Abstract
Bone conduction implants transfer sound to the inner ear through direct vibration of the skull. In patients with skull base tumors and infections, these devices can bypass a dysfunctional ear canal and/or middle ear. Though not all skull base surgery patients opt for bone conduction hearing rehabilitation, a variety of these devices have been developed and marketed over time. This article reviews the evolution and existing state of bone conduction technology.
Keywords: bone conduction implant, skull base surgery, single-sided deafness
Introduction
The ability to transfer sound energy through bone can provide an important means of hearing rehabilitation for patients with skull base tumors. Bone conduction directly and efficiently stimulates the cochlea, obviating the need for an ear canal, tympanic membrane and ossicles ( Fig. 1 ). Furthermore, the relative lack of impedance that exists when transmitting a sound signal through the skull can allow for contralateral cochlear stimulation in patients with unilateral deafness (single-sided deafness, SSD; Fig. 2 ). Thus, bone conduction hearing systems or bone conduction implants (BCIs) can potentially be useful for patients with skull base disease who have conductive hearing loss (CHL), sensorineural hearing loss (SNHL), and both simultaneously (mixed hearing loss, MHL). This article is an overview of technical aspects, indications for use and published outcomes of BCI systems available to patients with lateral skull base disease.
Fig. 1.
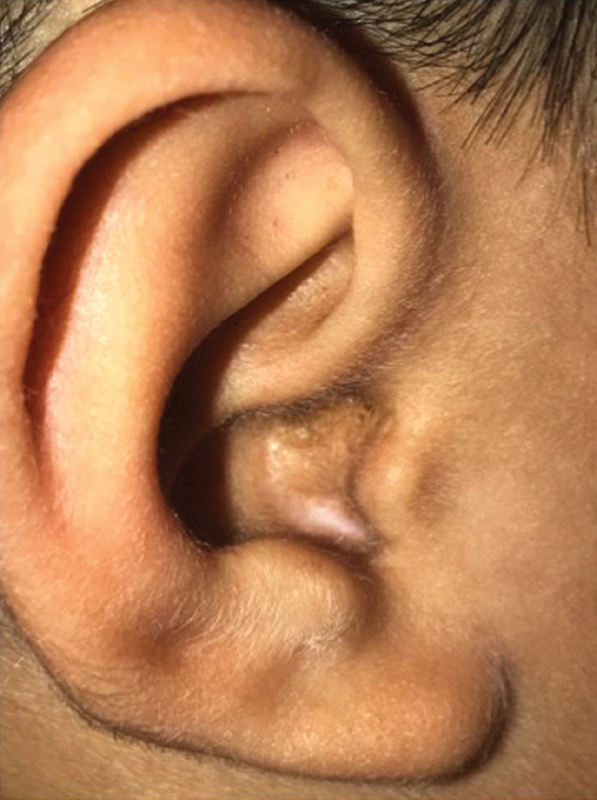
Clinical photograph of an ear canal overclosure following a transtemporal approach to the skull base.
Fig. 2.
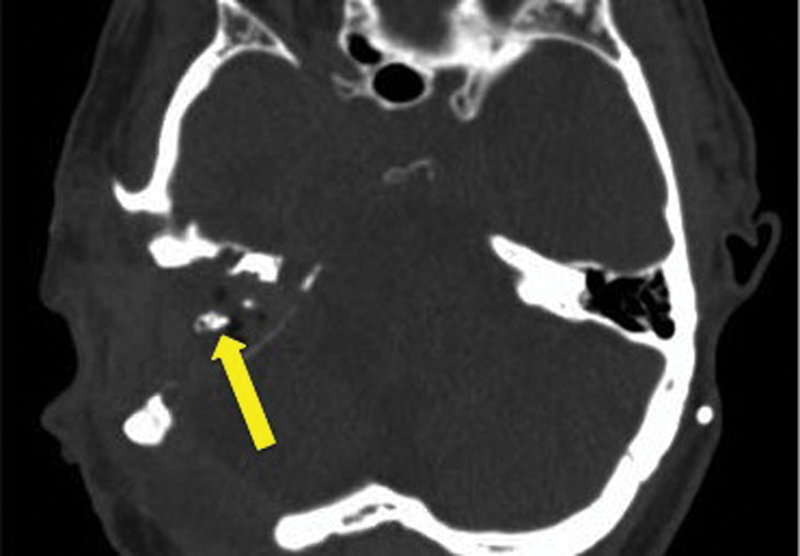
Axial computed tomography (CT) image demonstrating the postoperative appearance of a right transotic approach to an intracranial tumor. In this case, the ear canal has been obliterated and overclosed. The arrow points to the bone of the vertical fallopian canal.
History and Mechanism of Bone Conduction Hearing
The notion of bone conduction hearing was evident at least as early as the Italian renaissance period. “Ingrassia's Phenomenon,” named after anatomist Felippo Ingrassia (1510–1580 AD), described the perception of sound when a vibrating table fork was placed onto the teeth. 1 Over the time, a variety of physicians and musicians contributed to the development of the so-called “tuning fork,” which along with the pocket watch, utilized principles of bone conduction for diagnostic purposes around the turn of the 20th century. 1 Concurrently, the therapeutic nature of bone conduction hearing also became more widely evident and devices such as the Dentaphone, Osteophone, and Audiphone emerged. 2
Contemporary bone conduction devices used for auditory rehabilitation still follow the same general principal that inner ear stimulation can be achieved by transferring sound energy directly into the bone of the skull; however, more sophisticated technology and theories of osseointegration, a phenomenon that describes the actual incorporation of the implant into the surrounding bone, have allowed devices to become considerably less conspicuous and more effective. In the 1980s, Brånemark and colleagues described surgical techniques that would allow a BCI to be firmly rooted into the skull, able to withstand the vibration associated with sound conduction. 3 4 Modern BCIs generally involve the placement of an implant which is coupled to an abutment or a magnet that directly communicates with a sound processor either through a percutaneous (i.e., post going directly through the skin) or a transcutaneous (i.e., magnetic connection between the skin and bone without a direct physical connection) mechanism ( Fig. 3 ).
Fig. 3.
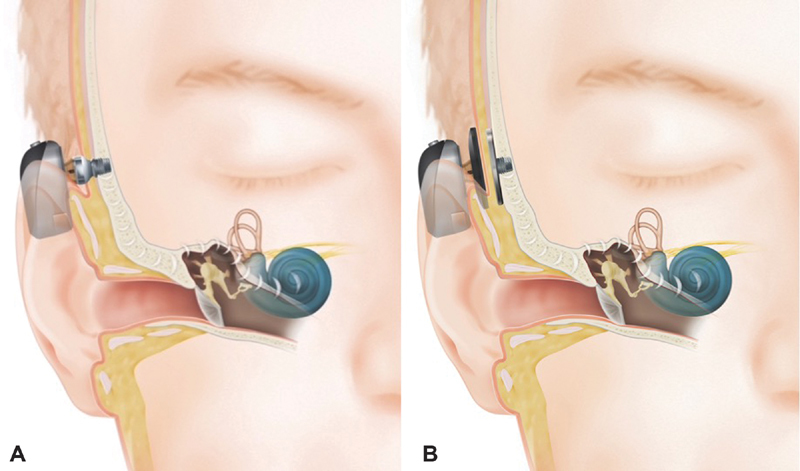
( A ) Percutaneous bone conduction and ( B ) transcutaneous bone conduction. Images provided courtesy of Cochlear Americas, 2014 Cochlear Americas.
General Surgical Technique
Bone conduction implant surgery has evolved significantly over time. Conceptually, BCI use relies on two fundamental concepts: an external hearing aid processor captures and amplifies the vibrations of sound energy, and an implant that is firmly rooted to the skull transfers these vibrations to the cochlea. Thus, BCI surgery, in theory, must simply involve an implant, that is, both able to osseointegrate with the skull and also communicate with an external device. However, the reality of either maintaining a permanent, percutaneous foreign body (percutaneous BCI), or stimulating a completely implanted device through intact skin (transcutaneous BCI) is more complex. Initially, all devices were percutaneous, and procedures were planned in two stages to maximize the probability of implant osseointegration and minimize soft tissue reactions to the implant. 3 5 For a period of time in the history of percutaneous BCIs, skin graft placement and one-stage surgery was felt to provide a balance between osseointegration, skin thinning, surrounding soft tissue viability, and inflammation. 6 However, it became recognized that smaller incisions and less soft tissue manipulation could be effective in conjunction with long percutaneous abutments attached to the actual implant. 7 8 In contemporary BCI surgery, each specific device has distinct procedural nuances but in all cases, minimizing trauma and invasiveness is preferred where possible.
When a percutaneous device is placed, the incision can be designed as either a 1 to 1.5 cm vertical line or a 5 mm diameter punch biopsy circle. Generally, it is the authors' preference that the surgical site to be centered at the same approximate height as the superior aspect of the auricle, approximately 5.5 cm in linear distance from the ear canal. Hair is removed from the immediate surgical site only. A small gauge needle is placed through the soft tissue of the planned implant site, down to the bony cortex. The depth of the soft tissue is measured by grasping the needle at the point of entry to the skin with care to not compress the skin which could result in an inappropriately sized abutment. Three millimeters are added onto this measurement and the resulting number is considered to be the preferred abutment size. In the case that the latter value does not match one of the manufactured abutment lengths, rounding up to the next-nearest size is recommended. Thereafter, local anesthetic is injected into the surgical site. The incision is carried down to bone and the periosteum is elevated away from the location planned for the implant. After hemostasis is achieved, depth gauging drill bits are used sequentially to ensure that appropriate bone thickness is present in the site intended for the BCI. Once the appropriate depth of the implant has been determined, a countersink drill bit is used to create a well for the implant. Subsequently, the implant is threaded into this location, preferably with a drill that has the capacity to apply force in a controlled manner (i.e., 30–40 N/cm). If the implant does not have a precoupled abutment in place, the abutment can be placed separately onto the implant. The soft tissue around the abutment is then closed in a multilayer fashion. Alternatively, the implant can be offset within the surgical site which can then be closed in its entirety and a punch biopsy tool can be used to remove the skin over the abutment outside. 9 As noted above, a form of percutaneous placement of the abutment in which a 5 mm punch biopsy replaces an open incision has recently gained interest among some surgeons. When compared with traditional techniques, this form of surgery has been shown to improve periincisional numbness, cosmetic results, and operative time. 10 11
In the case of a transcutaneous BCI, incisions are usually larger to account for the increased size of the implant ( Fig. 4 ). Though the procedure for placement can vary depending on the device manufacturer, generally, a subcutaneous pocket is created to house the device and a periosteal incision is used to expose the cortex. The device is then fastened to the cortex in either a piecemeal fashion (i.e., implant first and magnet second), or the entire device is placed at one time and locked into place with multiple self-drilling, self-tapping screws. Thinning the skin flap over the device is required in some cases to optimize the transcutaneous mechanism and surgical site closure is performed in a multilayer fashion.
Fig. 4.
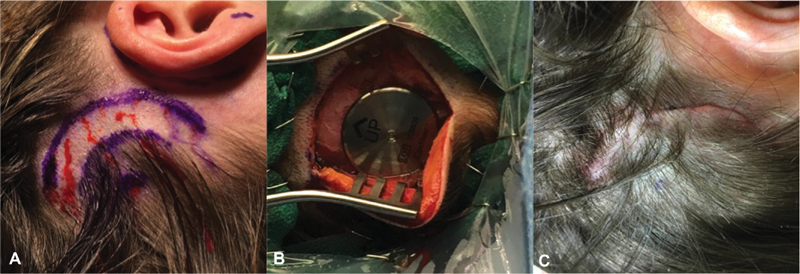
( A ) Preoperative, ( B ) intraoperative, and ( C ) postoperative appearance of a transcutaneous BCI surgical site. BCI, bone conduction implants.
Indications and Contraindications
Indications
Most bone conduction implants have been approved by the United States Food and Drug Administration (FDA) for use in individuals older than 5 years of age with CHL, MHL, and SSD who cannot benefit from traditional hearing aid amplification ( Fig. 5 ). As discussed below, the MED-EL Bonebridge is approved for use in patients age 12 years and older. In a skull base surgery clinic, candidates for a BCI generally present with hearing loss despite multiple corrective surgeries, without favorable anatomy for a repair, with ear canal stenosis, with radical mastoid cavities, or with ear canal overclosure following a skull base surgical approach. 12 13 In most cases, candidates are able to simulate the effectiveness of a bone conduction hearing aid prior to surgery by utilizing a bone conduction device held in place with a headband.
Fig. 5.
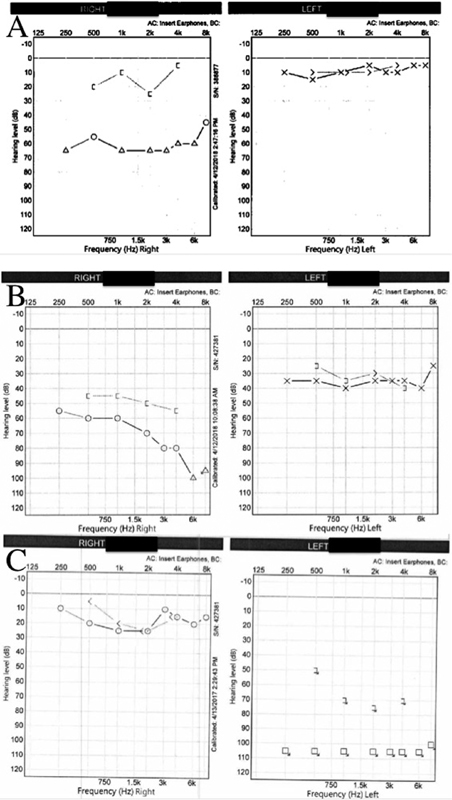
Audiogram examples of ( A ) right conductive hearing loss, ( B ) right mixed hearing loss, and ( C ) left single-sided deafness.
As previously mentioned, the advantage of the BCI in these populations lies in the ability of the technology to bypass the conductive element of the hearing loss and to provide high fidelity sound quality by direct stimulation of the ipsilateral cochlea, thereby restoring binaural hearing. Additionally, individuals with SSD can also be BCI candidates if traditional air conduction amplification or contralateral routing of sound (CROS) hearing aids are ineffective. 13 14 15 16 17 In this population, the sound signal is transmitted to the contralateral, functional cochlea, though it is noteworthy that this mechanism does not recreate binaural hearing.
Specific Audiologic Criteria: Conductive Hearing Loss
Candidates with CHL can generally benefit from a BCI system. Specifically, patients with a CHL consisting of air conduction thresholds that are greater than 30 dB HL are shown to have better outcomes with the use of a BCI when compared with traditional hearing aids. 14 18 Clinicians should consider bilateral BCI placement if the CHL is symmetrical and the interaural difference is less than 15 dB. Furthermore, more favorable benefit for spatial perception (localization) and release of masking has been shown with bilateral BCI use in the setting of CHL as compared with unilateral use. 18 19 The most common conditions resulting in unilateral CHL in patients with skull base disease include lateral temporal bone resection for cutaneous malignancy involving the ear canal or periauricular area, or subtotal petrosectomy with ear canal closure commonly performed for large jugular paraganglioma resections or refractory cerebrospinal fluid leak.
Specific Audiologic Criteria: Mixed Hearing Loss
Patients with MHL may potentially benefit from a BCI system. Presently, the most powerful BCI on the market can be fit on patients with a SNHL pure tone average (PTA) of up to 65 dB HL (averaged across 0.5, 1, 2, and 3 kHz). However, various manufacturer technologies have individual limits on power output; therefore, accurate assessment of the sensorineural component of the hearing loss is necessary for proper selection of the sound processor and implant (percutaneous versus transcutaneous). Typically, candidates with a mild-to-moderate SNHL and with > 30 dB CHL can benefit from the use of a BCI system.
Specific Audiologic Criteria: Single Sided Deafness
Bone conduction hearing systems can be used for patients with SSD when air conduction thresholds are within the normal hearing range (≥ 20 dB HL) in the contralateral ear.
Contraindications
The clearest contraindications for a bone conduction hearing system are evident in patients who do not satisfy the audiologic criteria for a specific device. Also, as noted above, age-based contraindications are present for most devices in the pediatric population, predicated on the notion that bone may lack sufficient structural integrity and thickness for proper BCI placement before a certain age. With most manufacturers, a bone conduction device can be attached to a headband when a candidate patient is too young, essentially leading to transcutaneous stimulation though the scalp. However, implantation with traditional percutaneous devices has been studied prior to age 5 years using additional sites of implant fixation, or using a staged surgical approach allowing for an extended length of osseointegration time to account for potentially decreased implant stability. 20
Other potential contraindications are worth considering in the candidate population. Patients may have suboptimal performance or outright failure with a BCI in the setting of poor bone or soft tissue quality in the surgical site. 14 As such, patients diagnosed with intrinsic bone abnormalities (i.e., osteogenesis imperfecta, Paget's disease, severe osteoporosis, or osteopenia) or those with medical comorbidities that would affect bone and soft tissue (i.e., external beam radiation, smoking, and chronic corticosteroid use) should be thoroughly counseled regarding the possibility of bone or soft tissue problems that could occur with a BCI. Recipients with altered bone density or quality may require alteration of standard implantation procedure, such as constant cooling irrigation during drilling to minimize thermal injury and limited torque to avoid stripping the implant. 21 Shorter abutments can also be used in this population to potentially reduce protrusion from the scalp, thus minimizing the risk of impact trauma. As above, staged placement of the abutment and allowance for extra osseointegration time (beyond 3 months) can also be used to mitigate the impact of suboptimal bone and soft tissue quality. 20 22
Other medical conditions that may become problematic include keloid and hypertrophic scar tendencies, or immune deficiencies in which soft tissue infections are probable. 14 The ability to provide hygiene to an abutment site can also be important, as a relative contraindication exists in patients who lack the personal ability or caregiver support to care for the device on a daily basis. 13 17 23 The need for future magnetic resonance imaging (MRI) studies can also be a relative contraindication to a ferromagnetic transcutaneous system, as is discussed in more detail below. 24
Current BCI Processor Systems/Manufacturers
Multiple FDA approved BCI systems are available for use in the United States: the Cochlear Baha 5 Implant System, the Oticon Medical Ponto 3 System, and the Medtronic Sophono Bone Conduction Hearing System. A fourth system, the MED-EL Bonebridge, was recently granted de novo FDA clearance in July 2018. These devices will be briefly reviewed below, and further information can be found in Table 1 .
Table 1. A comparison of bone conduction implant systems.
| Manufacturer | Model | Recommended bone conduction PTA threshold in ear to be implanted for CHL (dB) | Recommended air conduction PTA in the better ear for SSD (dB) | Frequency range (Hz) | Abutment type | Battery | Max battery life (h) |
|---|---|---|---|---|---|---|---|
| Cochlear | Baha 5 | ≤ 45 | ≤ 20 | 250–7,000 | Percutaneous, transcutaneous or Softband | 312 ZA | 100 |
| Baha 5 Power | ≤ 55 | 250–7,000 | 675 ZA | 160 | |||
| Baha 5 Superpower | ≤ 65 | 250–7,000 | RLI | 32 | |||
| Medtronic | Sophono Alpha 2 MPO Processor | ≤ 35 | ≤ 20 | 125–8,000 | Transcutaneous (retention dual magnet) or Softband | 13 ZA | 320 |
| Oticon | Ponto 3 | ≤ 45 | ≤ 20 | 200–9,500 | Percutaneous or Softband | 13 ZA | 130 |
| Ponto 3 Power | ≤ 55 | 200–9,600 | 675 ZA | 150 | |||
| Ponto 3 Superpower | ≤ 65 | 200–9,600 | 675 P ZA | 80 | |||
| MED-EL | Bonebridge | ≤ 45 | ≤ 20 | 250–8,000 | Transcutaneous (floating mass transducer) | 675 | 120 |
Abbreviations: CHL, conductive hearing loss; PTA, pure tone average; RLI, Rechargable Lithium Ion; SSD, single-sided deafness; ZA, Zinc Air.
Cochlear Baha Implant System
Cochlear'scurrent systems provide three sound processor options, Baha 5, Baha 5 Power, and the Baha 5 SuperPower, which are available for use with the Cochlear Baha magnetic implants (transcutaneous) and Dermalock abutments (percutaneous; BIA400; Fig. 6 ). All three sound processors are available for use with a percutaneous abutment system, transcutaneous magnetic system, a soft elastic headband, and a steel spring behind the headband. The portfolio of sound processors can use bluetooth smart technology and can support direct streaming to the sound processors for audio and data. All sound processors are compatible with a smartphone app which allows patients to control their sound processors. Accessories including a remote control, TV streaming device, microphone, and phone pairing device are available using 2.4 GHz wireless signal technology. The devices are compatible with standard frequency modulation (FM) systems and wireless assistive listening device (ALD) systems with the use of a receiver connected to the mini microphone accessory. 25 26
Fig. 6.

( A ) Cochlear Baha Connect (percutaneous) and ( B ) Baha Attract (transcutaneous) implants. Images provided courtesy of Cochlear Americas, 2014 Cochlear Americas.
Medtronic Sophono Bone Conduction Hearing System
Medtronic offers the Sophono Alpha 2 MPO Processor, which is compatible with the low profile Sophono Magnetic Implant ( Fig. 7 ). This system uses a retention dual magnet implant that is fixated to the temporal bone using five titanium screws and an externally worn sound processor that couples to the implant using magnetic force. Audio transmission through the Sophono processor is possible using a direct audio input with a standard earplug. The Sophono Alpha MPO processor uses dual microphones with Omni and directional abilities, and accessories permit use with mobile phones, standard FM systems, and personal music devices. 27
Fig. 7.
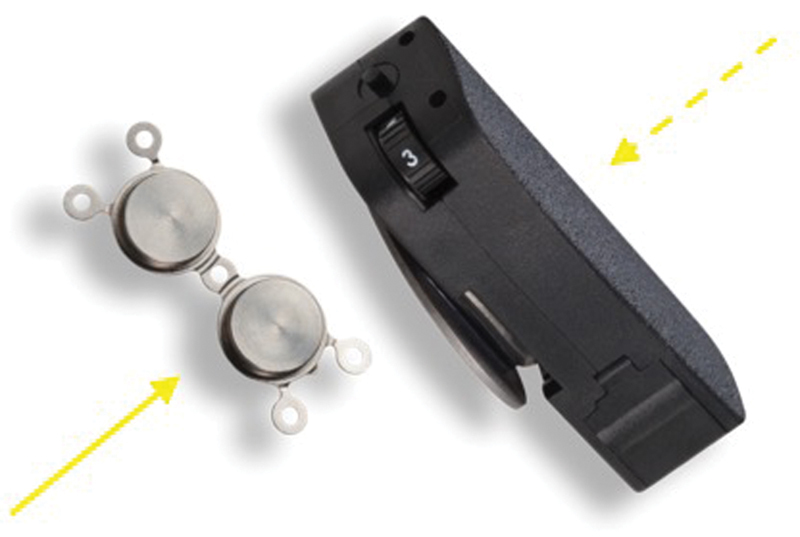
The Medronic Sophono implant (solid arrow) and audio processor (dashed arrow). Image provided courtesy of Medtronic PLC.
Oticon Medical Ponto System
The Oticon Medical BCI system is the Ponto 3 sound processor family, consisting of the single unit Ponto 3, Ponto 3 Power and Ponto 3 SuperPower ( Fig. 8 ). All three sound processors utilize a percutaneous abutment. Device control and audio streaming are possible using the Oticon Medical Streamer to connect any of the Ponto family sound processors with the Oticon Medical ConnectLine App available for certain smartphones. The system uses direct sound transmission and signal procession with a 6 ms delay. 28
Fig. 8.
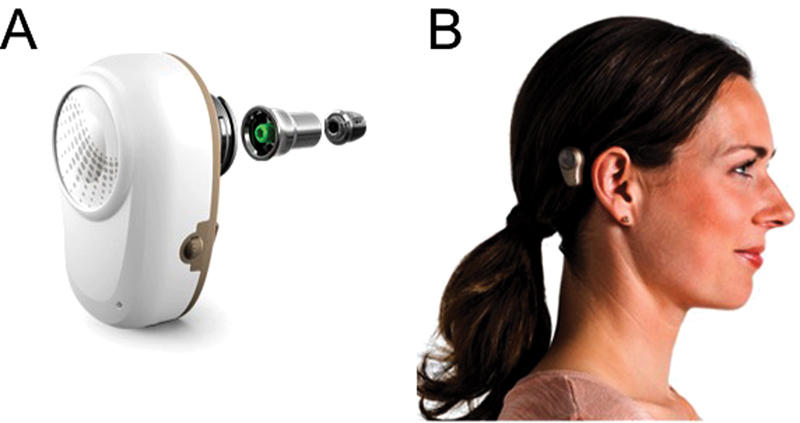
( A ) Oticon Ponto implant system components and ( B ) a clinical picture of the device in use. Images provided courtesy of Oticon Medical AB.
MED-EL Bonebridge System
The MED-EL Bonebridge implant was recently granted de novo clearance by the FDA for patients who are 12 years of age or older ( Fig. 9 ). The Bonebridge is completely implantable and works via transcutaneous conduction of sound energy. However, distinct from other transcutaneous devices, the Bonebridge involves active bone conduction by using a demodulator to drive a floating mass transducer. Recent publications would suggest that this device is safe and well tolerated, and compared with passive transcutaneous BCIs, hearing outcomes are potentially better. Further research will be needed to establish the role of the Bonebridge in hearing rehabilitation surgery for skull base surgery patient, though initial clinical results are promising. 29 30
Fig. 9.

The MED-EL Bonebridge implant. ( A ) Profile of the implant and of the ( B ) Samba audio processor. ( C ) Illustration of the Bonebridge placement in vivo. Images provided courtesy of MED-EL GmbH.
Nonimplantable Bone Conduction Devices
Conventional, nonimplantable bone conduction devices worn on a steel spring headband, soft headband, or attached to spectacles have been available for use since the beginning of the 20th century. 17 Today, similar concepts remain a valuable part of bone conduction hearing aid technology, especially in children who do not qualify for candidacy for a percutaneous or transcutaneous BCI due to their age. 31 Similar to transcutaneous BCIs, one major drawback to bone conduction hearing without an implant is the high amount of static pressure required for adequate transmission of sound which can be causing skin compression and discomfort. Also, it has previously been suggested that functional gain measures of hearing sensitivity using a bone conduction device on a soft headband or steel spring headband can be associated with a decrease in thresholds of 8 to 20 dB in the high-frequency range (1–4 kHz). 32 Frequent feedback due to microphone placement are is also cited as an issue. 17 Recently approved by the FDA, the MED-EL ADHEAR device ( Fig. 10 ) is a new addition to the realm of nonimplantable devices that can reportedly facilitate bone conduction without the potential discomfort of static pressure, though more evidence will be needed to establish the role of this device in clinical practice. 33
Fig. 10.

The MED-EL ADHEAR system. ( A ) The adhesive adapter (solid arrow) and the audio processor (dashed arrow) and ( B ) a clinical picture of the device in use. Images provided courtesy of MED-EL GmbH.
Outcome Considerations
Comparing Transcutaneous and Percutaneous Devices
Percutaneous BCIs are generally accepted as the traditional gold standard for bone conduction hearing rehabilitation in patients with CHL. 13 15 34 Compared with transcutaneous devices, some advantages to percutaneous bone conduction systems are a shorter, less invasive surgical procedure, significant improvement in speech reception thresholds (SRT), and superior amplification in the higher frequencies, resulting in an improvement in functional gain thresholds of 5 to 10 dB with the most substantial increase at 4,000 Hz (4.5 dB). 13 Specifically in patients with MHL and a more severe SNHL, the percutaneous implant may be more appropriate to overcome the soft tissue attenuation, particularly in the high frequencies. 16 17
There are disadvantages to the percutaneous systems, which are largely consist of cosmetic concerns and skin complications around the implant, which can include skin overgrowth, chronic wound infection, implant extrusion, and loss due to trauma. Most of the literature on this subject has identified soft tissue reactions to the protruding titanium skin-piercing coupler to be the most common complication of a percutaneous BCI. 13 15 17 34 Children are particularly susceptible, with research showing that those who receive percutaneous implants are more likely to suffer skin complications (7.8%) and trauma to the implant resulting in device removal (15.2%) when compared with the adult population. 15 34
Transcutaneous bone conduction hearing systems are an alternative to the traditional percutaneous devices. Benefits of the transcutaneous implant include less required care and maintenance of the surrounding hair and skin tissue, improved cosmesis, a reduced need for daily cleaning and maintenance, and reportedly lower revision surgery rates. 15 23 35 36 37 Most notably, a significant percentage of patients who fall within candidacy guidelines for a BCI system may reject the skin-piercing abutment for aesthetic reasons. 13 15 23 36 A review of the published outcomes from transcutaneous BCI system use shows the devices to be generally safe and effective. Along with some functional improvements that are comparable to percutaneous devices, there is a relatively low rate of fixture loss and high rates of patient satisfaction. 23 35 36
However, the potential benefits of the transcutaneous BCIs also come with obvious costs. Two noteworthy concerns are that these devices are generally less efficient due to the complexity of sound transmission through an intact skin layer when compared with direct bone conduction transmission, and that sound processors associated with these devices need to maintain significant pressure on the underlying soft tissue for effective coupling. With regard to the former, transcutaneous BCI systems provide adequate functional gain at all frequencies up to 3,000 Hz. 23 However, there is significant attenuation beyond that point. With regard to the second challenge, the coupling strength required for proper sound transmission through the implant may cause discomfort or skin/soft tissue complications. 15 23 36 The magnet strength should provide reliable retention of the sound processor but clinicians should exercise care when selecting magnet strength and fitting the magnetic sound processor to the implant to reduce discomfort for patients. A multicenter retrospective study revealed that as many as 25% of patients may complain of skin irritation and pain around the magnet site. 13
Special Considerations in Single-Sided Deafness
The FDA first approved BCI systems for the rehabilitation of SSD in 2002. 38 In patients with acquired SSD, the BCI systems can help to eliminate the head-shadow effect by transmitting sound from the poorer hearing ear/side to the contralateral intact cochlea via bone conduction. 14 18 39 An analysis of four controlled trials comparing the benefit of contralateral BCI systems to the CROS hearing aids and the unaided condition found no benefit to localization ability with either aid. However, all four studies showed an advantage with the BCI to speech discrimination in noise over the CROS and unaided conditions. 39 In the authors' opinion, the patient benefit with BCI for SSD is variable relative to what is seen with CHL. As previously noted, the benefit of binaural hearing is not restored with BCI for SSD, and a higher nonuse rate is seen in this setting relative to other indications for BCI use.
Complications
The incidence of major complications in BCI systems is generally low, with a majority of difficulties arising from soft tissue issues at the implant site, such as infections or skin reactions, as noted above. 12 13 36 37 40 41 Though there is a relative paucity of high-powered prospective studies that consider BCIs complications with uniform standards of reporting, a few specific points from the extant literature are noteworthy. 12 Dun et al published a large single-center experience highlighting the incidence of adverse reactions in 1,132 implants, and it was shown that some degree of skin reaction, ranging from mild erythema to complete skin overgrowth of the implant was seen in 14.6% of cases. However, only 8.3% of complications involved removal or “loss” of the device. 34 A meta-analysis of complications associated with osseointegrated hearing aids from 2013 compared 20 distinct publications of BCI complications, concluding that revision surgery or complete removal of the osseointegrated implant occurred in 0.0 to 25% in pediatric patients and 1.6 to 17.4% in adults. 12 It was also noted that negative prognostic factors can include patient demographics, incision technique, graft thickness, postoperative dressings, and one-stage versus two-stage procedure.
Specific Skull Base Surgery Considerations
As noted above, surgical approaches can lead to CHL or SNHL, and in some instances, bone conduction is an excellent rehabilitative option. Certainly, cases that spare the inner ear but involve ear canal overclosure would be expected to benefit from a BCI. SSD after vestibular schwannoma surgery can also present an opportunity for BCI use. 41 Following either translabyrinthine and retrosigmoid surgery, BCIs have been shown to be effective, though care should be taken to select a surgical site that gives access to healthy bone outside the prior craniotomy. 42 43 Similarly, consideration should be given to performing implantation as a staged procedure to mitigate the risk of cerebrospinal fluid (CSF) leak through a percutaneous BCI site. In terms of surgical timing, it has also been demonstrated that BCI placement is safe to perform simultaneous with tumor resection, if desired. 43 In all cases, patients should undergo extensive presurgical counseling regarding the risks and benefits of immediate hearing rehabilitation with a BCI, especially in patients with intact hearing prior to tumor resection.
The use of BCIs in the setting of external beam radiation deserves special mention. As noted above in the section on contraindications, external beam radiation in the treatment of skull base malignancy, though not incompatible with BCI use, can create an unfavorable environment for osseointegration and soft-tissue healing. In 2016, Nader et al published a report of 32 BCI patients, 19 of whom had radiotherapy for head and neck malignancy. 21 It was noted that the incidence of BCI-related complications was lower in cases where the implant surgery occurred prior to irradiation, and specifically, it was recommended that the BCI be placed at the time of a primary oncologic resection, if possible. While radiation scattering has not been previously investigated with BCIs, studies of similar titanium dental and craniofacial implants show minimal scattering effect at the implant site itself. 44
Another consideration for a skull base surgery team is the magnetic resonance imaging (MRI) compatibility of the implanted device. If MRI surveillance is necessary following BCI placement, a ferromagnetic implant could become problematic. 24 In terms of percutaneous devices, it has been demonstrated that implanted components (the implant and the abutment) create little artifact and are safe in terms of movement in the magnetic field, even at significant flux density (9.4T). 45 In terms of transcutaneous devices, the Sophono has been shown to be MRI conditional to 3 T, while the Cochlear Baha Attract is MRI conditional to 1.5 T. 27 46 47 Both transcutaneous devices would be expected to create significant image artifact, which depending on device placement, could blanket a significant portion of the lateral skull base. Additionally, pain or discomfort at the implant site induced from magnetic field exposure can limit patient tolerance. 48
Innovations
Though not currently available for clinical use, the SoundBite was an innovative, nonsurgical option for transmitting acoustic information to a healthy cochlea by dental bone conduction from a transmitter placed on the maxillary molars. 17 Clinical trials demonstrated improved sound quality, better spatial hearing, and improvement in understanding of speech in noise for users. 49 Murray et al also demonstrated that the SoundBite system was a safe and effective bone conduction intervention for patients with SSD. 50 51 Limitations of the SoundBite technology included acoustic feedback, softer output for low frequencies, and discomfort or distortion when eating, and ultimately, the device is no longer commercially available.
Conclusion
Bone conduction hearing implants can be an important means of hearing rehabilitation for specific patients with skull base disease. Some patients do not elect to purse hearing rehabilitation following skull base surgery, though for those who do, percutaneous and transcutaneous devices exist, each with their own respective set of potential benefits and challenges. Multiple BCI systems are currently available for use, and patient specific factors can largely dictate which device, if any, is most appropriate.
Conflict(s) of Interest to Declare Author A.D.S. has recent consultancy relationships with Advanced Bionics Corp., Cochlear Corp., Oticon Corp.
Financial Material and Support
Internal departmental funding was utilized without commercial sponsorship or support.
Disclosure
This article discusses off-label uses of bone-conduction hearing devices.
References
- 1.Ng M, Jackler R K. Early history of tuning-fork tests. Am J Otol. 1993;14(01):100–105. [PubMed] [Google Scholar]
- 2.Berger K W. Early bone conduction hearing aid devices. Arch Otolaryngol. 1976;102(05):315–318. doi: 10.1001/archotol.1976.00780100101017. [DOI] [PubMed] [Google Scholar]
- 3.Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark P I. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. 1981;2(04):304–310. [PubMed] [Google Scholar]
- 4.Bonding P, Jønsson M H, Salomon G, Ahlgren P. The bone-anchored hearing aid. Osseointegration and audiological effect. Acta Otolaryngol Suppl. 1992;492:42–45. doi: 10.3109/00016489209136807. [DOI] [PubMed] [Google Scholar]
- 5.Mylanus E A, Cremers C W, Snik A F, van den Berge N W. Clinical results of percutaneous implants in the temporal bone. Arch Otolaryngol Head Neck Surg. 1994;120(01):81–85. doi: 10.1001/archotol.1994.01880250071010. [DOI] [PubMed] [Google Scholar]
- 6.Mylanus E A, Cremers C W. A one-stage surgical procedure for placement of percutaneous implants for the bone-anchored hearing aid. J Laryngol Otol. 1994;108(12):1031–1035. doi: 10.1017/s002221510012883x. [DOI] [PubMed] [Google Scholar]
- 7.den Besten C A, Bosman A J, Nelissen R C, Mylanus E A, Hol M K. Controlled clinical trial on bone-anchored hearing implants and a surgical technique with soft-tissue preservation. Otol Neurotol. 2016;37(05):504–512. doi: 10.1097/MAO.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 8.Høgsbro M, Agger A, Johansen L V. Bone-anchored hearing implant surgery: randomized trial of dermatome versus linear incision without soft tissue reduction--clinical measures. Otol Neurotol. 2015;36(05):805–811. doi: 10.1097/MAO.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 9.A bone conduction hearing solution: surgery guide. Available from:https://www.cochlear.com/66b43e66-3e0b-453b-9751-bc904f3961fd/BUN128+ISS4+NOV30+-+Baha+Connect+Surgery+Guide+FINAL.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-66b43e66-3e0b-453b-9751-bc904f3961fd-llOXgkw. Accessed May, 2018
- 10.Gordon S A, Coelho D H. Minimally invasive surgery for osseointegrated auditory implants: a comparison of linear versus punch techniques. Otolaryngol Head Neck Surg. 2015;152(06):1089–1093. doi: 10.1177/0194599815571532. [DOI] [PubMed] [Google Scholar]
- 11.Calon T GA, Johansson M L, de Bruijn A JG et al. Minimally invasive ponto surgery versus the linear incision technique with soft tissue preservation for bone conduction hearing implants: a multicenter randomized controlled trial. Otol Neurotol. 2018;39(07):882–893. doi: 10.1097/MAO.0000000000001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiringoda R, Lustig L R. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. 2013;34(05):790–794. doi: 10.1097/MAO.0b013e318291c651. [DOI] [PubMed] [Google Scholar]
- 13.Iseri M, Orhan K S, Tuncer U et al. Transcutaneous bone-anchored hearing aids versus percutaneous ones: multicenter comparative clinical study. Otol Neurotol. 2015;36(05):849–853. doi: 10.1097/MAO.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 14.Cass S P, Mudd P A. Bone-anchored hearing devices: indications, outcomes, and the linear surgical technique. Oper Tech Otolaryngol--Head Neck Surg. 2010;21(03):197–206. [Google Scholar]
- 15.Hol M K, Nelissen R C, Agterberg M J, Cremers C W, Snik A F. Comparison between a new implantable transcutaneous bone conductor and percutaneous bone-conduction hearing implant. Otol Neurotol. 2013;34(06):1071–1075. doi: 10.1097/MAO.0b013e3182868608. [DOI] [PubMed] [Google Scholar]
- 16.Dimitriadis P A, Hind D, Wright K et al. Single-center experience of over a hundred implantations of a transcutaneous bone conduction device. Otol Neurotol. 2017;38(09):1301–1307. doi: 10.1097/MAO.0000000000001529. [DOI] [PubMed] [Google Scholar]
- 17.Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl) 2015;8:79–93. doi: 10.2147/MDER.S39691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hol M K, Snik A F, Mylanus E A, Cremers C W. Does the bone-anchored hearing aid have a complementary effect on audiological and subjective outcomes in patients with unilateral conductive hearing loss? Audiol Neurotol. 2005;10(03):159–168. doi: 10.1159/000084026. [DOI] [PubMed] [Google Scholar]
- 19.Stenfelt S. Acoustic and physiologic aspects of bone conduction hearing. Adv Otorhinolaryngol. 2011;71:10–21. doi: 10.1159/000323574. [DOI] [PubMed] [Google Scholar]
- 20.Davids T, Gordon K A, Clutton D, Papsin B C. Bone-anchored hearing aids in infants and children younger than 5 years. Arch Otolaryngol Head Neck Surg. 2007;133(01):51–55. doi: 10.1001/archotol.133.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Nader M E, Beadle B M, Roberts D B, Gidley P W. Outcomes and complications of osseointegrated hearing aids in irradiated temporal bones. Laryngoscope. 2016;126(05):1187–1192. doi: 10.1002/lary.25592. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd S, Almeyda J, Sirimanna K S, Albert D M, Bailey C M. Updated surgical experience with bone-anchored hearing aids in children. J Laryngol Otol. 2007;121(09):826–831. doi: 10.1017/S0022215107003714. [DOI] [PubMed] [Google Scholar]
- 23.Briggs R, Van Hasselt A, Luntz M et al. Clinical performance of a new magnetic bone conduction hearing implant system: results from a prospective, multicenter, clinical investigation. Otol Neurotol. 2015;36(05):834–841. doi: 10.1097/MAO.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 24.Doshi J, Schneiders S, Foster K, Reid A, McDermott A L. Magnetic resonance imaging and bone anchored hearing implants: pediatric considerations. Int J Pediatr Otorhinolaryngol. 2014;78(02):277–279. doi: 10.1016/j.ijporl.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Cochlear Baha 5 Power datasheet. Availablr from:https://www.cochlear.com/94d60676-a488-44da-a683-3202d592a300/BUN446_Rev1_Baha_5_Power_Comparison_Chart.pdf?MOD=AJPERES&CVID=llPYtaQ&CVID=llPYtaQ. Accessed April, 2018
- 26.Cochlear Baha Connect System and Cochlear Baha Attract system datasheet. Available from:https://www.cochlear.com/b9c07811-205c-4c38-98f4-4f539315ab35/BUN397+ISS1+JAN16+SuperPower+Connect+Attract+Datasheet.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-b9c07811-205c-4c38-98f4-4f539315ab35-llOYl50. Accessed April, 2018
- 27.Product Specification: Sophono Alpha 2 MPO Processor.http://professionals.sophono.com/hubfs/May_2016_-_Product_Literature/UC201603289aEN_Sophono_Magnetic_ProductSpecs_LR.pdf?t=1472051684526&hsCtaTracking=9f95e98f-d725-4fc0-8ac0-652901a8f2ca%7Cba067687-e951-45eb-9867-35b6ebde3381. Accessed April, 2018
- 28.Ponto 3, Ponto 3 Power and Ponto 3 SuperPower product information.https://www.oticonmedical.com/-/media/medical/main/files/bahs/products/ponto-3/pi/eng/ponto-3-product-information—m52682—english.pdf?la=en. Accessed April, 2018
- 29.Zernotti M E, Di Gregorio M F, Galeazzi P, Tabernero P. Comparative outcomes of active and passive hearing devices by transcutaneous bone conduction. Acta Otolaryngol. 2016;136(06):556–558. doi: 10.3109/00016489.2016.1143119. [DOI] [PubMed] [Google Scholar]
- 30.Schmerber S, Deguine O, Marx M et al. Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol. 2017;274(04):1835–1851. doi: 10.1007/s00405-016-4228-6. [DOI] [PubMed] [Google Scholar]
- 31.Doshi J, Sheehan P, McDermott A L. Bone anchored hearing aids in children: an update. Int J Pediatr Otorhinolaryngol. 2012;76(05):618–622. doi: 10.1016/j.ijporl.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Verstraeten N, Zarowski A J, Somers T, Riff D, Offeciers E F. Comparison of the audiologic results obtained with the bone-anchored hearing aid attached to the headband, the testband, and to the “snap” abutment. Otol Neurotol. 2009;30(01):70–75. doi: 10.1097/MAO.0b013e31818be97a. [DOI] [PubMed] [Google Scholar]
- 33.MED-EL ADHEAR. Available from:http://www.medel.com/us/adhear/. Accessed May, 2018
- 34.Dun C A, Faber H T, de Wolf M J, Mylanus E A, Cremers C W, Hol M K. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol. 2012;33(02):192–198. doi: 10.1097/MAO.0b013e318241c0bf. [DOI] [PubMed] [Google Scholar]
- 35.Reddy-Kolanu R, Gan R, Marshall A H. A case series of a magnetic bone conduction hearing implant. Ann R Coll Surg Engl. 2016;98(08):552–553. doi: 10.1308/rcsann.2016.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezdjian A, Bruijnzeel H, Daniel S J, Grolman W, Thomeer H GXM. Preliminary audiologic and peri-operative outcomes of the Sophono transcutaneous bone conduction device: a systematic review. Int J Pediatr Otorhinolaryngol. 2017;101:196–203. doi: 10.1016/j.ijporl.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Johansson M L, Stokroos R J, Banga R et al. Short-term results from seventy-six patients receiving a bone-anchored hearing implant installed with a novel minimally invasive surgery technique. Clin Otolaryngol. 2017;42(05):1043–1048. doi: 10.1111/coa.12803. [DOI] [PubMed] [Google Scholar]
- 38.Snik A F, Bosman A J, Mylanus E A, Cremers C W. Candidacy for the bone-anchored hearing aid. Audiol Neurotol. 2004;9(04):190–196. doi: 10.1159/000078388. [DOI] [PubMed] [Google Scholar]
- 39.Baguley D M, Bird J, Humphriss R L, Prevost A T. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol. 2006;31(01):6–14. doi: 10.1111/j.1749-4486.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 40.Nelissen R C, Mylanus E A, Cremers C W, Hol M K, Snik A F. Long-term compliance and satisfaction with percutaneous bone conduction devices in patients with congenital unilateral conductive hearing loss. Otol Neurotol. 2015;36(05):826–833. doi: 10.1097/MAO.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 41.House J W, Kutz J W., Jr Bone-anchored hearing aids: incidence and management of postoperative complications. Otol Neurotol. 2007;28(02):213–217. doi: 10.1097/MAO.0b013e31802c74c4. [DOI] [PubMed] [Google Scholar]
- 42.Boucek J, Vokral J, Cerny L et al. Baha implant as a hearing solution for single-sided deafness after retrosigmoid approach for the vestibular schwannoma: surgical results. Eur Arch Otorhinolaryngol. 2017;274(06):2429–2436. doi: 10.1007/s00405-017-4505-z. [DOI] [PubMed] [Google Scholar]
- 43.McRackan T R, Goddard J C, Wilkinson E P, Slattery W H, Brackmann D E. Bone-anchored hearing device placement with translabyrinthine tumor removal. Otolaryngol Head Neck Surg. 2015;152(02):314–318. doi: 10.1177/0194599814558038. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Pillai K, Jones P K. Dosimetric measurement of scattered radiation from dental implants in simulated head and neck radiotherapy. Int J Oral Maxillofac Implants. 1998;13(02):197–203. [PubMed] [Google Scholar]
- 45.Fritsch M H, Naumann I C, Mosier K M. BAHA devices and magnetic resonance imaging scanners. Otol Neurotol. 2008;29(08):1095–1099. doi: 10.1097/MAO.0b013e31818201fd. [DOI] [PubMed] [Google Scholar]
- 46.Cochlear Baha Connect System: radiographer's instructions. Available from:https://www.cochlear.com/107fc39f-bf96-47b6-9527-7d603b654344/BUN380+ISS1+AUG15+Radiographers+Instructions.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-107fc39f-bf96-47b6-9527-7d603b654344-l5vfKmC. Accessed May, 2018
- 47.Cochlear Baha Attract System: radiographer's instructions. Available from:https://www.cochlear.com/f5917ef2-bb35-4307-b330-8c15ffdd993c/BUN264+ISS2+APR15+Baha+Attract+Radiographers+Instructions+for+MRI.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-f5917ef2-bb35-4307-b330-8c15ffdd993c-l5veV.x. Accessed 11/2018
- 48.Kim B G, Kim J W, Park J J, Kim S H, Kim H N, Choi J Y. Adverse events and discomfort during magnetic resonance imaging in cochlear implant recipients. JAMA Otolaryngol Head Neck Surg. 2015;141(01):45–52. doi: 10.1001/jamaoto.2014.2926. [DOI] [PubMed] [Google Scholar]
- 49.Syms M J, Hernandez K E. Bone conduction hearing: device auditory capability to aid in device selection. Otolaryngol Head Neck Surg. 2014;150(05):866–871. doi: 10.1177/0194599814524530. [DOI] [PubMed] [Google Scholar]
- 50.Murray M, Popelka G R, Miller R. Efficacy and safety of an in-the-mouth bone conduction device for single-sided deafness. Otol Neurotol. 2011;32(03):437–443. doi: 10.1097/MAO.0b013e3182096b1d. [DOI] [PubMed] [Google Scholar]
- 51.Murray M, Miller R, Hujoel P, Popelka G R. Long-term safety and benefit of a new intraoral device for single-sided deafness. Otol Neurotol. 2011;32(08):1262–1269. doi: 10.1097/MAO.0b013e31822a1cac. [DOI] [PubMed] [Google Scholar]


