Abstract
Pterostilbene is a natural polyphenol compound found in small berries that is related to resveratrol, but has better bioavailability and a longer half-life. The purpose of this study was to assess the potential inhibitory effect of pterostilbene on in vitro drug metabolism. The effect of pterostilbene on cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) enzyme activities were studied using the enzyme-selective substrates amodiaquine (CYP2C8), midazolam (CYP3A4), estradiol (UGT1A1), serotonin (UGT1A6) and mycophenolic acid (UGT1A8/9/10). The IC50 value was used to express the strength of inhibition. Further, a volume per dose index (VDI) was used to estimate the potential for in vivo interactions. Pterostilbene significantly inhibited CYP2C8 and UGT1A6 activities. The IC50 (mean ± SE) values for CYP2C8 and UGT1A6 inhibition were 3.0 ± 0.4 µM and 15.1 ± 2.8 µM, respectively; the VDI exceeded the predefined threshold of 5 L/dose for both CYP2C8 and UGT1A6, suggesting a potential for interaction in vivo. Pterostilbene did not inhibit the metabolism of the other enzyme-selective substrates. The results of this study indicate that pterostilbene inhibits CYP2C8 and UTG1A6 activity in vitro and may inhibit metabolism by these enzymes in vivo. Clinical studies are warranted to evaluate the in vivo relevance of these interactions.
Abbreviations: CYP, cytochrome P450; DEAQ, desethylamodiaquine; HIM, human intestine microsomes; HLM, human liver microsomes; HPLC, high-performance liquid chromatography; IC50, concentration of inhibitor that results in 50% inhibition of reaction; LC-MS/MS, liquid chromatography/tandem mass spectrometry; M-IV, hydroxypioglitazone; RDI, recommended daily intake; UDPGA, uridine diphosphate glucuronic acid; UGT, UDP-glucuronosyltransferase; V/D, volume per dose index
Keywords: Pterostilbene, CYP2C8, UGT1A6, Amodiaquine, N-desethylamodiaquine, Pioglitazone, Hydroxypioglitazone, Serotonin, Serotonin glucuronide, Enzyme inhibition
1. Introduction
The consumption of herbal supplements and phytochemicals for purported health benefits increases annually in the U.S and around the world. In 2013, annual sales in the U.S exceeded $6 billion (Lindstrom et al., 2014). In 2006, studies showed that nearly 20% of Americans used at least one herbal product in the previous 12 months (Bardia et al., 2007). Compared to the general population, patients with chronic diseases, who are already ingesting multiple prescription drugs, tend to consume herbal products and phytochemicals more frequently (White et al., 2007, Engdal et al., 2009). Given the high rate of herbal supplement consumption, particularly among more frequent traditional medicine users, further studies are needed to clarify the potential of herb-drug interactions.
Pterostilbene is a natural polyphenol compound (Fig. 1) found in small berries, such as blueberries, huckleberries and grapes, belonging to the plant families Vitis and Vaccinium. Pterostilbene has exhibited anti-hypertensive, anti-cancer, anti-hypercholesterolemic, and anti-diabetic properties in animal studies, in addition to antioxidant and anti-inflammatory properties. A similar compound, resveratrol (Fig. 1), is known as a potential contributor to the “French Paradox,” which associates red wine consumption with lower coronary heart disease (McCormack and McFadden, 2013). Pterostilbene taken at a 250 mg daily dose was found to be associated with a reduction in blood pressure (Riche et al., 2013). Pterostilbene's physicochemical properties confer a higher bioavailability and a longer elimination half-life compared to resveratrol (McCormack and McFadden, 2013).
Fig. 1.
Chemical structures of pterostilbene and resveratrol.
Herbal extracts and phytochemicals have the potential to interact with co-administered drugs through the inhibition or induction of drug metabolism mediated by UDP glucuronosyltransferase (UGT) and cytochrome P450 (CYP) enzymes, leading to unwanted adverse effects or therapeutic failure. The CYP enzymes are the most important phase I xenobiotic-metabolizing enzymes (Zanger and Schwab, 2013); similarly, the (UGT) enzymes are the most important phase II xenobiotic-metabolizing enzyme (Li et al., 2012). More than 90% of prescribed drugs are metabolized by CYPs and UGTs enzymes (Rowland et al., 2013).
The purpose of this study was to examine the inhibitory effects of pterostilbene on the activities of CYP2C8, CYP3A4/5 (CYP3A), UGT1A1, UGT1A6, UGT1A8/10, and UGT1A9. Pterostilbene is available over-the-counter as a phytochemical alone or in combination with other ingredients, which increases the potential for it be co-administered with other drugs. The effect of pterostilbene on CYP and UGT enzyme activity was studied in human liver microsomes (HLM) or human intestinal microsomes (HIM) using enzyme-selective substrates (Table 1) for CYP2C8 (amodiaquine), CYP3A (midazolam), UGT1A1 (estradiol), UGT1A6 (serotonin), UGT1A8/10 and UGT1A9 (mycophenolic acid).
Table 1.
Enzyme selective substrates used for each enzyme, with the corresponding metabolite and positive control inhibitor.
| Enzyme | Substrate | Metabolite | Inhibitor |
|---|---|---|---|
| CYP2C8 | Amodiaquine Pioglitazone | Desethylamodiaquine, Hydroxypioglitazone | Montelukast |
| CYP3A4 | Midazolam | 1-Hydroxymidazolam | Ketoconazole |
| UGT1A1 | β-Estradiol | β-Estradiol Glucuronide | Niflumic acid |
| UGT1A6 | Serotonin | Serotonin Glucuronide | Naphthol |
| UGT1A9 | Mycophenolic Acid | Mycophenolic Acid Glucuronide | Niflumic acid |
2. Materials and methods
2.1. Chemicals and reagents
Amodiaquine, serotonin, β-estradiol (≥98%), glucose-6-phosphate, glucose-6-phosphate dehydrogenase, β-nicotinamide adenine dinucleotide phosphate (β-NADP), ammonium acetate and magnesium chloride, potassium phosphate dibasic, UDP-glucuronic acid, alamethicin, niflumic acid, bovine serum albumin and glacial acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pioglitazone hydrochloride (>98% purity), pioglitazone-d4, hydroxypioglitazone (M-IV), hydroxypioglitazone-d4, desethylamodiaquine (DEAQ; 99.3% purity) and DEAQ-d3 (>98% purity) were obtained from Toronto Research Chemicals Inc. (North York, Ontario, Canada). Montelukast was purchased from LKT Labs (St. Paul, MN, USA). Glucose-6-phosphate dehydrogenase solution was prepared by dissolving lyophilized enzyme in 5 mM of sodium citrate, and subsequently stored at −20 °C. Acetonitrile, methanol, potassium phosphate, sodium citrate, formic acid, dimethyl sulfoxide (DMSO) and 1-naphthol were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Serotonin-O-β-D-glucuronide was provided by RTI International (Research Triangle Park, NC) through the National Institute of Mental Health Chemical Synthesis Program. Midazolam, 1-hydroxymidazolam and the internal standard 1-hydroxymidazolam-d4 were obtained from Cerilliant Corporation (Round Rock, TX, USA). Pterostilbene (≥98%), ketoconazole (≥95%) and Estradiol 3-(β-D-glucuronide) (≥95%) were purchased from Cayman Chemical Company (Ann Arbor, Michigan, USA). Barnstead Nanopure Diamond UV Ultrapure water system was the source of the deionized water. Pooled human liver (HLM) and intestinal microsomes (HIM) were purchased from BD Biosciences (Woburn, MA, USA).
2.2. Preparation of pterostilbene
Pterostilbene stock solution was prepared by dissolving pterostilbene powder in methanol purged with nitrogen according to manufacturer’s instructions. Working solutions were freshly prepared by diluting pterostilbene stock solution with water to keep the organic solvent content present in the working solution less than 10%. Thus, the final concentration of methanol in the incubation samples, including controls, was less than 1%. The initial screening was conducted with three concentrations. For confirmation experiments, a wide range of concentrations around the rough IC50 of pterostilbene was used in incubations.
2.3. Analytical instrumentation
Analyses of CYP and UGT enzyme activities were performed with validated HPLC-MS/MS or HPLC-fluorescence methods. The HPLC-MS/MS system included a Surveyor HPLC auto-sampler and a Surveyor MS quaternary pump; the mass spectrometer was a TSQ quantum discovery triple quadrupole mass spectrometer (Thermo Corp., San Jose, CA, USA). The HPLC-fluorescence system was comprised of a Shimadzu LC-10AD Pump linked to a Waters 717 auto-sampler and Waters 2475 fluorescence detector.
2.4. CYP enzyme incubation methods
Briefly, the enzyme-selective substrate (amodiaquine 25 µM; pioglitazone 7.5 µM; or midazolam 50 µM) and HLM (0.1 mg/ml for amodiaquine and pioglitazone, 0.24 mg/ml for midazolam) were mixed in the presence of a phosphate buffer (50 mM, pH 7.4), NADPH regeneration system and various concentrations of pterostilbene. The NADPH regeneration system consisted of: MgCl2 (assay concentration, 3.3 mM), NADP+ (1.25 mM), glucose 6-phosphate (3.3 mM) and glucose 6-phosphate dehydrogenase (0.32 U/ml) in 5 mM sodium citrate solution. The final total volume was 250 µl. Incubations were performed for 10 min at 37 °C and the reaction was stopped by adding ice-cold acetonitrile containing a deuterated internal standard, hydroxypioglitazone-d4 or desethylamodiaquine-d3 or 1-hydroxymidazolam-d4. Samples tubes were vortex-mixed for two minutes and then centrifuged for ten minutes at 14,000g. An aliquot of the supernatant was injected into the LC-MS/MS system. The microsomal incubation experiments and analytical measurements were conducted as previously described for amodiaquine (Dravid & Frye 2008), pioglitazone (Albassam et al., 2015), and midazolam (Nolin et al., 2009).
2.5. UGT enzyme incubation methods
2.5.1. Serotonin and UGT1A6
To study the effect of pterostilbene on UGT1A6 activity, serotonin, a UGT1A6 probe substrate was incubated with pterostilbene in the presence of HLM, as described by Krishnaswamy et al. (2003), with modifications. In brief, the incubation mixture (final volume 100 μl) consisted of serotonin at a concentration close to the reported Km value (8 mM), 50 mM Tri-HCL buffer, 5 mM MgCl2, 0.5 mg/ml microsomal protein and alamethicin (μg/mg protein). The mixture was pre-incubated for ten minutes and the reaction was started by adding UDPGA (final concentration, 5 mM). The mixture then was incubated for 60 min at 37 °C, and the reaction was terminated by adding 20 μl of 24% perchloric acid-acetonitrile (1:1,v/v), vortex-mixing, and then centrifuging for ten minutes at 20,000g. The supernatant was transferred to HPLC autosampler vials. The positive control for inhibition was the UGT1A6 inhibitor 1-Naphthol (50 μM) (Fujiwara et al., 2008). Samples were analyzed by HPLC-fluorescence as described previously (Mohamed & Frye, 2011).
2.5.2. Mycophenolic acid and UGT enzymes
The incubation of mycophenolic acid was conducted according to Mohamed et al (2008) with certain modifications. The incubation mixture (total volume 100 μl) contained HLM or HIM (protein concentration, 0.16 mg/ml), alamethicin (100 μg/mg protein), MgCl2 (5 mM), 2% BSA and 100 μM phosphate buffer, pH7.4. The concentration of mycophenolic acid was equivalent to the reported Km value in HLM (240 μM) and HIM (70 μM). A 15 min pre-incubation of HLM with alamethicin was performed before initializing the reaction by UDPGA (1 mM) and maintaining the incubation tubes in a water bath held at 37 °C for 30 min. The reaction was terminated by adding 300 µl of ice-cold acetonitrile, followed by 20 µl of internal standard (10 µg/ml mycophenolic acid-d3-glucuronide). The incubation tubes were then vortexed for two minutes and centrifuged for ten minutes at 20,000g. The supernatant was diluted with purified water twelve-fold. In the positive control samples, niflumic acid (70 µM) was used as a UGT1A9 inhibitor (Vietri et al., 2002). Samples were analyzed by HPLC-MS/MS as described previously (Mohamed, et al., 2008).
2.5.3. β-estradiol and UGT1A1
The microsomal incubation assay was performed as described by Alkharfy & Frye (2002). The total volume of the incubation mixture was 250 μl, and contained HLM (protein concentration, 0.5 mg/ml), MgCl2 (1 mM), β-estradiol (25 μM), alamethicin (30 μg/ml microsomal protein) and different concentrations of pterostilbene in a 100 μM phosphate buffer, pH 7.4. A five minute pre-incubation time was done for the HLM with alamethicin to activate the UGT enzyme. The reaction was initiated by adding UDPGA (6 mM) and placing the incubation tube in a water bath held at 37 °C for 30 min. The reaction was terminated through the addition of 25 μl of 6% perchloric acid. The incubation tubes were then vortexed for two minutes and centrifuged for ten minutes at 20,000g. A 75 μl aliquot was injected into the HPLC system. In the positive control sample, niflumic acid (250 µM) was used as it is known to inhibit UGT1A1 activity (Mano et al., 2005). The amount of glucuronide metabolite produced was measured by HPLC-fluorescence (Alkharfy & Frye, 2002).
2.6. Data analysis
The remaining enzyme activity was measured by comparing the oxidized or the glucuronide metabolite formation with pterostilbene in the incubation samples and the negative control incubation samples. The remaining enzyme activity was expressed as a percent of control. A sigmoidal concentration-response model was used to fit the data of pterostilbene (inhibitor) and the remaining enzyme activity data using Eq. (1) to predicate IC50 values. The determination of the IC50 values were done using GraphPad Prism 6 (GraphPad, Software Inc., San Diego, CA)
To obtain an estimate of the potential to achieve the IC50 concentration in vivo, the volume per dose index (VDI) was calculated as presented by Eq. (2). This method was described by Strandell et al. (2004). The VDI was defined as the volume in which one dose should dissolve to obtain the corresponding IC50 concentration.
| (1) |
(Y: remaining enzyme activity (percent of control), [I]; Concentration of pterostilbene. H: Hill coefficient).
| (2) |
RDI: recommended daily intake.
3. Results
The effect of pterostilbene on the activity of five enzymes was tested with at least three different concentrations. The rough IC50 and VDI values were estimated according to the concept of the remaining enzyme activity data, which were obtained after testing three concentrations of pterostilbene with an enzyme –selective substrate.
The results from the initial screening are reported in Table 2. The VDI was used to prioritize which pterostilbene-enzyme interactions should be studied further. The VDI cut-off value was based on the site of interaction. The VDI cut-off value for enzymes present in the intestine, such as CYP3A, UGT1A1, UGT1A6 and UGT1A8/10, was 2 L, and for enzymes present mainly in the liver, such as CYP2C8 and UGT1A9, was 5 L (Zanger and Schwab, 2013, Court et al., 2012). A pterostilbene-enzyme interaction that showed potential inhibition with a VDI value exceeding the determined cut-off value at the initial screening phase was selected for more intensive screening with a wide range of pterostilbene concentration to get an accurate assessment of the IC50 and VDI values. The goodness of fit for the IC50 curves (non-linear regression) were determined based on the coefficient of determination (r2), which was greater than 0.9.
Table 2.
Rough IC50 and volume/dose index values for inhibition of enzyme-selective substrates metabolites formation by pterostilbene.
| Enzyme | RDI of Pterostilbene (mg) | IC50 (µM) | V/D index (L/dose) |
|---|---|---|---|
| CYP2C8 | 250 | 26.9 ± 5.6 | 36.30 |
| CYP3A4 | 250 | >100 | 4.70 |
| UGT1A1 | 250 | >500 | <2 |
| UGT1A6 | 250 | 26.7 ± 40.7 | 36.50 |
| UGT1A9 | 250 | >100 | 3.89 |
| UGT1A8/10 | 250 | 92.7 ± 18.4 | 10.53 |
Enzyme selective substrates and pooled HLM or HIM were incubated with at least four different concentrations of pterostilbene. All incubations were performed in duplicate. Using nonlinear regression, rough IC50 values were calculated by fitting the IC50 equation to percent of activity remaining (Material and Methods-Data analysis). Values are reported as best fit IC50 (mean ± standard error). All resulting values had r2 value for goodness of fit of at least 0.9. Volume per dose index (VDI) values were calculated as mentioned in materials and methods. The recommended daily intake values were determined based on the commercially available products and a previous clinical study (Riche, 2012).
3.1. The effect of pterostilbene on CYP2C8 and CYP3A enzymes
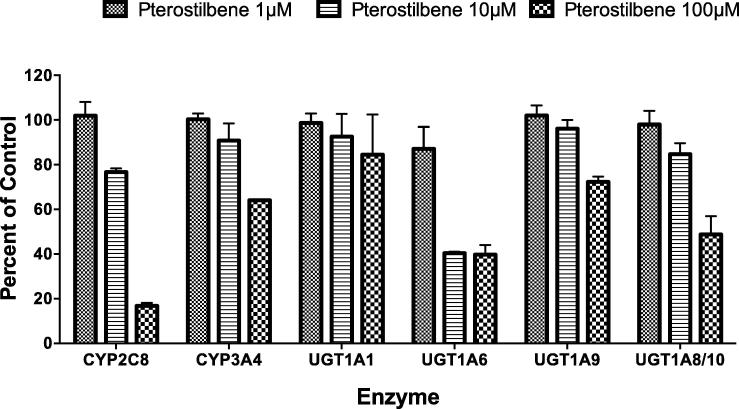
The effect of pterostilbene on CYP2C8 and CYP3A enzyme activity was measured after incubating the pooled HLM with amodiaquine and midazolam, respectively. The formation of the amodiaquine metabolite, desethylamodiaquine, and midazolam metabolite, 1-hydroxymidazolam, was used as an indicator of CYP2C8 and CYP3A enzyme activity, respectively. Pterostilbene inhibited desethylamodiaquine formation more than 50% (Fig. 2), and the rough IC50 values of pterostilbene (mean ± standard error) was 26.9 ± 5.6 μM. The inhibition of CYP3A-mediated metabolism by pterostilbene was <50% at 100 μM. The VDI values of pterostilbene for inhibiting CYP2C8 and CYP3A were 36.3 L and 4.7 L, respectively (Table 2).
Fig. 2.
Effects of pterostilbene on metabolite formation are shown as indexes of CYP and UGT activity in HLM (CYP2C8, CYP3A4, UGT1A1, UGT1A6 and UGT1A9) and in HIM (UGT1A8/10). Each enzyme-selective substrate, CYP2C8 (amodiaquine), CYP3A4 (midazolam), UGT1A1 (estradiol), UGT1A6 (serotonin) and UGT1A8/9/10 (mycophenolic acid) was incubated with HLM or HIM and three concentrations of pterostilbene. The three concentrations of pterostilbene were 1 µM (dotted bars), 10 µM (striped bars) and 100 µM (checkered bars). Error bars represent SE of the mean of duplicate incubations.
3.2. The effect of pterostilbene on UGT1A1, 1A6, 1A8/10, and 1A9 enzymes
UGT probe substrates (Table 1) were used to investigate the influence of pterostilbene on UGT enzymes activity. The formation of the metabolites, estradiol-3-glucuronide, serotonin glucuronide and mycophenolic acid glucuronide in HLM, were used as indicators of UGT1A1, UGT1A6 and UGT1A9 enzyme activity, respectively. The formation of mycophenolic acid glucuronide in HIM was used as an indicator of UGT1A8/10 enzyme activity. Pterostilbene inhibited serotonin glucuronide formation with an estimated IC50 value of 26.7 ± 40.7 μM (Table 2 & Fig. 2). Pterostilbene also modestly inhibited mycophenolic acid glucuronidation in HIM with an IC50 value of 92.7 ± 18.4 μM. The inhibition by pterostilbene observed with estradiol (UGT1A1) and mycophenolic acid in HLM was less than 50% (IC50 > 100 μM). The calculated VDI values for UGT1A1, UGT1A6, UGT1A9 and UGT1A8/10 were <2 L, 36.5 L, 3.8 L and 10.5 L, respectively.
3.3. Confirmatory inhibition experiment and determination of precise IC50
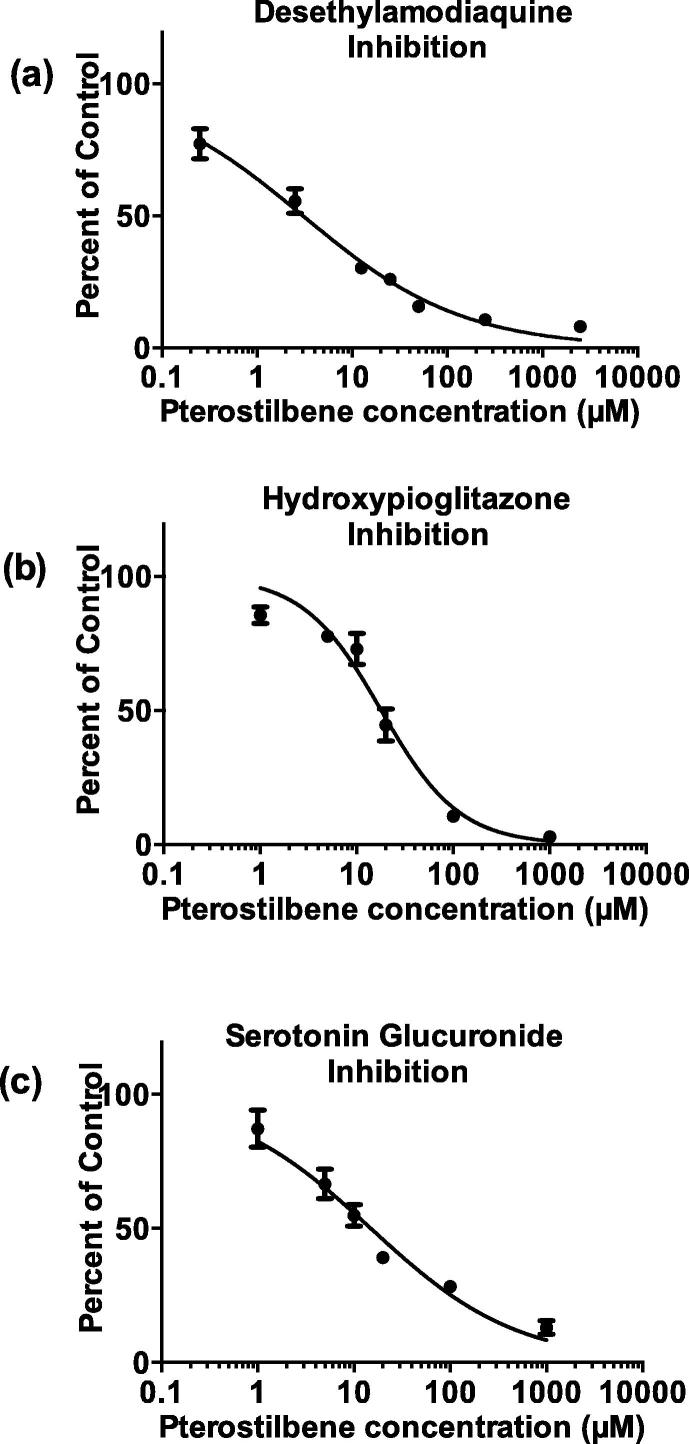
The enzymes CYP2C8 and UGT1A6 were the most inhibited exhibiting rough IC50 estimates of 26.9 μM and 26.7 μM, respectively. The VDI for both exceeded the pre-determined cut-off, and the VDI value was over 36 L. Thus, we selected CYP2C8 and UGT1A6 for advanced investigation, and a wide range of pterostilbene concentrations were used to define the precise IC50 values. The precise IC50 values of pterostilbene (mean ± SE) inhibiting CYP2C8 and UGT1A6 were 3.0 ± 0.4 μM (0.8 ± 0.10 μg/ml) and 15.1 ± 2.8 μM (3.85 ± 0.7 μg/ml), and the VDI values were >50 L (Table 3 & Fig. 3). A confirmatory experiment was conducted with another CYP2C8 probe substrate, pioglitazone, and the determined precise IC50 value with pterostilbene was 17.9 ± 2.2 μM and the VDI value was more than 50 L (Table 3 & Fig. 3).
Table 3.
Precise IC50 and volume/dose index values for pterostilbene showing strong inhibition of DEAQ, hydroxypioglitazone and serotonin glucuronide formation.
| Enzyme | IC50 (µM) | V/D index (L/dose) |
|---|---|---|
| CYP2C8 (Amodiaquine) | 3.0 ± 0.4 | >50 |
| CYP2C8 (Pioglitazone) | 18.0 ± 2.2 | >50 |
| UGT1A6 (Serotonin) | 15.1 ± 2.8 | >50 |
Amodiaquine, pioglitazone and serotonin and pooled HLM were incubated with different concentrations of pterostilbene as mentioned under materials and methods. All incubations were performed in duplicate and the data represent the best-fit IC50 values ± standard error. The r2 values for goodness of fit were >0.9. The recommended daily intake values (250 mg) were determined based on the commercially available products and a previous clinical study (Riche, 2012).
Fig. 3.
Inhibition of (a) DEAQ, (b) hydroxypioglitazone and (c) serotonin glucuronide formation by pterostilbene. Amodiaquine (CYP2C8), pioglitazone (CYP2C8) and serotonin (UGT1A6) were incubated with HLM and multiple concentrations of pterostilbene. The data points in (a) and (b) represent CYP2C8 activity remaining as a percent of control. The data points in (c) represent UGT1A6 activities remaining as a percent of control. Data analysis was performed by nonlinear regressions as discussed in methods. Error bars represent SE of the mean of duplicate incubations.
4. Discussion
In the current study, pterostilbene was screened for its effect on the oxidation activity of CYP2C8 and CYP3A, and on the glucuronidation activity of UGT1A1, UGT1A6, UGT1A9 and UGT1A8/10 in pooled HLM and HIM. The activities were measured using an in vitro system with enzyme selective substrates: amodiaquine and pioglitazone for CYP2C8, midazolam for CYP3A, β-estradiol for UGT1A1, serotonin for UGT1A6 and mycophenolic acid for UGT1A8/10 and UGT1A9. Based on the estimated IC50 and the calculated VDI values, pterostilbene has the highest inhibitory effect toward CYP2C8 and UGT1A6 activity in vitro, and may have the potential to inhibit metabolism by these enzymes in vivo. The IC50 values of pterostilbene towards the CYP2C8 substrates amodiaquine and pioglitazone were 3.0 μM and 17.9 μM and the IC50 value of pterostilbene toward serotonin was 15.1 μM. The calculated VDI values of pterostilbene exceeded 50L per dose unit with amodiaquine, pioglitazone, and serotonin. Of the enzyme activity tested, the results of our study suggest that pterostilbene has the potential to inhibit CYP2C8 and UGT1A6 enzyme activities in vitro, leading to possible drug interactions with drugs metabolized by these enzymes. Clinical studies are needed to determine whether pterostilbene affects drug metabolism in vivo.
The CYP enzymes are a major group of enzymes involved in the metabolism of approximately 75% of all drugs (Lamb et al., 2007). The CYP2C enzyme subfamily metabolizes about 20% of all clinically administered drugs (Evans and Relling, 2004). Compared to other CYP2C subfamily enzyme members such as 2C9 and 2C19, much less is known about factors that modulate CYP2C8 activity, including natural products. However, CYP2C8 is a major enzyme involved in the metabolism of antihyperglycemic agents such as repaglinide, rosiglitazone and pioglitazone. CYP2C8 is a major hepatic CYP enzyme, and represents approximately 7% of the total microsomal CYP content in the liver (Lai et al., 2009, Naraharisetti et al., 2010). The reported IC50 value of quercetin, a well-known strong CYP2C8 inhibitor, after being incubated with amodiaquine, was 0.59 µg/ml (3.94 µM) (Walsky et al., 2005). In addition, the IC50 values of moderate CYP2C8 inhibitors, such as gemfibrozil and trimethoprim, were 18.92 µg/ml and 11.78 µg/ml (75.6 µM and 40.6 µM), respectively (Walsky et al., 2005, Parikh et al., 2007). Pioglitazone was inhibited by montelukast and gemfibrozil, and the IC50 values were 0.29 µg/ml and 24.53 µg/ml (0.18 µM and 59 µM) (Jaakkola et al., 2006). In our study, pterostilbene inhibited amodiaquine and pioglitazone with IC50 values of 3.0 μM and 17.9 μM, which are close to the IC50 of reported with quercetin and montelukast. Additionally, the VDI value exceeded 50 L, indicating that the 250 mg dose can be diluted in up to 50 L and still attain a concentration adequate to inhibit CYP2C8 activity up to 50%. Therefore, depending on systemic concentrations achieved, pterostilbene may inhibit CYP2C8 systemic enzyme activity, leading to a potential adverse effect when it is co-administered with CYP2C8 substrates.
The UGT enzymes are a superfamily of 22 proteins divided into four families and six subfamilies based on the sequence identity. Much of the metabolism, (with subsequent renal elimination) of non-polar (lipophilic) drugs is mediated through the UGT 1A and 2B subfamilies (Rowland et al., 2013). UGT1A6 is considered a low-affinity enzyme that conjugates many drugs, e.g., acetaminophen, naproxen and deferiprone (Mohamed and Frye, 2011). The UGT1A6 hepatic expression ranges from 0.7 to 6.8% of the total UGT expression in the liver. The expression of UGT1A6 in the stomach, small intestine and colon are 19.2%, 8.7% and 6.1% of the total expression within each tissue, respectively (Ohno and Nakajin, 2009, Court et al., 2012). 1-Naphthol, a well-known inhibitor of UGT1A6 activity, was found to inhibit the UGT1A6 glucuronidation of serotonin with an IC50 value between 18 and 21 μM (Fujiwara et al., 2008). Silybin, an active constituent in Milk thistle (Silybum marianum) was found to inhibit UGT1A6 with an IC50 of 28 μM (Sridar et al., 2004). In addition, another study investigated the effect of hypericin, a major active component in St. John’s wort (Hypericum perforatum), on UGT1A6 activity. Hypericin was found to inhibit UGT1A6 glucuronidation of acetaminophen in a human cell line as well as inhibit UGT1A6 glucuronidation of serotonin in UGT expressed in insect cells with IC50 values of 7.1 μM and 0.59 μM (Volak and Court, 2010). Compared to the inhibitory abilities of 1-Napthol, silybin and hypericin toward UGT1A6, pterostilbene falls somewhere in the middle; with an IC50 value of 15.1 μM, higher than hypericin and lower than both 1-Naphthol and silybin. The VDI was more than 50 L, indicating that the typical 250 mg dose of pterostilbene can be diluted in more than 50 L and still inhibit UGT1A6 activity up to 50%. Taken together, these results suggest that pterostilbene has the potential to inhibit the systemic metabolism of UGT1A6 and/or the first-pass metabolism of UGT1A6 substrates in vivo.
In Ayurvedic medicine, Indian kino tree (Pterocarpus marsupium), rich with pterostilbene, has long been used for its health benefits, as an anti-inflammatory, anti-diabetic and astringent (Maurya et al., 2004, Manickam et al., 1997). Recently, in animal studies, pterostilbene has shown anti-hypertensive, anti-cancerous, anti-hypercholesterolemic, and anti-diabetic activity, in addition to its antioxidant and anti-inflammatory properties (McCormack and McFadden, 2013). In a recent clinical study evaluating safety of the long term use of pterostilbene in humans, pterostilbene was found to be safe at 250 mg/day (Riche et al., 2013). In addition, researchers have found that a this dose of pterostilbene was associated with a reduction in blood pressure (Riche, 2012).
After oral administration, most polyphenols go through extensive and rapid conjugation in the intestinal tract in both humans and animals. Therefore, free polyphenols are only absorbed in limited amounts prior to accessing the blood stream. In a bioavailability study conducted in rats, it was shown that pterostilbene has better bioavailability at 80%, compared with resveratrol at 20% (Kapetanovic et al., 2011). Additional animal pharmacokinetic studies have consistently shown a greater bioavailability for pterostilbene compared with resveratrol. Thus, taking the cumulative results of these studies into consideration, it is clear that pterostilbene has a different pharmacokinetics profile; this suggests greater biological availability than resveratrol at the same dose (Lin et al., 2009).
The structure of polyphenolic compounds makes them more susceptible to Phase II metabolism through glucuronidation, sulfation and methylation. The metabolites of polyphenols are mainly eliminated through urine and to a lesser extent in the bile (Asensi et al., 2011, Gao and Hu, 2010). Dellinger et al. (2014) identified the UGT enzymes involved in the metabolism pathway of pterostilbene using human liver microsomes (HLM). UGT1A1 and UGT1A3 were found to be the enzymes predominately responsible for pterostilbene glucuronidation. In addition, UGT1A8, UGT1A9 and UGT1A10 played a minor role in the glucuronidation of pterostilbene (Dellinger et al., 2014). Notably, pterostilbene is glucuronidated to a lesser extent than resveratrol (Kapetanovic et al., 2011), which suggests that the metabolism of pterostilbene will be less in humans.
Even though pterostilbene has been found to have a greater bioavailability than resveratrol, its poor water solubility and stability still affect its bioavailability, and therefore its biological activity. Recently, researchers have been working to improve the solubility and stability of pterostilbene to overcome its poor bioavailability through specific dosage forms and delivery systems. One study investigated the use of pterostilbene in nanoemulsion formulations. The study found that using nanoemulsion delivery systems of pterostilbene significantly improved both solubility and stability (Zhang et al., 2014). Another study investigated the influence of aqueous solubility, fasting, dose escalation and dosing route on pterostilbene bioavailability in rats. Pterostilbene clearance was rapid after an intravenous dose of 2.5 mg/kg and the clearance decreased with higher intravenous doses of pterostilbene. Furthermore, fasting decreased the pterostilbene bioavailability by almost 55%, and using pterostilbene in a solution made of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) improved its bioavailability from 15% with oral aqueous solution to approximately 60% (Yeo et al., 2013). These methods to improve pterostilbene bioavailability may increase the chance of it interacting with drugs metabolized through the inhibited enzymes.
4.1. Conclusion
In conclusion, the main findings of this study suggest that pterostilbene has an inhibitory effect on CYP2C8 and UGT1A6 activity in vitro, and may have the potential to inhibit metabolism by these enzymes in vivo. Pterostilbene’s physicochemical properties confer it with high bioavailability and a long elimination half-life, and efforts to improve pterostilbene solubility and stability increase the potential for interaction with CYP2C8 and UGT1A6 substrates. The calculated volume per dose index (VDI) exceeded 50 L, suggesting a potential for interaction. Thus, clinical studies are warranted to determine whether pterostilbene affects drug metabolism in vivo.
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albassam A.A., Mohamed M.E., Frye R.F. Inhibitory effect of six herbal extracts on CYP2C8 enzyme activity in human liver microsomes. Xenobiotica. 2015;45:406–412. doi: 10.3109/00498254.2014.989935. [DOI] [PubMed] [Google Scholar]
- Alkharfy K.M., Frye R.F. Sensitive liquid chromatographic method using fluorescence detection for the determination of estradiol 3- and 17-glucuronides in rat and human liver microsomal incubations: formation kinetics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;774:33–38. doi: 10.1016/s1570-0232(02)00188-5. [DOI] [PubMed] [Google Scholar]
- Asensi M., Ortega A., Mena S., Feddi F., Estrela J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011;48:197–216. doi: 10.3109/10408363.2011.631268. [DOI] [PubMed] [Google Scholar]
- Bardia A., Nisly N.L., Zimmerman M.B., Gryzlak B.M., Wallace R.B. Use of herbs among adults based on evidence-based indications: findings from the National Health Interview Survey. Mayo Clin. Proc. 2007;82:561–566. doi: 10.4065/82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court M.H., Zhang X., Ding X., Yee K.K., Hesse L.M., Finel M. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica. 2012;42:266–277. doi: 10.3109/00498254.2011.618954. [DOI] [PubMed] [Google Scholar]
- Dellinger R.W., Garcia A.M., Meyskens F.L. Differences in the glucuronidation of resveratrol and pterostilbene: altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014;29:112–119. doi: 10.2133/dmpk.dmpk-13-rg-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid P.V., Frye R.F. Determination of N-desethylamodiaquine by hydrophilic interaction liquid chromatography with tandem mass spectrometry: application to in vitro drug metabolism studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;863:129–134. doi: 10.1016/j.jchromb.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Engdal S., Klepp O., Nilsen O.G. Identification and exploration of herb-drug combinations used by cancer patients. Integr. Cancer Ther. 2009;8:29–36. doi: 10.1177/1534735408330202. [DOI] [PubMed] [Google Scholar]
- Evans W.E., Relling M.V. Moving towards individualized medicine with pharmacogenomics. Nature. 2004;429:464–468. doi: 10.1038/nature02626. [DOI] [PubMed] [Google Scholar]
- Fujiwara R., Nakajima M., Yamanaka H., Katoh M., Yokoi T. Product inhibition of UDP-glucuronosyltransferase (UGT) enzymes by UDP obfuscates the inhibitory effects of UGT substrates. Drug. Metab. Dispos. 2008;36:361–367. doi: 10.1124/dmd.107.018705. [DOI] [PubMed] [Google Scholar]
- Gao S., Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev. Med. Chem. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola T., Backman J.T., Neuvonen M., Niemi M., Neuvonen P.J. Montelukast and zafirlukast do not affect the pharmacokinetics of the CYP2C8 substrate pioglitazone. Eur. J. Clin. Pharmacol. 2006;62:503–509. doi: 10.1007/s00228-006-0136-9. [DOI] [PubMed] [Google Scholar]
- Kapetanovic I.M., Muzzio M., Huang Z., Thompson T.N., McCormick D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Can. Chemother. Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy S., Duan S.X., Von Moltke L.L., Greenblatt D.J., Sudmeier J.L., Bachovchin W.W., Court M.H. Serotonin (5-hydroxytryptamine) glucuronidation in vitro: assay development, human liver microsome activities and species differences. Xenobiotica. 2003;33:169–180. doi: 10.1080/0049825021000048809. [DOI] [PubMed] [Google Scholar]
- Lai X.S., Yang L.P., Li X.T., Liu J.P., Zhou Z.W., Zhou S.F. Human CYP2C8: structure, substrate specificity, inhibitor selectivity, inducers and polymorphisms. Curr. Drug Metab. 2009;10:1009–1047. doi: 10.2174/138920009790711832. [DOI] [PubMed] [Google Scholar]
- Lamb D.C., Waterman M.R., Kelly S.L., Guengerich F.P. Cytochromes P450 and drug discovery. Curr. Opin. Biotechnol. 2007;18:504–512. doi: 10.1016/j.copbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Li L., Hu H., Xu S., Zhou Q., Zeng S. Roles of UDP-glucuronosyltransferases in phytochemical metabolism of herbal medicines and the associated herb-drug interactions. Curr. Drug. Metab. 2012;13:615–623. doi: 10.2174/1389200211209050615. [DOI] [PubMed] [Google Scholar]
- Lin H.S., Yue B.D., Ho P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009;23:1308–1315. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- Lindstrom A.O.C., Lynch M.E., Blumenthal M., Kawa K. Sales of herbal dietary supplements increase by 7.9% in 2013, marking a decade of rising sales: turmeric supplements climb to top ranking in natural channel. HerbalGram. 2014:52–56. [Google Scholar]
- Manickam M., Ramanathan M., Jahromi M.A., Chansouria J.P., Ray A.B. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997;60:609–610. doi: 10.1021/np9607013. [DOI] [PubMed] [Google Scholar]
- Mano Y., Usui T., Kamimura H. In vitro inhibitory effects of non-steroidal antiinflammatory drugs on UDP-glucuronosyltransferase 1A1-catalysed estradiol 3beta-glucuronidation in human liver microsomes. Biopharm. Drug. Dispos. 2005;26:35–39. doi: 10.1002/bdd.430. [DOI] [PubMed] [Google Scholar]
- Maurya R., Singh R., Deepak M., Handa S.S., Yadav P.P., Mishra P.K. Constituents of Pterocarpus marsupium: an ayurvedic crude drug. Phytochemistry. 2004;65:915–920. doi: 10.1016/j.phytochem.2004.01.021. [DOI] [PubMed] [Google Scholar]
- McCormack D., McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell Longev. 2013;2013 doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.E., Frye R.F. Inhibitory effects of commonly used herbal extracts on UDP-glucuronosyltransferase 1A4, 1A6, and 1A9 enzyme activities. Drug. Metab. Dispos. 2011;39:1522–1528. doi: 10.1124/dmd.111.039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.F., Harvey S.S., Frye R.F. Determination of mycophenolic acid glucuronide in microsomal incubations using high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;870:251–254. doi: 10.1016/j.jchromb.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Naraharisetti S.B., Lin Y.S., Rieder M.J., Marciante K.D., Psaty B.M., Thummel K.E., Totah R.A. Human liver expression of CYP2C8: gender, age, and genotype effects. Drug. Metab. Dispos. 2010;38:889–893. doi: 10.1124/dmd.109.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin T.D., Frye R.F., Le P., Sadr H., Naud J., Leblond F.A., Pichette V., Himmelfarb J. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J. Am. Soc. Nephrol. 2009;20:2269–2276. doi: 10.1681/ASN.2009010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug. Metab. Dispos. 2009;37:32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- Parikh S., Ouedraogo J.B., Goldstein J.A., Rosenthal P.J., Kroetz D.L. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin. Pharmacol. Ther. 2007;82:197–203. doi: 10.1038/sj.clpt.6100122. [DOI] [PubMed] [Google Scholar]
- Riche, D.M., 2012. Impact of pterostilbene on metabolic parameters in humans. In: Deschamp, D., G.M., McEwen, C.L., Riche, K.D., Sherman, J.J., Wofford, M.R. (Ed.), Poster presentation at: American Heart Association 2012 Scientific Sessions on High Blood Pressure Research.
- Riche D.M., McEwen C.L., Riche K.D., Sherman J.J., Wofford M.R., Deschamp D., Griswold M. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol. 2013;2013 doi: 10.1155/2013/463595. 463595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland A., Miners J.O., Mackenzie P.I. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int. J. Biochem. Cell. Biol. 2013;45:1121–1132. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Sridar C., Goosen T.C., Kent U.M., Williams J.A., Hollenberg P.F. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug. Metab. Dispos. 2004;32:587–594. doi: 10.1124/dmd.32.6.587. [DOI] [PubMed] [Google Scholar]
- Strandell J., Neil A., Carlin G. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomedicine. 2004;11:98–104. doi: 10.1078/0944-7113-00379. [DOI] [PubMed] [Google Scholar]
- Vietri M., Pietrabissa A., Mosca F., Pacifici G.M. Inhibition of mycophenolic acid glucuronidation by niflumic acid in human liver microsomes. Eur. J. Clin. Pharmacol. 2002;58:93–97. doi: 10.1007/s00228-001-0407-4. [DOI] [PubMed] [Google Scholar]
- Volak L.P., Court M.H. Role for protein kinase C delta in the functional activity of human UGT1A6: implications for drug-drug interactions between PKC inhibitors and UGT1A6. Xenobiotica. 2010;40:306–318. doi: 10.3109/00498251003596817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky R.L., Gaman E.A., Obach R.S. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharmacol. 2005;45:68–78. doi: 10.1177/0091270004270642. [DOI] [PubMed] [Google Scholar]
- White C.P., Hirsch G., Patel S., Adams F., Peltekian K.M. Complementary and alternative medicine use by patients chronically infected with hepatitis C virus. Can. J. Gastroenterol. 2007;21:589–595. doi: 10.1155/2007/231636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.C., Ho P.C., Lin H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013;57:1015–1025. doi: 10.1002/mnfr.201200651. [DOI] [PubMed] [Google Scholar]
- Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shang Z., Gao C., Du M., Xu S., Song H., Liu T. Nanoemulsion for solubilization, stabilization, and in vitro release of pterostilbene for oral delivery. AAPS. 2014 doi: 10.1208/s12249-014-0129-4. PharmSciTech. [DOI] [PMC free article] [PubMed] [Google Scholar]