Abstract
Objectives Hearing rehabilitation is an important management aspect of patients undergoing excision of vestibular schwannomas. Studies have shown cochlear implantation (CI) is possible at the time of tumor excision via a translabyrinthine approach. Primary objectives of this report are (1) to review prospective studies pertaining to outcomes of concurrent CI and translabyrinthine tumor removal in detail and (2) perform an aggregate analysis of outcomes for case reports and series.
Design Systematic review based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Setting Review of literature using PubMed and Cochrane databases.
Participants Eligibility included patients undergoing translabyrinthine excision of vestibular schwannoma with concurrent CI.
Main Outcome Measures Open-set speech discrimination scores, sound localization, patient-reported outcome measures.
Results Forty-one subjects were identified. Two prospective studies have been performed, which showed improvement in speech localization and patient-reported outcome measures. While the majority of patients achieved open set speech recognition, data pertaining to improvement in speech perception were variable. Approximately 85% of subjects had audibility with their CI. Of those that achieved open-set speech discrimination, 75% could be classified as either intermediate or high performers. The majority of low performers in open-set speech either endorsed subjective benefit or demonstrated improvement compared to preoperative measures. There was a high risk of selection and reporting bias.
Conclusions The majority of patients undergoing translabyrinthine excision of vestibular schwannoma with concurrent CI achieve open set speech perception, with 75% of these patients meeting criteria for being intermediate to high performers. Additional benefits include improved subjective hearing measures, decreased tinnitus, and improved sound localization.
Keywords: cochlear implantation, schwannoma, hearing rehabilitation, translabyrinthine, hearing outcomes, review
Introduction
Vestibular schwannomas are benign tumors that develop from Schwann cells of the vestibular divisions of the eighth cranial nerve. 1 They can be either sporadic in nature or manifest as part of neurofibromatosis type 2 (NF2). Patients with sporadic tumors often present with the chief complaint of hearing loss or tinnitus. Hearing loss can be rendered nonfunctional secondary to either the natural history of disease, or interventions including microsurgery and radiation. Studies have shown that patients undergoing vestibular schwannoma resection often have significant subjective hearing deficits postoperatively. 2 3 As such, the implications of unilateral hearing loss are extremely relevant to this patient population, and an understanding of related auditory deficits and hearing rehabilitative options is important for both clinicians and patients.
Patients with unilateral hearing loss lack binaural cues which negatively impact auditory performance in complex listening environments. 4 5 Commonly cited benefits of binaural hearing include the head shadow effect, binaural squelch, and binaural summation. 5 6 The head shadow effect and binaural squelch make it possible to use interaural time and level difference (ITD and ILD, respectively) cues to separate a signal from background noise. ITD cues are the primary cues for localization and squelch effects in the lower frequencies, while ILDs predominate in the higher frequencies. Binaural summation provides two redundant signals to the central nervous system, which aids in producing a more complete auditory picture and is beneficial for loudness. These binaural effects are especially important for complex listening environments where speech and noise are spatially separate, such as the classroom, workplace, and other social situations.
While traditional means of hearing rehabilitation for unilateral hearing loss entailed contralateral routing of sound, cochlear implantation (CI) has emerged as a promising option for restoration of hearing in a deafened ear. Studies have generally shown that CI in this setting results in improved objective measures of listening including speech perception in noise, spatial hearing, localization, and listening effort. 7 8 9 10 CI for unilateral hearing loss also significantly improves subjective quality of life measures and portends benefit with respect to subjective tinnitus severity in appropriately selected patients. 7 8 9
Given the aforementioned benefits of CI for nontumor patients with unilateral hearing loss, groups have begun to explore outcomes of CI in patients with vestibular schwannomas. Implantation at the time of tumor extirpation is possible if a translabyrinthine approach is performed and the integrity of the cochlear nerve is preserved. There have been numerous case reports as well as two prospective studies that report outcomes of CI performed at the time of translabyrinthine tumor resection. Given the above, the primary objectives of this report are as follows (1) to review higher-level data (prospective studies) pertaining to outcomes of concurrent CI and translabyrinthine tumor removal in detail and (2) perform an aggregate analysis of outcomes for case reports and series.
Methods
A systematic review of the literature was performed based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 11 12 A literature search was performed using the PubMed database ( www.ncbi.nlm.nih.gov/pubmed ). Inclusion criteria included any report detailing patients undergoing translabyrinthine excision of a vestibular schwannoma and CI during the same operation. Searches were completed using the terms “schwannoma translabyrinthine cochlear implant” and “neurofibromatosis type 2 cochlear implant.” No filters were used with the aim of maximizing search results. There were no year restrictions placed. Published studies written or transcribed in English were reviewed. This strategy was adopted for use in the Cochrane database ( http://www.cochranelibrary.com/ ) with the broader terms of “schwannoma” and “cochlear implantation.” Titles and abstracts were reviewed, and any possible relevant papers were examined ( Fig. 1 ). Any references cited in these papers that appeared applicable were also reviewed for possible inclusion into the study.
Fig. 1.
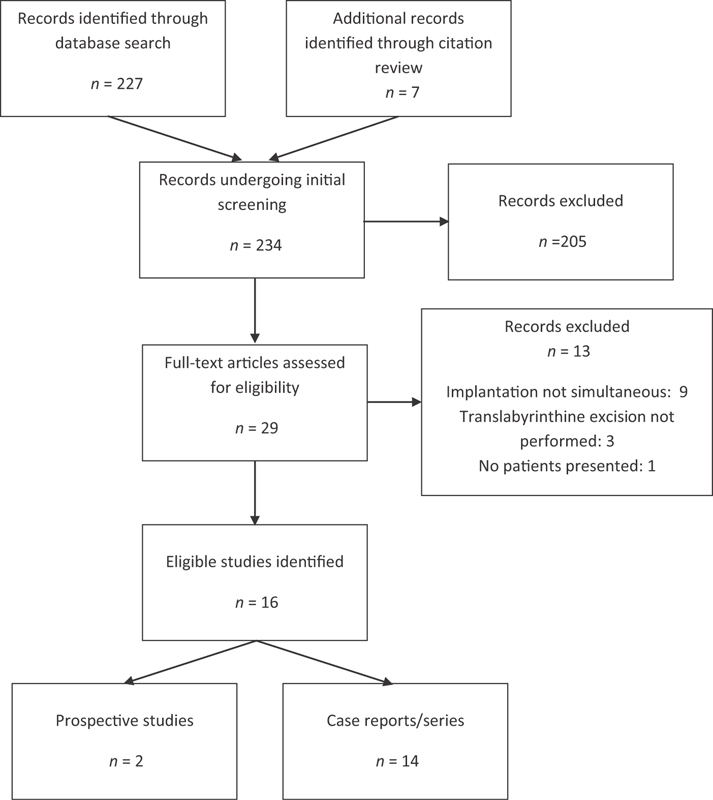
Flow diagram showing search strategy and numbers of studies identified.
Available demographic data from applicable studies were collected in a table format. Variables examined include tumor characteristics, laterality, neurofibromatosis status, implant characteristics, hearing outcome measures, and patient reported outcome measures.
Hearing outcome measures were examined and there was noted to be a large amount of heterogeneity in the data reported across studies. Due to this heterogeneity in combination with the relative infrequency of these procedures, it was not possible to perform a meaningful meta-analysis. A systematic review of the literature was therefore conducted, with particular attention given to the two prospective studies. The primary outcome measure of interest was open-set speech recognition. As used per convention published in previous studies, high performance was defined as achievement of open-set recognition scores ranging from 67 to 100%, intermediate performance from 34 to 66%, and low performance was characterized by scores between 0 and 33% correct. 13 14 We recognize the difference in difficulty and complexity between tests, but given the heterogeneity of open-set speech outcomes reported, this convention will be used to summarize results herein. Although there are limitations, it allowed for an aggregate analysis of speech outcomes pooled from case series and reports.
Assessment of the risk of bias in the prospective studies was completed using methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions. 15 Areas of bias assessed included selection bias, performance bias, detection bias, attrition bias, and reporting bias. Table 1 details the assessment for each study. Case reports and case series were assessed and were felt to have a high risk of bias across all subgroups of bias. The risk of publication bias is noted across the cumulative body of literature.
Table 1. Risk of bias assessment for prospective studies.
| Rooth et al 17 | Sanna et al 16 | |
|---|---|---|
| Selection bias | ||
| Random sequence generation | + | + |
| Allocation concealment | + | + |
| Performance bias | ||
| Blinding of participants and personnel | + | + |
| Detection bias | ||
| Blinding of outcome assessment | + | + |
| Attrition bias | ||
| Incomplete outcome data addressed | – | ? |
| Reporting bias | ||
| Selective reporting | – | – |
+High risk; –Low risk; ? Unclear risk.
Results
A total of 234 articles were initially screened, and 29 full-text articles were assessed for eligibility ( Fig. 1 ). Sixteen eligible studies were identified, with two prospective studies and 14 case reports/series published. This resulted in a total of 41 subjects identified.
Patients
Overall, 41 patients who underwent concurrent translabyrinthine vestibular schwannoma resection and CI were identified in the literature ( Table 2 ). The mean age at the time of CI was 33 years (range, 15–76); the majority of patients were male (56%). The average tumor size assessed with imaging immediately preceding surgery was 1.3 cm (range, 0.2–4.0 cm). Sporadic schwannomas were present in 29 patients, while 12 patients had a diagnosis of NF2. There were 25 implants manufactured by Cochlear (Sydney, Australia), and 15 by MED-EL (Innsbruck, Austria). There were five studies from the United States, three from Italy, two from Brazil, and one each from France, Germany, Korea, Spain, Turkey, and the United Kingdom.
Table 2. Patient demographics.
| Age (years) | Sex | Tumor size (cm) | NF2 a status | Laterality | Implant characteristics d | ||
|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||
| Rooth et al 17 | United States | 49 | M | 1.5 | Spor b | NR c | MED-EL Synchrony Standard |
| 76 | F | 1.4 | Spor | NR | MED-EL Synchrony Standard | ||
| 59 | F | 1.5 | Spor | NR | MED-EL Synchrony Standard | ||
| 71 | M | 1 | Spor | NR | MED-EL Synchrony Standard | ||
| 49 | F | 0.4 | Spor | NR | MED-EL Synchrony Standard | ||
| 52 | F | 1.2 | Spor | NR | MED-EL Synchrony Standard | ||
| 44 | M | 0.5 | Spor | NR | MED-EL Synchrony Standard | ||
| Sanna et al 16 | Italy | Average of 50; range 33–71 | 8M, 5 F | 11 Intrameatal tumors, 2 extending into cerebellopontine angle | All 13 sporadic | All 13 NR | All 13 Cochlear Nucleus Freedom Contour Advance |
| Case studies | |||||||
| Ahsan et al 19 | United States | 53 | M | Intracanalicular | NF2+ | L | Cochlear Nucleus 24 Contour Advance |
| Aristegui and Denia 22 | Spain | 53 | M | 4 | NF2+ | L | MED-EL Combi 40+ |
| 45 | M | 1.2 | Spor | L | Cochlear Nucleus 24 Contour Advance | ||
| Carlson et al 13 | United States | 67 | M | 1.1 | NF2+ | NR | Cochlear Nucleus Contour Advance |
| Cruz and Vellutini 23 | Brazil | 23 | M | NR | NF2+ | L | NR |
| DeHart et al 14 | United States | 76 | M | 0.7 | Spor | R | MED-EL Synchrony Flex 28 |
| Tran Ba Huy et al 24 | France | 17 | F | <1 | NF2+ | L | Cochlear Nucleus Freedom |
| Kim et al 20 | Korea | 72 | F | 0.8 | Spor | L | MED-EL Sonata TI100 |
| 58 | M | 1.3 | Spor | L | MED-EL Concerto | ||
| Lloyd et al 18 | United Kingdom | 15 | M | 1.3 | NF2+ | L | Cochlear Nucleus Contour Advance |
| 36 | M | 1.5 | NF2+ | R | Cochlear Nucleus Freedom Contour Advance | ||
| 35 | F | 0.9 | NF2+ | R | Cochlear Nucleus Contour Advance | ||
| 30 | F | 1.1 | NF2+ | R | Cochlear Nucleus Slim Straight | ||
| Neff et al 25 | United States | 37 | F | NR | NF2+ | R | Cochlear Nucleus 24 Cochlear Advance |
| Dos Santos Neto et al 21 | Brazil | 43 | M | 0.2 | Spor | L | Cochlear Freedom Contour Advance |
| Ozdek et al 29 | Turkey | 57 | F | 1.2 | NF2+ | R | MED-EL Sonata |
| Plontke et al 28 | Germany | 38 | M | NR | Spor | NR | MED-EL Sonata |
| 39 | M | NR | Spor | NR | MED-EL Synchrony Flex28 | ||
| 25 | F | NR | Spor | NR | MED-EL Synchrony Flex28 | ||
| Vincenti et al 27 | Italy | 24 | F | 2 | NF2+ | NR | Cochlear Nucleus 24 Contour Advance |
| Zanetti et al 26 | Italy | 65 | F | NR | Spor | R | Cochlear Nucleus Contour Advance |
Neurofibromatosis type 2.
Sporadic.
Not reported.
Not all manufacturers, processors, or electrode arrays were detailed.
Only available data is reported.
Prospective Studies
There were two prospective studies examining concurrent CI with translabyrinthine excision of sporadic vestibular schwannoma. Sanna et al studied 13 patients with sporadic vestibular schwannomas and normal contralateral hearing. 16 Eleven of the subjects had intrameatal tumors, and the remaining two subjects had tumors extending into the cerebellopontine angle.
The authors analyzed the following postoperative performance measures with masking to the normal hearing side: pure-tone average (PTA), vowel identification, disyllabic word recognition, sentence recognition (Bocca and Pellegrini sentence list), and common phrases comprehension with a monitored live voice through the sound field at a level of 70 dB sound pressure level (SPL). All subjects reported auditory sensation; however, two had unsatisfactory results with mean PTA of roughly 90 dB. One of these subjects had no open or closed set speech perception and was lost to follow-up; the other had relatively poor speech perception outcomes and stopped using the CI after 9 months. Mean speech perception scores ranged from around 60 to 90% for all analyzed variables at the 6-month timepoint for the 12 subjects tested. There were five subjects tested at the 14-month timepoint. Performance with disyllabic word recognition, speech recognition, and common phrases all improved slightly, though differences were not statistically significant when compared to the 6-month timepoint.
Speech recognition in noise as well as sound localization tasks was performed in these patients to evaluate binaural benefit. To assess speech recognition in noise, adaptive speech recognition threshold testing was used with varying spatial configurations to evaluate for summation (speech front and noise front), head shadow effect (speech from CI side and noise front), and squelch effects (speech front and noise from CI side). At 6 months postoperatively, improved performance was noted in all spatial conditions, though these differences were not significant when compared to the unaided condition. At 14 months postoperatively, there were further improvements in each condition with squelch effect difference achieving statistical significance. Sound localization was performed with four loudspeakers at 90-degree intervals, and patients were tested in the aided and unaided conditions. Results showed a significant improvement in sound localization ability with the aided condition for each timepoint.
These objective improvements in speech perception and localization were corroborated with patient-reported outcome measures. The Bern Benefit for Single-Sided Deafness Questionnaire results showed that all but one subject reported a subjective improvement with their CI for the 10 common daily situations that were evaluated. Results of the Single-Sided Deafness Questionnaire also showed that 90% of subjects used their implant between 5 and 7 days per week, with 60 to 80% benefiting in the five conditions that were evaluated.
Table 1 details the assessment for risk of bias as previously described. Patients were counseled on different management options and those wishing to pursue the surgical approach with CI were recruited for the study, resulting in a high amount of selection bias. Given the implantation, blinding was not possible. There was a moderate amount of loss to follow-up in the study. It was reported that two of the three subjects that were unable to have the initial follow-up had poor outcomes with their implant. There was also loss to follow-up between the two testing visits, though the reported analysis between the two timepoints automatically excluded these subjects. For these reasons, the attrition bias was given an unclear risk. It was felt that this study detailed all outcomes well despite significance levels, resulting in a low risk of selective reporting bias.
Rooth et al also performed a prospective Food and Drug Administration-approved feasibility study analyzing patients undergoing concurrent CI and translabyrinthine excision of sporadic vestibular schwannomas. 17 Seven subjects were included in the study, with a mean tumor size of 1.1 cm (range, 0.4–1.5 cm). Speech perception was assessed using consonant-nucleus-consonant (CNC) words and AzBio sentences in a 10-talker babble in the following configurations: (1) speech front, noise front; (2) speech front with noise to the CI ear; (3) speech front with noise to the better hearing non-CI ear. Sound localization was assessed with root mean square error using an 11-speaker array from –90 to +90 degrees. The aforementioned tests were performed at 1, 3, and 6 months postimplantation.
Five of the seven subjects had auditory perception at the time of activation; the two subjects without audibility were excluded in the following reported speech perception outcomes. Postoperative CNC scores with the CI alone were 24, 19, and 20% at 1, 3, and 6 months, respectively. There were no differences when comparing these scores to the preoperative word scores (mean 35%). Sentence testing in noise did demonstrate improvement at all test intervals with the implant-on compared to the implant-off. Specifically, AzBio in noise at 0 dB signal-to-noise ratio with speech at 0 degrees and noise to contralateral ear (most difficult condition) showed scores of 22, 12, and 18% with the implant-on compared to 12, 7, and 8% with the implant-off.
While there was modest improvement in speech perception in complex listening environments, there was considerable benefit for sound localization at all three timepoints conferred by the implant-on condition. Root mean square error at 1 month with the implant off was 78 degrees compared to 41 degrees with the implant on. Error remained low at 3 and 6 months postoperatively, with mean scores of 40 and 38 degrees.
Patient-reported outcome measures were also completed for this study. The Speech, Spatial, and Qualities of Hearing Scale questionnaires were completed which showed subjective improvement over the course of the 6 months in both speech and spatial hearing. No improvement was seen with the qualities of hearing measure. Tinnitus Handicap Inventory questionnaires were completed preoperatively as well as 1, 3, and 6 months postoperatively. Preoperative measures had an average score of 23, which improved to 7 at 1 month. Tinnitus measures continued to improve and at 6 months were an average of 3, indicating significantly improved symptoms with CI. Data-logging from the devices show that all subjects were daily users, and they wore their implants for an average of 10 hours per day at the 6-month timepoint. This provides additional evidence to support the subjective benefit of the implants, though it is noted that the possibility of selection bias exits given participation in the study.
Assessment for bias risk is again presented in Table 1 . Similar with the study by Sanna et al, the risk for selection bias, performance bias, and detection bias was felt to be high given the nature of subject selection as well as lack of ability for blinding. There were less problems with attrition, and outcomes were reported regardless of significance, so attrition and reporting biases were felt to be low.
Case Reports
Speech Perception Outcomes
There was a total of 21 subjects reported in case reports and case series. Significant heterogeneity in reporting hearing and speech outcomes was present across the case studies ( Table 2 ). Of the 20 subjects with some form of speech perception documented, 18 (90%) had audibility with their cochlear implant. The additional subject without objective speech perception documented reported good subjective sound quality and pitch, suggesting the presence of audibility. 18
Open-set recognition was tested using a variety of measures. Table 3 details specific tests utilized. Data were available for 16 of the 21 patients. As described in the Methods section, high performance has previously been defined as scores from 67 to 100% correct, intermediate performance of 34 to 66%, and low performance as 0 to 33% correct. 13 14
Table 3. Postoperative outcome measures reported.
| Prospective studies | Audibility of cochlear implant | Daily implant user | Postoperative measures | |
|---|---|---|---|---|
| Rooth et al 17 | Y | Y | Consonant-nucleus-consonant AZ-Bio sentences Sound localization Spatial and qualities of hearing scale Tinnitus handicap inventory |
|
| Y | Y | |||
| N | Y | |||
| Y | Y | |||
| Y | Y | |||
| Y | Y | |||
| N | Y | |||
| Sanna et al 16 | 11 y; 2 n | 90% wore 5–7 days per week | Vowel identification, disyllabic word recognition, sentence recognition with Bocca–Pellegrini sentence list, common phrases comprehension; binaural benefit- summation, squelch, and head shadow effects; sound localization; Bern benefit in single-sided deafness questionnaire; single-sided deafness questionnaire | |
| Case Studies | Audibility of cochlear implant | Daily implant user | Performance status a | Postoperative measures |
| Ahsan et al 19 | Y | NR b | Subjective | Subjective improvement |
| Aristegui and Denia 22 | Y | NR | HP | Vowel identification, disyllabic word recognition, daily words, sentences |
| Y | N | HP | ||
| Carlson et al 13 | Y | Y | LP | Consonant-nucleus-consonant; AzBio sentences; Bamford-Kowal-Bench sentence in noise test |
| Cruz and Vellutini 223 | Y | Y | HP | CI-aided PTA c ; monosyllable discrimination; open set sentence discrimination |
| DeHart et al 14 | Y | Y | HP | CI-aided PTA; consonant-nucleus-consonant; AzBio sentences |
| Tran Ba Huy et al 24 | Y | Y | HP | Open-set word; open-set sentences; phone with/without contextual clues |
| Kim et al 20 | Y | Y | NR | CI-aided PTA; Korean hearing in noise test |
| NR | Y | NR | CI-aided PTA | |
| Lloyd et al 18 | Y | Y | HP | CI-aided PTA; Bamford-Kowal-Bench sentences |
| Y | Y | LP | CI-aided PTA; Bamford-Kowal-Bench sentences | |
| Y | Y | IP | CI-aided PTA; Bamford-Kowal-Bench sentences; City University of New York sentences | |
| NR | NR | NR | Subjective improvement | |
| Neff et al 25 | Y | y | HP | Central Institute for the Deaf Sentences; hearing in noise sentences; four-choice Spondee |
| Dos Santos Neto et al 21 | Y | Y | NR | Ling sound test; vocabulary extension |
| Ozdek et al 29 | N | N | LP | CI-aided PTA; closed-set disyllabic words recognition; sentence recognition |
| Plontke et al 28 | Y | Y | IP | Monosyllable word recognition score |
| Y | Y | IP | ||
| Y | Y | LP | ||
| Vincenti et al 27 | Y | Y | IP to HP | Vowel identification, consonant identification, disyllabic word recognition, sentences, common phrases |
| Zanetti et al 26 | Y | Y | HP | Disyllabic word recognition |
Abbreviations: CI, cochlear implantation; PTA, pure tone average.
Open-set speech discrimination scores with percent correct, HP, high performance (67–100%), IP, intermediate performance (34–66%), LP, low performance (0–33%).
Not reported.
Cochlear implant-aided PTA.
There were 5 of the 21 subjects who either did not have open-set recognition tested or were not reported. 18 19 20 21 One of these subjects had subjective recognition, but formal testing was deferred due to the patient only speaking Spanish. 19 A subject detailed by dos Santos Neto et al showed improvement in both Ling Sound Test and identification of vocabulary extension compared to baseline measures. 21
High Performers
Fifty percent (8/16) of subjects with open-set recognition scores were identified as high performers during their first documented postoperative open-set speech testing. 14 18 22 23 24 25 26 Four of these subjects had open-set sentence recognition of > 95% at 6 months postoperatively. Three additional subjects were tested at longer term follow-up showing good open-set sentence recognition. One subject scored 72% at 12 months postoperatively, another scored 100% at 5 years, and the last scored 94% at 7 years postimplantation. 14 24 25 The final subject had open-set speech discrimination scores in the CI ear that improved from 70% at 1 month, to 90% at 3 months, and 100% at 6 months postoperatively.
Vincenti et al detailed a subject who improved to the high-performance category over time. 27 Disyllabic word recognition at 1, 6, and 12 months postoperatively were 32, 50, and 72%, respectively, and sentence recognition was 40, 59, and 81%, respectively. This subject improved from low-intermediate performance to high performance at the 1-year timepoint.
Intermediate Performers
Plontke et al reported two subjects that could be categorized to the intermediate performance group. 28 German Freiburger monosyllable test was performed at 65 dB SPL. Both subjects had scores of 0% preoperatively. At 6 and 12 months after implantation, word scores improved to 55 and 45%, respectively. Lloyd et al detail a subject who achieved a Bamford-Kowal-Bench (BKB) sentence score in quiet at 35%, and a City University of New York University sentence score of 94% at 2 years postoperatively. 18
Low Performers
There were four subjects included in the case studies that were identified as low performers. 13 18 28 29 Carlson et al detailed a subject with a postoperative CNC score of 22% and AzBio in quiet score of 32%. 13 One of the subjects detailed in the case series by Lloyd et al showed speech discrimination scores at 12 months postoperatively of 30% for BKB in quiet and 16% in noise, though gains in subjective benefit and daily implant use were noted by the authors. 18 One subject reported by Ozdek et al had preoperative speech discrimination scores of 0%, which improved on closed-set disyllabic word recognition testing to 25% at 3 months, and 67% at 6 months. 29 This patient did not gain improvement in open-set speech scores, but reportedly was very satisfied with her cochlear implant, uses it daily, and has improvement with the implant in conjunction with lip reading. The final subject in the low-performance category was reported by Plontke et al. 28 Word recognition score using the German Freiburger monosyllable test in quiet at 65 dB preoperatively was 5% which improved modestly to 25% postoperatively.
Summary
Of the 16 subjects with open-set recognition reported, 12 (75%) demonstrated either intermediate- to high-performance scores. Four patients (25%) were in the low-performance category, though most still noted subjective benefit of their implants. In addition, at least two of these patients had improved performance from their preoperative state, though still fell within the low-performance category. 28 29 Seven out of the 10 (70%) NF2 patients with open-set recognition reported had at least intermediate to high performance, compared with 5 out of 6 (83%) of those with sporadic tumors.
Discussion
Management strategies for vestibular schwannomas include observation, radiosurgery, and microsurgical resection. 17 30 Decisions as to which management strategy to utilize aim to minimize morbidity to the patient; hearing status is an important consideration in this algorithm. The translabyrinthine approach to tumor resection has benefits including adequate exposure to a wide array of tumor sizes and locations, early identification of the facial nerve, and relatively low rates of cerebrospinal fluid leak and headache. An obvious disadvantage is the fact that hearing preservation is not possible with this approach. As such, in patients with nonfunctional hearing, this is commonly selected as the surgical approach.
The deficits of single-sided hearing loss in both the general population and those with vestibular schwannoma are well documented. 2-10 The placement of a CI concomitant to translabyrinthine resection of vestibular schwannomas has recently been explored by various authors as a means for hearing rehabilitation in this patient cohort. The results from this systematic review support the notion that CI during a translabyrinthine tumor resection generally results in improved speech perception, better speech localization, and patient-reported benefit even in those that may not objectively demonstrate high performance.
Including both subjects from the prospective studies as well as case reports, 85% of subjects had audibility with their cochlear implant. Intermediate- to high performance in open-set recognition was achieved in at least 75% of patients. Many subjects that were considered low performers in the open-set recognition tasks still gained subjective benefit from their implant. Specifically, even though average open-set recognition performance in the prospective study by Rooth et al was in the low-performance category, patients had a significant improvement in sound localization with their implant. 17 It should be noted that speech testing was assessed with CNC scores in that study, which is typically a more difficult test than other means of assessing open set speech (e.g., AzBio in quiet, HINT). Furthermore, patient-reported outcome measures also showed subjective improvement in speech, spatial hearing, and tinnitus measures. Similar to Rooth et al, the prospective study by Sanna et al also showed a significant improvement in sound localization as well as subjective improvement in hearing outcomes in daily situations. 16 Taken together, these studies indicate that while open-set recognition is an important measure to consider when evaluating the efficacy of this intervention, additional outcome measures such as localization and quality of life should need to be considered.
Patients with both sporadic tumors and NF2-related tumors were included herein; this deserves further mention given the differences in tumor biology and prognosis of these cohorts. Subjects with sporadic tumors had a similar, though slightly higher, percentage performing in the intermediate- to high-performance categories when compared to those with a diagnosis of NF2. These sample sizes were small, precluding statistical analysis. This finding may be secondary to the differing intraoperative characteristics of the tumors, as NF2-related tumors are often associated with poor surgical planes and greater adherence to neurovascular structures. 13 31 This likely increases the risk for inadvertent injury to the cochlear nerve. Additionally, the increased likelihood of remnant tumor growth or de novo tumorigenesis in the surgical bed after resection of NF2-related tumors could lead to progressive decline in performance in this group over time.
There are important imaging considerations when deciding whether or not to place a CI in patients undergoing vestibular schwannoma resection. Postoperative magnetic resonance imagings (MRIs) are routinely performed for tumor surveillance after surgery. 16 30 The receiver–stimulator and magnet distort radiographic images obtained after CI. Some considerations raised in included studies include altering placement of internal hardware to avoid distortion and avoidance of CI if there is a reasonable suspicion for residual tumor or possibility of recurrence. 14 16 17 In the authors' opinion, CI should only be considered if gross total resection of tumor is achieved given the challenges associated with imaging surveillance of the cerebellopontine angle ipsilateral to a CI. Further, with respect to device selection, implants compatible with MRI that do not require magnet removal prior to imaging are preferred.
An additional consideration regarding the decision to proceed with CI at the time of tumor resection relates to cochlear nerve integrity. In the majority of studies included in the analysis, the decision to proceed with implantation was based on the surgeon's subjective analysis of an intact cochlear nerve after tumor resection. Some surgeons have suggested intraoperative cochlear nerve testing using methods such as promontory stimulation, though studies have called into question this method due to false negative results. 17 25 32 The benefits of implantation at the time of resection include the avoidance of a second procedure as well as the possibility of cochlear fibrosis or ossification which may limit future implantation. The development of a more reliable intraoperative testing method would be beneficial to accurately assess nerve integrity and guide the decision-making process.
The favorable complication profile of concomitant CI and translabyrinthine tumor resection deserves mention. While no complications could be directly attributed to the presence of the CI, one subject developed a hematoma at the operative site postoperatively. This was treated with needle aspiration and a pressure dressing and resolved. Notably, there were no cases of cerebrospinal fluid leak, wound breakdown, or infection attributed to the presence of the CI.
A limitation of this study on the review-level is the incomplete retrieval of identified research in the literature. There is also a high risk of publication bias, especially regarding case studies/series, given the file cabinet effect. The overall risk of bias was high across all studies given the nature of subject selection. An additional limitation of this study includes the inability to perform a meta-analysis. The wide range of outcome measures as well as the differences in language, sentence lists, and sound level used resulted in a large amount of heterogeneity in the data reported. Many studies reported open-set discrimination scores, though only the prospective studies reported additional measures such as sound localization and questionnaire data. Further prospective studies and standardization of reporting would be useful to further quantify the potential benefits of CI during translabyrinthine excision of vestibular schwannoma.
Conclusion
Patients undergoing translabyrinthine excision of vestibular schwannoma with concurrent CI generally have favorable audiologic outcomes. The vast majority of patients (85%) achieve audibility with their cochlear implant. Of those patients with open-set speech recognition, most (75%) were classified as either intermediate or high performers. Access to binaural cues also resulted in improved sound localization. Lastly, subjective benefit is noted in the majority of patients.
Footnotes
Conflict of Interest None.
References
- 1.Frisch C D, Eckel L J, Lane J I, Neff B A. Intralabyrinthine schwannomas. Otolaryngol Clin North Am. 2015;48(03):423–441. doi: 10.1016/j.otc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Andersen H T, Schrøder S A, Bonding P. Unilateral deafness after acoustic neuroma surgery: subjective hearing handicap and the effect of the bone-anchored hearing aid. Otol Neurotol. 2006;27(06):809–814. doi: 10.1097/01.mao.0000227900.57785.ec. [DOI] [PubMed] [Google Scholar]
- 3.Douglas S A, Yeung P, Daudia A, Gatehouse S, O'Donoghue G M. Spatial hearing disability after acoustic neuroma removal. Laryngoscope. 2007;117(09):1648–1651. doi: 10.1097/MLG.0b013e3180caa162. [DOI] [PubMed] [Google Scholar]
- 4.Feuerstein J F. Monaural versus binaural hearing: ease of listening, word recognition, and attentional effort. Ear Hear. 1992;13(02):80–86. [PubMed] [Google Scholar]
- 5.Corbin N E, Buss E, Leibold L J. Spatial release from masking in children: effects of simulated unilateral hearing loss. Ear Hear. 2017;38(02):223–235. doi: 10.1097/AUD.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limb C F, Francis H W, Niparko J K. Philadelphia, PA: Elsevier Saunders; 2015. Cochlear implantation: results, outcomes, rehabilitation, and education; pp. 2455–2471. [Google Scholar]
- 7.Hansen M R, Gantz B J, Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière's disease. Otol Neurotol. 2013;34(09):1681–1687. doi: 10.1097/MAO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon M T, Buss E, Rooth M Aet al. Effect of cochlear implantation on quality of life in adults with unilateral hearing loss Audiol Neurotol 201722(4-5):259–271. [DOI] [PubMed] [Google Scholar]
- 9.Amoodi H A, Mick P T, Shipp D B et al. The effects of unilateral cochlear implantation on the tinnitus handicap inventory and the influence on quality of life. Laryngoscope. 2011;121(07):1536–1540. doi: 10.1002/lary.21851. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein J GW, Schuchman G I, Rivera A L. Head shadow and binaural squelch for unilaterally deaf cochlear implantees. Otol Neurotol. 2017;38(07):e195–e202. doi: 10.1097/MAO.0000000000001469. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman D G, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman D G; PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement J Clin Epidemiol 200962101006–1012. [DOI] [PubMed] [Google Scholar]
- 13.Carlson M L, Breen J T, Driscoll C L et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol. 2012;33(05):853–862. doi: 10.1097/MAO.0b013e318254fba5. [DOI] [PubMed] [Google Scholar]
- 14.DeHart A N, Broaddus W C, Coelho D H. Translabyrinthine vestibular schwannoma resection with simultaneous cochlear implantation. Cochlear Implants Int. 2017;18(05):278–284. doi: 10.1080/14670100.2017.1337665. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J PT, Green S.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]The Cochrane Collaboration 2011. Available at:www.handbook.cochrane.org. Accessed January 16, 2019
- 16.Sanna M, Medina M D, Macak A, Rossi G, Sozzi V, Prasad S C. Vestibular schwannoma resection with ipsilateral simultaneous cochlear implantation in patients with normal contralateral hearing. Audiol Neurotol. 2016;21(05):286–295. doi: 10.1159/000448583. [DOI] [PubMed] [Google Scholar]
- 17.Rooth M A, Dillon M T, Brown K D. Prospective evaluation of patients undergoing translabyrinthine excision of vestibular schwannoma with concurrent cochlear implantation. Otol Neurotol. 2017;38(10):1512–1516. doi: 10.1097/MAO.0000000000001570. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd S K, Glynn F J, Rutherford S A et al. Ipsilateral cochlear implantation after cochlear nerve preserving vestibular schwannoma surgery in patients with neurofibromatosis type 2. Otol Neurotol. 2014;35(01):43–51. doi: 10.1097/MAO.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 19.Ahsan S, Telischi F, Hodges A, Balkany T. Cochlear implantation concurrent with translabyrinthine acoustic neuroma resection. Laryngoscope. 2003;113(03):472–474. doi: 10.1097/00005537-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Kim J W, Han J H, Kim J W, Moon I S. Simultaneous translabyrinthine tumor removal and cochlear implantation in vestibular schwannoma patients. Yonsei Med J. 2016;57(06):1535–1539. doi: 10.3349/ymj.2016.57.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dos Santos Neto P H, Zamponi J O, Jr, Hamerschmidt R, Wiemes G RM, Rassi M S, Borba L AB. Simultaneous cochlear implantation as a therapeutic option in vestibular schwannoma surgery: case report. Neurosurg Focus. 2018;44(03):E9. doi: 10.3171/2017.12.FOCUS17670. [DOI] [PubMed] [Google Scholar]
- 22.Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma) Otol Neurotol. 2005;26(02):205–210. doi: 10.1097/00129492-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Cruz O L, Vellutini E A. Cochlear implant in type 2 neurofibromatosis: an option for better hearing rehabilitation. Rev Bras Otorrinolaringol (Engl Ed) 2011;77(04):538. doi: 10.1590/S1808-86942011000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran Ba Huy P, Kania R, Frachet B, Poncet C, Legac M S. Auditory rehabilitation with cochlear implantation in patients with neurofibromatosis type 2. Acta Otolaryngol. 2009;129(09):971–975. doi: 10.1080/00016480802510202. [DOI] [PubMed] [Google Scholar]
- 25.Neff B A, Wiet R M, Lasak J M et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117(06):1069–1072. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti D, Campovecchi C B, Pasini S, Nassif N. Simultaneous translabyrinthine removal of acoustic neuroma and cochlear implantation. Auris Nasus Larynx. 2008;35(04):562–568. doi: 10.1016/j.anl.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: cochlear versus auditory brainstem implantation. Audiol Neurotol. 2008;13(04):273–280. doi: 10.1159/000115437. [DOI] [PubMed] [Google Scholar]
- 28.Plontke S K, Rahne T, Pfister M et al. Intralabyrinthine schwannomas: surgical management and hearing rehabilitation with cochlear implants. HNO. 2017;65 02:136–148. doi: 10.1007/s00106-017-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozdek A, Bayır O, Dönmez T et al. Hearing restoration in NF2 patients and patients with vestibular schwannoma in the only hearing ear: report of two cases. Am J Otolaryngol. 2014;35(04):538–541. doi: 10.1016/j.amjoto.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Halliday J, Rutherford S A, McCabe M G, Evans D G. An update on the diagnosis and treatment of vestibular schwannoma. Expert Rev Neurother. 2018;18(01):29–39. doi: 10.1080/14737175.2018.1399795. [DOI] [PubMed] [Google Scholar]
- 31.Linthicum F H, Jr, Brackmann D E. Bilateral acoustic tumors. A diagnostic and surgical challenge. Arch Otolaryngol. 1980;106(12):729–733. doi: 10.1001/archotol.1980.00790360007003. [DOI] [PubMed] [Google Scholar]
- 32.Nikolopoulos T P, Mason S M, Gibbin K P, O'Donoghue G M. The prognostic value of promontory electric auditory brain stem response in pediatric cochlear implantation. Ear Hear. 2000;21(03):236–241. doi: 10.1097/00003446-200006000-00007. [DOI] [PubMed] [Google Scholar]


