Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) is a growth factor for lung cancer cells. PACAP-27 or PACAP-38 binds with high affinity to non-small cell lung cancer (NSCLC) cells, causing elevated cytosolic Ca2+, increased proliferation and increased phosphorylation of extracellular regulated kinase (ERK) and the epidermal growth factor receptor (EGFR). The role of reactive oxygen species (ROS) was investigated in these processes. Addition of PACAP-38 to NCI-H838 or A549 cells increased the tyrosine phosphorylation of the EGFR, HER2 and ERK significantly by 4-, 3-, and 2-fold, respectively. The transactivation of the EGFR and HER2 was inhibited by gefitinib or lapatinib (tyrosine kinase inhibitors), PACAP (6–38) (PAC1 antagonist), N-acetylcysteine (NAC is an anti-oxidant) or dipheyleneiodonium (DPI is an inhibitor of Nox and Duox enzymes). PACAP-38 addition to NSCLC cells increased ROS which was inhibited by PACAP (6–38), NAC or DPI. Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2 mRNA was present in many NSCLC cell lines. PACAP-38 stimulated the growth of NSCLC cells whereas PACAP (6–38), gefitinib or DPI inhibited proliferation. The results show that ROS are essential for PAC1 to regulate EGFR and HER2 transactivation as well as proliferation of NSCLC cells.
Keywords: PAC1, transactivation, EGFR, HER2, ERK, lung cancer, ROS, Nox, Duox
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP), a 27 amino acid peptide, and vasoactive intestinal peptide (VIP), a 28 amino acid peptide, have 67% sequence homology [3,30,42]. PACAP-27 binds with high affinity to the class B/secretin-like G protein-coupled receptors (GPCR) VPAC1, VPAC2 and PAC1. In contrast, VIP binds with high affinity to VPAC1 and VPAC2, but not PAC1 [40]. VPAC1, VPAC2 and PAC1 interact with Gs, resulting in increased adenylyl cyclase activity and elevated cAMP [48]. Also, PAC1 interacts with Gq causing phosphatidyl inositol (PI) turnover [31,38]. The resulting inositol-1,4,5-trisphosphate causes elevation of cytosolic Ca2+, whereas the diacylglycerol activates protein kinase (PK)-C.
Addition of PACAP-27 to lung cancer cells increases ERK phosphorylation, c-fos expression and vascular endothelial growth factor expression [10,33]. PACAP-27 addition to colon cancer, lung cancer or prostate cancer cells increases their proliferation [6,11,32]. PACAP (6–38) is an antagonist which inhibits the growth of breast, lung and prostate cancer cells [22,34,52]. Approximately 160,000 lung cancer patients in the U.S.A die annually, mostly from non-small cell lung cancer (NSCLC). NSCLC is characterized by mutations in p53, k-Ras and the epidermal growth factor receptor (EGFR) [43]. PACAP addition to NSCLC cells increases the tyrosine phosphorylation of the EGFR [35]. The PAC1 regulation of EGFR transactivation is impaired by PACAP (6–38), gefitinib (EGFR tyrosine kinase inhibitor (TKI)), PP2 (Src inhibitor), GM6001 (MMP inhibitor) and N-acetylcysteine (NAC is an inhibitor of reactive oxygen species (ROS)). ROS are products of the electron transport chain and numerous enzymes including NADPH oxidases (Nox) [41]. ROS inactivate protein tyrosine phosphatases (PTP) impairing degradation of phosphotyrosine [20]. Alternatively, Cys797 of the EGFR reacts with ROS to form sulfenic acid after recruitment of Nox enzymes resulting in increased EGFR tyrosine kinase activity [14,37]. Here the effects of ROS inhibitors were investigated on NSCLC cells.
PACAP-38 addition to NSCLC cells significantly increased ROS which was inhibited by NAC or diphenyleneiodonium (DPI). DPI inhibited the ability of PACAP-38 to transactivate the EGFR and HER2. PACAP-27 stimulated whereas PACAP (6–38) or DPI inhibited the growth of NSCLC cells. By RT-PCR, Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2 mRNA was present in most NSCLC cell lines tested. By Western blot, Nox2, Nox3 and Nox4 protein was present in NSCLC cells. Because DPI inhibits all Nox and Duox enzymes, it may be a useful inhibitor of NSCLC proliferation.
2. Materials and methods
2.1. Cell Culture.
Human NSCLC cell lines were adherent and were split 1:10 weekly with trypsin/EDTA after washing in PBS. The cells were cultured in a T175 flask in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine serum (FBS Invitrogen, Grand Island, NY). The cells were used when they were in exponential growth phase after incubation at 37°C in 5%CO2/95% air.
2.2. RT-PCR.
Total RNA was isolated from 10 human NSCLC cell line using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA samples were treated with DNase Digestion (Qiagen, Valencia, CA, USA) to remove contaminating DNA, and total RNA (1 μg) was reverse transcribed using a SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen,Waltham, MA, USA) according to the manufacturer’s instructions for complementary DNA (cDNA) synthesis. PCR amplifications for Nox and Duox were performed using the HotStarTaq® Master Mix Kit (Qiagen) following the manufacturer’s instructions. Amplification conditions for PCR-reactions included an initial cycle of 95 °C for 15 min, followed by 35-cycles of denaturation at 94 °C for 30s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min. After the final-cycle, all PCR-reactions concluded with a 10 min extension at 72 °C. The primer sequences were listed in Table 1. The PCR products were analyzed on a 3% agarose gel and visualized by ethidium bromide staining. β-actin was used as internal control. cDNA from Ht29 (colon cancer cell), human thyroid and fetal kidney were used as positive controls [7,8,49]. In addition, each PCR included water only and no RT samples (fetal kidney and thyroid) as negative controls. At least one PCR product for each primer was extracted from agarose gel using a QIAquick Gel Extraction Kit (Qiagen) and sequenced at Eurofins Genomics (Louisville, KY, USA).
Table 1.
Primers used in RT-PCR
| Gene | Forward (5’-3’) | Reverse (5’-3’) | Size (bp) |
|---|---|---|---|
| Nox 1 | TTGTTTTGAGGTTGTGGTCTGC | GAACAACTATATTTCTGGGAGGATGTTTGA | 242 |
| Nox 2 | ACATTTTTGTCAAGTGCCCAAAGG | CCATTTTTCCTGAACTCATATACGTTGGTA | 230 |
| Nox 3 | AGAGTAAGAGATCAGCAAACCACTC | AT CACAGTGTGACTGTTTGTTCACT | 228 |
| Nox 4 | GCAGAGGCTGACCTCATAGTTC | TTTGCTACTCCTTTAGGCTAATCACTATG | 226 |
| Nox 5 | GGTCACTATGGACCTCCTTTCC | TGGACTTGCCAGCAGAGTG | 234 |
| Duox1 | CACTGGGCTTGTCTCCCATT | ATCCTGATATGTAATATTGGGAAAAGTGATCTG | 228 |
| Duox2 | GGGAGGCGAAGACATACATGAT | TTTCTAGCCTTCAGGACAAGCC | 226 |
| β-actin | CCTCGCCTTTGCCGATCC | GGAATCCTTCTGACCCATGC | 205 |
2.3. Western Blot.
The ability of PACAP-38 (Bachem Inc., Torrence, CA) to stimulate tyrosine phosphorylation of the EGFR, HER2 or ERK (p42/p44 MAP kinase) was investigated by Western blot. NCI-H838 or A549 cells were placed in 10 cm dishes. After they became confluent they were placed in SIT media for 3 hr. Routinely, NSCLC cells were treated with DPI, NAC (Sigma-Aldrich, St. Louis, MO) gefitinib or PACAP (6–38) (Tocris Bioscience, Bristol, UK), for 30 minutes. Then cells were incubated with 0.1 μM PACAP-38 for 2 min, washed twice with PBS and treated with 0.5 ml of lysis buffer. The lysate was sonicated for 5 s at 4°C and centrifuged at 10,000 x g for 15 min. Protein concentration was determined using the BCA reagent (Pierce Chemical Co., Rockford, IL), and 600 μg of protein was incubated with 4 μl of anti-phosphotyrosine (BD Biosciences), 1 ul of anti-EGFR or 1 ul of anti-HER2 (Cell Signaling Technologies, Danvers, MA). and 15 μl of immobilized protein A/G PLUS agarose added (Santa Cruz Biotech, Santa Cruz, CA) overnight at 4°C. The immunoprecipitates were washed 3 times with RIPA buffer and fractionated using 4–20% polyacrylamide gels (Novex, San Diego, CA). Proteins were transferred to nitrocellulose membranes and after washing the blot was incubated with enhanced chemiluminescence detection reagent for 5 min and exposed to Biomax XAR film (Carestream, Rochester, NY). The band intensity was determined using a densitometer.
Alternatively, 40 μg of cellular extract was loaded onto a 15 well 4–20% polyacrylamide gels. After transfer to nitrocellulose, the blot was probed with anti PY1068-EGFR, anti EGFR, anti PY204ERK, anti ERK, anti PY1248-HER2, anti-HER2, anti-tubulin (Cell Signaling Technologies, Danvers, MA), anti-Nox2, anti-Nox3 or anti-Nox4 (Abcam).
2.4. Reactive oxygen species.
NCI-H838 cells were placed in black 96 well plates (30,000 cells/well) and cultured overnight. The cells were treated with 10μM dichlorofluorescein diacetate (H2DCF) for 1 h and washed 3 times with serum- free SIT medium. Some of the cells were treated with DPI or NAC for 30 min and then stimuli such as 10 nM PACAP-38 or 10 μM H2O2 added. Fluorescence measurements were taken at the various times using an excitation wavelength of 485 nm and emission wavelength of 585 nm.
2.5. Proliferation.
Growth studies in vitro were conducted using the 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO) and clonogenic assays. In the MTT assay, NCI-H838 or A549 cells were placed in SIT medium and various concentrations of DPI added (Sigma-Aldrich, St. Louis, MO) added. After 2 days, 15 μl of 0.1 % MTT solution was added. After 4 h, 150 μl of dimethylsulfoxide was added and the optical density at 570 nm was determined. In the clonogenic assay, the effects of PACAP-38, PACAP (6–38), DPI or gefitinib were investigated on NCI-H838 cells. The bottom layer contained 0.5% agarose in SIT medium containing 5% FBS in 6 well plates. The top layer consisted of 3 ml of SIT medium in 0.3% agarose, PACAP-38, PACAP (6–38), DPI, lapatinib and/or gefitinib using 5 × 104 lung cancer cells. Triplicate wells were plated and after 2 weeks, 1 ml of 0.1% p-iodonitrotetrazolium violet was added and after 16 hours at 37°C, the plates were screened for colony formation; the number of colonies larger than 50 μm in diameter were counted using an Omnicon image analysis system.
2.6. Statistical analysis
The results are expressed as means ± S.D. Differences among the means were determined by a two-tailed Student’s t-test (paired or unpaired) or a one-way ANOVA followed by Student-Newman-Keuls posthoc analysis.
3. Results
3.1. EGFR, ERK and HER2
PACAP-38 (100 nM) addition to NCI-H838 cells increased tyrosine phosphorylation of the EGFR (Mr = 170 kDal), HER2 (Mr = 190 kDal), and ERK (Mr = 36–40 kDal) by 377, 299 and 216 %, respectively (Fig. 1A). PACAP had no effect on total α/β Tubulin (Mr = 56 kDal). In contrast, 0.1 or 1 μM DPI (an inhibitor of Nox and Duox) inhibited significantly the ability of PACAP to increase tyrosine phosphorylation of EGFR, HER2 and ERK (Fig.1B). Similar results were obtained using A549 cells (data not shown). Previously, we demonstrated that NAC (antioxidant) or Tiron (superoxide scavenger), impaired the ability of PAC1 to transactivate the EGFR [35]. These results indicate that ROS are essential for PAC1 to transactivate RTK in NSCLC cells.
Fig. 1.
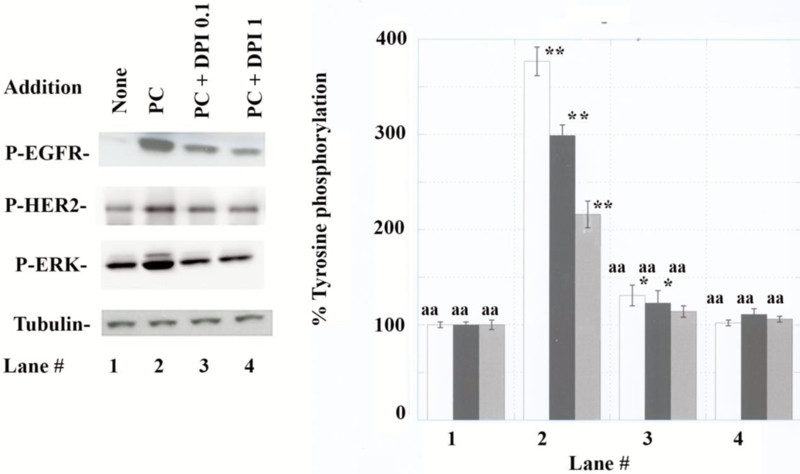
EGFR, HER2 and ERK tyrosine phosphorylation. (A) P-EGFR, P-HER2, P-ERK and tubulin were determined 2 min after addition of drugs. (B) The P-EGFR (white), P-HER2 (black) and P-ERK (gray) were determined after no additions (lane 1), 100 nM PACAP-38 (lane 2), PC + 0.1 μM DPI (lane 3) or PC + 1μM DPI (lane 4) to NCI-H838 cells. The mean value ± S.D. of 4 determinations is indicated; p < 0.05, *; p < 0.01, ** relative to lane 1 by ANOVA; p < 0.01 aa, relative to lane 2 by ANOVA.
TGFα is released from NSCLC cells after addition of PACAP-27 [35]. TGFα binds with high affinity to the EGFR but not HER2 [21]. While HER2 has no known ligand, it can form heterodimers with the EGFR resulting in increased tyrosine phosphorylation [36]. Figure 2A shows that when NCI-H838 lysis extracts are immunoprecipitated with anti-HER2, PACAP-38 in a dose-dependent manner increased P-EGFR and total EGFR. Using 100 nM PACAP-38, P-EGFR and EGFR significantly increased to 350 and 422% (Fig. 2B). Similarly, if NCI-H838 extracts are immunoprecipitated with EGFR, PACAP-38 increased P-HER2 and HER2 in a dose-dependent manner. PACAP-38 (100 nM) maximally increased P-HER2 and HER2 to 215 and 238%, respectively. These results suggest that PACAP increases the formation of EGFR and HER2 heterodimers in NSCLC cells.
Fig. 2.
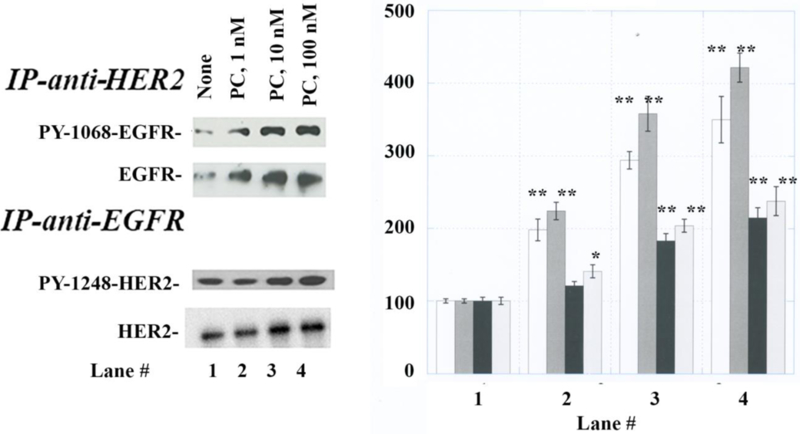
Immunoprecipitation experiments. (A) NCI-H838 cells were incubated with no additions (lane 1), 1 nM PACAP-38 (lane 2), 10 nM PACAP-38 (lane 3) or 100 nM PACAP-38 (lane 4) for 2 min and the lysates immunoprecipitated with anti-HER2 or anti-EGFR. (B) The immunoprecipitates analyzed for PY1068-EGFR (white), EGFR (dark grey), PY1248-HER2 (black) and HER2 (light grey) The mean value ± S.D. of 4 determinations is indicated; p < 0.05, *; p < 0.01, ** relative to lane 1.
The effects of gefitinib were investigated using A549 cells (Fig. 3A). Gefitinib, an EGFR tyrosine kinase inhibitor (TKI) impaired the ability of 100 nM PACAP-38 to increase the tyrosine phosphorylation of EGFR, HER2 and ERK to 385, 314 and 189%, respectively (Fig. 3B). Gefitinib, an EGFR tyrosine kinase inhibitor (TKI) decreased significantly the ability of PAC1 to regulate EGFR. ERK and HER2 transactivation. As a control PACAP-38 or Gefitinib had no effect on Tubulin. The results demonstrate that the EGFR tyrosine phosphorylation is essential for the tyrosine phosphorylation of HER2 and ERK.
Fig. 3.
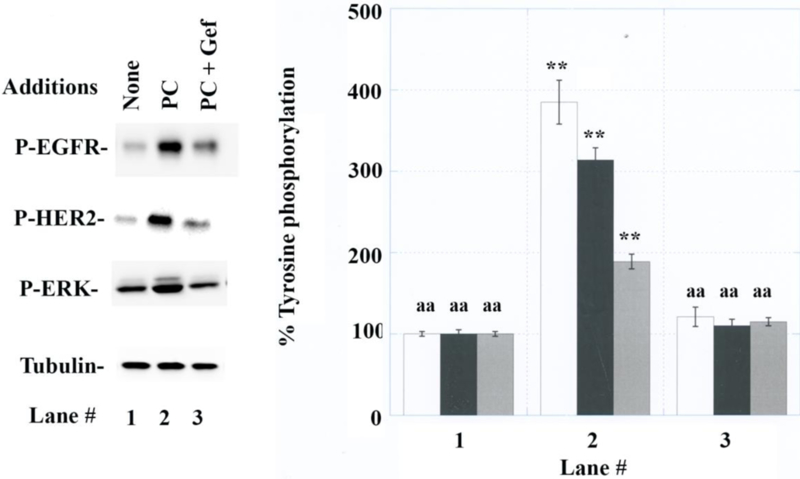
Effects of gefitinib. (A) Gefitinib (1 ug/ml) impaired the ability of PACAP-38 to increase tyrosine phosphorylation of EGFR (white), HER2 (black) and ERK (grey) using A549 cells. (B) A549 cells were treated with no additions (lane 1), 100 nM PACAP-38 (lane 2) or PACAP + 1 μg/ml gefitinib (lane 3). The mean value ± S.D. of 4 experiments in indicated; p < 0.01, ** relative to lane 1; p < 0.01, aa relative to lane 2.
3.2. ROS.
The ability of PACAP to increase ROS was investigated. Table II shows that PACAP increased significantly ROS levels by 51% after addition to NCI-H838 cells. The increase caused by PACAP-38 was inhibited by addition of DPI or NAC. As a control, NAC or DPI had little effect on basal ROS, where H2O2 strongly increased ROS. The increase in ROS caused by PACAP was inhibited significantly by NAC or DPI.
Table II.
Reactive oxygen species
| Addition | % Fluorescence intensity |
|---|---|
| None | 100 ± 5 |
| PACAP-38, 100 nM | 151 ±19* |
| PACAP-38 + 5 mM NAC | 103 ± 11 |
| PACAP-38 + 1000 nM DPI | 95 ± 5 |
| DPI | 98 ± 7 |
| NAC | 101 ± 6 |
| H2O2, 10 μM | 367 + 27** |
The relative fluorescence was determined 0.5 hr after the addition of PACAP-38 or H2O2 to H2DCF labeled NCI-H838 cells. DPI was added 0.5 h before the addition of PACAP-38 or H2O2. The mean value ± S.D. of 8 determinations is indicated. This experiment is representative of 4 others;
p <0.05
p < 0.01 relative to no additions; by ANOVA.
3.3. Nox and Duox
The expression of Nox and Duox was investigated in 10 lung cancer cell lines. Fig. 4 shows that several cell lines including NCI-H28 (mesothelioma), H358 (bronchoalveolar) and A549 (adenocarcinoma) had mRNA for Nox1, Nox2, Nox3, Nox4, Nox5, Duox1and Duox2. NCI-H838 (adenocarcinoma) and H727 (carcinoid) had mRNA for Nox1, Nox3, Nox4, Nox5, Duox1 and Duox2. NCI-H520 (squamous) had mRNA for Nox1, Nox5 and Duox1. NCI-H2122 (adenocarcinoma) had mRNA for Duox1 and Duox2. NCI-H322 (bronchoalveolar) had mRNA for Nox2 and Duox1. NCI-H1299 (large cell carcionoma) had mRNA for Nox1. For each of the 7 Nox and Duox enzymes 50–80% of the cell lines were positive. Equal amounts of β-Actin were present in each of the cell lines. PCR products were not detected in the negative controls. PCR products were present in the positive controls, which were HT29 colon cancer cells, fetal kidney and normal thyroid [7,8,49]. The results indicate that lung cancer cells have an abundance of Nox and Duox enzymes which are capable of producing ROS.
Fig. 4.
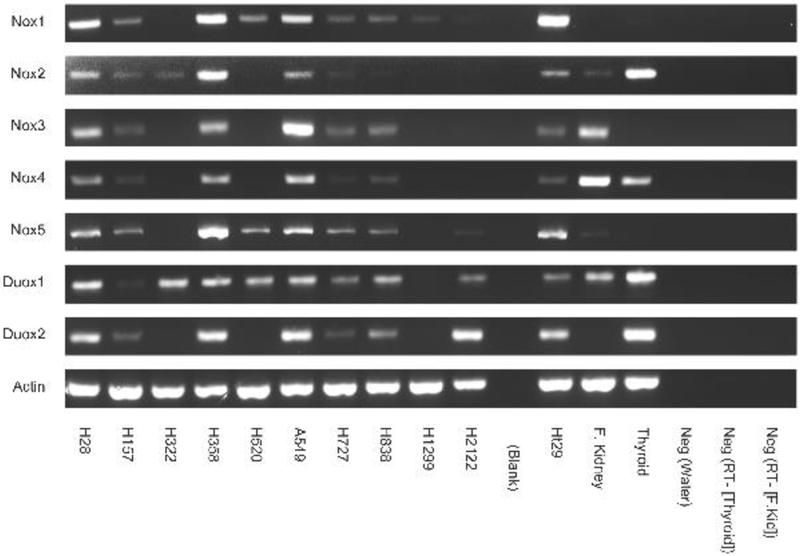
RT-PCR of Nox and Duox. RNA was isolated from frozen pellets of 10 lung cancer cells and cDNA prepared. RT-PCR was performed using the primers shown in Table I and experimental conditions described in Methods. PCR products were analyzed on a 3% agarose gel and visualized by ethidium bromide staining. HT29 (colon cancer cells), fetal kidney and human thyroid were positive controls whereas water, no RT samples (kidney and thyroid) were used as negative controls. This experiment is representative of 2 others.
Nox protein was investigated by Western blot. Figure 5 shows that Nox2, Nox3 and Nox 4 immunoreactivity was present in extracts derived from NCI-H358, A549, and NCI-H838 cells. High levels of Nox2/gp91 phox protein (Mr = 65 kDal) were present in NCI-H358, A549 and H838 cells. Nox3 was present in high concentrations in NCI-H358 (Mr = 66 kDal). Nox 4 was present in moderate concentrations in NCI-H358, A549 and NCI-H838 (Mr = 67 kDal). Equal amounts of Tubulin were present in NCI-H358, A549 and H838 cells.
Fig. 5.
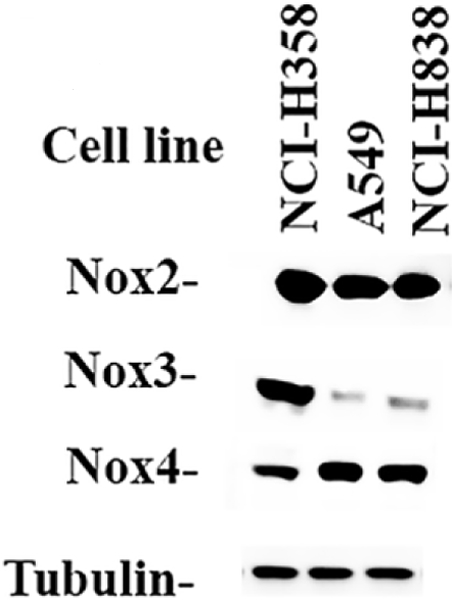
Western blot of Nox2, Nox3 and Nox4. Extracts from NCI-H358, A549 and NCI-H838 cells were analyzed for Nox2, Nox3, Nox4 and Tubulin. This experiment is representative of 3 others.
3.7. Proliferation of NSCLC cells.
The ability of PACAP and ROS inhibitors to alter NSCLC proliferation was investigated. In the clongenic assay, Table III shows that addition of PACAP-38 to NCI-H838 cells increased colony number by 72%. In contrast, PACAP (6–38) decreased colony number by 33%. Also, lapatinib or gefitinib (TKI) decreased colony number by 41 and 47%, respectively. GKT137831 (inhibits Nox1 and Nox4) or DPI decreased colony number by 44 and 57% respectively. In the MTT assay, DPI inhibited NCI-H838 and A549 growth in a dose-dependent manner. Figure 6 shows that 0.2 μM DPI had little effect, whereas at 20 μM DPI almost all growth was inhibited. The IC50 for DPI using NCI-H838 and A549 cells was 2 and 1 μM, respectively.
Table III.
Colony number.
| Addition | Colony number |
|---|---|
| None | 100 ± 6 |
| PACAP-38, 10 nM | 172 ± 16* |
| PACAP (6–38), 1000 nM | 67 ± 8* |
| Gefitinib, 1 ug/ml | 53 ± 5** |
| Lapatinib, 1 ug/ml | 59 ± 6** |
| GKT137831, 10000 nM | 56 ± 7** |
| DPI, 1000 nM | 43 + 6** |
The mean number of NCI-H838 colonies ± S.D. of 3 determinations is indicated. This experiment is representative of 2 others
p < 0.05,
p < 0.01, relative to control by ANOVA.
This experiment is representative of 3 others.
Fig. 6.
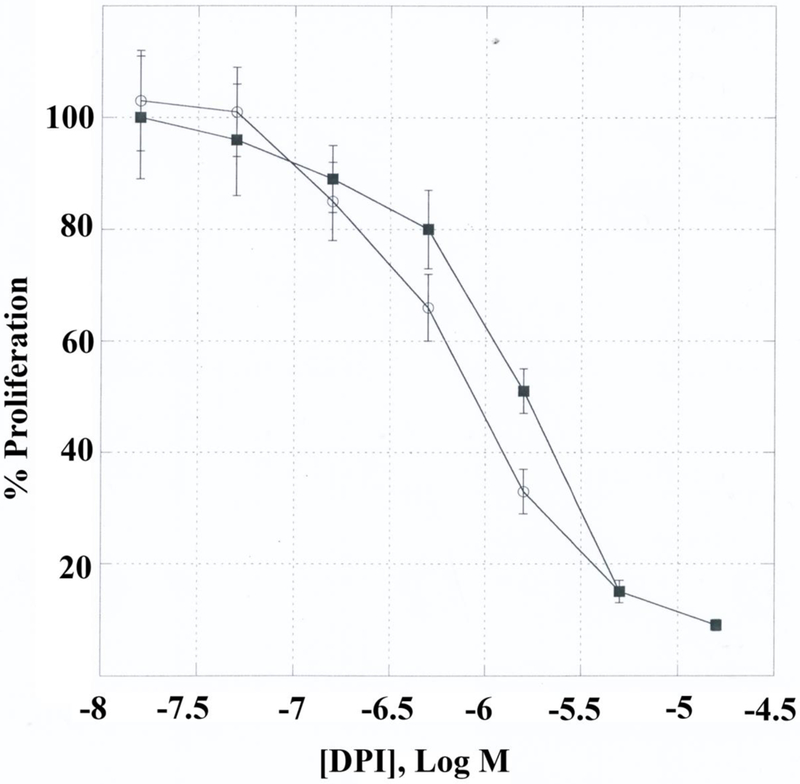
NSCLC proliferation. Using the MTT assay the growth of A549 (▀) and H838 (○) cells is indicated as a function of DPI concentration. The mean value ± S.D. of 8 determinations is indicated. This experiment is representative of 4 others.
4. Discussion
PAC1 is a class B/secretin-like GPCR which contains 468 amino acids and is localized to chromosome 7p14 [12]. It has a large N-terminal of 125 amino acids which contains antiparallel β-sheets and binds to the C-terminal of PACAP-38 [18,44]. PAC1 has an open state (G4) and 3 closed transition states (G1-G3) [23]. PAC1 splice variants (SV) have been detected in the N-terminal and intracellular loop (IL) 3 [26]. PAC1 has 18 exons and deletion of exons 5,6 or 4–6 reduces the size of the N-terminal by 7, 21 or 57 amino acids [4]. Addition of a 28 amino acid segment to IL3 results in PAC1 hip SV. Addition of a different 28 amino acid segment to PAC1null results in the PAC1 hop SV. Addition of both segments results in the PAC1 hip-hop SV [46]. PAC1 has been detected in breast, colon, liver, lung, neuroblastoma, pancreatic and prostate cancers [32]. The role of the PAC1 SV on cancer cellular proliferation in unknown.
PACAP addition to NSCLC cells increases ERK phosphorylation in a MEK-dependent manner [33]. Addition of PACAP (6–38) or PD98059 (MEK inhibitor) impaired the ability of PACAP-27 to increase ERK phosphorylation in NSCLC cells. Using HEK cells transfected with PAC1 hop1, bisindoylmaleimide x inhibited PKC activity and the ability of PACAP to cause ERK tyrosine phosphorylation [28]. Also, PAC1 internalization played a role in ERK phosphorylation. In NSCLC cells, the ability of PACAP to increase ERK phosphorylation was impaired by gefitinib. In NSCLC cells the EGFR may activate the Ras-Raf-MEK-ERK pathway leading to ERK phosphorylation after addition of PACAP to NSCLC cells.
Gefitinib impaired the ability of PACAP to increase tyrosine phosphorylation of the ERK, EGFR and HER2 in NSCLC cells. Ligands which activate HER2 have not been identified, however, it can be tyrosine phosphorylated after forming heterodimers with the EGFR [21]. PACAP-38 addition to NSCLC cells increased tyrosine phosphorylation of the EGFR and HER2 4- and 3-fold, respectively. The increase in EGFR transactivation caused by PACAP-38 was impaired by PACAP (6–38) or gefitinib [35]. Also, PACAP-38 increased EGFR and HER2 phosphorylation in a dose-dependent manner. When NSCLC lysates were immunoprecipitated with anti-HER2, P-EGFR was increased in a dose-dependent manner by PACAP-38. Similarly, when NSCLC lysates were immunoprecipitated with anti-EGFR, P-HER2 increased in a dose-dependent manner by PACAP-38. These results suggest that P-HER2 and P-EGFR form heterodimers after addition of PACAP-38 to NSCLC cells. Addition of VIP to breast cancer cells caused transactivation of the EGFR and HER2 [47]. Addition of PACAP-38 to PC12 pheochromocytoma cells increased tyrosine phosphorylation of TrkA [19, 39]. Addition of K252a (TKI inhibitor) or PP1 (Src inhibitor) impaired the ability of PACAP-38 to increase TrkA and Akt phosphorylation. Addition of PACAP-38 to PC12 cells promoted survival and neuritogenesis in a NF-kB-dependent manner [29]. The results indicate that PAC1 regulates the transactivation of numerous RTK.
ROS are essential for EGFR tyrosine phosphorylation to occur [21]. ROS (hydrogen peroxide or superoxide) can be produced by Nox and Duox enzymes. NSCLC cells, such as A549, have NOX1–5 and Duox 1–2 mRNA. The presence of Nox2, Nox3 and Nox4 protein was confirmed by Western blot. Knockdown of Nox1 in HT-29 colon cancer cells reduced ROS and ERK reducing cellular proliferation [16]. Toll-like receptor signaling which increases NSCLC metastasis is enhanced by overexpression of Nox1 [25]. Previously, Nox1, Nox2, Nox4, Nox5, Duox1 and Duox2 were detected by quantitative RT-PCR on A549 cells [17]. Nox4 expression was increased by addition of TGFβ to NCI-H1299 cells [5]. In A549 cells, Nrf2 expression was associated with decreased Nox4 [50] whereas Nox 4 promotes PI3K activity [51]. Nox5 immunoreactivity was detected inUACC-257 melanoma cells and biopsy specimens from breast, colon, lung, ovarian cancer patients [2]. Nox overexpression is associated with increased lung cancer cellular invasion and migration [13]. Duox1 silencing in lung cancer A549 cells promotes epithelial-mesenchymal transitions in and invasive properties [24]. Many lung cancer cells are hypermethylated resulting in a reduction of Doux1 and Duox2 [27]. A surprising finding is that high levels of Nox3 mRNA and protein are present in NCI-H358, H838 and A549 cells. The results indicate that Nox and Duox enzymes are abundant in NSCLC cells resulting excessive production of ROS.
The advantage of DPI is that it inhibits all Nox and Duox enzymes by formation of a covalent complex with the flavin-containing component of the oxidase [9]. DPI inhibited the proliferation of colon cancer HT-29 cells in a dose-dependent manner by reducing ROS. DPI decreased phosphorylation of STAT1, STAT3, ERK and Akt induced by interleukin (IL) addition to HT29 cells. Using lung cancer cells, DPI in a dose-dependent manner inhibited the ability of PACAP-38 to increase EGFR, HER2 and ERK tyrosine phosphorylation. Also, DPI inhibits the ability of PACAP-38 to increase ROS in NSCLC cells.
High levels of Nox1 and Nox2 but not Nox3 mRNA are present in colorectal cancer cell line such as HT-29 [15]. A surprising result is that Nox 3 mRNA was detected in 50% of the lung cancer cell lines tested and cell line A549 had high levels of Nox3 mRNA. In general, lung cancer cells lines have an abundance of Nox and Duox enzymes which can generate ROS. By Western blot, Nox2, Nox3 and Nox4 proteins were detected in 3 lung cancer cell lines tested including cell line NCI-H358, H838 and A549. DPI inhibits the growth of NCI-H838 cells and impairs the ability of PAC1 to regulate EGFR transactivation. Similar results were obtained using GKT137831 which is a selective inhibitor for Nox and Nox4 [1]. ROS and proliferation of malignant mesothelioma cells was impaired by DPI and Nox4 siRNA [45]. Precision medicine techniques may be required to determine which Nox enzymes are important in lung cancer.
In summary, addition of PACAP-38 to NSCLC cells increased tyrosine phosphorylation of the EGFR, ERK and HER2. The increase in tyrosine phosphorylation of EGFR, ERK and HER2 caused by PACAP-38 was inhibited by PACAP (6–38) and DPI. DPI inhibited the ability of PACAP-38 to increase ROS species in NSCLC cells. PACAP-38 stimulated the growth of NSCLC cells whereas PACAP (6–38), gefitinib and DPI inhibited the growth of NSCLC cells. The results indicate that PAC1 regulates the transactivation of EGFR and HER2 in a ROS- dependent manner.
Highlights.
PACAP-38 addition to NSCLC cells causes tyrosine phosphorylation of EGFR, HER2 and ERK.
PAC1 transactivation of the EGFR or HER2 is inhibited by diphenyleneiodonium (DPI), a Nox and Duox inhibitor.
PACAP-38 stimulates the production of ROS which is inhibited by DPI.
PACAP-38 stimulates the growth of NSCLC cells, whereas PACAP (6–38), gefitinib and DPI inhibit growth.
Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2 mRNA is present in many lung cancer cells.
Acknowledgments
This research is supported by the intramural program of NIDDK, NIAID and NCI of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Altenhofer S, Radermacher KA, Kleikers PWM, Wingler K, Schmidt HHHW. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxidants and Redox Signaling 2015; 23: 406–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anthony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G et al. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 2013; 65: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arimura A Pituitary adenylate cyclase activating polypeptide (PACAP): Discovery and current status of research. Regul Pept 1993; 37: 287–303. [PubMed] [Google Scholar]
- [4].Blechman J, Levkowitz G. Alternative splicing of pituitary adenylate cyclase-activating polypeptide receptor PAC1. Mechanisms of fine tuning of brain activity. Front Endocrinol (Lusanne) 2013; 4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-B-mediated migration of human lung and breast epithelial cells. Br J Cancer 2014; 110: 2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buscail L, Cambillau C, Seva C, Scemama JL, DeNeef P, Robberecht P et al. Stimulation of rat pancreatic tumoral AR4–2J cell proliferation by pituitary adenylate cyclase- activating polypeptide. Gastroenterology 1992; 103:1002–8. [DOI] [PubMed] [Google Scholar]
- [7].Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4 and Nox 5. Gene 2001; 269: 131–40. [DOI] [PubMed] [Google Scholar]
- [8].De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 2000; 275: 23227–33. [DOI] [PubMed] [Google Scholar]
- [9].Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu Jiamo, Antony S. et al. Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem Pharmacol 2012; 83: 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Draoui M, Hida T, Birrer M, Zia F, Moody TW. PACAP stimulates c-fos mRNAs in small cell lung cancer cells. Life Sci. 1996; 59:307–13. [DOI] [PubMed] [Google Scholar]
- [11].Germano PM, Lieu SN, Xue J, Cooke HJ, Christoff GL, Ly Y, Pisegna JR. PACAP induces signaling and stimulation of 5-hydroxytryptamine release and growth in neuroendocrine tumor cells. J Mol Neurosci 2009; 39:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna J et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase- activating polypeptide: IUPHAR review 1. Br J Pharmacol 2012; 166: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han M, Zhang T, Yang L, Wang Z, Ruan J, Cheng X. Association between NADPH oxidase (NOX) and lung cancer: A systematic review and meta-analysis. J Thorac Dis 2016; July i(7): 1704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heppner DE, Van der Vliet A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol 2016; 8: 24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J et al. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radical Res 2009; 43: 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Juhasz A, Markel S, Gaur S, Liu H, Lu J, Jiang G et al. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. J Biol Chem 2017; 292: 7866–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kolarova H, Bino L, Pejchalova K, Kubala L. The expression of NADPH oxidases and production of reactive oxygen species by human lung adenocarcinoma epithelial cell line A549. Folia Biol 2010; 56: 211–7. [DOI] [PubMed] [Google Scholar]
- [18].Kumar S, Ploszak A, Zhang C, Swaminathan K, Xu HE. Crystal structure of the PACC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. Plos ONE (2011) 6: e19682 Doi: 10.11371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotropin receptor signaling by pituitary adenylate cyclase-activating polypeptide. J Biol Chem 2002; 277: 9096–102. [DOI] [PubMed] [Google Scholar]
- [20].Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 1998; 273: 15366–72. [DOI] [PubMed] [Google Scholar]
- [21].Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: Not so prototypical receptor tyrosine kinase. Cold Spring Harbor Perspect Biol 2014; 6: a020768 Doi: 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leyton J, Coelho T, Coy DH, Jakowlew S, Birrer MJ, Wank S, Moody TW. PACAP (6–38) inhibits the growth of prostate cancer cell lines. Cancer Let. 1999; 125:131–139. [DOI] [PubMed] [Google Scholar]
- [23].Liao C, Zhao X, Brewer M, May V, Li J. Conformational transitions of the pituitary adenylate cyclase-activating polypeptide receptor, a human class B GPCR. Scientific Reports 2017; 7: 5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Little AC, Sham D, Hristova M, Danjal K, Hellner DE, Bauer RA et al. Duoxl silencing in lung cancer promotes EMT, cancer stem cell characteristics and invasive properties. Oncogenesis 2016; 5:e261.doi. 10.1038/oncsis.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X, Pei C, Yan S, Liu G, Liu G, Chen W et al. NADPH oxidase 1-dependent ROS is crucial for TLR4 signaling to promote tumor metastasis of non-small cell lung cancer. Tumour Biol 2015; 36: 1493–502. [DOI] [PubMed] [Google Scholar]
- [26].Lutz EM, Ronaldson E, Shaw P, Johnson MS, Holland PJ, Mitchell R Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: Consequences for signaling by VIP and PACAP. Mol Cell Neurosci 2006; 31:193–209. [DOI] [PubMed] [Google Scholar]
- [27].Luxen S, Belinski SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 2008; 68: 1037–45 [DOI] [PubMed] [Google Scholar]
- [28].May V, Clason TA, Buttolph TR, Girard BM, Patsons RL. Calcium influx, but not intracellular calcium release, supports PACAP-mediated ERK activation in HEK PAC1 receptor cells. J Mol Neurosci 2014; 54: 342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Manecka DL, Mahmood SF, Grumolato L, Lihrmann I, Anouar Y. Pituitary adenylate cyclase-activating polypeptide (PACAP) promotes both survival and neuritogenesis in PC12 cells through activation of nuclear factor KB (NF-kB) pathway: Involvement of extracellular signal regulated kinase (ERK), calcium and c-REL. J Biol Chem 2013; 288: 14936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miyata A, Arimura A, Cahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue hypothalamic polypeptide which stimulated adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989; 164:567–74. [DOI] [PubMed] [Google Scholar]
- [31].Moody TW, Jensen RT. VIP/PACAP receptors “Handbook of Biologically active peptides,” second edition Kastin A (Ed.), Elsevier Press; (2013) 556–61. [Google Scholar]
- [32].Moody TW, Jensen RT. PACAP and Cancer In “Pituitary adenylate cyclase activating polypeptide-PACAP”. Reglodi D, Tamas A (Eds.) Basel, Springer; (2016) 795–814. [Google Scholar]
- [33].Moody TW, Leyton J, Casibang M, Pisegna J, Jensen RT. PACAP-27 tyrosine phosphorylates mitogen activated protein kinase and increases VEGF mRNAs in human lung cancer cells. Reg. Peptides 2002; 109:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moody TW, Nuche-Berenguer B, Jensen RT. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide and their receptors in cancer. Curr Opin Endocrinol Diabetes Obes 2016; February 23: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moody TW, Osefo N, Nuche-Berenguer B, Ridnour L, Wink D, Jensen RT. Pituitary adenylate cyclase activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells. J Pharmacol Exp Ther 2012; 34: 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moody TW, Nuche-Berenguer B, Nakamura T, Jensen RT. EGFR transactivation by peptide G protein-coupled receptors in cancer. Current Drug Targets 2016; 17:520–8. [DOI] [PubMed] [Google Scholar]
- [37].Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE et al. Peroxide- dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol 2012; 8: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pisegna JR, Wank S. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J. Biol. Chem. 1996; 271: 17267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rajagopal R, Chem ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci 2004; 24: 6650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reubi JC, Laderach U, Waser B, Gebbers JD, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 2000; 60:3105–12. [PubMed] [Google Scholar]
- [41].Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G et al. NADPH oxidases and cancer. Clin Sci 2016; 128: 863–75. [DOI] [PubMed] [Google Scholar]
- [42].Said SI, Mutt V. Polypeptide with broad biological activity: Isolation from small intestine. Science 1970; 169: 1217–18. [DOI] [PubMed] [Google Scholar]
- [43].Schrump DS, Carter D, Kelsey CR, Marks LB, Giaccone G. Non-small cell lung cancer In: DeVita V Jr, Lawrence TS, Rosenber SA Editors, Cancer: Principles and practice on oncology, Philadelphia, Lippincott, Williams & Wilkins; (2011) 799–847. [Google Scholar]
- [44].Sun C, Song D, Davis-Taber R, Barrett LW, Scott V, Richardson PL et al. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to extracellular domain of PAC1-Rs. Proc Natl Acad Sci USA 2007; 104: 7875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tanaka M, Miura Y, Numanami H, Karnan S, Ota A, Konishi H et al. Inhibition of NADPH oxidase 4 induces apoptosis in malignant mesothelioma: Role of reactive oxygen species. Oncol Rep 2015; 34: 1726–32 [DOI] [PubMed] [Google Scholar]
- [46].Usiyama M, Ikelda R, Sugawara H, Yoshida M, Mori K, Kangawa K et al. Differential molecular signaling through PAC1 isoforms as a result of alternative splicing in the first extracellular domain and the third intracellular loop. Mol Pharmacol 2007; 72: 103–11. [DOI] [PubMed] [Google Scholar]
- [47].Valdehita A, Bajo AM, Schally AV, Vargfa JL, Carmena MJ, Prieto JC. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol Cell Endocrinol; 2009; 302:41–8. [DOI] [PubMed] [Google Scholar]
- [48].Vaudry D, Falluel Morel A, Bourgault S, Basille M, Burel D, Wurtz O et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharm. Rev. 2009; 61: 283–357 [DOI] [PubMed] [Google Scholar]
- [49].Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase Nox4 in normal and cancer thyroid tissues. Endocr Relat Cancer 2010; 17: 27–37. [DOI] [PubMed] [Google Scholar]
- [50].Wu Q, Yao B, Li N, Deng Y, Yang Y Zeng C et al. Nrf2 mediates redox adaptation in NOX4-overexpressed non-small cell lung cancer cells. Exp Cell Res 2017; 352: 245–54. [DOI] [PubMed] [Google Scholar]
- [51].Zhang C, Lan T, Hou J, Li J, Fang R, Zhang M et al. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation. Oncotarget 2014; 5: 4392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zia F, Fagarason M, Bitar K, Coy DH, Pisegna JR, Wank SA et al. Pituitary adenylate cyclase activating polypeptide receptors regulate the growth of non-small cell lung cancer cells. Cancer Res 1995; 55: 4886–91. [PMC free article] [PubMed] [Google Scholar]


