Abstract
An auditory brainstem implant (ABI) is a surgically implanted central neural auditory prosthesis for the treatment of profound sensorineural hearing loss in children and adults who are not cochlear implant candidates due to a lack of anatomically intact cochlear nerves or implantable cochleae. The device consists of a multielectrode surface array which is placed within the lateral recess of the fourth ventricle along the brainstem and directly stimulates the cochlear nucleus, thereby bypassing the peripheral auditory system. In the United States, candidacy criteria for ABI include deaf patients with neurofibromatosis type 2 (NF2) who are 12 years or older undergoing first- or second-side vestibular schwannoma resection. In recent years, several non-NF2 indications for ABI have been explored, including bilateral cochlear nerve avulsion from trauma, complete ossification of the cochlea due to meningitis, or a severe cochlear malformation not amenable to cochlear implantation. In addition, growing experience with ABI in infants and children has been documented with encouraging outcomes. While cochlear implantation generally remains the first-line option for hearing rehabilitation in NF2 patients with stable tumors or post hearing preservation surgery where hearing is lost but a cochlear nerve remains accessible for stimulation, an ABI is the next alternative in cases where the cochlear nerve is absent and/or if the cochlea cannot be implanted. Herein, we review ABI device design, clinical evaluation, indications, operative technique, and outcomes as it relates to lateral skull base pathology.
Keywords: auditory brainstem implant, hearing loss, auditory prosthesis, electric stimulation, auditory nerve, neurofibromatosis type 2
Introduction
Options for hearing rehabilitation are dictated by the nature and etiology of the hearing loss. While multichannel cochlear implantation is an extremely effective method of hearing rehabilitation for congenital and acquired sensorineural hearing loss, there remains a population of patients with conditions that involve the cochlea or cochlear nerve which make peripheral cochlear stimulation ineffective or impossible, and such patients may not receive adequate benefit from a cochlear implant (CI). Most commonly, this situation occurs in patients with neurofibromatosis type 2 (NF2), in which bilateral vestibular schwannoma growth or surgical removal results in loss of cochlear nerve function. In this situation, direct electric stimulation of the cochlear nucleus at the brainstem using an auditory brainstem implant (ABI) is possible.
House and Hitselberger reported the first results of an ABI in 1979 after placing the ABI electrode via translabyrinthine craniotomy at the time of vestibular schwannoma removal. 1 2 The first implant was a platinum electrode pair with 0.5 mm balls separated by 1.5 mm that was placed within the brainstem in the region of the cochlear nucleus. Initially, the patient did very well and reported benefit with enhanced lip reading and environmental sound awareness. Over time, however, her performance deteriorated which was attributed to electrode migration. As a result, future iterations of device design were aimed at reducing electrode migration.
In 2000, the Food and Drug Administration (FDA) approved the use of a multichannel ABI in patients 12 years of age and older diagnosed with NF2. In more recent years, particularly in Europe and countries outside of the United States, growing experience with ABIs in non-NF2 indications (bilateral total ossified cochlea, inner ear malformations, bilateral temporal bone fractures) has been reported. 3 4 5 6 Furthermore, studies to expand ABI indications to children and infants who are not CI candidates are underway. Colletti et al performed the first pediatric ABI surgery for auditory nerve aplasia in 2001 which established the feasibility of this device in the pediatric population. 7
To date, over 1,000 ABI procedures have been performed worldwide. 8 Results have been mixed, but the ABI continues to offer hope for a population of patients who otherwise do not have access to the auditory world. While the speech perception benefits are usually modest at best, the auditory information can serve to aid with lip reading, as well as provide protective and quality of life benefits from hearing environmental sounds. 9 10
ABI Design and Function
Similar to a CI, the ABI device includes both external and internal components. The external components include the microphone, battery, speech processor, external magnet, and transmitter antenna. The internal components include the internal magnet, antenna, receiver–stimulator, and an electrode array ( Fig. 1 ). Sound is first detected by a microphone (worn on the ear) and converted into an electrical signal. This signal is then sent to an external sound processor where it is transformed into an electronic code. This code is transmitted via radiofrequency through the skin by a transmitting coil that is held externally over the receiver–stimulator by a magnet. Subsequently, this code is translated by the receiver–stimulator into rapid electrical impulses distributed to individual wires which travel along the array to the electrode contacts which are housed within a soft silicone paddle that is placed along the surface of the brainstem, thereby allowing direct stimulation of the cochlear nucleus.
Fig. 1.
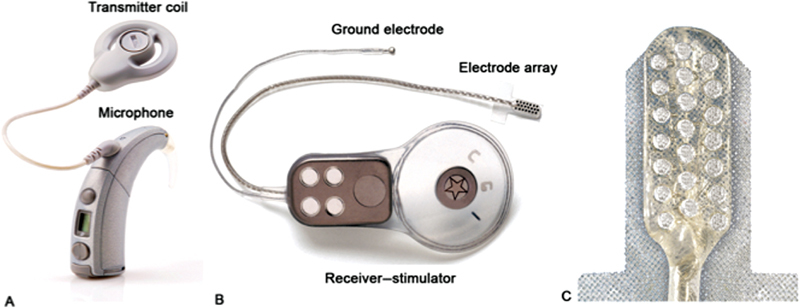
Cochlear corporation auditory brainstem implant device. ( A ) External component including microphone, sound processor, and magnet. ( B ) Internal component including the receiver–stimulator and electrode array. ( C ) Close-up view of the electrode contact paddle which interfaces with the brainstem. Image courtesy of Cochlear Americas, © 2019.
Since the tonotopicity of the cochlear nucleus is arranged obliquely through the pons, an ABI with penetrating electrodes has been designed to selectively stimulate frequencies that are located in areas deep to the surface. However, results of the penetrating ABI implant have not demonstrated an advantage over surface electrodes, in part due to the difficulty in definitively identifying the cochlear nucleus prior to placement. Midbrain implants placed on the dorsal surface of the inferior colliculus have also been reported for patients with a damaged or dysfunctional cochlear nucleus, to stimulate the auditory pathway more proximally.
In the United States, the only FDA-approved ABI device is by Cochlear Corporation (Sydney, Australia). The latest model (ABI541) features 21 platinum electrode contacts along the paddle, which measures 8.5 × 3.0 mm and is designed to work with the Nucleus 6 sound processor. MED-EL Corporation (Innsbruck, Austria) and Oticon Medical (Vallauris, France) also manufacture ABI devices which are used outside the United States.
Candidacy Evaluation
Evaluation for candidacy should be performed by a multidisciplinary team involving neurotology, neurosurgery, audiology, speech therapy, and neuropsychology. An ABI is indicated for patients with bilateral profound hearing loss due to an absent or nonfunctional cochlear nerve and/or an absent or un-implantable cochlea. The most common candidates are NF2 patients who have undergone tumor resection with known loss of their cochlear nerve. However, in nontumor patients, candidacy evaluation should first ensure that a CI is not a feasible option, due to the superior audiometric outcomes with cochlear implantation compared with ABI. A magnetic resonance imaging (MRI) of the brain is used to evaluate the patency of the cochlea and the presence or absence of the cochlear nerve. Heavily T2-weighted MRI is superior to high-resolution computed tomography (CT) for detecting subtle cochlear fibrosis. In patients without vestibular schwannoma, parasagittal thin-slice MRI through the bilateral internal auditory canals can be used to determine the presence and size of the cochlear nerves. Additionally, CT may complement MRI by evaluating for a patent cochlear nerve aperture between the internal auditory canal and modiolus. Radiographic evidence of an absent cochlea is a straightforward indication for ABI. However, radiographic evidence of cochlear nerve aplasia is less straightforward since resolution can be limited in identifying very thin cochlear nerves or those running with the facial nerve, even when using high-resolution T2-weighted MRI sequences (e.g., CISS and FIESTA). Several publications have shown an occult connection between the peripheral and central auditory pathways in the presence of an otherwise absent cochlear nerve radiographically. 11 12 13 For these reasons, functional tests of the auditory pathway—including conventional audiometry or promontory stimulation testing—remain the gold standard for determining the presence of a cochlear nerve. 12 Patients with gross sound perception or sound perception after promontory stimulation should first undergo cochlear implantation.
There are several conditions whereby an NF2 patient should also undergo cochlear implantation prior to considering ABI, including those with stable or radiated tumors and in those who have previously undergone microsurgical tumor resection with preservation of the cochlear nerve. In patients with a history of tumor removal whereby the status of the cochlear nerve is uncertain, use of promontory stimulation may be performed. 14 15 This should be done no sooner than 6 to 8 weeks after surgery to allow for neuropraxia to resolve. 16 Of note, an absent promontory stimulation waveform does not exclude the possibility that the patient will derive benefit with a CI. 15 Since CI surgery is performed on an outpatient basis and has a favorable risk profile when compared with ABI surgery, it is often attempted prior to ABI when feasible. If successful, a CI could provide open-set speech recognition in up to 70% of cases, which is significantly better than what can be achieved with an ABI. 17 18
In NF2 patients, obtaining an MRI prior to placement of an implant also provides them with their last opportunity to obtain a high-quality brain image without artifact or the need to remove a magnet. Additional MRI of the entire neuroaxis is important in NF2 patients since the presence of tumors along the spine may result in neurologic changes during surgery or may complicate the ability to perform a lumbar puncture during the postoperative period in the event of a cerebrospinal fluid (CSF) leak or suspected meningitis.
Indications
Indications for ABI can broadly be classified into two categories: NF2 patients and nontumor patients. The current criteria for an ABI in the United States are NF2 patients 12 years or older with poor hearing bilaterally and large vestibular schwannomas that do not allow for preservation of the cochlear nerve. In these patients, an ABI may be considered during first- or second-side tumor removal. The major benefit of implanting the ABI at the time of first tumor removal is to give the patient more time and experience using the device before they become reliant on it once their contralateral hearing decreases. Additionally, placement of the device may be more straightforward due to less anatomic distortion and scarring which may occur over time and result in obscuring of brainstem landmarks. 19 20
The nontumor indications for ABI include those with deafness secondary to bilateral temporal bone fractures in which the cochlear nerve has been avulsed or resulted in labyrinthine ossification, and in patients who suffered from meningitis which caused complete ossification of the cochlea. In addition, some patients with severe congenital inner ear malformation (e.g., complete labyrinthine aplasia, cochlear aplasia or cochlear nerve aplasia) are candidates for ABI since there is no receiving cavity to house a CI electrode array and/or a nerve to propagate the signal to the brainstem. The majority of experience with ABI in this cohort of patients stems from the international literature, and audiometric outcomes appear to be superior compared with those with NF2. 3 21
Once a patient has met criteria for undergoing ABI surgery, thorough counseling with regard to expectations after implantation is critical. Patients should understand that an ABI does not provide normal sound quality and achieving open-set speech recognition is not achieved in most patients. Sound awareness may be a reasonable goal, however. Nevertheless, among the surgical risks, there are risks that the ABI fails to provide auditory sensations. Candidates should also understand the necessity of following through with postimplant speech/auditory rehabilitation to gain the maximum benefit from their implant.
Operative Technique
General anesthesia without long-term paralytics should be administered to allow for nerve monitoring. The patient is positioned in the supine position with the head turned to the contralateral side. Continuous electromyography facial nerve monitor electrodes are applied in standard fashion. Subdermal electrodes are also placed for measuring an electrical auditory brainstem response (EABR) once the implant is in place. These electrodes are placed at the vertex of the head, over the seventh cervical vertebrae and the hairline of the occiput. An endotracheal tube with recurrent laryngeal nerve monitoring electrodes is also used for intubation to monitor cranial nerve X (CN X).
An ABI can be placed using either a translabyrinthine approach or a retrosigmoid approach. In the NF2 population in which tumor removal is being performed simultaneously, a translabyrinthine approach is often preferred as it allows a more lateral view of the brainstem and a better view into the foramen of Luschka. If a retrosigmoid approach is performed, the craniotomy should be placed as far forward and inferiorly as possible, skeletonizing and retracting the sigmoid sinus anteriorly, which will give the most direct access with the least cerebellar retraction.
After the skin is incised, an anteriorly based periosteal (Palva) flap is made and a superior/posterior subperiosteal pocket under the temporalis muscle is dissected ( Fig. 2 ). A bony well is drilled to secure the receiver/stimulator. A silicone replica of the receiver/stimulator is helpful in contouring the shape and size of the well. A trough between the receiver/stimulator and craniotomy opening is also drilled to house and protect the wires.
Fig. 2.
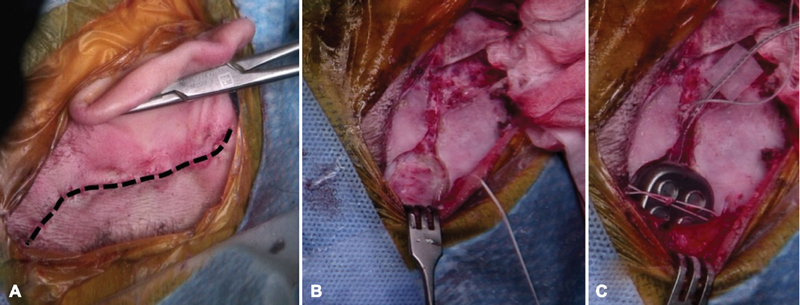
( A ) Postauricular incision for placing an auditory brainstem implant through a translabyrinthine approach. The postauricular incision is curved posteriorly at the superior aspect. An anteriorly based periosteal (Palva) flap provides exposure to the mastoid cortex. ( B ) A bony well contoured to the shape of the device is drilled posteriorly and superiorly to the lateral sinus. This may be taken down to the dura if necessary. A trough is also drilled for placement of the electrode array. Inferior and superior tie down holes are drilled. ( C ) After exposure and identification of the lateral recess of the fourth ventricle, the device is then placed into the bony well and sutured tightly into place. The lead wires are placed in the trough, and free ground wire is placed medial to the temporalis muscle.
The approach to the cerebellopontine angle (CPA) is performed in the standard fashion and the lower CNs as well as CN VII and VIII (if present) are identified. The foramen of Luschka is the pathway to the cochlear nucleus intraoperatively ( Fig. 3 ). The foramen of Luschka projects into the CPA at the lateral border of the pontomedullary sulcus and can be found between the roots of CN VII and CN IX, where the choroid plexus is identified. Alternatively, if a remnant of CN VIII is present, this can be followed back to the brainstem where the choroid plexus can then be identified. The choroid can be gently spread to enlarge the opening into the foramen of Luschka. Often the tenia (a soft tissue arachnoid band) is opened to allow access to the foramen. Additionally, a vein over the foramen can be dissected away from the opening. To verify correct identification of the lateral recess of the fourth ventricle, a Valsalva can be performed and CSF outflow should be noted.
Fig. 3.
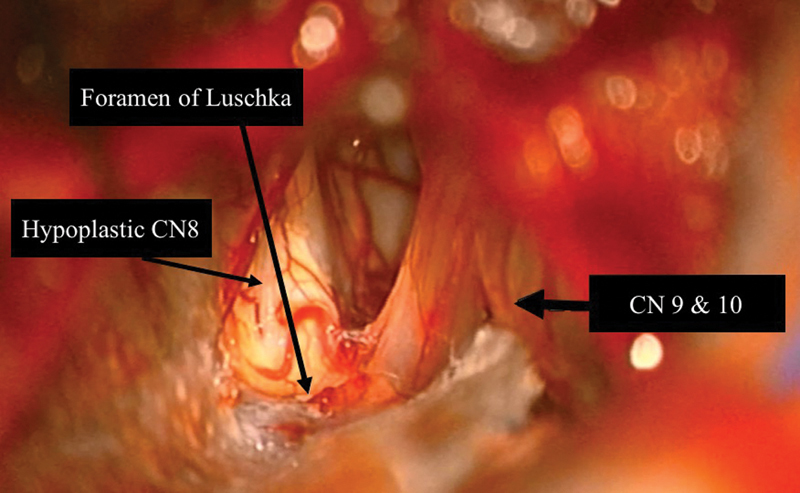
Exposure of the brainstem via the translabyrinthine approach. Note the foramen of Luschka—where auditory brainstem implant is inserted—is identified at the lateral border of the pontomedullary sulcus. The junction of the glossopharyngeal and vagus nerves with the brainstem is just ventral to the foramen and the junction of the facial and vestibulocochlear nerves with the brainstem is anteroinferior to it. The junction of the accessory and hypoglossal nerves with the brainstem is anteroinferior to the foramen as well. The cerebellar flocculus is directly superior to the foramen. Also note the severely hypoplastic eighth cranial nerve in this patient.
The device is then brought onto the field and secured in the bony well and the soft tissue pocket is sutured closed to help prevent device migration. The lead wires are placed into the trough and the free ground wire is placed medial to the temporalis muscle periosteum. The paddle is inserted into the lateral recess of the fourth ventricle with electrodes facing superiorly and anteriorly. The Dacron wings on the electrode paddle may need to be carefully trimmed to allow proper positioning. Audiologists are present intraoperatively and test the device by sending electrical stimulation to individual electrodes. This can determine whether an EABR is evoked or whether nonauditory stimuli patterns are noted such as facial nerve stimulation, myogenic responses, or changes in pulse rate or hemodynamics. Once the electrode is in appropriate position as confirmed by electrical testing, a piece of Teflon felt is placed posterior to the electrode paddle to stabilize the device. The matrix of Dacron mesh on the electrode paddle provides a scaffold for ingrowth of fibrous tissue that will further stabilize the electrode position. The craniotomy is closed in standard fashion for the given approach.
Postoperatively, patients are monitored in the intensive care unit for 24 hours and subsequently transferred to the floor and begin mobilizing on postoperative day 1. The majority of patients are discharged home on postoperative day 3. The implant is activated 6 weeks after implantation. During initial activation, monitoring for vagal nerve stimulation and significant nonauditory side effects is performed since bradycardia, motor long tract stimulation, vertigo, throat tightening, and fainting may occur. For these reasons, cardiac monitoring and physician attendance are part of the protocol for initial stimulation. The programming audiologist can “program out” any of nonauditory symptoms that the patient experiences.
The most common postoperative complications are CSF leak, implant migration, and nonauditory stimuli. CSF leaks may be treated with lumbar drain placement for CSF diversion. Rarely, reoperation for leak repair is necessary. Less common complications include cerebellar contusion, permanent facial palsy, meningitis, damage of the lower CNs, hydrocephalus, pseudomeningocele, headache, and tinnitus. These complications are significantly less in the nontumor patients than in the NF2 population. 22
Outcomes
Auditory performance with ABIs remains highly variable. Although the speech outcomes are poorer compared with cochlear implantation, the restoration of some auditory input is encouraging. Variability in performance is attributable to variations in surgical technique, surgeon experience, postimplant programming, and signal-coding strategies. In addition, the tonotopic organization of the cochlear nucleus is much more complex than what is observed in the cochlea. Specifically, in the cochlear nucleus, frequencies are encoded from superficial-to-deep as opposed to along the surface, which is suboptimal for surface electrode stimulation.
Perhaps the most significant predictor of postoperative speech performance is related to the etiology of the hearing loss, specifically whether it was due to an NF2-related tumor versus a nontumor condition (e.g., ossified cochlea, cochlear nerve avulsion from trauma). In NF2 patients, a multi-institutional study in the United States reported that overall (adults and children) 81% of implants received auditory sensations. 23 Unfortunately, this implies that nearly 20% fail to respond to stimulation altogether. 24 25 In addition, significant open-set word recognition is rare (around 10%) and therefore speech outcomes are much poorer with ABI compared with CI. 19 25 26 The greatest benefit attributable to the ABI comes in the form of enhanced lip reading, as it helps with determining the rhythm, stress, timing, and intensity of speech. When combined with lip reading, 93% of patients demonstrate improved sentence understanding at 3 to 6 months postimplant. 23
Outcomes in nontumor patients appear to be superior when compared with those with NF2. 21 27 Colletti et al reported that postlingual adults without NF2 achieved an average of 59% open-set sentence recognition in the audition-alone mode, compared with 10% in NF2 patients. 27 These differences in auditory performance between NF2 patients and those without tumors suggest that the NF2 condition itself may adversely affect the cochlear nucleus or auditory pathway. While a large tumor may damage or distort the cochlear nucleus resulting in a poor outcome, this alone does not explain the discrepancy because even patients with small tumors that do not contact the brainstem have demonstrated poor performance. 28 Colletti and Shannon compared 10 NF2 patients with 10 non-NF2 patients and examined the electric stimulation thresholds, electrode selectivity, and amplitude modulation and speech perception. 21 27 They found that NF2 patients had significantly worse modulation detection and speech discrimination than the non-NF2 cohorts. The physiologic reason for this is unclear but postulated to be due to damage to a specific cell type or region within the cochlear nucleus.
Given these promising outcomes in non-NF2 ABI recipients, studies to expand ABI indications to children and infants who are not CI candidates are underway. Colletti et al reported on a long-term prospective analysis of 64 deaf children implanted with ABIs and followed up for up to 12 years. 7 All children in the study showed improvement in auditory perception, with 11% being able to converse on the telephone and 31.3% realizing open-set speech recognition. Early experiences with pediatric ABI in the United States also show promise but long-term studies have not been published. 29 30
Conclusion
ABIs provide a safe and effective way to provide some degree of auditory rehabilitation to patients who are not candidates for a CI or who have failed to benefit from a CI. However, the degree of auditory rehabilitation can vary significantly and patients should be counseled with regard to realistic expectations and risks of the surgery. The functional aspect of hearing restored by ABIs is rarely comparable to the benefit received by the majority of CI recipients. Multimodal language access should be provided, especially early in the rehabilitation, for all patients where ABI placement is being considered. At present, ABIs have been shown to provide auditory benefit to a group of patients who otherwise would be completely isolated from the auditory world, such as in patients with bilateral skull base lesions resulting in nonviable cochlear nerves. Future research and iterations of the device design and signal processing strategies are being conducted in hopes to further improve outcomes.
Conflictof Interest JTR is a consultant for Cochlear Americas and receives research grant money.
Financial Material and Support
Internal departmental funding was utilized without commercial sponsorship or support.
References
- 1.Hitselberger W E, House W F, Edgerton B J, Whitaker S. Cochlear nucleus implants. Otolaryngol Head Neck Surg. 1984;92(01):52–54. doi: 10.1177/019459988409200111. [DOI] [PubMed] [Google Scholar]
- 2.House W F, Hitselberger W E. Twenty-year report of the first auditory brain stem nucleus implant. Ann Otol Rhinol Laryngol. 2001;110(02):103–104. doi: 10.1177/000348940111000201. [DOI] [PubMed] [Google Scholar]
- 3.Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino F. Auditory brainstem implant (ABI): new frontiers in adults and children. Otolaryngol Head Neck Surg. 2005;133(01):126–138. doi: 10.1016/j.otohns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Grayeli A B, Bouccara D, Kalamarides M et al. Auditory brainstem implant in bilateral and completely ossified cochleae. Otol Neurotol. 2003;24(01):79–82. doi: 10.1097/00129492-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino F G. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115(03):445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- 6.Colletti L. Beneficial auditory and cognitive effects of auditory brainstem implantation in children. Acta Otolaryngol. 2007;127(09):943–946. doi: 10.1080/00016480601110253. [DOI] [PubMed] [Google Scholar]
- 7.Colletti L, Shannon R V, Colletti V. The development of auditory perception in children after auditory brainstem implantation. Audiol Neurotol. 2014;19(06):386–394. doi: 10.1159/000363684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.House Research Institute.FDA approves clinical trial of auditory brainstem implant procedure for children in U.S.”Available at:www.sciencedaily.com/releases/2013/01/130122101334.htm
- 9.Lundin K, Stillesjö F, Nyberg G, Rask-Andersen H. Self-reported benefit, sound perception, and quality-of-life in patients with auditory brainstem implants (ABIs) Acta Otolaryngol. 2016;136(01):62–67. doi: 10.3109/00016489.2015.1079925. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes N F, Goffi-Gomez M V, Magalhães A T, Tsuji R K, De Brito R V, Bento R F. Satisfaction and quality of life in users of auditory brainstem implant. CoDAS. 2017;29(02):e20160059. doi: 10.1590/2317-1782/20172016059. [DOI] [PubMed] [Google Scholar]
- 11.Buchman C A, Teagle H F, Roush P A et al. Cochlear implantation in children with labyrinthine anomalies and cochlear nerve deficiency: implications for auditory brainstem implantation. Laryngoscope. 2011;121(09):1979–1988. doi: 10.1002/lary.22032. [DOI] [PubMed] [Google Scholar]
- 12.Warren F M, III, Wiggins R H, III, Pitt C, Harnsberger H R, Shelton C. Apparent cochlear nerve aplasia: to implant or not to implant? Otol Neurotol. 2010;31(07):1088–1094. doi: 10.1097/MAO.0b013e3181eb3272. [DOI] [PubMed] [Google Scholar]
- 13.Song M H, Kim S C, Kim J, Chang J W, Lee W S, Choi J Y. The cochleovestibular nerve identified during auditory brainstem implantation in patients with narrow internal auditory canals: can preoperative evaluation predict cochleovestibular nerve deficiency? Laryngoscope. 2011;121(08):1773–1779. doi: 10.1002/lary.21791. [DOI] [PubMed] [Google Scholar]
- 14.Roehm P C, Mallen-St Clair J, Jethanamest D et al. Auditory rehabilitation of patients with neurofibromatosis type 2 by using cochlear implants. J Neurosurg. 2011;115(04):827–834. doi: 10.3171/2011.5.JNS101929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng K A, Lorenz M B, Otto S R, Brackmann D E, Wilkinson E P. Cochlear implantation and auditory brainstem implantation in neurofibromatosis type 2. Laryngoscope. 2018;128(09):2163–2169. doi: 10.1002/lary.27181. [DOI] [PubMed] [Google Scholar]
- 16.Neff B A, Wiet R M, Lasak J M et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117(06):1069–1072. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- 17.Pai I, Dhar V, Kelleher C et al. Cochlear implantation in patients with vestibular schwannoma: a single United Kingdom center experience. Laryngoscope. 2013;123(08):2019–2023. doi: 10.1002/lary.24056. [DOI] [PubMed] [Google Scholar]
- 18.Carlson M L, Breen J T, Driscoll C L et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol. 2012;33(05):853–862. doi: 10.1097/MAO.0b013e318254fba5. [DOI] [PubMed] [Google Scholar]
- 19.Brackmann D E, Hitselberger W E, Nelson R A et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108(06):624–633. doi: 10.1177/019459989310800602. [DOI] [PubMed] [Google Scholar]
- 20.Colletti V, Sacchetto L, Giarbini N, Fiorino F, Carner M. Retrosigmoid approach for auditory brainstem implant. J Laryngol Otol Suppl. 2000;•••(27):37–40. doi: 10.1258/0022215001904707. [DOI] [PubMed] [Google Scholar]
- 21.Colletti V, Shannon R V. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115(11):1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- 22.Colletti V, Shannon R V, Carner M, Veronese S, Colletti L. Complications in auditory brainstem implant surgery in adults and children. Otol Neurotol. 2010;31(04):558–564. doi: 10.1097/MAO.0b013e3181db7055. [DOI] [PubMed] [Google Scholar]
- 23.Ebinger K, Otto S, Arcaroli J, Staller S, Arndt P. Multichannel auditory brainstem implant: US clinical trial results. J Laryngol Otol Suppl. 2000;•••(27):50–53. doi: 10.1258/0022215001904743. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M S, Otto S R, Shannon R V, Hitselberger W E, Brackmann D E. Auditory brainstem implants. Neurotherapeutics. 2008;5(01):128–136. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto S R, Brackmann D E, Hitselberger W E, Shannon R V, Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96(06):1063–1071. doi: 10.3171/jns.2002.96.6.1063. [DOI] [PubMed] [Google Scholar]
- 26.Toh E H, Luxford W M. Cochlear and brainstem implantation. Otolaryngol Clin North Am. 2002;35(02):325–342. doi: 10.1016/s0030-6665(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 27.Colletti V, Shannon R, Carner M, Veronese S, Colletti L. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years' experience. Otol Neurotol. 2009;30(05):614–618. doi: 10.1097/MAO.0b013e3181a864f2. [DOI] [PubMed] [Google Scholar]
- 28.Behr R, Colletti V, Matthies C et al. New outcomes with auditory brainstem implants in NF2 patients. Otol Neurotol. 2014;35(10):1844–1851. doi: 10.1097/MAO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 29.Puram S V, Barber S R, Kozin E D et al. Outcomes following pediatric auditory brainstem implant surgery: early experiences in a North American Center. Otolaryngol Head Neck Surg. 2016;155(01):133–138. doi: 10.1177/0194599816637599. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson E P, Eisenberg L S, Krieger M D et al. Initial results of a safety and feasibility study of auditory brainstem implantation in congenitally deaf children. Otol Neurotol. 2017;38(02):212–220. doi: 10.1097/MAO.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]


