Abstract
Radiation and chemotherapy are frequently used in the treatment of head and neck malignancies. Ototoxicity is a common adverse effect of these treatment modalities. This article discusses the patterns of hearing loss following chemotherapy and radiation therapy. Specific issues related to hearing rehabilitation in oncological patients will also be covered, such as controversies regarding treatment of radiation-induced serous otitis media, risks of otologic surgery, and the use of osseointegrated hearing aids and cochlear implants.
Keywords: hearing loss, ototoxicity, chemotherapy, radiation therapy, otitis media, osseointegrated implant, cochlear implant, temporal bone, cancer, myringotomy
Introduction
Radiation and chemotherapy have become common tools in the armamentarium against tumors of the head and neck. However, the use of these treatment modalities can be complicated by hearing loss. Despite significant advances in radiation oncology and research efforts in prevention of chemotherapy-induced ototoxicity, a significant portion of patients still suffer from this complication. The effects of radiation therapy (RT) and chemotherapy on the inner ear and temporal bone are complex. Both treatment modalities can lead to sensorineural hearing loss (SNHL), and RT can cause additional conductive hearing loss. This article will cover the mechanisms and patterns of hearing loss secondary to chemotherapy and RT. The issues and controversies relating to hearing rehabilitation in cancer patients will be explored.
Chemotherapeutic Ototoxicity
Ototoxicity is characterized by high-frequency SNHL and tinnitus. Some patients may also exhibit dizziness and vertigo. Auditory symptoms tend to be bilateral, dose-dependent, progressive, and irreversible. More than 300 different medications have been associated with hearing loss. Platinum-based chemotherapeutic drugs are widely used in the treatment of solid tumors, including head and neck and some skull base malignancies. 1 2 The three most commonly used platinum agents, namely cisplatin, carboplatin, and oxaliplatin, differ in their antineoplastic and toxicity profiles, but cisplatin stands alone as a major cause of treatment-related hearing loss. 1 The potential risk of hearing loss secondary to cisplatin was first described by Piel in 1974. 3
Prevalence of hearing loss with platinum-based chemotherapy has been reported anywhere between 4 and 90%. 4 The wide range of ototoxicity depends on the specific agent used, the dosage, the patient's age, the patient's preexisting hearing loss, the criteria used to define treatment-related hearing loss, and the rigorous nature of a surveillance program. Variability in the prevalence and in the degree of hearing loss in subjects receiving similar treatments raises the question of genetic predisposition for platinum-based ototoxicity. Several genes have been identified that may modulate the degree of hearing loss caused by platinum agents. 4
Many risk factors for platinum-related ototoxicity have been identified such as age less than 15 years old, renal insufficiency, combination therapy using more than one platinum-based drug, temporal bone irradiation, and concomitant use of other ototoxic agents. When cumulative doses exceed 400 mg/m 2 , up to 90% of young children may suffer moderate to severe hearing loss, requiring rehabilitation with hearing aids. 5 6 Children under 5 years of age are more sensitive to the effects of cisplatin than children over 15, even controlling for cumulative dose. 7
Cisplatin ototoxicity is related to the production of reactive oxygen species (ROS), which triggers apoptosis in cochlear hair cells. 8 Histologic examination of temporal bones treated with cisplatin shows loss of inner and outer hair cells, atrophy of the stria vascularis, and degeneration of the spiral ganglion and cochlear nerve. 9 Cisplatin accumulates in the cochlea and is retained for several months or perhaps years following treatment. 10 This retention helps to explain delayed hearing loss and represents a challenge to preventing ototoxicity.
Patterns of Hearing Loss after Chemotherapy
Cisplatin generally produces bilateral, irreversible, progressive high frequency (2 kHz and above) SNHL ( Fig. 1 ). Clinically, word discrimination scores are often preserved. Given the high incidence of hearing loss following treatment with cisplatin, patients who receive this chemotherapy are enrolled in an ototoxicity surveillance program. These patients undergo a baseline audiogram, otoacoustic emission testing, and otologic evaluation to uncover preexisting hearing loss and ear diseases. Audiograms are repeated monthly, prior to additional rounds of chemotherapy, until treatment concludes. Audiograms are then repeated at 3 months, 6 months, 12 months, and 24 months. Several grading systems have been developed to grade the severity of hearing loss associated with chemotherapy in children and adults. 11 12 13 14
Fig. 1.
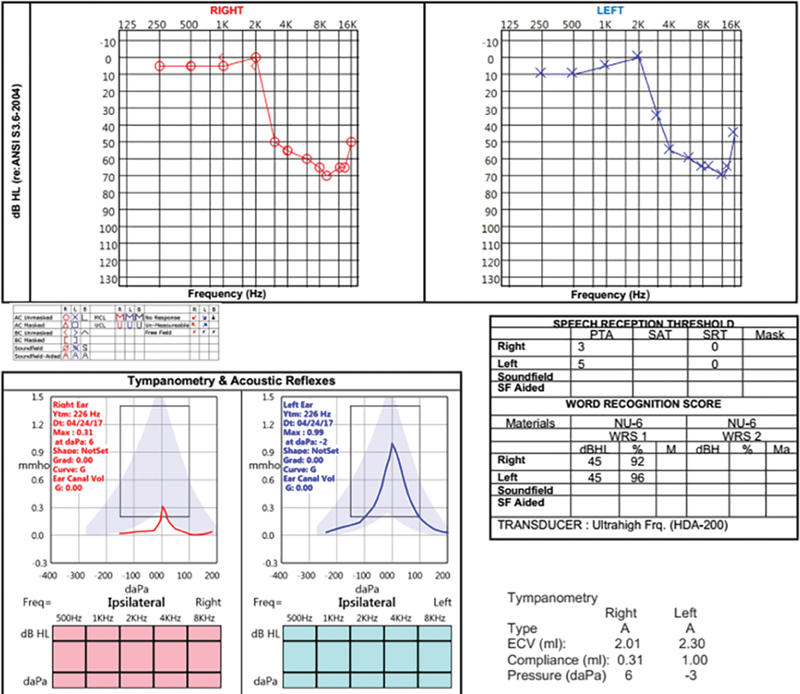
Audiogram of a 48-year-old man who developed hearing loss and tinnitus 1 month after starting induction chemotherapy with cisplatin and docetaxel. He had no preexisting hearing loss or significant occupational or recreational noise exposure. Note the precipitous drop in hearing above 2 kHz bilaterally and the preserved word recognition scores.
Management of Chemotherapy Ototoxicity
The first step in treating ototoxicity related to chemotherapy is recognizing the problem and discontinuance of the medication. The development of high-pitched tinnitus or a drop in high-frequency hearing signals the onset of ototoxicity. 15 16 Changes of >10 dB in one or more frequencies are worrisome. When these symptoms occur, the benefit of the medication should be weighed against its harmful effects. When less ototoxic options are available, then the decision should be made to switch medications to avoid further hearing loss. 17 Cancer is a life-threatening illness; hearing loss is not. The nature of the cancer may require an ototoxic medication despite its risk for hearing loss (e.g., childhood medulloblastoma, high-risk neuroblastoma). 18 Hearing loss has a significant impact on communication, socialization, and quality of life 19 20 ; however, there are many options available for hearing rehabilitation depending on the degree of hearing loss.
Hearing aids are the main form of rehabilitation for chemotherapy-associated ototoxic hearing loss. 4 Referral is made to the audiology service for hearing aid consultation. Patients are counselled about brands, styles, and costs of the devices. Affordability, accessibility, and stigma associated with the use of hearing aids represent important obstacles to seeking hearing rehabilitation. 21 Care is taken to avoid a device that will exert pressure in the ear canal, especially in patients who have received radiation to the head and neck. Generally, head and neck cancer patients are fitted with behind-the-ear devices using an open-fit dome. Hearing loss may tend to worsen with time, and a programmable device is necessary for this reason.
Preventing the ototoxic effects of chemotherapy has been an area of intense research. Multiple animal studies and a few human studies have examined both systemic and intratympanic medications, such as thiosulfate, N-acetylcysteine, dexamethasone, vitamin E, and even lactate. 22 23 24 25 The systemic medications are unattractive because they have the potential to diminish the antineoplastic effect of cisplatin. The intratympanic therapies have to be given prior to each round of chemotherapy, making this form of prevention clinically impracticable. An emerging field of research focuses on sustained drug delivery systems for the inner ear. 26 This approach might help to counteract the retention of cisplatin in the cochlea.
Radiation Associated Ototoxicity
RT plays an integral part in the treatment of head and neck cancers. 27 Given their central location, the ear and temporal bone are often in radiation fields for nasopharyngeal, oropharyngeal, parotid, and periauricular skin cancers. Acute and late toxicities due to radiation are seen in all parts of the ear. 28 Hearing loss may occur when the temporal bone is included in radiation fields. 29 It can present as conductive loss in cases of middle ear or eustachian tube (ET) injuries or as SNHL when the inner ear is involved. 30
Patterns of Hearing Loss after Radiation Therapy
Conductive Hearing Loss
Radiation can produce conductive hearing loss through stenosis of the ear canal, thickening of the tympanic membrane (TM), or middle ear and ET changes producing serous otitis media ( Fig. 2 ). 30 Radiation-induced otitis media with effusion (OME) is the most frequent cause of conductive hearing loss. 31 32 Apart from OME, other middle ear complications leading to hearing loss include acute or chronic suppurative otitis media, chronic perforation, middle ear fibrosis, and ossicular necrosis. 31 33
Fig. 2.
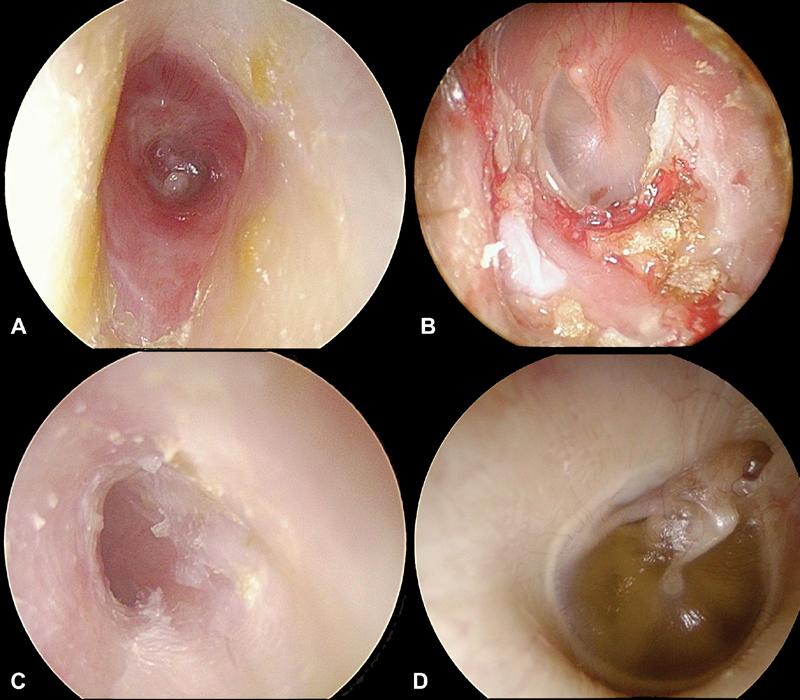
Effects of radiation on the ear canal, TM, and middle ear. ( A ) Ear canal stenosis due to radiotherapy. ( B ) Exposed bone in the ear canal, the chief sign of osteoradionecrosis. ( C ) Effacement of the TM due to radiotherapy. ( D ) Radiation otitis media. TM, tympanic membrane.
OME is a frequent finding among patients receiving RT for nasopharyngeal cancer (NPC). Studies have reported OME rates of up to 48% with conventional RT 31 32 34 35 36 and between 29 and 53% with intensity-modulated RT (IMRT) 37 38 39 in NPC patients. Almost one-third of these patients have persistent OME 5 years following completion of IMRT. 39
The underlying pathophysiology explaining the development of OME following head and neck RT seems to be multifactorial. Mucosal damage and altered ciliary function of the middle ear epithelium are likely to play a role. 40 41 Irradiation of the ET isthmus, the narrowest portion of the canal, may lead to fibrosis and mechanical obstruction. 30 Late fibrosis and dysfunction of the ET musculature may also prevent effective tube opening. 39
Radiation dose over the middle ear and ET isthmus seems to correlate with the risk of developing OME and, consequently, conductive hearing loss. Wang et al found an increased rate of middle ear morbidity in patients receiving more than 46 Gy to the middle ear and 52 Gy to the ET isthmus. 42 Similarly, Walker et al determined that nasopharyngeal or mastoid irradiation above 30 Gy was associated with an increased rate of otomastoid opacification on imaging. 43
Sensorineural Hearing Loss
Radiation induced SNHL is a dose-dependent, progressive, permanent, and late effect. Patients undergoing head and neck RT can develop SNHL, most often as a result of cochlear injury. The precise characteristics of that hearing loss are difficult to ascertain due to significant heterogeneity in prior studies. Differences in these studies pertain to the patient population, follow-up duration, hearing loss criteria, and RT protocols and technologies. The rate of SNHL following temporal bone irradiation has been reported between 14 and 67%. 44 45 46 The incidence can increase up to 90% in patients concomitantly treated with cisplatin. 47 Onset of SNHL is typically delayed and can occur 3 months to 13 years post-RT. 44 48 Progressive deterioration of hearing and increased incidence rates of hearing loss may be observed with longer follow-up. 44 48
Radiation-induced ototoxicity is dose-dependent. Most studies show an increased risk of hearing loss with cochlear doses above 45 Gy. 49 50 Some authors have determined that a dose between 20 and 30 Gy may be sufficient to cause SNHL. 51 The cochlea may be sensitive to radiation doses as low as 10 Gy in patients simultaneously receiving platinum-based chemotherapy. 52 Other risk factors for RT-induced SNHL include age <3 or >50 years and presence of OME. 31 44 53
The cochlea is more sensitive to radiotherapy than the brain or the auditory nerve. 54 55 Histopathologic examination of the irradiated cochlea reveals damage to the organ of Corti and atrophy of the basilar membrane, spiral ligament, and stria vascularis. 9 56 57 58 These changes are greater than those seen in age-matched controls. Outer hair cell loss in the basal turn corresponds to the high frequency loss seen in these patients, particularly the 4 kHz frequency. 58
The mechanism of cell death appears to be related to ROS and necrosis. Dose-dependent production of ROS has been demonstrated at 1 hour postirradiation in a cochlear inner ear cell line. 59 ROS might explain the high frequency loss seen in postirradiation patients, since outer hair cells in the basal turn have less antioxidant capacity than do cells in the apical turn. 60
Treatment of Radiation Associated Hearing Loss
Treatment of Postradiation OME and Conductive Hearing Loss
The treatment of postradiation OME remains challenging and controversial. Several management options have been studied: clinical observation, conventional hearing aids, tympanocentesis, myringotomy and aspiration, ventilation tube insertion, intratympanic steroid injections, and implantation of osseointegrated hearing aids (OIHAs). 35 61 62 63 64 65 66 67 68 The goals are to create an aerated middle ear and to eliminate conductive hearing loss without producing a chronic perforation or chronically draining ear.
Most of the controversy relates to ventilation tube surgery compared with more conservative alternatives. Studies have reported conflicting results regarding the resolution rate of OME in patients treated with or without ventilation tubes. On the one hand, Morton et al did not observe any statistically significant difference in the rate of resolution of OME when patients underwent ventilation tube surgery as opposed to those observed (54 vs. 38%, respectively). 66 Chen et al determined that ventilation tubes were no more effective than simple myringotomy. 64 Similarly, other authors did not find any differences in cure rates when comparing observation, myringotomy, and tube insertion. 38 61 On the other hand, through a prospective quasi-randomized trial including 96 patients, Xu et al compared tympanocentesis, myringotomy with cauterization, and myringotomy and tube, and they showed a significantly higher cure rate with ventilation tubes compared with tympanocentesis (51.1 vs. 37.8%, p = 0.011). Tube insertion showed comparable results to myringotomy though (51.1 vs. 46.7%). 62 They noted that ventilation tubes offered a much longer period of symptom relief as compared with the other two methods. Ventilation tube insertion prior to initiation of RT has not been found to confer an advantage. 63 66
The risks of otologic complications must also be considered when discussing the treatment options with patients suffering from radiation-induced OME. The risk of otorrhea and tympanic perforation remains high in this patient population. Otorrhea has been reported in 32 to 60% of patients following ventilation tube insertion compared with 6.7 to 20% after myringotomy. 38 61 62 64 66 Chronic otorrhea and infection might be associated with progressive SNHL in this patient population. Tympanic perforation was found in 8.5 to 18% following ventilation tube insertion and in 16% following myringotomy. 62 66 In light of the increased risk of otorrhea and ear drum perforation, some authors have argued against the use of ventilation tubes. 64 65 66 Others have advocated a stepwise approach to treat postradiation OME, with initial conservative management, followed by repeated tympanocentesis or myringotomies and, as a last resort, ventilation tube insertion if bothersome symptoms persist. 62 69
Interest in balloon dilation for ET dysfunction and chronic otitis media has been growing since 2010. 70 71 Although a recent systematic review reported a low rate of self-limiting complications in the general population, the safety of this procedure has yet to be confirmed in patients who have undergone temporal bone irradiation. 72 Concern has been raised that ET balloon dilation (ETBD) might convert a radiated ET from obstructed to patulous. Furthermore, an irradiated carotid artery might become dehiscent, and ETBD could be disastrous in this situation.
OIHAs may be considered when conventional aids cannot be used, such as with chronic otorrhea or osteoradionecrosis (ORN) of the ear canal. 67 The possibility of developing progressive sensorineural or mixed hearing loss in an irradiated ear might preclude OIHA candidacy. Nonetheless, more powerful OIHA processors are now available, allowing patients with moderate SNHL up to 65 dB to benefit from OIHAs.
Functional Otologic Surgery Following RT
Functional surgery of the TM and of the middle ear may sometimes be contemplated in patients receiving RT. Reasons to consider this option include tympanic perforation, otorrhea preventing the use of hearing aids, and underlying chronic ear disease. RT may cause damage to the osteocytes and to the blood supply of the temporal bone, which can impair its healing capabilities. 73 74 The benefits of this treatment modality must therefore be weighed against the increased risks of complications, such as ORN of the external auditory canal (EAC) and persistent TM perforation.
Definitive conclusions are difficult to obtain regarding the safety and success rate of middle ear surgery following RT due to the limited medical literature. Only three studies have reported results following tympanoplasty with or without mastoidectomy in irradiated temporal bones. 75 76 77 These studies have included a limited number of patients. Variable delays between completion of RT and otologic surgery may explain the discrepancies in their results. Radiation dosage to the temporal bone is not available in any of these reports. In addition, the available data do not allow proper evaluation of the impact of middle ear surgery on conductive hearing loss. Successful closure of TM perforation following simple tympanoplasty was found between 52.6 and 88.9%. 75 76 Resolution of otorrhea was found between 50 and 58% in patients who had undergone RT for NPC. 76 77 Bennett et al found that tympanoplasty with canal-intact mastoidectomy was the procedure that caused the highest rate of complications as compared with simple tympanoplasty or tympanoplasty with canal-wall down mastoidectomy (complication rates of 75, 11.1, and 37.5%, respectively). They speculated that removal of the bone surrounding the EAC during mastoidectomy could affect the blood supply of the bony ear canal and TM. The overall rate of EAC ORN was 9% in their series. No case of ORN was found following simple tympanoplasty as compared with 15.4% in patients undergoing any type of mastoidectomy. 75 Clear guidelines cannot be obtained from these findings. The rate of ORN especially after mastoidectomy remains high. Resolution of tympanic perforation is variable and possibly dependent on total radiation dose to the EAC and TM. Resolution of otorrhea may allow some patients to use conventional hearing aids.
Osseointegrated Hearing Aids in Cancer Patients
Indications of Osseointegrated Hearing Aids
OIHAs are prosthetic devices that bypass the conductive mechanism of the external and middle ear. They directly stimulate the cochlea by bone conduction. OIHAs are indicated in patients with conductive and mixed hearing loss who cannot benefit from either traditional hearing aids or functional middle ear surgery. In the oncologic setting, examples of pathologies causing conductive hearing loss amenable to OIHAs include chronic otitis media, chronic otitis externa, ORN, and absence of the auricle or of the EAC. 2 OIHAs can also be offered to patients with single-sided deafness who may not be able to use Contralateral Routing of Signal hearing aids. This situation may occur in cancer patients undergoing subtotal or total temporal bone resection with sacrifice of the auricle.
Effects of RT and Chemotherapy on Osseointegration
The primary concern related to OIHA utilization in patients undergoing cancer treatments is failure of osseointegration. RT effects on bone are complex. Injury to the vascular endothelium can lead to hypovascularity and altered wound healing. Bone remodeling may also be affected due to damaged osteoblasts and osteoclasts. 78 Data regarding failure rate of osseointegration specifically for OIHAs in irradiated patients are rather limited. For all craniofacial implants regardless of subsite, the risk of failed osseointegration may be increased up to 12 times following RT. 79 One study found a rate 6.6 times higher for temporoparietal implants (33 vs. 5% in nonirradiated patients). 80
The mastoid seems to be a more favorable location for implantation in the context of RT compared with other craniofacial sites, such as the orbital and nasal bones. 79 80 Combined, available studies have examined fewer than 60 cases of implants in irradiated mastoid bones. The overall survival rate has varied between 83 and 100%. 81 82 83 Nader et al performed the largest study on OIHAs in irradiated temporal bones and reported a survival rate of 89.5% in 19 subjects. Interestingly, no failures were noted when implants were inserted prior to RT while those placed secondarily had a 15% failure rate (0/6 vs. 2/13, respectively). 82 In comparison, a meta-analysis looking at a nononcological pediatric and adult population reported an extrusion rate of OIHAs between 0 and 18%. 84 Therefore, the survival rate of OIHAs in irradiated patients seems comparable to that of the general population.
It has been difficult to determine a radiation dose threshold above which survival of OIHAs is in jeopardy. Nader et al could not identify such a relationship between RT dosages and failure rate. 82 Studies looking at craniofacial implants at multiple sites found RT dose thresholds between 30 and 55 Gy. 80 85
Similar to RT, chemotherapy may also interfere with osseointegration. The effects of chemotherapy on osseointegration have not been as extensively examined as those of RT. Nonetheless, both animal and clinical studies have pointed at the negative impact of chemotherapy on implant integration and survival. In a rabbit model, titanium implants were found to have a lower percentage of bone–implant contact in the group receiving cisplatin. 86 Granström et al performed a retrospective clinical study that evaluated factors affecting survival of various craniofacial implants. 80 Chemotherapy, given either pre- or postimplantation, was associated with a significantly higher failure rate. However, it is unclear if confounding variables, such as concomitant RT, were accounted for in their statistical analysis.
OIHA Complications
In addition to failure of osseointegration, other soft tissue and bony complications need to be considered. Various grading systems have been proposed to report complications occurring after implantation of OIHAs. 82 87 88 In general, these complications can be divided as minor and major. Nader et al described minor complications as local inflammation, skin growth over the abutment not requiring surgical resection, or contact between processor and skin. Major complications included soft tissue growth requiring surgery, bone exposure, or implant extrusion. 82
Implantation of OIHAs in irradiated bones may lead to an increased risk of major complications, especially when the implants are placed after RT ( Fig. 3 ). In the largest study evaluating OIHAs in relation to RT, major complications were found in 26.3% ( n = 5/19) of irradiated bones versus 3.4% ( n = 1/29) of controls. Timing of implantation with regards to RT and to oncologic resection also correlated with outcome. Patients implanted prior to RT did not have any complications. Similarly, OIHAs inserted during the primary oncologic resection had significantly fewer minor and major complications compared with those implanted secondarily (0/8 vs. 8/11, p < 0.05). 82
Fig. 3.
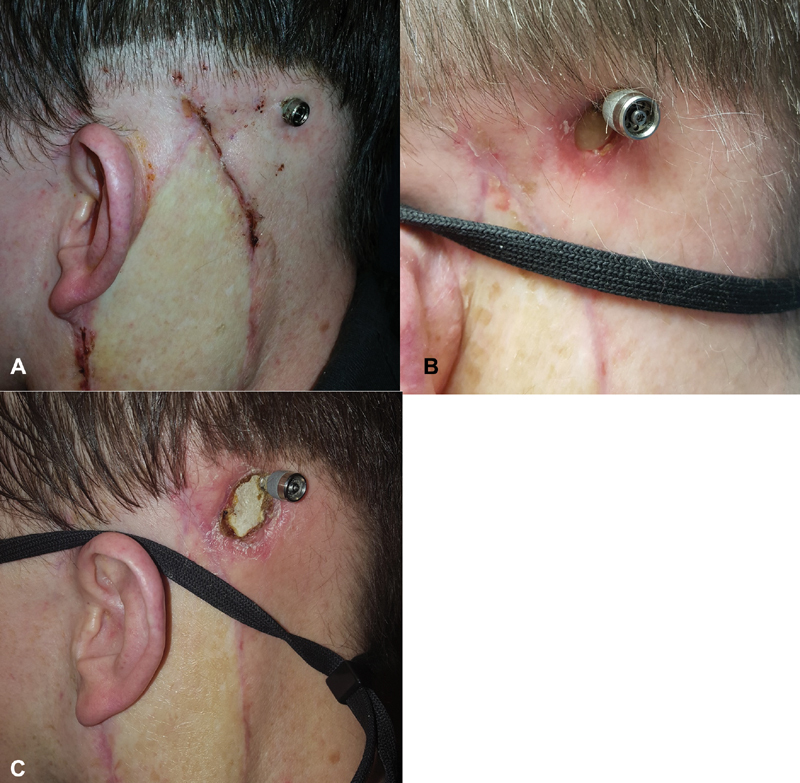
Exposed bone around osseointegrated implant. This patient underwent lateral temporal bone resection and stage 1 implantation of an osseointegrated implant. The second stage was performed along with free flap revision approximately 6 months after radiotherapy. ( A ) One month follow-up, showing slight pallor to the skin between the abutment and the free flap incision. Note the loss of hair due to the radiation. ( B ) Six-month follow-up showing exposed bone around the abutment and implant. ( C ) Fourteen-month follow-up showing progressive loss of skin.
Surgical Technique and Other Considerations
Several recommendations can be made based on our institutional experience and results from prior studies. One should exercise (1) careful manipulation of soft tissue, (2) use of a high torque drill at a low speed (2,000 RPM), (3) use of lower torque of 30 N/cm when inserting implants in irradiated bone and a torque of 20 N/cm in children or adults with softer bone, and (4) use of sharp drill bits and abundant irrigation to prevent heat and damage to the surgical bed.
In regards to surgical timing, implantation should ideally be performed prior to initiation of RT and preferably at the time of the primary cancer resection procedure. Presently, we prefer implanting OIHAs in two stages to minimize the risk of soft tissue infection and to allow proper osseointegration. In patients not undergoing any RT, we wait 3 months before placing the abutment as per the manufacturers' recommendations (Cochlear and Oticon Medical). In irradiated patients, we favor a 6-month waiting period although no consensus is available on the optimal loading time. Our group has favored the use of a percutaneous abutment and has avoided the use of magnetic systems. These systems have been associated with skin necrosis in patients without a prior history of radiation. 89
Nonsurgical options allow patients to use the OIHA processor without its implant and abutment. Soft bands, consisting of elastic materials, are available from all three OIHA manufacturers. A variant of the soft band, consisting of a plastic and steel spring band, is also offered by Cochlear Corporation. These bands are placed around the head and allow the processor to sit over the temporal area. They may be used following initial implantation while patients are waiting to undergo placement of the abutment. This alternative can also be considered when patients prefer not to undergo a second stage procedure or in cases of implant extrusion. However, one must be careful not to put excessive pressure on fresh wounds when using these soft bands, especially on microvascular free flap repairs. Another nonsurgical alternative offered by MED-EL consists of a bone conduction device held in place with an adhesive adapter, which avoids any cutaneous pressure but might harm irradiated skin.
Cochlear Implantation in Cancer Patients
Cancer patients may develop bilateral severe to profound hearing loss for which conventional hearing aids do not provide sufficient benefits. This hearing loss may be secondary to their oncologic treatments or to unrelated otologic pathologies. Cochlear implantation becomes the only option for hearing rehabilitation in these patients. However, several considerations must be taken into account for patients undergoing chemotherapy or RT who are potential candidates for cochlear implantation.
Cochlear Implants and Radiotherapy
The first concern relates to the performance of cochlear implants (CIs) in recipients who have had temporal bone or brainstem irradiation. This issue arises from the possible impact of RT on retrocochlear pathways. An early animal study showed that the cochlear nerve could be damaged by RT at a dose as low as 40 Gy. 54 However, results from subsequent animal and human studies suggest that the retrocochlear pathways remain functional following irradiation. Greene et al found no changes in auditory brainstem response (ABR) thresholds in guinea pigs exposed up to 70 Gy. 90 Similarly, Low et al did not detect any changes in ABR interwave latencies in 27 patients treated by RT for NPC. 91 The outcome following CI surgery in RT patients has been reported in only small retrospective series or case reports, including in total 25 patients. 92 93 94 95 96 97 98 99 All the case control series showed good performance in RT patients, which was similar to the control groups. 92 94 95 Pediatric patients treated for medulloblastoma were also found to benefit from CIs. 93 Some authors have suggested preoperative brain imaging to evaluate for the presence of central nervous system pathologies, such as demyelination, which could predict poorer outcome. 96 Overall, the available literature suggests that irradiated patients with profound hearing loss may still benefit from CIs.
Radiation-induced complications, such as chronic suppurative otitis media or ORN, may further complicate CI candidacy and surgery ( Fig. 4 , Fig. 5 ). Chronic middle ear disease causing conductive hearing loss may make auditory assessment more difficult. 94 Middle ear and mastoid disease may need to be treated prior to undergoing safe cochlear implantation. 95 In addition, the surgical technique may need to be adapted. Low et al described a case that required a modified canal-wall down mastoidectomy with blind sac closure during CI implantation. The patient had a tympanic perforation with chronic otorrhea in a context of severe ET dysfunction post NPC treatment. 94
Fig. 4.

Cochlear implantation in a patient 10 years after chemotherapy and radiotherapy for nasopharyngeal cancer. ( A ) Preoperative audiogram. ( B ) Cochlear implant results showing near-normal pure tone thresholds and 68% HINT sentences. HINT, hearing in noise test.
Fig. 5.
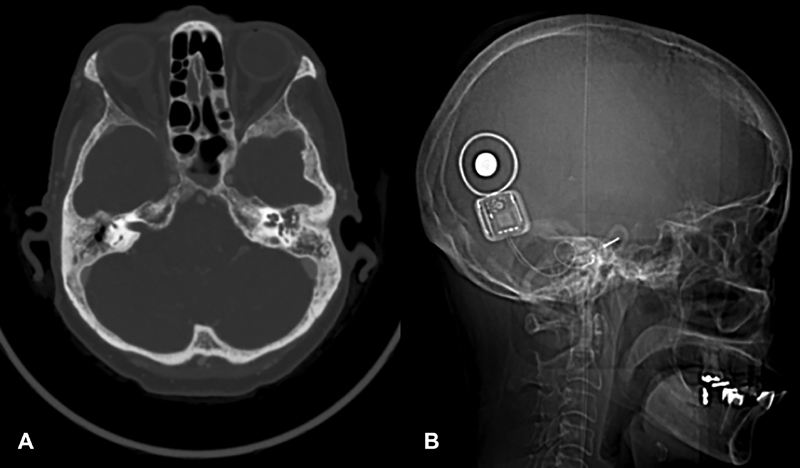
Cochlear implantation in that same patient 10 years after chemotherapy and radiotherapy. ( A ) Preoperative axial CT scan. The right middle ear appears aerated due to chronic perforation with otorrhea. The left mastoid and middle ear are opacified. The left ear was chosen to avoid the need for a two-stage procedure. ( B ) Complete insertion of the device is seen on the lateral radiograph.
Radiation effects on bone and on the structures of the middle and inner ear may increase the technical difficulty during CI surgery. Greater caution should be exercised if softer bone is encountered, especially near the facial nerve. 94 95 Dehiscence of the mastoid portion of the facial nerve and increased mucosal inflammation may further complicate the dissection. 95 96 99 Fibrosis over the round window and sclerosis of the cochlear lumen may make electrode insertion more challenging. 94 99 Shorter electrode arrays may sometimes be needed. 99 There may also be an increased risk of wound dehiscence and infection. Delicate handling of soft tissue should be exercised, and tension should be avoided during closure. Peri- and postoperative antibiotics should also be considered. 93 94
Cancer patients with CI in place may be required to undergo magnetic resonance imaging (MRI) as part of their oncologic surveillance protocol. Traditionally, options have included inserting a device with no magnet or temporary removal of the magnet prior to imaging. MED-EL, Advanced Bionics, and Cochlear Corporation offer devices that are approved for MRI up to 1.5 T with magnet in place. More recently, MED-EL has received U.S. Food and Drug Administration (FDA) approval for MRI up to 3 T for their newer generation model. Retrospective and in vitro studies have evaluated the risk of magnet displacement and device malfunction following MRI without magnet removal. Using a tight head wrap with a solid splint placed over the receiver–transmitter effectively minimized the risk of magnet movement, and no device failure was reported. 100 101 102 Furthermore, artifacts caused by magnets should also be considered when planning cochlear implantation. Adequate imaging of the contralateral brain hemisphere and of the ipsilateral cerebellopontine angle is still possible even with a magnet in place. 100
Lastly, CI recipients may sometimes require RT following implantation. Challenges may be encountered during radiosurgical planning with MRI due to artifacts as discussed above. Other concerns relate to damage to the device and scattering effects. Thankfully, the receiver–transmitter component is often quite remote from the primary cancer site and is not frequently subject to irradiation. In vitro testing showed no device dysfunction or failure in different CI models subjected to clinically relevant radiation doses. 103 104 Similarly, two case reports noted normal CI functioning in patients who had received a cumulative dose of 12 Gy at the level of their implants. 105 106
Cochlear Implants and Chemotherapy
Less is known about the impact of chemotherapy on CI outcome. Only two case reports have commented on this issue. Ryu et al reported good performance in three patients implanted following multimodal therapy for neuroblastoma that included platinum-based agents. 107 On the other hand, Harris et al reported a case of a patient who had been implanted prior to chemotherapy and who subsequently loss benefits from his CI. The authors presumed that damage to the spiral ganglion cells could explain that finding. 108
Conclusion
Hearing loss is a significant side effect from chemotherapy and radiotherapy used to treat head and neck cancer. Chemotherapy and RT can affect the temporal bone physiology and function of the inner ear. Conductive hearing loss following RT is most often secondary to radiation-induced serous otitis media, but other pathologies of the external and middle ear can also occur. Functional otologic surgery remains controversial in irradiated temporal bones due to altered wound healing and increased surgical risks. OIHA is presently the best option in patients with temporal bone cancers who have sufficient sensorineural reserve. In those patients, OIHAs are best implanted prior to RT, during the primary oncologic surgery. Patients with severe or profound SNHL who cannot use conventional aids may still be candidates for CIs even following RT. During both OIHA and CI surgery, gentle handling of soft tissue and great care during dissection must be exercised to minimize the risk of complications. More research is required to better understand the impact of chemotherapy on the outcome of OIHA and CI.
Footnotes
Conflict of Interest Dr. Nader reports other from Amgen, other from Cardinal Health, other from Johnson & Johnson, other from Medtronic, other from Pfizer, outside the submitted work. Dr. Gidley reports other from Eli Lily, other from Amgen, other from Merck, other from Medtronic, other from Pfizer, outside the submitted work.
References
- 1.Hilal-Dandan R, Brunton L L.Cytotoxic Agents New York, NY: McGraw-Hill Education; 2016. Available at:hemonc.mhmedical.com/content.aspx?aid=1127554849 [Google Scholar]
- 2.Gidley P W, DeMonte F. Temporal bone malignancies. Neurosurg Clin N Am. 2013;24(01):97–110. doi: 10.1016/j.nec.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Piel I J, Meyer D, Perlia C P, Wolfe V I. Effects of cis-diamminedichloroplatinum (NSC-119875) on hearing function in man. Cancer Chemother Rep. 1974;58(06):871–875. [PubMed] [Google Scholar]
- 4.Landier W. Ototoxicity and cancer therapy. Cancer. 2016;122(11):1647–1658. doi: 10.1002/cncr.29779. [DOI] [PubMed] [Google Scholar]
- 5.Bertolini P, Lassalle M, Mercier G et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 6.Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010;125(04):e938–e950. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Womer R B, Silber J H. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40(16):2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res Int. 2015;2015:617207. doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoistad D L, Ondrey F G, Mutlu C, Schachern P A, Paparella M M, Adams G L. Histopathology of human temporal bone after cis-platinum, radiation, or both. Otolaryngol Head Neck Surg. 1998;118(06):825–832. doi: 10.1016/S0194-5998(98)70276-1. [DOI] [PubMed] [Google Scholar]
- 10.Breglio A M, Rusheen A E, Shide E D et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8(01):1654. doi: 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock P R, Bellman S C, Yeomans E C, Pinkerton C R, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19(04):295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt C M, Bartholomäus E, Deuster D, Heinecke A, Dinnesen A G. The “Muenster classification” of high frequency hearing loss following cisplatin chemotherapy [in German] HNO. 2007;55(04):299–306. doi: 10.1007/s00106-005-1368-1. [DOI] [PubMed] [Google Scholar]
- 13.Chang K W, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28(10):1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 14.Theunissen E A, Dreschler W A, Latenstein M N et al. A new grading system for ototoxicity in adults. Ann Otol Rhinol Laryngol. 2014;123(10):711–718. doi: 10.1177/0003489414534010. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122(06):828–833. doi: 10.1016/S0194-59980070009-X. [DOI] [PubMed] [Google Scholar]
- 16.Reddel R R, Kefford R F, Grant J M, Coates A S, Fox R M, Tattersall M H. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep. 1982;66(01):19–23. [PubMed] [Google Scholar]
- 17.Crabb S J, Martin K, Abab J et al. COAST (cisplatin ototoxicity attenuated by aspirin trial): a phase II double-blind, randomised controlled trial to establish if aspirin reduces cisplatin induced hearing-loss. Eur J Cancer. 2017;87:75–83. doi: 10.1016/j.ejca.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landier W, Knight K, Wong F L et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group. J Clin Oncol. 2014;32(06):527–534. doi: 10.1200/JCO.2013.51.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalton D S, Cruickshanks K J, Klein B E, Klein R, Wiley T L, Nondahl D M. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(05):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 20.Gurney J G, Tersak J M, Ness K K, Landier W, Matthay K K, Schmidt M L; Children's Oncology Group.Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group Pediatrics 200712005e1229–e1236. [DOI] [PubMed] [Google Scholar]
- 21.Blazer D G, Domnitz S, Liverman C T, eds.Hearing Health Care for Adults: Priorities for Improving Access and Affordability Washington, DC: National Academies Press; 2016 [PubMed] [Google Scholar]
- 22.Ishikawa E, Sugimoto H, Hatano M et al. Protective effects of sodium thiosulfate for cisplatin-mediated ototoxicity in patients with head and neck cancer. Acta Otolaryngol. 2015;135(09):919–924. doi: 10.3109/00016489.2015.1035797. [DOI] [PubMed] [Google Scholar]
- 23.Villani V, Zucchella C, Cristalli G et al. Vitamin E neuroprotection against cisplatin ototoxicity: preliminary results from a randomized, placebo-controlled trial. Head Neck. 2016;38 01:E2118–E2121. doi: 10.1002/hed.24396. [DOI] [PubMed] [Google Scholar]
- 24.Marshak T, Steiner M, Kaminer M, Levy L, Shupak A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: a randomized controlled study. Otolaryngol Head Neck Surg. 2014;150(06):983–990. doi: 10.1177/0194599814524894. [DOI] [PubMed] [Google Scholar]
- 25.Nader M E, Théorêt Y, Saliba I. The role of intratympanic lactate injection in the prevention of cisplatin-induced ototoxicity. Laryngoscope. 2010;120(06):1208–1213. doi: 10.1002/lary.20892. [DOI] [PubMed] [Google Scholar]
- 26.Lavigne P, Lavigne F, Saliba I. Sustained inner ear steroid delivery via bioabsorbable stent: a tolerability and feasibility study on guinea pigs. Otolaryngol Head Neck Surg. 2016;155(04):649–653. doi: 10.1177/0194599816651262. [DOI] [PubMed] [Google Scholar]
- 27.Adelstein D, Gillison M L, Pfister D G et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(06):761–770. doi: 10.6004/jnccn.2017.0101. [DOI] [PubMed] [Google Scholar]
- 28.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47(01):1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 29.Ondrey F G, Greig J R, Herscher L.Radiation dose to otologic structures during head and neck cancer radiation therapy Laryngoscope 2000110(2, Pt 1):217–221. [DOI] [PubMed] [Google Scholar]
- 30.Lambert E M, Gunn G B, Gidley P W. Effects of radiation on the temporal bone in patients with head and neck cancer. Head Neck. 2016;38(09):1428–1435. doi: 10.1002/hed.24267. [DOI] [PubMed] [Google Scholar]
- 31.Bhandare N, Antonelli P J, Morris C G, Malayapa R S, Mendenhall W M. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67(02):469–479. doi: 10.1016/j.ijrobp.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Jereczek-Fossa B A, Zarowski A, Milani F, Orecchia R. Radiotherapy-induced ear toxicity. Cancer Treat Rev. 2003;29(05):417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 33.Gyorkey J, Pollock F J. Radiation necrosis of the ossicles. AMA Arch Otolaryngol. 1960;71:793–796. doi: 10.1001/archotol.1960.03770050053008. [DOI] [PubMed] [Google Scholar]
- 34.Tang N L, Choy A T, John D G, van Hasselt C A. The otological status of patients with nasopharyngeal carcinoma after megavoltage radiotherapy. J Laryngol Otol. 1992;106(12):1055–1058. doi: 10.1017/s0022215100121747. [DOI] [PubMed] [Google Scholar]
- 35.Low W K, Fong K W. Long-term post-irradiation middle ear effusion in nasopharyngeal carcinoma. Auris Nasus Larynx. 1998;25(03):319–321. doi: 10.1016/s0385-8146(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 36.Kew J, King A D, Leung S F et al. Middle ear effusions after radiotherapy: correlation with pre-radiotherapy nasopharyngeal tumor patterns. Am J Otol. 2000;21(06):782–785. [PubMed] [Google Scholar]
- 37.Hsin C H, Chen T H, Liang K L, Tseng H C, Liu W S. Postirradiation otitis media with effusion in nasopharyngeal carcinoma patients treated by intensity-modulated radiotherapy. Laryngoscope. 2013;123(09):2148–2153. doi: 10.1002/lary.23215. [DOI] [PubMed] [Google Scholar]
- 38.Liang K L, Su M C, Twu C W, Jiang R S, Lin J C, Shiao J Y. Long-term result of management of otitis media with effusion in patients with post-irradiated nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2011;268(02):213–217. doi: 10.1007/s00405-010-1381-1. [DOI] [PubMed] [Google Scholar]
- 39.Hsin C H, Tseng H C, Lin H P, Chen T H. Post-irradiation otitis media, rhinosinusitis, and their interrelationship in nasopharyngeal carcinoma patients treated by IMRT. Eur Arch Otorhinolaryngol. 2016;273(02):471–477. doi: 10.1007/s00405-015-3518-8. [DOI] [PubMed] [Google Scholar]
- 40.Elwany S. Delayed ultrastructural radiation induced changes in the human mesotympanic middle ear mucosa. J Laryngol Otol. 1985;99(04):343–353. doi: 10.1017/s002221510009681x. [DOI] [PubMed] [Google Scholar]
- 41.Panossian D, Jung T T, Weeks D et al. Effect of the radioprotector WR2721 on irradiation-induced injury to ciliated cells of eustachian tube. Ann Otol Rhinol Laryngol. 1992;101(05):395–402. doi: 10.1177/000348949210100504. [DOI] [PubMed] [Google Scholar]
- 42.Wang S Z, Wang W F, Zhang H Y, Guo M, Hoffman M R, Jiang J J. Analysis of anatomical factors controlling the morbidity of radiation-induced otitis media with effusion. Radiother Oncol. 2007;85(03):463–468. doi: 10.1016/j.radonc.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Walker G V, Ahmed S, Allen P et al. Radiation-induced middle ear and mastoid opacification in skull base tumors treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(05):e819–e823. doi: 10.1016/j.ijrobp.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 44.Bass J K, Hua C H, Huang J et al. Hearing loss in patients who received cranial radiation therapy for childhood cancer. J Clin Oncol. 2016;34(11):1248–1255. doi: 10.1200/JCO.2015.63.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J J, Guo Y K, Tang Q L et al. Prospective study of sensorineural hearing loss following radiotherapy for nasopharyngeal carcinoma. J Laryngol Otol. 2010;124(01):32–36. doi: 10.1017/S0022215109991435. [DOI] [PubMed] [Google Scholar]
- 46.Chi F H, Young Y H. Inner ear deficits in irradiated nasopharyngeal carcinoma survivors. Laryngoscope. 2015;125(11):2565–2571. doi: 10.1002/lary.25329. [DOI] [PubMed] [Google Scholar]
- 47.Scobioala S, Parfitt R, Matulat P et al. Impact of radiation technique, radiation fraction dose, and total cisplatin dose on hearing : Retrospective analysis of 29 medulloblastoma patients. Strahlenther Onkol. 2017;193(11):910–920. doi: 10.1007/s00066-017-1205-y. [DOI] [PubMed] [Google Scholar]
- 48.Wang L F, Kuo W R, Ho K Y, Lee K W, Lin C S. A long-term study on hearing status in patients with nasopharyngeal carcinoma after radiotherapy. Otol Neurotol. 2004;25(02):168–173. doi: 10.1097/00129492-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Theunissen E A, Bosma S C, Zuur C L et al. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck. 2015;37(02):281–292. doi: 10.1002/hed.23551. [DOI] [PubMed] [Google Scholar]
- 50.Mujica-Mota M, Waissbluth S, Daniel S J. Characteristics of radiation-induced sensorineural hearing loss in head and neck cancer: a systematic review. Head Neck. 2013;35(11):1662–1668. doi: 10.1002/hed.23201. [DOI] [PubMed] [Google Scholar]
- 51.Herrmann F, Dörr W, Müller R, Herrmann T. A prospective study on radiation-induced changes in hearing function. Int J Radiat Oncol Biol Phys. 2006;65(05):1338–1344. doi: 10.1016/j.ijrobp.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Hitchcock Y J, Tward J D, Szabo A, Bentz B G, Shrieve D C. Relative contributions of radiation and cisplatin-based chemotherapy to sensorineural hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(03):779–788. doi: 10.1016/j.ijrobp.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 53.Pan C C, Eisbruch A, Lee J S, Snorrason R M, Ten Haken R K, Kileny P R. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61(05):1393–1402. doi: 10.1016/j.ijrobp.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 54.Bohne B A, Marks J E, Glasgow G P.Delayed effects of ionizing radiation on the ear Laryngoscope 198595(7, Pt 1):818–828. [PubMed] [Google Scholar]
- 55.Low W K, Tan M G, Chua A W, Sun L, Wang D Y. 12th Yahya Cohen Memorial Lecture: the cellular and molecular basis of radiation-induced sensori-neural hearing loss. Ann Acad Med Singapore. 2009;38(01):91–94. [PubMed] [Google Scholar]
- 56.Leach W. Irradiation of the ear. J Laryngol Otol. 1965;79(10):870–880. doi: 10.1017/s0022215100064495. [DOI] [PubMed] [Google Scholar]
- 57.Schuknecht H F, Karmody C S. Radionecrosis of the temporal bone. Laryngoscope. 1966;76(08):1416–1428. doi: 10.1288/00005537-196608000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Asenov D R, Kaga K, Tsuzuku T. Changes in the audiograms of a nasopharyngeal cancer patient during the course of treatment: a temporal bone histopathological study. Acta Otolaryngol. 2007;127(10):1105–1110. doi: 10.1080/00016480601127026. [DOI] [PubMed] [Google Scholar]
- 59.Low W K, Tan M G, Sun L, Chua A W, Goh L K, Wang D Y. Dose-dependant radiation-induced apoptosis in a cochlear cell-line. Apoptosis. 2006;11(12):2127–2136. doi: 10.1007/s10495-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 60.Rybak L P, Whitworth C A. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10(19):1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 61.Shah J O, Herrera S J, Roberts D B, Gunn G B, Gidley P W. Complications of tympanostomy tubes in head and neck cancer patients. Am J Otolaryngol. 2016;37(04):356–361. doi: 10.1016/j.amjoto.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y D, Ou Y K, Zheng Y Q, Chen Y, Ji S F. The treatment for postirradiation otitis media with effusion: a study of three methods. Laryngoscope. 2008;118(11):2040–2043. doi: 10.1097/MLG.0b013e31818208d6. [DOI] [PubMed] [Google Scholar]
- 63.Ho W K, Wei W I, Kwong D L et al. Randomized evaluation of the audiologic outcome of ventilation tube insertion for middle ear effusion in patients with nasopharyngeal carcinoma. J Otolaryngol. 2002;31(05):287–293. doi: 10.2310/7070.2002.34311. [DOI] [PubMed] [Google Scholar]
- 64.Chen C Y, Young Y H, Hsu W C, Hsu M M. Failure of grommet insertion in post-irradiation otitis media with effusion. Ann Otol Rhinol Laryngol. 2001;110(08):746–748. doi: 10.1177/000348940111000809. [DOI] [PubMed] [Google Scholar]
- 65.Young Y H, Lu Y C. Mechanism of hearing loss in irradiated ears: a long-term longitudinal study. Ann Otol Rhinol Laryngol. 2001;110(10):904–906. doi: 10.1177/000348940111001002. [DOI] [PubMed] [Google Scholar]
- 66.Morton R P, Woollons A C, McIvor N P. Nasopharyngeal carcinoma and middle ear effusion: natural history and the effect of ventilation tubes. Clin Otolaryngol Allied Sci. 1994;19(06):529–531. doi: 10.1111/j.1365-2273.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 67.Soo G, Tong M C, Tsang W S et al. The BAHA hearing system for hearing-impaired postirradiated nasopharyngeal cancer patients: a new indication. Otol Neurotol. 2009;30(04):496–501. doi: 10.1097/MAO.0b013e31819d34ab. [DOI] [PubMed] [Google Scholar]
- 68.Kuo C L, Wang M C, Chu C H, Shiao A S. New therapeutic strategy for treating otitis media with effusion in postirradiated nasopharyngeal carcinoma patients. J Chin Med Assoc. 2012;75(07):329–334. doi: 10.1016/j.jcma.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 69.Schwarz Y, Manogaran M, Daniel S J. Ventilation tubes in middle ear effusion post-nasopharyngeal carcinoma radiation: To insert or not? Laryngoscope. 2016;126(12):2649–2651. doi: 10.1002/lary.26149. [DOI] [PubMed] [Google Scholar]
- 70.Silvola J, Kivekäs I, Poe D S. Balloon dilation of the cartilaginous portion of the eustachian tube. Otolaryngol Head Neck Surg. 2014;151(01):125–130. doi: 10.1177/0194599814529538. [DOI] [PubMed] [Google Scholar]
- 71.Kapadia M, Tarabichi M. Feasibility and safety of transtympanic balloon dilatation of eustachian tube. Otol Neurotol. 2018;39(09):e825–e830. doi: 10.1097/MAO.0000000000001950. [DOI] [PubMed] [Google Scholar]
- 72.Huisman J ML, Verdam F J, Stegeman I, de Ru J A. Treatment of eustachian tube dysfunction with balloon dilation: a systematic review. Laryngoscope. 2018;128(01):237–247. doi: 10.1002/lary.26800. [DOI] [PubMed] [Google Scholar]
- 73.O'Neill J V, Katz A H, Skolnik E M. Otologic complications of radiation therapy. Otolaryngol Head Neck Surg (1979) 1979;87(03):359–363. doi: 10.1177/019459987908700314. [DOI] [PubMed] [Google Scholar]
- 74.Stone H B, Coleman C N, Anscher M S, McBride W H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4(09):529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 75.Bennett M, Kaylie D, Warren F, Jackson C G. Chronic ear surgery in irradiated temporal bones. Laryngoscope. 2007;117(07):1240–1244. doi: 10.1097/MLG.0b013e318060305c. [DOI] [PubMed] [Google Scholar]
- 76.Hsu Y C, Su C Y. Tympanoplasty for chronic otitis media in post-irradiated nasopharyngeal carcinoma patients. Ann Otol Rhinol Laryngol. 2006;115(05):330–333. doi: 10.1177/000348940611500502. [DOI] [PubMed] [Google Scholar]
- 77.Yuen P W, Wei W I. Tympanomastoidectomy for chronic suppurative otitis media of irradiated ears of nasopharyngeal carcinoma patients. J Otolaryngol. 1994;23(04):302–304. [PubMed] [Google Scholar]
- 78.Jegoux F, Malard O, Goyenvalle E, Aguado E, Daculsi G. Radiation effects on bone healing and reconstruction: interpretation of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(02):173–184. doi: 10.1016/j.tripleo.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Ihde S, Kopp S, Gundlach K, Konstantinović V S. Effects of radiation therapy on craniofacial and dental implants: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(01):56–65. doi: 10.1016/j.tripleo.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Granström G. Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. J Oral Maxillofac Surg. 2005;63(05):579–585. doi: 10.1016/j.joms.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Abu-Serriah M M, McGowan D A, Moos K F, Bagg J. Extra-oral craniofacial endosseous implants and radiotherapy. Int J Oral Maxillofac Surg. 2003;32(06):585–592. doi: 10.1054/ijom.2003.0429. [DOI] [PubMed] [Google Scholar]
- 82.Nader M E, Beadle B M, Roberts D B, Gidley P W. Outcomes and complications of osseointegrated hearing aids in irradiated temporal bones. Laryngoscope. 2016;126(05):1187–1192. doi: 10.1002/lary.25592. [DOI] [PubMed] [Google Scholar]
- 83.Wilkie M D, Lightbody K A, Salamat A A, Chakravarthy K M, Luff D A, Temple R H. Stability and survival of bone-anchored hearing aid implant systems in post-irradiated patients. Eur Arch Otorhinolaryngol. 2015;272(06):1371–1376. doi: 10.1007/s00405-014-2932-7. [DOI] [PubMed] [Google Scholar]
- 84.Kiringoda R, Lustig L R. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. 2013;34(05):790–794. doi: 10.1097/MAO.0b013e318291c651. [DOI] [PubMed] [Google Scholar]
- 85.Beumer J, III, Roumanas E, Nishimura R. Advances in osseointegrated implants for dental and facial rehabilitation following major head and neck surgery. Semin Surg Oncol. 1995;11(03):200–207. doi: 10.1002/ssu.2980110305. [DOI] [PubMed] [Google Scholar]
- 86.Al-Mahalawy H, Marei H F, Abuohashish H, Alhawaj H, Alrefaee M, Al-Jandan B. Effects of cisplatin chemotherapy on the osseointegration of titanium implants. J Craniomaxillofac Surg. 2016;44(04):337–346. doi: 10.1016/j.jcms.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Wazen J J, Young D L, Farrugia M C et al. Successes and complications of the Baha system. Otol Neurotol. 2008;29(08):1115–1119. doi: 10.1097/MAO.0b013e318187e186. [DOI] [PubMed] [Google Scholar]
- 88.Holgers K M, Tjellström A, Bjursten L M, Erlandsson B E. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol. 1988;9(01):56–59. [PubMed] [Google Scholar]
- 89.Chen S Y, Mancuso D, Lalwani A K. Skin necrosis after implantation with the BAHA attract: a case report and review of the literature. Otol Neurotol. 2017;38(03):364–367. doi: 10.1097/MAO.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 90.Greene J S, Giddings N A, Jacobson J T.Effect of irradiation on guinea pig ABR thresholds Otolaryngol Head Neck Surg 1992107(6, Pt 1):763–768. [DOI] [PubMed] [Google Scholar]
- 91.Low W K, Burgess R, Fong K W, Wang D Y. Effect of radiotherapy on retro-cochlear auditory pathways. Laryngoscope. 2005;115(10):1823–1826. doi: 10.1097/01.mlg.0000175061.59315.58. [DOI] [PubMed] [Google Scholar]
- 92.Soh J M, D'Souza V D, Sarepaka G K, Ng W N, Ong C S, Low W K. cochlear implant outcomes: a comparison between irradiated and non-irradiated ears. Clin Exp Otorhinolaryngol. 2012;5 01:S93–S98. doi: 10.3342/ceo.2012.5.S1.S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roland J T, Jr, Cosetti M, Liebman T, Waltzman S, Allen J C. Cochlear implantation following treatment for medulloblastoma. Laryngoscope. 2010;120(01):139–143. doi: 10.1002/lary.20669. [DOI] [PubMed] [Google Scholar]
- 94.Low W K, Gopal K, Goh L K, Fong K W. Cochlear implantation in postirradiated ears: outcomes and challenges. Laryngoscope. 2006;116(07):1258–1262. doi: 10.1097/01.mlg.0000225935.80559.11. [DOI] [PubMed] [Google Scholar]
- 95.Yue V, Leung E K, Wong T K, Tong M C, Van Hasselt C A. Cochlear implantation for post-irradiation deafness. Cochlear Implants Int. 2004;5 01:165–168. doi: 10.1179/cim.2004.5.Supplement-1.165. [DOI] [PubMed] [Google Scholar]
- 96.Adunka O F, Buchman C A. Cochlear implantation in the irradiated temporal bone. J Laryngol Otol. 2007;121(01):83–86. doi: 10.1017/S0022215106002180. [DOI] [PubMed] [Google Scholar]
- 97.Chua D Y, Tan H K. Successful rehabilitation with cochlear implant in post-irradiation induced hearing loss in nasopharyngeal carcinoma patient. Ann Acad Med Singapore. 2007;36(01):74–77. [PubMed] [Google Scholar]
- 98.Biggs N D, Ramsden R T. Cochlear implantation in a previously irradiated temporal bone--a case report. Cochlear Implants Int. 2001;2(02):129–134. doi: 10.1179/cim.2001.2.2.129. [DOI] [PubMed] [Google Scholar]
- 99.Formanek M, Czerny C, Gstoettner W, Kornfehl J. Cochlear implantation as a successful rehabilitation for radiation-induced deafness. Eur Arch Otorhinolaryngol. 1998;255(04):175–178. doi: 10.1007/s004050050038. [DOI] [PubMed] [Google Scholar]
- 100.Carlson M L, Neff B A, Link M J et al. Magnetic resonance imaging with cochlear implant magnet in place: safety and imaging quality. Otol Neurotol. 2015;36(06):965–971. doi: 10.1097/MAO.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 101.Walton J, Donnelly N P, Tam Y C et al. MRI without magnet removal in neurofibromatosis type 2 patients with cochlear and auditory brainstem implants. Otol Neurotol. 2014;35(05):821–825. doi: 10.1097/MAO.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 102.Gubbels S P, McMenomey S O. Safety study of the cochlear nucleus 24 device with internal magnet in the 1.5 tesla magnetic resonance imaging scanner. Laryngoscope. 2006;116(06):865–871. doi: 10.1097/01.MLG.0000216807.03225.CE. [DOI] [PubMed] [Google Scholar]
- 103.Guevara N, Gérard A, Dupré J et al. Influence of ionizing radiation on two generations of cochlear implants. BioMed Res Int. 2015;2015:609607. doi: 10.1155/2015/609607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klenzner T, Knapp F, Röhner F et al. Influence of ionizing radiation on nucleus 24 cochlear implants. Otol Neurotol. 2005;26(04):661–667. doi: 10.1097/01.mao.0000178134.96977.f5. [DOI] [PubMed] [Google Scholar]
- 105.Markiewicz M, Giebel S, Wyleioł Iet al. Allogeneic bone marrow transplantation with total body irradiation-based conditioning in acute lymphoblastic leukemia patient with Cochlear implant Ann Transplant 2006110221–22., discussion 32–43 [PubMed] [Google Scholar]
- 106.Reddy K, Cook B, Shaw C et al. Intact performance of a cochlear implant following radiotherapy in a child with acute lymphoblastic leukemia. Pract Radiat Oncol. 2012;2(03):233–236. doi: 10.1016/j.prro.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 107.Ryu N G, Moon I J, Chang Y S et al. Cochlear implantation for profound hearing loss after multimodal treatment for neuroblastoma in children. Clin Exp Otorhinolaryngol. 2015;8(04):329–334. doi: 10.3342/ceo.2015.8.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harris M S, Gilbert J L, Lormore K A, Musunuru S A, Fritsch M H. Cisplatin ototoxicity affecting cochlear implant benefit. Otol Neurotol. 2011;32(06):969–972. doi: 10.1097/MAO.0b013e3182255893. [DOI] [PMC free article] [PubMed] [Google Scholar]


