Abstract
Frequent maternal use of acetaminophen in pregnancy has been linked to attention-deficit/hyperactivity disorder (ADHD) in children, but concerns regarding uncontrolled confounding remain. In this article, we illustrate use of the negative control exposure (NCE) approach to evaluate uncontrolled confounding bias in observational studies on pregnancy drug safety and explain the causal assumptions behind the method. We conducted an NCE analysis and evaluated the associations between maternal acetaminophen use during different exposure periods and ADHD among 8,856 children born in 1993–2005 to women enrolled in the Nurses’ Health Study II cohort. Information on regular maternal acetaminophen use was collected prospectively in biennial questionnaires. A total of 721 children (8.1%) in the cohort had been diagnosed with ADHD as reported by the mothers. Our NCE analysis suggested that only acetaminophen use at the time of pregnancy was associated with childhood ADHD (odds ratio = 1.34, 95% confidence interval: 1.05, 1.72), and the effect estimates for the 2 NCE periods (about 4 years before and 4 years after the pregnancy) were null. Our findings corroborate those of prior reports suggesting that prenatal acetaminophen exposure may influence neurodevelopment. The lack of an association between acetaminophen use in the pre- and postpregnancy exposure periods and ADHD provides assurance that uncontrolled time-invariant factors do not explain this association.
Keywords: acetaminophen, attention-deficit/hyperactivity disorder, negative control exposure analysis, neurological development, pregnancy, prenatal exposure delayed effects, uncontrolled confounding
Observational studies are useful for drug safety research, especially when it comes to evaluation of the safety of medication use during pregnancy (1, 2). Some adverse effects of medication exposure on the offspring might be hidden from clinical detection until years after the exposure occurs (3). Confounding, however, is a major threat to the validity of findings from observational data (4). Newer analytical methods such as marginal structural models and propensity score calibrations have been proposed for control of complex confounding (5, 6), but these methods do not address unmeasured or unknown confounding factors. Depending on data structure and availability, some novel study designs or approaches, such as sibling-controlled analysis (7), case-crossover and case-time-control designs (8), and negative control exposures (NCEs) or outcomes (9), have been utilized to evaluate the influence of uncontrolled confounding.
Recently, several reports based on large European birth cohorts suggested a positive association between frequent acetaminophen use during pregnancy and neurobehavioral deficits in offspring, particularly hyperactivity and conduct disorders (10–16). Acetaminophen (or paracetamol) is an over-the-counter medication commonly used to treat pain and fever in pregnancy. Most previous studies were able to control for maternal fever and infections in pregnancy that are also suspected to affect neurological development (17, 18), but possible influences of unmeasured factors, such as maternal genetics, illnesses, and familial and social factors, in the findings are difficult to address. In this article, we introduce NCE analysis as a useful approach with which to evaluate uncontrolled confounding bias in observational research on pregnancy drug safety. We then demonstrate NCE analysis using an example from Nurses’ Health Study II (NHS II)—a large, US-wide cohort study with longitudinally collected data—and investigate the association between regular maternal use of acetaminophen and attention-deficit/hyperactivity disorder (ADHD) in offspring.
METHODS
The Partners Human Research Committee (Partners HealthCare, Boston, Massachusetts) approved this research and determined that completion and return of the questionnaires constituted implied consent.
Causal assumptions of NCE analysis
The use of negative controls to detect suspected and unsuspected threats to causal inference from confounding in observational epidemiology studies have been introduced and discussed (9, 19–21). In NCE analysis, the effect estimates from variable(s) that do not causally affect the outcome but share a similar confounding structure with the exposure variable of interest are evaluated. As illustrated in Figure 1, the basic principle relies on an NCE variable (N) that does not cause the outcome D (depicted as no arrow from N to D) but shares the same unmeasured variables (U) that could potentially introduce confounding to the exposure (E)-outcome (D) relationship of interest. Such N variables are referred to as “U-comparable” to E. The variables C represent variables that can confound either the E-D relationship or the N-D relationship, but these factors are measured and can be controlled in analyses. In the figure, the exposure E is drawn as affecting the outcome D, but this is the unknown relationship under investigation and so in reality may not be present. Because N does not affect D directly, if we find an association between N and D after controlling for C and E, this would then be evidence that U variables may exist and therefore might also introduce confounding to the E-D association. In contrast, if analyses show no association between N and D, this suggests that the U variables are not introducing confounding to the N-D association and therefore are also not confounding the E-D association.
Figure 1.
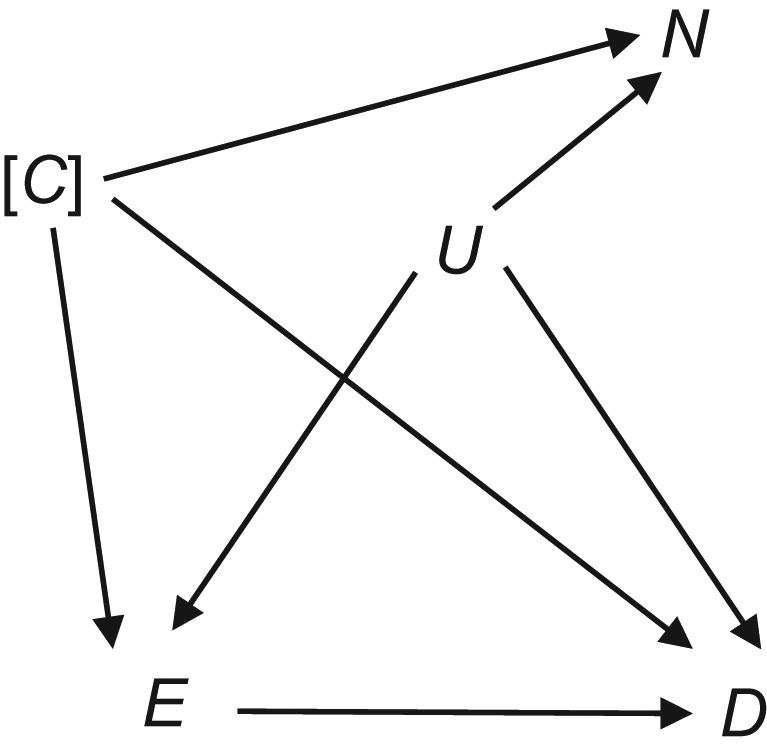
Directed acyclic graph illustrating the basic principles of negative control exposure analysis. E and D are the exposure and outcome of interest, respectively. The directed acyclic graph is drawn under the assumption of a true causal association between E and D, although there may not be one (this is the question being studied). C represents a set of measured and controlled confounding variables, and U represents a set of unknown or unmeasured confounding variables. The brackets around C suggest that the variable(s) is/are controlled. N is the “negative control” variable.
Some examples of studies that have utilized NCE analysis to evaluate associations between pregnancy exposure and child neurodevelopment include studies that have used paternal behaviors or exposures in pregnancy as NCEs for maternal behaviors or exposures in pregnancy (22, 23) and studies that have examined exposures before, during, or after pregnancy or exposures in different gestational periods if a critical or sensitive period of exposure effects is known or assumed (13, 15, 24–27).
Acetaminophen exposure and ADHD in NHS II
NHS II is a longitudinal cohort study of 116,430 female nurses who were recruited in 1989 at 25–42 years of age, were followed up biennially every odd calendar year, and are now living in all US states and territories. Questionnaires are mailed midyear, and most nurses have returned them before the end of that year. In the NHS II questionnaires, nurse mothers were asked to report whether they had used acetaminophen (e.g., Tylenol (Johnson & Johnson, New Brunswick, New Jersey)) regularly (defined as ≥2 times/week in the 1989 and 1993 questionnaires and ≥1 day/week from 1995 onwards) during the previous 2 years. Regular maternal acetaminophen use (yes/no) reported on the questionnaire during the year of the child’s birth was analyzed as the index exposure variable of interest. We refer to this regular acetaminophen use as use “at the time of pregnancy.” Although this question was not about use specifically during pregnancy, we considered regular use, which would be more likely than occasional use to continue through pregnancy, particularly since there was no recommendation against acetaminophen use during pregnancy in the study periods.
Importantly, because these data were collected prospectively, any error in capturing use specifically during pregnancy should have been nondifferential and therefore should have introduced random errors. However, as a sensitivity analysis, in order to reduce pregnancy-specific exposure misclassification, we also conducted analyses restricted to the subset of women who reported that they were pregnant when responding to the questionnaire. For the NCEs, we considered the same maternal regular use variables from the questionnaires completed 2 cycles (i.e., 4 years) before and after the one completed at the time of pregnancy (the 1991 questionnaire did not ask about acetaminophen use; therefore, 1989 use was used as the prepregnancy NCE for births that took place in 1995). These exposure periods make good NCE variables because we assume that they do not affect the offspring’s risks of ADHD in a pregnancy 4 years before or later and that time-invariant variables that could have introduced confounding in other studies—such as genetic factors, maternal chronic illnesses, family and social factors, and/or general medication use behaviors and choices—would affect these other exposure periods in the same way as they would during the pregnancy.
On the 2013 questionnaire, nurse mothers were asked, “Have any of your biological children been diagnosed with attention-deficit/hyperactivity disorder (ADHD)?” and the year of birth of any child diagnosed with ADHD. Maternal reports of ADHD have been found to be reliable (28). In a previous small validation study, Gao et al. (29) found that 92 children reported as having ADHD in NHS II also scored high on ADHD Rating Scale-IV (30), which is an 18-item questionnaire assessing the 2 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (31), factors comprising ADHD, including inattention and hyperactivity-impulsivity. All girls scored above 90%, and 81.1% of boys scored above 80%; 63.8% of boys scored above 90% (29).
In our main analyses, we included singleton children born during the years in which the questionnaires were mailed in order to increase the chance that the acetaminophen reporting included the pregnancy period. We restricted this to the years 1993 through 2005 (i.e., 1993, 1995, 1997, 1999, 2001, 2003, and 2005) to have NCE periods both 4 years before and 4 years after all pregnancies and so that all children would be at least 8 years old when ADHD in children was queried about in 2013. Multiple births were not included because in these cases, if the mother reported a child with ADHD, she was not asked to report whether 1 or more than 1 of the children had ADHD. These analyses included 8,856 children (see Web Figure 1, available at https://academic.oup.com/aje, for subject selection), and the sensitivity analyses carried out among those nurse mothers who indicated that they were pregnant when answering the questionnaire (the “pregnancy subset”) included 3,716 children.
Statistical analysis
We used generalized linear models to estimate odds ratios and 95% confidence intervals for ADHD. Generalized estimating equations were used to account for potentially correlated outcomes among children born to the same nurses. We adjusted analyses for time-varying variables that could possibly introduce confounding, including maternal age at the child’s birth (<30, 30–34, 35–40, or >40 years), child’s birth order (first, second, third, or fourth or later), child’s birth year (continuous), maternal gestational diabetes (yes/no), preeclampsia (yes/no), and self-reported regular maternal use of aspirin or aspirin-containing medication (Anacin (Pfizer Inc., New York, New York), Bufferin (Novartis Consumer Health, Inc., Parsippany, New Jersey), Alka-Selzer (Bayer Healthcare LLC, Morristown, New Jersey), etc.) or other nonsteroidal antiinflammatory drugs (ibuprofen, Advil (Wyeth Consumer HealthCare, Richmond, Virginia, and other companies), Midol (Bayer Healthcare), Aleve (Bayer Healthcare and other companies), Naprosyn (Genentech, Inc., South San Francisco, California; and Hoffman-La Roche, Basel, Switzerland), Relafen (Glaxo SmithKline Pharmaceuticals, Memphis, Tennessee), ketoprofen, Anaprox (Genentech, Hoffman-La Roche, and other companies), etc.). Information regarding use of aspirin and other nonsteroidal antiinflammatory drugs was collected in a manner similar to that described above for acetaminophen.
The estimates for maternal acetaminophen use before and after pregnancy (the negative control periods) might be confounded by exposure during pregnancy, which is hypothesized as the critical window of exposure, and acetaminophen use during that time is correlated with use during the time periods before and after pregnancy. Therefore, we estimated the associations with exposure in each period alone and additionally fitted a model that included acetaminophen use in all exposure periods together.
We conducted sex-stratified analyses to evaluate potential effect modification by child’s sex (14). In sensitivity analyses, we also performed analyses restricted to nurses who had never been diagnosed with depression, rheumatoid arthritis, or migraine headache, as these conditions might increase use of acetaminophen and, separately, be related to ADHD in the child. We also performed analyses that adjusted for maternal social factors, including nurses’ self-reported household income, subjective social standing in the community (32), and education of the husband/partner, as well as lifestyle factors such as maternal smoking (yes/no) and alcohol drinking (yes/no) during each pregnancy, which was reported on a separate questionnaire (33), with information provided by approximately 75% of the nurses. Lastly, on the 2005 questionnaire, nurses were also asked whether they had any children diagnosed with ADHD, but the specific child(ren) were not identified. To improve precision in ADHD diagnosis, we conducted additional analyses using births taking place during 1993–1999 but excluded ADHD cases that were reported on either the 2005 questionnaire or the 2013 questionnaire but not on both. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
The demographic characteristics of the study sample are presented in Table 1 by acetaminophen use. Approximately 14% of nurse mothers reported using acetaminophen regularly at the time of the pregnancy. Approximately 8% children in the cohort had ADHD. The demographic characteristics of children with and without ADHD are presented in Table 2.
Table 1.
Selected Demographic Characteristics (%) of Mothers According to Acetaminophen Use During Pregnancy in an Analysis of Attention-Deficit/Hyperactivity Disorder in Offspring, Nurses’ Health Study II, 1993–2005
| Characteristic | Acetaminophen Use at Time of Pregnancy (Yes/No) | |||
|---|---|---|---|---|
| Main Analysis (n = 8,856) | Pregnancy Subset (n = 3,716) | |||
| Yes (n = 1,230) | No (n = 7,626) | Yes (n = 513) | No (n = 3,203) | |
| Maternal age at child’s birth, years | ||||
| <30 | 1.7 | 2.8 | 2.3 | 2.9 |
| 30–34 | 28.3 | 39.0 | 29.0 | 40.2 |
| 35–40 | 50.2 | 43.2 | 47.8 | 43.9 |
| >40 | 19.8 | 15.0 | 20.9 | 13.0 |
| Child’s birth order | ||||
| 1 | 23.4 | 24.8 | 25.7 | 25.7 |
| 2 | 38.0 | 38.3 | 33.3 | 37.4 |
| 3 | 24.7 | 24.8 | 26.1 | 24.5 |
| ≥4 | 13.9 | 12.2 | 14.8 | 12.4 |
| Pregnancy complications | ||||
| Gestational diabetes | 5.9 | 5.1 | 7.2 | 5.6 |
| Preeclampsia | 4.2 | 2.8 | 3.9 | 2.6 |
| Maternal use of acetaminophen | ||||
| Before pregnancy | 41.8 | 12.2 | 45.2 | 11.3 |
| After pregnancy | 51.0 | 18.1 | 52.4 | 18.1 |
| Maternal medication use at time of pregnancy | ||||
| Aspirin | 6.7 | 2.1 | 5.3 | 2.6 |
| Other NSAIDs | 37.9 | 5.2 | 31.6 | 3.0 |
| Maternal diagnosis of chronic illness | ||||
| Rheumatoid arthritis | 3.2 | 1.6 | 3.5 | 1.4 |
| Depression | 25.2 | 16.0 | 26.1 | 15.6 |
| Migraine | 29.8 | 13.6 | 31.2 | 12.9 |
| Maternal lifestyle during pregnancya | ||||
| Cigarette smoking | 7.1 | 4.4 | 7.9 | 4.2 |
| Alcohol drinking | 8.0 | 6.5 | 8.7 | 6.5 |
| Annual household incomea | ||||
| <$50,000 | 15.4 | 12.6 | 15.0 | 12.7 |
| $50,000–$100,000 | 25.3 | 24.1 | 28.9 | 24.0 |
| >$100,000 | 59.3 | 63.4 | 56.1 | 63.3 |
Abbreviation: NSAIDs, nonsteroidal antiinflammatory drugs.
a Information on these factors was available for approximately 75% of the participants.
Table 2.
Selected Demographic Characteristics of Offspring With and Without Attention-Deficit/Hyperactivity Disorder (n = 8,856), Nurses’ Health Study II, 1993–2005
| Characteristic | ADHD Diagnosis | |||
|---|---|---|---|---|
| Yes (n = 721) | No (n = 8,135) | |||
| No. of Children | % | No. of Children | % | |
| Maternal age at child’s birth, years | ||||
| <30 | 21 | 2.9 | 214 | 2.6 |
| 30–34 | 268 | 37.2 | 3,052 | 37.5 |
| 35–40 | 324 | 44.9 | 3,587 | 44.1 |
| >40 | 108 | 15.0 | 1,282 | 15.8 |
| Child’s birth order | ||||
| 1 | 209 | 29.0 | 1,967 | 24.2 |
| 2 | 289 | 40.1 | 3,096 | 38.1 |
| 3 | 170 | 23.6 | 2,026 | 24.9 |
| ≥4 | 53 | 7.4 | 1,046 | 12.9 |
| Pregnancy complications | ||||
| Gestational diabetes | 42 | 5.8 | 421 | 5.2 |
| Preeclampsia | 27 | 3.7 | 236 | 2.9 |
| Maternal medication use at time of pregnancy | ||||
| Aspirin | 24 | 3.3 | 216 | 2.7 |
| Other NSAIDs | 83 | 11.5 | 780 | 9.6 |
| Maternal diagnosis of chronic illness | ||||
| Rheumatoid arthritis | 16 | 2.2 | 143 | 1.8 |
| Depression | 201 | 27.9 | 1,326 | 16.3 |
| Migraine | 145 | 20.1 | 1,258 | 15.5 |
| Maternal lifestyle during pregnancya | ||||
| Cigarette smoking | 23 | 4.3 | 290 | 4.8 |
| Alcohol drinking | 41 | 7.7 | 401 | 6.6 |
| Annual household incomea | ||||
| <$50,000 | 63 | 11.1 | 803 | 13.2 |
| $50,000–$100,000 | 133 | 23.3 | 1,482 | 24.4 |
| >$100,000 | 374 | 65.6 | 3,797 | 62.4 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; NSAIDs, nonsteroidal antiinflammatory drugs.
a Information on these factors was available for approximately 75% of the participants.
Regular maternal acetaminophen use during the 3 defined exposure periods was moderately correlated (Spearman’s r = 0.28–0.32). In models with acetaminophen use in all exposure periods included together, we found that only maternal acetaminophen use at the time of pregnancy was associated with elevated odds of ADHD in offspring (odds ratio = 1.34, 95% confidence interval: 1.05, 1.72) (Table 3). The associations were null for maternal acetaminophen use in the pre- or postpregnancy periods (Table 3). In the pregnancy subset, the association with use in the pregnancy period was somewhat larger (odds ratio = 1.46, 95% confidence interval: 1.01, 2.09), while use in the other periods remained null (Table 3). The effect estimates were less precise in sex-stratified analyses, and we did not find strong evidence to suggest effect measure modification by child’s sex (Web Table 1).
Table 3.
Odds Ratios for Attention-Deficit/Hyperactivity Disorder in Offspring According to Regular Maternal Use of Acetaminophen During Different Exposure Periods, Nurses’ Health Study II, 1993–2005
| Regular Acetaminophen Use | Main Analysis (n = 8,856) | Pregnancy Subset (n = 3,716) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Children | No. of Cases | Adjusted OR 1a | 95% CI | Adjusted OR 2b | 95% CI | No. of Children | No. of Cases | Adjusted OR 1a | 95% CI | Adjusted OR 2b | 95% CI | |
| At time of pregnancy | ||||||||||||
| Unexposed | 7,626 | 596 | 1.00 | Referent | 1.00 | Referent | 3,203 | 266 | 1.00 | Referent | 1.00 | Referent |
| Exposed | 1,230 | 125 | 1.35 | 1.07, 1.71 | 1.34 | 1.05, 1.72 | 513 | 57 | 1.39 | 0.99, 1.95 | 1.46 | 1.01, 2.09 |
| Before pregnancyc | ||||||||||||
| Unexposed | 7,410 | 588 | 1.00 | Referent | 1.00 | Referent | 3,123 | 266 | 1.00 | Referent | 1.00 | Referent |
| Exposed | 1,446 | 133 | 1.12 | 0.91, 1.38 | 1.06 | 0.85, 1.32 | 593 | 57 | 1.12 | 0.82, 1.53 | 1.08 | 0.78, 1.50 |
| After pregnancyd | ||||||||||||
| Unexposed | 6,849 | 548 | 1.00 | Referent | 1.00 | Referent | 2,867 | 253 | 1.00 | Referent | 1.00 | Referent |
| Exposed | 2,007 | 173 | 1.05 | 0.88, 1.26 | 0.97 | 0.80, 1.18 | 849 | 70 | 0.91 | 0.69, 1.20 | 0.82 | 0.60, 1.11 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for maternal age, child’s birth year, child’s birth order, gestational diabetes, preeclampsia, and regular maternal use of aspirin or other nonsteroidal antiinflammatory drugs at the time of the pregnancy.
b Adjusted for all of the above variables; additionally, acetaminophen use during all 3 periods was included in the same model.
c Maternal acetaminophen use reported on the questionnaire completed 2 cycles before the one from which the pregnancy exposure was derived.
d Maternal acetaminophen use reported on the questionnaire completed 2 cycles after the one from which the pregnancy exposure was derived.
Our main results were not materially different in any of the sensitivity analyses. The positive association between acetaminophen use at the time of pregnancy but not in the other exposure periods and ADHD in offspring remained largely unchanged in analyses restricted to mothers with no depression, rheumatoid arthritis, or migraine headache (Web Table 2) and in analyses excluding mothers who used aspirin and other nonsteroidal antiinflammatory drugs at the time of the pregnancy (Web Table 3). The estimates became less precise but the magnitude of associations also did not change in analysis of a subset of data in which we further adjusted for maternal social demographic and lifestyle factors (Web Table 4). The effect estimates were slightly strengthened when we included the ADHD cases reported on both the 2005 and 2009 questionnaires (Web Table 5).
DISCUSSION
We found an association between maternal use of acetaminophen at the time of pregnancy and risk of ADHD diagnosis in offspring using acetaminophen use a few years before pregnancy and a few years after pregnancy as NCEs to test for residual confounding by time-invariant factors. The correlation between acetaminophen use in the different time periods was modest, which suggests only modest U→E and U→N correlations (Figure 1) that are a first indication that confounding by time-invariant factors is probably weak (since a weak U→E correlation would contribute to weaker E←U→D confounding). However, the absences of associations with acetaminophen use in the prepregnancy and postpregnancy periods are the true NCE tests (9, 21), and they suggest that variables that do not vary over a few years—such as genetics, maternal chronic diseases, or socioeconomic status—do not explain the association observed for acetaminophen exposure at the time of pregnancy. This is because such time-invariant variables would result in U-comparability between acetaminophen use in pregnancy and use before and after pregnancy. That is, U→E and U→N are the same, so the confounding paths N←U→D and E←U→D would be the same. Thus, not finding an N→D association implies that N←U→D is not present, and therefore neither is E←U→D. Our findings in this large US cohort are also consistent with the few prior European cohort studies suggesting that in utero exposure to acetaminophen might affect neurodevelopment (10–16).
In contrast to time-invariant variables, our NCE analyses do not inform possible time-varying confounding that might differ for the different exposure periods used. This does not have to be the case in NCE analyses, but it is in the current study because we assessed exposures during different time periods as NCEs. We therefore adjusted for several time-varying variables that might have introduced confounding, such as lifestyle factors and use of other pain and fever medications during the pregnancy period. However, we cannot rule out the possibility of other uncontrolled risk factors for ADHD that are uniquely correlated with the use of acetaminophen during the pregnancy period. One possible candidate could be conditions like fever, infections, or mild pain. However, such conditions should have led to sporadic use of acetaminophen, if any; our exposure variable was regular use of acetaminophen. Thus, confounding from this source would seem less likely.
Another important consideration in NCE analysis is exposure measurement error. For a null finding for the NCEs to imply an absence of unmeasured confounding, the NCEs must be measured at least as accurately as the exposure of interest. Otherwise, larger errors in the measures of the NCEs could possibly account for an absence of association with the NCEs but not the exposure of interest. In our study setting, the question on regular acetaminophen use was phrased slightly differently in the 1989 and 1993 questionnaires, which might have contributed to some exposure misclassification, especially for the prepregnancy exposure variable, but the postnatal exposure variables were not affected because the first postpregnancy exposure year was 1997. Moreover, it is expected that mothers might have underreported their over-the-counter medication use in general (34, 35). Any measurement error due to flawed recall of medication use was expected to be similar in the different study periods, since the exposure data were collected prospectively before the outcome. Thus, the finding of an association specific to exposure around the time of pregnancy suggests that maternal reporting bias on medication use is unlikely to explain the finding.
Strengths of our NCE analysis included the fact that the information on acetaminophen use was prospectively collected in a large cohort study of nurse mothers and their children across the United States, and the medical information reported by the nurses in NHS II is expected to have greater accuracy compared with general population cohorts because the participants are all medically trained. NHS II has a retention rate greater than 90% for more than 20 years of follow-up, limiting any possible influence of selection bias. Moreover, the wealth of prospectively collected data in NHS II allowed us to adjust for many potentially confounding factors in the NCE analyses. The findings were also robust in several subgroup and sensitivity analyses we conducted.
There were several important limitations of our study. First, as mentioned above, although several measured confounders were accounted for in the analyses, we cannot rule out the possibility of residual confounding by another unaccounted-for time-varying factor that is specific to the pregnancy period. Second, we did not have detailed information regarding the exact timing of acetaminophen use or on the frequency and dosage. However, previous studies have found that the prevalence of acetaminophen use is stable across pregnancy trimesters (2, 36). Thus, we expect that the nurse mothers who indicated regular acetaminophen use in the questionnaires would likely have taken acetaminophen during pregnancy, particularly since during our study period acetaminophen was largely considered safe and its use during pregnancy was not contraindicated. Thus, potential misclassification of exposure is likely to have been nondifferential, which typically leads to underestimation of the true effect size (37, 38). The NHS II questionnaires only asked about regular acetaminophen use, and there was no additional information with which to explore dose-response relationships.
We did not have data with which to evaluate possible confounding by prescription medication use in pregnancy. However, use of prescribed pain medications such as triptans and opioids was rare (<0.6% in the first trimester) among pregnant women during the study period and thus less likely to have confounded the results observed for acetaminophen, for which the exposure prevalence was much higher (1, 2). Lastly, ADHD diagnoses were self-reported by the nurse mothers without clinical verification. However, maternal reports of ADHD have been found to be reliable (28), all of the mothers were nurses, and the overall ADHD prevalence reported in the study cohort was comparable to Centers for Disease Control and Prevention estimates (37).
The underlying biological mechanisms that may explain the increased risk of ADHD with acetaminophen exposure are not known, but possibilities have been suggested, such as frequent acetaminophen use in pregnancy affecting maternal-fetal hormonal functions (36, 38, 39) or inducing oxidative stress leading to neuronal death (40, 41). Acetaminophen exposure has been repeatedly linked to male reproductive outcomes such as cryptorchidism (24, 42) and reduced anogenital distance (43), which may be attributable to its endocrine-disrupting effects via inhibition of androgen or prostaglandin synthesis (36, 38, 39). Exposure to endocrine disruptors during sensitive periods of development has been associated with neurobehavioral consequences (44). Future mechanistic studies are needed to strengthen causal inference for possible effects of acetaminophen exposure on neurodevelopmental outcomes.
In conclusion, uncontrolled confounding bias remains a major challenge in drug safety research using observational data. NCE analyses can be a useful tool for evaluating whether uncontrolled confounding bias contributes to any observed association. The success of NCE analyses largely depends on whether one can find negative exposure variable(s) that are expected to share a similar confounding structure with the index exposure variable of interest, but the negative exposure variable(s) must not have a causal effect on the outcome (21). In this example, the longitudinal data collection waves of NHS II, in which nurse mothers were repeatedly queried about regular acetaminophen use, provided a unique opportunity allowing the comparison of associations with exposures in different time periods. The findings of our NCE analyses corroborate those of prior reports suggesting that prenatal acetaminophen exposure may influence neurodevelopment (10–17). While several prior studies based on pregnancy cohort data were better suited to ruling out potential confounding by time-varying factors specific to the pregnancy, using the NHS II data here we obtained further evidence that this association is also unlikely to be explained by other time-invariant factors. Future investigations are still needed, especially studies with improved exposure and outcome assessment and studies with the ability to address known and possibly unknown confounding factors in the analyses.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health Sciences, School of Public Health, Yale University, New Haven, Connecticut (Zeyan Liew); Yale Center for Perinatal, Pediatric, and Environmental Epidemiology, School of Public Health, Yale University, New Haven, Connecticut (Zeyan Liew); Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York (Marianthi-Anna Kioumourtzoglou); Department of Environmental Health, T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Andrea L. Roberts, Marc G. Weisskopf); School of Public Health, College of Medicine, University College Cork, Cork, Ireland (Éilis J. O’Reilly); Department of Nutrition, T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Éilis J. O’Reilly, Alberto Ascherio); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts (Alberto Ascherio); and Department of Epidemiology, T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Alberto Ascherio, Marc G. Weisskopf).
Nurses’ Health Study II is supported by infrastructure grant UM1 CA176726 from the National Cancer Institute. This study was partially supported by the National Institute of Environmental Health Sciences (NIEHS) (grant P30 ES000002). Z.L. was partly supported by a National Institutes of Health/NIEHS career development award (K99ES026729 and R00ES026729), and M.-A.K. was partially supported by the National Institutes of Health (training grant T32 ES 007069 and NIEHS center grant P30 ES09089).
The funders played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest: none declared.
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- NCE
negative control exposure
- NHS II
Nurses’ Health Study II
REFERENCES
- 1. Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e1–51.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werler MM, Mitchell AA, Hernandez-Diaz S, et al. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3):771–777. [DOI] [PubMed] [Google Scholar]
- 3. Savitz DA, Hertz-Picciotto I, Poole C, et al. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol Rev. 2002;24(2):91–101. [DOI] [PubMed] [Google Scholar]
- 4. Wood ME, Lapane KL, van Gelder MMHJ, et al. Making fair comparisons in pregnancy medication safety studies: an overview of advanced methods for confounding control. Pharmacoepidemiol Drug Saf. 2018;27(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. [DOI] [PubMed] [Google Scholar]
- 6. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 7. Frisell T, Öberg S, Kuja-Halkola R, et al. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. [DOI] [PubMed] [Google Scholar]
- 8. Schneeweiss S, Sturmer T, Maclure M. Case-crossover and case-time-control designs as alternatives in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf. 1997;6(suppl 3):S51–S59. [DOI] [PubMed] [Google Scholar]
- 9. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liew Z, Ritz B, Rebordosa C, et al. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168(4):313–320. [DOI] [PubMed] [Google Scholar]
- 11. Brandlistuen RE, Ystrom E, Nulman I, et al. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liew Z, Ritz B, Virk J, et al. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish National Birth Cohort study. Autism Res. 2016;9(9):951–958. [DOI] [PubMed] [Google Scholar]
- 13. Stergiakouli E, Thapar A, Davey Smith G. Association of acetaminophen use during pregnancy with behavioral problems in childhood evidence against confounding. JAMA Pediatr. 2016;170(10):964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avella-Garcia CB, Julvez J, Fortuny J, et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol. 2016;45(6):1987–1996. [DOI] [PubMed] [Google Scholar]
- 15. Ystrom E, Gustavson K, Brandlistuen RE, et al. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140(5):e20163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gervin K, Nordeng H, Ystrom E, et al. Long-term prenatal exposure to paracetamol is associated with DNA methylation differences in children diagnosed with ADHD. Clin Epigenetics. 2017;9:Article 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liew Z, Ritz B, Virk J, et al. Prenatal use of acetaminophen and child IQ: a Danish cohort study. Epidemiology. 2016;27(6):912–918. [DOI] [PubMed] [Google Scholar]
- 18. Zerbo O, Iosif AM, Walker C, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flanders WD, Klein M, Darrow LA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology. 2011;22(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flanders WD, Klein M, Darrow LA, et al. A method to detect residual confounding in spatial and other observational studies. Epidemiology. 2011;22(6):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisskopf MG, Tchetgen-Tchetgen EJ, Raz R. On the use of imperfect negative control exposures in epidemiological studies. Epidemiology. 2016;27(3):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mikkelsen SH, Hohwü L, Olsen J, et al. Parental body mass index and behavioral problems in their offspring: a Danish National Birth Cohort study. Am J Epidemiol. 2017;186(5):593–602. [DOI] [PubMed] [Google Scholar]
- 23. Zhu JL, Olsen J, Liew Z, et al. Parental smoking during pregnancy and ADHD in children: the Danish National Birth Cohort. Pediatrics. 2014;134(2):e382–e388. [DOI] [PubMed] [Google Scholar]
- 24. Jensen MS, Rebordosa C, Thulstrup AM, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology. 2010;21(6):779–785. [DOI] [PubMed] [Google Scholar]
- 25. Kalkbrenner AE, Windham GC, Serre ML, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26(1):30–42. [DOI] [PubMed] [Google Scholar]
- 26. Raz R, Levine H, Pinto O, et al. Traffic related air pollution and autism spectrum disorder: a population based nested case-control study in Israel. Am J Epidemiol. 2018;187(4):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raz R, Roberts AL, Lyall K, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II cohort. Environ Health Perspect. 2015;123(3):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faraone SV, Biederman J, Milberger S. How reliable are maternal reports of their children’s psychopathology? One-year recall of psychiatric diagnoses of ADHD children. J Am Acad Child Adolesc Psychiatry. 1995;34(8):1001–1008. [DOI] [PubMed] [Google Scholar]
- 29. Gao XA, Lyall K, Palacios N, et al. RLS in middle aged women and attention deficit/hyperactivity disorder in their offspring. Sleep Med. 2011;12(1):89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 31. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Arlington, VA: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- 32. Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56(6):1321–1333. [DOI] [PubMed] [Google Scholar]
- 33. Roberts AL, Liew Z, Lyall K, et al. Association of maternal exposure to childhood abuse with elevated risk for attention deficit hyperactivity disorder in offspring. Am J Epidemiol. 2018;187(9):1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olesen C, Sondergaard C, Thrane N, et al. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497–501. [DOI] [PubMed] [Google Scholar]
- 35. van Gelder MMHJ, Vorstenbosch S, te Winkel B, et al. Using Web-based questionnaires to assess medication use during pregnancy: a validation study in two prospectively enrolled cohorts. Am J Epidemiol. 2018;187(2):326–336. [DOI] [PubMed] [Google Scholar]
- 36. Kristensen DM, Mazaud-Guittot S, Gaudriault P, et al. Analgesic use—prevalence, biomonitoring and endocrine and reproductive effects. Nat Rev Endocrinol. 2016;12(7):381–393. [DOI] [PubMed] [Google Scholar]
- 37. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Albert O, Desdoits-Lethimonier C, Lesné L, et al. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898. [DOI] [PubMed] [Google Scholar]
- 39. Mazaud-Guittot S, Nicolas Nicolaz C, Desdoits-Lethimonier C, et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J Clin Endocrinol Metab. 2013;98(11):E1757–E1767. [DOI] [PubMed] [Google Scholar]
- 40. Nuttall SL, Khan JN, Thorpe GH, et al. The impact of therapeutic doses of paracetamol on serum total antioxidant capacity. J Clin Pharm Ther. 2003;28(4):289–294. [DOI] [PubMed] [Google Scholar]
- 41. Posadas I, Santos P, Blanco A, et al. Acetaminophen induces apoptosis in rat cortical neurons. PLoS One. 2010;5(12):e15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snijder CA, Kortenkamp A, Steegers EA, et al. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: the Generation R Study. Hum Reprod. 2012;27(4):1191–1201. [DOI] [PubMed] [Google Scholar]
- 43. Lind DV, Main KM, Kyhl HB, et al. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: a cohort study of 1027 mother-child pairs. Hum Reprod. 2017;32(1):223–231. [DOI] [PubMed] [Google Scholar]
- 44. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13(3):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


