinylidene difluoride membranes (#1620175, BioRad) with constant current (0.6 A, 2 h, 4 °C); membranes were blocked in 0.1% casein solution (1 h, room temperature) before probing with primary antibodies. Primary antibodies specific to GSK3β (#9315) or p-GSK3β-S9 (#8566) (Cell Signaling Technology) and CaMKIIβ (RRID:AB_2275072) or p-CaMKII-T287 (ab182647) (Abcam, Cambridge, MA) were used at 1:1000 per manufacturer suggestion. Since p-CaMKII-T287 antibodies recognize all T286/7 phosphorylated CaMKII isozymes, p-CaMKIIβ-T287 was distinguished by its larger size. Bound antibodies were detected by appropriate IRDye secondary antibodies (RRID:AB_10956389, RRID:AB_10956589, 1:3000, LI-COR, Lincoln, NE), and imaged using an Odyssey Imager (LI-COR). Fluorescent signal intensity of targeted protein bands was analyzed using LI-COR Image Studio software. The p-GSK3β-S9 antibody also has a weak affinity to p-GSK3α-S21. However, p-GSK3β-S9 (46 kDa) is easily distinguished from p-GSK3α-S21 (51 kDa) given differences in protein size (Logie et al. 2017, Gupta et al. 2017). Western blots on brain tissue were analyzed in an investigator-blinded manner based on eartag numbers.
Immunostaining
Immature or mature OLs cultured on glass coverslips were fixed with 4% paraformaldehyde for 10 min before being permeabilized with Triton-X 100 for 20 min. Cells were stained with the O4 monoclonal antibody (1:20) and antibodies specific against CaMKIIβ (#PA5–67640, 1:1000, Life Technologies), diluted in a PBS buffer containing bovine serum albumin and normal goal serum. O4 antibody, which primarily detects the sulfated galactolipid sulfatide, is grown in our lab from hybridoma cells (Knapp & Hauser 1996). Sulfatide is expressed on both immature and mature OLs, and not found on other CNS cell types. Secondary antibodies conjugated to Alexa 594 (#A-11032) or 488 (#A11001) (1:2000, Life Technologies) were used to detect primary antibodies. Hoechst 33342 (#62249, Life Technologies) was used at 1:2000 to visualize nuclei. Coverslips were mounted onto Superfrost-Plus slides (Fisher) using Prolong Gold Antifade reagent (RRID:SCR_015961, Life Technologies). Z-stacks of fluorescent images were acquired using a Zeiss LSM 700 laser scanning confocal microscope and compiled using maximum projection.
Statistics
OL cell survival was assessed using time-lapse imaging (Figs. 1A, 5D, and 7B), for which a repeated measures two-way ANOVA with time and treatment as factors, and subsequent Bonferroni’s post-hoc testing was used. For each treatment group, 4 individual cell cultures acquired from mice coming from different litters were used (N=4). After 96 h of time-lapse imaging, these same cells were used to perform Live/Dead assays (Fig. 1B–C), and were analyzed using Student’s t-test. Western blot experiments comparing in vivo expression or activity of GSK3β or CaMKIIβ in Fig. 2, or in vitro experiments assessing protein levels in vehicle or Tat-treated OL cultures (Figs. 3A–B, 4D, and 6A) also were analyzed using Student’s t-test. Western blot experiments that involved multiple treatment concentrations or time points in dissociated murine OL cultures (Figs. 4A–C, 5A–C, 6B, 7A, and 7C–D) were analyzed using one-way ANOVA followed by Bonferroni’s post-hoc testing. For all Western blot experiments comparing brain homogenates from different brain regions, three Tat− mice and three Tat+ mice, each from a different litter, were used (N=3). For Western blot experiments using OL cultures, three or four individual cultures were derived from tissue samples from different litters (N=3 or 4). The sample sizes used here are commensurate with our a priori power analyses indicating large differences exceeding power = 0.80 detected between Tat− and Tat+ samples on Western blots of myelin basic protein (power = 1.00) and myelin-associated glycoprotein (power = 0.89) using equivalent observations per group (Zou et al. 2015, Masvekar et al. 2015). Similarly, sample sizes for repeated measures experiments and live/dead assays (Fig. 1A-B; 7C) are based on power analyses from previous similar experiments with equal or fewer observations per group (Zou et al. 2011, Zou et al. 2015, Masvekar et al. 2015). Dixon’s two-tailed test (95% confidence level) was utilized to identify and exclude outliers from analyses. Analyses were considered significant if p < 0.05. Data were tested for equal variance and homoscedasticity using Bartlett’s test and the Brown-Forsythe test. Normal distribution was verified via the Shapiro-Wilk test with visual assessment of Q-Q plots.
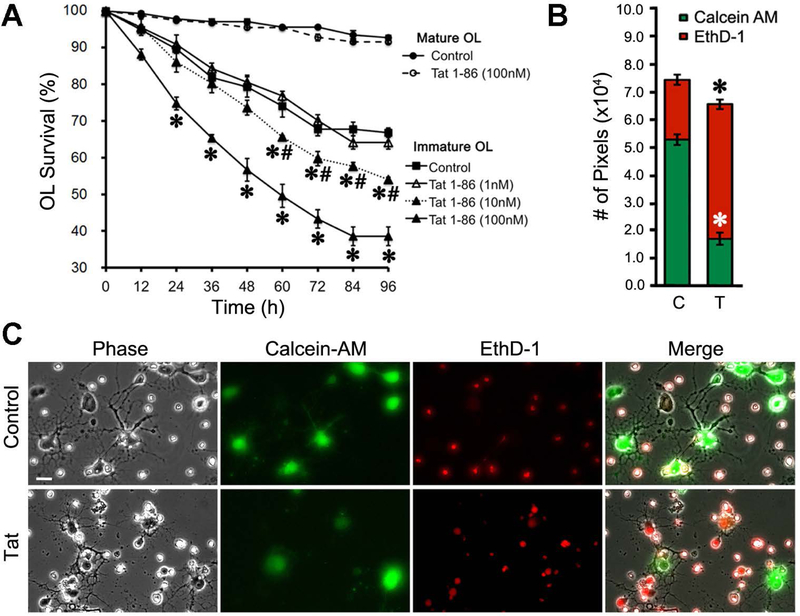
Figure 1. Effects of Tat on OL survival.
(A) Tat treatment led to a dose-dependent decrease of immature OL survival, but did not affect survival of mature OLs. In 4 independent experiments, ≥ 50 OLs were tracked for every treatment during the course of the entire 96 h time period; survival effects were analyzed by repeated measures two-way ANOVA. (B-C) Some results from (A) were confirmed by a fluorescent viability assay using Calcein-AM (green) and EthD-1 (red), and quantified with ImageJ software. Sample images in (C) were chosen from vehicle and Tat-treated immature OL groups assayed at 96 h. (* p < 0.05 vs. corresponding control group; # p < 0.05 vs. corresponding 100 nM Tat group; Scale bar: 10 μm; C: control; T: 100 nM Tat). For each experiment, N=4 independent cell cultures prepared from mice of different litters.
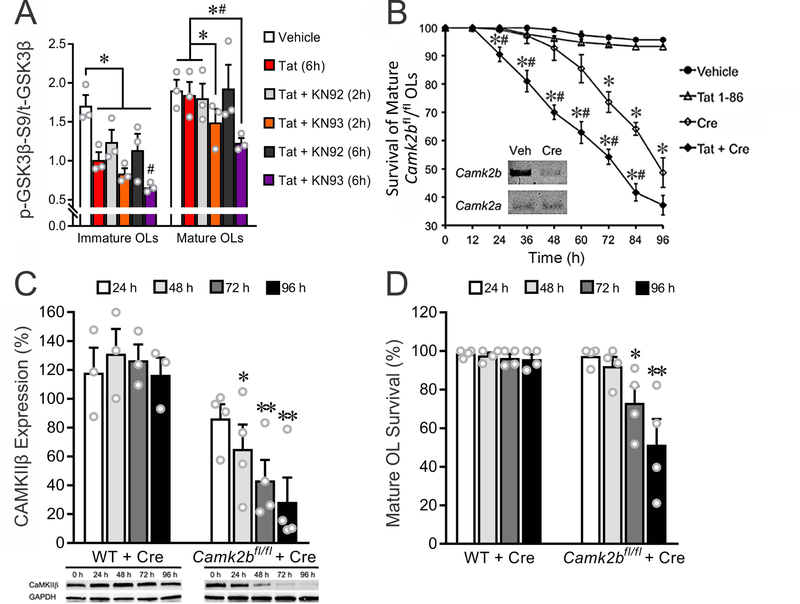
Figure 7. CaMKIIβ inhibits GSK3β activity and underlies the survival of mature OLs exposed to Tat.
(A) Inhibiting CaMKIIβ with KN93 for 2 h and/or 6 h significantly increased Tat (100 nM)-induced GSK3β activation in cultured OLs. (B) In OLs cultured from Camk2bfl/fl mice, 24 h treatment with 50 nM Cre recombinase reduced Camk2b (PCR result, inset) and led to increased death in mature OL cultures treated with Tat. (C) Western blotting showed that 50 nM Cre recombinase decreases CaMKIIβ in mature Camk2bfl/fl, but not WT, OL cultures, between 48–96 h. (D) 50 nM Cre recombinase decreases mature Camk2bfl/fl OL survival at 72 and 96 h, but does not affect WT OL viability. (A: *, #: p < 0.05, vs. corresponding vehicle control or 2 h Tat + KN92, respectively; B: *, #: p < 0.05, vs. vehicle control or Cre (50 nM), respectively; C-D: * p < 0.05, ** p < 0.01, vs. 0 h). Experiments in A, C, and D represent results from N=3 or N=4 independent cultures prepared from mice of different litters. For each of N=4 independent experiments in B, ≥ 50 OLs were tracked for every treatment during the course of the entire 96 h time period and survival effects were analyzed by repeated measures, two-way ANOVA.
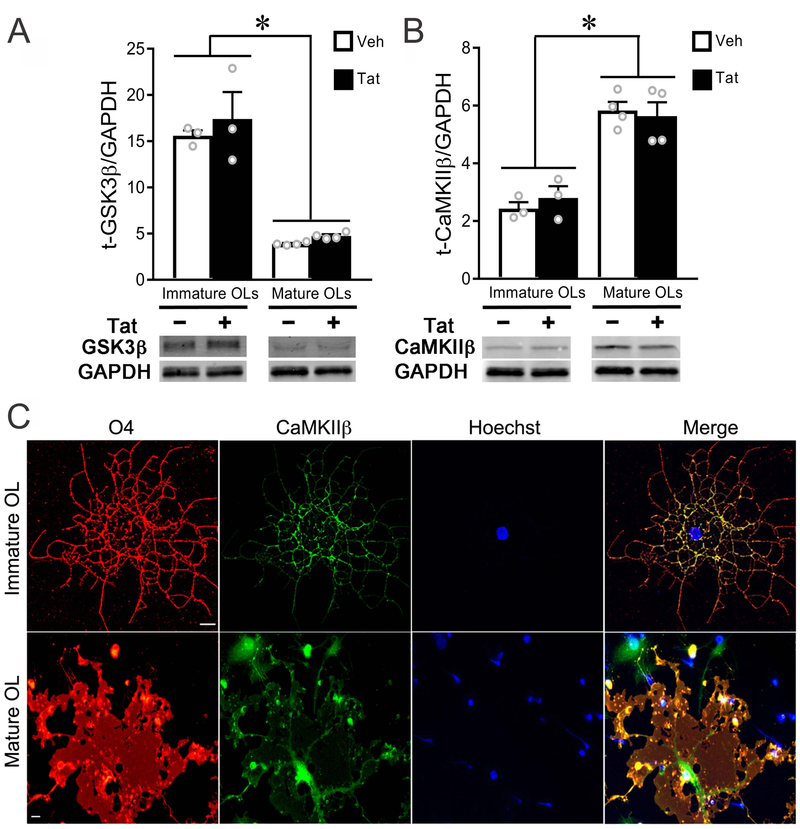
Figure 3. Expression level and localization of CaMKIIβ differ between immature and mature OLs.
(A) Western blotting showed that GSK3β levels were significantly (~3 fold) higher in immature versus mature OLs in vitro, and unaffected by Tat at either stage. (B) Expression of CaMKIIβ was significantly lower in immature versus mature OLs in vitro, and not affected by 96 h Tat treatment. (C) Confocal images (maximum projection) of fluorescent immunostaining showed that CaMKIIβ was expressed in OLs in vitro. In immature OLs, the majority of CaMKIIβ was found in cellular processes. In mature OLs, CaMKIIβ was found both in cell body cytoplasm and along larger processes. (Scale bar: 10 μm; *: p < 0.05, vs. corresponding control). Experiments in A and B represent results from N=3 (immature) or N=4 (mature) independent cultures prepared from mice of different litters.
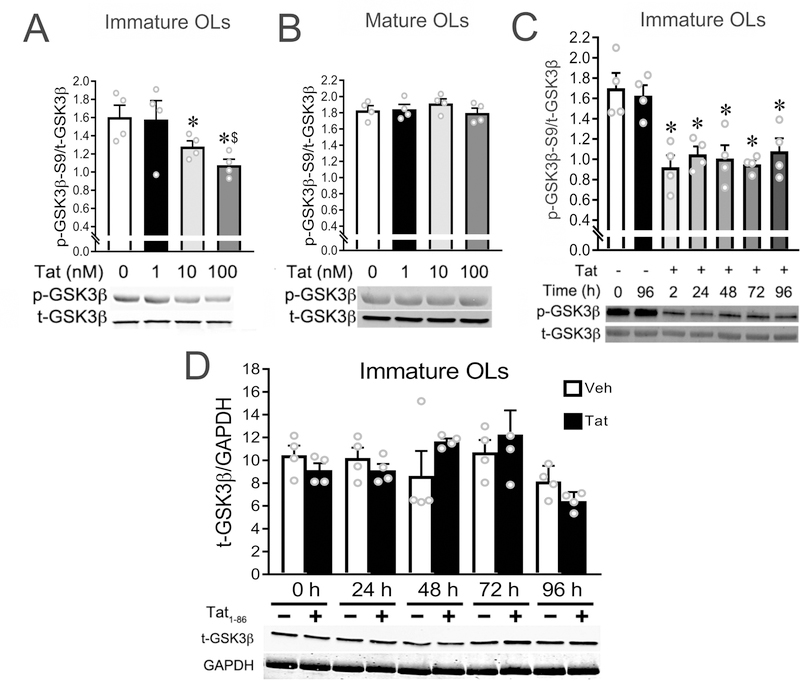
Figure 4. Tat upregulates GSK3β activity in immature OLs in vitro.
(A) Tat induced a dose-dependent upregulation of GSK3β activity (decreased ratio of p-GSK3β-S9/t-GSK3β) in immature OLs. (B) GSK3β activity in mature OLs was not affected by Tat. (C) The upregulated GSK3β activity induced by 100 nM Tat in immature OLs persists over 96 h. (D) Total GSK3β levels in immature OLs were not affected by 100 nM Tat treatment within 96 h. (* p < 0.05 vs. controls lacking Tat; $ p < 0.05 vs. 10 nM Tat). Experiments in A–D represent results from N=4 independent cultures prepared from mice of different litters.
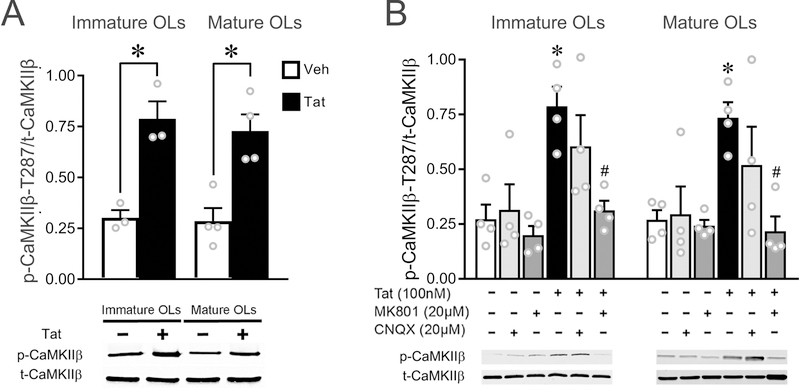
Figure 6. Tat activates OL CaMKIIβ in vitro via iGluRs in both immature and mature OLs.
(A) Western blot results indicate that CaMKIIβ activity was significantly upregulated in both immature and mature OLs after 1 h Tat (100 nM) treatment. (B) Tat-induced upregulation of CaMKIIβ activity can be completely inhibited by MK801, and partially reversed by CNQX (not different from either control or Tat; p=0.113 vs Tat). (* p < 0.05 vs. corresponding vehicle control; # p < 0.05 vs 100 nM Tat.) Results represent N=3 or N=4 independent cultures prepared from mice of different litters.
RESULTS:
Effects of Tat on OL survival.
We first confirmed that HIV-1 Tat caused OL degeneration using morphologic criteria in phase contrast digital photomicrographs, and assessed the time course of cell losses. As noted previously, loss of immature OLs in untreated cultures exceeds that of mature OLs over the 3 day experimental period (Zou et al. 2015). The survival of mature OLs (7 d in culture, O4+/MBP+) was unaffected by Tat at 96 h, while the viability of immature OLs (2 d in culture, O4+/MBP−) was significantly reduced by Tat in a concentration-dependent manner (Fig. 1A). The effects of 100 nM Tat on immature OL survival reached statistical significance as early as 24 h post-treatment, quite a bit earlier than observed with 10 nM Tat (~60 h, Fig. 1A). Live/dead assays were performed on cells after these experiments (at 96 h) to verify that the degenerating OLs identified by phase contrast microscopy had lost cell membrane integrity, confirming cell death. The Tat-treated (100 nM) group exhibited significantly more red fluorescent signal (EthD-1+, dead), and less green fluorescent signal (Calcein-AM+, live), when compared to control groups (Figs. 1B, C).
Tat expression in vivo activates GSK3β and CaMKIIβ in multiple brain regions.
Since expression of Tat in vivo leads to increased caspase-3 activity in O4+ oligodendroglial lineage cells (Hauser et al. 2009), we investigated Tat effects on GSK3β or CaMKIIβ signaling by Western blot using a DOX-inducible Tat-transgenic mouse model. We examined white matter-containing regions where morphological and functional changes are prevalent in both animal models of HIV and in HIV-infected individuals (cortex, striatum, hippocampus) and compared them to the cerebellum, a region that is less affected. A significantly decreased ratio of pGSK3β-S9 to total GSK3β (p-GSK3β/t-GSK3β) was detected in cortex, striatum, and hippocampus, suggesting that GSK3β activity was upregulated in these areas by Tat (Fig. 2A,B). GSK3β activity in cerebellum showed an opposing trend towards a decrease (Fig. 2B, p = 0.077). CaMKIIβ protein levels were significantly increased in three of the four brain regions examined in Tat+ mice (Fig. 2C, cerebellum: p = 0.09). Further, total CaMKIIβ activity, as assessed by phosphorylation at CaMKIIβ’s T287 site, was increased in all 4 regions (cortex, cerebellum, striatum, and hippocampus) in Tat+ mice (Fig. 2D). Since other cells types express both GSK3β and CaMKIIβ, these changes may not be unique to OLs.
Expression and localization of CaMKIIβ varies with developmental stage
OLs at different developmental stages show distinct patterns of expression and subcellular distribution of iGluRs, GSK3β and CaMKIIβ (Zhang et al. 2014, Karadottir et al. 2005, De Biase et al. 2010). We thus assessed the level of GSK3β and CaMKIIβ proteins using Western blotting and CaMKIIβ localization by immunocytochemistry. Total GSK3β levels in immature OLs were significantly higher than in mature OLs, independent of Tat treatment (Fig. 3A). In contrast, total CaMKIIβ levels were significantly higher in mature OLs compared to immature OLs, also independent of Tat treatment (Fig. 3B). In immature OLs, CaMKIIβ localized mainly to cellular processes, while in mature OLs CaMKIIβ was found in both the cytoplasm of larger processes and the cell body (Fig. 3C). A varying ratio of GSK3β to CaMKIIβ, and distinct subcellular distribution of CaMKIIβ may contribute to the differential vulnerability of immature and mature OLs to Tat. The activation of GSK3β versus CaMKIIβ as a function of OL development is explored in experiments described below.
Tat upregulates GSK3β activity in immature, but not mature OLs.
It is well-established that GSK3β-S9-phosphorylation reduces GSK3β activity (Doble & Woodgett 2003, Hur & Zhou 2010, Masvekar et al. 2015). Thus, we and others have used the ratio of p-GSK3β-S9:t-GSK3β to indicate GSK3β activity (decreased ratio indicating increased GSK3β activity). The p-GSK3β-S9:t-GSK3β ratio was significantly decreased in immature OLs treated with 10 or 100 nM Tat as compared with vehicle or 1 nM Tat-treated groups (Fig. 4A), indicating upregulated GSK3β activity. Total GSK3β was not affected by Tat treatment over 96 h (Fig. 4D). The upregulation of GSK3β activity by Tat (100 nM) was detected as early as 2 h, and persisted over 96 h (Fig. 4C). In comparison, Tat did not affect GSK3β activity in mature OL cultures (Fig. 4B).
Inhibiting GSK3β activation rescues immature OLs from Tat-induced death in vitro.
We previously reported that the immature OL death induced by Tat could be reversed by the N-methyl-d-aspartate receptor (NMDA-R) antagonist, MK801, or the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor (AMPA/KA-R) antagonist, CNQX (Zou et al. 2015). Since iGluR activation has also been shown to regulate GSK3β activity (De Montigny et al. 2013), we assessed the effects of MK801/CNQX on GSK3β in Tat-treated OLs. Western blots showed that the up-regulated GSK3β activity caused by Tat was completely abolished when MK801 or CNQX were added concurrently (Fig. 5A), implicating GSK3β in mediating the toxic effect of Tat on immature OLs.
We next investigated whether blocking GSK3β could reverse the effect of Tat on immature OL survival, using two drugs that block GSK3β activity via different mechanisms. VPA is used widely as a GSK3β inhibitor, working through activation of its upstream WNT signaling pathway (Hall et al. 2002), but has numerous other actions including HDAC, nitric oxide synthase, and aromatase inhibition. SB415286 is a more specific small molecule inhibitor that competitively binds to an ATP binding domain and adjacent sites, inhibiting activities downstream of GSK3β activation (Liang & Chuang 2007, Coghlan et al. 2000). Both VPA (0.1–1 mM) and SB415286 (0.01–0.05 mM) effectively blocked Tat induction of GSK3β activity in immature OLs after 24 h treatment (Fig. 5B and C). Continuous VPA (100 μM) and SB415286 (10 μM) treatment reduced the GSK3β activity (Fig. 5C) and reversed OL death induced by 96 h Tat (Fig. 5D).
Tat activates CaMKIIβ in vitro via iGluRs in both immature and mature OLs.
Given that Tat activates iGluRs in both immature and mature OLs, it was intriguing that Tat did not increase GSK3β activity in mature OLs. Activated CaMKII has been reported to phosphorylate GSK3β-S9 and thereby inhibit GSK3β, and to promote neuronal survival (Song et al., 2010). Our previous studies have also shown that Tat activates OL iGluRs and up-regulates CaMKIIβ activity (Zou et al. 2015). Since CaMKIIβ is the predominant CaMKII isoform in OLs, based on the neuronal results, we hypothesized that Tat-induced CaMKIIβ activation may maintain higher levels of GSK3β phosphorylation (reduced GSK3β activity) in mature OLs, thus making them less vulnerable to Tat. To test this hypothesis, we first verified the effects of Tat on CaMKIIβ activities in OLs. Western blots showed that CaMKIIβ activity was significantly increased in both immature and mature OLs treated with Tat (100 nM, 1h), as demonstrated by an increase in the p-CaMKIIβ-T287/t-CaMKIIβ ratio (Hudmon & Schulman 2002b) (Fig. 6A). The Tat-induced CaMKIIβ activation in OLs was mediated through iGluRs activation, since MK801 completely reversed, and CNQX partially blocked, the upregulated CaMKIIβ activity in both immature and mature OL treated with 100 nM Tat (Fig. 6B).
CaMKIIβ suppresses Tat-induced GSK3β activity in mature OLs
To test the effects of inhibiting CaMKIIβ on Tat-induced GSK3β activation, we added the CaMKIIβ inhibitor KN93 and its inactive derivative KN92 concurrent with Tat. Although Tat by itself did not affect GSK3β activity in mature OLs (Fig. 7A), KN93 + Tat significantly increased GSK3β activity at 2 h and 6 h. This suggests that normal levels of CaMKIIβ activity were sufficient to suppress Tat-induced GSK3β activation in mature OLs. In contrast, Tat alone increased GSK3β activity in immature OLs, and this was further enhanced by KN93 (Fig. 7A). KN92 had no effect on GSK3β activities at either stage of maturity.
CaMKIIβ-deficient OLs have enhanced vulnerability to Tat
Longer-term applications of KN93 were toxic to OLs in vitro, so we used primary OLs cultured from Camk2bfl/fl mice to test the hypothesis that knocking down CaMKIIβ would make mature OLs vulnerable to Tat. In cell culture, loxP-flanked genes can be excised by adding cell-permeable Cre recombinase to the media (O’Meara et al. 2013). RT-PCR showed that 50 nM Cre significantly decreased the mRNA level of Camk2b in mature OL cultures at 24 h (Fig. 7B, inset). Importantly, the viability of mature OLs exposed to Tat was significantly decreased between 24 and 96 h when Camk2b was knocked down (Fig. 7B). Knockdown of Camk2b by itself decreased mature OL viability after 72 h, although always to a lesser extent than with co-exposure to Tat. To further clarify that these effects on viability were mediated by knockdown of CaMKIIβ, Western blot and live/dead assays were performed at 0–96 h on mature, wild-type, or Camk2bfl/fl OLs treated with Cre. Adding Cre to the media significantly decreased CaMKIIβ protein levels (48 – 96 h) and cell viability (72 – 96 h) in Camk2bfl/fl, but not wild-type, mature OLs (Fig. 7C-D).
DISCUSSION
Myelin disruptions are a common finding in the CNS of HIV-infected individuals, and indications of OL dysfunction have been noted both in post-mortem tissue and experimentally. While blood-brain barrier leakage and edema probably contribute to these outcomes, we have also shown that OLs are directly injured by exposure to HIV-1 Tat, which increases [Ca2+]i levels coincident with increased CaMKIIβ activity in both immature OLs, and mature OLs that are producing myelin-like membranes (Zou et al. 2015). Viability of immature OLs was reduced by Tat and was rescued by attenuating [Ca2+]i increases. However, mature OLs survived very high [Ca2+]i increases and instead reduced their production of myelin-like membranes (Zou et al. 2015). Here we have explored the basis for these quite different responses of immature versus mature OLs to Tat-induced [Ca2+]i increases.
The finding that [Ca2+]i was increased and CaMKIIβ activated at both developmental stages suggested that different pathways downstream of CaMKIIβ might be activated in OLs as they matured. GSK3β activity is upregulated by iGluR-mediated [Ca2+]i increases (Hartigan & Johnson 1999), and negatively regulates OL proliferation and myelination (Azim & Butt 2011). It also has been invoked as a cause of Tat-induced neurotoxicity in numerous studies (Masvekar et al. 2015, Chao et al. 2014). Since CaMKII activation can inhibit GSK3β, we hypothesized that the relative levels of total and activated CaMKII versus GSK3β might be altered to favor cell survival in more mature OLs.
As GSK3β is ubiquitously expressed among CNS cell types, we used highly purified cultures to assess whether Tat regulates GSK3β activity in OLs. Tat caused concentration-dependent GSK3β activation in immature, but not mature OLs, in parallel with decreased cell viability (Fig. 1A, 4A-C). MK801 or CNQX, which attenuated the Tat-induced [Ca2+]i increase and rescued immature OLs from Tat-induced death (Zou et al. 2015), also reversed GSK3β activation by Tat (Fig. 5A). This finding is consistent with the idea that GSK3β activation can be caused by iGluR-mediated [Ca2+]i increases (Hartigan & Johnson 1999). Further, VPA and SB415286, two GSK3β inhibitors that block GSK3β activity via different mechanisms, both reversed Tat-induced immature OL death (Fig. 5D). Together, these data strongly support a Tat-induced signaling mechanism whereby interactions between Tat and iGluRs lead to [Ca2+]i increase, GSK3β activation in immature OLs, and subsequent cell death. Theoretically, VPA and SB415286 also inhibit GSK3α (Coghlan et al. 2000). However, Tat did not alter GSK3α activity in OLs (data not shown), suggesting that GSK3β is the principal target.
Since Tat elevates [Ca2+]i similarly in mature and immature OLs (Zou et al. 2015), it was intriguing that Tat did not activate GSK3β in mature OLs. Based on our previous finding that Tat activates CaMKIIβ (Zou et al. 2015), the predominant isoform of CaMKII in OLs (Waggener et al. 2013), and that CaMKIIβ has been reported to inhibit GSK3β (Song et al. 2010), we hypothesized that [Ca2+]i-mediated GSK3β activation in OLs might be determined by the balance between CaMKIIβ and GSK3β signaling. The relatively higher levels of CaMKIIβ versus GSK3β measured in mature OLs may contribute to greater inhibition of GSK3β activity and less vulnerability to the toxic effects of Tat. Independent of kinase activities, CaMKIIβ also can function structurally by stabilizing the actin cytoskeleton (Hudmon & Schulman 2002a), which has been shown to influence OL maturation and myelination (Waggener et al. 2013). Persistent cytoskeletal disruption may thus contribute to mature OL loss after 72 h of Camk2b knockdown. This might be further clarified by experiments using the Camk2bA303R mice, in which the mutated CaMKIIβ (CaMKIIβA303R) results in lost kinase catalytic activity, with preservation of actin-binding capacity (Lin & Redmond 2008, O’Leary et al. 2006).
NMDA-R stimulation has been reported to influence both GSK3β and CaMKII activity, inhibiting GSK3β (De Montigny et al. 2013) and persistently activating CaMKII, due to stable interactions with the NR2B subunit (Bayer et al. 2006, Lee et al. 2009). Since mature OLs express higher levels of NMDA-Rs than immature OLs (De Biase et al. 2010, Micu et al. 2006, Salter & Fern 2005), the Tat-induced NMDA-R activation that we have previously shown in OLs (Zou et al. 2015), and that others have observed in neurons (Haughey et al. 2001, Kim et al. 2008), might also contribute to downregulated GSK3β activity in mature OLs.
In vivo expression of HIV-1 Tat expression altered overall CaMKIIβ and GSK3β activities in multiple brain regions. Although CaMKIIβ activity was consistently elevated by Tat (Fig. 2D), it did not suppress overall GSK3β activity, except perhaps in cerebellum (Fig. 2B). In vivo measures reflect the combined responses of multiple cell types in the brain that express both enzymes, not all of which will respond equally to Tat. In addition, GSK3β expression is quite high compared to CaMKIIβ expression in many CNS cells (Zhang et al. 2014), suggesting that CaMKIIβ suppression of GSK3β activation may be unique to OLs. The in vitro studies likely provide greater insight into OL-specific responses.
Dysregulation of GSK3β activity has been reported to be involved in multiple neurodegenerative processes, including HAND (Jacobs et al. 2012, Crews et al. 2009, Schifitto et al. 2006). Among the regions we examined, GSK3β activity was upregulated in all except the cerebellum (Fig. 2B). This observation is intriguing for several reasons. Firstly, clinical observations of HIV-related neuropathology are rarely reported in cerebellum, but frequently reported in the other regions examined. Secondly, CaMKIIβ is the predominant isoform of CaMKII in both OLs and cerebellar granule neurons (Waggener et al. 2013, Burgin et al. 1990) and our experiments reveal CaMKIIβ activity to be significantly upregulated by Tat expression in cerebellum (Fig. 2D). We have also previously shown that cerebellar astrocytes secrete significantly less cytokines/chemokines than astrocytes from cortex or spinal cord when exposed to Tat in vitro (Fitting et al. 2010). Together, these findings suggest that the more limited glial inflammatory response and high levels of CaMKIIβ in cerebellar neurons may protect that region from GSK3β-induced damage due to Tat or other HIV proteins.
Data reported here suggest that the relative levels of CaMKIIβ and GSK3β may be critical in determining OL vulnerability to Tat (summarized in Fig. 8). Exposure to Tat leads to Ca2+ influx into the cytoplasm, which activates both GSK3β (p-GSK3β-S9 dephosphorylation) and CaMKIIβ (CaMKIIβ-T287 phosphorylation). In mature OLs, a large amount of activated cytoplasmic CaMKIIβ inhibits the activated GSK3β, which protects cells from death. In immature OLs, the amount of activated CaMKIIβ is not sufficient to inhibit GSK3β, and consequently, downstream cell death signaling pathways are triggered.
Figure 8. Summary.
HIV-1 Tat-dependent increases in [Ca2+]i activate both GSK3β (by dephosphorylating p-GSK3β at S9) and CaMKIIβ (by phosphorylating CaMKIIβ at T287). In mature OLs, large amounts of activated, cytoplasmic CaMKIIβ inhibit GSK3β (┴), which protects OLs from death. Conversely, in immature OLs, higher levels of activated GSK3β are present. The low levels of activated CaMKIIβ, which are preferentially distributed within OL cellular processes but not the cell body (┴), are inadequate to sufficiently inhibit GSK3β activation. Overactivation of GSK3β triggers signaling events leading to immature OL death.
Our results with HIV-1 Tat are in accord with the finding that conditional depletion of GSK3β protected myelinating OLs from caspase-dependent death in the cuprizone demyelination model (Xing et al. 2018). Although CaMKIIβ levels or activity were not explored in the cuprizone study, it is striking that GSK3β activation is implicated in OL death resulting from these disparate insults. Survival during development is closely linked to differentiation, and GSK3β activation has been observed to positively (Zhou et al. 2014) or negatively (Azim & Butt 2011, Azim et al. 2014) affect OL maturation, in part depending on the dynamics of Wnt/β-catenin signaling. GSK3β activation appears to be at the crossroads of multiple developmental events whose outcomes may depend upon multiple factors related to specific stages in the OL lineage.
White matter injuries, such as decreased volume (Sarma et al. 2014), increased occurrence of abnormal myelinated tracts (Gongvatana et al. 2009), and degenerated OLs (Gyorkey et al. 1987, Jayadev et al. 2007), contribute to HAND pathogenesis and can occur during early, asymptomatic stages of HIV infection. cART itself has also been recently shown to impact myelin integrity and maintenance (Jensen et al. 2015), suggesting that these injuries may be extremely difficult to prevent in HIV-infected patients. Thus, the ability of immature OLs to survive, differentiate and remyelinate may be an important factor in limiting CNS damage due to HIV exposure. Our findings suggest that immature OLs are vulnerable to Tat-induced death because CaMKIIβ levels are insufficient to restrict GSK3β activation. This vulnerability at an early developmental stage may compromise myelin repair efforts. Therapeutic strategies that directly target GSK3β activity in OLs may be difficult to design since GSK3β is ubiquitous. However, an indirect approach by modulating NMDA-R and/or increasing CaMKIIβ to overcome any deleterious effects of Tat-induced GSK3β activation might provide important adjunctive therapy to HIV-infected individuals afflicted with HAND.
Acknowledgements:
Funding support from the NIH: F31 NS084838 (SZ), R01 DA024461 (PEK), R01 DA044939 (KFH and PEK), R21 NS084335 (BF), and K02 DA027374 (KFH) are greatly appreciated. Electron microscopy was performed at the VCU-Dept. Anatomy and Neurobiology Microscopy Facility, supported in part with funding from NIH-NINDS Center core grant 5P30NS047463.
Abbreviations
- ANOVA
Analysis of variance
- cART
Combined antiretroviral therapy
- [Ca2+]I
Intracellular calcium concentration
- CaMKIIβ
Ca2+/calmodulin-dependent kinase IIβ
- Camk2bfl/fl
Transgenic mouse with a loxP-modified Camk2b allele
- CNQX
Cyanquixilene
- CNS
Central nervous system
- CNTF
Ciliary neurotrophic factor
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- DOX
Doxycycline
- EthD-1
Ethidium homodimer-1
- GSK3β
Glycogen synthase kinase 3β
- GSK3α
Glycogen synthase kinase 3α
- HAND
HIV-associated neurocognitive disorders
- HIV
Human immunodeficiency virus
- IGluR
ionotropic glutamate receptor
- NAC
N-acetyl cysteine
- NMDA-R
N-methyl-D-aspartate receptor
- OL
Oligodendrocyte
- p-CaMKII-T287
CaMKII phosphorylated at Threonine 287
- p-GSK3β-S9
GSK3β phosphorylated at Serine 9
- RIPA
Radioimmunoprecipitation assay
- RRID
Research Resource Identifier
- Tat
Transactivator of transcription
- VPA
Valproic acid
Footnotes
Conflict of interest: The authors declare no competing financial interests. B.Fuss is a former editor of The Journal of Neurochemistry and an ISN council member.
References:
- Albright AV, Strizki J, Harouse JM, Lavi E, O’Connor M and Gonzalez-Scarano F (1996) HIV-1 infection of cultured human adult oligodendrocytes. Virology, 217, 211–219. [DOI] [PubMed] [Google Scholar]
- Azim K and Butt AM (2011) GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia [DOI] [PubMed]
- Azim K, Rivera A, Raineteau O and Butt AM (2014) GSK3beta regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-beta-catenin signaling. Glia, 62, 778–779. [DOI] [PubMed] [Google Scholar]
- Bagashev A and Sawaya BE (2013) Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J, 10, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H and De Koninck P (2006) Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci, 26, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC and Kelly PT (1990) In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci, 10, 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Yang L, Yao H and Buch S (2014) Platelet-derived growth factor-BB restores HIV Tat -mediated impairment of neurogenesis: role of GSK-3beta/beta-catenin. Journal of Neuroimmune Pharmacol, 9, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard C, Tong PBV, Toth P et al. (2018) Cyclophilin A enables specific HIV-1 Tat palmitoylation and accumulation in uninfected cells. Nat Commun, 9, 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA et al. (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol, 7, 793–803. [DOI] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, Everall IP and Masliah E (2009) Molecular pathology of neuro-AIDS (CNS-HIV). Int J Mol Sci, 10, 1045–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Juge L, Lennon MJ et al. (2018) HIV brain latency as measured by CSF BcL11b relates to disrupted brain cellular energy in virally suppressed HIV infection. AIDS [DOI] [PubMed]
- Dayton AI, Sodroski JG, Rosen CA, Goh WC and Haseltine WA (1986) The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell, 44, 941–947. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A and Bergles DE (2010) Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci, 30, 3600–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Montigny A, Elhiri I, Allyson J, Cyr M and Massicotte G (2013) NMDA reduces Tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3beta, and PKC activities. Neural Plast, 2013, 261593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW and Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci, 116, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P and Gallo RC (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol, 67, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AG, Feinberg MB, Josephs SF et al. (1986) The trans-activator gene of HTLV-III is essential for virus replication. Nature, 320, 367–371. [DOI] [PubMed] [Google Scholar]
- Fitting S, Zou S, Chen W, Vo P, Hauser KF and Knapp PE (2010) Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J Proteome Res, 9, 1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD and Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell, 55, 1189–1193. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ et al. (2009) White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol, 15, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL and Purcell DF (2003) Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res, 1, 463–473. [DOI] [PubMed] [Google Scholar]
- Gupta A, Anjomani-Virmouni S, Koundouros N and Poulogiannis G (2017) PARK2 loss promotes cancer progression via redox-mediated inactivation of PTEN. Mol Cell Oncol, 4, e1329692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkey F, Melnick JL and Gyorkey P (1987) Human immunodeficiency virus in brain biopsies of patients with AIDS and progressive encephalopathy. J Infect Dis, 155, 870–876. [DOI] [PubMed] [Google Scholar]
- Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR and Salinas PC (2002) Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol Cell Neurosci, 20, 257–270. [DOI] [PubMed] [Google Scholar]
- Hartigan JA and Johnson GV (1999) Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem, 274, 21395–21401. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT and Geiger JD (2001) HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem, 78, 457–467. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ and Knapp PE (2009) HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia, 57, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr. et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A and Schulman H (2002a) Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem, 71, 473–510. [DOI] [PubMed] [Google Scholar]
- Hudmon A and Schulman H (2002b) Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J, 364, 593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM and Zhou FQ (2010) GSK3 signalling in neural development. Nature Rev Neurosci, 11, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE and Thotala D (2012) GSK-3beta: A Bifunctional Role in Cell Death Pathways. Int J Cell Biol, 2012, 930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Yun B, Nguyen H, Yokoo H, Morrison RS and Garden GA (2007) The glial response to CNS HIV infection includes p53 activation and increased expression of p53 target genes. J Neuroimmune Pharmacol, 2, 359–370. [DOI] [PubMed] [Google Scholar]
- Jensen BK, Monnerie H, Mannell MV et al. (2015) Altered Oligodendrocyte Maturation and Myelin Maintenance: The Role of Antiretrovirals in HIV-Associated Neurocognitive Disorders. J Neuropathol Exp Neurol, 74, 1093–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP and Nath A (2016) Protocol for Detection of HIV-Tat Protein in Cerebrospinal Fluid by a Sandwich Enzyme-Linked Immunosorbent Assay. Methods Mol Biol, 1354, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH and Attwell D (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature, 438, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA and Thayer SA (2008) Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci, 28, 12604–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE and Hauser KF (1996) mu-Opioid receptor activation enhances DNA synthesis in immature oligodendrocytes. Brain Res, 743, 341–345. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM and Yasuda R (2009) Activation of CaMKII in single dendritic spines during long-term potentiation. Nature, 458, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH and Chuang DM (2007) Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. The Journal of biological chemistry, 282, 3904–3917. [DOI] [PubMed] [Google Scholar]
- Lin YC and Redmond L (2008) CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA, 105, 15791–15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie L, Van Aalten L, Knebel A et al. (2017) Rab-GTPase binding effector protein 2 (RABEP2) is a primed substrate for Glycogen Synthase kinase-3 (GSK3). Sci Rep, 7, 17682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masvekar RR, El-Hage N, Hauser KF and Knapp PE (2015) GSK3beta-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity. Mol Cell Neurosci, 65, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N and Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol, 67, 699–714. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E et al. (2006) NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature, 439, 988–992. [DOI] [PubMed] [Google Scholar]
- O’Leary H, Lasda E and Bayer KU (2006) CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Molec Biol Cell, 17, 4656–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara RW, Michalski JP, Anderson C, Bhanot K, Rippstein P and Kothary R (2013) Integrin-linked kinase regulates process extension in oligodendrocytes via control of actin cytoskeletal dynamics. J Neurosci, 33, 9781–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R et al. (2001) HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology, 56, 257–260. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Miyahara S, Deng L et al. (2011) Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology, 77, 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG and Fern R (2005) NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature, 438, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, Deville J, Church JA and Thomas MA (2014) Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage Clin, 4, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau QJ, Dalgleish AG, Weiss RA and Beverley PC (1986) Epitopes of the CD4 antigen and HIV infection. Science, 234, 1120–1123. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Peterson DR, Zhong J, Ni H, Cruttenden K, Gaugh M, Gendelman HE, Boska M and Gelbard H (2006) Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology, 66, 919–921. [DOI] [PubMed] [Google Scholar]
- Song B, Lai B, Zheng Z, Zhang Y, Luo J, Wang C, Chen Y, Woodgett JR and Li M (2010) Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J Biol Chem, 285, 41122–41134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL and McArthur JC (2012) HIV-associated neurological disorders: a guide to pharmacotherapy. CNS Drugs, 26, 123–134. [DOI] [PubMed] [Google Scholar]
- Waggener CT, Dupree JL, Elgersma Y and Fuss B (2013) CaMKIIbeta Regulates Oligodendrocyte Maturation and CNS Myelination. J Neurosci, 33, 10453–10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Brink LE, Maers K, Sullivan ML, Bodnar RJ, Stolz DB and Cambi F (2018) Conditional depletion of GSK3b protects oligodendrocytes from apoptosis and lessens demyelination in the acute cuprizone model. Glia [DOI] [PubMed]
- Yilmaz A, Svennerholm B, Hagberg L and Gisslen M (2006) Cerebrospinal fluid viral loads reach less than 2 copies/ml in HIV-1-infected patients with effective antiretroviral therapy. Antivir Ther, 11, 833–837. [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci, 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Shao CY, Xu SM et al. (2014) GSK3beta promotes the differentiation of oligodendrocyte precursor cells via beta-catenin-mediated transcriptional regulation. Mol Neurobiol, 50, 507–519. [DOI] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF and Knapp PE (2011) Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain, 134, 3613–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fuss B, Fitting S, Hahn YK, Hauser KF and Knapp PE (2015) Oligodendrocytes Are Targets of HIV-1 Tat: NMDA and AMPA Receptor-Mediated Effects on Survival and Development. J Neurosci, 35, 11384–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]