Abstract
Background
Oral poisoning is a major cause of mortality and disability worldwide, with estimates of over 100,000 deaths due to unintentional poisoning each year and an overrepresentation of children below five years of age. Any effective intervention that laypeople can apply to limit or delay uptake or to evacuate, dilute or neutralize the poison before professional help arrives may limit toxicity and save lives.
Objectives
To assess the effects of pre‐hospital interventions (alone or in combination) for treating acute oral poisoning, available to and feasible for laypeople before the arrival of professional help.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL, ISI Web of Science, International Pharmaceutical Abstracts, and three clinical trials registries to 4 December 2018, and we also carried out reference checking and citation searching.
Selection criteria
We included randomized controlled trials comparing interventions (alone or in combination) that are feasible in a pre‐hospital setting for treating acute oral poisoning patients, including but potentially not limited to activated charcoal (AC), emetics, cathartics, diluents, neutralizing agents and body positioning.
Data collection and analysis
Two review authors independently performed study selection, data collection and assessment. Primary outcomes of this review were incidence of mortality and adverse events, plus incidence and severity of symptoms of poisoning. Secondary outcomes were duration of symptoms of poisoning, drug absorption, and incidence of hospitalization and ICU admission.
Main results
We included 24 trials involving 7099 participants. Using the Cochrane 'Risk of bias' tool, we assessed no study as being at low risk of bias for all domains. Many studies were poorly reported, so the risk of selection and detection biases were often unclear. Most studies reported important outcomes incompletely, and we judged them to be at high risk of reporting bias.
All but one study enrolled oral poisoning patients in an emergency department; the remaining study was conducted in a pre‐hospital setting. Fourteen studies included multiple toxic syndromes or did not specify, while the other studies specifically investigated paracetamol (2 studies), carbamazepine (2 studies), tricyclic antidepressant (2 studies), yellow oleander (2 studies), benzodiazepine (1 study), or toxic berry intoxication (1 study). Twenty‐one trials investigated the effects of activated charcoal (AC), administered as a single dose (SDAC) or in multiple doses (MDAC), alone or in combination with other first aid interventions (a cathartic) and/or hospital treatments. Six studies investigated syrup of ipecac plus other first aid interventions (SDAC + cathartic) versus ipecac alone. The collected evidence was mostly of low to very low certainty, often downgraded for indirectness, risk of bias or imprecision due to low numbers of events.
First aid interventions that limit or delay the absorption of the poison in the body
We are uncertain about the effect of SDAC compared to no intervention on the incidence of adverse events in general (zero events in both treatment groups; 1 study, 451 participants) or vomiting specifically (Peto odds ratio (OR) 4.17, 95% confidence interval (CI) 0.30 to 57.26, 1 study, 25 participants), ICU admission (Peto OR 7.77, 95% CI 0.15 to 391.93, 1 study, 451 participants) and clinical deterioration (zero events in both treatment groups; 1 study, 451 participants) in participants with mixed types or paracetamol poisoning, as all evidence for these outcomes was of very low certainty. No studies assessed SDAC for mortality, duration of symptoms, drug absorption or hospitalization.
Only one study compared SDAC to syrup of ipecac in participants with mixed types of poisoning, providing very low‐certainty evidence. Therefore we are uncertain about the effects on Glasgow Coma Scale scores (mean difference (MD) −0.15, 95% CI −0.43 to 0.13, 1 study, 34 participants) or incidence of adverse events (risk ratio (RR) 1.24, 95% CI 0.26 to 5.83, 1 study, 34 participants). No information was available concerning mortality, duration of symptoms, drug absorption, hospitalization or ICU admission.
This review also considered the added value of SDAC or MDAC to hospital interventions, which mostly included gastric lavage. No included studies investigated the use of body positioning in oral poisoning patients.
First aid interventions that evacuate the poison from the gastrointestinal tract
We found one study comparing ipecac versus no intervention in toxic berry ingestion in a pre‐hospital setting. Low‐certainty evidence suggests there may be an increase in the incidence of adverse events, but the study did not report incidence of mortality, incidence or duration of symptoms of poisoning, drug absorption, hospitalization or ICU admission (103 participants).
In addition, we also considered the added value of syrup of ipecac to SDAC plus a cathartic and the added value of a cathartic to SDAC.
No studies used cathartics as an individual intervention.
First aid interventions that neutralize or dilute the poison
No included studies investigated the neutralization or dilution of the poison in oral poisoning patients.
Authors' conclusions
The studies included in this review provided mostly low‐ or very low‐certainty evidence about the use of first aid interventions for acute oral poisoning. A key limitation was the fact that only one included study actually took place in a pre‐hospital setting, which undermines our confidence in the applicability of these results to this setting. Thus, the amount of evidence collected was insufficient to draw any conclusions.
Plain language summary
First aid treatments for oral poisoning
Review question
We reviewed the evidence on the effects of first aid treatments for poisoning that could be feasibly given by people who are not health professionals.
Background
Many first aid treatments are recommended for treating people who have ingested poisonous substances. Some treatments, such as activated charcoal (AC), bind to the poison, limiting the body's absorption of it. Others may induce vomiting (such as syrup of ipecac) or dilute or neutralize the poison (such as drinking water, milk or juices). Adjusting the person's body position may also have an effect.
Study characteristics
In December 2018 we searched for high‐quality studies (randomly dividing participants into different treatment groups) investigating treatments for poisoning that laypeople can perform. We found 24 studies with 7099 participants. All but one study took place in hospitals; the remaining one was in a home setting.
Fourteen studies either did not specify the type of poison or studied different kinds. The others investigated overdoses of specific medicines (paracetamol, carbamazepine, antidepressant, benzodiazepine) or poisonous plants (yellow oleander or poisonous berries).
Twenty‐one trials studied different treatments with activated charcoal: as a single dose or multiple doses, with or without other first aid treatments (a substance to speed up bowel transit), and with or without hospital treatments. Six studies compared syrup of ipecac, with or without other first aid treatments (single‐dose activated charcoal plus bowel transit enhancing substance) versus no treatment. We found no studies that investigated the neutralization or dilution of the poison or the use of certain body positions.
Key results
Two studies compared a single dose of activated charcoal to no treatment following poisoning with paracetamol or different kinds of poisoning. We are uncertain about the treatment's side effects, admission to intensive care or worsening of the patient, and there was no information about effects on death, symptom duration, poison uptake or hospitalization.
One study compared a single dose of activated charcoal to ipecac in mixed types of poisoning. We are uncertain about the effect of activated charcoal compared to ipecac, on the patient's level of coma or the number of unwanted effects. There was no information about effects on death, symptom duration, poison uptake, hospitalization or intensive care admission.
One study compared ipecac to no treatment in children who ate poisonous berries at home. There may be an increase in the number of unwanted effects for ipecac. There was no information about effects on death, poisoning symptoms, symptoms duration, poison uptake, hospitalization or intensive care admission.
We also investigated the use of single‐dose or multi‐dose activated charcoal, with or without hospital treatment, compared to each other or no treatment. Furthermore, we investigated the added value of ipecac to single‐dose activated charcoal and the added value of adding bowel transit enhancing substances to AC.
Certainty of the evidence
All but one study took place in a hospital setting, which means that the results cannot be directly applied to the lay setting. Because studies did not always report the methods they used, we are uncertain about the quality of the research conduct for many. Outcomes important to patients and pre‐specified by us as important outcomes for this review were often absent or incompletely reported. Our certainty about the results of this review is mostly low to very low. Therefore future research is highly likely to change the findings.
Conclusion
Based on the identified evidence, we cannot draw any conclusions about the effects of any of the investigated first aid treatments in a lay setting.
Summary of findings
Background
Description of the condition
Poisoning can be defined as exposure of the body to exogenous substances, in sufficiently large amounts to cause harm to the individual. This can happen through chronic exposure to low doses of a substance, or more acutely through sudden exposure to a harmful dose. Acute poisoning can happen either accidentally or voluntarily, as a way to end one's own or another's life or as a 'cry for help'.
Poisoning inflicts a major burden of morbidity and mortality worldwide. The World Health Organization (WHO) estimates that 108,000 deaths a year are caused by unintentional poisoning (WHO 2016), accompanied by the loss of a staggering 6,558,000 disability adjusted life years (DALYs) (WHO 2016). In addition to this, auto‐intoxication is one of the most common methods to attempt suicide. Yearly, around 800,000 people worldwide commit suicide, and around 30% of their attempts occur through the intake of pesticides, a phenomenon typically occurring in rural areas in lower‐ and middle‐income countries (WHO 2018). As most attempted suicides are unsuccessful, the actual burden will be much higher (Albert 2015). Poisoning can happen via different routes of exposure, such as through inhalation, injection or dermal absorption, but by far the most common is through deliberate or accidental ingestion of a toxic substance (Mowry 2016), which is the focus of this review. An important patient group to suffer from unintentional poisoning are young children. Roughly 20% of all accidental poisonings are thought to occur in children aged under 5 years (WHO 2016). In high‐income countries, this proportion is even larger: up to 47% of the incoming calls to the American Poison Control Centers concern exposures in this age group (Mowry 2016). This is most likely because young children are curious to explore their environment and do not realize the dangers of putting unknown and potentially harmful things in their mouth. Indeed, large numbers of exposures are to cosmetics and household products (25% of all reported exposures in children aged 5 years or younger; Mowry 2016).
Hospital treatment of acute oral poisoning focuses initially on supportive therapy: hypertonic glucose infusion, maintaining the victim's vital parameters and keeping poison‐induced symptoms under control (Isbister 2016; Nelson 2011). If practitioners can identify a toxin syndrome, they can administer a poison‐specific antidote, for example N‐acetylcysteine for a paracetamol overdose or naloxone for an opioid overdose (Chiew 2018; Wilkerson 2016). Third‐line treatment options include gastrointestinal decontamination procedures: activated charcoal can adsorb the poisonous substance (Corcoran 2016), while gastric lavage or whole bowel irrigation are procedures that attempt to eliminate the poison out of the gastrointestinal tract before absorption into the blood (Donkor 2016; Thanacoody 2015). A final treatment strategy is to eliminate toxins that have already been absorbed through multiple doses of activated charcoal, haemodialysis or blood/urinary alkalinization (Decker 2015; Gaudreault 2005; Proudfoot 2003; Roberts 2005).
In cases of acute oral poisoning, a swift reaction is crucial. For activated charcoal (AC), experimental studies have shown that its efficacy in limiting drug absorption decreases dramatically over time (Chyka 2005). Therefore, treatment guidelines recommend using AC within an hour after ingestion of the poison, although AC may still produce effects after that time, especially in drugs administered in a delayed release formula (Chyka 2005; Juurlink 2015). However, it is difficult to adhere to these guidelines in emergency services, mainly due to the delay between ingestion and presentation at the emergency department (Karim 2001; LoVecchio 2007; Tuuri 2009). Thus, any effective first aid measure that would neutralize, limit or delay uptake, or promote evacuation from the gastrointestinal tract in case of acute oral poisoning, could save precious time for professionals, potentially making the difference between life and death, or serious morbidity, for the poisoned patient.
First aid, as defined by the International Liaison Committee On Resuscitation (ILCOR), is the immediate help provided to a sick and injured person until professional help arrives. First aid interventions seek to preserve life, alleviate suffering, prevent further illness or injury and promote recovery (Zideman 2015). This definition implies that a first aid intervention must be both available to and feasible for a layperson in a pre‐hospital setting. Of the previously mentioned hospital interventions, only activated charcoal, which is relatively easy to administer orally and available without prescription, is feasible. In addition to these, other suggested first aid techniques include administering emetics, such as syrup of ipecac (or ipecacuanha) (Quang 2000); using cathartics, such as sorbitol (Keller 1990), drinking water, milk, vinegar or citrus juice to dilute and/or neutralize the poison (Rumack 1977); or adjusting the poisoned victim's body position to slow down the uptake of the poison (Vance 1992).
In case of ingestion of toxic alcohols (e.g. methanol, ethylene glycol), ethanol could be considered a potential home remedy due to its wide availability. However, the use of large volumes of ethanol is dangerous and needs to be monitored carefully (Rietjens 2014). Therefore, it is not recommended for use in a lay setting without professional guidance. Current recommendations for laypeople are limited to placing the victim in the lateral decubitus position and seeking professional help (e.g. contacting poison control centres if available) and following their advice (IFRC 2016).
Description of the intervention
The focus of this Cochrane Review is any intervention that is readily available to and administrable by laypeople before professional help arrives, targeted at neutralizing, limiting or delaying the absorption, or promoting the evacuation of a poison.
Limiting the absorption of a poison can be achieved by administering an adsorbent, such as activated charcoal. This black powder is produced through pyrolysis of carbon‐rich materials and activation by steam to remove already adsorbed substances (Olson 2010). This process results in a material with a very large surface area and hence adsorbing capacity. It needs to be mixed with water to form a slurry that can be ingested.
Placing the poisoning victim on their left side might be another method to decrease absorption of the poison (Vance 1992).
Substances that can promote the evacuation of a poison from the gastrointestinal tract firstly include emetics. The best known and most recommended is syrup of ipecacuanha, or ipecac. This syrup is derived from the roots and rhizome of Cephaelis ipecacuanha (Lee 2008). Other suggested emetics are apomorphine and copper sulphate. Apomorphine is believed to induce vomiting faster than ipecac (MacLean 1973), but it is not feasible to administer in a home setting and can cause central nervous depression, so it is not recommended, especially in children (MacLean 1973). Copper sulphate has also been used to induce vomiting in people with oral poisoning due to its action as a local irritant in the stomach (Karlsson 1965). However, it is a common source of intoxication itself, hence its use is also discouraged (Nastoulis 2017).
A second class of substances that can theoretically speed up the evacuation of an ingested poison from the gastrointestinal tract are cathartics. Patients who have ingested slow‐absorbing materials might benefit most from these treatments, although current guidelines suggest not using cathartics without activated charcoal (American Academy of Clinical Toxicology 2004). Examples of suggested cathartics include sugars, such as mannitol, lactulose and sorbitol, or salts, including magnesium sulphate, magnesium citrate and sodium sulphate.
In addition, diluting and neutralizing poisons, especially caustic substances such as lye, could occur through the intake of water, milk, vinegar or citrus juice (Rumack 1977). Milk might also have some adsorbing capacity (Chin 1969).
How the intervention might work
First aid interventions to treat poisoning can be categorized in four groups:
those that either limit or delay absorption of the poison in the body, such as activated charcoal or certain body positions;
interventions that evacuate the poison from the gastrointestinal tract, either by vomiting or by defecation;
combinations of first aid interventions that limit uptake and promote evacuation of the poison, e.g. sorbitol and activated charcoal;
first aid interventions that neutralize or dilute the poison, such as drinking water, milk, vinegar or citrus juice.
Furthermore, other combinations of first aid interventions may also be used.
A. First aid interventions that limit or delay the absorption of the poison in the body
One way to limit the absorption of a poison is to administer a substance that binds to the poison, thus preventing it from being absorbed by the body. Activated charcoal (AC) is one such adsorbent. Its enormous surface area can adsorb large quantities of drugs through the generation of Van der Waals forces between the charcoal and the adsorbed molecule (Olson 2010). Not all substances are equally effectively bound by AC. For example, lithium, iron, cyanide or alcohols bind to AC only to a minor extent, which means its appropriateness needs to be carefully considered in these cases (Bateman 1999; Juurlink 2015; Olson 2010). The optimal dose regimen for activated charcoal administration is not entirely clear, but 25 g to 100 g is considered to be a standard dose for adults (Chyka 2005). In practice, ingesting more than 50 g seems to be difficult to achieve for patients.
A certain body position might also slow down the uptake of the poison. The primary site of absorption for most pharmacologic substances is the small intestine, because of its large surface area and thin epithelium. Therefore, any factor that would delay gastric emptying into the small intestine should decrease the rate of absorption and limit the potential toxic effects of the ingested drug. Studies indicate that laying on the right side accelerates gastric emptying (Loots 2013; Valeur 2015; Van Wijk 2007). In contrast, placing the patient in the left lateral decubitus position might slow the rate of absorption of the ingested poison, because the anatomy of the stomach, combined with gravity, would allow the gastric content to stay in the greater curvature of the stomach (Vance 1992).
B. First aid interventions that evacuate the poison from the gastrointestinal tract
Evacuation of the poison from the gastrointestinal tract as quickly as possible can be achieved by inducing vomiting or accelerating defecation. Two types of drugs can be considered: emetics induce vomiting, while cathartics accelerate defecation.
As mentioned before, syrup of ipecac is the best known type of emetic. The main active substances of the ipecacuanha plant are emetine and cephaeline, which induce emesis and diarrhoea by acting both as a local irritant in the upper gastrointestinal tract and by targeting the chemoreceptor trigger zone in the medulla oblongata of the brain, the body's vomiting centre (Lee 2008). A potential risk associated with the use of emetics is lung injury through vomit aspiration (Höjer 2013).
Cathartics draw water into the large intestine, thereby stimulating bowel movements and thus accelerating defecation (American Academy of Clinical Toxicology 2004).
C. First aid interventions that limit uptake and promote evacuation of the poison from the gastrointestinal tract
Cathartics can be used with activated charcoal. This combination is thought to reduce drug uptake by accelerating evacuation out of the small bowel (Moon 2015). Furthermore, cathartics counteract the constipating effects of AC (James 1995). On the other hand, in vitro studies have suggested that cathartics might influence the adsorbing capacity of AC (Orisakwe 2001).
D. First aid interventions that neutralize or dilute the poison
A commonly used home remedy for poisoning by caustic substances is drinking large amounts of fluids, such as water, milk, vinegar or citrus juice (Rumack 1977). The rationale behind this is not only to dilute the poison, but also to change the pH in the stomach, thereby neutralizing the caustic effects of the ingested poison. Considerations that need to be made when using this approach are the chemical properties of the ingested substance (acidic or basic), the heat production that might occur during neutralization and sufficient availability of the neutralizing substance. In addition to its potential neutralizing effect, in vitro data suggest that milk has some adsorbing capacity (Chin 1969). However, increasing the volume of fluids in the stomach might also increase the rate of emptying into the small bowel, where the absorption of the poison takes place (Blain 2011). Furthermore, drinking large amounts of water might cause water intoxication (Lai 2016). A final consideration is that drinking large volumes of fluids might increase the risk of vomiting, which could be problematic in cases of caustic poisoning, as the caustic substance would contact the oesophagus for a second time.
Why it is important to do this review
There are several Cochrane Reviews concerning the treatment and prevention of poisoning. Kendrick 2012 provided evidence on interventions to prevent injuries at home, including cases of oral poisoning, while Hawton 2015 investigated potential interventions to decrease self‐harm in children, adolescents and adults. A review by Nussbaumer‐Streit 2016 documented potential household interventions to prevent domestic lead exposure in children. In addition, numerous Cochrane Reviews have investigated the use of hospital interventions to treat a range of specific intoxications, such as for example paracetamol or lithium poisoning (Chiew 2018; Lavonas 2015).
This Cochrane Review fills the gap between prevention and hospital treatment of poisoning, by investigating which pre‐hospital interventions, available and feasible for a lay person in a non‐healthcare setting, are effective in cases of acute oral poisoning. Identified interventions can be used in first aid guidelines targeted at lay people in settings such as nightclubs, childcare centres or the workplace, to be applied before arrival of professional help. As time is a crucial factor in acute oral poisoning, effective interventions conducted by laypeople would save valuable time and could therefore be crucial to survival (Chyka 2005; Juurlink 2015).
Objectives
To assess the effects of pre‐hospital interventions (alone or in combination) for treating acute oral poisoning, available to and feasible for laypeople before the arrival of professional help.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials (RCTs) in actual poisoning patients. We excluded studies involving healthy volunteers and preclinical studies (animal studies, in vitro research).
In order to be eligible for inclusion in the review, all RCTs taking place after 2010 must have been prospectively registered (Roberts 2015). All RCTs conducted prior to 2010 were eligible for inclusion.
Types of participants
We included participants poisoned via oral ingestion, both deliberately and accidentally. In addition to studies in a community setting, we considered studies conducted in a healthcare setting, including a hospital setting or ambulatory care, as most studies identified would likely have been performed in a controlled setting. Although this may be a source of indirectness, we feel that excluding these studies would result in selection bias.
Types of interventions
All identified first aid interventions, alone or in combination and feasible for a layperson in a pre‐hospital setting, were eligible. These included, among others, activated charcoal and other adsorbents (single‐ or multi‐dose); syrup of ipecac and other emetics (single or multi‐dose); cathartics (single or multi‐dose); body positioning; and water, milk, vinegar or citrus juice.
We compared the interventions to each other or to no intervention. We did not compare them to typical hospital interventions such as gastric lavage, whole bowel irrigation or the use of antidotes. However, if pre‐hospital treatments were used in adjuvant to an established hospital treatment, we included these studies. The reason for not considering established hospital treatments as comparisons is that we are interested in the most efficacious treatments in a non‐healthcare setting. It is likely that these would be less efficient than a hospital treatment, but they might still be useful as a first aid measure, which typically takes place before presentation to a healthcare facility.
Co‐interventions were allowed if all groups received them in equal doses. We separately explored interventions aiming to limit or delay absorption of poison, evacuate poison, limit uptake and evacuate poison, and neutralize or dilute poison.
Types of outcome measures
Timings of outcomes are defined as early (within 24 h after poisoning), intermediate (24 h to one week after poisoning) and late (more than one week and less than one year after poisoning).
Primary outcomes
Incidence of mortality
Incidence of adverse events due to the intervention
Incidence and severity of symptoms of poisoning, reported for example with the Poisoning Severity Score (PSS) (Persson 1998)
Secondary outcomes
Duration of toxic symptoms
Drug absorption: measured as maximal concentration of drug in the blood (Cmax), time to Cmax (Tmax) or area under the curve (AUC) of drug concentration versus time
Incidence of hospitalization
Incidence of intensive care unit (ICU) admission
Search methods for identification of studies
Electronic searches
We searched the following databases on 4 December 2018, without any language restrictions or date limits.
-
The Cochrane Library (2018, Issue 11, searched 4 December 2018; www.cochranelibrary.com), including the following databases.
The Cochrane Database of Systematic Reviews.
The Cochrane Central Register of Controlled Trials (CENTRAL), for reports of RCTs from MEDLINE, Embase and records submitted from Cochrane Specialized Registers, including the Cochrane Injuries Group.
DARE (Database of Abstracts of Reviews of Effect).
MEDLINE, using the PubMed interface (1966 to 4 December 2018).
Embase, using the Embase.com interface (1947 to 4 December 2018).
CINAHL, using the EBSCO host interface (1982 to 4 December 2018).
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐Science (CPCI‐S) (1900 to 4 December 2018).
International Pharmaceutical Abstracts, using the Ovid interface (1970 to 4 December 2018).
Clinicaltrials.gov (clinicaltrials.gov).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
For each of the articles included, we did a search in MEDLINE (via the PubMed interface) and screened the first 20 similar articles for additional relevant publications. Search strategies can be found in Appendix 1. Furthermore, we searched previously published systematic reviews and evidence‐based guidelines that were identified during the database searches (Table 7).
1. Sources of individual studies.
| Author and year of publication | Title |
| Abrass 2012 | The evidence for activated charcoal in resource poor settings: a systematic review |
| American Academy of Clinical Toxicology 1999 | Position statement and practice guidelines on the use of multi‐dose activated charcoal in the treatment of acute poisoning |
| American Academy of Clinical Toxicology 2004 | Position paper: cathartics |
| Chiew 2018 | Interventions for paracetamol (acetaminophen) overdoses |
| Chyka 2005 | Position paper: single‐dose activated charcoal |
| Eddleston 2003 | Does gastric lavage really push poisons beyond the pylorus? A systematic review of the evidence |
| Blain 2011 | Organophosphorus poisoning (acute) |
| Höjer 2013 | Position paper update: ipecac syrup for gastrointestinal decontamination. |
| Jones 2002 | Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Activated charcoal and gastric absorption of iron compounds |
| Manoguerra 2005 | Guideline on the use of ipecac syrup in the out‐of‐hospital management of ingested poisons |
| Qureshi 2011 | Adverse effects of activated charcoal used for the treatment of poisoning |
| Roberts 2011 | Enhanced elimination in acute barbiturate poisoning ‐ a systematic review |
We included relevant conference abstracts retrieved from searches in the above‐mentioned databases in the review.
Searching other resources
We searched the reference list of included articles, retrieved with the above searches, to identify other studies.
Data collection and analysis
Selection of studies
Two authors (BA and VB or AV) independently screened the titles and abstracts of all references yielded by the search. Subsequently, we retrieved full texts of selected articles, using a study selection form to assess eligibility. We resolved any discrepancies between authors through discussion. In cases where no consensus could be reached, we consulted a third author (EDB or AV). We documented the included studies in the appropriate sections within the review and summarized studies that were excluded after full‐text assessment in the Characteristics of excluded studies table, together with the reason for exclusion. We describe identified studies that were selected based on study design, study population and intervention of interest, but which reported no outcome of interest or outcome data, in the Results section of the review. We tried to contact the authors to ascertain whether the data for our outcomes of interest were unavailable due to lack of measurement or lack of reporting.
Data extraction and management
Two authors (BA and VB or AV) independently extracted data from all studies using a standardized and piloted data extraction form.
They extracted the following information from each study.
General information: author, year of publication, year of study, country of study lead author.
-
Study characteristics.
Study design.
Information on study population: number of participants, age, sex, country of study and poisoning characteristics (type and dose of intoxication, deliberate or accidental intoxication, time elapsed between intoxication and intervention, experimental or community setting).
Details of the intervention and the comparison: type of intervention, dose, route of administration, duration of the treatment.
Outcome(s) measured.
-
Study findings.
Effects of the intervention on the outcome: effect measure, confidence interval, P value.
Number of events and participants in intervention and comparison groups.
Assessment of risk of bias in included studies
Two authors (BA and VB or AV) independently assessed risk of bias in the included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). They assessed the domains of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data addressed, selective reporting and possible other bias, rating each domain as being at low, high or unclear risk of bias.
Measures of treatment effect
We used Review Manager 5 (RevMan 5) to manage data and conduct analyses (RevMan 2014). We reported continuous outcomes as mean differences (MD) with 95% confidence intervals (CIs) and dichotomous outcomes as risk ratios (RR) with 95% CIs, or Peto odds ratios (OR) when events were rare and criteria were satisfied.
Unit of analysis issues
We identified studies that had a multi‐arm design. We were cautious during the analysis of these data, ensuring that the same group of participants was not included twice in the meta‐analysis. We achieved this by ensuring that separate interventions were not included in a single meta‐analysis. Secondly, if multiple doses or administration times of an intervention were compared to a control group, we combined groups to create a single pair‐wise comparison in the case of dichotomous outcomes, according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions on the analysis of multi‐arm trials (Higgins 2011). We did not identify multi‐arm trials reporting continuous outcomes.
Dealing with missing data
In case of missing data, we attempted to contact the authors to obtain these data at least twice, if contact details were available.
Where possible, we calculated missing values (such as SDs) from the available data (P values, t values, CIs or standard errors) (Higgins 2011).
If insufficient data were available to calculate missing values, we only analysed the available data. We narratively described results from studies with missing data. We addressed the issue of the missing data and their potential impact on the findings of the study in the Discussion.
Assessment of heterogeneity
The target population of this review, patients with oral poisoning, is inherently heterogeneous with respect to the type, dose and timing of poison intake. However, the target audience for delivering this intervention, laypeople, are likely not capable of differentiating between these differences. Therefore, a certain degree of heterogeneity in the results is unavoidable.
Our analyses are stratified based on type of intervention. We assessed heterogeneity by inspection of the forest plot and by using the Chi²‐test and the I² statistic. We considered the Chi² statistic to be significant at P < 0.10. For interpretation of the I², we followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
When I² was at least 80% and the P value of the Chi² test was less than 0.1, we considered heterogeneity to be substantial, whereas for I² values below 40%, we considered heterogeneity to be unimportant. When heterogeneity was substantial, we examined the direction of the effects before making a decision whether to report the pooled result or describe the effects narratively.
Assessment of reporting biases
We planned methods for assessing reporting biases, but we could not perform them (New Reference). See Differences between protocol and review section.
Data synthesis
Where possible, we performed meta‐analyses. We pooled data if there were two or more studies on the same intervention that assessed the same outcome and provided sufficient data. We did not combine outcomes with different timings into a single meta‐analysis. We analysed different comparisons as separate analyses. We performed meta‐analyses using a random‐effects model, given the anticipated variation between studies. For dichotomous outcomes, we used the Mantel‐Haenszel method, while for continuous outcomes, we used the inverse variance method. In case of dichotomous outcomes with no or few events in one of the test groups, we used the Peto OR method, if criteria were met according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Given the large number of interventions in this review, we considered the possibility of a network meta‐analysis (NMA). However, due to the paucity of data and the heterogeneity in reported outcomes, this was not an option. In future updates of this review, we will consider this possibility again if there are sufficient data.
Subgroup analysis and investigation of heterogeneity
To investigate potential heterogeneity, we could have theoretically performed four possible subgroup analyses.
Different drugs taken. We hypothesized that for the intervention activated charcoal, drugs with a higher or a lower affinity for activated charcoal would be taken up to a lesser or higher extent in the body, while for cathartics, drugs that are absorbed faster would be less effectively flushed out of the body than drugs with a slower absorption rate.
Time point of the intervention. We hypothesized that the later an intervention is performed, the less efficacious it is in lowering the uptake of the drug.
Co‐interventions administered. We hypothesized that differing co‐interventions, such as the administration of a hospital treatment (e.g. gastric lavage), could influence the efficacy of the intervention investigated.
Type of adverse event experienced. We hypothesized that for the combined outcome 'occurrence of adverse events', different types of adverse events might be experienced to a different degree for a certain intervention.
Of these potential analyses, we could perform only the latter two because of the paucity of data.
Sensitivity analysis
We had planned to perform a sensitivity analysis by excluding studies at high or unclear risk of bias for sequence generation, allocation concealment, incomplete outcome reporting or other sources of bias, and then comparing the results with the initial analysis. However, we were not able to combine sufficient studies into a meta‐analysis for this analysis based on risk of bias of the individual studies.
We had also planned to perform sensitivity analyses in case we were required to impute data for some studies to enable meta‐analysis. We would have excluded the studies with imputed data and compared the results to the initial analysis. However, we were not able to impute data.
See Differences between protocol and review section.
'Summary of findings' table
We assessed the certainty of the body of evidence from the included studies according to the methodology described by the GRADE working group (Atkins 2004). The GRADE approach assesses the certainty of evidence for separate outcomes across the different studies in five domains: limitations in study design, consistency, imprecision, indirectness and publication bias. RCTs start with a level of high‐certainty evidence, which can be downgraded by one (serious limitations) or two (very serious limitations) levels for each of these domains. The certainty of evidence can therefore be high, moderate, low or very low. For the assessment of the GRADE domain 'limitations in study design', we decided to downgrade the certainty of evidence for an outcome if we judged one of the studies contributing to this outcome to be at high risk of bias in one of following domains: selection bias, detection bias, attrition bias or other bias. We decided not to take into account domains with unclear risk of bias to make this judgment. For the assessment of the GRADE domain 'imprecision' according to the guidance of the GRADE working group (Guyatt 2011), we decided to downgrade the certainty of evidence for an outcome:
if the optimal information size criterion was not met and total sample size of studies contributing to the outcome was low (fewer than 400 participants) for continuous outcomes or there was a low number of events (fewer than 300 events) for dichotomous outcomes;
if the CIs were wide (including both the line of no effect and an appreciable benefit or harm, i.e. a 25% increase or decrease in risk for dichotomous outcomes or a 50% increase or decrease in mean difference for continuous outcomes); or
if there was a lack of data to judge the prior two criteria.
We created a 'Summary of findings' table, using the online GRADEpro Guideline Development Tool (GRADEpro GDT 2015), for the most relevant comparison of interventions in a first aid setting: single‐dose activated charcoal (SDAC) versus no intervention. We created additional 'Summary of findings' tables for the other most clinically relevant comparisons involving single‐ and multi‐dose activated charcoal: SDAC plus hospital intervention versus hospital intervention alone, MDAC plus hospital intervention versus SDAC plus hospital intervention, MDAC plus hospital intervention versus hospital intervention alone, and syrup of ipecac versus no intervention. We also created 'Summary of findings' tables for the other identified comparisons, but we placed these in the Appendices.
We have included all primary and secondary outcomes of this review in our 'Summary of findings' tables. For outcomes such as severity of symptoms, studies reported multiple outcomes (e.g. incidence of clinical improvement, incidence of intubation requirement, incidence of convulsions etc.). As 'Summary of findings' tables should include no more than seven outcomes, we chose the clinically most relevant outcomes together with a clinical expert (PD).
Results
Description of studies
Results of the search
Our search strategies identified a total of 11,582 references. After removing 1859 duplicates and screening titles and abstracts, we assessed 78 full‐text records for eligibility. At this stage we included 20 studies, reported in 27 records, and we then included an additional four studies after screening reference lists of included studies and systematic reviews retrieved with the database searches and similar articles in PubMed. Figure 1 shows the flowchart of the study selection.
1.
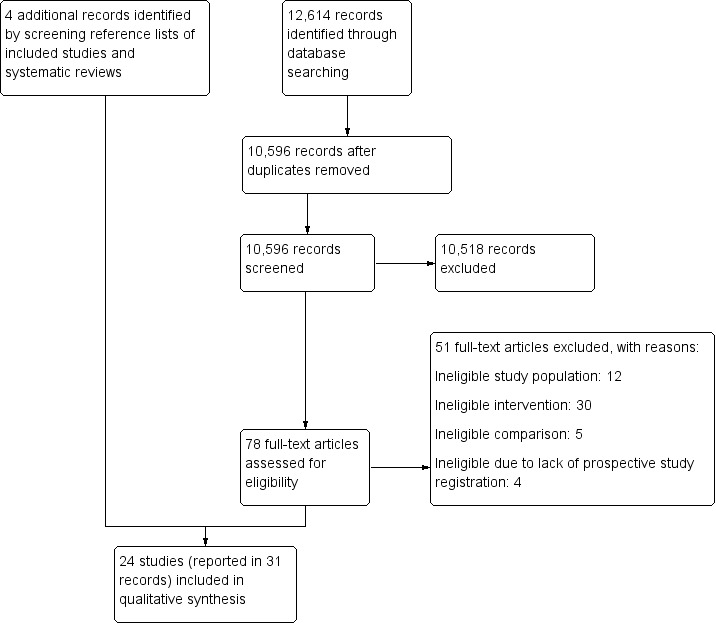
Study selection flow diagram.
Included studies
We included 24 studies reported in 31 publications and involving a total of 7099 participants randomized to different treatment groups. Only one study took place in a pre‐hospital setting (Wax 1999), whereas the rest were in hospitals.
Nineteen studies assessed the effects of single‐dose activated charcoal (SDAC), either administered alone (Amigó Tadín 2002; Merigian 1990; Underhill 1990), in adjuvant to hospital treatment (Behnoush 2009; Brahmi 2006; Comstock 1982; Cooper 2005; Crome 1983; De Silva 2003; Eddleston 2008; Hultén 1988; Merigian 2002; Roberts 2006), combined with a cathartic (James 1995; Passeron 1989), or combined with syrup of ipecac (Albertson 1989; Kornberg 1991; Kulig 1985; Pond 1995).
Seven studies looked at the effect of multi‐dose activated charcoal (MDAC) either in adjuvant to hospital treatment (Behnoush 2009; Bouget 1989; Brahmi 2006; De Silva 2003; Eddleston 2008; Roberts 2006), or combined with cathartics and in adjuvant to hospital treatment (Montoya‐Cabrera 1999).
Six studies investigated syrup of ipecac alone (Amigó Tadín 2002; Wax 1999), or followed by SDAC and a cathartic (Albertson 1989; Kornberg 1991; Kulig 1985; Pond 1995).
Table 8 contains an overview of the comparisons made in the different studies.
2. Overview of comparisons.
| Comparison | Type of poisoning | Study |
| A. First aid interventions that limit or delay the absorption of the poison in the body | ||
| SDAC vs no intervention | Not specified | Merigian 1990 |
| Paracetamol | Underhill 1990 | |
| SDAC + hospital intervention vs hospital intervention | Not specified | Comstock 1982 |
| Benzodiazepines + paracetamol or other drug combinations | Cooper 2005 | |
| Tricyclic antidepressants | Crome 1983 | |
| Yellow oleander, organophosphorus/carbamate pesticide, organochlorine, other/unknown pesticide or paraquat, medicine or unknown | Eddleston 2008 | |
| Amitriptyline, clomipramine, mianserin, imipramine, dothiepin, doxepin, nortriptyline, mixed overdoses with most commonly benzodiazepines or alcohol | Hultén 1988 | |
| Not specified | Merigian 2002 | |
| Yellow oleander | Roberts 2006 | |
| MDAC + hospital intervention vs SDAC + hospital intervention | Carbamazepine | Behnoush 2009 |
| Carbamazepine | Brahmi 2006 | |
| Yellow oleander | De Silva 2003 | |
| Yellow oleander, organophosphorus/carbamate pesticide, organochlorine, other/unknown pesticide or paraquat, medicine or unknown | Eddleston 2008 | |
| Yellow oleander | Roberts 2006 | |
| SDAC vs syrup of ipecac | Anti‐inflammatory drugs, analgesics or psychotropic drugs | Amigó Tadín 2002 |
| MDAC + hospital intervention vs hospital intervention | Benzodiazepine | Bouget 1989 |
| Yellow oleander, organophosphorus/carbamate pesticide, organochlorine, other/unknown pesticide or paraquat, medicine or unknown | Eddleston 2008 | |
| Yellow oleander | Roberts 2006 | |
| B. First aid interventions that evacuate the poison from the gastrointestinal tract | ||
| Emetics | ||
| Syrup of ipecac vs no intervention | Toxic berries | Wax 1999 |
| Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic | Not specified | Albertson 1989 |
| Wide variety, most commonly paracetamol | Kornberg 1991 | |
| Not specified | Kulig 1985 | |
| Paracetamol, salicylate, phenothiazines or ethanol, or other drugs | Pond 1995 | |
| Syrup of ipecac 15 mL vs syrup of ipecac 30 mL (dose) | Benzodiazepine tranquillizers or hypnotics, other tranquillizers, other hypnotics, antidepressants, analgesics, antihistamines, miscellaneous drugs and chemicals | Ilett 1977 |
| Cathartics | ||
| SDAC + cathartic vs SDAC | Not specified | Sue 1994 |
| SDAC + cathartic vs SDAC | Analgesics, anticonvulsants, antihistamines and decongestants, asthma therapies, automotive products, cardiovascular drugs, gastrointestinal preparations, insecticides, mushrooms, psychotropic drugs, rodenticides, topicals, miscellaneous drugs | James 1995 |
| SDAC + cathartic vs SDAC + cathartic (dose) | Not specified | Sue 1994 |
| SDAC + cathartic vs SDAC + cathartic (type) | Analgesics, anticonvulsants, antihistamines and decongestants, asthma therapies, automotive products, cardiovascular drugs, gastrointestinal preparations, insecticides, mushrooms, psychotropic drugs, rodenticides, topicals, miscellaneous drugs | James 1995 |
| C. Combined first aid interventions that limit uptake and promote evacuation of the poison from the gastrointestinal tract | ||
| SDAC + cathartic + hospital intervention vs hospital intervention | Benzodiazepines, barbiturates or imipramine | Passeron 1989 |
| MDAC + cathartic + hospital intervention vs hospital intervention | Paracetamol | Montoya‐Cabrera 1999 |
| D. First aid interventions that neutralize or dilute the poison | ||
| No studies were identified | ||
APF: Australian Pharmaceutical Formulary; MDAC: multi‐dose activated charcoal; SDAC: single‐dose activated charcoal; USP: United States Pharmacopeia.
Excluded studies
We excluded 47 studies after full‐text evaluation (Characteristics of excluded studies). We excluded 11 studies because of an ineligible study population (not oral poisoning patients or patients with chronic poisoning), 30 studies because of an intervention that did not meet our selection criteria and 5 because of an inappropriate comparison. Furthermore, we excluded one recent study, published as an abstract only (Escalante 2016), because of a lack of prospective trial registration, in accordance to the editorial policies of the Cochrane Injuries review group.
Risk of bias in included studies
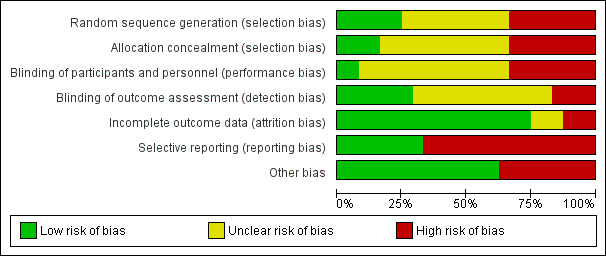
We did not judge any study to be at low risk of bias on all domains investigated. We scored two studies as having a low risk of bias for all but one domain: in one study there was a risk of selective reporting (De Silva 2003), and in the other there was a risk of performance bias (Eddleston 2008). All other studies were at high or unclear risk of bias for two or more domains. Six studies were at high risk of bias in at least four domains (Albertson 1989; Crome 1983; Kornberg 1991; Merigian 2002; Pond 1995; Wax 1999), whereas 12 studies were at unclear risk of bias in three or more domains (Amigó Tadín 2002; Behnoush 2009; Bouget 1989; Brahmi 2006; Comstock 1982; Crome 1983; Hultén 1988; Ilett 1977; Montoya‐Cabrera 1999; Passeron 1989; Sue 1994; Underhill 1990). Figure 2 and Figure 3 provide an overview of the risk of bias across domains and studies, and detailed judgments by domain can be found for each included study in the Characteristics of included studies table.
2.
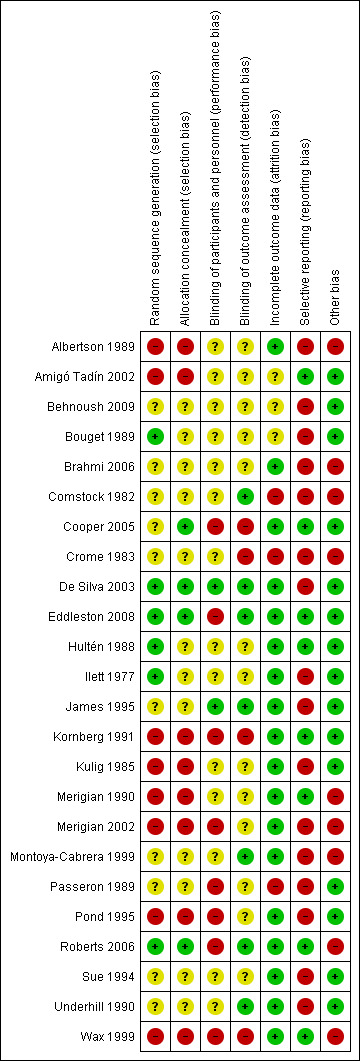
3.
Allocation
In general, randomization and allocation concealment was inadequately performed or poorly reported. The population was sufficiently randomized and adequately reported in six studies only (Bouget 1989; De Silva 2003; Eddleston 2008; Hultén 1988; Ilett 1977; Roberts 2006). In four studies the allocation concealment was adequate (Cooper 2005; De Silva 2003; Eddleston 2008; Roberts 2006).
Blinding
Most studies either did not blind or did not report on blinding of the participants and personnel. This is likely due to the nature of the interventions, which makes it difficult to perform adequate blinding. However, this might lead to performance bias, for example, because of differential administration of co‐interventions. One study that combined activated charcoal with different cathartics reported blinding both participants and personnel (James 1995), while another study testing multiple versus single doses of activated charcoal blinded the treating physicians by making sure research assistants cleaned the participants and their bedclothes after each activated charcoal treatment (De Silva 2003). Blinding of outcome assessors was not common, but seven studies did take this step (Comstock 1982; De Silva 2003; Eddleston 2008; James 1995; Montoya‐Cabrera 1999; Roberts 2006; Underhill 1990).
Incomplete outcome data
Only three studies were at high risk of attrition bias (Comstock 1982; Crome 1983; Passeron 1989), and three were at unclear risk (Amigó Tadín 2002; Behnoush 2009; Bouget 1989). All other studies showed no evidence of incomplete outcome data.
Selective reporting
Overall there was a high risk of reporting bias. Only a third of the studies were at low risk (Amigó Tadín 2002; Cooper 2005; Eddleston 2008; Hultén 1988; Kornberg 1991; Merigian 1990; Roberts 2006; Wax 1999).
Other potential sources of bias
Fifteen studies were at low risk of other potential sources of bias, and we assessed nine studies as being at high risk of bias for reasons other than those mentioned above.
In Albertson 1989, actual poisoning was not verified for 25% of the participants by means other than history. Furthermore, in Wax 1999, there was no confirmation of actual ingestion or uptake of the drug.
In another study, investigators suspected a clinical difference between the groups receiving the MDAC intervention versus the SDAC control, based on divergent carbamazepine kinetics during the initial six hours of the treatment period, when both groups had received only one dose of activated charcoal (Brahmi 2006). Also in Montoya‐Cabrera 1999, the hepatic toxicity marker values suggest there might be a clinically meaningful difference between the two treatment groups. This could create a bias in effectiveness of the treatment, because of differences in degree and type of poisoning.
In Comstock 1982 there was a potential bias in the selection of the study population since participants were selected at the discretion of the attending physician.
Crome 1983 did not find significant amounts of any drugs in 11 of the 48 participants, and 7 of them had not taken any tricyclic antidepressant (although this was a criterion for inclusion). Furthermore, the role of the study funder was not clear.
Two studies included only asymptomatic participants, who are less likely to experience a benefit from any treatment (Merigian 1990; Wax 1999).
Merigian 2002 performed only post hoc analyses according to clinical severity, and there was no follow‐up after discharge from the hospital.
In Roberts 2006 it is not entirely clear, even to the authors, what exactly is measured with the digoxin assay used in the study. The fact that both active cardenolides and metabolites might be detected by the assay compromise the results of these analyses, potentially explaining the wide variability observed. Furthermore, only participants with mild intoxication were included in this analysis, as the severe cases were treated with Fab antitoxin or transferred to a tertiary hospital, but these patients might have shown the biggest effect (Roberts 2006).
In Wax 1999, the authors reported dichotomous outcomes but performed measurement using an ordinal scale.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. SDAC versus no intervention for first aid in patients with acute oral poisoning.
| SDAC versus no intervention for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (paracetamol or not specified) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with SDAC | |||||
| Incidence of mortality | No studies collected or reported this outcome | |||||
| Incidence of adverse events | Control group: 0/236; intervention group: 4/240 (Peto OR 4.17, 95% CI 0.30 to 57.26) | — | 476 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of SDAC on the incidence of adverse events. | |
| Incidence and severity of symptoms of poisoning: incidence of clinical deterioration during stay in the hospital | — | — | 451 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | The relative effect was not estimable due to the absence of events in the intervention (0/220) and the control group (0/231). We are uncertain of the effect of SDAC on incidence and severity on poisoning. | |
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption | No studies collected or reported this outcome | |||||
| Incidence of hospitalization | No studies collected or reported this outcome | |||||
| Incidence of ICU admission | Control group: 0/231; intervention group: 1/220 (Peto OR 7.77, 95% CI 0.15 to 391.93) | — | 451 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of SDAC on the incidence of ICU admission. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SDAC: single‐dose activated charcoal; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious limitations in study design: high risk of selection bias. bDowngraded one level for serious indirectness: study conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals. dDowngraded one level for serious imprecision: low number of events.
Summary of findings 2. SDAC + hospital intervention versus hospital intervention alone for first aid in patients with acute oral poisoning.
| SDAC + hospital intervention versus hospital intervention alone for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (not specified, tricyclic antidepressants, combinations of different drugs or yellow oleander) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) + hospital intervention Comparison: hospital intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hospital intervention | Risk with SDAC + hospital intervention | |||||
| Incidence of mortality | Study population | Peto OR 1.04 (0.79 to 1.37) | 3425 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | SDAC in addition to hospital treatments may make little or no difference on incidence of mortality. | |
| 62 per 1000 | 64 per 1000 (49 to 85) | |||||
| Incidence of adverse events | Incidence of vomiting: intervention group: 118/570 and control group: 163/1236 (RR 1.44, 95% CI 0.88 to 2.37; 1806 participants; 2 studies). Incidence of absent bowel sounds: intervention group: 7/1544 and control group: 17/1554 (RR 0.41, 95% CI 0.17 to 1.00, 1 study, 3098 participants). |
— | 4904 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | Statistically significant heterogeneity was found, which may be explained partially by subgroup analyses per type of adverse event. We are uncertain about the effect of SDAC in addition to hospital treatments on incidence of adverse events. | |
| Incidence and severity of symptoms of poisoning: incidence of need for intubation | Patients that received gastric lavage prior to SDAC: intervention group: 80/1578 and control group: 87/1597 (RR 0.95, 95% CI 0.70 to 1.27, 2 studies, 3175 participants). Patients that did not receive gastric lavage prior to SDAC: intervention group: 24/194 and control group: 10/193 (RR 2.61, 95% CI 1.38 to 4.93, 1 study, 387 participants). |
— | 3562 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Statistically significant heterogeneity was found, which may be explained by subgroup analyses in patients receiving or not receiving gastric lavage. We are uncertain about the effect of SDAC on incidence of need for intubation. | |
| Duration of toxic symptoms: duration of intubation (h) |
Eddleston 2008: intervention group median (IQR): 112.0 (36.6 to 234.9) h and control group median (IQR): 88.5 (38.5 to 203.1) h (median difference: 23.5 h, P > 0.05). Merigian 2002: intervention group mean: 54.6 h and control group mean: 39.9 h (MD: 14.7 h, P = 0.70). |
— | (2 RCTs) | ⊕⊕⊝⊝ Lowa,e | Data were reported as median with IQR in one or means without measure of spread in another study, without information on participant numbers. SDAC in addition to hospital treatments may make little or no difference on the duration of intubation. |
|
| Drug absorption: cardenolide: AUC (µg/L) × h Follow‐up: 1 days | The median (IQR) in intervention group was 17.7 (11.1 to 21.8) (µg/L) × h and in the control group 19.0 (13.7 to 24.3) (µg/L) × h (median difference: −1.3 h, P > 0.05) | — | 68 (1 RCT) | ⊕⊝⊝⊝ Very lowa,f,g | We are uncertain about the effect of SDAC in addition to hospital treatments on cardenolide absorption. | |
| Incidence of hospitalization | 125 per 1000 | 196 per 1000 (152 to 252) | RR 1.57 (1.22 to 2.02) | 1479 (1 RCT) | ⊕⊝⊝⊝ Very lowa,g,h | We are uncertain about the effect of SDAC in addition to hospital treatments on incidence of hospitalization. |
| Incidence of ICU admission | 30 per 1000 | 69 per 1000 (42 to 114) | RR 2.33 (1.42 to 3.82) | 1479 (1 RCT) | ⊕⊝⊝⊝ Very lowa,g,h | We are uncertain about the effect of SDAC in addition to hospital treatments on incidence of ICU admission. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; RCT: randomized controlled trial; RR: risk ratio; SDAC: single‐dose activated charcoal; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious indirectness: study conducted in a hospital setting. bDowngraded one level for serious imprecision: low number of events and wide confidence intervals. cDowngraded one level for serious inconsistency: large and statistically significant heterogeneity present (I² > 60%, P < 0.10). dDowngraded one level for serious imprecision: wide confidence intervals. eDowngraded one level for serious imprecision: lack of data on the number of patients analysed. fDowngraded one level due to serious limitations in study design: high risk of other bias: it is not entirely clear what is measured with the assay used. The fact that both active cardenolides and (inactive) metabolites might be detected by the assay compromise the results of these analyses, as they might explain the wide variability observed. gDowngraded one level for serious imprecision: low number of events. hDowngraded one level for serious limitations in study design: high risk of selection bias.
Summary of findings 3. MDAC + hospital intervention versus SDAC + hospital intervention for first aid in patients with acute oral poisoning.
| MDAC + hospital intervention versus SDAC + hospital intervention for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (carbamazepine, yellow oleander, or combinations of different drugs) Setting: hospital setting Intervention: multiple dose of activated charcoal (MDAC) + hospital intervention Comparison: single‐dose activated charcoal (SDAC) + hospital intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SDAC + hospital intervention | Risk with MDAC + hospital intervention | |||||
| Incidence of mortality | Study population | RR 0.59 (0.21 to 1.63) | 3476 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Combining the studies resulted in statistically significant heterogeneity, for which explanations remain speculative. We are uncertain about the effects of MDAC in addition to hospital treatment, compared to SDAC, in addition to hospital treatment. |
|
| 72 per 1000 | 42 per 1000 (15 to 117) |
|||||
| Incidence of adverse events | Study population | Peto OR 3.55 (1.85 to 6.79) | 3476 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | There was statistically significant heterogeneity, which may be attributable to different adverse events measured in individual studies. MDAC in addition to hospital treatment may increase abdominal discomfort/diarrhoea and absent bowel sounds, compared to SDAC in addition to hospital treatment. |
|
| 4 per 1000 | 14 per 1000 (7 to 27) |
|||||
| Incidence and severity of symptoms of poisoning: incidence of need for intubation | Study population | RR 1.01 (0.75 to 1.38) | 3097 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | MDAC in addition to hospital treatment may make little or no difference in the incidence of need for intubation, compared to SDAC in addition to hospital treatment. | |
| 49 per 1000 | 49 per 1000 (37 to 67) | |||||
| Duration of toxic symptoms: duration of intubation (h) |
Brahmi 2006: intervention group: 24.1 (SD 4.2 h and control group 36.4 (SD 3.6 h (MD: 12.30 h lower, 95% CI −18.56 to −6.04, 6 participants). Eddleston 2008: intervention group median (IQR): 83.8 (35.0 to 173.0) h and control group median (IQR): 112.0 (36.6 to 234.9) h (median difference: 28.2 h), unclear number of participants |
— | (2 RCTs) | ⊕⊝⊝⊝ Very lowb,d,e | Data were reported as means with SD in one study or medians with IQR in another study, without information on participant numbers or statement of significance. We are uncertain about the effects of MDAC in addition to hospital treatment on duration of intubation, compared to SDAC in addition to hospital treatment. |
|
| Drug absorption: cardenolide: AUC (µg × L/h) Follow‐up: 1 days | The median (IQR) in intervention group was 17.3 (12.8 to 21.7) (µg/L) × h and in the control group 17.7 (11.1 to 21.8) (µg/L) × h (median difference −0.4, P > 0.05). | — | 64 (1 RCT) | ⊕⊝⊝⊝ Very lowb,e,f | We are uncertain about the effects of MDAC in addition to hospital treatment on cardenolide drug absorption, compared to SDAC in addition to hospital treatment. | |
| Incidence of hospitalization | No studies collected or reported this outcome | |||||
| Incidence of ICU admission | Study population | RR 0.31 (0.12 to 0.83) | 401 (1 RCT) | ⊕⊕⊝⊝ Lowb,g | MDAC in addition to hospital treatment may result in a decreased incidence of ICU admission, compared to SDAC in addition to hospital treatment. | |
| 80 per 1000 | 25 per 1000 (10 to 66) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AUC: area under the receiver operating curve; CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MDAC: multi‐dose activated charcoal; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation; SDAC: single‐dose activated charcoal; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious inconsistency: combining results resulted in a considerable and statistically significant degree of heterogeneity (I² > 60%, P < 0.10). bDowngraded one level for serious indirectness: study conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals. dDowngraded one level for other limitations: inconsistent conclusions made by the studies. eDowngraded one level for serious imprecision: low sample size and lack of data. fDowngraded one level for serious study limitations: high risk of other bias: it is not entirely clear what is measured with the assay used. The fact that both active cardenolides and (inactive) metabolites might be detected by the assay compromise the results of these analyses, as they might explain the wide variability observed. gDowngraded one level for serious imprecision: low number of events.
Summary of findings 4. SDAC versus syrup of ipecac for first aid in patients with acute oral poisoning.
| SDAC versus syrup of ipecac for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (anti‐inflammatory drugs, analgesics or psychotropic drugs) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) Comparison: syrup of ipecac | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with syrup of ipecac | Risk with SDAC | |||||
| Incidence of mortality | No studies collected this outcome | |||||
| Incidence of adverse events | Study population | RR 1.24 (0.26 to 5.83) | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain about the effect of SDAC, compared to syrup of ipecac on incidence of adverse events. | |
| 154 per 1000 | 191 per 1000 (40 to 897) | |||||
| Incidence and severity of symptoms of poisoning: level of coma assessed with Glasgow Coma Scale Scale from: 3 to 15 Follow‐up: 1 h | The mean incidence and severity of symptoms of poisoning: level of coma was 14.91 | MD 0.15 lower (0.43 lower to 0.13 higher) | — | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain about the effect of SDAC, compared to syrup of ipecac on the level of coma. |
| Duration of toxic symptoms | No studies collected this outcome | |||||
| Drug absorption | No studies collected this outcome | |||||
| Incidence of hospitalization | No studies collected this outcome | |||||
| Incidence of ICU admission | No studies collected this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SDAC: single‐dose activated charcoal. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations: high risk of selection bias. bDowngraded one level for serious indirectness: study conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals. dDowngraded one level for serious imprecision: low sample size.
Summary of findings 5. MDAC + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning.
| MDAC + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (benzodiazepines, yellow oleander or combinations of different drugs) Setting: hospital setting Intervention: multi‐dose activated charcoal (MDAC) + hospital intervention Comparison: hospital intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hospital intervention | Risk with MDAC + hospital intervention | |||||
| Incidence of mortality | Study population | RR 0.94 (0.72 to 1.22) | 3085 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | MDAC in addition to hospital treatment may make little or no difference in incidence of mortality. | |
| 68 per 1000 | 64 per 1000 (49 to 82) | |||||
| Incidence of adverse events | Study population | RR 1.02 (0.52 to 1.98) | 3085 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | MDAC in addition to hospital treatment may make little or no difference in incidence of adverse events. | |
| 11 per 1000 | 11 per 1000 (6 to 22) | |||||
| Incidence and severity of symptoms of poisoning: incidence of need for intubation | Study population | RR 0.97 (0.71 to 1.33) | 3085 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | MDAC in addition to hospital treatment may make little or no difference in incidence of need for intubation. | |
| 49 per 1000 | 47 per 1000 (35 to 65) | |||||
| Duration of toxic symptoms: length of intubation (h) | The median (IQR) length of intubation in the intervention group was 83.8 (35.0 to 173.0) h and 88.5 (38.5 to 203.1) h in the control group and was reported not to differ significantly (P > 0.05); unclear number of participants | — | (1 RCT) | ⊕⊕⊝⊝ Lowa,c | The number of participants analysed was not reported. MDAC in addition to hospital treatment may make little or no difference in length of intubation |
|
| Drug absorption: cardenolide: AUC (µg/L × h) Follow‐up: 1 day | The median (IQR) cardenolide AUC in the intervention group was 17.3 (12.8 to 21.7) (µg/L) × h and 19.0 (13.7 to 24.3) (µg/L) × h in the control group. | — | 76 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | We are uncertain about the effects of MDAC in addition to hospital treatment on cardenolide drug absorption. | |
| Incidence of hospitalization | No studies collected or reported this outcome | |||||
| Incidence of ICU admission | No studies collected or reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AUC: area under the receiver operating curve; CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MDAC: multi‐dose activated charcoal; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious indirectness: study conducted in a hospital setting. bDowngraded one level for serious imprecision: low number of events and wide confidence interval. cDowngraded one level for serious imprecision: low sample size and lack of data. dDowngraded one level for serious study limitations: high risk of other bias: it is not entirely clear what is measured with the assay used. The fact that both active cardenolides and (inactive) metabolites might be detected by the assay compromise the results of these analyses, as they might explain the wide variability observed.
Summary of findings 6. Syrup of ipecac versus no intervention for first aid in patients with acute oral poisoning.
| Syrup of ipecac versus no intervention for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (toxic berries) Setting: pre‐hospital setting Intervention: syrup of ipecac Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with Syrup of ipecac | |||||
| Incidence of mortality | No studies collected this outcome | |||||
| Incidence of adverse events: diarrhoea Follow‐up: 1 day | Study population | RR 4.08 (1.66 to 10.04) | 103 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Syrup of ipecac may result in an increased incidence of diarrhoea. | |
| 96 per 1000 | 392 per 1000 (160 to 965) | |||||
| Incidence and severity of symptoms of poisoning | No studies collected this outcome | |||||
| Duration of toxic symptoms | No studies collected this outcome | |||||
| Drug absorption | No studies collected this outcome | |||||
| Hospitalization: incidence of hospitalization Follow‐up: 1 days | — | — | 103 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | The effect was not estimable due to the absence of events in the intervention (0/52) and the control group (0/51). | |
| ICU admission | No studies collected this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations: high risk of selection bias, high risk of detection bias, and high risk of other bias (no confirmation of actual ingestion or uptake, reporting of dichotomous outcomes while measuring with an ordinal scale). bDowngraded one level for serious imprecision: low number of events.
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7.
A. First aid interventions that limit or delay the absorption of the poison in the body
1. Single‐dose activated charcoal versus no intervention
Two studies compared a single dose of activated charcoal versus no intervention (Merigian 1990; Underhill 1990). Underhill 1990 included 25 participants presenting in the emergency department with acute paracetamol overdose. Recruitment in the control group was stopped early for ethical reasons, as blood levels of paracetamol kept rising over time. Merigian 1990 included 820 participants presenting at the emergency department with self‐reported oral overdose in general. This study subdivided participants into a symptomatic and an asymptomatic group, for which treatments differed. Only the 451 asymptomatic participants, who received either a single dose of activated charcoal or were kept for observation, were within scope of this review. For a detailed summary of the outcomes, we refer to the Data and analyses section. Below we provide a narrative overview. See Table 1.
Primary outcomes
Incidence of mortality
The identified studies either did not collect or did not report outcomes related to mortality.
Adverse events
The only adverse event Underhill 1990 reported in response to SDAC was vomiting, which occurred in 4/20 participants compared to 0/5 in the control group (Peto OR 4.17, 95% CI 0.30 to 57.26; Analysis 1.1). Merigian 1990 reported no adverse events in any treatment group (451 participants). We assessed this evidence as being of very low certainty because of limitations in study design, imprecision due to a low event number and indirectness.
1.1. Analysis.
Comparison 1 SDAC vs no intervention, Outcome 1 Incidence of adverse events.
Incidence and severity of poisoning symptoms
Only Merigian 1990 reported an outcome related to symptom severity. In 451 asymptomatic participants presenting to the emergency department, no participants experienced events of clinical deterioration in either group (Table 9). We assessed this evidence as being of very low certainty because of limitations in study design, imprecision due to a low event number and indirectness.
3. Additional pre‐defined outcomes reported in the included studies.
| A. First aid interventions that limit or delay the absorption of the poison in the body | |||||
| SDAC vs no intervention | |||||
| Incidence of clinical deterioration | |||||
| SDAC | No intervention | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Merigian 1990 | 0 | 220 | 0 | 231 | Not estimable |
| Incidence of ICU admission | |||||
| SDAC | No intervention | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Merigian 1990 | 0 | 220 | 0 | 231 | 7.77 (0.15 to 391.93) |
| SDAC + hospitalintervention vs hospitalintervention | |||||
| Incidence of clinical deterioration | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Merigian 2002 | 0 | 455 | 0 | 1075 | Not estimable |
| Grade of coma (4 h after admission) | |||||
| SDAC + hospital treatment (N = 9) | Hospital treatment (N = 7) | ||||
| Study | Median | IQR | Median | IQR | Median difference (P value) |
| Crome 1983 | 2 | (1 to 3) | 2 | (1 to 2.5) | 0 (P = 0.55) |
| Grade of coma (8 h after admission) | |||||
| SDAC + hospital treatment (N = 9) | Hospital treatment (N = 7) | ||||
| Study | Median | IQR | Median | IQR | Median difference (P value) |
| Crome 1983 | 2 | (1 to 3) | 1 | (0.5 to 2) | 1 (P = 0.38) |
| Grade of coma (24 h after admission) | |||||
| SDAC + hospital treatment (N = 9) | Hospital treatment (N = 7) | ||||
| Study | Median | IQR | Median | IQR | Median difference (P value) |
| Crome 1983 | 1 | (0 to 2) | 0 | (0 to 0.5) | 1 (P = 0.27) |
| Incidence of coma grade III (4 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 7 | 34 | 6 | 43 | 1.48 (0.55 to 3.98) |
| Incidence of coma grade IV (4 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 1 | 34 | 7 | 43 | 0.18 (0.02 to 1.40) |
| Incidence of coma grade III (8 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 2 | 34 | 2 | 43 | 1.26 (0.19 to 8.52) |
| Incidence of coma grade IV (8 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 0 | 34 | 2 | 43 | 0.16 (0.01 to 2.70) |
| Incidence of coma grade III (24 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Hultén 1988 | 0 | 34 | 0 | 43 | Not estimable |
| Incidence of coma grade IV (24 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Hultén 1988 | 0 | 34 | 0 | 43 | Not estimable |
| Incidence of need for cardiac pacing/antitoxin | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Eddleston 2008 | 101 | 549 | 101 | 555 | 1.01 (0.79 to 1.30) |
| Incidence of need for respirator | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 2 | 34 | 9 | 43 | 0.28 (0.06 to 1.22) |
| Incidence of systolic blood pressure < 100 mmHg (4 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 4 | 34 | 8 | 43 | 0.63 (0.21 to 1.92) |
| Incidence of systolic blood pressure < 100 mmHg (8 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 1 | 34 | 5 | 43 | 0.25 (0.03 to 2.06) |
| Incidence of systolic blood pressure < 100 mmHg (24 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 0 | 34 | 2 | 43 | 0.16 (0.01 to 2.70) |
| Incidence of heart rate > 100 bpm (4 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 10 | 34 | 15 | 43 | 0.84 (0.44 to 1.63) |
| Incidence of heart rate > 100 bpm (8 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 8 | 34 | 10 | 43 | 1.01 (0.45 to 2.28) |
| Incidence of heart rate > 100 bpm (24 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 10 | 34 | 10 | 43 | 1.26 (0.60 to 2.68) |
| Incidence of cardiac arrhythmias (4 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 1 | 34 | 4 | 43 | 0.32 (0.04 to 2.70) |
| Incidence of cardiac arrhythmias (8 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 1 | 34 | 3 | 43 | 0.42 (0.05 to 3.87) |
| Incidence of cardiac arrhythmias (24 h after admission) | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 1 | 34 | 2 | 43 | 0.63 (0.06 to 6.68) |
| Incidence of intubation > 8 h | |||||
| SDAC + hospital treatment | Hospital treatment | ||||
| Study | Events | Total | Events | Total | RR (95% CI) |
| Hultén 1988 | 4 | 34 | 9 | 43 | 0.56 (0.19 to 1.67) |
| B. First aid interventions that evacuate the poison from the gastrointestinal tract | |||||
| Syrup of ipecac vs no intervention | |||||
| Incidence of referrals to the emergency department | |||||
| Syrup of ipecac | No intervention | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Wax 1999 | 1 | 51 | 0 | 52 | 7.54 (0.15 to 378.83) |
| Incidence of hospitalizations | |||||
| Syrup of ipecac | No intervention | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Wax 1999 | 0 | 51 | 0 | 52 | Not estimable |
| SDAC + cathartic vs SDAC (higher dose) | |||||
| Incidence of adverse events (6 mL vs 4 mL) | |||||
| SDAC + 6 mL cathartic | SDAC + 4 mL cathartic | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Sue 1994 | 0 | 16 | 0 | 16 | Not estimable |
| Incidence of adverse events (8 mL vs 4 mL) | |||||
| SDAC + 8 mL cathartic | SDAC + 4 mL cathartic | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Sue 1994 | 0 | 18 | 0 | 16 | Not estimable |
| Incidence of adverse events (8 mL vs 6 mL) | |||||
| SDAC + 8 mL cathartic | SDAC + 6 mL cathartic | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Sue 1994 | 0 | 18 | 0 | 16 | Not estimable |
| Incidence of hospitalization (6 mL vs 4 mL) | |||||
| SDAC + 6 mL cathartic | SDAC + 4 mL cathartic | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Sue 1994 | 1 | 16 | 0 | 16 | 7.39 (0.15 to 372.38) |
| Incidence of hospitalization (8 mL vs 4 mL) | |||||
| SDAC + 8 mL cathartic | SDAC + 4 mL cathartic | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Sue 1994 | 2 | 18 | 0 | 16 | 7.01 (0.42 to 117.63) |
| C. Combined first aid interventions that limit uptake and promote removal of the poison | |||||
| MDAC + cathartic + hospitalintervention vs hospitalintervention | |||||
| Incidence of adverse events | |||||
| MDAC + cathartic + hospital intervention | Hospital intervention | ||||
| Study | Events | Total | Events | Total | Peto OR (95% CI) |
| Montoya‐Cabrera 1999 | 0 | 7 | 0 | 7 | Not estimable |
| T1/2 (h) | |||||
| MDAC + cathartic + hospital intervention (N = 7) | Hospital intervention (N = 7) | ||||
| Study | Mean | SD | Mean | SD | MD (95% CI) |
| Montoya‐Cabrera 1999 | 10 | N/A | 17 | N/A | −7 (not estimable) |
bpm: beats per minute; CI: confidence interval; IQR: interquartile range; OR: odds ratio; RR: risk ratio; MDAC; multi‐dose activated charcoal; SDAC: single‐dose activated charcoal.
Secondary outcomes
Duration of symptoms
The identified studies either did not collect or did not report outcomes related to symptom duration.
Drug absorption
Underhill 1990 measured drug levels of paracetamol before treatment and at several time points after treatment. However, the study reported none of the pre‐defined outcomes of interest in our protocol, nor could we derive them from the data provided; therefore we could make no reliable estimation of drug absorption from the available data.
Incidence of hospitalization
The identified studies either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
Merigian 1990 reported the number of participants admitted to the ICU department, which was 1/220 in the intervention group and 0/231 participants in the control group (Peto OR 7.77; 95% CI 0.15 to 391.93; Table 9). We assessed this evidence as being of very low certainty, downgraded for limitations in study design, imprecision due to a low number of events and indirectness.
Summary for this comparison
We were uncertain about the effect of SDAC compared to no intervention on the incidence of ICU admission or the incidence of clinical deterioration (very low certainty due to limitations in study design, imprecision and indirectness). One study described a single type of adverse event in response to the treatment, vomiting, but we are uncertain about the effect (very low‐certainty evidence due to limitations in study design, imprecision and indirectness).
2. Single‐dose activated charcoal plus hospital intervention versus hospital intervention alone
Seven trials used single‐dose activated charcoal (SDAC) in adjuvant to established hospital treatments (Comstock 1982; Cooper 2005; Crome 1983; Eddleston 2008; Hultén 1988; Merigian 2002; Roberts 2006). These hospital treatments consisted of supportive treatments to maintain vital parameters plus poison‐specific treatments, but in most studies this also included gastric lavage (Comstock 1982; Crome 1983; Eddleston 2008; Hultén 1988; Roberts 2006). Crome 1983 and Hultén 1988 specifically included participants with tricyclic antidepressant overdose, while the other studies did not define a specific toxic syndrome. Roberts 2006 investigated drug uptake in a subpopulation of participants entering the Eddleston 2008 study, with yellow oleander seed poisoning. For a detailed summary of the outcomes, we refer to the Data and analyses section. Below we provide a narrative overview. See Table 2.
Primary outcomes
Incidence of mortality
Two studies reported the impact of SDAC on mortality (Cooper 2005; Eddleston 2008). Both studies included participants with a variety of toxic syndromes, so we considered it appropriate to pool these results. Moreover, from a layperson's perspective, it is usually impossible to distinguish different toxic syndromes, let alone to decide on the appropriateness of administering SDAC in case of a specific syndrome. The statistical results were mainly determined by the large study, Eddleston 2008 (Peto OR 1.04, 95% CI 0.79 to 1.37; 3425 participants; 2 studies; Analysis 2.1). We did not identify important heterogeneity (P = 0.30, I² = 7%). Evidence was of low certainty, downgraded for imprecision due to wide confidence intervals and indirectness.
2.1. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 1 Incidence of mortality.
Adverse events
Three studies reported on adverse events in relation to administering SDAC (Cooper 2005; Eddleston 2008; Merigian 2002). Two studies reported the occurrence of vomiting (Cooper 2005; Merigian 2002), while Eddleston 2008 reported the absence of bowel sounds as a proxy for constipation. We considered a combined estimate of adverse events to be appropriate, given the wide variety in toxic syndromes included in the different studies and the inability of laypeople to distinguish between different toxic syndromes. However, this resulted in considerable heterogeneity (P = 0.002, I² = 83%) with different directions of effect. We performed a subgroup analysis by reported symptom, showing between‐group differences (P = 0.02, I² = 83%), which decreased but did not fully eliminate the heterogeneity. The risk ratio for the outcome occurrence of vomiting was 1.44 (95% CI 0.88 to 2.37; 1806 participants; 2 studies) and still showed substantial unexplained heterogeneity (P = 0.08, I² = 68%). The RR for the sub‐outcome, absence of bowel sounds, was 0.41 (95% CI 0.17 to 1.00; 3098 participants; 1 study; Analysis 2.2). The evidence on adverse events was of very low certainty because of inconsistency between studies, imprecision (low number of events) and indirectness.
2.2. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 2 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
One study evaluated the incidence of clinical deterioration, which was absent in both an intervention group of 455 participants and a control group of 1075 participants (Merigian 2002; Table 9). This evidence was of very low certainty, downgraded due to limitations in study design, imprecision (low number of events) and indirectness.
Crome 1983 and Hultén 1988 expressed the grade of coma using the Matthew‐Lawson coma scale at 4 h, 8 h and 24 h after hospital admission. The median coma score scales of intervention and control groups were similar in the study of 16 participants (Crome 1983; Table 9). The proportion of participants with a coma scale of III or IV were also similar in Hultén 1988, with 77 participants (Table 9). Evidence was of very low certainty, downgraded for limitations in study design, imprecision due to a low number of events and a low sample size and indirectness.
Eddleston 2008 reported the incidence of participants with yellow oleander seed poisoning, who needed specialized treatment, namely cardiac pacing or Fab antitoxin treatment (Eddleston 2008). The risk ratio was 1.01 (95% CI 0.79 to 1.30, P = 0.93, 1104 participants; Table 9). This evidence was of low certainty, downgraded for imprecision due to a low number of events and indirectness.
Four studies reported on the need for intubation and/or ventilation (Cooper 2005; Eddleston 2008; Hultén 1988; Merigian 2002). In Merigian 2002 these data were only available for participants admitted to the ICU. We considered it appropriate to combine the results, given the multiple or unspecified toxic syndromes included in three out of four individual studies, and the inability of laypeople to distinguish between ingested toxins.The combined result showed substantial heterogeneity and different directions of effect, so we do not present it (P = 0.04; I² = 63%; Analysis 2.3). A possible reason for the observed heterogeneity may have been the co‐interventions, i.e. whether participants received gastric lavage as part of the hospital treatments. A subgroup analysis suggested there may be between‐group differences between participants who received gastric lavage prior to receiving SDAC and those who did not (P = 0.005, I² = 87.6%). The summary estimate in the subgroup with gastric lavage was RR 0.95 (95% CI 0.70 to 1.27, 3175 participants, 2 studies, P = 0.71). In the subgroup without gastric lavage the RR was 2.61 (95% CI 1.38 to 4.93, 387 participants, 2 studies, P = 0.003), in favour of not receiving SDAC. In addition, Hultén 1988 made a subcomparison of the need for ventilation with a respirator (RR 0.28, 95% CI 0.06 to 1.22, 77 participants, P = 0.09; Table 9). Evidence on ventilation was of low certainty, downgraded for imprecision (low number of events and wide confidence intervals) and indirectness.
2.3. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 3 Incidence of need for intubation.
Two studies, Eddleston 2008 and Hultén 1988, studied the incidence of convulsions. We considered combining these outcomes appropriate, given the inability of laypeople to make a distinction between different toxic syndromes. A combined estimate of these studies, however, had substantial heterogeneity (P = 0.03; I² = 79%) and a different direction of effect, so we only report the individual study results. The individual estimates were RR 1.87 (95% CI 0.75 to 4.67, 3098 participants; Eddleston 2008) and RR 0.28 (95% CI 0.06 to 1.22, 77 participants; Hultén 1988; Analysis 2.4). Exploring heterogeneity in a meta‐analysis with only two studies is difficult, so explanations for the observed differences between studies remain speculative. Possible reasons might be the small study sample in Hultén 1988. Alternatively, true differences in patient population might explain the differences, as Hultén 1988 specifically recruited participants with tricyclic antidepressant poisoning, while most participants in Eddleston 2008 took an overdose of pesticides or yellow oleander seeds. This evidence was of very low certainty, downgraded for inconsistency between studies, imprecision (wide confidence intervals and low number of events) and indirectness.
2.4. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 4 Incidence of convulsions.
Hultén 1988 recorded some additional clinical parameters in their 77 participants, as measures of poisoning severity: systolic blood pressure, heart rate and incidence of cardiac arrhythmias, at 4 h, 8 h and 24 h after treatment (Table 9). These numbers were not shown to differ between treatments. The certainty of evidence for these parameters was rated as very low, due to limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
Secondary outcomes
Duration of symptoms
Three studies measured length of intubation (Eddleston 2008; Hultén 1988; Merigian 2002). Combination of the studies' results in a meta‐analysis was not feasible, due to differences in reporting. Eddleston 2008 reported medians with interquartile ranges, and there was no demonstrable difference. Authors did not report the number of participants in this analysis. Merigian 2002 reported length of intubation as means without a measure of spread or the number of participants in the analysis, and likewise, authors could not show a difference between intervention and control (Analysis 2.5). Hultén 1988 reported the proportion of participants that were intubated for longer than 8 h, which was similar between intervention and control (RR 0.56, 95% CI 0.19 to 1.67, 77 participants, P = 0.30; Hultén 1988; Table 9). This evidence was rated to be of low certainty, downgraded for imprecision due to a low number of events or lack of data, plus indirectness.
2.5. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 5 Duration of intubation (h).
| Duration of intubation (h) | ||||
|---|---|---|---|---|
| Study | SDAC | No SDAC | Summary estimate (P value) | # participants |
| Eddleston 2008 | median (IQR): 112.0 (36.6–234.9) | median (IQR): 88.5 (38.5–203.1) | median difference: 23.5 (P > 0.05) | No information |
| Merigian 2002 | mean: 54.6 | mean: 39.9 | mean difference: 14.7 (P = 0.70) | No information |
Drug absorption
Three included studies measured drug absorption (Comstock 1982; Hultén 1988; Roberts 2006). Comstock 1982, with 339 participants, only reported increases in blood drug concentrations over time, without reporting any of our pre‐specified outcomes of interest. Hultén 1988 presented the course of tricyclic antidepressant levels in the blood of 77 participants graphically, and reported narratively that there was no demonstrable difference in AUC, Cmax or T1/2 between treatments (Hultén 1988). Therefore, the only numeric data available were on the AUC, Cmax and Tmax of cardenolides from yellow oleander seeds, measured by Roberts 2006 in a subset of 68 participants from the Eddleston study. Authors reported results as median with interquartile ranges (IQR) and could not show a difference between treatments (Analysis 2.6; Analysis 2.7; Analysis 2.8). The evidence on the pharmacokinetic parameters was of very low certainty, due to limitations in study design, imprecision (low sample size) and indirectness.
2.6. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 6 AUC ((µg/L) × h).
| AUC ((µg/L) × h) | ||||
|---|---|---|---|---|
| Study | SDAC (median (IQR)) | no SDAC (median (IQR)) | Summary estimate (P value) | # participants |
| Roberts 2006 | 17.7 (11.1;21.8) | 19.0 (13.7;24.3) | ‐1.3 (P > 0.05) | 28 vs 40 |
2.7. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 7 Cmax (µg/L).
| Cmax (µg/L) | ||||
|---|---|---|---|---|
| Study | SDAC (median (IQR)) | no SDAC (median (IQR)) | Summary estimate (P value) | # participants |
| Roberts 2006 | 0.98 (0.72;1.50) | 1.05 (0.75;1.40) | ‐0.07 (P > 0.05) | 28 vs 40 |
2.8. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 8 Tmax (h).
| Tmax (h) | ||||
|---|---|---|---|---|
| Study | SDAC (median (IQR)) | no SDAC (median (IQR)) | summary estimate (P value) | # participants |
| Roberts 2006 | 7.2 (5.7;13.8) | 12.1 (5.4;17.4) | ‐4.9 (P > 0.05) | 28 vs 40 |
Incidence of hospitalization
Merigian 2002 reported the incidence of hospitalization. The results favoured no treatment over SDAC (RR 1.57, 95% CI 1.22 to 2.02, 1479 participants, P < 0.001; Analysis 2.9). We assessed this evidence as being of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
2.9. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 9 Incidence of hospitalization.
Incidence of ICU admission
Merigian 2002 reported the incidence of ICU admission in favour of no treatment with SDAC (RR 2.33, 95% CI 1.42 to 3.82, 1479 participants, P < 0.001; Analysis 2.10). This evidence was of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
2.10. Analysis.
Comparison 2 SDAC + hospital intervention vs hospital intervention, Outcome 10 Incidence of ICU admission.
Summary for this comparison
SDAC as adjuvant to supportive hospital treatments may have little or no influence on one of our primary outcomes, incidence of mortality, while we are uncertain about its effect on another primary outcome, adverse events due to the intervention. In addition, SDAC plus hospital treatments may have little or no influence on the primary outcomes of need for intubation, need for cardiac pacing or antitoxin treatment in cases of yellow oleander poisoning, or the secondary outcome, length of intubation. We are uncertain about the effect of SDAC in addition to hospital treatments on the incidence of clinical deterioration, Matthew‐Lawson coma scale scores, incidence of convulsions, blood pressure, heart rate, cardiac arrhythmias, and the secondary outcomes of drug absorption and incidence of hospital or ICU admission. The evidence collected is of low to very low certainty, due to limitations in study design, indirectness and/or imprecision.
3. Multi‐dose activated charcoal plus hospital intervention versus single‐dose activated charcoal plus hospital intervention
Five trials compared single‐dose (SDAC) versus multi‐dose activated charcoal (MDAC), in adjuvant to hospital treatments (Behnoush 2009; Brahmi 2006; De Silva 2003; Eddleston 2008; Roberts 2006). The identified trials studied the following toxic overdoses: carbamazepine (Behnoush 2009; Brahmi 2006), yellow oleander (De Silva 2003; Roberts 2006), and a combination of toxic syndromes (Eddleston 2008). In all studies except for Brahmi 2006, supportive treatments included gastric lavage. Behnoush 2009 did not report any outcomes of interest for this review. See Table 3.
Primary outcomes
Incidence of mortality
Incidence of mortality was an outcome of interest in De Silva 2003 and Eddleston 2008. We considered it appropriate to combine the findings given the similar populations studied. Nevertheless, the meta‐analysis resulted in an estimate with substantial heterogeneity (P = 0.04, I² = 76%), albeit the same direction of effect. The pooled risk ratio was 0.59 (95% CI 0.21 to 1.63; 3476 participants; 2 studies; Analysis 4.1). Reasons for the observed heterogeneity were not immediately clear, as both the provided interventions and the population studied are remarkably similar. Eddleston 2008 included a broader range of toxic syndromes; however, including only the subpopulation of participants with yellow oleander poisoning (SDAC: 26/549 and MDAC: 23/541) would not change the conclusions made (P = 0.06, I² = 71%). There are some factors that might explain the differences observed, such as a longer treatment in De Silva 2003 (activated charcoal up to 72 h) compared to Eddleston 2008 (activated charcoal up to 24 h) or differences in the compliance rate with the treatment (reported to drop to 66% by the final dose by Eddleston 2008 but claimed to be ensured in all cases by De Silva 2003). However, these explanations remain speculative, and if there are more studies in a future update, we may be able to show more robust evidence and clarify the heterogeneity issue. We considered the evidence here to be of very low certainty, due to inconsistency between studies, imprecision (wide confidence intervals) and indirectness.
4.1. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 1 Incidence of mortality.
Adverse events
Two studies reported the incidence of adverse events in response to the intervention (De Silva 2003; Eddleston 2008). We considered combining the results of these studies appropriate, given the similar study population. The combined result (Peto OR 3.55, 95% CI 1.85 to 6.79; 3476 participants; 2 studies) contained a substantial degree of heterogeneity (P = 0.08, I² = 66.8%), but with the same direction of effect. Reasons for this heterogeneity remain speculative, but it could be due to differing definitions and diagnostic methods for adverse events, for example absent bowel sounds in Eddleston 2008 (Peto OR 2.34, 95% CI 1.05 to 5.21; 3075 participants, P = 0.04) versus abdominal discomfort/diarrhoea in De Silva 2003 (Peto OR 7.82, 95% CI 2.59 to 23.58; 401 participants, P < 0.001; Analysis 4.2). Both studies suggest that the number of adverse events may increase in case of MDAC, compared to SDAC. This evidence was of low certainty, downgraded for imprecision (low number of events and wide confidence intervals) and indirectness.
4.2. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 2 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
Both De Silva 2003 and Eddleston 2008 reported on the need for cardiac pacing or administration of a Fab antitoxin, treatments used in severe cases of yellow oleander poisoning. We considered it appropriate to combine results, given the similar patient populations studied. The combined effect estimate (RR 0.26, 95% CI 0.02 to 4.18; 1490 participants; 2 studies) resulted in considerable heterogeneity (P = 0.005, I² = 87%; Analysis 4.3). As for mortality, the reasons for the observed heterogeneity are not immediately clear and remain speculative. We considered evidence to be of very low certainty, due to imprecision (low numbers of events and wide confidence intervals), inconsistency between studies and indirectness.
4.3. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 3 Incidence of need for cardiac pacing/antitoxin treatment.
De Silva 2003 also recorded the incidence of life‐threatening arrhythmias after 24 h, which may be lower for the group receiving SDAC (RR 0.21, 95% CI 0.06 to 0.71, 385 participants, P = 0.01; Analysis 4.4). Evidence was of low certainty, downgraded for imprecision due to a low number of events and indirectness.
4.4. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 4 Incidence of life‐threatening arrhythmias.
Another outcome De Silva 2003 reported in their study with 401 participants was the need for atropine, expressed as both the amount of atropine administered and the number of boluses administered. Both the amount (mg) of atropine administered (MD −1.60, 95% CI −2.25 to −0.95, P < 0.001; Analysis 4.5) and the median number of boluses (Analysis 4.6) were higher in the group receiving SDAC. We assessed the evidence as being of moderate certainty, downgraded for indirectness.
4.5. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 5 Amount of atropine administered (mg).
4.6. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 6 Number of atropine boluses administered.
| Number of atropine boluses administered | |||||
|---|---|---|---|---|---|
| Study | MDAC (median (range)) | SDAC (median (range)) | Median difference [95% CI] | P value | # participants |
| De Silva 2003 | 1 (1‐6) | 2 (1‐12) | 0.0 (0.0‐1.0) | P < 0.0001 | 201 vs 200 |
Two studies reported the need for intubation (Brahmi 2006; Eddleston 2008). We decided to combine these results, as from the point of view of laypeople, the focus of this review, it is usually impossible to distinguish between toxic syndromes or adapt the provided intervention accordingly. There may be little or no effect on the need for intubation (RR 1.01, 95% CI 0.75 to 1.38, 3097 participants, P = 0.93; Analysis 4.7). There was no heterogeneity between studies (P = 0.98, I² = 0%). The evidence was of low certainty, downgraded due to imprecision (low number of events and wide confidence intervals) and indirectness.
4.7. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 7 Incidence of need for intubation.
Finally, Eddleston 2008 reported the incidence of convulsions (RR 1.09, 95% CI 0.52 to 2.32, 3085 participants, P = 0.82; Analysis 4.8). Evidence was of low certainty, downgraded due to imprecision (low number of events and wide confidence intervals) and indirectness.
4.8. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 8 Incidence of convulsions.
Secondary outcomes
Duration of symptoms
One study in six participants reported the duration of coma as a measure of symptom duration (Brahmi 2006). The mean difference was −9.00 h (95% CI −14.79 to −3.21, P = 0.002), in favour of MDAC (Analysis 4.9). We considered this to be evidence of very low certainty, downgraded for limitations in study design, imprecision (small sample size) and indirectness.
4.9. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 9 Duration of coma (h).
Brahmi 2006 and Eddleston 2008 also reported duration of intubation. Due to differences in reporting, it was not possible to combine the estimates. The mean difference reported by Brahmi 2006 is −12.30 h (95% CI −18.56 to −6.04, 6 participants, P < 0.001). Eddleston 2008 reported medians with IQR; however, authors made no statement of effectiveness. The number of participants in the analysis was not clear either (Analysis 4.10). This evidence was of very low certainty, downgraded for inconsistency between studies, imprecision (low sample size and lack of data) and indirectness.
4.10. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 10 Duration of intubation (h).
| Duration of intubation (h) | |||||
|---|---|---|---|---|---|
| Study | MDAC | SDAC | Summary estimate | P value | # participants |
| Brahmi 2006 | mean±SD: 24.1±4.2 | mean±SD: 36.4±3.6 | MD: ‐12.30, 95%CI [‐18.56;‐6.04] | P = 0.0001 | 3 vs 3 |
| Eddleston 2008 | median [IQR]: 83.8 (35.0–173.0) | median [IQR]: 112.0 (36.6–234.9) | median difference: ‐28.2 | No information | No information |
Drug absorption
Two studies, Brahmi 2006 and Roberts 2006, reported on pharmacokinetic parameters as measures of drug absorption. Differences in reporting precluded meta‐analysis.
Both studies reported the Cmax. The mean difference reported by Brahmi 2006 was 0.40 mg/L (95% CI −4.89 to 5.69, 6 participants, P = 0.88). Roberts 2006 reported Cmax, as medians with IQR, demonstrating no difference between intervention and control (participants = 64, P > 0.05; Analysis 4.11). AUC and Tmax were similar between treatments (64 participants, P > 0.05; Analysis 4.12; Analysis 4.14). In addition, Brahmi 2006 measured the T1/2 which was in favour of MDAC (MD −15.32 h (95% CI −21.84 to −8.80, 6 participants, P < 0.001; Analysis 4.13). The evidence was of very low certainty, downgraded for limitations in study design, imprecision (low sample size) and indirectness.
4.11. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 11 Cmax (µg/L).
| Cmax (µg/L) | |||||
|---|---|---|---|---|---|
| Study | MDAC | SDAC | Summary estimate | P value | # participants |
| Brahmi 2006 | mean±SD: 33±3.46 | mean±SD: 32.6±5.63 | MD: 0.40, 95%CI [‐4.89;5.69] | P = 0.88 | 6 vs 6 |
| Roberts 2006 | median (IQR): 1.13 (0.86;1.47) | median (IQR): 0.98 (0.72;1.50) | median difference: 0.15 | P > 0.05 | 36 vs 28 |
4.12. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 12 Tmax (h).
| Tmax (h) | |||||
|---|---|---|---|---|---|
| Study | MDAC | SDAC | Summary estimate | P value | # participants |
| Roberts 2006 | median (IQR): 8.3 (4.8;15.0) | median (IQR): 7.2 (5.7;13.8) | median difference: 1.1 | P > 0.05 | 36 vs 28 |
4.14. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 14 AUC ((µg/L) × h).
| AUC ((µg/L) × h) | |||||
|---|---|---|---|---|---|
| Study | MDAC | SDAC | Summary estimate | P value | # participants |
| Roberts 2006 | median (IQR): 17.3 (12.8;21.7) | median (IQR): 17.7 (11.1;21.8) | median difference: ‐0.4 | P > 0.05 | 36 vs 28 |
4.13. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 13 T1/2 (h).
Incidence of hospitalization
The identified studies either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
De Silva 2003 reported the incidence of ICU admissions. The risk ratio suggests MDAC may have a strong effect on reducing ICU admissions (RR 0.31, 95% CI 0.12 to 0.83, 401 participants, P = 0.02; Analysis 4.15). The presented evidence was of low certainty, downgraded for imprecision (low number of events) and indirectness.
4.15. Analysis.
Comparison 4 MDAC + hospital intervention vs SDAC + hospital intervention, Outcome 15 Incidence of ICU admission.
Summary for this comparison
The evidence that we have collected concerning the use of single‐ versus multi‐dose activated charcoal in adjuvant to hospital treatments is of moderate to very low certainty. There may be little or no difference in the incidence of convulsions and the need for intubation between MDAC plus hospital treatments and SDAC plus hospital treatments. On the other hand, there may be a favourable effect for MDAC on the incidence of life‐threatening cardiac arrhythmias and ICU admissions, while it probably decreases the number of atropine boluses and total amount of atropine administered. Low‐certainty evidence suggests, however, that MDAC may come with an increased risk of adverse events. We are uncertain about the effects of MDAC plus hospital treatments on mortality, the need for cardiac pacing or antitoxin treatment, symptom duration and drug absorption.
4. Single‐dose activated charcoal versus syrup of ipecac
One study with 34 participants compared SDAC versus syrup of ipecac in oral poisoning participants with mild levels of intoxication (defined as a Glasgow Coma Scale score of more than 12; Amigó Tadín 2002). The study specifically included participants presenting with oral overdoses of anti‐inflammatory, psychotropic or analgesic drugs. Participants received no additional treatments. See Table 4.
Primary outcomes
Incidence of mortality
The identified study did not collect outcomes related to mortality.
Adverse events
Amigó Tadín 2002 reported the incidence of adverse events encountered (RR 1.24, 95% CI 0.26 to 5.83, 34 participants, P = 0.79; Analysis 3.1). Evidence was of very low certainty, downgraded for limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
3.1. Analysis.
Comparison 3 SDAC vs syrup of ipecac, Outcome 1 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
The identified study measured participants' poisoning symptoms 1 h after treatment (Amigó Tadín 2002). The mean difference in Glasgow Coma Scale scores between treatments was −0.15 (95% CI −0.43 to 0.13, 34 participants, P = 0.29; Analysis 3.2). Furthermore, the study reported mean arterial blood pressure (MD 7.00 mmHg, 95% CI −3.56 to 17.56, 34 participants, P = 0.19; Analysis 3.3), heart rate (MD −2.39 bpm, 95% CI −15.58 to 10.80, 34 participants, P = 0.72; Analysis 3.4) and respiratory rate (MD 1.12 breaths/min, 95% CI −1.69 to 3.93, 34 participants, P = 0.44; Analysis 3.5) as measures of intoxication. The collected evidence was of very low certainty, downgraded for limitations in study design, imprecision (low sample size) and indirectness.
3.2. Analysis.
Comparison 3 SDAC vs syrup of ipecac, Outcome 2 Glasgow Coma Scale score.
3.3. Analysis.
Comparison 3 SDAC vs syrup of ipecac, Outcome 3 Mean arterial blood pressure (mmHg).
3.4. Analysis.
Comparison 3 SDAC vs syrup of ipecac, Outcome 4 Heart rate (bpm).
3.5. Analysis.
Comparison 3 SDAC vs syrup of ipecac, Outcome 5 Respiratory rate (breaths/min).
Secondary outcomes
Duration of symptoms
The identified study did not collect outcomes related to symptom duration.
Drug absorption
The identified study did not collect outcomes related to drug absorption.
Incidence of hospitalization
The identified study did not collect outcomes related to incidence of hospitalization.
Indicence of ICU admission
The identified study did not collect outcomes related to incidence of ICU admission.
Summary for this comparison
We identified evidence of very low certainty, originating from one study (Amigó Tadín 2002). We are uncertain about any difference between SDAC and syrup of ipecac concerning poisoning symptoms or incidence of adverse events.
5. Multi‐dose activated charcoal plus hospital treatment versus hospital treatment alone
We found three studies comparing the administration of MDAC in adjuvant to hospital treatments versus hospital treatments alone (Bouget 1989; Eddleston 2008; Roberts 2006). For most participants in Eddleston 2008, who had a variety of intoxications, hospital treatments included gastric lavage. Roberts 2006 studied a subgroup of participants from the Eddleston study, those with yellow oleander seed poisoning. Bouget 1989 included 36 participants with benzodiazepine poisoning, but no numeric outcomes were reported in this study. See Table 5.
Primary outcomes
Incidence of mortality
Eddleston 2008 reported the incidence of mortality, which may not differ between intervention and control (RR 0.94, 95% CI 0.72 to 1.22, 3085 participants, P = 0.64; Analysis 5.1). The evidence was of low certainty, downgraded for imprecision (low number of events and wide confidence intervals) and indirectness.
5.1. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 1 Incidence of mortality.
Adverse events
Eddleston 2008 reported one adverse event, incidence of absent bowel sounds (RR 1.02, 95% CI 0.52 to 1.98, 3085 participants, P = 0.97; Analysis 5.2). Evidence was of low certainty, downgraded for imprecision (low number of events and wide confidence intervals) and indirectness.
5.2. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 2 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
Eddleston 2008 reported several markers of intoxication, including the need for intubation (RR 0.97, 95% CI 0.71 to 1.33, 3085 participants, P = 0.87; Analysis 5.3), seizures (RR 2.03, 95% CI 0.82 to 5.02, 3085 participants, P = 0.12; Analysis 5.4) and need for cardiac pacing/Fab antitoxin treatment in participants with yellow oleander poisoning (RR 0.86, 95% CI 0.66 to 1.13, 1095 participants, P = 0.28; Analysis 5.5). Evidence was of low certainty, downgraded for imprecision (low number of events and wide confidence intervals) and indirectness.
5.3. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 3 Incidence of need for intubation.
5.4. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 4 Incidence of seizures.
5.5. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 5 Incidence of need for cardiac pacing/antitoxin treatment.
Secondary outcomes
Duration of symptoms
Eddleston 2008 reported length of intubation as a measure of symptom duration. It was expressed as a median plus IQR, and it may not be different between groups (Analysis 5.6). The evidence was of low certainty, downgraded for imprecision (lack of data) and indirectness.
5.6. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 6 Length of intubation (h).
| Length of intubation (h) | |||||
|---|---|---|---|---|---|
| Study | MDAC (median IQR) | no intervention (median IQR) | Summary estimate | P value | # participants |
| Eddleston 2008 | 83.8 (35.0–173.0) | 88.5 (38.5–203.1) | median difference: ‐4.7 | P > 0.05 | No information |
Drug absorption
Roberts 2006 analysed cardenolide pharmacokinetic parameters in a subgroup of 76 participants from the Eddleston study, with yellow oleander poisoning. AUC, Cmax and Tmax were reported as medians with IQR, and there was no demonstrable difference between treatment groups (Analysis 5.7; Analysis 5.8; Analysis 5.9). Evidence was of very low certainty due to limitations in study design, imprecision (low sample size) and indirectness.
5.7. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 7 AUC.
| AUC | |||||
|---|---|---|---|---|---|
| Study | MDAC (median IQR) | no intervention (median IQR) | Summary estimate | P value | # participants |
| Roberts 2006 | 17.3 (12.8;21.7) | 19.0 (13.7;24.3) | median difference: ‐1.7 | P > 0.05 | 36 vs 40 |
5.8. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 8 Cmax.
| Cmax | |||||
|---|---|---|---|---|---|
| Study | MDAC (median IQR) | no intervention (median IQR) | Summary estimate | P value | # participants |
| Roberts 2006 | 1.13 (0.86;1.47) | 1.05 (0.75;1.40) | median difference: 0.08 | P > 0.05 | 36 vs 40 |
5.9. Analysis.
Comparison 5 MDAC + hospital intervention vs hospital intervention, Outcome 9 Tmax.
| Tmax | |||||
|---|---|---|---|---|---|
| Study | MDAC (median IQR) | no intervention (median IQR) | Summary estimate | P value | # participants |
| Roberts 2006 | 8.3 (4.8;15.0) | 12.1 (5.4;17.4) | median difference: ‐3.8 | P > 0.05 | 36 vs 40 |
Incidence of hospitalization
The identified studies either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
The identified studies either did not collect or did not report outcomes related to incidence of ICU admission.
Summary for this comparison
The identified evidence on the use of MDAC in addition to hospital treatment is of low to very low certainty and originates from two studies, one of which is a subgroup analysis of the larger study (Eddleston 2008; Roberts 2006). MDAC plus hospital treatments may not be better than hospital treatments alone for mortality, symptoms of intoxication (need for intubation, seizures, cardiac pacing or antitoxin treatment), duration of intubation or absence of bowel sounds as adverse events. We are uncertain about the effects of MDAC on pharmacokinetic parameters of cardenolides.
B. First aid interventions that evacuate the poison from the gastrointestinal tract
B1. Emetics
6. Syrup of ipecac versus no intervention
One study compared ipecac versus observation in 103 cases of paediatric, asymptomatic toxic berry ingestion (Wax 1999). This study took place in a pre‐hospital setting and did not include any other treatments. See Table 6.
Primary outcomes
Incidence of mortality
The identified study did not collect outcomes related to incidence of mortality.
Adverse events
The identified study reported on the incidence of several adverse events separately. As it was likely that one patient could encounter multiple adverse events, it was not possible to combine these into a composite outcome. Therefore, we present the risk ratios for the individually described adverse events. Participants receiving ipecac may show an increased risk of diarrhoea (RR 4.08, 95% CI 1.66 to 10.04, 103 participants, P = 0.002; Analysis 6.1) and sedation (RR 5.10, 95% CI 1.17 to 22.13, 103 participants, P = 0.03; Analysis 6.3), while there may be little or no difference for abdominal pain (RR 1.02, 95% CI 0.07 to 15.87, 103 participants, P = 0.99; Analysis 6.2) or agitation (RR 1.53, 95% CI 0.27 to 8.77, 103 participants, P = 0.63; Analysis 6.4). The evidence was of low certainty, downgraded for limitations in study design and imprecision (low number of events and wide confidence intervals).
6.1. Analysis.
Comparison 6 Syrup of ipecac vs no intervention, Outcome 1 Incidence of diarrhoea.
6.3. Analysis.
Comparison 6 Syrup of ipecac vs no intervention, Outcome 3 Incidence of sedation.
6.2. Analysis.
Comparison 6 Syrup of ipecac vs no intervention, Outcome 2 Incidence of abdominal pain.
6.4. Analysis.
Comparison 6 Syrup of ipecac vs no intervention, Outcome 4 Incidence of agitation.
Incidence and severity of symptoms of poisoning
The identified study did not collect outcomes related to incidence and severity of poisoning symptoms.
Secondary outcomes
Duration of symptoms
The identified study did not collect outcomes related to duration of poisoning symptoms.
Drug absorption
The identified study did not collect outcomes related to drug absorption.
Incidence of hospitalization
One patient in the intervention group was referred to the emergency department, compared to none in the control group (Wax 1999). The Peto OR was 7.54 (95% CI 0.15 to 379.83, 103 participants, P = 0.31; Table 9). None of the 103 participants were hospitalized (Table 9). The evidence was of low certainty, downgraded for limitations in study design and imprecision due to a small sample size and wide confidence intervals.
Incidence of ICU admission
As none of the participants were hospitalized, none could have been admitted to the ICU.
Summary for this comparison
We identified one study that compared syrup of ipecac versus home observation (Wax 1999). Authors did not report any clinical outcomes, but there may be little or no difference in emergency department referrals. In contrast, the incidence of adverse events (diarrhoea and sedation) may be larger. Evidence is of low certainty (downgraded for limitations in study design and imprecision).
7. Syrup of ipecac plus single‐dose activated charcoal plus cathartics versus single‐dose activated charcoal plus cathartics
We identified four studies that compared SDAC plus a cathartic (sorbitol or magnesium sulphate), preceded or not preceded by syrup of ipecac in participants presenting to an emergency department (Albertson 1989; Kornberg 1991; Kulig 1985; Pond 1995). None of the studies selected participants on a specific toxic syndrome. Kornberg 1991 focused on children under 6 years old, while Albertson 1989 and Pond 1995 included adults (specified as more than 18 years old or more than 13 years old, respectively). Kulig 1985 did not specify a certain age range. See Appendix 2.
Primary outcomes
Incidence of mortality
Two of the included studies reported on incidence of mortality, but neither study noted any events in the 573 participants across treatment groups (Kornberg 1991; Kulig 1985; Analysis 7.1). We considered this evidence to be of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
7.1. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 1 Incidence of mortality.
Adverse events
Two studies reported the incidence of adverse events (Albertson 1989; Pond 1995), while one study reported the number of activated charcoal that was vomited as an adverse event (Kornberg 1991). We combined these outcomes, as all studies included a wide variety of toxic syndromes. The meta‐analysis favoured not using ipecac (RR 2.59, 95% CI 1.37 to 4.91, 764 participants, 3 studies, P = 0.003). We found no important heterogeneity (P = 0.29, I² = 19%; Analysis 7.2). This was evidence of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
7.2. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 2 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
Three studies reported on the incidence of participants with clinical improvement during their stay in the emergency department (Kornberg 1991; Kulig 1985; Pond 1995). We considered a meta‐analysis appropriate, given the wide variety of toxic syndromes included in the individual studies. The combined risk ratio was 1.00 (95% CI 0.83 to 1.21, 989 participants, 3 studies, P = 0.98), without evidence of important heterogeneity (P = 0.21, I² = 36%; Analysis 7.3). The evidence was of low certainty, downgraded for limitations in study design and indirectness.
7.3. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 3 Incidence of clinical improvement.
Two trials studied the incidence of clinical deterioration during the emergency department stay (Kulig 1985; Pond 1995); we combined these in a meta‐analysis, given the wide variety of toxic syndromes included in the individual studies. The pooled RR was 0.88 (95% CI 0.46 to 1.69, 970 participants, 2 studies, P = 0.70), with no apparent heterogeneity (P = 0.38, I² = 0%; Analysis 7.4). We considered this to be evidence of very low certainty, downgraded for limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
7.4. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 4 Incidence of clinical deterioration.
Secondary outcomes
Duration of symptoms
The identified studies either did not collect or did not not report outcomes related to duration of poisoning symptoms.
Drug absorption
The identified studies either did not collect or did not report outcomes related to drug absorption.
Incidence of hospitalization
The incidence of hospitalization was an outcome of interest in three studies (Albertson 1989; Kornberg 1991; Kulig 1985). Given the wide variety of toxic syndromes included in the individual studies, and the inability of laypeople to distinguish between ingested toxins, we considered a meta‐analysis appropriate. The Peto OR was 1.17 (95% CI 0.69 to 1.98, 746 participants, 3 studies, P = 0.56), without any important heterogeneity (P = 0.15, I² = 47%; Analysis 7.5). We considered this evidence to be of very low certainty, due to limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
7.5. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 5 Incidence of hospitalization.
Incidence of ICU admission
One trial, Albertson 1989, reported the incidence of ICU admission (RR 1.38, 95% CI 0.44 to 4.38, 200 participants, 1 study, P = 0.58; Analysis 7.6). We considered this evidence to be of very low certainty, downgraded for limitations in study design, imprecision (low number of events or low sample size, and wide confidence intervals) and indirectness.
7.6. Analysis.
Comparison 7 Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic, Outcome 6 Incidence of ICU admission.
Summary for this comparison
Evidence from four studies suggests that adding syrup of ipecac to SDAC plus cathartics may make little difference for clinical improvement. On the other hand, we are uncertain about its impact on mortality, adverse events, clinical deterioration, hospitalization or ICU admission. Evidence was of low to very low certainty, downgraded for limitations in study design, imprecision and/or indirectness.
8. Syrup of ipecac versus syrup of ipecac (different doses)
We found one study using different types of syrup of ipecac, manufactured according to the American Pharmacopeia (USP) or the Australian Pharmaceutical Formulary (AFP) (Ilett 1977). More interestingly, this study also compared two different doses of the AFP syrup of ipecac: 15 mL or 30 mL, followed by 200 mL water. This study involved 120 participants presenting to the emergency department of a hospital with various intoxications.
Primary outcomes
Incidence of mortality
The identified study either did not collect or did not report on outcomes related to incidence of mortality.
Adverse events
The identified study either did not collect or did not report on outcomes related to adverse events due to the intervention.
Incidence and severity of symptoms of poisoning
The identified study either did not collect or did not report on outcomes related to incidence and severity of poisoning symptoms.
Secondary outcomes
Duration of symptoms
The identified study either did not collect or did not report on outcomes related to duration of poisoning symptoms.
Drug absorption
The identified study either did not collect or did not report on outcomes related to drug absorption.
Incidence of hospitalization
The identified study either did not collect or did not report on outcomes related to incidence of hospitalization.
Incidence of ICU admission
The identified study either did not collect or did not report on outcomes related to incidence of ICU admission.
Summary for this comparison
We identified one study that compared the use of different doses of syrup of ipecac (Ilett 1977), but it did not report any outcome of interest to our review.
B2. Cathartics
9. Single‐dose activated charcoal plus cathartics versus single‐dose activated charcoal alone
Two trials compared SDAC plus cathartics versus SDAC alone (James 1995; Sue 1994). Both trials studied children presenting to the emergency department following various or unspecified toxic ingestions, requiring SDAC. See Appendix 3.
Primary outcomes
Incidence of mortality
The identified studies either did not collect or did not report outcomes related to incidence of mortality.
Adverse events
In Sue 1994 the only measured adverse event due to the intervention was lethargy during follow‐up. Authors reported no cases of lethargy in the SDAC plus magnesium citrate group (50 participants) or in the SDAC group (14 participants). James 1995 recorded the incidence of participants vomiting upon receiving activated charcoal with or without sorbitol, magnesium citrate or magnesium sulphate. The pooled RR was 1.46 (95% CI 0.61 to 3.49, 116 participants, P = 0.39; Analysis 8.1). We considered the evidence to be of very low certainty, due to limitations in study design, imprecision (low number of events) and indirectness.
8.1. Analysis.
Comparison 8 SDAC + cathartic vs SDAC, Outcome 1 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
The identified studies either did not collect or did not report outcomes related to occurrence and severity of symptoms of poisoning.
Secondary outcomes
Duration of symptoms
The identified studies either did not collect or did not report outcomes related to symptom duration.
Drug absorption
The identified studies either did not collect or did not report outcomes related to drug absorption.
Incidence of hospitalization
Sue 1994 studied the incidence of hospitalization. Three participants required hospitalization in the group receiving SDAC plus either 4 mL/kg, 6 mL/kg or 8 mL/kg of magnesium citrate, versus one patient in the activated charcoal group (RR 0.84, 95% CI 0.09 to 7.46, 64 participants, P = 0.88; Analysis 8.2). The evidence was of very low certainty, downgraded for limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
8.2. Analysis.
Comparison 8 SDAC + cathartic vs SDAC, Outcome 2 Incidence of hospitalization.
Incidence of ICU admission
The identified studies did not report outcomes related to incidence of ICU admission.
Summary for this comparison
We are uncertain if adding SDAC to a cathartic has an effect on adverse events or hospitalization. The evidence was of very low certainty, downgraded for limitations in study design, imprecision and indirectness.
10. Single‐dose activated charcoal plus different doses of cathartics
Sue 1994 assessed SDAC with different doses of cathartics. This trial studied 64 children presenting to the emergency department following an unspecified toxic ingestion requiring SDAC. See Appendix 4.
Primary outcomes
Incidence of mortality
The identified study either did not collect or did not report outcomes related to incidence of mortality.
Adverse events
The only measured adverse event due to the intervention was lethargy during follow‐up. Authors reported no cases of lethargy in any of the treatment groups (Table 9). We considered this evidence to be of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
Incidence and severity of symptoms of poisoning
The identified study either did not collect or did not report outcomes related to incidence and severity of symptoms of poisoning.
Secondary outcomes
Duration of symptoms
The identified study either did not collect or did not report outcomes related to symptom duration.
Drug absorption
The identified study either did not collect or did not report outcomes related to drug absorption.
Incidence of hospitalization
No patient required hospitalization in the group receiving 4 mL/kg of magnesium citrate plus SDAC compared to one participant in the 6 mL/kg magnesium citrate plus SDAC group and two participants in the 8 mL/kg magnesium citrate plus SDAC group. When comparing 6 mL/kg or 8 mL/kg magnesium citrate versus 4 mL/kg, the ORs were 7.39 (95% CI 0.15 to 372.38, 32 participants, P = 0.32; Table 9) and 7.01 (95% CI 0.42 to 117.63, 34 participants, P = 0.18; Table 9), respectively. The RR when comparing 6 mL/kg to 8 mL/kg magnesium citrate was 1.78 (95% CI 0.18 to 17.80, 34 participants, P = 0.62; Analysis 9.1). We considered this to be evidence of very low certainty, due to limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
9.1. Analysis.
Comparison 9 SDAC + cathartic vs SDAC + cathartic (higher dose), Outcome 1 Incidence of hospitalization (8 mL vs 6 mL).
Incidence of ICU admission
The identified study either did not collect or did not report outcomes related to incidence of ICU admission.
Summary for this comparison
We are uncertain about the effects of higher doses of magnesium citrate combined with SDAC, compared to lower doses combined with SDAC, with respect to the incidence of adverse events or hospitalization. These results were of very low certainty, downgraded for limitations in study design, imprecision and indirectness.
11. Single‐dose activated charcoal plus different types of cathartics
One trial compared SDAC plus different types of cathartics, namely sorbitol, magnesium citrate and magnesium sulphate (James 1995). This trial studied 119 children who ingested a variety of toxins (analgesics, anticonvulsants, antihistamines and decongestants, asthma therapies, automotive products, cardiovascular drugs, gastrointestinal preparations, insecticides, mushrooms, psychotropic drugs, rodenticides, topicals, or miscellaneous drugs). See Appendix 5.
Primary outcomes
Incidence of mortality
The identified study either did not collect or did not report outcomes related to incidence of mortality.
Adverse events
Emesis occurred in 13 of 32 children receiving SDAC plus sorbitol; 6 of 33 children receiving SDAC plus magnesium citrate, and 4 of 23 children receiving SDAC plus magnesium sulphate.
When comparing sorbitol with magnesium sulphate, the RR was 2.34 (95% CI 0.87 to 6.25, 55 participants, P = 0.09; Analysis 10.1). Sorbitol versus magnesium citrate resulted in an RR of 2.23 (95% CI 0.97 to 5.16, 55 participants, P = 0.06; Analysis 10.2). For magnesium sulphate versus magnesium citrate, the RR is 0.96 (95% CI 0.30 to 3.01, 55 participants, P = 0.94; Analysis 10.3). We considered this evidence to be of very low certainty, due to limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
10.1. Analysis.
Comparison 10 SDAC + cathartic vs SDAC + cathartic (different type), Outcome 1 Incidence of vomiting (sorbitol vs magnesium sulphate.
10.2. Analysis.
Comparison 10 SDAC + cathartic vs SDAC + cathartic (different type), Outcome 2 Incidence of vomiting (sorbitol vs magnesium citrate).
10.3. Analysis.
Comparison 10 SDAC + cathartic vs SDAC + cathartic (different type), Outcome 3 Incidence of vomiting (magnesium sulphate vs magnesium citrate).
Incidence and severity of symptoms of poisoning
The identified study either did not collect or did not report outcomes related to incidence and severity of symptoms of poisoning.
Secondary outcomes
Duration of symptoms
The identified study either did not collect or did not report outcomes related to symptom duration.
Drug absorption
The identified study either did not collect or did not report outcomes related to drug absorption.
Incidence of hospitalization
The identified study either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
The identified study either did not collect or did not report outcomes related to incidence of ICU admission.
Summary for this comparison
Only one study assessed the effect of different types of cathartics in combination with SDAC in the treatment of poisoning. The only relevant outcome measured is incidence of emesis as an adverse event, for which any effect of using different types of cathartics is uncertain. Evidence is of very low certainty, downgraded for limitations in study design, imprecision and indirectness.
C. Combined first aid interventions that limit uptake and promote evacuation of the poison from the gastrointestinal tract
12. Single‐dose activated charcoal plus cathartic plus hospital intervention versus hospital intervention alone
One study compared SDAC plus a cathartic plus hospital interventions versus hospital interventions alone (Passeron 1989). Passeron 1989 included participants presenting with a confirmed overdose of benzodiazepines, barbiturates or imipramine. All participants in this study received gastric lavage prior to the SDAC plus sorbitol or no additional intervention. See Appendix 6.
Primary outcomes
Incidence of mortality
The identified study either did not collect or did not report outcomes related to incidence of mortality.
Adverse events
The included study reported the incidence of vomiting (Passeron 1989). The Peto OR was 9.94 (95% CI 1.52 to 65.02, 32 participants; Analysis 11.1). The level of evidence was very low, downgraded due to limitations in study design, imprecision (low number of events and wide confidence intervals) and indirectness.
11.1. Analysis.
Comparison 11 SDAC + cathartic + hospital intervention vs hospital intervention, Outcome 1 Incidence of adverse events.
Incidence and severity of symptoms of poisoning
Passeron 1989 monitored participants' symptoms using the Glasgow Coma Scale. They did not report any numeric data but reported no difference for the course of the Glasgow Coma Scale scores between treatments (P = 0.49). The evidence was of very low certainty, downgraded for limitations in study design, imprecision due to a lack of data and indirectness.
Secondary outcomes
Duration of symptoms
The identified study either did not collect or did not report outcomes related to symptom duration.
Drug absorption
Passeron 1989 measured drug levels in their participants, but did not report any of our pre‐defined outcomes of interest (AUC, Cmax, Tmax), so we could not make a reliable estimate of effect on drug absorption.
Incidence of hospitalization
The identified study either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
The identified study either did not collect or did not report outcomes related to incidence of ICU admission.
Summary for this comparison
We found only evidence of very low certainty (downgraded for limitations in study design, imprecision and indirectness) concerning the use of SDAC plus a cathartic, in adjuvant to established hospital treatments. Thus we are uncertain about the effect on Glasgow Coma Scale scores or incidence of vomiting.
13. Multi‐dose activated charcoal plus cathartic plus hospital intervention versus hospital intervention alone
One study in 14 participants compared MDAC plus magnesium sulphate as an adjuvant to oral N‐acetylcysteine versus N‐acetylcysteine alone for paediatric paracetamol overdose (Montoya‐Cabrera 1999). See Appendix 7.
Primary outcomes
Incidence of mortality
The identified study either did not collect or did not report outcomes related to incidence of mortality.
Adverse events
Montoya‐Cabrera 1999 reported that no adverse events occurred in any treatment group (Table 9). This is evidence of very low certainty, downgraded for limitations in study design, imprecision (low number of events) and indirectness.
Incidence and severity of symptoms of poisoning
The identified study either did not collect or did not report outcomes related to occurrence and severity of poisoning symptoms.
Secondary outcomes
Duration of symptoms
The identified study either did not collect or did not report outcomes related to symptom duration.
Drug absorption
Montoya‐Cabrera 1999 measured T1/2 of paracetamol in the plasma, reporting a decrease (P < 0.05; Table 9). However, they failed to report any measure of spread, so we cannot report any summary estimate with 95% CI. We consider the evidence to be of very low certainty due to limitations in study design, imprecision (low sample size and lack of data) and indirectness.
Incidence of hospitalization
The identified study either did not collect or did not report outcomes related to incidence of hospitalization.
Incidence of ICU admission
The identified study either did not collect or did not report outcomes related to incidence of ICU admission.
Summary for this comparison
We identified one study, which provided evidence of very low certainty (downgraded for limitations in study design, imprecision and indirectness) (Montoya‐Cabrera 1999). Any effect on the incidence of adverse events or plasma half‐life of paracetamol after receiving MDAC in adjuvant to oral administration of N‐acetylcysteine, compared to oral administration of N‐acetylcysteine alone is uncertain.
D. First aid interventions that neutralize or dilute the poison
We identified no studies comparing interventions aiming at neutralizing or diluting orally ingested poisons in a patient setting.
Discussion
Summary of main results
Out of a total of 11,582 potentially relevant references, we identified 24 studies reported in 31 publications. All but one study took place in a hospital setting (Wax 1999 was in a pre‐hospital setting). A total of 7099 participants were randomized to different treatment groups.
A. First aid interventions that limit or delay the absorption of the poison in the body
Activated charcoal
A commonly used intervention in poisoning is activated charcoal. Due to its enormous surface area it can adsorb large quantities of drugs, thus preventing the absorption of the poison by the body. Included studies made different comparisons, either using single‐dose activated charcoal (SDAC) alone, combined with other pre‐hospital treatments, or in adjuvant to hospital interventions. Furthermore, multi‐dose activated charcoal (MDAC) was used in adjuvant to hospital interventions.
We found very low‐certainty evidence from two studies, involving 476 participants and comparing single‐dose activated charcoal to no intervention, which is our main comparison (Merigian 1990; Underhill 1990). These studies included participants with unspecified exposures in Merigian 1990 or paracetamol overdoses in Underhill 1990. Any effect on the incidence of adverse events, clinical deterioration or ICU admission is uncertain. See Table 1.
Seven trials, providing evidence of low to very low certainty and including 5383 participants, investigated the effect of SDAC in adjuvant to established hospital interventions such as supportive treatment and in most cases also gastric lavage (Comstock 1982; Cooper 2005; Crome 1983; Eddleston 2008; Hultén 1988; Merigian 2002; Roberts 2006). All but three studies either did not specify a toxic syndrome or recruited participants with different toxic syndromes (Crome 1983; Hultén 1988;Roberts 2006). There may be little or no difference in the incidence of mortality or the need for and length of intubation. We are uncertain about the effects of SDAC in addition to hospital treatments with regard to adverse events, drug absorption and incidence of hospitalization or ICU admission. See Table 2.
Five trials including 3568 participants compared MDAC versus SDAC, all in adjuvant to hospital interventions (Behnoush 2009; Brahmi 2006; De Silva 2003; Eddleston 2008; Roberts 2006). Two trials included participants with carbamazepine overdose (Behnoush 2009; Brahmi 2006), and two trials studied participants with yellow oleander poisoning (De Silva 2003; Roberts 2006). Eddleston 2008 included participants with different types of overdose, including yellow oleander and pesticide poisoning. There were some discrepancies between studies, preventing us from drawing any conclusions regarding incidence of mortality. This is evidence of very low certainty. In addition, low‐certainty evidence suggests that MDAC may result in decreased incidence of ICU admissions and an increase in abdominal discomfort or diarrhoea, but it may have no influence on the need for intubation. Furthermore, we are uncertain about the effects of MDAC on drug absorption or length of intubation, evidence of very low certainty. See Table 3.
We identified one study, involving 34 participants, with very low‐certainty evidence that compared SDAC with syrup of ipecac in participants with anti‐inflammatory, analgesic or psychotropic drug overdose (Amigó Tadín 2002). We are uncertain about the effect of SDAC versus ipecac on the incidence of adverse events or Glasgow Coma Scale scores. See Table 4.
We identified three studies comparing MDAC in adjuvant to hospital intervention versus hospital intervention alone in 3121 participants (Bouget 1989; Eddleston 2008; Roberts 2006). This low‐ to very low‐certainty evidence suggests there may be no difference in the incidence of mortality, symptoms of intoxication or length of intubation, while we are uncertain about its effects on pharmacokinetic parameters. See Table 5.
None of the above‐mentioned evidence could show added value for the use of activated charcoal, either administered as a single dose or as multiple doses, or in adjuvant or not to hospital interventions.
Body position
Another possible intervention to slow down the uptake of the poison is a certain body position. The theory is that placing a patient on the left lateral decubitus position would allow the gastric content to stay in the greater curvature of the stomach, due to the combination of gravity and the anatomy of the stomach, which might slow down the rate of absorption of the poison. However, we did not identify any studies performed in poisoning participants that compared different kinds of body position.
B. First aid interventions that evacuate the poison from the gastrointestinal tract
Vomiting or accelerated defecation might induce the quick evacuation of the poison from the gastrointestinal tract. Interventions that might obtain this effect are emetics, which induce vomiting, or cathartics, for the acceleration of defecation.
Emetics
Five included studies looked at the effectiveness of syrup of ipecac as a first aid measure for poisoning.
One study including 103 participants provided evidence of low certainty on the use of ipecac versus no intervention in asymptomatic participants with toxic berry ingestion (Wax 1999). This study took place in a pre‐hospital setting and reported no clinical outcomes. While there may be little or no difference in emergency department referral, there may be an increase in adverse events. See Table 6.
Four studies, involving 1240 participants, assessed the addition of syrup of ipecac to SDAC plus a cathartic (Albertson 1989; Kornberg 1991; Kulig 1985; Pond 1995). All studies either did not specify or included multiple types of overdose. Low‐certainty evidence suggests there may be little or no difference in the incidence of clinical improvement. On the other hand, we are uncertain about any effect on the incidence of mortality, adverse events, clinical deterioration, hospitalization or ICU admission. See Appendix 2.
We identified one study with 120 participants comparing different doses of ipecac (Ilett 1977), but it did not report any outcomes of interest.
One study compared syrup of ipecac versus SDAC. We describe this study above (interventions that limit or delay the absorption of the poison in the body). See Table 4.
None of the evidence on the use of syrup of ipecac as a first aid intervention shows any benefit, and it may even cause harm.
Cathartics
Cathartics are often used in combination with activated charcoal, where activated charcoal is used to adsorb the poison and the cathartic to accelerate the evacuation from the gastrointestinal tract. We identified two studies in 183 participants that looked at the combination of different types or doses of cathartic with SDAC in children with unspecified or various intoxications (James 1995; Sue 1994).
These trials provided evidence of very low certainty comparing the use of SDAC plus a cathartic versus SDAC alone in either unspecified or multiple toxic syndromes. We are uncertain whether adding a cathartic to the treatment influences the incidence of adverse events or the incidence of hospitalization. See Appendix 3.
Sue 1994 assessed SDAC plus different doses of magnesium citrate in 64 participants, but we are uncertain whether this would result in a difference regarding the incidence of hospitalization. See Appendix 4.
James 1995 studied the effects of different types of cathartics (sorbitol, magnesium citrate or magnesium sulphate) in combination with SDAC, in 119 participants. We are uncertain whether a different type of cathartic in adjuvant to SDAC influences the incidence of vomiting as an adverse event. See Appendix 5.
In summary, we did not identify any trials that looked at the use of cathartics alone compared with no intervention. Cathartics were always used in combination to SDAC. From the limited evidence available, we are not able to draw conclusions regarding the use of cathartics in addition to SDAC.
C. Combinations of first aid interventions
One study including 32 participants looked at the effects of combining SDAC with cathartics as an adjuvant to hospital intervention, compared to hospital intervention alone in participants with overdoses of benzodiazepines, barbiturates or imipramine (Passeron 1989). The study provided evidence of very low certainty, so we are uncertain about the impact of adding SDAC plus cathartics in adjuvant to a hospital intervention on Glasgow Coma Scale scores or the incidence of vomiting. See Appendix 6.
One study in 14 participants used MDAC in combination with magnesium sulphate as a cathartic, in adjuvant to hospital treatment (Montoya‐Cabrera 1999). The evidence of very low certainty precluded us from drawing conclusions about the effects of MDAC and magnesium sulphate in adjuvant to hospital treatments on the plasma half‐life of paracetamol. See Appendix 7.
D. First aid interventions that neutralize or dilute the poison
We did not identify any studies in poisoning patients that looked at the effects of commonly used home remedies such as drinking milk, water, vinegar or citrus juice to neutralize or dilute the poison.
Overall completeness and applicability of evidence
The objective of this review was to assess the effects of pre‐hospital interventions, alone or in combination, that laypeople could feasibly provide victims of acute oral poisoning before professional help arrives. We identified only one study from a pre‐hospital setting (Wax 1999). As we anticipated that this would be the case in advance, we also included studies performed in a hospital setting as indirect evidence. Furthermore, half of the studies compared the intervention of interest in adjuvant to a hospital intervention versus hospital interventions alone. These considerations limit the applicability of our findings.
With regard to the interventions of interest, about two‐thirds of the identified studies looked at SDAC and MDAC alone or in combination with cathartics, and sometimes in adjuvant to hospital treatment. We identified six studies assessing syrup of ipecac. We found little evidence on the use of cathartics, and in the two studies we did identify cathartics were used in adjuvant to another treatment (i.e. SDAC), making it difficult to make a judgment on the use of cathartics by themselves for oral poisoning. Finally, we found no studies of the effect of body position or interventions that might dilute or neutralize the poison, such as drinking water or milk.
The 24 identified studies described a wide range of outcomes. However, the primary outcomes of interest, mortality, severity of symptoms due to poisoning and adverse events, were very variably and often incompletely reported. Useable data on these outcomes were thus limited. This also precluded combining the different interventions in this review in a network meta‐analysis (NMA), which would have allowed us to compare the relative efficacy of different interventions and potentially rank the interventions for efficacy. We will perform an NMA for future updates of this review, if more useable data becomes available.
Furthermore, most studies were over 10 years old, with the oldest study being performed in 1977 (Ilett 1977). Only two studies took place in the past decade (Behnoush 2009; Eddleston 2008).
A major limitation in most of the identified studies is the substantial heterogeneity of the included participants. This might obscure potential benefits in subgroups of participants, for example participants with severe poisoning, specific toxic syndromes or those presenting early (Juurlink 2015). On the other hand, in a first aid setting it might often be unclear what type of patient a caregiver is dealing with, with regard to the type, dose and timing of intoxication, further complicating conclusions with respect to the lay setting.
Overall, the identified evidence is scarce and of low to very low certainty, which precludes any firm conclusions about the added value of any of the first aid interventions discussed in this review. However, almost all of these studies were performed in a hospital setting, which means there is a delay in presentation and thus treatments are started at a later time than when given in a home setting. It could therefore be possible that treatments were not effective because of their delayed administration.
In addition, evidence is too scarce to be able to draw conclusions about the safety of most of the first aid interventions. The exception is syrup of ipecac, for which low‐certainly evidence suggests that the number of complications increases when using it compared to no intervention.
Quality of the evidence
A key methodological limitation in the included studies is that most studies used inappropriate or unclear methods of randomization. Furthermore, most studies reported outcomes poorly, and the reporting format was highly heterogeneous. This makes the studies difficult to compare with one another. The variation between and within studies with respect to the population further complicates the comparison of different studies.
First aid interventions that limit or delay the absorption of the poison in the body
For most of the comparisons including single‐ or multi‐dose activated charcoal, the evidence is of low to very low certainty. In most cases, we downgraded the evidence for indirectness (since most studies were performed in a hospital setting), imprecision (limited sample size, low number of events and/or wide confidence intervals) and limitations in study design.
In the comparison of SDAC versus MDAC (both in adjuvant to hospital interventions), we identified two studies at low risk of bias; however, there was inconsistency between the studies' findings, which makes it difficult to draw any conclusions. There is no clear cause of this inconsistency, but possible explanations might be that De Silva 2003 included participants up to 72 h after poison ingestion, whereas Eddleston 2008 included participants only up to 24 h after ingestion. Furthermore, Eddleston 2008 included less severely poisoned participants than De Silva 2003 (Glasgow Coma Scale of less than 13).
First aid interventions that evacuate the poison from the gastrointestinal tract
For interventions that promote the evacuation of the poison from the gastrointestinal tract, we found the most evidence on the use of syrup of ipecac. However, all studies were at high risk of selection bias, and most were at high or unclear risk of detection bias. We further downgraded studies due to imprecision and indirectness, leading to low or very low certainty evidence.
The evidence on the use of cathartics was limited and of very low certainty due to a high risk of reporting bias, indirectness and imprecision. The identified studies always used cathartics in combination with other interventions, making it difficult to draw conclusions about cathartics alone as a treatment for oral poisoning.
First aid interventions that neutralize or dilute the poison
There are no available data that we identified on interventions that neutralize or dilute the poison.
Potential biases in the review process
This review intended to assess interventions that are feasible for laypeople to use in situations of oral poisoning. This means that the interventions should be feasible to use in a pre‐hospital setting by people without any medical knowledge. According to these criteria, we excluded interventions such as gastric lavage or intravenous drug administrations.
Most identified studies took place in a hospital setting, which means that we had to downgrade the level of evidence due to indirectness. Only one included study was in a pre‐hospital community setting, but it included only asymptomatic poisoning patients.
Although the interventions were mostly in a hospital setting, we included only studies that used interventions feasible by laypeople. We only allowed comparisons with hospital interventions if the treatment group received the same hospital interventions in adjuvant to the possible first aid treatment under investigation.
We did not include the many available volunteer studies. These are studies in which healthy volunteers receive a drug in a therapeutic or supratherapeutic dose in an attempt to simulate oral poisoning, in a controlled setting such as a laboratory environment, mostly without co‐ingestion of other drugs or alcohol, on an empty stomach. We believed this was even more indirect than including studies performed in actual oral poisoning patients, although they were performed in a hospital setting.
As mentioned earlier, most studies were over 10 years old, with the oldest study from 1977. Only two studies took place within 10 years of our literature search. Many of the studies were poorly reported: data were missing, and our attempts to contact the authors were often unsuccessful because no contact details were available, authors did not respond, or data were no longer available. This could introduce a bias on the completeness of the data and the risk of bias assessment, leading to perhaps a more strict judgment of bias for some studies.
Agreements and disagreements with other studies or reviews
An existing Cochrane Review on interventions for paracetamol (acetaminophen) overdose included not only possible first aid interventions such as activated charcoal or syrup of ipecac, but also hospital interventions such as gastric lavage, charcoal haemoperfusion, antidotes such as N‐acetylcysteine or cimetidine, and liver transplantations (Chiew 2018). The review included randomized controlled trials as well as observational studies, and studies performed in healthy volunteers as well as in patients. In our review, the focus is on first aid interventions feasible for laypeople. This excludes all types of hospital interventions. Furthermore, we did not focus on a specific toxin, and since sufficient studies in patients were available, we decided to exclude studies performed in healthy volunteers. We agree with the conclusions of Chiew 2018 that the use of activated charcoal seems a safer option than syrup of ipecac to reduce uptake, although research still needs to demonstrate a clear clinical benefit.
There might be some overlap with the different position papers published on the use of SDAC (American Academy of Clinical Toxicology 2005), MDAC (American Academy of Clinical Toxicology 1999), cathartics (American Academy of Clinical Toxicology 2004), and syrup of ipecac (Höjer 2013). These papers give a comprehensive overview of the interventions and discuss the published literature, from preclinical to clinical research. However, most are out of date, and it is not clear if the literature search was systematic. Our systematic review does highlight that in the decade preceding publication, there has been very little research on this important topic.
Furthermore, the position papers only give a description of the different identified studies, whereas in our review we combined studies in a meta‐analysis where possible, to give an overall effect size. In any case, our conclusions are largely similar: there is insufficient evidence for a clinical benefit and thus for the routine use of any of the investigated treatments. Few studies have been published since the publication of these position papers, and while our review includes them, all fail to show a clear clinical benefit for the use of these first aid treatments in a hospital setting. However, one important difference is that the recommendations made in these position papers were designed for a professional care setting. The relevance for a pre‐hospital setting remains unclear.
A systematic literature search and meta‐analysis on the effect of activated charcoal in healthy volunteers showed that administration of activated charcoal was most effective when administered immediately after drug intake, but it was still effective up until four hours after drug ingestion (Jürgens 2009). There was no information on adverse events due to the intervention. This meta‐analysis demonstrates the theoretical capacity of activated charcoal to reduce uptake of a variety of toxins. However, the actual clinical benefit for oral poisoning patients remains speculative, as demonstrated by the studies included in our review, which fail to show a clear clinical benefit. Reasons for the discrepancy between data collected from healthy volunteers and actual patients might include the time passed between ingestion of the drugs and the start of the treatment. Also, the meta‐analysis looked at activated charcoal as the only treatment. It did not assess any combination treatments, such as activated charcoal plus a cathartic. Furthermore, the controlled setting where studies in healthy volunteers take place excludes certain confounding variables, for example ingesting the drugs with alcohol, intake of a cocktail of different kinds of drugs or not knowing which drugs were taken. These considerations imply that the use of activated charcoal is still a therapeutic option in emergency departments, but clinicians should carefully consider its use for individual patients (Juurlink 2015). From the available evidence, it is unclear whether a layperson would be capable of making these considerations in a pre‐hospital setting.
Authors' conclusions
Implications for practice.
We are unsure about the effects of activated charcoal, syrup of ipecac or cathartics for pre‐hospital management by laypeople of acute oral poisoning, due to the low‐ or very low‐certainty evidence. Data mostly came from emergency care departments, where the added value of first aid interventions is uncertain. Given the indirectness of these results, it is not possible to draw any conclusions concerning the use of these interventions for the pre‐hospital setting.
Implications for research.
There are many studies available on the use of activated charcoal, cathartics, syrup of ipecac or combinations of these interventions. Studies are performed either in oral poisoning patients or in healthy volunteers, mostly in a healthcare or controlled setting. However, there is very little up‐to‐date evidence. Researchers may feel hampered by practical issues to further investigate these interventions; nevertheless, the most recent studies show that high methodological quality can be feasible and ethical. The identified evidence, however, is indirect.
On the other hand, the clinical benefit of the one recommendation that is being made in practice in a pre‐hospital setting (IFRC 2016), the use of the left lateral decubitus position, remains to be demonstrated. If researchers are designing future studies on the effectiveness of first aid measures for acute oral poisoning, these could take place in a pre‐hospital setting, for example by collaborating with poisoning centres, to avoid the delay that is inherent to hospital studies. This delay precludes firm conclusions about interventions whose effectiveness decreases over time, as is clearly the case for interventions that try to limit the uptake of a poisonous substance.
What's new
| Date | Event | Description |
|---|---|---|
| 2 July 2019 | Amended | Author affiliations amended |
History
Review first published: Issue 12, 2018
| Date | Event | Description |
|---|---|---|
| 19 December 2018 | Amended | Minor changes made as requested by a copy editor |
Acknowledgements
We would like to thank all authors that provided additional data or information for this review. We would also like to thank the Cochrane Injuries group Editors, Helen Wakeford, Marialena Trivella and Emma Sydenham, and the peer referees Rose Cairns, Jim Mowry and Jay Schauben for their comments.
This project was supported by the UK National Institute for Health Research (NIHR), through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
The Cochrane Library
1. [mh "poisoning"] OR [mh "poisons"] OR poison*:ti,ab,kw OR (toxic NEXT/1 ingestion*):ti,ab,kw OR intoxica*:ti,ab,kw OR overdos*:ti,ab,kw OR [mh "drug overdose"]
2. (active NEXT/1 charcoal):ti,ab,kw OR (activated NEXT/1 charcoal):ti,ab,kw OR (active NEXT/1 carbon):ti,ab,kw OR (activated NEXT/1 carbon):ti,ab,kw OR [mh "charcoal"]
3. [mh "vomiting"] OR vomit*:ti,ab,kw OR emesis:ti,ab,kw OR (gastric NEXT/1 evacuation*):ti,ab,kw OR (gastrointestinal NEXT/1 decontamination*):ti,ab,kw OR [mh "ipecac"] OR ipecac*:ti,ab,kw OR emetic*:ti,ab,kw OR [mh "emetics"] OR [mh "cathartics"] OR cathartic*:ti,ab,kw OR purgative*:ti,ab,kw OR bowel evacuant*:ti,ab,kw OR [mh "sorbitol"] OR sorbitol:ti,ab,kw OR [mh "mannitol"] OR mannitol:ti,ab,kw OR [mh "lactulose"] OR lactulose:ti,ab,kw OR [mh "magnesium sulfate"] OR (magnesium NEXT/1 sulphate):ti,ab,kw OR (magnesium NEXT/1 sulfate):ti,ab,kw OR (magnesium NEXT/1 citrate):ti,ab,kw OR (sodium NEXT/1 sulphate):ti,ab,kw OR (sodium NEXT/1 sulfate):ti,ab,kw
4. ([mh "Drinking"] OR drink*:ti,ab,kw OR intake:ti,ab,kw OR consum*:ti,ab,kw OR ingest*:ti,ab,kw) AND ([mh "drinking water"] OR [mh "water"] OR water:ti,ab,kw)
5. (left NEXT/1 side):ti,ab,kw OR (body NEXT/1 position*):ti,ab,kw OR [mh "posture"] OR posture:ti,ab,kw OR (lateral NEXT/1 decubitus):ti,ab,kw
6. [mh "Milk"] OR milk:ti,ab,kw OR [mh "acetic acid"] OR vinegar:ti,ab,kw OR (acetic NEXT/1 acid):ti,ab,kw OR [mh "citrus"] OR citr*:ti,ab,kw OR orange*:ti,ab,kw OR grapefruit*:ti,ab,kw OR lemon*:ti,ab,kw
7. 2‐6 OR
8. 1 AND 7
MEDLINE, using the PubMed interface
1. "poisoning"[MeSH] OR "poisons"[MeSH] OR poison*[TIAB] OR toxic ingestion*[TIAB] OR intoxica*[TIAB] OR overdos*[TIAB] OR "drug overdose"[MeSH]
2. "active charcoal"[TIAB] OR "activated charcoal"[TIAB] OR "active carbon"[TIAB] OR "activated carbon"[TIAB] OR "charcoal"[MeSH]
3. "vomiting"[MeSH] OR vomit*[TIAB] OR emesis[TIAB] OR gastric evacuation*[TIAB] OR gastrointestinal decontamination*[TIAB] OR "ipecac"[MeSH] OR ipecac*[TIAB] OR emetic*[TIAB] OR "emetics"[MeSH] OR "cathartics"[MeSH] OR cathartic*[TIAB] OR purgative*[TIAB] OR bowel evacuant*[TIAB] OR "sorbitol"[MeSH] OR sorbitol[TIAB] OR "mannitol"[MeSH] OR mannitol[TIAB] OR "lactulose"[MeSH] OR lactulose[TIAB] OR "magnesium sulfate"[MeSH] OR "magnesium sulphate"[TIAB] OR "magnesium sulfate"[TIAB] OR "magnesium citrate"[TIAB] OR "sodium sulphate"[TIAB] OR "sodium sulfate"[TIAB]
4. ("Drinking"[Mesh] OR drink*[TIAB] OR intake[TIAB] OR consum*[TIAB] OR ingest*[TIAB]) AND ("drinking water"[MeSH] OR "water"[MeSH] OR water[TIAB])
5. "left side"[TIAB] OR body position*[TIAB] OR "posture"[Mesh] OR posture[TIAB] OR "lateral decubitus"[TIAB]
6. "Milk"[Mesh] OR milk[TIAB] OR "acetic acid"[MeSH] OR vinegar[TIAB] OR "acetic acid"[TIAB] OR "citrus"[MeSH] OR citric*[TIAB] OR citrus*[TIAB] OR orange*[TIAB] OR grapefruit*[TIAB] OR lemon*[TIAB]
7. 2‐6 OR
8. (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized[TIAB] OR placebo[TIAB] OR drug therapy[sh] OR randomly[TIAB] OR trial[TIAB] OR groups[TIAB]) NOT (Animals[MeSH] NOT Humans[MeSH])
9. 1 AND 7 AND 8
Embase, using the Embase.com interface
1. 'intoxication'/exp OR 'poison'/exp OR poison*:ab,ti OR (toxic NEXT/1 ingestion*):ab,ti OR intoxica*:ab,ti OR overdos*:ab,ti OR 'drug overdose'/exp
2. 'active charcoal':ab,ti OR 'activated charcoal':ab,ti OR 'active carbon':ab,ti OR 'activated carbon':ab,ti OR 'activated carbon'/exp
3. 'vomiting'/exp OR vomit*:ab,ti OR emesis:ab,ti OR (gastric NEXT/1 evacuation*):ab,ti OR (gastrointestinal NEXT/1 decontamination*):ab,ti OR 'ipecac'/exp OR ipecac*:ab,ti OR emetic*:ab,ti OR 'emetic agent'/exp OR 'laxative'/exp OR cathartic*:ab,ti OR purgative*:ab,ti OR (bowel NEXT/1 evacuant*):ab,ti OR 'sorbitol'/exp OR sorbitol:ab,ti OR 'mannitol'/exp OR mannitol:ab,ti OR 'lactulose'/exp OR lactulose:ab,ti OR 'magnesium sulfate'/exp OR 'magnesium sulphate':ab,ti OR 'magnesium sulfate':ab,ti OR 'magnesium citrate':ab,ti OR 'sodium sulphate':ab,ti OR 'sodium sulfate':ab,ti
4. ('Drinking'/exp OR drink*:ab,ti OR intake:ab,ti OR consum*:ab,ti OR ingest*:ab,ti) AND ('drinking water'/exp OR 'water'/exp OR water:ab,ti)
5. 'left side':ab,ti OR (body NEXT/1 position*):ab,ti OR 'body position'/exp OR posture:ab,ti OR 'lateral decubitus':ab,ti
6. 'milk'/exp OR milk:ab,ti OR 'acetic acid'/exp OR vinegar:ab,ti OR 'acetic acid':ab,ti OR 'citrus'/exp OR citr*:ab,ti OR orange*:ab,ti OR grapefruit*:ab,ti OR lemon*:ab,ti
7. 2‐6 OR
8. ('randomized controlled trial'/exp OR 'controlled clinical trial'/exp OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR (double NEXT/1 blind*):ab,ti OR (single NEXT/1 blind*):ab,ti OR trial:ab,ti OR groups:ab,ti) NOT ('animal'/exp NOT 'human'/exp)
9. 1 AND 7 AND 8
CINAHL, using the EBSCO host interface
1. MH "poisoning+" OR MH "poisons+" OR TI "poison*" OR AB "poison*" OR TI "toxic ingestion*" OR AB "toxic ingestion*" OR TI "intoxica*" OR AB "intoxica*" OR TI "overdos*" OR AB "overdos*" OR MH "overdose"
2. TI "active charcoal" OR AB "active charcoal" OR TI "activated charcoal" OR AB "activated charcoal" OR TI "active carbon" OR AB "active carbon" OR TI "activated carbon" OR AB "activated carbon" OR MH "charcoal"
3. MH "vomiting+" OR TI "vomit*" OR AB "vomit*" OR TI emesis OR AB emesis OR TI "gastric evacuation*" OR AB "gastric evacuation*" OR TI "gastrointestinal decontamination*" OR AB "gastrointestinal decontamination*" OR MH "ipecac" OR TI "ipecac*" OR AB "ipecac*" OR TI "emetic*" OR AB "emetic*" OR MH "emetics+" OR MH "cathartics+" OR TI "cathartic*" OR AB "cathartic*" OR TI "purgative*" OR AB "purgative*" OR TI "bowel evacuant*" OR AB "bowel evacuant*" OR MH "sorbitol" OR TI sorbitol OR AB sorbitol OR MH "mannitol" OR TI mannitol OR AB mannitol OR TI lactulose OR AB lactulose OR MH "magnesium sulfate" OR TI "magnesium sulphate" OR AB "magnesium sulphate" OR TI "magnesium sulfate" OR AB "magnesium sulfate" OR TI "magnesium citrate" OR AB "magnesium citrate" OR TI "sodium sulphate" OR AB "sodium sulphate" OR TI "sodium sulfate" OR AB "sodium sulfate"
4. (TI "drink*" OR AB "drink*" OR TI intake OR AB intake OR TI "consum*" OR AB "consum*" OR TI "ingest*" OR AB "ingest*") AND (MH "Water supply" OR MH "water+" OR TI water OR AB water)
5. TI "left side" OR AB "left side" OR TI "body position*" OR AB "body position*" OR MH "posture+" OR TI posture OR AB posture OR TI "lateral decubitus" OR AB "lateral decubitus"
6. MH "Milk+" OR TI milk OR AB milk OR TI "vinegar" OR AB "vinegar" OR MH "acetic acid" OR MH "citrus+" OR TI "citr*" OR AB "citr*" OR TI "orange*" OR AB "orange*" OR TI "grapefruit*" OR AB "grapefruit*" OR TI "lemon*" OR AB "lemon*"
7. 2‐6 OR
8. ((MH "Random Assignment") or (MH "Random Sample+") or (MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies") or (MH "Control (Research)+") or (MH "Control Group") or (MH "Factorial Design") or (MH "Quasi‐Experimental Studies+") or (MH "Placebos") or (MH "Meta Analysis") or (MH "Sample Size") or (MH "Research, Nursing") or (MH "Research Question") or (MH "Research Methodology+") or (MH "Evaluation Research+") or (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") or (MH "Nursing Practice, Research‐Based") or (MH "Solomon Four‐Group Design") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Static Group Comparison") or (MH "Study Design") or (MH "Clinical Research+")) or (clinical nursing research or random* or cross?over or placebo* or control* or factorial or sham* or meta?analy* or systematic review* or blind* or mask* or trial*)
9. 1 AND 7 AND 8
ISI Web of Science
1. TS=("poison*") OR TS=("toxic ingestion*") OR TS=("intoxica*") OR TS=("overdos*")
2. TS=("active charcoal") OR TS=("activated charcoal") OR TS=("active carbon")
3. TS=("vomit*") OR TS=("emesis") OR TS=("gastric evacuation*") OR TS=("gastrointestinal decontamination*") OR TS=("ipecac") OR TS=("emetic*") OR TS=("cathartic*") OR TS=("purgative*") OR TS=("bowel evacuant*") OR TS=("sorbitol") OR TS=("mannitol") OR TS=("lactulose") OR TS=("magnesium sulphate") OR TS=("magnesium sulfate") OR TS=("magnesium citrate") OR TS=("sodium sulphate") OR TS=("sodium sulfate")
4. (TS=("drink*") OR TS=("intake") OR TS=("consum*") OR TS=("ingest*")) AND (TS=("water"))
5. TS=("left side") OR TS=("body position") OR TS=("posture") OR TS=("lateral decubitus")
6. TS=("milk") OR TS=("acetic acid") OR TS=("vinegar") OR TS=("citr*") OR TS=("orange*") OR TS=("grapefruit*") OR TS=("lemon*")
7. 2‐6 OR
8. TS=(clinical trial*) OR TS=(research design) OR TS=(comparative stud*) OR TS=(evaluation stud*) OR TS=(controlled trial*) OR TS=(follow‐up stud*) OR TS=(prospective stud*) OR TS=(random*) OR TS=(placebo*) OR TS=(single blind*) OR TS=(double blind*)
9. 1 AND 7 AND 8
International Pharmaceutical Abstracts, using the Ovid interface
1. Poisoning.sh. OR poisons.sh. OR poison*.ti,ab. OR toxic ingestion*.ti,ab. OR intoxica*.ti,ab. OR overdos*.ti,ab. OR (drug overdose).ti,ab.
2. Charcoal.sh. OR carbon.sh. OR active charcoal.ti,ab. OR activated charcoal.ti,ab. OR active carbon.ti,ab. OR activated carbon.ti,ab.
3. Vomiting.sh. OR vomit*.ti,ab. OR emesis.sh. OR emesis.ti,ab. OR gastric evacuation*.ti,ab. OR gastrointestinal decontamination*.ti,ab. OR ipecac.sh. OR ipecac*.ti,ab. OR emetic*.ti,ab. OR emetics.sh. OR cathartics.sh. OR cathartic*.ti,ab. OR purgative*.ti,ab. OR bowel evacuant*.ti,ab. OR sorbitol.sh. OR sorbitol.ti,ab. OR mannitol.sh OR mannitol.ti,ab. OR lactulose.sh. OR lactulose.ti,ab. OR magnesium sulfate.sh. OR magnesium sulphate.ti,ab. OR magnesium sulfate.ti,ab. OR magnesium citrate.sh. OR magnesium citrate.ti,ab. OR sodium sulfate.sh. OR sodium sulphate.ti,ab. OR sodium sulfate.ti,ab.
4. (drink*.ti,ab. OR intake.ti,ab. OR consum*.ti,ab. OR ingest*.ti,ab.) AND (water.sh. OR water.ti,ab.)
5. left side.ti,ab. OR body position*.ti,ab. OR posture.sh. OR posture.ti,ab. OR lateral decubitus.ti,ab.
6. Milk.sh. OR milk.ti,ab. OR acetic acid.sh. OR vinegar.sh. OR vinegar.ti,ab. OR acetic acid.ti,ab. OR citrus.sh. OR citr*.ti,ab. OR orange*.ti,ab. OR grapefruit*.ti,ab. OR lemon*.ti,ab.
7. 2‐6 OR
8. 1 AND 7
Clinicaltrials.gov
poisoning OR poison OR poisons OR "toxic ingestion" OR intoxication OR overdose OR overdoses OR overdosing
EU Clinical Trials Register
(poisoning OR poison OR poisons OR "toxic ingestion" OR intoxication OR overdose OR overdoses OR overdosing) AND ("active charcoal" OR "activated charcoal" OR "active carbon" OR Vomiting OR emesis OR emetic OR "gastric evacuation" OR "gastrointestinal decontamination" OR ipecac OR cathartic OR cathartics OR purgative OR purgatives OR "bowel evacuant" OR "bowel evacuants" OR sorbitol OR mannitol OR lactulose OR "magnesium sulphate" OR "magnesium sulfate" OR "magnesium citrate" OR "sodium sulphate" OR "sodium sulfate" OR ((drinking OR drink OR intake OR consuming OR consumption OR ingestion OR ingesting) AND water) OR "left side" OR "body position" OR "body positions" OR posture OR postures OR "lateral decubitus" OR Milk OR "acetic acid" OR vinegar OR citrus OR citric OR orange OR oranges OR grapefruit OR grapefruits OR lemon OR lemons)
WHO International Clinical Trials Registry Platform (ICTRP)
Poison* OR toxic ingestion* OR intoxica* OR overdos*
Appendix 2. Syrup of ipecac + SDAC + cathartic versus SDAC + cathartic for first aid in patients with acute oral poisoning
| Syrup of ipecac + SDAC + cathartic versus SDAC + cathartic for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (combination of different drugs or not specified) Setting: hospital setting Intervention: syrup of ipecac + single‐dose activated charcoal (SDAC) + Cathartic Comparison: SDAC + Cathartic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SDAC + cathartic | Risk with syrup of ipecac + SDAC + cathartic | |||||
| Incidence of mortality | — | — | 573 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The effect was not estimable due to zero events in intervention group (0/300) and control group (0/273). We are uncertain about the effect of syrup of ipecac in addition to SDAC + cathartic on incidence of mortality. | |
| Incidence of adverse events | Study population | RR 2.59 (1.37 to 4.91) | 764 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain about the effect of syrup of ipecac in addition to SDAC + cathartic on incidence of adverse events. | |
| 41 per 1000 | 105 per 1000 (56 to 199) | |||||
| Incidence and severity of symptoms of poisoning: incidence of clinical improvement | Study population | RR 1.00 (0.83 to 1.21) | 989 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Syrup of ipecac in addition to SDAC + cathartic may make little or no difference in incidence of clinical improvement. | |
| 548 per 1000 | 548 per 1000 (455 to 663) | |||||
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption | No studies collected or reported this outcome | |||||
| Incidence of hospitalization | 76 per 1000 | 89 per 1000 (53 to 151) |
Peto OR 1.17 (0.69 to 1.98) | 746 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain about the effect of Syrup of ipecac in addition to SDAC + cathartic on incidence of hospitalization |
| Incidence of ICU admission | 47 per 1000 | 64 per 1000 (21 to 205) |
RR 1.38 (0.44 to 4.38) | 200 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain about the effect of Syrup of ipecac in addition to SDAC + cathartic on incidence of ICU admission |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; RCT: randomized controlled trial; RR: risk ratio; OR: odds ratio; SDAC: single‐dose activated charcoal. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Footnotes
aDowngraded one level for serious limitations in study design: high risk of selection bias and high or unclear risk of detection bias. bDowngraded one level for serious indirectness: study conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events. dDowngraded one level for serious imprecision: low number of events and wide confidence interval.
Appendix 3. SDAC + cathartic versus SDAC for first aid in patients with acute oral poisoning
| SDAC + cathartic versus SDAC for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (not specified or a combination of different drugs) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) + cathartic Comparison: SDAC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SDAC | Risk with SDAC + cathartic | |||||
| Incidence of mortality | No studies collected or reported this outcome | |||||
| Incidence of adverse events | Study population | RR 1.46 (0.61 to 3.49) | 180 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain about the effect of SDAC + cathartic, compared to SDAC alone on the incidence of adverse events. | |
| 119 per 1000 | 174 per 1000 (73 to 415) | |||||
| Incidence and severity of symptoms of poisoning ‐ not reported | No studies looked at this outcome | |||||
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption | No studies collected or reported this outcome | |||||
| Incidence of hospitalization | Study population | RR 0.84 (0.09 to 7.46) | 64 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain about the effect of SDAC + cathartic, compared to SDAC alone on the incidence of ICU admission. | |
| 71 per 1000 | 60 per 1000 (6 to 533) | |||||
| Incidence of ICU admission | No studies collected or reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; RCT: randomized controlled trial; RR: risk ratio; SDAC: single‐dose activated charcoal. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Footnotes
aDowngraded one level for serious study limitations: high risk of reporting bias. bDowngraded one level for serious indirectness: study was conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals.
Appendix 4. SDAC + cathartic compared to SDAC + cathartic (higher dose) for first aid in patients with acute oral poisoning
| SDAC + cathartic compared to SDAC + cathartic (higher dose) for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (not specified) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) + Cathartic Comparison: SDAC + Cathartic (higher dose) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SDAC + cathartic (higher dose) | Risk with SDAC + cathartic | |||||
| Incidence of mortality | No studies collected or reported this outcome | |||||
| Incidence of adverse events: 8 mL vs 4 mL magnesium citrate | — | — | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The effect was not estimable due to zero events in intervention group (0/18) and control group (0/16). We are uncertain about the effects of SDAC + cathartic (higher dose) on the incidence of adverse events. |
|
| Occurrence and severity of symptoms of poisoning | No studies collected or reported this outcome | |||||
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption | No studies collected or reported this outcome | |||||
| Incidence of hospitalization (8 mL vs 4 mL magnesium citrate) | control:0/16 and intervention 2/18 (Peto OR 7.01, 95% CI 0.42 to 117.63). | — | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain about the effects of SDAC + cathartic (higher dose) on the incidence of hospitalization. | |
| Incidence of ICU admission | No studies collected or reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; OR: odds ratio; SDAC: single‐dose activated charcoal; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Footnotes
aDowngraded one level for serious limitations in study design: high risk of reporting bias. bDowngraded one level for serious indirectness: study was conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events. dDowngraded one level for serious imprecision: low number of events and wide confidence intervals.
Appendix 5. SDAC + cathartic compared to SDAC + cathartic (different type) for first aid in patients with acute oral poisoning
| SDAC + cathartic compared to SDAC + cathartic (different type) for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (combination of different drugs) Setting: hospital setting Intervention: single‐dose activated charcoal (SDAC) + cathartic Comparison: SDAC + cathartic (different type) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SDAC + Cathartic (different type) | Risk with SDAC + Cathartic | |||||
| Incidence of mortality | No studies collected or reported this outcome | |||||
| Incidence of adverse events: vomiting (sorbitol vs magnesium citrate) | Study population | RR 2.23 (0.97 to 5.16) | 65 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain about the effect of different types of cathartics combined with SDAC on the incidence of adverse events. | |
| 182 per 1000 | 405 per 1000 (176 to 938) | |||||
| Incidence and severity of symptoms of poisoning ‐ not reported | No studies collected or reported this outcome | |||||
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption | No studies collected or reported this outcome | |||||
| Incidence of hospitalization admission | No studies collected or reported this outcome | |||||
| Incidence of ICU admission | No studies collected or reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; RR: risk ratio; RCT: randomized controlled trial; SDAC: single‐dose activated charcoal. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Footnotes
aDowngraded one level for serious study limitations: high risk of reporting bias. bDowngraded one level for serious indirectness: study was conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals.
Appendix 6. SDAC + cathartic + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning
| SDAC + cathartic + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning | |||
| Patient or population: first aid in patients with acute oral poisoning Setting: hospital setting Intervention: SDAC + cathartic + hospital intervention Comparison: hospital intervention | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Incidence of mortality | No studies collected or reported this outcome | ||
| Incidence of adverse events | Control group: 0/16 and intervention group: 5/16 (Peto OR 9.94, 95% CI 1.52 to 65.02). We are uncertain of the effect of SDAC + cathartic on the incidence of adverse events. | 32 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c |
| Incidence and severity of symptoms of poisoning: level of coma assessed with: Glasgow Coma Scale Follow‐up: 2 days | No numeric data were provided about Glasgow Coma Scale scores, but the course of the scores was reported not to differ significantly between treatments (P = 0.49). We are uncertain of the effect of SDAC + cathartic on the level of coma. | 32 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d |
| Duration of toxic symptoms | No studies collected or reported this outcome | ||
| Drug absorption | No studies collected or reported this outcome | ||
| Hospitalization | No studies collected or reported this outcome | ||
| ICU admission | No studies collected or reported this outcome | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; OR: odds ratio; RCT: randomized controlled trial; SDAC: single‐dose activated charcoal. | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
Footnotes
aDowngraded one level for serious study limitations: high risk of selection bias. bDowngraded one level for serious indirectness: study conducted in a hospital setting. cDowngraded one level for serious imprecision: low number of events and wide confidence intervals. dDowngraded one level for serious imprecision: lack of data.
Appendix 7. MDAC + cathartic + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning
| MDAC + cathartic + hospital intervention versus hospital intervention for first aid in patients with acute oral poisoning | ||||||
| Patient or population: first aid in patients with acute oral poisoning (paracetamol) Setting: hospital setting Intervention: multiple dose of activated charcoal (MDAC) + Cathartic + Hospital intervention Comparison: hospital intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hospital intervention | Risk with MDAC + cathartic + hospital intervention | |||||
| Incidence of mortality | No studies collected or reported this outcome | |||||
| Incidence of adverse events | — | — | 14 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The effect was not estimable due to the absence of events in the intervention (0/7) and the control group (0/7). We are uncertain about the effect of MDAC with a cathartic on the incidence of adverse events. |
|
| Incidence and severity of symptoms of poisoning | No studies collected or reported this outcome | |||||
| Duration of toxic symptoms | No studies collected or reported this outcome | |||||
| Drug absorption: paracetamol: elimination half‐life T1/2 (h) | The mean drug absorption: paracetamol: elimination half‐life T1/2 (h) was 17 h | MD 7 h lower | — | 14 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | The study reported no standard deviations or other measures of data spread. We are uncertain about the effect of MDAC with a cathartic on paracetamol elimination half‐life. |
| Incidence of hospitalization | No studies collected or reported this outcome | |||||
| Incidence of ICU admission | No studies collected or reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MD: mean difference; MDAC: multi‐dose activated charcoal; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Footnotes
aDowngraded one level due to serious limitations in study design: high risk of other bias: hepatic toxicity marker values suggest a clinically meaningful difference between the two treatment groups. bDowngraded one level due to serious indirectness: study conducted in a hospital setting. cDowngraded one level due to serious imprecision: low number of events. dDowngraded one level due to serious imprecision: lack of data.
Data and analyses
Comparison 1. SDAC vs no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adverse events | 2 | 476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.17 [0.30, 57.26] |
| 1.1 Incidence of adverse events | 1 | 451 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Incidence of vomiting | 1 | 25 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.17 [0.30, 57.26] |
Comparison 2. SDAC + hospital intervention vs hospital intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of mortality | 2 | 3425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.79, 1.37] |
| 2 Incidence of adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Incidence of vomiting | 2 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.88, 2.37] |
| 2.2 Incidence of absent bowel sounds | 1 | 3098 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 1.00] |
| 3 Incidence of need for intubation | 4 | 3562 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.76, 2.47] |
| 3.1 Gastric lavage prior to SDAC | 2 | 3175 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 3.2 No gastric lavage prior to SDAC | 2 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.38, 4.93] |
| 4 Incidence of convulsions | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Duration of intubation (h) | Other data | No numeric data | ||
| 6 AUC ((µg/L) × h) | Other data | No numeric data | ||
| 7 Cmax (µg/L) | Other data | No numeric data | ||
| 8 Tmax (h) | Other data | No numeric data | ||
| 9 Incidence of hospitalization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Incidence of ICU admission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. SDAC vs syrup of ipecac.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Glasgow Coma Scale score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Mean arterial blood pressure (mmHg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Heart rate (bpm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Respiratory rate (breaths/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. MDAC + hospital intervention vs SDAC + hospital intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of mortality | 2 | 3476 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.63] |
| 2 Incidence of adverse events | 2 | 3476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.55 [1.85, 6.79] |
| 3 Incidence of need for cardiac pacing/antitoxin treatment | 2 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.02, 4.18] |
| 4 Incidence of life‐threatening arrhythmias | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Amount of atropine administered (mg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Number of atropine boluses administered | Other data | No numeric data | ||
| 7 Incidence of need for intubation | 2 | 3097 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.75, 1.38] |
| 8 Incidence of convulsions | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Duration of coma (h) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Duration of intubation (h) | Other data | No numeric data | ||
| 11 Cmax (µg/L) | Other data | No numeric data | ||
| 12 Tmax (h) | Other data | No numeric data | ||
| 13 T1/2 (h) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 AUC ((µg/L) × h) | Other data | No numeric data | ||
| 15 Incidence of ICU admission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. MDAC + hospital intervention vs hospital intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of need for intubation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of seizures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Incidence of need for cardiac pacing/antitoxin treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Length of intubation (h) | Other data | No numeric data | ||
| 7 AUC | Other data | No numeric data | ||
| 8 Cmax | Other data | No numeric data | ||
| 9 Tmax | Other data | No numeric data |
Comparison 6. Syrup of ipecac vs no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of abdominal pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of sedation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of agitation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of mortality | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Incidence of adverse events | 3 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.37, 4.91] |
| 3 Incidence of clinical improvement | 3 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 4 Incidence of clinical deterioration | 2 | 970 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.46, 1.69] |
| 5 Incidence of hospitalization | 3 | 746 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.69, 1.98] |
| 6 Incidence of ICU admission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. SDAC + cathartic vs SDAC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Lethargy during follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of hospitalization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. SDAC + cathartic vs SDAC + cathartic (higher dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of hospitalization (8 mL vs 6 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. SDAC + cathartic vs SDAC + cathartic (different type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of vomiting (sorbitol vs magnesium sulphate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of vomiting (sorbitol vs magnesium citrate) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of vomiting (magnesium sulphate vs magnesium citrate) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. SDAC + cathartic + hospital intervention vs hospital intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albertson 1989.
| Methods |
Study design: randomized controlled trial Study duration: 24 months ending December 1987 Setting: emergency department (ED) at UC Davis Medical Center Country: USA Number of individuals randomized: 200 Number of individuals receiving the intervention: 93 Number of individuals receiving the control: 107 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: 113 women, 87 men Age: 30.1 (SEM 0.8), range 18‐77 years Country (if different from study authors'): NA Type, dose and timing of poisoning: all participants with mild or moderate oral overdose, 56% had mixed overdoses, most frequently with ethanol, timing could not be reliably obtained in most participants Inclusion criteria: awake with gag reflex, > 18 years, cooperative Exclusion criteria: rapidly deteriorating level of consciousness, previous vomiting, received ipecac syrup at home or en route, ingested substance with contraindication for ipecac syrup, ingested strong acids or bases, camphor, volatile petroleum distillates and strychnine, ingested large amounts of iron and lithium alone |
|
| Interventions |
Intervention arm: Type: syrup of ipecac followed with activated charcoal‐sorbitol after vomiting subsided Timing: Syrup of ipecac: no information AC‐sorbitol: after induced vomiting Dose: 30 mL ipecac syrup + 1 g/kg AC (50 g AC‐sorbitol‐water suspension Frequency: 1× ipecac unless no response then repeated after 30 min + 1× AC Integrity: no information Control arm: Type: activated charcoal‐sorbitol Timing: no information Dose: 1 g/kg AC (50 g AC‐sorbitol‐water suspension) Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): Mean time in the emergency department (h) (see Table 21) Proportion requiring hospitalization Number of days hospitalized (see Table 21) Proportion admitted to the intensive care unit Number of days in ICU (see Table 21) Proportion of complications Timing: during time in the hospital (early) |
|
| Funding | No information | |
| Notes | 75% had toxicology screen done but was not a criterion for inclusion or exclusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "were randomized by hospital unit numbers to two treatment groups" Comment: not an adequate randomisation method |
| Allocation concealment (selection bias) | High risk | Quote: "were randomized by hospital unit numbers to two treatment groups" Comment: allocation scheme does not allow allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied (e.g. mean time spend in the emergency department) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No specific information, but no failure to adhere to intervention reported |
| Selective reporting (reporting bias) | High risk | No assessment of symptom severity |
| Other bias | High risk | Quote: "Most patients (75%) had partial or complete toxicological analysis of blood and/or urine performed, although this was not a requirement of the study." Comment: for 25% of the participants, actual poisoning was not verified by means other than history. |
Amigó Tadín 2002.
| Methods |
Study design: randomized controlled trial Study duration: 11 December 2000 to 12 March 2001 Setting: emergency department of a tertiary healthcare facility (El Hospital Clinic, Barcelona) Country: Spain Number of individuals randomized: 34 Number of individuals receiving the intervention: 21 Number of individuals receiving the control: 13 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: Ipecac: 17 females, 4 males AC: 10 females, 3 males Age: Ipecac: 35 (SD 13) years AC: 27 (SD 6) years Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants presenting at the emergency department with an oral overdose of either anti‐inflammatory drugs, analgesics or psychotropic drugs. 91% were psychotropics, mostly benzodiazepines, followed by tricyclic antidepressants. In 35% of cases, more than 1 drug was taken. 18% were taken with alcohol. Selection criteria: Inclusion criteria: > 15 years and Glasgow score > 12 Exclusion criteria: participants with a medical indication for gastric lavage or if it had previously been performed by a medical service in an out‐of‐hospital setting. Presenting more than 2 h after intoxication unless they had taken antidepressants, neuroleptics, salicylates or opioids in which the interval was extended to 4 h. Participants for whom, due to their potential severity, was presumed that 1‐2 doses of SOI or AC would be insufficient to effectively decontaminate the digestive tract |
|
| Interventions |
Intervention arm: Type: syrup of ipecac Timing: 8.65 (SD 8.4) min after arriving at the ED or 113.46 (SD 80.29) min after ingestion Dose: 30 mL, followed by 240 mL of water Frequency: 1× Integrity: dose was repeated after 20 minutes if no vomiting occurred in that time Time to vomiting: 32 (SD 25.17) min, mean number of vomiting episodes: 2.05 (SD 1.68). 38% of vomits contained rests of tablets. 3 people did not vomit. Control arm: Type: activated charcoal Timing: 10.68 (SD 9.48) min after arriving at the ED or 112.35 (SD 81.48) min after ingestion Dose: 25 g in 200 mL of water Frequency: 1× Integrity: in order to mask the black color of the AC and so that its oral administration did not pose problems of acceptability, it was given in the same jar in which it is marketed or in a glass with a cane |
|
| Outcomes |
Type (unit): Mean arterial blood pressure (mmHg) Heart rate (bpm) Breathing rate (breaths/min) Glasgow Coma Scale score Length of stay ED (min) (see Table 21) Workload nurses (data not extracted) Adverse events Timing: Clinical parameters: 1 h after initial assessment of patient (early) Rest: during ED stay (early) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients entering on even days receives syrup of ipecac and patients entering on uneven days received activated charcoal." Comment: no adequate randomization |
| Allocation concealment (selection bias) | High risk | Quote: "Patients entering on even days receives syrup of ipecac and patients entering on uneven days received activated charcoal." Comment: randomization scheme does not allow allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied (e.g. Glasgow Coma Scale assessment) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The rest were excluded because they had a Glasgow coma score < 12, did not meet the inclusion criteria or because caregivers were not able to complete the forms". Comment: of the 97 potentially eligible participants, only 34 were included, due to justified reasons as "Glasgow coma score <12" or "not meeting inclusion criteria", but also due to staff business. Not clear how many participants were lost because of this |
| Selective reporting (reporting bias) | Low risk | No indication of a risk of reporting bias |
| Other bias | Low risk | No indication of other risk of bias |
Behnoush 2009.
| Methods |
Study design: randomized controlled trial Study duration: July 2003 to September 2004 Setting: hospital setting (poisoning ward of the Loghman Hospital, Tehran) Country: Iran Number of individuals randomized: 68 Number of individuals receiving the intervention: 38 Number of individuals receiving the control: 30 Number of individuals lost to follow‐up: unclear, as loss to follow‐up was considered an exclusion criterion for the study Sample size calculated: yes, but methods are not clearly reported: "The sample size was measured according to the descriptive studies formula, and the P value was calculated based on the number of controls with carbamazepine poisoning in Loghman Hospital, in previous years." |
|
| Participants |
Sex: 28 males and 40 females Age: 24.2 years, range 13‐65 years Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants admitted to the poisoning ward with history of carbamazepine poisoning: Dose average (range): 6.8 g (1.2‐24 g) Delay between drug intake and admission average (range): 7.44 h (0.5 h to 15 h) Inclusion criteria: poisoning confirmed by clinical examination and paraclinical tests Exclusion criteria: taken other drugs or unknown drugs, hospitalization not needed, left hospital before completion of treatment, not possible to confirm poisoning by carbamazepine |
|
| Interventions |
Intervention arm: Type: MDAC via nasogastric tube + supportive treatment (including gastric lavage) Timing: every 4 h Dose: 100 g AC per dose Frequency: several, but unknown number of doses Integrity: no information Control arm: Type: SDAC via nasogastric tube + supportive treatment (including gastric lavage) Timing: no information provided, presumably after poisoning confirmation Dose: not specified, but likely 100 g AC Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): duration of hospitalization (h) (see Table 21) Timing: no information |
|
| Funding | No information | |
| Notes | All 8 ICU participants, with grade III or IV level of unconsciousness received the multi‐dose treatment, thus seem not to have been randomized and were therefore not included in the analysis of hospitalization duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In all patients admitted to I.C.U. and 30 patients of the ward, multiple doses of charcoal were administered, whereas the resting 30 patients ‐who were chosen randomly‐ received single doses of charcoal" Comment: not enough information to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In all patients admitted to I.C.U. and 30 patients of the ward, multiple doses of charcoal were administered, whereas the resting 30 patients ‐who were chosen randomly‐ received single doses of charcoal" Comment: not enough information to make a judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The following patients were excluded from the study: those who left the hospital before the treatment process was completed" Comment: participants that did not complete the treatment process were excluded and no data is available on the number of people |
| Selective reporting (reporting bias) | High risk | Occurrence of complications not clearly described: unclear in which group they occurred. Drug and symptom monitoring are crucial outcomes that are lacking in this study. Especially for symptom monitoring, which was described as being the criterion for hospital discharge, this is problematic. |
| Other bias | Low risk | No other risk of bias detected |
Bouget 1989.
| Methods |
Study design: randomized controlled trial Study duration: one month Setting: hospital setting (emergency department of a regional hospital) Country: France Number of individuals randomized: 36 Number of individuals receiving the intervention: 19 Number of individuals receiving the control: 17 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: MDAC group: 16 female and 3 male Control group: 12 female and 5 male Age: MDAC group: 31 (SD 3.6) years Control group: 30 (SD 3.5) years Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants presenting with a deliberate overdose of benzodiazepines, with or without concomitant alcohol ingestion Inclusion criteria: overdose of benzodiazepines of any sort, with or without alcohol Exclusion criteria: pregnant women, participants < 18 years old, concomitant ingestion of other drugs |
|
| Interventions |
Intervention arm: Type: MDAC (Carbomix) + supportive treatment including gastric lavage and infusion of a an isotonic solution (5% glucose, enriched with 2 g/L KCl & 4 g/L NaCl) Timing: after gastric lavage, and after 4 h, 8 h, 12 h Dose: 50 g Frequency: 4× Integrity: no information Control arm: Type: supportive treatment including gastric lavage and infusion of a an isotonic solution (5% glucose, enriched with 2 g/L KCl and 4 g/L NaCl) Timing: gastric lavage: upon admission. Infusion: every 12 h Dose: Gastric lavage: 15 L Infusion: 1 L/time Frequency: Gastric lavage: 1× Infusion: every 12 h Integrity: no information |
|
| Outcomes |
Type (unit): Glasgow Coma Scale score Heart rate Blood pressure Diuresis Temperature Benzodiazepine concentration Timing: At 0 h and 12 h after intervention (early) Blood samples were drawn every 4 h until discharge (early) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By drawing of a lot" Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "By drawing of a lot" Comment: not enough information to make a judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect certain outcomes at study (e.g. Glasgow Coma Scale scores) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about incomplete outcomes |
| Selective reporting (reporting bias) | High risk | Outcomes are not reported in such a way that any interpretation is possible |
| Other bias | Low risk | No other risk of bias detected |
Brahmi 2006.
| Methods |
Study design: randomized controlled trial Study duration: January to June 2004 Setting: 8 men and 4 women Country: hospital setting (intensive care and toxicological unit) Number of individuals randomized: 12 Number of individuals receiving the intervention: 6 Number of individuals receiving the control: 6 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: 8 men and 4 women Age: 27.6 (SD 12.2) years Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants admitted with a history of carbamazepine (CBZ) poisoning. Participants did not receive gastric lavage and received the same symptomatic and supportive treatment, as needed. Poisoning symptoms at admission: SAPS II score: 16.37 (SD 8.46) APACHE II score: 8 (SD 3.96) Glasgow coma score of the comatose participants (6): 8.28 (SD 1.6) CBZ concentration: 29.42 (SD 6.68) mg/L Inclusion criteria: history of CBZ ingestion, clinical features of poisoning, and laboratory findings using gas chromatography Exclusion: children, mixed poisoning |
|
| Interventions |
Intervention arm: Type: MDAC via nasogastric tube Timing: every 6 h Dose: 50 g AC Frequency: variable, until carbamazepine blood levels drop below 12 mg/L Integrity: no information Control arm: Type: SDAC via nasogastric tube Timing: no information Dose: 1 g/kg AC Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): Cmax (mg/L) T1/2 (h) Ventilation required Duration of ventilation (h) Duration of coma (h) Length of stay (h) (see Table 21) Timing: blood levels measured every 3 h until the peak and then every 6 h. (intermediate) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Once CBZ poisoning was retained, no gastric lavage was done, and patients were randomized in 2 groups." Comment: not enough information to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Once CBZ poisoning was retained, no gastric lavage was done, and patients were randomized in 2 groups." Comment: not enough information to make a judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect certain outcomes studied (e.g. decision to discharge) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up described |
| Selective reporting (reporting bias) | High risk | No AUC or potential adverse events reported |
| Other bias | High risk | A clinical difference is suspected between intervention and control group, based on the divergent carbamazepine kinetics |
Comstock 1982.
| Methods |
Study design: randomized controlled study Study duration: October 1975 to June 1976 Setting: hospital (emergency department of the Ben Taub hospital, Houston, Texas) Country: USA Number of individuals randomized: 339 Number of individuals receiving the intervention: 131 Number of individuals receiving the control: 208 Number of individuals lost to follow‐up:277 at the start of follow‐up, 308 at the 3‐5 h sample Sample size calculated: no information |
|
| Participants |
Sex: no information Age: no information Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants presenting with illness associated with acute oral drug overdose, and at the discretion of the attending physician selected for gastric lavage. Of these, chemical evidence of intake of sedative‐hypnotics or aspiring in the blood and at least 2 blood samples (1 at lavage and 1 afterwards) were available for 62 participants (25 AC and 37 control participants) Inclusion criteria: taken a sedative‐hypnotic or aspirin Degree of functional decompensation: moderate ‐ slight impairment to unconscious, normal gag reflex and deep tendon reflexes, responsive to superficial pain; severe ‐ unconscious with depressed or absent pain response, gag reflex and deep tendon reflexes to respiratory arrest Chemical proof of intake of sedative‐hypnotics or aspirin |
|
| Interventions |
Intervention arm: Type: gastric lavage + activated charcoal via nasogastric tube Timing: no information Dose: gastric lavage followed by 100 g AC Frequency: 1× Integrity: no information Control arm: Type: gastric lavage Timing: no information Dose: no information Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): percentage of participants showing increased blood drug concentrations (%) (data not extracted) Timing: blood samples were taken at the time of lavage and at 2 h to 4 h intervals thereafter when possible (early) |
|
| Funding | Supported by grant from the National Institute of Drug Abuse: grant 1 H81 DA 0175301 | |
| Notes | Of the initially 339 selected participants, only 62 had chemical proof of sedative‐hypnotics or aspirin at at least 1 blood sample available, which constituted the study sample | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following gastric lavage, 131 patients were randomly chosen to receive a slurry of 100 g of activated charcoal (Norit A) via the gastric tube." Comment: not enough information to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Following gastric lavage, 131 patients were randomly chosen to receive a slurry of 100 g of activated charcoal (Norit A) via the gastric tube." Comment: not enough information to make a judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information to support judgment, but lack of blinding is not likely to affect measurement of the outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the total population, 25 AC treated patients and 37 control patients had chemical evidence for the presence of one of the sedative‐hypnotics listed in Tables la and lb or aspirin in the blood, and at least one blood sample in addition to the sample taken at lavage. These 62 patients constituted the population under study." Comment: only data from 62 out 339 randomized patients (18%) is presented. Loss to follow‐up increases over time |
| Selective reporting (reporting bias) | High risk | Several clinically relevant outcomes (symptoms, adverse events) not reported |
| Other bias | High risk | Quote: "995 were ingestions by history and 339 or 34% were selected for gastric lavage at the discretion of the attending physician." Comment: potential bias during selection of the study population |
Cooper 2005.
| Methods |
Study design: randomized controlled trial Study duration: July 1999 to October 2000 Setting: hospital (emergency department at tertiary referral teaching hospital, the Canberra Hospital) Country: Australia Number of individuals randomized: 327 Number of individuals receiving the intervention: 166 Number of individuals receiving the control: 161 Number of individuals lost to follow‐up: 0 Sample size calculated: yes, a power of 80% to detect a 33% reduction in length of stay at the 5% level was anticipated |
|
| Participants |
Sex: Control: 48 males and 113 females Intervention: 50 males and 116 females Age: Control: median age 28.5 years, IQR: 21.5‐42.5 years Intervention: median age 31.5 years, IQR: 21‐42 years Country (if different from study authors'): NA Type, dose and timing of poisoning: participants presenting at the emergency department with a history of oral drug overdose. Benzodiazepines and paracetamol combined accounted for most of the overdoses (62‐66%). 31‐35% ingested more than one drug. Most subjects (57‐59%) presented within 2 h after overdose. Glasgow Coma Scale was < 15 in 27‐30% of cases. Inclusion criteria: ≥16 years, within 12 h following a deliberate oral overdose, thought to have ingested a substance adsorbed by AC Exclusion criteria (at discretion of treating physician): ingested a potentially toxic modified release preparation, presentation within 1 h of ingestion of a highly lethal substance (e.g. large doses of tricyclic antidepressants, antineoplastic medications, aspirin, cardioactive agents) Exclusion criteria: transferred, ingested substances not significantly adsorbed by AC (hydrocarbons, acids, alkalis), contraindications (unprotected airway, non‐intact gastrointestinal tract) |
|
| Interventions |
Intervention arm: Type: activated charcoal orally or via nasogastric tube + other treatment appropriate to the substances ingested Timing: after randomization Dose: 50 g (Norit‐C) in 200 mL of water as slurry Frequency: 1× Integrity: 3 participants refused charcoal Control arm: Type: no activated charcoal, only other treatment appropriate to the substances ingested Timing: after randomization Dose: NA Frequency: NA Integrity: 1 received charcoal |
|
| Outcomes |
Type (unit): Primary: Medical length of hospital stay (h) (see Table 21) Secondary: Requirement for ventilation Vomiting after admission Occurrence of aspiration Occurrence of death Timing: during hospital admission (intermediate) |
|
| Funding | The study was funded by the Australian Rotary Foundation and the Private Practice Trust Fund of The Canberra Hospital. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to AC or no gastrointestinal decontamination, as indicated by the sealed sequentially numbered envelope contents." Comment: not enough information to make a judgment |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized to AC or no gastrointestinal decontamination, as indicated by the sealed sequentially numbered envelope contents." Comment: adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The treating medical staff were not blinded to the administration of AC, as this would be very difficult to achieve." Comment: not blinded, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This decision was made by a senior member of the medical staff, but this was not usually a toxicologist or any other member of the study team... The coordinator and data manager of the study was never involved in the decision to medically discharge the patient." Comment: suggests that members of the study team are at least in some cases involved in outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis was based on intention to treat..." Comment: analysis was appropriate, and there was very little deviation from protocol |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes are reported |
| Other bias | Low risk | No other risk of bias detected |
Crome 1983.
| Methods |
Study design: randomized controlled trial Study duration: no information Setting: hospital: accident departments of multiple hospitals Country: UK Number of individuals randomized: 48 Number of individuals receiving the intervention: 20 Number of individuals receiving the control: 28 Number of individuals lost to follow‐up: 33 Sample size calculated: no information |
|
| Participants |
Sex: 10 males and 38 females Age: 10‐21 years: n = 7 22‐31 years: n = 12 32‐41 years: n = 8 42‐51 years: n = 7 52‐61 years: n = 4 62‐71 years: n = 2 72‐81 years: n = 1 unknown: n = 7 Country (if different from study authors'): NA Type, dose and timing of poisoning: Presenting at the accident department with suspected overdose of tricyclic antidepressants and considered required to be hospitalized. Mixed dose were okay if tricyclic antidepressants were considered responsible for the symptoms. Of these 48 cases, 17 had taken tricyclic antidepressants alone, 13 in combination with other drugs, 7 had not taken antidepressants and 11 did not have significant amounts of any drug in their blood Inclusion criteria: symptoms considered to be caused by tricyclic antidepressants, patient will be admitted to hospital |
|
| Interventions |
Intervention arm: Type: activated charcoal in water suspended (+ supportive care), given through a nasogastric tube after gastric lavage in obtunded patients and as a drink in conscious and co‐operative patients Timing: no information Dose: 10 g in 200 mL water Frequency: 1× Integrity: no information Control arm: supportive care, which might include gastric lavage, otherwise not specified Type: NA Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Grade of coma Presence of convulsions/movement disorders Presence of pyramidal signs Anticholinergic signs Airway inserted, intubated and/or ventilated Heart rate, rhythm, ECG Blood pressure Respiratory rate Timing: Clinical information was recorded on admission and at 4 h, 8 h and 24 h and at discharge (intermediate) Blood samples were collected on admission and at 4, 8 and 24 h (intermediate) Urine and gastric washings were collected. |
|
| Funding | Grant from Leo Research Foundation pharmaceutical company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Local randomisation by sealed envelopes" (personal communication) Comment: not enough information to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Local randomisation by sealed envelopes" (personal communication) Comment: sealed envelopes were kept in emergency departments of participating hospitals, but not specified whether these were opaque and sequentially opened |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Were outcome assessors blinded? No" (personal communication) Comment: lack of blinding might affect measurement of the outcomes at study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the main outcome reported, coma, only data for pure tricyclic antidepressant were reported and not for the other subgroups. Although 17 participants are reported to make up this subgroup, data for 15 participants is reported. Participants that refused to ingest AC were not included in the analysis, no intention‐to‐treat analysis (personal communication) |
| Selective reporting (reporting bias) | High risk | Pre‐specified outcomes presence of convulsions/movement disorders, presence of pyramidal signs, anticholinergic signs, airway inserted, intubated and/or ventilated, heart rate, heart rhythm, ECG, blood pressure and respiratory rate were not reported |
| Other bias | High risk | In 11 of the 48 participants no significant amounts of any drugs were found and 7 of them had not taken any tricyclic antidepressant Role of the study funder not clarified |
De Silva 2003.
| Methods |
Study design: randomized controlled trial Study duration: November 2001 to June 2002 Setting: hospital setting (accident and emergency department of the Kurunegala Teaching Hospital) Country: Sri Lanka Number of individuals randomized: 401 Number of individuals receiving the intervention: 201 Number of individuals receiving the control: 200 Number of individuals lost to follow‐up: 23 Sample size calculated: yes, a sample size of 376 was calculated to detect a decrease in death rate from 10% to 2.5% with 80% power at the 5% level |
|
| Participants |
Sex: SDAC: 111 males and 89 females MDAC: 87 males and 114 females Age: SDAC: 24.1 (SD 8.7) years MDAC: 23.5 (SD 9.6) years Country (if different from study authors'): NA Type, dose and timing of poisoning: yellow oleander seed poisoning, presenting within 24 h of ingestion Inclusion criteria: yellow oleander tree poisoning; 12‐70 years old; presenting within 24 h of poisoning Exclusion criteria: taken another drug (e.g. alcohol, organophosphates, paracetamol, or sedatives); debilitating disease (diabetes mellitus, hepatic or renal disease, heart failure, or malignant disease); abdominal surgery within the past year; known hypersensitivity to AC; severe infection; pregnant and lactating women |
|
| Interventions |
Intervention arm: Type: MDAC and supportive care (which included gastric lavage, followed by a first dose of AC, atropine and metoclopramide as required) Timing: initial dose on admission, after gastric lavage, additional doses every 6 h for 3 days: 0 h, 6 h, 12 h, 18 h, 24 h, 30 h, 36 h, 42 h, 48 h, 54 h, 60 h, 66 h, 72 h Dose: 50 g in 400 mL water Frequency: 13× Integrity: 16 discharged themselves before end of treatment. Although most participants found the charcoal unpalatable, none refused to take it. Control arm: Type: SDAC + water and supportive care (which included gastric lavage, atropine and metoclopramide as required) Timing: on admission, after gastric lavage Dose: 50 g dose AC + 400 mL, followed by 400 mL water every 6 h as placebo Frequency: 1× Integrity: 10 discharged themselves before end of treatment |
|
| Outcomes |
Type (unit): Primary outcome: Death Secondary outcomes: ICU admission Participants given anti‐digoxin antibody Fab fragments Cardiac pacing Life‐threatening arrhythmias at 24 h Atropine administered (mg) Boluses of atropine administered Time in hospital (days) (see Table 21) Patient response to treatment Bowel sounds Timing: data collected until death or discharge from hospital (intermediate) |
|
| Funding | The University of Kelaniya gave financial support for the study. | |
| Notes | Sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors declared no conflict of interest 23/26 participants that left the hospital before end of treatment were confirmed to be alive and well within 1 week of leaving the hospital. 3 that could not be contacted were considered and analysed as being alive. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "6 h after admission, an investigator (AP) used a computer‐generated random‐allocation table to allocate patients ... This investigator was not involved in care or assessment of patients." Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "6 h after admission, an investigator (AP) used a computer‐generated random‐allocation table to allocate patients ... This investigator was not involved in care or assessment of patients." Comment: adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators were unaware of patients' treatment allocation. Three medically qualified research assistants supervised administration of activated
charcoal or sterile water, but they did not participate in clinical assessment or management of patients. To facilitate blinding, research assistants also ensured that patients and their bedclothes were cleaned thoroughly after each treatment." Comment: participants were not blinded, but lack of blinding is not likely to affect the outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators were unaware of patients' treatment allocation. Three medically qualified research assistants supervised administration of activated charcoal or sterile water, but they did not participate in clinical assessment or management of patients. To facilitate blinding, research assistants also ensured that patients and their bedclothes were cleaned thoroughly after each treatment." Comment: adequate, outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis was by intention to treat.", "26 (16 in the treatment group) patients discharged themselves within 72 h of admission; all had normal heart rates at the time they left hospital, and 23 (16 in the treatment group) reported being well when contacted at their homes within 1 week" Comment: low attrition rate (6%), which was accounted for and analysed as intention‐to‐treat |
| Selective reporting (reporting bias) | High risk | Quote: "The most frequent adverse effects of treatment with multiple doses of activated charcoal were diarrhoea and abdominal discomfort. Three patients had diarrhoea and 13 complained of abdominal discomfort." Comment: potential adverse events not clearly described for the control group and for the outcome 'boluses of atropine administered' statistical analyses and reported summary effect were not clear |
| Other bias | Low risk | No other risk of bias detected |
Eddleston 2008.
| Methods |
Study design: randomized controlled trial Study duration: Total: 31 March 2002 to September 2004 Anuradhapura: 31 March 2002 to September 2004 Polonnaruwa: 4 June 2002 to September 2004 Kurunegala: 23 November 2002 to 3 Febrary 2003 Setting: hospital setting (medical wards of 3 Sri Lankan secondary hospitals) Country: UK Number of individuals randomized: 4632 Number of individuals receiving the intervention: SDAC: 1545 MDAC: 1533 Number of individuals receiving the control: 1554 Number of individuals lost to follow‐up: 3 Sample size calculated: yes, a total sample size of 4200 was calculated to measure a decrease in mortality from 10% to 7%, with 80% power at the 5% level |
|
| Participants |
Sex: No AC: 915 men and 639 women SDAC: 883 men and 662 women MDAC: 960 men and 573 women Age: No AC: 25 (19‐35) SDAC: 25 (19‐35) MDAC: 25 (19‐36) Country (if different from study authors'): Sri Lanka Type, dose and timing of poisoning: participants admitted to the medical ward of 3 secondary referral hospitals with a history of oral poisoning Median time between ingestion and admission (h, mean (IQR)) Usual care: 4.2 (2.7 to 7.0) SDAC: 4.2 (2.7 to 7.1) MDAC: 4.3 (2.7 to 7.1) Type of poisoning: Usual care: Oleander: 555 Organophosphorus/carbamate pesticide: 441 Organochlorine: 4 Other/unknown pesticide or paraquat: 343 Medicine or unknown: 211 SDAC: Oleander: 550 Organophosphorus/carbamate pesticide: 440 Organochlorine: 3 Other/unknown pesticide or paraquat: 340 Medicine or unknown: 212 MDAC: Oleander: 542 Organophosphorus/carbamate pesticide: 429 Organochlorine: 3 Other/unknown pesticide or paraquat: 345 Medicine or unknown: 214 Participants were stabilized upon admission by resuscitation, by airway stabilization and providing oxygen, atropine, fluid and antidotes, as necessary, before intervention started Exclusion criteria: < 14 years, prior treatment with AC during this episode of poisoning, known pregnancy, ingestion of corrosives or hydrocarbons alone, requirement for oral medication, inability to intubate the patient with a Glasgow coma score < 13, presentation > 72 h after ingestion, previous recruitment, < 16 years old or unconscious without relatives present to give consent |
|
| Interventions |
Intervention arm: Type: SDAC or MDAC orally or via nasogastric tube + supportive care (see control) Timing: SDAC: "soon" after admission MDAC: every 4 h Dose: 50 g superactivated charcoal (Carbomix) in 300 mL water per dose Frequency: SDAC: 1× MDAC: 6× Integrity: first protocol intended to deliver 18 doses of AC in the multi‐dose group, this however was not feasible, so protocol was adapted to 6 doses. Compliance was not anticipated a problem as was given while patient was under supervision; however participants were not forced. An analysis of compliance was done in 2 of the 3 hospitals, involving 1103 participants, showing that compliance decreased to 66% by the 6th dose of AC. Furthermore, an estimated 8% of the first dose of AC was vomited. This amount decreased to 1% by the sixth dose. < 5% did not receive allocated intervention (reasons included damaged throat and refusal) Control arm: Type: supportive care: resuscitated if needed, stabilized and given oxygen and antidotes as necessary. Atropine (usually 0.3–0.6 mg/h) and intravenous fluids were administered as needed to maintain a heart rate > 70 bpm and systolic blood pressure > 80 mm Hg. Participants with severe cardiotoxicity either were administered antidigoxin Fab antitoxin or treated with temporary pacing. Most participants, 54% and 7.5% respectively, received forced emesis or gastric lavage prior to arriving at the study hospital. Initially gastric lavage was never performed at study hospital. However, after patient 1905, in participants presenting less than 2 h with significant poisonings gastric lavage was performed (3 × 300 mL) Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Primary outcome: All‐cause mortality Secondary outcomes (per ingested poison): For organophosphorus or carbamate pesticide: Intubation Time ventilated Time to first ventilation Seizures For oleander poisoning: Cardiac dysrhythmias needing digoxin‐specific antibody fragments, serum potassium > 6.0 mmol/L or temporary pacing Timing: participants were seen at least every 3 h and more if needed. Condition of participants was recorded twice per day at 8:30 and 20:30. Significant events (intubation, seizures, death) were recorded at time of the event (intermediate) |
|
| Funding | Grant 063560 from the Wellcome Trust's Tropical Interest Group to ME. The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant 071669. | |
| Notes | The funding source had no role in study design, data collection, data analysis, and data interpretation; or writing of the report; or in the decision to submit for publication. The authors declared no conflict of interest. Trial registration number: ISRCTN02920054 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence was generated by computer and incorporated into a Microsoft Access programme written for patient recruitment, randomisation and event recording (Figure 1). Stratified block randomisation was performed using the following strata:" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequences were generated independently by the study statistician (EJ) and implemented by the programmer (SA), neither of whom had a role in patient recruitment, treatment or assessment. Variable block sizes of 3, 6 and 9 were used to allocate patients in equal numbers to each treatment group... Randomisation occurred after the patient's baseline data had been entered and receipt of consent noted, and could not be manipulated by study doctors. The recruiting doctor was unable to predict allocation before randomisation." Comment: adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Another limitation was the absence of masking. We believed that masking was difficult because of the impossibility to conceal from a reviewing doctor whether a patient had received any charcoal. An absence of masking might have allowed for performance bias for the secondary outcomes. To counter this potential bias, the medical team made decisions about intubation and transfer of patients independently of the study doctors." Comment: participants and personnel were not blinded, which may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Another limitation was the absence of masking. We believed that masking was difficult because of the impossibility to conceal from a reviewing doctor whether a patient had received any charcoal. An absence of masking might have allowed for performance bias for the secondary outcomes. To counter this potential bias, the medical team made decisions about intubation and transfer of patients independently of the study doctors." Comment: outcome assessors were not blinded, but primary outcome was unambiguous |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We did an intention‐to‐treat analysis on all patients with available outcomes data (loss to follow‐up of three (< 1%) patients) analysed in the groups to which they were allocated." Comment: adequate |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported |
| Other bias | Low risk | No other risk of bias detected |
Hultén 1988.
| Methods |
Study design: randomized controlled trial Study duration: no information Setting: hospital setting Country: Sweden Number of individuals randomized: 91 Number of individuals receiving the intervention: 34 Number of individuals receiving the control: 43 Number of individuals lost to follow‐up: 14 Sample size calculated: no information |
|
| Participants |
Sex: no information Age: older than 14 years Country (if different from study authors'): UK, Belgium, Sweden Type, dose and timing of poisoning: 32 participants took amitriptyline, 16 clomipramine, 10 mianserin, 9 imipramine, 6 dothiepin, 2 doxepin, 2 nortriptyline, mixed overdoses in 67% with most commonly benzodiazepines or alcohol Inclusion criteria: participants with self‐poisoning with 1 or more of 7 different TCA (mixed overdoses also included if clinician considered 1 of 7 TCA drugs was major cause of participants' symptoms) Exclusion criteria: participants < 14 years old, taken significant amount of other drugs Plasma TCA concentration < 0.3 µg/L |
|
| Interventions |
Intervention arm: Type: activated charcoal (MedicoalR) after gastric lavage Timing: no information Dose: 20 g Frequency: 1× Integrity: no information Control arm: Type: gastric lavage Timing: no information Dose: no information Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): AUC ((mg/L) × h) Blood pressure (mmHg) Heart rate (bpm) Coma grade (Matthew‐Lawson coma scale) Symptoms: e.g. convulsions, arrhythmias, muscle twitching Number of participants intubated Time spent intubated Time admitted to ICU (see Table 21) Time admitted to hospital (see Table 21) Timing: Plasma drug concentration at 0 h, 1 h, 2 h, 4 h, 8 h and 24 h (intermediate) Blood pressure, heart rate, coma grade and symptoms at 0 h, 4 h, 8 h and 24 h (intermediate) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by using rand numbers and equilibration made by groups of 10." Comment: adequate randomization |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by using rand numbers and equilibration made by groups of 10." Comment: not enough information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "14 patients were excluded because they had taken a significant amount of other drugs" Comment: adequate explanation |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported |
| Other bias | Low risk | No other risk of bias detected |
Ilett 1977.
| Methods |
Study design: randomized controlled trial Study duration: no information Setting: hospital setting (emergency centre at Royal Perth Hospital) Country: Australia Number of individuals randomized: 120 Number of individuals receiving the intervention: USP 15 mL: 38 APF 15 mL: 34 Number of individuals receiving the control: APF 30 mL: 33 Number of individuals lost to follow‐up: 15 Sample size calculated: no information |
|
| Participants |
Sex: 100 females, 20 males Age: Females: 27 (SD 10) years Males: 29 (SD 8.9) years Range: 13‐64 years Country (if different from study authors'): NA Type, dose and timing of poisoning: benzodiazepine tranquillizers or hypnotics (n = 37), other tranquillizers (n = 4), other hypnotics (n = 18), antidepressants (n = 7), analgesics (n = 30), antihistamines (n = 3), miscellaneous drugs and chemicals (n = 26) Exclusion criteria: only partial dose was taken (n = 2), left monitored field (n = 2), physician in charge ordered alternative treatment because of deterioration of patient's condition, insufficient data collected |
|
| Interventions |
Intervention arm 1: Type: syrup of ipecacuanha formulated according to the American Pharmacopeia (USP), 0.12% w/v alkaloid content. Timing: upon admission Dose: 15 mL followed by 200 mL of water Frequency: 1×, participants who did not vomit within 30 minutes of the first dose were given a second identical dose and an additional 200 mL of water Integrity: no information Intervention arm 2: Type: syrup of ipecacuanha formulated according to the Australian Pharmaceutical Formulary (APF) 0.14 w/v alkaloid content. Timing: upon admission Dose: 15 mL followed by 200 mL of water Frequency: 1×, participants who did not vomit within 30 minutes of the first dose were given a second identical dose and an additional 200 mL of water. Integrity: no information Intervention arm 3: Type: syrup of ipecacuanha APF Timing: upon admission Dose: 30 mL followed by 200 mL of water Frequency: 1×, participants who did not vomit within 30 minutes of the first dose were given a second identical dose and an additional 200 mL of water Integrity: no information |
|
| Outcomes |
Type (unit): Incidence of vomiting (data not extracted) Time to vomit (min) (data not extracted) Number of times vomiting occurred (data not extracted) Volume of vomitus (mL) (data not extracted) Timing: on occurrence (no information) |
|
| Funding | No information | |
| Notes | Data for syrup of ipecacuanha USP vs syrup of ipecacuanha APF not extracted, because not within scope of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated sequentially to the treatments, which had been previously randomized by means of a table of random numbers". Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were allocated sequentially to the treatments, which had been previously randomized by means of a table of random numbers". Comment: not enough information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The trial was conducted at a double blind design" Comment: not enough information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The trial was conducted at a double blind design" Comment: not enough information to support judgment, but lack of blinding may affect outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/120 (12.5%) is lost to follow‐up: 2 only took partial dose, 2 left monitored field, 6 received alternative treatment because of deterioration of condition, 5 with insufficient data. Not a high attrition rate, adequately explained |
| Selective reporting (reporting bias) | High risk | No reporting of potential adverse events. No data on symptom severity or drug absorption/dug recovery |
| Other bias | Low risk | No other risk of bias detected |
James 1995.
| Methods |
Study design: randomized controlled trial Study duration: February 1993 to January 1994 Setting: hospital setting (emergency department) Country: USA Number of individuals randomized: 119 Number of individuals receiving the intervention: Sorbitol: 32 Magnesium citrate: 33 Magnesium sulphate: 23 Number of individuals receiving the control: 28 Number of individuals lost to follow‐up:3 Sample size calculated: sample size calculations determined that a minimum of 25 participants were needed in each treatment group to detect a difference in mean time to the first stool of 4 h, using a power of 0.80 and an α of 0.10 |
|
| Participants |
Sex: no information Age: 25 (SD 8) months, range 1‐5 years Country (if different from study authors'): NA Type, dose and timing of poisoning: variety of toxins (analgesics, anticonvulsants, antihistamines and decongestants, asthma therapies, automotive products, cardiovascular drugs, gastrointestinal preparations, insecticides, mushrooms, psychotropic drugs, rodenticides, topicals, miscellaneous drugs) Inclusion criteria: suspected acute ingestions in which activated charcoal and a cathartic were indicated. Parents of participants had to have telephone access for follow‐up purposes. |
|
| Interventions |
Intervention arm 1: Type: sorbitol All treatments were administered as a slurry with 1 g/kg activated charcoal. Participants also received syrup of ipecac or gastric lavage. Timing: as soon as possible Dose: 50% solution, 2 g/kg. Administered as a slurry with 1 g/kg activated charcoal per nasogastric tube. Frequency: 1×, if emesis occurred after administration of the cathartic/charcoal slurry, additional doses were administered at the discretion of the attending physician in the emergency department Integrity: no information Intervention arm 2: Type: magnesium citrate All treatments were administered as a slurry with 1 g/kg activated charcoal. Participants also received syrup of ipecac or gastric lavage Timing: as soon as possible Dose: 233 mg/kg. Administered as a slurry with 1 g/kg activated charcoal per nasogastric tube. Frequency: 1×, if emesis occurred after administration of the cathartic/charcoal slurry, additional doses were administered at the discretion of the attending physician in the emergency department Integrity: no information Intervention arm 3: Type: magnesium sulphate All treatments were administered as a slurry with 1 g/kg activated charcoal. Participants also received syrup of ipecac or gastric lavage Timing: as soon as possible Dose: 6.25% solution, 250 mg/kg. Administered as a slurry with 1 g/kg activated charcoal per nasogastric tube Frequency: 1×, if emesis occurred after administration of the cathartic/charcoal slurry, additional doses were administered at the discretion of the attending physician in the emergency department Integrity: no information Control arm: Type: water Timing: as soon as possible Dose: no information Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): Mean time to stool (h) (data not extracted) Number of stools during 24 h (data not extracted) Occurrence of side effects Timing: Telephone follow‐ups at 1 h, 4 h, 8 h and 24 h after completion of cathartic administration (intermediate). |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Physicians, nurses and parents were blinded. Cathartics were formulated for delivery at a uniform volume in opaque bottles." Comment: adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Poison control centre staff who conducted telephone follow‐ups were blinded" Comment: adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were lost to follow‐up, and not included in the analysis. Low attrition rate: 2.5% |
| Selective reporting (reporting bias) | High risk | Important outcomes, clinical outcomes, are not measured |
| Other bias | Low risk | No other risk of bias detected |
Kornberg 1991.
| Methods |
Study design: randomized controlled trial Study duration: 2 years (November 1987 to November 1989) Setting: hospital setting (pediatric emergency department of the Children's Hospital of Buffalo) Country: USA Number of individuals randomized: 70 Number of individuals receiving the intervention: 32 Number of individuals receiving the control: 38 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: SOI: 39 boys, 31 girls; AC: 17 boys and 15 girls AC: 22 boys and 16 girls Age: SOI and AC group: 2.5 (SD 0.2) years AC alone group: 2.3 (SD 0.2) years Country (if different from study authors'): NA Type, dose and timing of poisoning: wide variety of ingested substances, most common was acetaminophen Inclusion criteria: orally poisoned participants less than 6 years old presenting to the ED Exclusion criteria: not awake or without a definite gag reflex, deteriorating level of consciousness, vomited or received SOI before ED arrival, or ingested hydrocarbons, corrosives, iron, ethanol alone or acetaminophen alone if more than 6 h before ED arrival |
|
| Interventions |
Intervention arm: Type: SOI + 160 mL tap water or apple juice + activated charcoal (Actidose) with sorbitol Timing: SOI: on admission, AC after vomiting occurred (mean time 2.1 h after SOI) Dose: SOI: 15 mL, AC: 1 g/kg premixed with 40% sorbitol Frequency: 1× but repeated if no emesis occurred after 30 min Integrity: no information. Control arm: Type: activated charcoal (Actidose) with sorbitol Timing: on admission Dose: 1 g/kg with 40% sorbitol Frequency: 1× Integrity: AC was presented orally, but if patient was unwilling or unable to take AC orally, it was given by nasogastric tube |
|
| Outcomes |
Type (unit): Time to ED (h) (see Table 21) Time in ED (h) (see Table 21) Time to receive AC (h) Hospital admission Improved in ED Emesis of AC Time in ED (if discharged) (h) (see Table 21) Timing: On occurrence (early) |
|
| Funding | Study was not funded (personal communication) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "They were randomized into two groups based on the date of arrival." Comment: randomization based on even/odd days |
| Allocation concealment (selection bias) | High risk | Quote: "They were randomized into two groups based on the date of arrival." Comment: allocation based on the date of arrival does not allow for adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding (personal communication), which might affect the subjective outcomes admission to ED, improvement in ED, time to discharge from ED |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding (personal communication), which might affect subjective outcome measures, such as admission to ED, improvement in ED, time to discharge from ED |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported, all subjects included in analysis |
| Selective reporting (reporting bias) | Low risk | No reason to believe there is reporting bias |
| Other bias | Low risk | No other risk of bias detected |
Kulig 1985.
| Methods |
Study design: randomized controlled trial Study duration: 18 months (June 1981 to December 1982) Setting: hospital setting (emergency department of Denver General Hospital) Country: USA Number of individuals randomized: 630 Number of individuals receiving the intervention: 214 ipecac + AC 72 gastric lavage + AC (data not extracted) Number of individuals receiving the control: 262 AC orally 44 AC via nasogastric tube (data not extracted) Number of individuals lost to follow‐up: 38 Sample size calculated: no information |
|
| Participants |
Sex: 268 male and 324 female (based on 592 finally included participants) Age: 29.3 (8 months to 80 years) (based on 592 finally included participants) Country (if different from study authors'): NA Type, dose and timing of poisoning: any kind over oral drug overdose not mentioned in exclusion criteria Exclusion criteria: emesis occurred spontaneously or after administration of activated charcoal; ipecac had been administered prior to arrival; ingested poison was a hydrocarbon, corrosive, iron, strychnine or if acetaminophen was ingested alone; ethanol alone had been ingested |
|
| Interventions |
Intervention arm: Type: syrup of ipecac + activated charcoal‐magnesium sulphate, in addition to vigorous supportive care if needed (including airway support, ventilation, antidotes, anticonvulsants, antiarrhythmic and pressors) Timing: on admission Dose: SOI: no information AC‐magnesium sulphate: 30‐50 g AC mixed with 20 g magnesium sulphate (250 mg/kg for a child) and water Frequency: 1× Integrity: no information Control arm: Type: activated charcoal + magnesium sulphate, in addition to vigorous supportive care if needed (including airway support, ventilation, antidotes, anticonvulsants, antiarrhythmic and pressors) Timing: on admission Dose: 30‐50 g mixed into a slurry with 20 g MgSO4 (or 250 mg/kg for a child) Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): Number of admissions Clinical deterioration Clinical improvement Mortality Timing: on occurrence: ED data were collected on a standard toxicology form created for the study, which detailed the patient's history, physical examination, laboratory data, and clinical course (early) |
|
| Funding | McNeil Consumer Products Company | |
| Notes | Data for gastric lavage vs AC administered via nasogastric tube were not extracted, because not within scope of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients arriving on odd‐numbered days were treated in the traditional manner by receiving syrup of ipecac. Patients presenting on even‐numbered days did not undergo gastric emptying procedures, but only received activated charcoal and the cathartic." Comment: no adequate randomization |
| Allocation concealment (selection bias) | High risk | Quote: "Patients arriving on odd‐numbered days were treated in the traditional manner by receiving syrup of ipecac. Patients presenting on even‐numbered days did not undergo gastric emptying procedures, but only received activated charcoal and the cathartic." Comment: randomization process allows to know in which group the next participants will be allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding, but not possible due to nature of interventions. May affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38 participants were excluded because they were not treated according to protocol. Low attrition rate: 38/630 = 6% |
| Selective reporting (reporting bias) | High risk | No reporting of adverse data such as nausea. Sample sizes of subgroups not reported |
| Other bias | Low risk | No other risk of bias detected |
Merigian 1990.
| Methods |
Study design: randomized controlled trial Study duration: October 1986 to March 1988 Setting: hospital setting Country: USA Number of individuals randomized:820 Number of individuals receiving the intervention: asymptomatic: 220 symptomatic: 163 (data not extracted) Number of individuals receiving the control: asymptomatic: 231 symptomatic: 194 (data not extracted) Number of individuals lost to follow‐up: 5 Sample size calculated: no information |
|
| Participants |
Sex: no significant difference in male/female ratio between AC and control group Age: no significant difference in age between AC and control group Country (if different from study authors'): NA Type, dose and timing of poisoning: self‐reported poisoning with substances other than described in exclusion criteria. Selection criteria: excluded if their presenting history included ingestion of any of the following: acetaminophen > 140 mg/kg, lithium, monoamine oxidase inhibitors, heavy metals, formaldehyde, mushrooms, digitalis, methanol, ethylene glycol, iron, or sustained release products. Diagnostic criteria: AMSE score ≥ 7, GCS of 15 and vital signs in the following ranges: systolic blood pressure between 110 mm Hg and 160 mm Hg, diastolic blood pressure between 60 mm Hg and 100 mm Hg, pulse rate between 60 beats/min and 110 beats/min, temperature between 36.4°C and 37.5°C (oral) |
|
| Interventions |
Intervention arm: Type: oral activated charcoal Timing: no information Dose: 50 g Frequency: 1× Integrity: no information Control arm: Type: observation only Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Clinical deterioration Time in ED (min) (see Table 21) Admission to ICU Intubation Duration of intubation (h) Timing: each patient was observed for 4 h (early) |
|
| Funding | No information | |
| Notes | Data for asymptomatic participants (receiving ipecac or gastric lavage) were not extracted, because these were analyzed as one group (gastric emptying) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients presenting with asymptomatic overdoses were given 50 grams of AC orally on even days and were simply observed without AC on odd days." Comment: alternation is not an adequate randomization method |
| Allocation concealment (selection bias) | High risk | Quote: "Patients presenting with asymptomatic overdoses were given 50 grams of AC orally on even days and were simply observed without AC on odd days." Comment: does not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No unexplained loss to follow‐up. Only small number of participants (5/451 = 1.1%) excluded from analysis due to receiving incorrect treatment |
| Selective reporting (reporting bias) | Low risk | No reason to believe there is reporting bias |
| Other bias | High risk | Study only observes the effects of AC in asymptomatic participants, which are more likely not to experience a benefit from a treatment |
Merigian 2002.
| Methods |
Study design: randomized controlled trial Study duration: inclusion period was 24 months, 1992‐1994, no follow‐up after hospital discharge Setting: hospital setting: emergency department of a regional medical center Country: USA Number of individuals randomized: 1479 Number of individuals receiving the intervention: 404 Number of individuals receiving the control: 1075 Number of individuals lost to follow‐up: 1 Sample size calculated: no information |
|
| Participants |
Sex: 688 males and 791 females Age: 30 (SD 10.4) years (range 22‐82 years) Country (if different from study authors'): USA (61% African‐American, 38% white, < 1% other) Type, dose and timing of poisoning: Participants with a history of recent oral drug overdose. Not specified further. 48% reported ingesting a single agent, 52% ingested 2 or more drugs Exclusion criteria: more than 140 mg/kg paracetamol ingested; inhalation/ingestion of crack; ingestion of mushrooms, volatiles, caustic agents, heavy metals, lithium, iron preparations; participants did not receive a gastric emptying or lavage procedure |
|
| Interventions |
Intervention arm: Type: oral activated charcoal + supportive therapy when necessary (including but not limited to: maintenance of airway, pulmonary hygiene, intubation, circulatory support, assurance of adequate urine output and renal function) Timing: no information Dose: 50 g Frequency: 1× Integrity: 1 patient was excluded from the analysis due to receiving lavage at the emergency department. 4 others received lavage but stayed in the study, 3 in the ICU and one before being transferred to the hospital. Control arm: Type: supportive therapy (including but not limited to: maintenance of airway, pulmonary hygiene, intubation, circulatory support, assurance of adequate urine output and renal function) Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Length of stay in the emergency department (ED) (h) (see Table 21) Length of stay in the intensive care unit (ICU) (h) (see Table 21) Clinical deterioration of symptoms (defined by presence of one of pre‐defined parameters: table 1) Proportion of intubation Duration of intubation (h) Adverse events/complications Incidence of vomiting Timing: on occurrence or at discharge (intermediate) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Our protocol required that patients given OAC be observed in the ED for a minimum of 4 hours on even days. On odd days, patients received supportive observation only, with no OAC administration, for a minimum of 4 hours." Comment: alternation is not an adequate randomization method |
| Allocation concealment (selection bias) | High risk | Quote: "Our protocol required that patients given OAC be observed in the ED for a minimum of 4 hours on even days. On odd days, patients received supportive observation only, with no OAC administration, for a minimum of 4 hours." Comment: randomization process does not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded, which may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors; could influence subjective outcomes studied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient was excluded from the analysis due to receiving lavage at the emergency department. 4 others received lavage but stayed in the study, 3 in the ICU and one before being transferred to the hospital. All received activated charcoal. Low attrition rate: 1/404 (0.25%) |
| Selective reporting (reporting bias) | High risk | No reporting of the amount of intubations in the ED or hospital groups, no reporting of the time of intubation in the ICU group, selectively grouping of the ED and hospital groups for the outcome time of intubation |
| Other bias | High risk | Post hoc analyses according to clinical severity No follow‐up after discharge from the hospital |
Montoya‐Cabrera 1999.
| Methods |
Study design: randomized controlled trial Study duration: blood sampling was done up to 48 h, mean duration between final and initial dose of treatment was 21 h (range 6‐36 h) Setting: hospital setting: toxicology department of a children's hospital Country: Mexico Number of individuals randomized: 14 Number of individuals receiving the intervention: 7 Number of individuals receiving the control: 7 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: no information, but from both sexes. Age: mean: 2 years and 5 months (range 8 months to 8 years and 2 months) Country (if different from study authors'): NA Type, dose and timing of poisoning: children admitted to the toxicology department with suspected overdose of paracetamol of 122 (SD 81) mg/kg (54‐247 mg/kg), with a delay of 60 h (10‐168 h) Inclusion criteria: overdose was defined as an administered dose that was higher than therapeutic (10‐15 mg/kg) and plasma levels of paracetamol were over 20 mg/mL for over 4 h |
|
| Interventions |
Intervention arm: Type: N‐acetylcysteine, as in the control group, followed by AC (with magnesium sulphate), also delivered via the nasogastric tube Timing: AC: every 4 h for 24 h MgSO4: every 12 h Dose: 1 g/kg AC, suspended in 120‐200 mL saline 1 g/kg MgSO4, added to the AC Frequency: 6× AC Integrity: no information Control arm: Type: N‐acetylcysteine, administered via a nasogastric tube Timing: upon admission and every 4 h Dose: initial dose of 140 mg/kg, followed by repeat doses of 70 mg/kg Frequency: 18× Integrity: no information |
|
| Outcomes |
Type (unit): Elimination half‐life: T1/2 (h) Total body clearance ClB (mL × kg × min) Prothrombin time Aminotransferases ASAT & ALAT (U/dL) (data not extracted) Timing: blood was sampled at 0 h, 24 h and 48 h (intermediate) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomization was achieved |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on blinding, but should not affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of incomplete outcomes. |
| Selective reporting (reporting bias) | High risk | No standard deviations for main outcomes of interest, no information about clinical course of the overdose, especially with regard to the obvious and not further investigated difference in hepatic toxicity between groups |
| Other bias | High risk | Hepatic toxicity marker values suggest a clinically meaningful difference between the 2 treatment groups |
Passeron 1989.
| Methods |
Study design: randomized controlled trial Study duration: no information on recruitment period, delay before presentation to the hospital was comparable between groups mean: intervention: 6.2 (SD 4.6); control: 7 (SD 4.6). Clinical follow‐up was done up to 48 h after administration/no administration of AC. Setting: hospital setting: emergency department of a university hospital Country: France Number of individuals randomized: 32 Number of individuals receiving the intervention: 16 Number of individuals receiving the control: 16 Number of individuals lost to follow‐up: 7 (44%) and 2 (13%) participants in control and intervention group did not have a blood sample at 9 h Sample size calculated: no information |
|
| Participants |
Sex: no information on proportion of males and females Age: intervention: 36.6 (SD: 18.7) years control: 36.6 (SD: 14) years Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants presenting at the emergency department with an overdose (confirmed by positive blood test) of benzodiazepines, barbiturates or imipramine. Participants in the intervention and control groups did not differ with regard to: their initial mean Glasgow Coma Scale score: intervention: 9 (SD 4.5); control: 10 (SD 4.5); drugs taken: intervention: 12 benzodiazepines, 2 barbiturates and 3 imipramine; control: 13 benzodiazepines, 1 barbiturates and 5 imipramine Inclusion criteria: overdose of benzodiazepines, barbiturates or imipramine. Confirmed blood toxicology test |
|
| Interventions |
Intervention arm: Type: AC‐sorbitol, in addition to usual care (gastric lavage), delivered via nasogastric tube Timing: immediately after gastric lavage Dose: 1 g/kg AC in a 70% sorbitol solution Frequency: 1× Integrity: no information Control arm: Type: usual care, consisting of gastric lavage, forced diuresis and supportive treatment of symptoms Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Glasgow Coma Scale Blood pressure Heart rhythm Serum levels of benzodiazepines, barbiturates and imipramine (µg/mL): evolution & proportion with increasing levels) (data not extracted) Side effects of the intervention: gastrointestinal issues, pulmonary complications, electrolyte balance (measured via ionogram, glycemia and acidosis) Timing: Glasgow Coma Scale: at 0 h, 3 h, 9 h, 24 h and 48 h after treatment (intermediate) Serum drug levels, blood pressure, heart rhythm, and side effects at 0 h, 3 h and 9 h after treatment (early) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomization process |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding, but blinding not possible due to differences in treatments. May affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding, might influence assessment of outcomes, such as Glasgow Coma Scale |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Certain patients did not have a blood sample taken at 9 h." Comment: 7 (44%) and 2 (13%) patients in control and intervention group did not have a blood sample at 9 h |
| Selective reporting (reporting bias) | High risk | Not reporting the normal pharmacokinetic outcomes, incomplete reporting of basically every outcome reported, no further reporting of the pre‐specified outcomes heart rhythm and pulse pressure |
| Other bias | Low risk | No other risk of bias detected |
Pond 1995.
| Methods |
Study design: randomized controlled trial Study duration: recruitment period from 4 January 1988 to 11 June 1990 (29 months) Setting: hospital setting: emergency department of a tertiary referral hospital (Princess Alexandra Hospital, Brisbane) Country: Australia Number of individuals randomized: 876 Number of individuals receiving the intervention: 459 (ipecac or lavage) Ipecac: 220 Gastric lavage: 209 Number of individuals receiving the control: 417 (charcoal, oral or nasogastrically) AC: 274 Nasogastric tube: 133 Number of individuals lost to follow‐up: 82 Sample size calculated: post hoc power calculation |
|
| Participants |
Sex: 377 males and 499 females Age: male: 30 (SD 11; range 14‐82 years); female: 30 (SD 1; range 13‐81 years) Intervention: 30 (SD 12 years (range 13‐76 years) Control: 31 (SD 13 years; range 13‐82 years) Country (if different from study authors'): NA Type, dose and timing of poisoning: Participants presenting within 12 h of drug overdose (adsorbing to AC) whether accidental, intended or during recreational use, at the emergency department. Most presented earlier (140 < 1 h). 59% ingested more than 1 drug Ingestion of paracetamol, salicylate, phenothiazines or ethanol, or other drugs Inclusion criteria: history of drug overdose, whether accidental, intended or recreational, > 13 years old Exclusion criteria: ingestion > 12 h before presentation, treated in a way breaching the protocol, gastric emptying contraindicated, gastric emptying indicated for diagnostic purposes, substance not adsorbed by AC. Confirmation of intoxication by measuring in serum/blood |
|
| Interventions |
Intervention arm: Type: gastric emptying, being via ipecac in conscious and gastric lavage in obtunted participants. All participants received activated charcoal (Norit "C" Extra) in a slurry with 200 mL sorbitol. AC was given after ipecac‐induced vomiting had ceased or after gastric lavage Timing: before receiving AC‐sorbitol Dose: 30‐50 mL ipecac followed by 200 mL water; at least 2 L tap water for gastric lavage, via nasogastric tube Frequency: 1×, repeated if no vomiting within 30 min Integrity: no information Control arm: Type: activated charcoal‐sorbitol + supportive and drug‐specific treatment, orally in conscious and via nasogastric tube in obtunted participants Timing: after diagnosis and allocation to treatment group Dose: 50 g AC in 200 mL 70% sorbitol slurry Frequency: 1× Integrity: no information |
|
| Outcomes |
Type (unit): Proportion with clinical deterioration in the first 6 h after treatment Number of days hospitalized (for medical indication related to overdose or its treatment and complications) Number of complications Admission to ward/ICU Timing: clinical course was assessed over the first 6 h at 1‐2 h intervals (early) |
|
| Funding | No information | |
| Notes | Data for gastric lavage was not extracted, because not within scope of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated to one of two groups: those who presented on odd‐numbered dates to the emptied (E) group; those on even‐numbered days to the not‐emptied (NE) group." Comment: no adequate randomization |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were allocated to one of two groups: those who presented on odd‐numbered dates to the emptied (E) group; those on even‐numbered days to the not‐emptied (NE) group." Comment: allocation was not concealed, as randomisation scheme is predictable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded (not possible due to difference in interventions); might influence outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding, but might affect assessment of subjective outcomes (clinical deterioration) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not all participants allocated were treated, but the number remains small (9%) and reasons are thoroughly justified |
| Selective reporting (reporting bias) | High risk | Certain outcomes (no. referred to wards/ICU, type of complication) only reported for gastric emptying group as a whole, not stratified per treatment |
| Other bias | Low risk | No other risk of bias detected |
Roberts 2006.
| Methods |
Study design: randomized controlled trial Study duration: recruitment period: 31 March 2002 to October 2004 Participants were followed up until death/discharge Setting: hospital setting: medical wards of 2 Sri Lankan secondary hospitals Country: Australia Number of individuals randomized: 104 Number of individuals receiving the intervention: 64 SDAC: 28 MDAC: 36 Number of individuals receiving the control: 40 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: Usual care group: 20 male and 20 female SDAC: 8 male and 20 female MDAC: 22 male and 14 female Age: median (IQR) Usual care group: 21.5 (17.5 to 28.5) SDAC: 22 (18.0 to 33.0) MDAC: 22.5 (17.5 to 28.0) Country (if different from study authors'): Sri Lanka Type, dose and timing of poisoning: participants with acute yellow oleander poisoning, admitted to the medical ward of 3 secondary referral hospitals in Sri Lanka Exclusion criteria: < 14 years, pregnant, ingestion of hydrocarbons alone or corrosives, requirement for oral medication, inability to intubate participants with Glasgow coma score < 13, presentation > 72 h postingestion, previous recruitment in the study, previous AC administration for the poisoning episode, < 16 years or unconscious without relatives present to give consent |
|
| Interventions |
Intervention arm: Type: SDAC or MDAC in water suspension, in addition to usual care Timing: "soon" after admission, for MDAC repeated at 4 h intervals. Dose: 50 g superactivated charcoal (Carbomix) in 300 mL water (per dose), administered orally or via nasogastric tube if unconscious Frequency: SDAC: 1× MDAC: 6× Integrity: first protocol intended to deliver 18 doses of AC in the multi‐dose group, this however was not feasible, so protocol was adapted to 6 doses Compliance was not anticipated a problem as was given while patient was under supervision, however participants was not forced. Analyses were performed intention‐to‐treat. Control arm: Type: usual care, consisting of atropine and intravenous fluids, where needed to maintain heart rate > 70 bpm and systolic blood pressure > 80 mmHg. Gastric lavage was initially not planned but upon request of treating physicians was included in standard treatment if participants presented within 2 h of a potentially serious poisoning (3 × 300 mL of water). Furthermore, forced emesis (ipecac) and lavage were mostly performed (54% and 7.5%, respectively) at primary hospitals before transfer to the secondary study hospitals Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Primary outcome: All‐cause hospital mortality Secondary outcome: Proportion cardiac dysrhythmias requiring anti‐digoxin Fab or transfer to tertiary care (3° heart block, Mobitz type II 2° block, sinus bradycardia with heart rate < 35 bpm and sinus arrest or block with sinus pauses > 3 s) Cmax (µg/L) Tmax (µg/L) AUC0‐24 (µg/L × h) Gradient of the linear regression time of the concentration/AUC0‐24 curve (representing elimination) (data not extracted) Mean residence time0‐24 (h) (data not extracted) Timing: blood samples were taken at 0 h, 1 h, 4 h, 12 h, 24 h after administration of the first charcoal dose and from then on every 24 h until discharge or death (intermediate) |
|
| Funding | National Health and Medical Research Council (Australia), The Welcome trust: grants GR063560MA and GR071669MA. | |
| Notes | This study is part of the Eddleston study, part of the info here comes from the protocol of Eddleston 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were recruited and randomised by a study doctor at the bedside using a dedicated handheld computer at each study hospital. Randomisation occurred after the patient's baseline data had been entered and receipt of consent noted, and could not be manipulated by study doctors. The recruiting doctor was unable to predict allocation before randomisation." Comment: adequate randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "The recruiting doctor was unable to predict allocation before randomisation." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the primary outcome, vital status at discharge, was unambiguous, and the secondary outcomes were objective; all outcomes were recorded systematically by the study team, not other hospital physicians" Comment: lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the primary outcome, vital status at discharge, was unambiguous, and the secondary outcomes were objective; all outcomes were recorded systematically by the study team, not other hospital physicians". Comment: outcome assessors were kept blinded from data analysis. They were not kept blinded from treatment, but objective outcomes are used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data from all eligible participants are reported. Non‐eligibility from other participants is thoroughly justified. Quote: "patient follow‐up was expected to be near 100% complete; and the analysis will be performed on an intention‐to‐treat basis." |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes are reported |
| Other bias | High risk | It is not entirely clear, even to the authors, what exactly is measured with the digoxin assay. The fact that both active cardenolides and (inactive?) metabolites might bind the assay compromise the results of these analyses, as they might explain the wide variability observed. Only participants with 'mild' intoxication were included in this analysis, as the severe cases were treated with Fab or transferred to a tertiary hospital, but these might have shown the biggest effect. |
Sue 1994.
| Methods |
Study design: randomized controlled trial Study duration: recruitment period from October 1990 to April 1992 Setting: hospital setting: emergency department of a children's hospital Country: USA Number of individuals randomized: 64 Number of individuals receiving the intervention: 4 mL/kg group: 16 6 mL/kg group: 16 8 mL/kg group: 18 Number of individuals receiving the control: 14 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: no information Age: median age: 25 months (range 3‐53 months) Country (if different from study authors'): NA Type, dose and timing of poisoning: children presenting to the emergency department, following a toxic ingestion requiring SDAC Exclusion criteria: dehydrated or renal dysfunction and those whose ingestions required MDAC |
|
| Interventions |
Intervention arm: Type: activated charcoal + MgCitrate (6%) Timing: after appropriate initial care (supportive care, gastric emptying if indicated and diagnostic laboratory evaluation) Dose: 50 g AC in 240 mL, combined with: 4 mL/kg of MgCitrate (6%) and 2 mL/kg water 6 mL/kg of MgCitrate (6%) 8 mL/kg of MgCitrate (6%) Frequency: 1× Integrity: no attempts were made to control the oral intake of the children following administration of the charcoal slurry Control arm: Type: activated charcoal Timing: after appropriate initial care (supportive care, gastric emptying if indicated and diagnostic laboratory evaluation) Dose: 1 g/kg: 50 g AC in 240 mL, combined with 6 mL/kg water Frequency: 1× Integrity: no attempts were made to control the oral intake of the children following administration of the charcoal slurry. |
|
| Outcomes |
Type (unit): Time to first stool (h) (data not extracted) Number requiring hospitalization Number of black‐colored stools (data not extracted) Potential adverse events (vomiting, diarrhoea, abdominal pain, lethargy) Timing: outcomes were measured during the subsequent 48 h after treatment, either by review of the hospital chart or telephone follow‐up (intermediate) |
|
| Funding | No information | |
| Notes | Only clinically relevant outcome is requirement of hospitalization Not clear when outcome hospitalization was measured: after 48 h or initially. Study reports "no difference" in diarrhoea, abdominal pain, but no numbers reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomization process |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to support judgment, but lack of blinding may affect outcomes studied |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding, but could affect outcomes studies (e.g. hospitalization) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No enrolled patient withdrew from the study, and follow‐up information was obtained for all children." Comment: adequate |
| Selective reporting (reporting bias) | High risk | Clinical outcome data are lacking. Adverse events incompletely reported |
| Other bias | Low risk | No other risk of bias detected |
Underhill 1990.
| Methods |
Study design: randomized controlled trial Study duration: recruitment period between April and October 1988 Setting: hospital setting: accident and emergency departments of two teaching hospitals Country: UK Number of individuals randomized: 60 Number of individuals receiving the intervention: Gastric lavage: 14 Acitvated charcoal: 20 Ipecac: 21 Number of individuals receiving the control: 5 Number of individuals lost to follow‐up: 0 Sample size calculated: |
|
| Participants |
Sex: 16 male and 44 female Age: mean (range): 25.7 (16‐62) years Country (if different from study authors'): NA Type, dose and timing of poisoning: participants presenting within 4 h after an overdose (mean delay: 123 min, range 30‐240 min) of at least 5 g paracetamol. 48 took paracetamol without another drug; 21 took paracetamol with alcohol Inclusion criteria: > 16 years, presenting < 4 h after intake, ingested > 5 g paracetamol Exclusion criteria: depressed conscious level, conditions that might preclude use of any one of the treatment methods |
|
| Interventions |
Intervention arm 1: Type: gastric lavage Timing: NA Dose: NA Frequency: NA Integrity: NA Intervention arm 2: Type: activated charcoal (Carbomix) Timing: no information Dose: AC:Drug ratio of 10:1 Frequency: 1× Integrity: 16 participants managed to take the recommended dose. 4 participants vomited and 1 refused to take more than one mouthful Intervention arm 3: Type: ipecac Timing: no information Dose: 30 mL Frequency: 1×, repeated if no vomiting after 30 min Integrity: mean time to emesis was 20 min (range 5‐50), 2 participants did not vomit until 50 min and 2 did not vomit at all Control arm: Type: no intervention Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): Plasma paracetamol levels Adverse events Timing: prior to treatment and 60 min, 90 min and 150 min after the first sample (early) |
|
| Funding | No information | |
| Notes | No treatment group was stopped for ethical reasons when the serum paracetamol levels increased between the first and last samples in 4 out of 5 participants. Data for gastric lavage was not extracted, because not within scope of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomization process |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded (not possible due to difference in interventions); might influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on blinding, but should not affect outcome measurements |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of incomplete outcomes |
| Selective reporting (reporting bias) | High risk | No clinical outcomes reported, adverse events incompletely reported |
| Other bias | Low risk | No other risk of bias detected |
Wax 1999.
| Methods |
Study design: randomized controlled trial Study duration: 27‐month recruitment period Setting: home setting, with telephone support from a poison centre Country: USA Number of individuals randomized: 103 Number of individuals receiving the intervention: 51 Number of individuals receiving the control: 52 Number of individuals lost to follow‐up: 0 Sample size calculated: no information |
|
| Participants |
Sex: 57 male and 46 female Age: median (range): 2 years (9 months‐5 years) Country (if different from study authors'): NA Type, dose and timing of poisoning: asymptomatic participants with suspected ingestion of a small number (< 6) of potentially toxic berries, including Taxus species (yew), Solanum americanus (nightshade), Ilex species (holly) or unknown berries Exclusion criteria: ingestion of a known other type of berry, > 5 berries ingested, symptomatic when calling poison centre, parents planning transport to healthcare facility regardless of the advice of the poison centre, ingestion of more than 1 type of berry/plant parts, contraindication for syrup of ipecac |
|
| Interventions |
Intervention arm: Type: syrup of ipecac (+ home observation) Timing: no information Dose: no information Frequency: no information Integrity: no information Control arm: Type: home observation Timing: NA Dose: NA Frequency: NA Integrity: NA |
|
| Outcomes |
Type (unit): symptom assessment (vomiting, diarrhoea, abdominal pain, drowsiness, agitation) and disposition assessment (ED referral, hospital admission) Timing: 24 h after telephone call to poison centre (intermediate) |
|
| Funding | No information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The group who called the poison centre on even days of the month received ipecac followed by parenteral/guardian HO. The group that called the poison centre on odd days of the month were assigned to the HO only group" Comment: not an adequate method of randomization |
| Allocation concealment (selection bias) | High risk | Randomization method does not allow for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind, but might affect subjective symptom outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded, which might influence assessment of subjective symptom outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of missing data |
| Selective reporting (reporting bias) | Low risk | Specified outcomes are reported |
| Other bias | High risk | Only asymptomatic participants included, no confirmation of actual ingestion and uptake, reporting dichotomous outcomes while measuring using an ordinal scale |
AC: activated charcoal; APACHE: acute physiology and chronic health evaluation; AUC: area under the receiver operating curve; bpm: beats per minute; CBZ: carbamazepine; ECG: electrocardiogram;ED: emergency department; ICU: intensive care unit; IQR: interquartile range; MDAC: multi‐dose activated charcoal; NA: not applicable; SAPS: simplified acute physiology score; SD: standard deviation; SDAC: single‐dose activated charcoal; SEM: standard error of the mean; SOI: syrup of ipecac; TCA: tricyclic antidepressant; UC: University of California.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afshari 2010a | Ineligible intervention: IV administration of intervention |
| Afshari 2010b | Ineligible intervention: IV administration of intervention |
| Auerbach 1986 | Ineligible intervention: comparison between gastric lavage and ipecac |
| Belon 2007 | Ineligible intervention: homeopathic remedy |
| Berg 1982 | Ineligible study population: not oral poisoning |
| Berlinger 1983 | Ineligible population: not oral poisoning |
| Bhalla 2014 | Ineligible intervention: IV administration of intervention |
| Bosse 1995 | Ineligible intervention: not possible to determine the additional effect of activated charcoal on top of hospital treatment |
| Boxer 1969 | Ineligible comparison: hospital treatment vs ipecac |
| Boyd 1999 | Ineligible comparison: 2 types of charcoal compared, no control group without charcoal |
| Campbell 1992 | Ineligible intervention: repeat dose of activated charcoal, no control group without activated charcoal |
| Chamberlain 1993 | Ineligible intervention: different groups received different doses of N‐acetylcysteine. Impossible to distinguish the effect of AC from this |
| Corby 1968 | Ineligible intervention: control is apomorphine |
| Crome 1976 | Ineligible intervention: methionine |
| Dorooshi 2016 | Recent trial that was not prospectively registered in a trials register |
| Eddleston 2009 | Ineligible intervention: pralidoxime |
| Ekins 1987 | Ineligible study population: not poisoned patients |
| Escalante 2016 | Recent trial that was not prospectively registered in a trials register |
| Espinosa 1987 | Ineligible intervention: feasibility of administration |
| Filippone 1987 | Ineligible intervention: pre‐absorbed durg‐charcoal mixture |
| Fischer 1999 | Ineligible comparison: 2 types of charcoal compared, no control group without charcoal |
| Frenia 1996 | Ineligible study population: not oral poisoning |
| Gomez 1997 | Ineligible intervention: pre‐absorbed durg‐charcoal mixture |
| Grierson 2000 | Ineligible intervention: gastric lavage |
| Hoegberg 2005 | Ineligible intervention: yoghourt |
| Hoegberg 2012 | Ineligible intervention: alcohol |
| Ilkhanipour 1992 | Ineligible study population: not oral poisoning |
| Ilkhanipour 1993 | Ineligible study population: not oral poisoning |
| IRCT138811142717N1 2010 | Ineligible intervention: IV administration of intervention |
| IRCT20180118038426N2 2018 | Recent trial that was not prospectively registered in a trials register. The trial was registered after recruitment had started |
| Isbister 2011 | Ineligible study population: not oral poisoning |
| ISRCTN50739829 2006 | Ineligible intervention: IV administration of treatment |
| Karim 2001 | Ineligible intervention: feasibility of administration |
| Krenzelok 1985a | Ineligible study population: not poisoning patients |
| Ly 2004 | Ineligible intervention: whole bowel irrigation |
| MacLean 1973 | Ineligible intervention: apomorphine |
| Mahutte 1983 | Ineligible study population: not oral poisoning |
| Merigian 1988 | Ineligible intervention: gastric emptying not specified |
| Navabi 2017 | Ineligible intervention: hospital treatments |
| Nogue 1987 | Ineligible intervention: two formulations of ipecac compared |
| Olsen 1993 | Ineligible intervention: whole bowel irrigation |
| Olsen 1995 | Ineligible intervention: whole bowel irrigation |
| Pond 1984 | Ineligible comparison: no suitable comparison |
| Roberts 1997 | Ineligible intervention: comparison of 2 brands of activated charcoal |
| Schofferman 1976 | Ineligible intervention: apomorphine |
| Skinner 2012 | Ineligible population: chronic poisoning patients |
| Smith 1967 | Ineligible intervention: montmorillonite |
| Tincu 2017 | Recent trial that was not prospectively registered in a trials register |
| Varipapa 1977 | Ineligible study population: not oral poisoning |
| Vijayakumar 2017 | Ineligible intervention: IV administration of intervention |
| Young 1993 | Inelgible comparison: hospital treatment vs ipecac |
IV: intravenous.
Differences between protocol and review
Changes from the protocol
In the protocol, we stated that we would include studies performed in poisoning patients as well as studies with healthy volunteers (Avau 2017). During screening of references, it became clear that there were sufficient patient studies available, which led to the decision to only include these. We believe that studies performed in actual oral poisoning patients give better insight into the reality of oral poisoning than studies performed in healthy volunteers, where poisoning is simulated in a controlled setting.
Furthermore, we described mult‐dose activated charcoal as the same intervention as single‐dose activated charcoal, but with multiple doses of the same intervention. The identified studies made us aware that we should analyse them as different interventions, which we did.
The pre‐defined secondary outcome 'drug recovery rate from the body' did not make it to the review, as we had anticipated finding this outcome in studies in healthy volunteers, not in patient studies. As studies in healthy volunteers were no longer within scope of this review, we did not report this outcome. Instead, we subdivided the primary outcome 'incidence and severity of symptoms of poisoning, including mortality' into two separate outcomes, 'incidence and severity of symptoms of poisoning' and 'mortality'.
We also left out two other pre‐defined secondary outcomes, 'length of hospital stay' and 'length of ICU stay'. The Cochrane Injuries review group does not consider these outcomes appropriate because they are prone to bias, as they depend on a lot of confounding factors, such as time of death, insurance coverage, patient income, distance from a hospital, hospital admission policy and bed availability, among other factors. As we extracted these outcomes, we present them in Table 21.
4. Pre‐defined outcomes that were extracted but not included in the review.
| A. First aid interventions that limit or delay the absorption of the poison in the body | |||||||
| SDAC vs no intervention | |||||||
| Length of stay in the emergency department (min) | |||||||
| SDAC | No intervention | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Merigian 1990 | 252 | 279 | 220 | 230 | 166 | 231 | 22.00 (−20.63 to 64.63) |
| SDAC + hospitalintervention vs hospitalintervention | |||||||
| Length of ICU stay (h) | |||||||
| SDAC + hospital intervention | Hospital intervention | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Merigian 2002 | 54.4 | 93.15 | 28 | 45.5 | 36.3 | 32 | 8.90 (−27.82 to 45.62) |
| Incidence of ICU stay > 3 days | |||||||
| SDAC + hospital intervention | Hospital intervention | ||||||
| Study | Events | Total | Events | Total | RR (95% CI) | ||
| Hultén 1988 | 0 | 34 | 5 | 43 | 0.11 (0.01 to 2.00) | ||
| Length of hospital stay (h) | |||||||
| SDAC + hospital intervention | Hospital intervention | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Merigian 2002 | 63.8 | 79.8 | 51 | 91.7 | 103.97 | 102 | −27.90 (−57.68 to 1.88) |
| Study | Median | IQR | N | Median | IQR | N | Median difference (P value) |
| Cooper 2005 | 6.8 | (4.0 to 14.0) | 166 | 5.5 | (3.0 to 12.0) | 161 | 1.3 (P = 0.11) |
| Incidence of hospital stay > 3 days | |||||||
| SDAC + hospital intervention | Hospital intervention | ||||||
| Study | Events | Total | Events | Total | RR (95% CI) | ||
| Hultén 1988 | 1 | 34 | 4 | 43 | 0.32 (0.04 to 2.70) | ||
| Length of stay in the emergency department (h) | |||||||
| SDAC + hospital intervention | Hospital intervention | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Merigian 2002 | 6.2 | 3.9 | 325 | 5.3 | 3.9 | 941 | 0.90 (0.41 to 1.39) |
| MDAC + hospitalintervention vs hospitalintervention | |||||||
| Length of hospital stay (h) | |||||||
| MDAC + hospital intervention | Hospital intervention | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Behnoush 2009 | 31.0 | 8.9 | 30 | 55.0 | 8.5 | 30 | −24.00 (−28.40 to −19.60) |
| Brahmi 2006 | 30.3 | 3.4 | 6 | 39.7 | 7.3 | 6 | −9.40 (−15.84 to −2.96) |
| Median | IQR | N | Median | IQR | N | Median difference (P value) | |
| De Silva 2003 | 3 | (0.25 to 24) | 201 | 3 | (0.5 to 10) | 200 | 0 (P = 0.90) |
| SDAC vs syrup of ipecac | |||||||
| Length of stay in the emergency department (min) | |||||||
| Syrup of ipecac | SDAC | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Amigó Tadín 2002 | 113.21 | 66.0 | 21 | 81.46 | 27.92 | 13 | 31.75 (−0.30 to 63.80) |
| B. First aid interventions that evacuate the poison from the gastrointestinal tract | |||||||
| Syrup of ipecac + SDAC + cathartic vs SDAC + cathartic | |||||||
| Length of hospital stay (days) | |||||||
| Syrup of ipecac + SDAC + cathartic | SDAC + cathartic | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Albertson 1989 | 2.4 | 5.8 | 13 | 1.7 | 5.2 | 12 | 0.70 (−3.61 to 5.01) |
| Length of stay in the emergency department (h) | |||||||
| Syrup of ipecac + SDAC + cathartic | SDAC + cathartic | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Albertson 1989 | 6.8 | 2.9 | 93 | 6.0 | 2.1 | 107 | 0.80 (0.09 to 1.51) |
| Kornberg 1991 | 4.1 | 1.1 | 29 | 3.4 | 1.2 | 38 | 0.70 (0.15 to 1.25) |
| Length of ICU stay (h) | |||||||
| Syrup of ipecac + SDAC + cathartic | SDAC + cathartic | ||||||
| Study | Mean | SD | N | Mean | SD | N | MD (95% CI) |
| Albertson 1989 | 1.8 | 3.9 | 6 | 1.0 | 0.0 | 5 | Not estimable |
CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; SD: standard deviation; SDAC: single‐dose activated charcoal.
For some comparisons, we identified more than 7 outcomes. The outcomes as stated in the protocol were more generally described; however, it became clear that outcomes such as occurrence and severity of poisoning symptoms could include a wide variety of specific outcomes (e.g. incidence of clinical improvement, incidence of intubation requirement, incidence of convulsions) which we could not combine in a meta‐analysis due to the differences in the symptoms. As 'Summary of findings' tables include only seven outcomes, we decided, together with a clinical expert (PD), to choose the clinically most relevant outcomes.
For the assessment of the GRADE domain 'limitations in study design', we decided to downgrade the level of the evidence if one of the studies contributing to the outcome was classified as a having a high risk of bias in one or more of the following domains: selection bias, detection bias, attrition bias or other bias. We did not consider domains with unclear risk of bias. The protocol did not clearly state this. Furthermore, we have expanded explanations on our considerations for the assessment of the GRADE domain 'imprecision'.
During the analysis of our data, we encountered dichotomous outcomes with zero events. We decided to analyse these with the Peto OR method instead of the Maentel‐Haenzel method in cases where this method is appropriate, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In addition, we have further expanded our Methods section concerning the interpretation of I² for the assessment of heterogeneity.
In the protocol we anticipated making subgroups of different drugs taken, single versus multiple doses of an intervention and different time points of the interventions. Based on the identified evidence, we decided to create the following subgroups: different drugs taken, different time points of the intervention, co‐interventions administered and type of adverse event experienced. We performed no subgroup analysis for single versus multiple doses of an intervention, as it became clear that these should be treated as two different interventions and are therefore different analyses. As for the different time points of the interventions, we did not identify any studies that compared against a control intervention, so we could not perform subgroup analyses.
For practical reasons, we decided to include an extra review author (AV) to help with data extraction of the studies. This person was included as third author of this review.
Methods not implemented
Selection of subsets of participants
Had we encountered a study where only a subset of participants met the eligibility criteria of our review, we would have only extracted data for this relevant subset, if separate data for this subset were available or could be obtained.
Assessment of reporting biases
Had we identified more than 10 studies for the same outcome, we would have used funnel plots to assess possible publication bias. In case of funnel plot asymmetry, we would have considered small‐study effects in the meta‐analysis (Higgins 2011).
Sensitivity analysis
We would have performed sensitivity analysis by excluding studies with high or unclear risk of bias for sequence generation, allocation concealment, incomplete outcome reporting or other sources of bias and comparing results with the initial analysis, had more than two studies been in the comparison.
We would also have carried out sensitivity analyses had we been required to impute data for some studies to be able to perform a meta‐analysis. We would then have excluded the studies with imputed data and compared the results to the initial analysis.
Contributions of authors
Bert Avau: co‐ordinated the review; selected studies; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; wrote to study authors; and approved the final review prior to submission.
Vere Borra: co‐ordinated the review; selected studies; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; wrote to study authors; and approved the final review prior to submission.
Anne‐Catherine Vanhove: extracted data; analysed and interpreted data; contributed to editing the review; made an intellectual contribution to the review; wrote to study authors; and approved the final review prior to submission.
Philippe Vandekerckhove: conceptualized the review; interpreted data; contributed to editing the review; made an intellectual contribution to the review; approved the final review prior to submission.
Peter De Paepe: interpreted data; contributed to editing the review; made an intellectual contribution to the review; approved the final review prior to submission.
Emmy De Buck: conceptualized the review; interpreted data; contributed to editing the review; made an intellectual contribution to the review; approved the final review prior to submission.
Sources of support
Internal sources
-
Foundation for Scientific Research of the Belgian Red Cross, Belgium.
This work was made possible through funding from the Foundation for Scientific Research of the Belgian Red Cross.
External sources
No sources of support supplied
Declarations of interest
BA, VB, AC, EDB and PV are employees of the Belgian Red Cross and have no further interests to declare. PDP is a specialist in internal medicine and emergency medicine, and is currently head of the emergency department of the Ghent University Hospital (Belgium). Furthermore, he is appointed by the Belgian Federal Health Authorities as Medical Director of East Flanders and a member of the National Council for emergency Medical Services. PDP is professor in clinical pharmacology at the Heymans Institute of Pharmacology at Ghent University (Belgium).
Edited (no change to conclusions)
References
References to studies included in this review
Albertson 1989 {published data only}
- Albertson TE, Derlet RW, Foulke GE, Minguillon MC, Tharratt SR. Superiority of activated charcoal alone compared with ipecac and activated charcoal in the treatment of acute toxic ingestions. Annals of Emergency Medicine 1989;18(1):56‐9. [DOI] [PubMed] [Google Scholar]
- Foulke GE, AIbertson TE, Derlet RW. Use of ipecac increases emergency department stays and patient complication rates. Annals of Emergency Medicine 1988;17:402. [Google Scholar]
Amigó Tadín 2002 {published data only}
- Amigó Tadín M, Faro Colomina J, Ambrós Ribó Á, Alves Vieta D, Ferró Ricart I, Mangirón Vásquez P, et al. Syrup of ipecac versus activated charcoal in acute drug poisoning [Jarabe de Ipecacuana versus carbon activado en las intoxicaciones medicamentosas agudas]. Metas de Enfermería 2002;46:6‐11. [Google Scholar]
Behnoush 2009 {published data only}
- Behnoush B, Bazmi E, Taghaddosinejad F. Carbamazepine poisoning and effect of multiple‐dose activated charcoal. Acta Medica Iranica 2009;47(1):9‐14. [Google Scholar]
Bouget 1989 {published data only}
- Bouget J, Breurec JY, Baert A, Yapo‐Ette H, Feuillu A, Curtes JP. Value of charcoal by oral route in patients admitted to emergency department for voluntary absorption of benzodiazepines [lnteret du charbon par voie orale chez des patients admis en service d'urgence pour absorption volontaire de benzodiazepines]. Journal of Clinical and Experimental Toxicology 1989;9(4):287‐9. [PubMed] [Google Scholar]
Brahmi 2006 {published data only}
- Brahmi N, Kouraichi N, Thabet H, Amamou M. Influence of activated charcoal on the pharmacokinetics and the clinical features of carbamazepine poisoning. American Journal of Emergency Medicine 2006;24(4):440‐3. [DOI] [PubMed] [Google Scholar]
Comstock 1982 {published data only}
- Comstock EG, Boisaubin EV, Comstock BS, Faulkner TP. Assessment of the efficacy of activated charcoal following gastric lavage in acute drug emergencies. Journal of Toxicology: Clinical Toxicology 1982;19(2):149‐65. [DOI] [PubMed] [Google Scholar]
Cooper 2005 {published data only}
Crome 1983 {published data only}
- Crome P. Contribution to a Cochrane review [personal communication]. Email to: Avau B. 7 August 2017.
- Crome P, Adams R, Ali C. Activated charcoal in tricyclic antidepressant poisoning: pilot controlled clinical trial. Human Toxicology 1983;2(2):205‐9. [DOI] [PubMed] [Google Scholar]
De Silva 2003 {published data only}
- Silva HA, Fonseka M, Pathmeswaran A, Alahakone DG, Ratnatilake GA, Gunatilake SB, et al. Multiple‐dose activated charcoal for treatment of yellow oleander poisoning: a single‐blind, randomised, placebo‐controlled trial. Lancet 2003;361(9373):1935‐8. [DOI] [PubMed] [Google Scholar]
- Silva HA, Fonseka M, Pathmeswaran A, Ratnatilake GA, Alahakone DS, Ranasinha CD, et al. Treatment of yellow oleander poisoning with multiple dose activated charcoal: a randomized controlled trial. British Journal of Clinical Pharmacology 2003;55(4):422‐3. [Google Scholar]
Eddleston 2008 {published data only}
- Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Allen S, et al. Study protocol: a randomised controlled trial of multiple and single dose activated charcoal for acute self‐poisoning. BMC Emergency Medicine 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. A randomised controlled trial of multiple dose activated charcoal in acute self‐poisoning. Clinical Toxicology 2008;46(5):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. Multiple‐dose activated charcoal in acute self‐poisoning: a randomised controlled trial. Lancet 2008;371(9612):579‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Sheriff MHS, Warrell DA. Randomized controlled trial of routine single or multiple dose superactivated charcoal for self‐poisoning in a region with high mortality. Clinical Toxicology 2005;43:442‐3. [Google Scholar]
- ISRCTN02920054. Acute organophosphate pesticide poisoning in Sri Lanka: management, complications and pharmacogenetics. www.isrctn.com/ISRCTN02920054 (first received 29 July 2002).
- Mohamed F, Sooriyarachchi M R, Senarathna L, Azhar S, Sheriff MH, Buckley NA, et al. Compliance for single and multiple dose regimens of superactivated charcoal: a prospective study of patients in a clinical trial. Clinical Toxicology 2007;45(2):132‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hultén 1988 {published data only}
- Hultén BA, Adams R, Askenasi R, Dallos V, Dawling S, Heath A, et al. Activated charcoal in tricyclic antidepressant poisoning. Human and Experimental Toxicology 1988;7(4):307‐10. [DOI] [PubMed] [Google Scholar]
Ilett 1977 {published data only}
- Ilett KF, Gibb SM, Unsworth RW. Syrup of ipecacuanha as an emetic in adults. Medical Journal of Australia 1977;2(3):91‐3. [DOI] [PubMed] [Google Scholar]
James 1995 {published data only}
- James LP, Nichols MH, King WD. A comparison of cathartics in pediatric ingestions. Pediatrics 1995;96(2 Pt 1):235‐8. [PubMed] [Google Scholar]
Kornberg 1991 {published data only}
- Kornberg A E, Dolgin J. Pediatric ingestions: charcoal alone versus ipecac and charcoal. Annals of Emergency Medicine 1991;20(6):648‐51. [DOI] [PubMed] [Google Scholar]
Kulig 1985 {published data only}
- Kulig K, Bar‐Or D, Cantrill SV, Rosen P, Rumack BH. Management of acutely poisoned patients without gastric emptying. Annals of Emergency Medicine 1985;14(6):562‐7. [DOI] [PubMed] [Google Scholar]
Merigian 1990 {published data only}
- Merigian KS, Woodard M, Hedges JR, Roberts JR, Stuebing R, Rashkin MC. Prospective evaluation of gastric emptying in the self‐poisoned patient. American Journal of Emergency Medicine 1990;8(6):479‐83. [DOI] [PubMed] [Google Scholar]
Merigian 2002 {published data only}
- Merigian KS, Blaho KE. Single‐dose oral activated charcoal in the treatment of the self‐poisoned patient: a prospective, randomized, controlled trial. American Journal of Therapeutics 2002;9(4):301‐8. [DOI] [PubMed] [Google Scholar]
Montoya‐Cabrera 1999 {published data only}
- Montoya‐Cabrera MA, Escalante‐Galindo P, Nava‐Juarez A, Terroba‐Larios VM, Teran‐Hernandez JA. Evaluation of the efficacy of N‐acetylcysteine administered alone or in combination with activated charcoal in the treatment of acetaminophen overdoses [Evaluación de la eficacia de la N‐acetilcisteina administrada sola o combinada con carbón activado en el tratamiento de la sobredosis por acetaminofén]. Gaceta Médica de México 1999;135(3):239‐43. [PubMed] [Google Scholar]
Passeron 1989 {published data only}
- Passeron D, Bermudez A, Riviere R. Efficacy of treatment with charcoal diluted in 70% sorbitol during acute poisonings [Etude de l'efficacité d'un traitement par du charbon dilué dans du sorbitol à 70% au cours des intoxications aiguës]. Journal de Toxicologie Clinique et Expérimentale 1989;9(4):277‐81. [PubMed] [Google Scholar]
Pond 1995 {published data only}
- Pond SM, Lewis‐Driver DJ, Williams GM, Green AC, Stevenson NW. Gastric emptying in acute overdose: a prospective randomised controlled trial. Medical Journal of Australia 1995;163(7):345‐9. [DOI] [PubMed] [Google Scholar]
Roberts 2006 {published data only}
- Roberts DM, Southcott E, Potter JM, Roberts MS, Eddleston M, Buckley NA. Pharmacokinetics of digoxin cross‐reacting substances in patients with acute yellow Oleander (Thevetia peruviana) poisoning, including the effect of activated charcoal. Therapeutic Drug Monitoring 2006;28(6):784‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sue 1994 {published data only}
- Sue YJ, Woolf A, Shannon M. Efficacy of magnesium citrate cathartic in pediatric toxic ingestions. Annals of Emergency Medicine 1994;24(4):709‐12. [DOI] [PubMed] [Google Scholar]
Underhill 1990 {published data only}
- Underhill TJ, Greene MK, Dove AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Archives of Emergency Medicine and Critical Care 1990;7(3):148‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wax 1999 {published data only}
- Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac‐induced emesis be routinely recommended in the management of toxic berry ingestions?. Veterinary and Human Toxicology 1999;41(6):394‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Afshari 2010a {published data only}
- Afshari R, Mohammadi M, Balali‐Mood M. Therapeutic effects of sodium bicarbonate in organophosphate poisoning in man is pH dependent: a randomized clinical trial. Toxicology Letters 2010;196(Suppl 1):S84‐S85. [EMBASE: L70444760] [Google Scholar]
Afshari 2010b {published data only}
- Afshari R, Balali‐Mood M. Therapeutic effects of sodium bicarbonate and magnesium sulphate in organophosphate poisoning: a randomized clinical trial. Clinical Toxicology 2010;48(3):244. [CENTRAL: CN‐00861678] [Google Scholar]
Auerbach 1986 {published data only}
- Auerbach PS, Osterloh J, Braun O, Hu P, Geehr EC, Kizer KW, et al. Efficacy of gastric emptying: gastric lavage versus emesis induced with ipecac. Annals of Emergency Medicine 1986;15(6):692‐8. [PUBMED: 2871787] [DOI] [PubMed] [Google Scholar]
Belon 2007 {published data only}
- Belon P, Banerjee A, Karmakar SR, Biswas SJ, Choudhury SC, Banerjee P, et al. Homeopathic remedy for arsenic toxicity? Evidence‐based findings from a randomized placebo‐controlled double blind human trial. Science of the Total Environment 2007;384(1‐3):141‐50. [PUBMED: 17628642] [DOI] [PubMed] [Google Scholar]
Berg 1982 {published data only}
- Berg MJ, Berlinger WG, Goldberg MJ, Spector R, Johnson GF. Acceleration of the body clearance of phenobarbital by oral activated charcoal. New England Journal of Medicine 1982;307(11):642‐4. [DOI] [PubMed] [Google Scholar]
Berlinger 1983 {published data only}
- Berlinger WG, Spector R, Goldberg MJ, Johnson GF, Quee CK, Berg MJ. Enhancement of theophylline clearance by oral activated charcoal. Clinical Pharmacology and Therapeutics 1983;33(3):351‐4. [PUBMED: 6337763] [DOI] [PubMed] [Google Scholar]
Bhalla 2014 {published data only}
- Bhalla A, Kumar M, Singh S, Sharma N, Kumar S. Role of magnesium sulphate in management of organophosphate poisoning. Clinical Toxicology 2014;52(Suppl 1):433. [DOI: 10.3109/15563650.2014.906213] [DOI] [Google Scholar]
Bosse 1995 {published data only}
- Bosse GM, Barefoot JA, Pfeifer MP, Rodgers GC. Comparison of three methods of gut decontamination in tricyclic antidepressant overdose. Journal of Emergency Medicine 1995;13(2):203‐9. [PUBMED: 7775792] [DOI] [PubMed] [Google Scholar]
Boxer 1969 {published data only}
- Boxer L, Anderson FP, Rowe DS. Comparison of ipecac‐induced emesis with gastric lavage in the treatment of acute salicylate ingestion. Journal of Pediatrics 1969;74(5):800‐3. [PUBMED: 4388519] [DOI] [PubMed] [Google Scholar]
Boyd 1999 {published data only}
- Boyd R, Hanson J. Prospective single blinded randomised controlled trial of two orally administered activated charcoal preparations. Journal of Accident and Emergency Medicine 1999;16(1):24‐5. [PUBMED: 9918281 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1992 {published data only}
- Campbell JW, Chyka PA. Physicochemical characteristics of drugs and response to repeat‐dose activated charcoal. American Journal of Emergency Medicine 1992;10(3):208‐10. [DOI] [PubMed] [Google Scholar]
Chamberlain 1993 {published data only}
- Chamberlain JM, Gorman RL, Oderda GM, Klein‐Schwartz W, Klein BL. Use of activated charcoal in a simulated poisoning with acetaminophen: a new loading dose for N‐acetylcysteine?. Annals of Emergency Medicine 1993;22(9):1398‐402. [DOI] [PubMed] [Google Scholar]
Corby 1968 {published data only}
- Corby DG, Decker WJ, Moran MJ, Payne CE. Clinical comparison of pharmacologic emetics in children. Pediatrics 1968;42(2):361‐4. [PubMed] [Google Scholar]
Crome 1976 {published data only}
- Crome P, Volans G N, Vale J A. The use of methionine for acute paracetamol poisoning. Journal of International Medical Research 1976;4(4, Suppl):105‐11. [DOI] [PubMed] [Google Scholar]
Dorooshi 2016 {published data only}
- Dorooshi G, Mesri M, Taheri S, Habibollahi S. Comparative study of gastrointestinal disposal facility following administration of magnesium hydroxide, lactulose and polyethylene glycol in poisoned patients. Journal of Isfahan Medical School 2016;33(366):2360‐7. [Google Scholar]
Eddleston 2009 {published data only}
- Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, et al. Pralidoxime in acute organophosphorus insecticide poisoning ‐ a randomised controlled trial. PLOS Medicine 2009;6(6):e1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekins 1987 {published data only}
- Ekins BR, Ford DC, Thompson MIB, Bridges RR, Rollins DE, Jenkins RD. The effect of activated charcoal on N‐acetylcysteine absorption in normal subjects. American Journal of Emergency Medicine 1987;5(6):483‐7. [DOI] [PubMed] [Google Scholar]
Escalante 2016 {published data only}
- Escalante PG, Gonzalez L, Perez JR. Cathartics, calcined magnesium sulfate vs mannitol plus activated carbon for the treatment of acute poisonings. Toxicology Letters 2017; Vol. 259:S147.
Espinosa 1987 {published data only}
- Espinosa Garcia M, Roca Massa M, Codina Jane C, Ribas Sala J, Marques J, et al. Effectiveness of ipecac syrup as an emetic. Farmacia e Clinica 1987;4(Jul):466‐73. [Google Scholar]
Filippone 1987 {published data only}
- Filippone GA, Fish SS, Lacouture PG. Reversible adsorption (desorption) of aspirin from activated charcoal. Archives of Internal Medicine 1987;147(8):1390‐2. [PubMed] [Google Scholar]
Fischer 1999 {published data only}
- Fischer TF, Singer AJ. Comparison of the palatabilities of standard and superactivated charcoal in toxic ingestions: a randomized trial. Academic Emergency Medicine 1999;6(9):895‐9. [DOI] [PubMed] [Google Scholar]
Frenia 1996 {published data only}
- Frenia ML, Schauben JL, Wears RL, Karlix JL, Tucker CA, Kunisaki TA. Multiple‐dose activated charcoal compared to urinary alkalinization for the enhancement of phenobarbital elimination. Journal of Toxicology: Clinical Toxicology 1996;34(2):169‐75. [DOI] [PubMed] [Google Scholar]
Gomez 1997 {published data only}
- Gomez HF, McClafferty HH, Flory D, Brent J, Dart RC. Prevention of gastrointestinal iron absorption by chelation from an orally administered premixed deferoxamine/charcoal slurry. Annals of Emergency Medicine 1997;30(5):587‐92. [DOI] [PubMed] [Google Scholar]
Grierson 2000 {published data only}
- Grierson R, Green R, Sitar DS, Tenenbein M. Gastric lavage for liquid poisons. Annals of Emergency Medicine 2000;35(5):435‐9. [PubMed] [Google Scholar]
Hoegberg 2005 {published data only}
- Hoegberg LC, Christophersen AB, Christensen HR, Angelo HR. Comparison of the adsorption capacities of an activated‐charcoal‐‐yogurt mixture versus activated‐charcoal‐‐water slurry in vivo and in vitro. Clinical Toxicology 2005;43(4):269‐75. [PubMed] [Google Scholar]
Hoegberg 2012 {published data only}
- Hoegberg LCG, Boegevig S, Filtenborg DK, Jakobsdóttir MR, Hansen NB, Henning M, et al. The effect of ethanol on paracetamol absorption and activated charcoal efficacy, in a simulated human paracetamol overdose. Clinical Toxicology 2012;50(4):276. [Google Scholar]
Ilkhanipour 1992 {published data only}
- Ilkhanipour K, Yealy DM, Krenzelok EP. The comparative efficacy of various multiple‐dose activated charcoal regimens. American Journal of Emergency Medicine 1992;10(4):298‐300. [DOI] [PubMed] [Google Scholar]
Ilkhanipour 1993 {published data only}
- Ilkhanipour K, Yealy DM, Krenzelok EP. Activated charcoal surface area and its role in multiple‐dose charcoal therapy. American Journal of Emergency Medicine 1993;11(6):583‐5. [DOI] [PubMed] [Google Scholar]
IRCT138811142717N1 2010 {published data only}
- IRCT138811142717N1. Effects of magnesium sulfate in patients poisoned with tricyclic antidepressants. en.irct.ir/trial/2565 (first received 29 May 2010).
IRCT20180118038426N2 2018 {published and unpublished data}
- IRCT20180118038426N2. Evaluation of therapeutic effect of echinacea extract on cardiovascular poisoning caused by aluminum phosphide. http://en.irct.ir/trial/29074 (first received 19 March 2018).
Isbister 2011 {published data only}
- Isbister GK, Kumar VV. Indications for single‐dose activated charcoal administration in acute overdose. Current Opinion in Critical Care 2011;17(4):351‐7. [DOI] [PubMed] [Google Scholar]
ISRCTN50739829 2006 {published data only}
- ISRCTN50739829. Is magnesium an effective treatment for organophosphate poisoning?. doi.org/10.1186/ISRCTN50739829 (first received 31 July 2006).
Karim 2001 {published data only}
- Karim A, Ivatts S, Dargan P, Jones A. How feasible is it to conform to the European guidelines on administration of activated charcoal within one hour of an overdose?. Emergency Medicine Journal 2001;18(5):390‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krenzelok 1985a {published data only}
Ly 2004 {published data only}
- Ly BT, Schneir AB, Clark RF. Effect of whole bowel irrigation on the pharmacokinetics of an acetaminophen formulation and progression of radiopaque markers through the gastrointestinal tract. Annals of Emergency Medicine 2004;43(2):189‐95. [DOI] [PubMed] [Google Scholar]
MacLean 1973 {published data only}
- MacLean WC Jr. A comparison of ipecac syrup and apomorphine in the immediate treatment of ingestion of poisons. Journal of Pediatrics 1973;82(1):121‐4. [DOI] [PubMed] [Google Scholar]
Mahutte 1983 {published data only}
- Mahutte CK, True RJ, Michiels TM. Increased serum theophylline clearance with orally administered activated charcoal. American Review of Respiratory Disease 1983;128(5):820‐2. [DOI] [PubMed] [Google Scholar]
Merigian 1988 {published data only}
- Merigian KS, Hedges JR, Pesce A, Stuebing RC, Roberts JR, Rashkin MC. Prospective evaluation of gastric emptying in the self‐poisoned patient. Annals of Emergency Medicine 1988;17:402. [DOI] [PubMed] [Google Scholar]
Navabi 2017 {published data only}
- Navabi J, Yaghoubi RH. Comparison of the prognosis of the new and old therapeutic protocols in poisoning by phosphide compounds. Journal of Kermanshah University of Medical Sciences 2017;21(1):23‐26. [Google Scholar]
Nogue 1987 {published data only}
- Nogue S, Campanya M, Espinosa M, Camp J, Urbano‐Marquez A, Corbella J. Efficiency and safety of ipecac syrup in the treatment of acute poisoning [Eficacia y seguridad del jarabe de ipecacuana en el tratamiento de las intoxicaciones agudas]. Medicina Clínica Facultad de Medicina de Barcelona 1987;88(20):795‐7. [PubMed] [Google Scholar]
Olsen 1993 {published data only}
- Olsen KM, Ma FH, Ackerman BH, Stull RE. Low‐volume whole bowel irrigation and salicylate absorption: a comparison with ipecac‐charcoal. Pharmacotherapy 1993;13(3):229‐32. [PubMed] [Google Scholar]
Olsen 1995 {published data only}
- Olsen KM, Gurley BJ, Davis GA, Archer SR, Ackerman BH. Comparison of fluid volumes with whole bowel irrigation in a simulated overdose of ibuprofen. Annals of Pharmacotherapy 1995;29(Mar):246‐50. [DOI] [PubMed] [Google Scholar]
Pond 1984 {published data only}
- Pond SM, Olson KR, Osterloh JD, Tong TG. Randomized study of the treatment of phenobarbital overdose with repeated doses of activated charcoal. JAMA 1984;251(23):3104‐8. [PubMed] [Google Scholar]
Roberts 1997 {published data only}
- Roberts JR, Gracely EJ, Schoffstall JM. Advantage of high‐surface‐area charcoal for gastrointestinal decontamination in a human acetaminophen ingestion model. Academic Emergency Medicine 1997;4(3):167‐74. [DOI] [PubMed] [Google Scholar]
Schofferman 1976 {published data only}
- Schofferman JA. A clinical comparison of syrup of ipecac and apomorphine use in adults. Journal of the American College of Emergency Physicians 1976;5(1):22‐5. [DOI] [PubMed] [Google Scholar]
Skinner 2012 {published data only}
- Skinner CG, Chang AS, Matthews AS, Reedy SJ, Morgan BW. Randomized controlled study on the use of multiple‐dose activated charcoal in patients with supratherapeutic phenytoin levels. Clinical Toxicology 2012;50(8):764‐9. [DOI] [PubMed] [Google Scholar]
Smith 1967 {published data only}
- Smith RP, Gosselin RE, Henderson JA, Anderson DM. Comparison of the adsorptive properties of activated charcoal and Alaskan montmorillonite for some common poisons. Toxicology and Applied Pharmacology 1967;10(1):95‐104. [DOI] [PubMed] [Google Scholar]
Tincu 2017 {published data only}
- Tincu RC, Cobilinschi C, Tomescu D, Coman L, Tincu I, Diaconu C, Macovei RA. Favourable results for L‐carnitine use in valproic acid acute poisoning. Farmacia 2017;65(3):396‐400. [Google Scholar]
Varipapa 1977 {published data only}
- Varipapa RJ, Oderda GM. Effect of milk on ipecac induced emesis. New England Journal of Medicine 1977;296(2):112‐113. [DOI] [PubMed] [Google Scholar]
Vijayakumar 2017 {published data only}
- Vijayakumar HN, Kannan S, Tejasvi C, Duggappa DR, Veeranna Gowda KM, Nethra SS. Study of effect of magnesium sulphate in management of acute organophosphorous pesticide poisoning. Anesthesia: Essays and Researches 2017;11(1):192‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 1993 {published data only}
- Young WF, Bivins HG. Evaluation of gastric‐emptying using radionuclides ‐ gastric lavage versus ipecac‐induced emesis. Annals of Emergency Medicine 1993;22(9):1423‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Abrass 2012
- Abrass A, Manini AF. Activated charcoal in resource poor settings: reviewing the evidence. Journal of Clinical Toxicology 2012;S1:006. [Google Scholar]
Albert 2015
- Albert M, McCaig LF, Uddin S. Emergency department visits for drug poisoning: United States, 2008‐2011. National Center for Health Statistics Data Brief (CDC) 2015;Apr(196):1‐8. [PubMed] [Google Scholar]
American Academy of Clinical Toxicology 1999
- American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. Position statement and practice guidelines on the use of multi‐dose activated charcoal in the treatment of acute poisoning. Journal of Toxicology: Clinical Toxicology 1999;37(6):731‐51. [DOI] [PubMed] [Google Scholar]
American Academy of Clinical Toxicology 2004
- American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. Position paper: cathartics. Journal of Toxicology: Clinical Toxicology 2004;42(3):243‐53. [0731‐3810: (Print)] [DOI] [PubMed] [Google Scholar]
American Academy of Clinical Toxicology 2005
- American Academy of Clinical Toxicology & European Association of Poisons Centres and Clinical Toxicologists. Position paper: single‐dose activated charcoal. Clinical Toxicology 2005;43(2):61‐87. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [1756‐1833: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 1999
- Bateman DN. Gastric decontamination‐‐a view for the millennium. Journal of Accident & Emergency Medicine 1999;16(2):84‐6. [1351‐0622: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blain 2011
- Blain PG. Organophosphorus poisoning (acute). BMJ Clinical Evidence 2011:2102. [1752‐8526: (Electronic)] [PMC free article] [PubMed] [Google Scholar]
Chiew 2018
- Chiew AL, Gluud C, Brok J, Buckley N. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD003328.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chin 1969
- Chin L, Picchioni AL, Duplisse BR. Comparative antidotal effectiveness of activated charcoal, Arizona montmorillonite, and evaporated milk. Journal of pharmaceutical sciences 1969;58(11):1353‐6. [PUBMED: 5349747] [DOI] [PubMed] [Google Scholar]
Chyka 2005
- Chyka PA, Seger D, Krenzelok EP, Vale JA, American Academy of Clinical T, European Association of Poisons C, et al. Position paper: single‐dose activated charcoal. Clinical Toxicology 2005;43(2):61‐87. [1556‐3650: (Print)] [DOI] [PubMed] [Google Scholar]
Corcoran 2016
- Corcoran G, Chan B, Chiew A. Use and knowledge of single dose activated charcoal: a survey of Australian doctors. Emergency Medicine Australasia 2016;28(5):578‐85. [1742‐6723: (Electronic)] [DOI] [PubMed] [Google Scholar]
Decker 2015
- Decker BS, Goldfarb DS, Dargan PI, Friesen M, Gosselin S, Hoffman RS, et al. Extracorporeal treatment for lithium poisoning: systematic review and recommendations from the EXTRIP workgroup. Clinical Journal of the American Society of Nephrology 2015;10(5):875‐87. [1555‐905X: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donkor 2016
- Donkor J, Armenian P, Hartman IN, Vohra R. Analysis of gastric lavage reported to a statewide poison control system. Journal of Emergency Medicine 2016;51(4):394‐400. [0736‐4679: (Print)] [DOI] [PubMed] [Google Scholar]
Eddleston 2003
- Eddleston M, Juszczak E, Buckley N. Does gastric lavage really push poisons beyond the pylorus? A systematic review of the evidence. Annals of Emergency Medicine 2003;42(3):359‐64. [DOI] [PubMed] [Google Scholar]
Gaudreault 2005
- Gaudreault P. Activated Charcoal Revisited. Clinical Pediatric Emergency Medicine 2005;6(2):76‐80. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 14 December 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Hawton 2015
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Townsend E, et al. Interventions for self‐harm in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 12. [1469‐493X: (Electronic); DOI: 10.1002/14651858.CD012013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [1756‐1833: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Höjer 2013
- Höjer J, Troutman WG, Hoppu K, Erdman A, Benson BE, Megarbane B, et al. Position paper update: ipecac syrup for gastrointestinal decontamination. Clinical Toxicology 2013;51(3):134‐9. [1556‐9519: (Electronic)] [DOI] [PubMed] [Google Scholar]
IFRC 2016
- International Federation of Red Cross and Red Crescent Societies (IFRC). International first aid and resuscitation guidelines 2016. www.ifrc.org/Global/Publications/Health/First‐Aid‐2016‐Guidelines_EN.pdf (accessed prior to 14 December 2018).
Isbister 2016
- Isbister GK, Buckley NA. Therapeutics in clinical toxicology: in the absence of strong evidence how do we choose between antidotes, supportive care and masterful inactivity. British Journal of Clinical Pharmacology 2016;81(3):408‐11. [1365‐2125: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2002
- Jones S, Ali B. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Activated charcoal and gastric absorption of iron compounds. Emergency Medicine Journal 2002;19(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juurlink 2015
- Juurlink DN. Activated charcoal for acute overdose: a reappraisal. British Journal of Clinical Pharmacology 2015;81(3):482‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jürgens 2009
- Jürgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta‐analysis. Clinical Pharmacology and Therapeutics 2009;85(5):501‐5. [DOI] [PubMed] [Google Scholar]
Karlsson 1965
- Karlsson B, Noren L. Ipecacuanha and copper sulphate as emetics in intoxications in children. Acta Paediatrica Scandinavica 1965;54:331‐5. [0001‐656X: (Print)] [DOI] [PubMed] [Google Scholar]
Kendrick 2012
- Kendrick D, Young B, Mason‐Jones AJ, Ilyas N, Achana FA, Cooper NJ, et al. Home safety education and provision of safety equipment for injury prevention. Cochrane Database of Systematic Reviews 2012, Issue 9. [1469‐493X: (Electronic); DOI: 10.1002/14651858.CD005014.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2016
- Lai J, Lu Q, Xu Y, Hu S. Severe water intoxication and secondary depressive syndrome in relation to delusional infestation. Neuropsychiatric Disease and Treatment 2016;12:517‐21. [1176‐6328: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavonas 2015
- Lavonas EJ, Buchanan J. Hemodialysis for lithium poisoning. Cochrane Database of Systematic Reviews 2015, Issue 9. [1469‐493X: (Electronic); DOI: 10.1002/14651858.CD007951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008
- Lee MR. Ipecacuanha: the South American vomiting root. Journal of the Royal College of Physicians of Edinburgh 2008;38(4):355‐60. [1478‐2715: (Print)] [PubMed] [Google Scholar]
Loots 2013
- Loots C, Smits M, Omari T, Bennink R, Benninga M, Wijk M. Effect of lateral positioning on gastroesophageal reflux (GER) and underlying mechanisms in GER disease (GERD) patients and healthy controls. Neurogastroenterology and Motility 2013;25(3):222‐9, e161‐2. [1365‐2982: (Electronic)] [DOI] [PubMed] [Google Scholar]
LoVecchio 2007
- LoVecchio F, Shriki J, Innes K, Bermudez J. The feasibility of administration of activated charcoal with respect to current practice guidelines in emergency department patients. Journal of Medical Toxicology 2007;3(3):100‐2. [1556‐9039: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manoguerra 2005
- Manoguerra AS, Cobaugh DJ. Guideline on the use of ipecac syrup in the out‐of‐hospital management of ingested poisons. Clinical Toxicology 2005;43(1):1‐10. [DOI] [PubMed] [Google Scholar]
Moon 2015
- Moon J, Chun B, Song K. An exploratory study; the therapeutic effects of premixed activated charcoal‐sorbitol administration in patients poisoned with organophosphate pesticide. Clinical Toxicology 2015;53(2):119‐26. [1556‐9519: (Electronic)] [DOI] [PubMed] [Google Scholar]
Mowry 2016
- Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 33rd Annual Report. Clinical Toxicology 2016;54(10):924‐1109. [1556‐9519: (Electronic)] [DOI] [PubMed] [Google Scholar]
Nastoulis 2017
- Nastoulis N, Karakasi MV, Couvaris CM, Kapetanakis S, Fiska A, Pavlidis P. Greenish‐blue gastric content: literature review and case report on acute copper sulphate poisoning. Forensic Science Review 2017;29(1):77‐91. [1042‐7201: (Print)] [PubMed] [Google Scholar]
Nelson 2011
- Nelson LS LN, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. Goldfrank's Toxicological Emergencies. 9. New York: McGraw Hill Education, 2011. [MCGRAW: Hill Medical] [Google Scholar]
Nussbaumer‐Streit 2016
- Nussbaumer‐Streit B, Yeoh B, Griebler U, Pfadenhauer LM, Busert LK, Lhachimi SK, et al. Household interventions for preventing domestic lead exposure in children. Cochrane Database of Systematic Reviews 2016, Issue 10. [1469‐493X: (Electronic); DOI: 10.1002/14651858.CD006047.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2010
- Olson KR. Activated charcoal for acute poisoning: one toxicologist's journey. Journal of Medical Toxicology 2010;6(2):190‐8. [1556‐9039: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orisakwe 2001
- Orisakwe OE, Afonne OJ, Agbasi PU, Ilondu NA, Ofoefule SI, Obi E. Adsorptive capacity of activated charcoal for rifampicin with and without sodium chloride and sodium citrate. Biological and Pharmaceutical Bulletin 2001;24(6):724‐6. [0918‐6158: (Print)] [DOI] [PubMed] [Google Scholar]
Persson 1998
- Persson HE, Sjoberg GK, Haines JA, Pronczuk de Garbino J. Poisoning severity score. Grading of acute poisoning. Journal of Toxicology: Clinical Toxicology 1998;36(3):205‐13. [0731‐3810: (Print)] [DOI] [PubMed] [Google Scholar]
Proudfoot 2003
- Proudfoot AT, Krenzelok EP, Brent J, Vale JA. Does urine alkalinization increase salicylate elimination? If so, why?. Toxicology Review 2003;22(3):129‐36. [1176‐2551: (Print)] [DOI] [PubMed] [Google Scholar]
Quang 2000
- Quang LS, Woolf AD. Past, present, and future role of ipecac syrup. Current Opinion in Pediatrics 2000;12(2):153‐62. [1040‐8703: (Print)] [DOI] [PubMed] [Google Scholar]
Qureshi 2011
- Qureshi Z, Eddleston M. Adverse effects of activated charcoal used for the treatment of poisoning. Adverse Drug Reaction Bulletin 2011;266:1023‐6. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rietjens 2014
- Rietjens SJ, Lange DW, Meulenbelt J. Ethylene glycol or methanol intoxication: which antidote should be used, fomepizole or ethanol?. Netherlands Journal of Medicine 2014;72(2):73‐9. [1872‐9061: (Electronic)] [PubMed] [Google Scholar]
Roberts 2005
- Roberts D, Buckley NA. Alkalinisation for organophosphorus pesticide poisoning. Cochrane Database of Systematic Reviews 2005, Issue 1. [1469‐493X: (Electronic); DOI: 10.1002/14651858.CD004897.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts DM, Buckley NA. Enhanced elimination in acute barbiturate poisoning ‐ a systematic review. Clinical Toxicology 2011;49(1):2‐12. [DOI] [PubMed] [Google Scholar]
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning health care is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rumack 1977
- Rumack BH, Burrington JD. Caustic ingestions: a rational look at diluents. Clinical Toxicology 1977;11(1):27‐34. [0009‐9309: (Print)] [DOI] [PubMed] [Google Scholar]
Thanacoody 2015
- Thanacoody R, Caravati EM, Troutman B, Hojer J, Benson B, Hoppu K, et al. Position paper update: whole bowel irrigation for gastrointestinal decontamination of overdose patients. Clinical Toxicology 2015;53(1):5‐12. [1556‐9519: (Electronic)] [DOI] [PubMed] [Google Scholar]
Tuuri 2009
- Tuuri RE, Ryan LM, He J, McCarter RJ, Wright JL. Does emergency medical services transport for pediatric ingestion decrease time to activated charcoal?. Prehospital Emergency Care 2009;13(3):295‐303. [1545‐0066: (Electronic)] [DOI] [PubMed] [Google Scholar]
Valeur 2015
- Valeur J, Berstad A, Hausken T. The effect of body position on postprandial perceptions, gastric emptying, and intragastric meal distribution: an ultrasonographic study in reclining healthy subjects. Scandinavian Journal of Gastroenterology 2015;50(2):170‐3. [1502‐7708: (Electronic)] [DOI] [PubMed] [Google Scholar]
Van Wijk 2007
- Wijk MP, Benninga MA, Dent J, Lontis R, Goodchild L, McCall LM, et al. Effect of body position changes on postprandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. Journal of Pediatrics 2007;151(6):585‐90, 590 e1‐2. [1097‐6833: (Electronic)] [DOI] [PubMed] [Google Scholar]
Vance 1992
- Vance MV, Selden BS, Clark RF. Optimal patient position for transport and initial management of toxic ingestions. Annals of Emergency Medicine 1992;21(3):243‐6. [PUBMED: 1536482] [DOI] [PubMed] [Google Scholar]
WHO 2016
- Wold Health Organization. World Health Statistics 2016: Monitoring health for the SDGs, sustainable development goals, 2016. www.who.int/gho/publications/world_health_statistics/2016/en. France: World Health Organization, (accessed prior to 14 December 2018).
WHO 2018
- World Health Organization. Fact sheet on suicide 2018. www.who.int/mediacentre/factsheets/fs398/en (accessed prior to 14 December 2018).
Wilkerson 2016
- Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The opioid epidemic in the United States. Emergency Medicine Clinics of North America 2016;34(2):e1‐e23. [1558‐0539: (Electronic)] [DOI] [PubMed] [Google Scholar]
Zideman 2015
- Zideman DA, Buck ED, Singletary EM, Cassan P, Chalkias AF, Evans TR, et al. European Resuscitation Council Guidelines for Resuscitation 2015 Section 9. First aid. Resuscitation 2015;95:278‐87. [1873‐1570: (Electronic)] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Avau 2017
- Avau B, Borra V, Vandekerckhove P, Paepe P, Buck E. First aid interventions by laypeople for acute oral poisoning [Protocol]. PROSPERO 2017; Vol. CRD42017065911:www.crd.york.ac.uk/prospero/display_record.php?RecordID=65911. [http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017065911]


