Abstract
Objective:
In this longitudinal multicenter cohort study, we evaluated the potential of a dedicated electrical impedance myography (EIM) device to assess ALS progression and the system’s basic reproducibility and diagnostic accuracy.
Methods:
Forty-six ALS patients underwent up to 5 sequential measurements of multiple muscles over a period of 8 months at 2-month intervals using the mView EIM device (Myolex, Inc). Standard measures of disease status were also obtained. A group of 30 healthy volunteers and 30 ALS-mimics were evaluated once to determine if the technique could assist with initial diagnosis. Several electrode arrays and EIM outcomes were assessed.
Results:
EIM tracked ALS progression; power analyses suggested a 5.2-fold reduction in sample size requirements compared to ALSFRS-R by utilizing 50 kHz phase value from the muscle with the greatest EIM decline in each subject. This progression rate correlated to total ALSFRS-R progression, with R=0.371, p=0.021. Reproducibility was high, with both intra- and inter-rater intraclass correlation coefficients for individual muscles mostly greater than 0.90. The mean 50 kHz phase distinguished between ALS patients and healthy controls (area-under-curve 0.78, 95% confidence intervals (CIs) 0.68,0.89), but not between mimics and ALS patients (area-under-curve 0.60, 95% CIs 0.47,0.73).
Conclusions:
While limited in its specificity to identify ALS versus disease mimics, these results support the hypothesis that single-muscle EIM can serve as a convenient, repeatable, and powerful outcome measure in ALS clinical trials.
Keywords: electrical impedance myography, biomarker, clinical trials, ALS, outcomes
INTRODUCTION
In recent ALS clinical trials, outcome measures have included survival [1], the ALS functional rating scale-revised (ALSFRS-R) [2], or a combination of the two [3]. A variety of secondary measures including handheld dynamometry (HHD), vital capacity, and several electrophysiological measures, including motor unit number estimation have also been used [4].
Electrical impedance myography (EIM) has shown promise as biomarker in assessing ALS progression [5]. Unlike standard electrophysiological measures, EIM involves application of a weak, high frequency electrical current that is passed between two outer electrodes with resulting voltages measured between two inner electrodes [5]. Alterations in muscle structure, including atrophy and fat infiltration, are detected as a change in muscle impedance. Two previous longitudinal clinical studies have demonstrated high sensitivity of the technique with respect to ALS progression when the 50 kHz phase value was assessed [6,7]. However, those studies utilized off-the-shelf impedance systems and adhesive electrodes not intended for this specific application.
Here we utilize the first dedicated multifrequency EIM system, the mView® (Myolex, Inc, San Francisco, CA) for the assessment of ALS progression [8]. We compare these results to standard measures of disease progression including vital capacity, HHD, and ALSFRS-R. In addition, we study whether EIM can distinguish ALS patients from healthy controls and disease mimics.
METHODS
Standard Protocol Approvals, Registrations, and Patient Consents:
Subjects were recruited from 6 clinical sites (Myolex, Inc., Barrow Neurological Institute, University of Miami, Wake Forest University, Massachusetts General Hospital, and Upstate Medical Center), over 28 months. Each individual institutional review board approved the study, and written informed consent was obtained from all participants. The study was registered with Clinicaltrials.gov (NCT02011204). Needed population size was based on the previous EIM in ALS longitudinal study [9]. The inclusion/exclusion criteria for each cohort were as follows:
ALS:
Inclusion: 1) Age 35–80 years; 2) ALS diagnosed as possible, probable, probable-laboratory supported, or definite as defined by revised El Escorial criteria [10]; 3) Symptom onset of ALS ≤36 months; 4) Slow vital capacity (SVC) ≥60% of predicted. Exclusion: Unstable psychiatric disease, cognitive impairment, or substance abuse.
ALS-Mimics:
Inclusion: 1) Age 35–80 years, 2) Diagnosis of multi-focal motor neuropathy, autoimmune motor neuropathy, cervical or lumbosacral radiculopathies with clinical weakness involving more than one extremity, Charcot-Marie-Tooth Disease, or any condition producing generalized or localized weakness without concomitant sensory symptoms. Exclusion: 1) Diagnosis of ALS or family history of ALS, 2) Unstable psychiatric disease, cognitive impairment, or substance abuse.
Healthy controls:
Inclusion: 1) Age 35–80 years; 2) Absence of known neurological disorder. Exclusion: 1) History of ALS, myopathy, neuropathy, ALS mimic disorder or other neurodegenerative disease; 2) Family history of ALS, 3) Unstable psychiatric disease, cognitive impairment, or substance abuse.
Study procedures:
After obtaining consent, all subjects underwent a screening and baseline evaluation. Mimics and healthy patients were only evaluated once. ALS patients returned for follow-up visits approximately every 2 months for up to 8 months after the baseline visit for a maximum of 5 visits.
EIM:
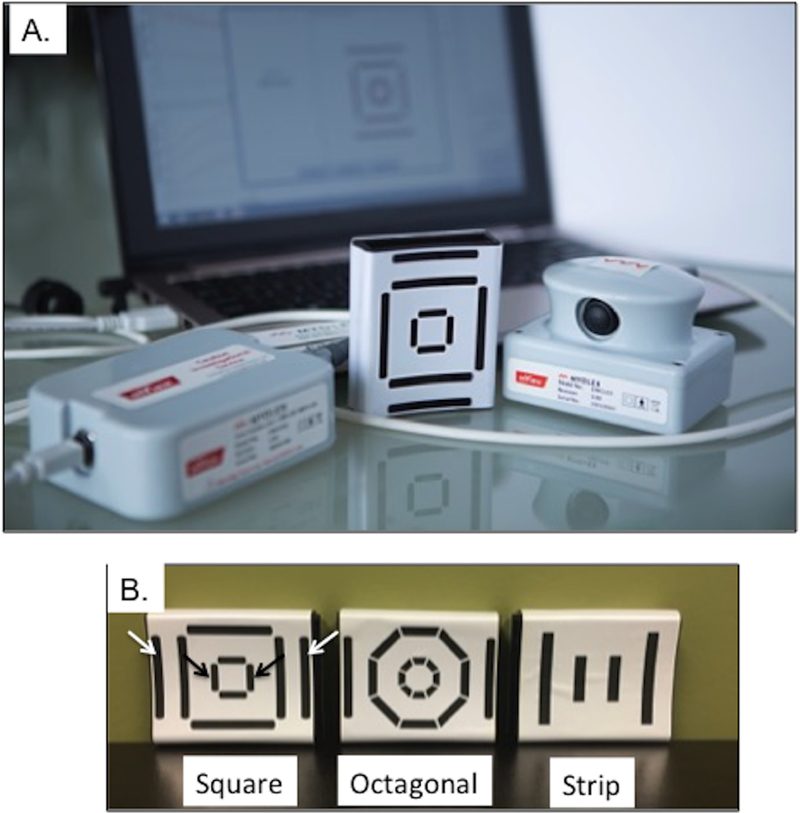
EIM was performed using the Myolex mView®. A handheld scanner attaches to a disposable electrode array and is connected to a laptop computer (see Figure 1A). The system provides a set of 41 discrete frequencies between 1 kHz and 10 MHz. To determine optimum efficacy, 3 different electrode array designs were included in the study (Figure 1B) —a “strip” design which provided a single output; a “square” design based on work that demonstrated that a greater distance between the voltage and current electrodes would provide better muscle penetration, and an “octagonal” design with smaller electrode surfaces that could provide more detailed orientation-dependent data. For each electrode array design, one or more stimulus configurations were employed (1 for the strip design, 3 for the square design, and 4 for the octagonal design).
Figure 1.
A. mView® EIM system and B. The three electrode arrays (i.e., square, octagonal, and strip) used in the study. Black arrows in the “square” sensor (far left) show the current electrodes and white arrows show the corresponding voltage measuring electrodes that provided the best outcome (lowest CoVRD).
EIM measurements were performed bilaterally on quadriceps, tibialis anterior, gastrocnemius, wrist extensor compartment, biceps, and deltoid, as well as the right thoracic paraspinal muscles at approximately T3–5. For each measurement, the surface of the skin overlying the muscle was moistened with saline and the device was moved over the muscle until good contact was confirmed via an automated detector. The device was briefly lifted, the skin remoistened, and the device was replaced and a second measurement was taken. A third measurement was also obtained. Based on the 50 kHz phase, the 2 closest measurements were averaged and used in the subsequent analyses.
In order to evaluate technique reproducibility, the first and then a second evaluator repeated the entire set of three measurements on each muscle.
Slow Vital Capacity (SVC):
Upright SVC was measured using the NDD EasyOne® Plus Diagnostic Spirometer (NDD Medical Technologies, Andover, MA). The SVC was recorded as percent predicted using the Knudsen (1983) algorithm. The highest of 3 SVC determinations was recorded.
Handheld Dynamometry (HHD):
HHD was performed using the MicroFet2® handheld dynamometer (Hoggan Health, Salt Lake City, UT) by trained examiners. Shoulder flexion, elbow flexion, elbow extension, hip flexion, knee flexion, knee extension, wrist extension, first dorsal interosseous contraction and ankle dorsiflexion were tested. Each muscle was measured twice unless the values fell outside of 15% repeatability, which prompted a third measurement. The highest value was utilized. For HHD, megascores were calculated for all muscles as well as separately for the upper and lower extremities [11].
ALS Functional rating scale-revised (ALSFRS-R) [2].
The ALSFRS-R was administered by trained personnel at each visit.
Data analysis:
Initial processing.
An artifact detection program was developed to automatically exclude EIM data that was of poor quality (most often due to poor electrode contact). The algorithm had been previously developed using an unrelated data set. Specifically, data were excluded if negative reactance values occurred at or above a frequency of 30 kHz, any phase values were noted above 25 degrees at or above 22 kHz, or oscillations were seen in any of the impedance data (the slope changing sign more than ten times). If one measurement from the set of three was removed due to artifact, the remaining two were used. If 2 sets were removed due to artifact, no data for that muscle on that visit was included.
Missing data.
No data imputation was utilized. If values were missing for a given muscle at a single time point (because the measurements were not performed or filtered out due to the automated artifact detection) that muscle was excluded from the entire analysis for both the mean and individual muscle assessments.
Primary analysis.
The primary aim of this study was to determine EIM’s potential value as a therapy-response biomarker in a clinical trial; intra-evaluator and inter-rater reproducibility and primary diagnostic accuracy were both secondary goals. Accordingly, we sought to complete an initial screen of the 3 electrode arrays to identify which was most sensitive to progression prior to any other analyses. To do this, we utilized the coefficient of variation in the rate of decline (CoVRD) of the 50 kHz phase, which had been used in earlier studies [6,9]. To do so, we first established a mean slope of decline by linear regression for each patient and each measure. We then calculated the standard deviation of this group of slopes and divided this by the mean slope. The lower the CoVRD the fewer patients will be needed in a clinical trial, and we used this relatively simple metric to identify the single best electrode configuration among all 3 sensors, evaluating both mean rate of decline and that of the muscle with the steepest slope.
Once the optimum electrode sensor and electrode configuration within that sensor was identified, we then sought to identify which set or sets of frequencies provided the lowest CoVRD. For this we restricted our analysis to 2 pre-determined frequencies: 50 kHz and 100 kHz as well as a 50/200 kHz phase ratio; the latter was chosen because we had previously identified that this ratio might be sensitive to disease status and help reduce the impact of subcutaneous fat on measurements [12].
We also determined whether data from a single muscle vs. multiple muscles provided a greater potential sensitivity to treatment effect [9]. Thus for each patient, we identified the muscle with the steepest rate of decline and compared those data to the data of all muscles averaged at a single time point.
Repeatability assessments:
We assessed intra- and inter-evaluator reproducibility. For intra-evaluator, we utilized the first two measurements of the first evaluator for each muscle. For inter-evaluator, we utilized the average of the two closest measurements of the first evaluator with the two-closest measurements of the second evaluator. Intraclass correlation coefficients were calculated for both measures.
Discrimination between disease types.
Standard one-way ANOVA was performed with post-hoc t-tests with Bonferonni correction for multiplicity. Receiver operating characteristic (ROC) analyses were also performed and the area under the curve (AUC) calculated as a measure of the test’s overall accuracy.
Formal longitudinal analysis and calculation of treatment effects and sample size estimations.
Power was calculated for a standard shared-baseline, random-slopes linear mixed model using variance components estimated from the study data. The sample size required for 80% power to detect a 30% reduction in the observed rate of progression was calculated for each outcome assuming a two-arm trial, randomized 1:1, with 5 visits (baseline and months 2, 4, 6, and 8), up to 10% loss to mortality or loss to follow-up, and testing at a two-tailed p < 0.05.
Correlation analyses.
Correlations between rates of change for EIM measures and HHD, ALSFRS-R, and SVC were calculated.
Adverse Events:
Given the novel nature of this system, adverse events were assessed after each testing session.
RESULTS
1. Demographics.
Forty-six ALS patients, 30 ALS mimics, and 30 healthy controls were enrolled and underwent baseline assessment. Table 1 provides the summary demographics for the three groups of patients. For the ALS patients, months from diagnosis ± standard deviation (SD) was 9.5±10.2, months from symptom onset was 18.6±11.3, baseline ALSFRS-R was 37.5±6.1, and the proportion of bulbar versus limb onset was 22%. Of the ALS patients who enrolled, 34 completed all 5 visits (average 209±75 days follow-up); all data were used in the longitudinal analyses whether or not the subject completed the study.
Table 1.
Demographics
| ALS patients (N=46) |
Mimics* (N=30) |
Healthy controls (N=30) |
p value | |
|---|---|---|---|---|
| Age (years) | 59.9±9.7 | 57.0±11.1 | 49.5±8.6 | p < 0.001 |
| Female | 37% (17) | 36.7% (11) | 43.3% (13) |
p = 0.826 |
| Male | 63% (29) | 63.3% (19) | 56.7% (27) |
Mimics included: multiple sclerosis (N = 8), hereditary spastic paraparesis (N = 4), multifocal motor neuropathy (N = 4), autoimmune motor neuropathy (N = 2), cervical/lumbosacral radiculopathies (N = 4), mononeuropathy (N = 1), and Charcot-Marie Tooth (N = 7).
2. EIM data completion and filtering.
Across all subjects, sensors and electrode configurations, 14.5% of muscle measurements were excluded by the automated artifact detection program. For the ALS patients, a total of 9% of data were rejected.
3. Identifying the best array design.
Using the CoVRD as described above, we compared the arrays shown in Figure 1B. The “square electrode” with the widest electrode spacing (see arrows on Figure 1B) provided the lowest CoVRD for both the mean of all muscles studied (1.15) and for the muscle with the steepest slope (0.544). Other sensors/electrodes ranged from 1.36–2.02 for mean and 0.622–1.01 for steepest slope.
4. Reproducibility in ALS patients using this design.
Intra- and inter-evaluator reproducibility intraclass correlation coefficients across individual muscles in the ALS patients showed mean values (range) of 0.95 (0.91–0.98) and 0.91 (0.78–0.96), respectively.
5. Discrimination across disease types.
Using mean sensor/muscle data, ANOVAs were significant for the 50 kHz phase, 100 kHz phase, and 50/200 kHz phase (Table 2); post-hoc t-tests and ROC analysis showed ALS patients were distinguishable from healthy controls (area-under-curve 0.78, 95% CIs 0.68,0.89), but not between mimics and ALS patients (area-under-curve 0.60, 95% CIs 0.47,0.73).
Table 2.
Baseline comparisons between ALS patients, mimics and healthy controls
| Healthy versus ALS |
Mimic versus ALS |
|||||||
|---|---|---|---|---|---|---|---|---|
| ALS Patients |
Mimics* | Healthy controls |
ANOVA p value |
Post-hoc t-test |
AUC from ROC analysis |
Post-hoc t-test |
AUC from ROC analysis |
|
|
Mean 50 kHz phase (degrees) |
10.9±3.3 |
12.0±3.0 |
14.4±3.0 |
<0.001 |
<0.001 |
0.785 |
0.253 (NS) |
0.601 |
|
Mean 100 kHz phase (degrees) |
13.1±3.1 |
13.9±2.8 |
16.2±2.9 |
<0.001 |
<0.001 |
0.772 |
0.405 (NS) |
0.582 |
|
Ratio of Mean 50/200 kHz phase |
0.76±0.1 |
0.81±0.1 |
0.87±0.1 |
<0.001 |
<0.001 |
0.780 |
0.079 (NS) |
0.619 |
Mimics included: multiple sclerosis (N = 8), hereditary spastic paraparesis (N = 4), multifocal motor neuropathy (N = 4), autoimmune motor neuropathy (N = 2), cervical/lumbosacral radiculopathies (N = 4), mononeuropathy (N = 1), and Charcot-Marie Tooth (N = 7).
6. Ability to detect potential treatment effects.
Figure 2A shows the average rate of progression (and 95% CIs) for several of the measures. Figure 2B shows the comparison of sample sizes per arm required to detect a 30% treatment effect with 80% power. As can be seen, sample size for ALSFRS-R and HHD megascore were 391 and 517 per treatment group; SVC required a larger sample size of 530. Mean EIM 50 kHz required 369 patients per arm; however analysis of the muscle with the steepest rate of decline in EIM 50 kHz phase required only 75 subjects per arm. The other frequency measures were slightly worse and added no value over the 50 kHz measure, either as a single muscle or whole-body metric.
Figure 2.
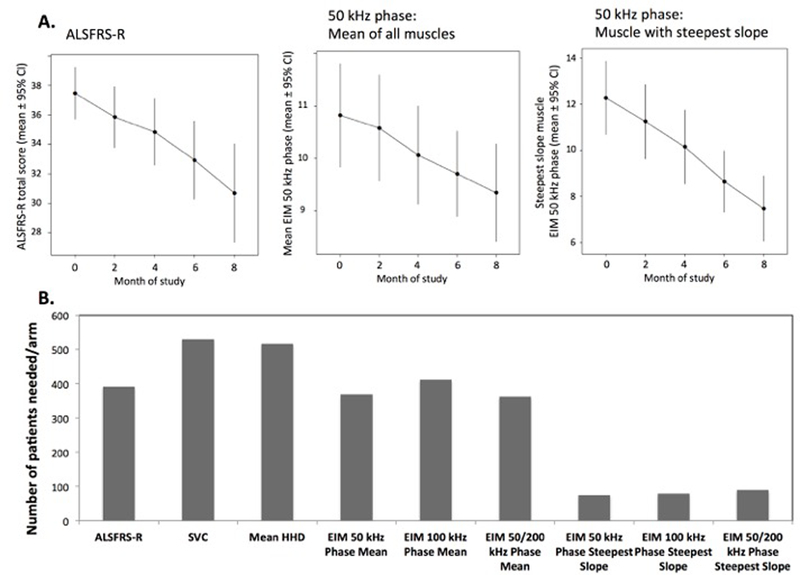
A. Average rate of change (and 95% confidence bounds) for ALSFRS-R, 50 kHz Phase (mean), and 50 kHz Phase (muscle with steepest slope). Note the marked linearity, especially for the single muscle data and the relatively smaller standard deviation in the data from the most rapidly progressing muscle. B. Associated sample size calculations.
7. Correlation to standard measures.
Of all EIM frequency metrics, the 50 kHz phase from the muscle with the steepest rate of EIM decline correlated best with all 3 standard measures (ALSFRS-R, r=0.371, p=0.021; whole-body HHD megascore, r=0.369, p=0.022; SVC, r=0.305, p=0.062). Whereas the mean muscle 50 kHz phase correlated better with whole-body HHD megascore (r=0.481, p=0.002), ALSFRS-R and SVC correlated worse (r=0.234, p=0.159 and r=0.106, p=0.528, respectively).
8. Adverse events.
No adverse events were recorded at any of the sites during the study procedures.
DISCUSSION
These results demonstrate that using this EIM device to track a single rapidly deteriorating muscle can provide excellent sensitivity to overall ALS progression. With this approach, we are able to achieve a 5.2-fold reduction in sample size requirements as compared to the ALSFRS-R (i.e., 81% fewer subjects needed) and an even greater reduction compared to whole-body HHD and SVC. This result is also far superior to that obtained in an earlier EIM study that used an off-the-shelf impedance device and adhesive electrodes in which sample size requirements were reduced only 2.3-fold as compared to ALSFRS-R (i.e., 47% fewer subjects needed) [9]. Importantly, despite evaluating only a single muscle, we still identified a moderate strength correlation between this measure and whole-body HHD and ALSFRS-R, supporting its construct validity. Moreover, EIM demonstrated high reproducibility in both healthy and diseased populations and distinguished healthy individuals from those with ALS. However, it could not distinguish ALS patients from disease mimics.
A major secondary goal of this study was to evaluate several different electrode array designs. The array on the square sensor in which the current electrodes were placed far from voltage electrodes proved the best, supporting previous theoretical work showing that such a configuration ensured better muscle penetration of current, reducing the impact of subcutaneous fat on the data [13]. We also identified that the arrays in which current flow was directed parallel to the muscle fibers performed better compared to those with 90° and 45° angles (in the square and octagonal sensors).
In terms of choice of frequencies, we studied both 50 and 100 kHz phase as well as the 50/200 phase ratio. Prior ALS studies focused on the 50 kHz phase data [6,7], as available devices were limited to that frequency. Since here we used a system with non-adhesive electrodes, we were concerned that contact impedance might cause inconsistencies in the 50 kHz data (generally a problem at lower frequencies). However, our consideration that the 100 kHz value would be more stable and sensitive to progression was not borne out by the analysis. Similarly, we chose the 50/200 phase ratio since in a separate study it was found to be less affected by subcutaneous fat than the single frequency 50 kHz phase [12]. However, it too appeared less sensitive to change than the 50 kHz phase.
Like the earlier multicenter study, we identified that following a single muscle’s EIM data outperformed the mean muscle EIM data. Since ALS does not affect the body homogeneously, there are clear advantages to evaluating the region that is progressing most rapidly in a given person. Using mean values of muscles that are not yet affected by the disease as well as those that are already reaching end stage will dilute the ability to detect decline over relatively short periods of time. By using a single muscle as a sentinel measure of disease progression, we can improve our ability to detect change. The muscle that is chosen uniquely for each person is presumably in a body region that is rapidly declining during the period of study. Thus, this strategy helps to amplify the signal and improve our ability to detect progression. Moreover, the fact that this single-muscle measure correlates to other measures of progression supports the concept that it has clinical meaning. The advantage of specifically using EIM for a single-muscle assessment is its high reproducibility at the single muscle level, which is not the case with HHD. In fact, data from two large ALS clinical trials (ceftriaxone [14] and dexpramipexole [15]) showed marked variability in individual muscle HHD data over time, negating the advantage of focusing on the body region showing the greatest rate decline (Shefner, unpublished results). Nevertheless, taking this single-muscle approach with other more quantitative strength measurement techniques, such as ATLIS [16], would be worth considering in future trials.
How such a single-muscle measure would be used in a clinical trial is yet to be determined. There are two straightforward possibilities. In one, a muscle would be selected a priori in each individual prior to initiation of therapy. This muscle would be one that would be expected to have the steepest slope of EIM decline in that patient in the coming months (e.g., in a limb where weakness is just becoming evident at study initiation); such an approach could also include a short lead-in phase. In point of fact, this is similar to how most motor unit number estimation (MUNE) or motor unit number index (MUNIX) techniques are applied to a clinical trial since generally only one muscle is studied with the results thought to represent the true rate of progression of the disease in that individual. The second approach would be to perform the analysis post-hoc, as we have essentially done here. Since the study would be presumably blinded, there would be no reason why such an approach should bias the results.
There were several limitations to this study. First, except for a brief introductory training session, the evaluators were not required to practice the technique or pass any certification test to demonstrate proficiency in EIM performance; such training and validation would be likely to further increase reliability and reduce the amount of unusable data. Moreover, anatomic landmarks for sensor placement were not defined in this study—evaluators were simply requested to place the device over the center of the muscle; we have since learned that careful positioning is likely to further improve reproducibility. Also, we did not evaluate any hand or tongue muscles since the sensors were too large and not designed for those regions. A hand- or tongue-specific sensor would allow study of additional muscles typically affected in both limb and bulbar-onset ALS. In addition, a direct comparison between EIM of a single muscle with MUNE/MUNIX from that same muscle would be interesting. In fact, such an analysis has already been completed in rats [17] and mice [18], revealing a strong relationship between the two measurements. Finally, slow recruitment resulted in sites with few subjects, reducing staff familiarity with the technique. The demonstration that, despite all these limitations, reliable and sensitive data were acquired strongly supports the concept that EIM may be an effective measure especially in early and mid-phase drug development.
In summary, whereas EIM has limited value in diagnosis, it is highly sensitive to ALS progression on a muscle-specific basis. The technique also correlates to standard measures of disease progression. However, as with any biomarker for assessing disease progression, the ultimate proof of EIM’s utility will depend on its ability to detect the effect of a therapy that meaningfully alters the clinical course of ALS.
Acknowledgments
Funding: This study was funded by SBIR grant (R44NS070385) from the NINDS at the National Institutes of Health to Skulpt Inc. N.B. Skulpt, Inc was renamed Myolex, Inc in 12/2016.
Disclosure statement:
Drs. Rutkove and Bohorquez hold equity in Myolex, Inc, receive consulting fees from the company and are named as inventors on relevant patents; they also received funding support for this study. All others received funding support only.
References
- 1.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996;347:1425–31. [DOI] [PubMed] [Google Scholar]
- 2.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 3.Berry JD, Miller R, Moore DH, Cudkowicz ME, Van Den Berg LH, Kerr DA, et al. The Combined Assessment of Function and Survival (CAFS): A new endpoint for ALS clinical trials. Amyotroph. Lateral Scler. Front. Degener 2013;14:162–8. [DOI] [PubMed] [Google Scholar]
- 4.Benatar M, Boylan K, Jeromin A, Rutkove SB, Berry J, Atassi N, et al. ALS biomarkers for therapy development: State of the field and future directions. Muscle Nerve 2016;53:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkove SB. Electrical Impedance Myography: Background, Current State, and Future Directions. Muscle Nerve 2009;40:936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, et al. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol 2007;118:2413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph. Lateral Scler 2012;13:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: A multicenter study of reliability and validity. Muscle Nerve 2015;52:592–7. [DOI] [PubMed] [Google Scholar]
- 9.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph. Lateral Scler 2012;13:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot. Neuron Disord 2000;1:293–9. [DOI] [PubMed] [Google Scholar]
- 11.Andres PL, Finison LJ, Conlon T, Thibodeau LM, Munsat TL. Use of composite scores (megascores) to measure deficit in amyotrophic lateralsclerosis. Neurology 1988;38:405–8. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz S, Geisbush TR, Mijailovic A, Pasternak A, Darras BT, Rutkove SB. Optimizing electrical impedance myography measurements by using a multifrequency ratio: A study in Duchenne muscular dystrophy. Clin. Neurophysiol 2015;126:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafarpoor M, Li J, White JK, Rutkove SB. Optimizing electrode configuration for electrical impedance measurements of muscle via the finite element method. IEEE Trans. Biomed. Eng 2013;60:1446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudkowicz ME, Titus S, Kearney M, Yu H, Sherman A, Schoenfeld D, et al. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet. Neurol 2014;13:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet. Neurol 2013;12:1059–67. [DOI] [PubMed] [Google Scholar]
- 16.Andres PL, Skerry LM, Munsat TL, Thornell BJ, Szymonifka J, Schoenfeld DA, et al. Validation of a new strength measurement device for amyotrophic lateral sclerosis clinical trials. Muscle Nerve 2012;45:81–5. [DOI] [PubMed] [Google Scholar]
- 17.Wang LL, Spieker AJ, Li J, Rutkove SB. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin. Neurophysiol 2011;122:2505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Sung M, Rutkove SB. Electrophysiologic biomarkers for assessing disease progression and the effect of riluzole in SOD1 G93A ALS mice. PLoS One 2013/June/14 2013;8:e65976. [DOI] [PMC free article] [PubMed] [Google Scholar]