Abstract
Seven fungal species were isolated from soil samples collected from the University of Sultan Qaboos, Muscat, Sultanate of Oman. The fungal isolates were identified as Aspergillus athecius, A. terreus var. africans, A. flavus, A. terreus, A. foetidus, Fusarium chlamydosporum and F. nygamai. Phytochemical and chromatographic investigation showed variety of secondary metabolites in all of the fugal extracts (extra and intra cellular). The antimicrobial activity of internal and external extracts of the isolated fungal species were screened against Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, Pseudomonas aeruginosa, Lactobacillus acidophilus, Streptococcus gordonii, S. mutans. The antimicrobial activity of external secondary metabolites was generally better than the internal metabolites. The highest antimicrobial activity (32 mm, 30 mm and 29 mm) was obtained from external secondary metabolites of Aspergillus flavus against Candida tropicals, Candida parapsilosis and Candida albicans, respectively.
Keywords: Aspergillus flavus, Phytochemical contents, Intra cellular, Extra cellular, Fusarium chlamydosporum, Candida albicans, Antimicrobial, Soil microbes, Aspergillus, Fusarium
1. Introduction
Antibiotic is a natural substance of biological origin used to treat diseases caused by microbes to eukaryotic organisms including human being (Pannapa and Pattra, 2017, Hacioglu and Dulger, 2011, Nikodinovic et al., 2003). According to their mode of action, antibiotics are known as wide- or narrow-spectrum. These include cell wall, protein and nucleic acid inhibition (Tortora et al., 2007, Brooks et al., 2001).
The necessity for new antibiotics increasing daily due to the emergence of drug-resistant pathogens. Despite the huge potential sources of antibiotics including medicinal plants, soil still the most important reservoir for novel antibiotics with pharmaceutical and biological activity (Rajaperumal et al., 2013). There are more than 500 antibiotics are discovered every year, however, almost 60% are obtained from the soil (Molinari, 2009). Interestingly, a few grams of soil contain numerous microorganisms (Makut and Owolewa, 2011).
Numerous species of fungi including Penicillium, Aspergillus, Fusarium, Cladosporium and Yeasts are able to produce enzymes and secondary metabolites including antibiotics (Hyde, 2001). Approximately 20% of antibiotics have been obtained from fungi isolated from soil (Berdy, 2005). Those antibiotics, produced by fungi, including fusidic acid (Akpotu et al., 2017), cephalosporin and penicillin are widely used for treatment of many diseases (Sethi et al., 2013) and they are the most important source of potentially significant pharmaceutical drugs (Wasser, 2002). The objective of this study was to isolate and identify soil fungi and to determine their ability to inhibit the growth of microorganisms.
2. Materials and methods
2.1. Fungal isolation and identification
2.1.1. Isolation
2.1.1.1. Soil samples collection
The soil samples were collected from different sites at Sultan Qaboos University, Muscat, Sultanate Oman. The direct inoculation method was used for sampling and isolation of fungal isolates. Soil samples were separately transferred onto the surface malt agar media and incubated for 5–7 day at 25 °C.
2.1.1.2. Fungal isolation
For isolation of all fungi four different media were used (yeast extract sucrose potato dextrose, czapek’s dox and yeast malt extract agar malt extract) were uses. The technique used was described by Al-Enazi et al. (2018).
2.1.2. Identification of fungal isolates
For identification of the isolated fungi, each fungal isolate was microscopically examined be by transferring fungal mycelia onto a glass slide containing a drop of lactophenol cotton blue stain, and then covered with the cover slip and was viewed under microscope. Also, the macroscopic observation was determined by comparing the fungal isolate characters with the Pictorial atlas of soil and seed fungi (Casero et al., 2017). However, identification of the obtained fungal isolates was confirmed by the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
2.2. Extraction
2.2.1. Internal fungal secondary metabolites extraction
The mycelial mat for each fungus (250 g) was obtained, washed with distilled water, extracted by refluxing in 2 liters of boiled ethanol for 120 min and re-extracted again for three times (as mentioned before). The obtained filtrates were then concentrated together and dried from ethanol under reduced pressure at low temperature and kept in the fridge for the investigation.
2.2.2. External fungal secondary metabolites extraction
One liter of each fungal growth medium was separately extracted using 2 L of n-butanol (saturated with water), and re-extracted again for four repetitive times. The obtained butanol extract was filtered using Buchner funnel contains anhydrous sodium sulphate. The collected butanol extracts was dried from solvent using rotatory evaporator at temperature not exceeding 35˚C and the dry extract was then kept in fridge for investigation (Zain et al., 2012).
2.3. Phytochemical screening and chromatographic investigation
2.3.1. Phytochemical screening were carried out for all both extra and intra fungal extracts to investigate their phytochemical contents of secondary metabolites, these were done according to the standard published methods (Tiwari et al., 2011).
All of Each fungal extracts (intra and extra) was tested chromatographically using three systems a- c [(a) ethyl acetate: methanol: water 90: 5: 4 v/v/v, (b) chloroform: methanol 95: 5 v/v & (c) benzene: ethyl acetate 86: 14 v/v]. Visualization of the spots were carried out under UV before and after spraying with Antimony trichloride (SbCl3).
2.4. Antimicrobial activity
2.4.1. Test organisms
The test organisms used were Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, Lactobacillus acidophilus, Pseudomonas aeruginosa, Streptococcus gordonii, and Streptococcus mutans were obtained from the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt as test organisms.
2.4.2. Antimicrobial screening
The antimicrobial activity of internal and external secondary metabolites of the isolated fungal strains grown on malt extract, yeast extract sucrose, and yeast-malt extract media were determined using disc-diffusion method (Al-Enazi et al., 2018). Petri dishes containing 20 mL of agar medium were seeded with test organisms. Standard discs (6 mm in diameter) loaded with 50 μL of fungal extract were placed onto the agar, and incubated at 37 °C for 24–48 h. The antimicrobial activity was recorded as the diameter of the inhibition zone formed around the disc.
3. Results and discussion
3.1. Isolation and identification of fungal strains
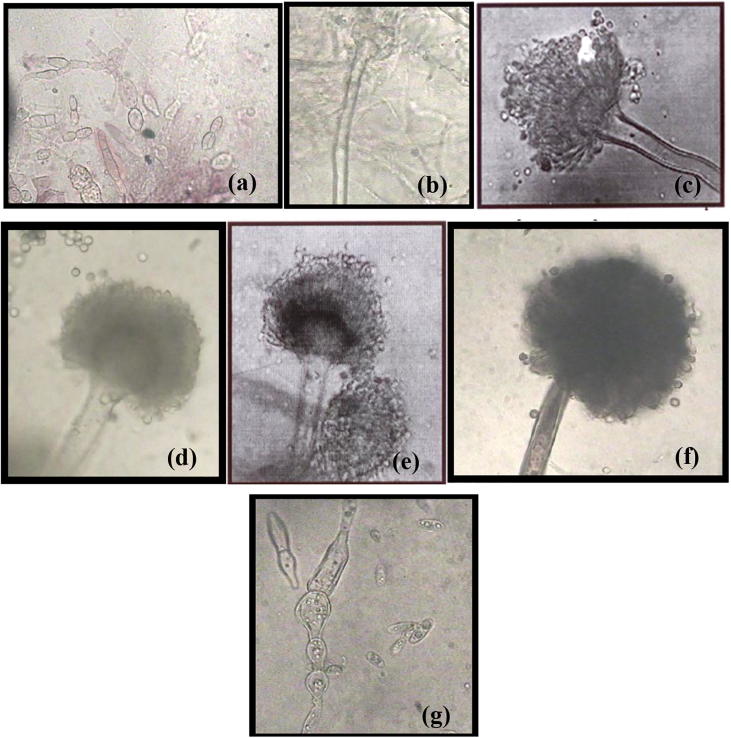
Fungal isolates were obtained from the analyses of different soil samples collected from the University of Sultan Qaboos, Muscat, and Sultanate of Oman. Seven fungal strains were isolated from malt extract agar plates incubated at 28 °C for 7 days. All fungal isolates were obtained in pure cultures by single conidial transfer onto beer agar plates. The fungal isolates were identified as Aspergillus athecius, A. terreus var. africans, A. flavus, A. terreus, A. foetidus Fusarium chlamydosporum and F. nygamai (Fig. 1).
Fig. 1.
The microscopic structures of fungal isolates; Aspergillus athecius (a), Aspergillus terreus var. africans (b), Aspergillus flavus (c), Fusarium chlamydosporum (d), Aspergillus terreus (e), Aspergillus foetidus (f) and Fusarium nygamai (g).
Similarly, Raja and others (Raja et al., 2017) isolated and identified different fungal strains from soil samples collected from Loyola College Campus, Chennai, India. Also, it was suggested that the number and frequencies of fungal species isolated from soil depend on the moisture content and/or level of organic carbon in the soil (Salar and Aneja, 2006).
3.2. Phytochemical and chromatographic screening
All extracts of each fungi (extra & intra cellular) were found to be quite similar to each other, they contain; Proteins and/or amino acids, unsaturated sterols and/or triterpens, carbohydrates and /or glycosides in addition to traces of coumarins and lactones, saponins, tannins > no alkaloids, flavonoids, Anthraquinones and Cardenolides were detected (Table 1).
Table 1.
Phytochemical Screening of all fungal extracts (extra and intra cellular).
| Test for |
Aspergillus athecius |
Aspergillus terreus var. africans |
Aspergillus flavus |
Fusarium chlamydo-sporum |
Aspergillus terreus |
Aspergillus toetidus |
Fusarium nygama |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | |
| Alkaloids | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Carbohydrates and /or glycosides | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Flavonoids | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Saponins | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| Tannins | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| Unsaturated sterols and/orTriterpens | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Anthraquinones | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Proteins and/or amino acids | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Coumarins and lactones | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| Cardenolides | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
(+): present; (−) absent, (±) trace.
Thin Layer Chromatography (TLC) investigation of all fungal extracts (extra and intra) showed that all extra cellular extracts of each fungi were similar to each other, and that all intra cellular extracts of each fungi were similar to each other. However, the intra cellular extracts contain more compounds than extra cellular extracts (Table 2).
Table 2.
TLC results for phytochemical Screening of all extracts (extra and intra cellular).
| Rf values |
Colour |
Aspergillus athecius |
Aspergillus terreus var. africans |
Aspergillus flavus |
Fusarium chlamydo-sporum |
Aspergillus terreus |
Aspergillus toetidus |
Fusarium nygama |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | UV | NH3 | SbCl3 | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra | Intra | Extra |
| – | 0.86 | – | bl. | bl. | bl. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | 0.86 | – | br. | br. | br. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | – | 0.73 | bl gr. | bl gr. | bl gr. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | – | 0.64 | bl gr. | bl gr. | bl gr. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | 0.85 | 0.55 | bl gr. | bl gr. | bl gr. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| – | 0.83 | 0.49 | bl gr. | bl gr. | bl gr. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | – | 0.43 | bl gr. | bl gr. | bl gr. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | – | 0.43 | br. | br. | br. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | – | 0.35 | bl. | bl. | bl. | ± | + | ± | + | ± | + | ± | + | ± | + | ± | + | ± | + |
| – | – | 0.31 | bl. | bl. | bl. | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± |
| – | – | 0.18 | bl. | bl. | bl. | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± |
| – | – | 0.15 | bl. | bl. | bl. | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± |
| – | – | 0.13 | bl gr. | bl gr. | bl gr. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
| – | 0.51 | 0.05 | bl. | bl. | bl. | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± |
| 0.80 | 0.28 | 0.02 | bl. | bl. | bl. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.77 | 0.03 | 0.18 | y. | br. | br. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.71 | 0.04 | 0.02 | y. | br. | br. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.66 | 0.22 | – | bl. | bl. | bl. | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ |
| 0.59 | 0.21 | – | bl. | bl. | bl. | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ |
| 0.53 | 0.14 | – | bl. | bl. | vi. | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| 0.40 | 0.12 | – | bl. | bl. | bl. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.37 | 0.08 | – | br. | br. | br. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.22 | 0.06 | – | br. | br. | br. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.16 | 0.04 | – | br. | br. | br. | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.13 | – | – | y. | y. | y. | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| 0.08 | – | – | y. | y. | y. | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ | ± | _ |
| 0.03 | – | – | br. | br. | br. | + | ± | + | ± | + | ± | + | ± | + | ± | + | ± | + | ± |
| – | 0.86 | – | bl. | bl. | bl. | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + | _ | + |
(−): absent; (+): present; (±): trace; (a): ethyl acetate: methanol: water 90:5:4 v/v/v; SbCl3: antimony trichloride; (b): chloroform: methanol 95:5 v/v; (c): benzene: ethyl acetate 86:14 v/v; bl: blue; br: brown; gr: green; y:yellow; NH3: ammonia; ex: extra cellular; In: intra cellular.
3.3. Antimicrobial activity
The internal and external secondary metabolites of the obtained fungal species were investigated for their antimicrobial activity against Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, Pseudomonas aeruginosa, Lactobacillus acidophilus, Streptococcus gordonii, S. mutans. Each fungal strain was grown on Malt Extract (ME), Yeast Extract Sucrose (YES) and Yeast-Malt Extract media at 28 ± 2 °C for 14 days. Both the intra- and extra-cellular metabolites of the fungal species were screened for their antimicrobial activity (Table 3, Table 4, Table 5). The isolated fungal species exhibited anticandidal and antibacterial activity against almost all the investigated test organisms. However, activity of secondary metabolites of fungi grown on malt extract media was the highest (Table 3). Moreover, the antimicrobial activity of external secondary metabolites was generally better than the internal metabolites.
Table 3.
Antimicrobial activity of cell extract of fungal isolates grown on malt extract medium.
| Test organism | Diameter of inhibition zone (mm) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Aspergillus athecius |
Aspergillus terreus var. africans |
Aspergillus flavus |
Fusarium chlamydo-sporum |
Aspergillus terreus |
Aspergillus foetidus |
Fusarium nygamai |
||||||||
| Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | |
| Unicellular fungi | ||||||||||||||
| Candida albicans | 17.0 | 19.0 | 21.0 | 22.0 | 16.0 | 16.0 | 14.0 | 14.0 | 27.0 | 29.0 | 17.0 | 18.0 | 15.0 | 16.0 |
| Candida glabrata | 15.0 | 15.0 | 19.0 | 19.0 | 14.0 | 17.0 | 13.0 | 15.0 | 26.0 | 25.0 | 16.0 | 16.0 | 14.0 | 17.0 |
| Candida parapsilosis | 17.0 | 18.0 | 18.0 | 17.0 | 15.0 | 17.0 | 13.0 | 13.0 | 28.0 | 30.0 | 17.0 | 17.0 | 16.0 | 21.0 |
| Candida tropicalis | 16.0 | 19.0 | 20.0 | 21.0 | 17.0 | 17.0 | 14.0 | 15.0 | 27.0 | 32.0 | 17.0 | 19.0 | 14.0 | 19.0 |
| Gram-Negative Bacteria | ||||||||||||||
| Pseudomonas aeruginosa | 15.0 | 18.0 | 16.0 | 20.0 | 16.0 | 18.0 | 10.0 | 10.0 | 22.0 | 24.0 | 16.0 | 18.0 | 17.0 | 17.0 |
| Gram-Positive Bacteria | ||||||||||||||
| Lactobacillus acidophilus | 15.0 | 15.0 | 17.0 | 17.0 | 19.0 | 20.0 | 12.0 | 15.0 | 21.0 | 27.0 | 19.0 | 20.0 | 14.0 | 15.0 |
| Streptococcus gordonii | 16.0 | 17.0 | 17.0 | 19.0 | 17.0 | 19.0 | 13.0 | 14.0 | 23.0 | 26.0 | 18.0 | 18.0 | 17.0 | 17.0 |
| Streptococcus mutans | 14.0 | 16.0 | 15.0 | 16.0 | 17.0 | 18.0 | 14.0 | 16.0 | 22.0 | 25.0 | 17.0 | 21.0 | 15.0 | 17.0 |
Int., intracellular metabolites; Ext., extracellular metabolites.
Table 4.
Antimicrobial activity of cell extract of fungal isolates grown on yeast extract sucrose medium.
| Test organism | Diameter of inhibition zone (mm) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Aspergillus athecius |
Aspergillus terreus var. africans |
Aspergillus flavus |
Fusarium chlamydo-sporum |
Aspergillus terreus |
Aspergillus foetidus |
Fusarium nygamai |
||||||||
| Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | |
| Unicellular fungi | ||||||||||||||
| Candida albicans | 12.0 | 12.0 | 11.0 | 10.0 | 10.0 | 10.0 | 00.0 | 00.0 | 13.0 | 11.0 | 09.0 | 09.0 | 10.0 | 09.0 |
| Candida glabrata | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 |
| Candida parapsilosis | 14.0 | 15.0 | 12.0 | 13.0 | 13.0 | 13.0 | 10.0 | 11.0 | 15.0 | 16.0 | 11.0 | 12.0 | 12.0 | 14.0 |
| Candida tropicalis | 10.0 | 12.0 | 10.0 | 10.0 | 11.0 | 10.0 | 07.0 | 00.0 | 12.0 | 12.0 | 00.0 | 00.0 | 10.0 | 11.0 |
| Gram-Negative Bacteria | ||||||||||||||
| Pseudomonas aeruginosa | 10.0 | 10.0 | 10.0 | 12.0 | 11.0 | 11.0 | 11.0 | 11.0 | 14.0 | 13.0 | 12.0 | 12.0 | 11.0 | 10.0 |
| Gram-Positive Bacteria | ||||||||||||||
| Lactobacillus acidophilus | 11.0 | 12.0 | 11.0 | 12.0 | 10.0 | 11.0 | 00.0 | 00.0 | 13.0 | 12.0 | 00.0 | 00.0 | 13.0 | 16.0 |
| Streptococcus gordonii | 11.0 | 11.0 | 12.0 | 10.0 | 00.0 | 00.0 | 00.0 | 00.0 | 12.0 | 15.0 | 09.0 | 10.0 | 11.0 | 11.0 |
| Streptococcus mutans | 10.0 | 10.0 | 10.0 | 11.0 | 10.0 | 11.0 | 00.0 | 00.0 | 11.0 | 13.0 | 00.0 | 00.0 | 10.0 | 11.0 |
Int., intracellular metabolites; Ext., extracellular metabolites.
Table 5.
Antimicrobial activity of cell extract of fungal isolates grown on yeast malt-extract medium.
| Test organism | Diameter of inhibition zone (mm) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Aspergillus athecius |
Aspergillus terreus var. africans |
Aspergillus flavus |
Fusarium chlamydo-sporum |
Aspergillus terreus |
Aspergillus foetidus |
Fusarium nygamai |
||||||||
| Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | Int. | Ext. | |
| Unicellular fungi | ||||||||||||||
| Candida albicans | 12.0 | 13.0 | 11.0 | 11.0 | 10.0 | 10.0 | 08.0 | 10.0 | 12.0 | 13.0 | 00.0 | 00.0 | 10.0 | 11.0 |
| Candida glabrata | 11.0 | 10.0 | 12.0 | 12.0 | 09.0 | 10.0 | 07.0 | 07.0 | 12.0 | 12.0 | 00.0 | 00.0 | 10.0 | 10.0 |
| Candida parapsilosis | 12.0 | 12.0 | 12.0 | 11.0 | 10.0 | 11.0 | 00.0 | 00.0 | 11.0 | 11.0 | 00.0 | 00.0 | 12.0 | 12.0 |
| Candida tropicalis | 00.0 | 00.0 | 12.0 | 12.0 | 11.0 | 11.0 | 08.0 | 09.0 | 11.0 | 11.0 | 00.0 | 00.0 | 11.0 | 11.0 |
| Gram-Negative Bacteria | ||||||||||||||
| Pseudomonas aeruginosa | 00.0 | 00.0 | 10.0 | 11.0 | 09.0 | 09.0 | 00.0 | 00.0 | 10.0 | 10.0 | 00.0 | 00.0 | 00.0 | 10.0 |
| Gram-Positive Bacteria | ||||||||||||||
| Lactobacillus acidophilus | 12.0 | 12.0 | 10.0 | 12.0 | 10.0 | 11.0 | 10.0 | 09.0 | 13.0 | 13.0 | 00.0 | 00.0 | 10.0 | 11.0 |
| Streptococcus gordonii | 13.0 | 12.0 | 12.0 | 12.0 | 09.0 | 10.0 | 00.0 | 00.0 | 11.0 | 11.0 | 00.0 | 00.0 | 12.0 | 10.0 |
| Streptococcus mutans | 13.0 | 13.0 | 00.0 | 09.0 | 11.0 | 10.0 | 00.0 | 00.0 | 10.0 | 11.0 | 00.0 | 00.0 | 12.0 | 12.0 |
Int., intracellular metabolites; Ext., extracellular metabolites.
The internal and external secondary metabolites of all the isolated fungi grown on malt extract media showed anticandidal and antibacterial activity against all the investigated candidal and bacterial species. However, the highest antimicrobial activity (32 mm, 30 mm, 29 mm) was obtained from external secondary metabolites of Aspergillus flavus against Candida tropicals, C. parapsilosis and C. albicans, respectively (Table 4).
On the other hand, when grown on yeast extract sucrose media, all the isolated fungi showed no activity against Candida glabrata (Table 4). Also, there was no antimicrobial activity against any of the test organisms for internal or external secondary metabolites of Aspergillus foetidus grown on yeast malt extract media (Table 5).
The differences in the antimicrobial activity between intra and extra cellular extracts of the investigated fungi are somewhat similar to each other’s but different in the concentration of the secondary metabolites (according to their TLC) in each of them.
Previous studies mentioned that different culture media could lead to variation in morphology, physiology and growth rate of the same fungal strain according to the components and nutrients of the growth media (Magaldi et al., 2004). Several studies have been carried out on the antimicrobial activity of fungi and their secondary metabolites and proved that the activities is depend on the nature of the secondary metabolites present in the fungi (Akpotu et al., 2017, Zain et al., 2014, Wang et al., 2013, Shen et al., 2013, Zhang et al., 2008, Effendi, 2004).
Acknowledgment
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akpotu M.O., Eze P.M., Abba C.C., Umeokoli B.O., Nwachukwu C.U., Okoye F.B.C., Charles O., Esimone C.O. Antimicrobial activities of secondary metabolites of endophytic fungi isolated from Catharanthus roseus. J. Health Sci. 2017;7(1):15–22. [Google Scholar]
- Al-Enazi Nouf M., Awaad Amani S, Al-Othman Monerah R, Al-Anazi Nour K, Alqasoumi Saleh I. Isolation, identification and anti-candidal activity of filamentous fungi from Saudi Arabia soil. Available online in Saudi Pharm. 2018;26:253–257. doi: 10.1016/j.jsps.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdy J. Bioactive metabolites, a personal view. J. Antibot. 2005;58(1):1–26. [Google Scholar]
- Brooks G.F., Butel J.S., Morse S.A. 22nd ed. The McGraw-Hill Companies Inc; San Francisco: 2001. Jawetz, Melnick, Adelberg’s Medical Microbiology. [Google Scholar]
- Casero M.C., Ascaso C., DiRuggiero J., Meslier V., Artieda O.A., Quesada, Wierzchos J. Book of Abstracts VII International Conference on nvironmental Industrial and Applied Microbiology – BMW2017 Madrid (Spain) 2017. Biogeography at microscopic scale: diversity of endolithic microbial communities in icrohabitats of gypcrete from the Atacama Desert; p. 31. [Google Scholar]
- Effendi H. Heinrich‑Heine – Universität Düsseldorf; 2004. Isolation and Structure Elucidation of Bioactive Secondary Metabolites of Sponge‑Derived Fungi Collected from the Mediterranean Sea (Italy) and Bali Sea (Indonesia) pp. 46–48. Doctoral dissertation. [Google Scholar]
- Hacioglu N., Dulger B. Occurence and antibiotic susceptibility of some bacteria in Saricay stream (Canakkale, Turkey) Eur. J. Exp. Biol. 2011;1(4):158–163. [Google Scholar]
- Hyde K.D. Increasing the likelihood of novel compound discovery from filamentous fungi. Bio-exploitation Filamentous Fungi. 2001:77–91. [Google Scholar]
- Magaldi S., Mata-Essayag S., Hartung de Capriles C., Perez C., Colella M.T., Olaizalo C., Ontiveroes Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004;8(1):39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Makut M., Owolewa O. Antibiotic-producing fungi present in the soil environment of Keffi metropolis, Nasarawa state, Nigeria. Eubacteria. 2011;10(18):19. [Google Scholar]
- Molinari G. Natural products in drug discovery, present status and perspectives. Pharm. Biotechnol. 2009;655:13–27. doi: 10.1007/978-1-4419-1132-2_2. [DOI] [PubMed] [Google Scholar]
- Nikodinovic J., Barrow K.D., Chuck J.A. High frequency transformation of the amphotericin-producing bacterium Streptomyces nodosus. J. Microbiol. Methods. 2003;55(1):273–277. doi: 10.1016/s0167-7012(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Pannapa P., Pattra S. Antimicrobial and enzyme activity produced by Bacillus Spp. Isolated from soil. Int. J. Pharm. Pharm. Sci. 2017;9(3):205–210. [Google Scholar]
- Raja M., Praveena G., William S.J. Isolation and identification of fungi from soil in Loyola college campus, Chennai, India. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(2):1789–1795. [Google Scholar]
- Rajaperumal S., Nimmi M., Kumari B.D.R. In vitro studies on antimicrobial and antioxidant effect of methanolic extract of Indigofera aspalathoides (Vahl ex DC) and its cytotoxic property against human lung cancer cell line NCI H460. Eur. J. Exp. Biol. 2013;3(3):18–29. [Google Scholar]
- Salar R.K., Aneja K.R. Thermophilous fungi from temperate soils of northern India. J. Agric. Technol. 2006;2(1):49–58. [Google Scholar]
- Sethi S., Kumar R., Gupta S. Antibiotic production by microbes isolated from soil. Int. J. Pharm. Sci. Res. 2013;4(8):2967. [Google Scholar]
- Shen S., Li W., Wang J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum. Nat. Prod. Res. 2013;27(24):2286–2291. doi: 10.1080/14786419.2013.827190. [DOI] [PubMed] [Google Scholar]
- Tiwari P., Kumar B., Kaur M., Kaur G., Kaur G. Phytochemical screening and extraction: a review. Int. Pharm. Sci. 2011:98–106. [Google Scholar]
- Tortora G.J., Funke B.R., Case C.L. 9th ed., Pearson Education Inc; San Franscisco: 2007. Microbiology: An Introduction; p. 192. [Google Scholar]
- Wang X., Radwan M.M., Taráwneh A.H., Gao J., Wedge D.E., Rosa L.H. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J. Agric. Food Chem. 2013;61:4551–4555. doi: 10.1021/jf400212y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60(3):258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Zain M.E., Awaad A.S., Al-Othman M.R., Alafeefy A.M., El-Meligy R.M. Biological activity of fungal secondary metabolites. Int. J. Chem. Appl. Biol. Sci. 2014;1(1):14–22. [Google Scholar]
- Zain M.E., Awaad A.S., Al-Outhman M.R., El-Meligy R.M. Antimicrobial activities of Saudi Arabia desert plants. Phytopharmacol. 2012;2:106–113. [Google Scholar]
- Zhang W., Krohn K., Draeger S., Schulz B. Bioactive iso-coumarins isolated from the endophytic fungus Microdochium bolleyi. J. Nat. Prod. 2008;71(6):1078–1081. doi: 10.1021/np800095g. [DOI] [PubMed] [Google Scholar]