Abstract
Modern imaging techniques allow early detection of small vestibular schwannomas (VSs) with minimal or no hearing impairment. While controversy surrounds the management of these tumors, given their benign nature and unpredictable natural history, microsurgical excision is the only modality that offers the opportunity to cure the tumor and preserve hearing. Hearing preservation in VS surgery may be accomplished via the middle fossa or retrosigmoid approaches. Appropriate patient selection and surgical approach is critical in achieving the best hearing outcomes. This article highlights the preoperative assessment, patient selection and prognostic factors, intraoperative monitoring of hearing, and surgical approaches to optimize hearing preservation during VS removal.
Keywords: vestibular schwannoma, hearing preservation, skull base, retrosigmoid, middle cranial fossa
Introduction
Management options for vestibular schwannomas (VSs) have not changed in the past few decades. Observation, radiotherapy, and microsurgical excision remain the three accepted therapeutic modalities. Modern magnetic resonance imaging (MRI), and widespread access to this technology, allowed early detection of small VSs with minimally impaired hearing. Management of these patients is still under debate due to the variable natural history of VSs. The majority of VSs will grow at a rate of ≤2 mm per year. Intracanalicular tumors may remain stable for years whereas other locations grow less predictably. 1 2 Additionally hearing loss can occur gradually or suddenly, and independent of tumor growth. 3 Applying population statistics to individual patients is therefore difficult, making patient counseling challenging.
Nonetheless, in patients with small VSs who have functional hearing, preservation of hearing should be a management priority. Numerous reports have examined the hearing preservation rates associated with the three management options for VSs. In a longitudinal study in which patients were observed with serial imaging, Stangerup et al demonstrated that only 55% of patients with serviceable hearing at diagnosis (American Academy of Otolaryngology—Head and Neck Surgery [AAO-HNS] class A or B) retained their hearing at 10 years. 4 A recent systematic review by Coughlin et al demonstrated that the long-term hearing preservation rate after radiotherapy is 23% at 10-year follow-up. 5 Moreover, neither observation nor radiotherapy allows for tumor cure. Conversely, microsurgical resection via the retrosigmoid or middle cranial fossa approach allows for tumor cure while preserving hearing in approximately 70 to 85% of small tumors 1 cm or less in size. 6 7 8 9 Hearing preservation microsurgery is only possible in select patients and tumors. This review highlights the clinical and technical considerations when attempting hearing preservation microsurgery for VSs.
Preoperative Assessment
History and Physical Examination
Patients with VS usually present with a combination of asymmetric hearing loss, unilateral tinnitus and less commonly imbalance. 10 Vertigo at presentation is uncommon but relevant as it may correlate with decreased postoperative dizziness due to central nervous system accommodation to tumor-related unilateral vestibular dysfunction. A history of frequent headaches or migraines should also be elicited particularly in patients being considered for the retrosigmoid approach. Such patients should be counseled that they may be at higher risk for lingering postoperative headaches.
Physical examination involves routine otologic and vestibular assessment, and a neurological examination focusing mainly on cranial nerve and cerebellar function.
Imaging
Gadolinium-enhanced MRI with dedicated thin cuts of the internal auditory canal (IAC) is the gold standard for the detection of small VSs. 11 MRI has replaced auditory brainstem response (ABR) in the work-up of asymmetric sensorineural hearing loss, the latter showing unsuitably low sensitivity and specificity. 12 Post-gadolinium T1 sequences offer the best tumor visualization, as VSs enhance with contrast. At our institution, we use the measurement along the tumor's longest axis to determine hearing preservation candidacy ( Fig. 1 ). The FIESTA (fast imaging employing steady-state acquisition) or CISS (constructive interference in steady-state) sequences provide the high resolution critical for evaluating the paths of the facial and cochlear nerves as they course around the tumor in the cerebellopontine angle (CPA) and IAC. Nerves travelling on the posterior aspect of the tumor are prone to manipulation in the retrosigmoid approach and therefore at risk of injury. In the middle fossa approach, a facial nerve coursing superiorly will be more susceptible to injury. The bright appearance of the cerebrospinal fluid (CSF) in the T2 or FIESTA/CISS sequences allows for accurate evaluation of fundal tumor extension. The presence of CSF between the lateral tumor margin and the fundus of the IAC (i.e., fundal fluid) has not been shown to be an independent predictor of hearing preservation after surgery. 13
Fig. 1.
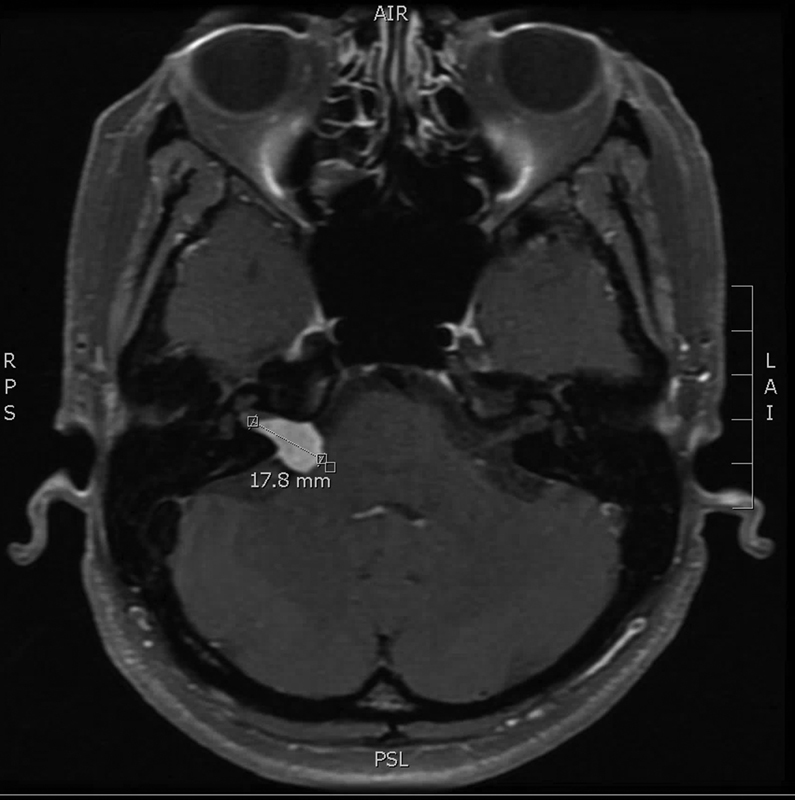
Vestibular schwannoma size is determined by measuring the tumor along its longest axis (post-gadolinium sequence, axial view).
Audiometry and Vestibular Function Tests
The audiometric evaluation consists of standard air and bone pure-tone thresholds along with word recognition scores (WRS). The AAO-HNS scale is the most commonly reported classification scheme for the evaluation of hearing preservation in acoustic neuroma. 14 According to this classification, serviceable hearing is defined as AAO-HNS class A (pure-tone average [PTA] < 30 dB, speech detection threshold [SDS] > 70%) or B (PTA: 30–50 dB, SDS: 50–70%). However, there is debate in the literature regarding the functional significance of this classification system. Some authors argue that preservation of word discrimination—independent of PTA—is a more relevant measure of successful hearing preservation, mainly because a preserved WRS can achieve socially useful hearing with amplification. 15 In addition to a standard audiogram, some centers perform a preoperative ABR to assess prognosis for hearing preservation and to confirm that adequate responses can be recorded for intraoperative monitoring. 16 In the senior author's practice ABR is not obtained routinely as use of direct cochlear nerve intraoperative monitoring is usually possible in the setting of absent preoperative ABR. 17 Vestibular function tests have also been suggested as topodiagnostic studies to predict the nerve of origin of the VS, but the results of these studies are inconclusive and are not routinely ordered in our practice. 18 19
Patient Selection and Prognostic Factors
Typical hearing preservation candidates are younger than 65 and have small tumors and serviceable hearing. Treatment should be individualized according to patient age, health status, tumor size, growth patterns and hearing status in both ears, and ultimately patient preferences and expectations.
A large body of evidence has shown tumor size to be the most consistent predictive factor for hearing preservation. 6 20 21 22 Hearing preservation may be attempted in tumors up to 2.5 cm, but the best outcomes (∼70–85%) are obtained with tumors smaller than 1 cm. 7 At our institution, we rarely attempt hearing preservation microsurgery for lesions larger than 2.5 cm. In addition, investigators previously demonstrated that preoperative hearing correlated with improved hearing preservation outcomes. 20 23 24 In light of these findings, clinicians generally offer hearing preservation for patients with AAO-HNS class A or B (i.e., PTA < 50 dB and SDS > 50%). Given the ongoing debate as to whether WRS may be a better indication of socially useful hearing, it could be reasonable to consider patients with preserved WRS independent of PTA (i.e., AAO-HNS class C). Such patients should be informed that their hearing preservation prognosis is lower. VSs occurring in the only-hearing ear present a management dilemma and have been discussed elsewhere. 25 26
Studies by Goddard et al and Mohr et al reported that preservation is generally more likely in tumors that only involve the medial aspect of the IAC as opposed to those with extension into the fundus. 24 27 Nguyen et al found evidence to the contrary of other published reports in that more lateral extent of the tumor predicted better hearing in patients undergoing retrosigmoid microsurgical tumor removal. 13 These authors hypothesized that fundal extension allows tumors to become clinically symptomatic at an earlier stage with smaller size and better preoperative hearing, which translates into better hearing preservation rates.
Generally, the middle fossa approach is suitable for intracanalicular tumors and tumors ≤2 cm overall size with minimal CPA extension, i.e., not contacting the brainstem. This approach should be reserved for patients ≤65 years old because of the fragility of the dura and the underlying temporal lobe in older individuals. When tumors contact the brainstem, appropriate visualization can be hard to achieve and the retrosigmoid approach is preferred due to its wide exposure of the CPA. The senior author performs retrosigmoid craniotomy for all VS hearing preservation cases. Ultimately, the decision to employ one approach over the other will depend on the surgeon's experience and comfort level with either or both approaches.
Intraoperative Monitoring of Hearing
The likelihood of successful preservation of cochlear nerve function during VS surgery has been improved by the advent of intraoperative monitoring techniques, the two most common being ABR and direct eighth nerve monitoring (DENM). Electrocochleography is rarely used and will not be discussed in this review.
ABR consists of the recording of the electrical activity of the central auditory pathways in response to an auditory signal, generally filtered clicks or tone bursts. 28 Because the recording is performed over the surface of the skull, ABR is a far-field technique. This is a limitation, as only small amplitude electrical activity can be recorded. Hundreds to thousands of responses therefore need to be averaged to generate a reliable tracing, which can take from 2 to 5 minutes. This inherent delay in obtaining a recording precludes real-time feedback to the surgeon, allowing irreversible damage to occur. Lastly, while the presence of good ABR waveforms is highly correlated with successful hearing outcomes, their absence does not particularly predict hearing loss. 29
DENM involves the electrical recording of sound-evoked compound eight nerve action potential. 28 The scalp electrode setup and auditory stimuli are the same as for performing ABR. The major difference is that the recording electrode is placed directly on the cochlear portion of CNVIII located on the inferior aspect of the bundle at the brainstem. This can be more easily performed with an atraumatic self-retaining electrode design. 30 As such, DENM is a near-field technique: large amplitude responses can be recorded, requiring less averaging to produce a reliable tracing within 1 or 2 seconds. This translates into near-instantaneous intraoperative feedback during tumor dissection. In addition, by placing the electrode proximal to the area of tumor involvement, the integrity of the entire cochlear nerve may be assessed during dissection. Preservation of the action potential after tumor dissection is correlated with positive hearing outcomes, independent of ABR results. The major limitation of DENM is that auditory monitoring can only be performed once the nerve has been exposed, usually after the CPA component of the tumor has been removed and the IAC drilled out. The recording is also impeded when the electrode is bathed in CSF or blood, requiring manipulation and sometimes repositioning.
Hearing Preservation Approaches
Retrosigmoid Craniectomy
The retrosigmoid approach for resection of VSs has traditionally been recommended for tumors with sizable CPA components or for intracanalicular tumors more medially located. This dogma has been challenged by Nguyen et al in their paper in which tumor size was found to be more important than location within the IAC—particularly presence or absence of fundal involvement. 13 While the retrosigmoid approach may be used to remove VSs of any size, attempting hearing preservation in tumors larger than 25 mm in the maximal dimension is seldom successful and places the patient at slightly higher risk for postoperative facial nerve dysfunction. 22 31 The best hearing results are in patients with tumor 10 mm or smaller in size in whom odds of hearing preservation may approach 80 to 85%. 8 13
The patient is positioned supine on the operating table. No shoulder roll is placed as this pushes the shoulder into the surgeon's way. Additionally, park bench positioning is almost never needed. Lumbar drainage is not employed. The head is placed in pin fixation with slight elevation of the head in a “sniff” position then rotation toward the opposite shoulder and finally nodded toward the clavicle. This brings the retromastoid area above and away from the ipsilateral shoulder in all but the most obese patients. The skin incision is placed roughly two finger-breadths behind the postauricular crease ( Fig. 2A ). The lower extent approximates the mastoid tip and the upper end the top of the auricle. The root of zygoma is marked as an approximation for the junction of the sigmoid and transverse sinuses. Local anesthetic with epinephrine is injected into the site for hemostasis. Once the site is prepared and draped the incision is taken down to the muscle layer. The muscles are divided along their longitudinal axis to reach the skull posterior to the mastoid. Periosteal elevation is undertaken anteriorly to identify the posterior extent of the digastric groove. This landmark is a reliable guide to estimate the posterior aspect of the sigmoid sinus. Limited posterior periosteal elevation is performed and a self-retraining retractor placed. Next a 2-cm craniectomy is created with the anterior limit of bone removal at the posterior edge of the sigmoid sinus ( Fig. 2B ). Superiorly the limit of bone removal is the transverse sinus. We do not decompress the sigmoid or transverse sinuses.
Fig. 2.
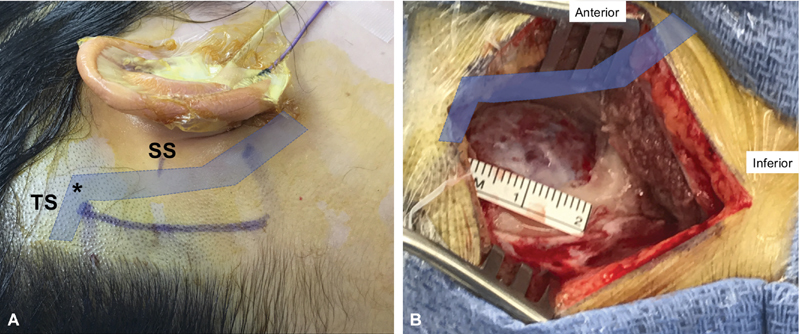
( A ) Skin incision marking during a right retrosigmoid approach. The projected course of the sigmoid (SS) and transverse (TS) sinuses is shadowed. The root of zygoma is marked to estimate the junction ( asterisk ) of the sigmoid and transverse sinuses. ( B ) Retrosigmoid craniectomy defect. The limits of bone removal are the posterior edge of the sigmoid sinus (anteriorly) and the transverse sinus (superiorly).
The dura is then opened in a cruciate fashion and the resulting triangular flaps are held aside with 4–0 braided nylon (Nurolon) sutures. Using a curved brain ribbon, the basal cistern is accessed and CSF is suctioned to achieve cerebellar relaxation. Gentle anterior to posterior cerebellar retraction is then undertaken to expose the CPA. A retractor is left gently in place more to protect the surface of the cerebellum during instrument insertion rather than to hold the cerebellum back. In smaller tumors the superior cerebellar emissary vein bridging from the upper aspect of the anterior cerebellum to the superior petrosal sinus may be left in situ. In larger tumors it may be necessary to sacrifice this vessel. Inspection of the CPA portion of the tumor is undertaken to identify the course of the facial and cochleovestibular nerves over the surface of the tumor. Use of the facial nerve stimulator on the posterior surface of the tumor prior to incising tumor capsule is routinely employed to confirm a safe entry point into the tumor. ABR monitoring is routinely used during this stage of surgery.
The capsule is then opened and internal debulking of the tumor is undertaken. This creates a more flexible tumor capsule that can then be more delicately separated from the vessels and nerves traveling on the tumor surface. Once the CPA portion of the tumor has been largely removed, attention turns to the posterior surface of the petrous bone. The operculum of the endolymphatic sac is identified and serves as the lateral limit of dural resection ( Fig. 3A ). A dural flap is excised anticipating the size of the superior and inferior bony corridors that will be removed around the IAC. Absorbable gelatin compressed sponge (Gelfoam) or preferably collagen sponge (Bicol) impregnated with papaverine is placed in the cisterns above and below the seventh and eighth nerves to limit spread of bone dust in these areas and to reduce chances of internal auditory artery spasm from cold saline irrigation during drilling. Using diamond burs of varied sizes (usually 2-, 3-, and 4-mm diameters), the IAC bone is dissected. This process starts by removing an arc of bone on the posterior petrous bone between the operculum and porus of the IAC. This bony removal is undertaken to identify the posterior surface of the IAC and define the superior and inferior limits of the canal. Once the more medial aspect of the IAC is identified, the superior and inferior corridors are further developed. The goal is to have the depth of these corridors to align with the anterior limit of the IAC.
Fig. 3.
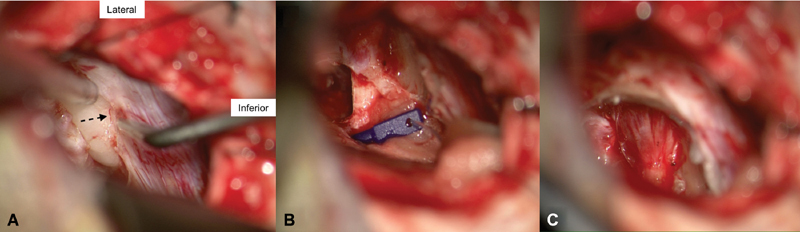
( A ) The operculum of the endolymphatic sac ( dotted arrow ) is identified and serves as a guide for the lateral limit of dural dissection and bony removal. ( B ) The direct eight-nerve monitoring electrode is applied over the inferior aspect of the cochleovestibular bundle. Notice how it is secured in place with collagen sponges medially at the brainstem and laterally at the craniotomy edges. ( C ) Drilled out internal auditory canal demonstrating the vestibular nerves splayed over the posterior surface of the tumor.
As the drilling is taken laterally it is imperative that the course of the endolymphatic sac/duct is carefully delineated and serves as a guide to the lateral limit of bony removal. Use of this landmark will maximize the amount of bone removal laterally while avoiding injury to the labyrinth. In practiced hands, bone surrounding the IAC may be removed to within 2 to 3 mm of the IAC fundus. Use of specially designed dissectors allows for confirmation of the extent of unexposed distal IAC (available from Grace Medical, Inc.). Once the bony dissection has been completed, the CPA is irrigated copiously to remove any remaining bone dust. The papaverine-soaked collagen sponge is replaced with fresh pieces and attention directed to the medial cochlear nerve.
Under direct vision the 2-mm cochlear nerve electrode (available from AD-Tech Medical, Inc.) is applied on the inferior aspect of the cochleovestibular nerve as it leaves the brainstem ( Fig. 3B ). The flange on the electrode wire is then secured to the drape with a clamp to reduce the chance for accidental displacement. The electrode wire is then connected to the lead wire that is next inserted into the ABR wiring harness. Small pieces of collagen sponge soaked in papaverine are placed medial and lateral to the cochlear nerve electrode to help keep it in position on the nerve. Cochlear nerve action potentials are routinely obtained within just seconds rather than the 2 to 3 minutes needed for an ABR waveform.
Once DENM is underway the dura of the IAC is opened and the canal portion of the tumor centrally debulked ( Fig. 3C ). As the intracanalicular portion of the tumor is dissected from the cochlear and facial nerves, care is taken to have dissection force proceed from medial to lateral. Additionally, any artery traveling along the surface of the tumor is preserved if at all possible. Cessation of dissection and application of additional papaverine is performed in response to prolongation of latency or drops in amplitude of the compound nerve action potential. Occasionally suctioning of excess CSF and/or blood from the area of the cochlear nerve electrode may be needed to optimize the tracing. If the tumor extends beyond the limit of bony exposure, the specially designed IAC dissectors facilitate separation of the tumor from the cochlear and facial nerves as well as tumor delivery from the fundus. Once complete tumor removal has felt to have been accomplished careful survey of the IAC fundus with the dissectors is done to confirm complete tumor removal. One may choose to inspect the fundus endoscopically but we have not found this particularly helpful as a thin layer of blood often obscures the view.
Once tumor removal is completed, hemostasis within the IAC may be achieved with the application of Microfibrillar Collagen Hemostat (Avitine) and/or very judicious use of bipolar cautery at lower settings. A papaverine and then a methylprednisolone acetate (Depomedrol) soaked collagen sponge is placed over the facial and cochlear nerves in the IAC while the areas of bone dissection are sealed with carefully placed bone wax. The latter serves to occlude any air cells that may have been opened during bone removal thereby reducing chances for CSF leak. Once the application of bone wax is complete, the cochlear nerve electrode is removed and ABR monitoring is resumed. Hemostasis of the CPA is achieved once all the medication soaked collagen sponge is removed. The dura is closed with a combination of interrupted and running 4–0 braided nylon sutures. Any mastoid air cells opened during creation of the craniectomy are sealed with bone wax. Next the bony craniectomy defect is filled with a Microfibrillar Collagen Hemostat blood patch. Two layers of muscular fascial closure are performed with 2–0 absorbable suture. The same suture is used to achieve a closure of the deep subcutaneous tissues in an inverted, interrupted fashion. Finally, stainless steel staples approximate the skin. A large adhesive island dressing is used to cover the closed incision. Pin fixation is removed and the patient allowed to awaken from their anesthetic and is extubated prior to transfer to the postanesthesia care unit. The patient is monitored overnight in the intensive care unit. Discharge from hospital is typically within 2 to 3 days.
Middle Fossa Craniotomy
The middle fossa approach provides complete exposure of the contents of the IAC from porus to fundus, allowing removal of laterally placed tumors under direct visualization. The entire tumor can be exposed with the exception of 1 to 2 mm under the transverse crest in some laterally impacted tumors. However, the surgical anatomy of the temporal bone as seen from above is complex, and the landmarks are not as apparent as in other approaches to the skull base.
The surgical technique is summarized below. The patient is placed in the supine position with the head turned to the opposite side and the surgeon seated at the head of the table. No pinning is necessary and appropriate neuromonitoring is set up. A “reverse question mark” incision is made starting in the pretragal area (at the root of the zygoma) and extended superiorly in the temporal area in a gently curving fashion. The incision is located two-thirds anterior and one-third posterior to the external auditory canal. The incision is carried down to the plane of the superficial temporalis fascia. Care is taken not to damage the frontal branch of the facial nerve close to the anterior end of the incision. The path of this nerve in the soft tissue can be estimated by Pitanguy's line, starting from a point 0.5 cm below the tragus and extending in the direction of the eyebrow, passing 1.5 cm above the lateral extremity of the eyebrow. 32 An inferiorly based U-shaped flap is fashioned in the temporalis muscle and fascia and is reflected inferiorly.
By use of a 4-mm cutting bur, a 4 × 4 cm craniotomy opening is made in the squamous portion of the temporal bone. Care is taken to avoid laceration of the underlying dura by reversing the drill motion or using a diamond bur once the bone is thinned. This bone flap is based on the root of the zygoma as close to the floor of the middle fossa as possible. It is not uncommon to encounter air cells in this area which can be waxed to prevent postoperative CSF leak. The bone flap is marked and set aside for later closure.
Elevation of the dura with a suction irrigator and a blunt dural elevator proceeds in a posterior-to-anterior fashion. This prevents damage to the geniculate ganglion, which can be dehiscent in 15% of cases. The tegmen is also frequently very thin or dehiscent, and care must be taken to avoid creating a defect into the epitympanum. The initial landmark during dural elevation is the arcuate eminence, which marks the relative position of the superior semicircular canal. It makes an approximately 45 to 60 degree angle with the IAC and may be readily apparent in some patients but obscure in others. The next landmark encountered while proceeding with anterior dural elevation is the greater superficial petrosal nerve. The middle meningeal artery forms the anterior limit of the dissection, although it is often not exposed when performing small tumor removal. Venous bleeding can be encountered from this area and is controlled with oxidized cellulose. The petrous ridge is identified, and care is taken not to injure the superior petrosal sinus. When the dura has been elevated, the House-Urban retractor is placed to support the temporal lobe. There often exists a bony overhang directly above the porus. This is the ideal location for retractor placement. The tip of the retractor engages the medial aspect of the petrous ridge, and teeth are locked against the bone margins of the craniotomy window ( Fig. 4 ).
Fig. 4.
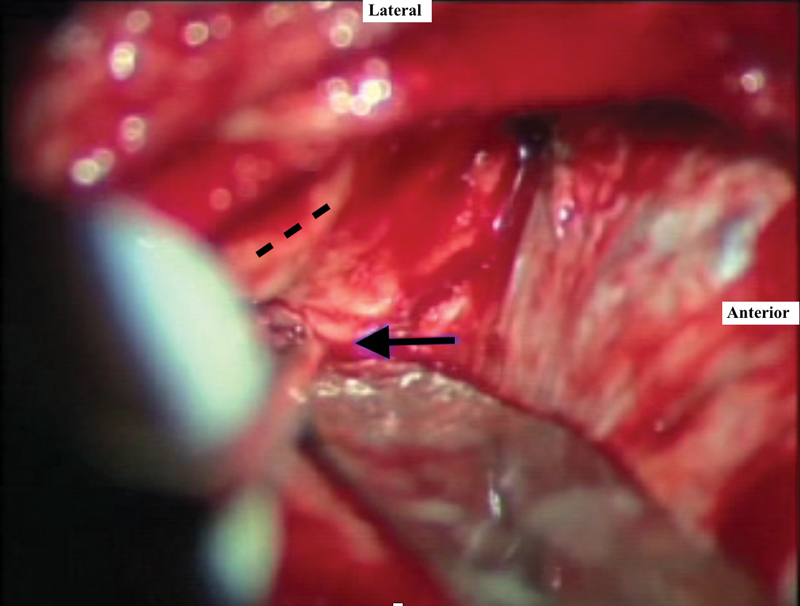
Ideal location for retractor placement, engaging the medial aspect of the true petrous ridge ( black arrow ). The arcuate eminence is shown in dashed line .
The arcuate eminence and greater superficial petrosal nerve are the two major landmarks for the subsequent intratemporal dissection. The IAC is located at the bisection of the angle formed by the superior semicircular canal and greater superficial petrosal nerve. The “ivory” bone of the semicircular canal can be identified near the arcuate eminence by the use of a large diamond bur. Having situated the location of the IAC in our mind's eye, bone removal is begun with a combination of diamond burs in the posterior triangle between the IAC, the arcuate eminence, and the petrous ridge. The posterior surface IAC is identified medially at the porus and traced laterally. This is the safest technique to initiate drilling, as the exposure narrows laterally due to the location of the inner ear—the cochlea anteriorly and the superior semicircular canal ampulla posteriorly. Bone is then removed on the superior and anterior aspects of the IAC ( Fig. 5 ). The lateral end of the IAC dura is exposed by careful drilling, identifying Bill's bar, the superior vestibular nerve, and the labyrinthine segment of the facial nerve. The labyrinthine facial nerve is followed to the geniculate ganglion. As a general rule, the dura overlying the superior vestibular nerve can be exposed for approximately one-half of the distance of the labyrinthine facial nerve. Medially, 270 degrees of bone can be removed from around the IAC to identify the posterior fossa dura. A small durotomy can be performed at that location to decompress some CSF which will relax the temporal lobe and reduce the need for retraction.
Fig. 5.
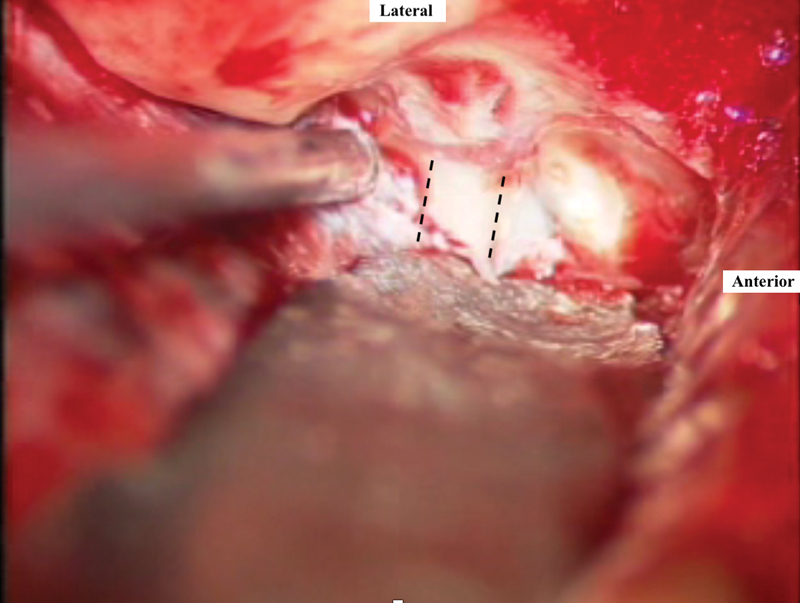
Bone removal posterior and anterior to the internal auditory canal (delineated in dashed lines ). The blue lining of the superior semicircular canal can be seen posteriorly.
Once adequate exposure is achieved, the dura is incised along the posterior aspect of the IAC to avoid injury to the facial nerve. At the time of dural opening, the facial nerve is identified immediately throughout its course with its relationship to the tumor. A surgical plane is then created between the facial nerve and the tumor. The superior vestibular nerve is then sectioned medially, and the tumor is dissected away from the facial nerve. All dissection within the IAC should be performed from medial to lateral. This prevents traction on the cochlear nerve and blood supply as they enter the modiolus. It is important to identify and divide the facial-vestibular anastomoses as dissection proceeds to avoid excessive tension on the facial nerve. With the use of a right-angled hook, the inferior vestibular nerve is sectioned, allowing for gentle tumor delivery from the lateral end of the IAC. This should be performed medial to Scarpa's ganglion, away from the fundus of the IAC. Sectioning of both vestibular nerves, independent of tumor involvement, is important to prevent persistent unsteadiness from a partial vestibulopathy. The tumor is then separated from the cochlear and facial nerves and removed. The labyrinthine artery generally travels between these two nerves within the IAC but may not be visible during the dissection.
Irrigation of the tumor bed is performed, followed by adequate hemostasis. Any air cells encountered during the petrous apex drilling are sealed with bone wax. Abdominal fat is then used to pack the IAC. The House-Urban retractor is removed, and the temporal lobe is allowed to re-expand. The craniotomy bone flap is replaced. The temporalis fascia and muscle are reapproximated. The deep dermal layer is closed with absorbable sutures. The skin is closed with a running nylon suture. A mastoid-type dressing is applied for 2 days postoperatively. Patients are observed in the intensive care unit overnight, with neurological vitals performed hourly. Discharge usually occurs on postoperative day 2 or 3.
Conclusion
Hearing preservation microsurgery is best suited for younger patients with small tumors and good hearing. Optimal management of these patients remains controversial, but surgical resection is the only modality that allows for complete tumor removal while possibly preserving hearing. The two available hearing preservation approaches are the middle fossa craniotomy and the retrosigmoid craniectomy. Both techniques achieve high rates of hearing preservation in properly selected patients in experienced centers. The decision to employ one approach over the other depends on various factors, such as tumor size, hearing quality, patient age as well as surgeon's experience.
Footnotes
Conflict of Interest None.
References
- 1.Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otol Neurotol. 2006;27(05):705–712. doi: 10.1097/01.mao.0000226302.59198.87. [DOI] [PubMed] [Google Scholar]
- 2.Stangerup S-E, Cayé-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27(04):547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 3.Stangerup S-E, Cayé-Thomasen P.Epidemiology and natural history of vestibular schwannomas Otolaryngol Clin North Am 20124502257–268., vii –vii [DOI] [PubMed] [Google Scholar]
- 4.Stangerup S-E, Thomsen J, Tos M, Cayé-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol. 2010;31(02):271–275. doi: 10.1097/MAO.0b013e3181c34bda. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin A R, Willman T J, Gubbels S P. Systematic review of hearing preservation after radiotherapy for vestibular schwannoma. Otol Neurotol. 2018;39(03):273–283. doi: 10.1097/MAO.0000000000001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arts H A, Telian S A, El-Kashlan H, Thompson B G. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using the middle cranial fossa approach. Otol Neurotol. 2006;27(02):234–241. doi: 10.1097/01.mao.0000185153.54457.16. [DOI] [PubMed] [Google Scholar]
- 7.Kutz J W, Jr, Scoresby T, Isaacson Bet al. Hearing preservation using the middle fossa approach for the treatment of vestibular schwannoma Neurosurgery 20127002334–340., discussion 340–341 [DOI] [PubMed] [Google Scholar]
- 8.Yamakami I, Yoshinori H, Saeki N, Wada M, Oka N. Hearing preservation and intraoperative auditory brainstem response and cochlear nerve compound action potential monitoring in the removal of small acoustic neurinoma via the retrosigmoid approach. J Neurol Neurosurg Psychiatry. 2009;80(02):218–227. doi: 10.1136/jnnp.2008.156919. [DOI] [PubMed] [Google Scholar]
- 9.Sameshima T, Fukushima T, McElveen J T, Jr, Friedman A H.Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach Neurosurgery 20106703640–644., discussion 644–645 [DOI] [PubMed] [Google Scholar]
- 10.Pinna M H, Bento R F, Neto R de B. Vestibular schwannoma: 825 cases from a 25-year experience. Int Arch Otorhinolaryngol. 2012;16(04):466–475. doi: 10.7162/S1809-97772012000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidman J D, Carrasco V N, Whaley R A, Pillsbury H C., III Gadolinium. The new gold standard for diagnosing cerebellopontine angle tumors. Arch Otolaryngol Head Neck Surg. 1989;115(10):1244–1247. doi: 10.1001/archotol.1989.01860340098026. [DOI] [PubMed] [Google Scholar]
- 12.Cueva R A. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114(10):1686–1692. doi: 10.1097/00005537-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Q T, Wu A P, Mastrodimos B J, Cueva R A. Impact of fundal extension on hearing after surgery for vestibular schwannomas. Otol Neurotol. 2012;33(03):455–458. doi: 10.1097/MAO.0b013e318245cf01. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113(03):179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 15.Woodson E A, Dempewolf R D, Gubbels S P et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol. 2010;31(07):1144–1152. doi: 10.1097/MAO.0b013e3181edb8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson R F, Hansen M R, Gantz B J. Hearing preservation surgery for vestibular schwannomas. Curr Otorhinolaryngol Rep. 2014;2(04):235–241. [Google Scholar]
- 17.Jackson L E, Roberson J B., Jr Acoustic neuroma surgery: use of cochlear nerve action potential monitoring for hearing preservation. Am J Otol. 2000;21(02):249–259. doi: 10.1016/s0196-0709(00)80018-6. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Yamada C, Inoue R, Kashio A, Saito Y, Nakanishi W. Analysis of vestibular testing in patients with vestibular schwannoma based on the nerve of origin, the localization, and the size of the tumor. Otol Neurotol. 2008;29(07):1029–1033. doi: 10.1097/MAO.0b013e3181845854. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi T, Tsunoda A, Noguchi Y, Komatsuzaki A. Prediction of the nerves of origin of vestibular schwannomas with vestibular evoked myogenic potentials. Am J Otol. 2000;21(05):712–715. [PubMed] [Google Scholar]
- 20.Meyer T A, Canty P A, Wilkinson E P, Hansen M R, Rubinstein J T, Gantz B J. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol. 2006;27(03):380–392. doi: 10.1097/00129492-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Jacob A, Robinson L L, Jr, Bortman J S, Yu L, Dodson E E, Welling D B. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope. 2007;117(12):2087–2092. doi: 10.1097/MLG.0b013e3181453a07. [DOI] [PubMed] [Google Scholar]
- 22.Yates P D, Jackler R K, Satar B, Pitts L H, Oghalai J S. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol. 2003;24(03):460–464. doi: 10.1097/00129492-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Brackmann D E, Owens R M, Friedman R A et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21(03):417–424. doi: 10.1016/s0196-0709(00)80054-x. [DOI] [PubMed] [Google Scholar]
- 24.Mohr G, Sade B, Dufour J-J, Rappaport J M. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of meatal filling. J Neurosurg. 2005;102(01):1–5. doi: 10.3171/jns.2005.102.1.0001. [DOI] [PubMed] [Google Scholar]
- 25.Thedinger B A, Cueva R A, Glasscock M E., III Treatment of an acoustic neuroma in an only-hearing ear: case reports and considerations for the future. Laryngoscope. 1993;103(09):976–980. doi: 10.1288/00005537-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma) Otol Neurotol. 2005;26(02):205–210. doi: 10.1097/00129492-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Goddard J C, Schwartz M S, Friedman R A. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31(07):1128–1134. doi: 10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 28.Youssef A S, Downes A E. Intraoperative neurophysiological monitoring in vestibular schwannoma surgery: advances and clinical implications. Neurosurg Focus. 2009;27(04):E9. doi: 10.3171/2009.8.FOCUS09144. [DOI] [PubMed] [Google Scholar]
- 29.Cueva R A.Preoperative, intraoperative, and postoperative auditory evaluation of patients with acoustic neuroma Otolaryngol Clin North Am 20124502285–290., vii –vii [DOI] [PubMed] [Google Scholar]
- 30.Danner C, Mastrodimos B, Cueva R A. A comparison of direct eighth nerve monitoring and auditory brainstem response in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2004;25(05):826–832. doi: 10.1097/00129492-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 31.Mamikoglu B, Esquivel C R, Wiet R J. Comparison of facial nerve function results after translabyrinthine and retrosigmoid approach in medium-sized tumors. Arch Otolaryngol Head Neck Surg. 2003;129(04):429–431. doi: 10.1001/archotol.129.4.429. [DOI] [PubMed] [Google Scholar]
- 32.Pitanguy I, Ramos A S. The frontal branch of the facial nerve: the importance of its variations in face lifting. Plast Reconstr Surg. 1966;38(04):352–356. doi: 10.1097/00006534-196610000-00010. [DOI] [PubMed] [Google Scholar]


