Abstract
Purpose
Ductal carcinoma in situ (DCIS) contributes to 20%–30% of newly diagnosed cases of breast cancer in China. Although the breast cancer-specific mortality of DCIS is extremely low, a small proportion of DCIS patients still show relapse or metastasis, leading to poor prognosis. Little is known about the molecular mechanism for DCIS metastasis, partly due to the limited number of poor prognosis patients. This study analyzed the clinicopathological features and screened key microRNAs (miRNAs) contributing to local or distant recurrence.
Methods
The clinicopathological features of DCIS were evaluated and survival analysis were performed to clarify risk factors associated with poor prognosis. Using miRNA arrays and real-time quantitative polymerase chain reaction (RT-qPCR) on DCIS formalin-fixed and paraffin-embedded samples with or without microinvasion with different clinical outcomes, potential DCIS metastasis-related miRNAs were screened out and further validated. The influence of one identified miRNA, miRNA-654-5p, on DCIS progression was analyzed.
Results
Poor prognosis was significantly associated with larger tumor size and higher lymph node metastasis rate (both p < 0.05). Both were independent prognostic factors for DCIS. According to RT-qPCR results, distinct miRNA expression profiles were identified between DCIS and DCIS with microinvasion (DCIS-Mi) patients. In the DCIS panel, miRNA-654-5p was significantly upregulated in the patients with poor prognosis. In vitro, miRNA-654-5p promoted MDA-MB-231 cell mobility in healing tests and metastasis in the Transwell study.
Conclusion
The panel of high-risk miRNAs in DCIS and DCIS-Mi differs markedly. miRNA-654-5p is significantly upregulated DCIS patients having poor prognosis and may be essential for local and distant recurrence in DCIS.
Keywords: Disease-free survival, Epithelial-mesenchymal transition, Noninfiltrating intraductal carcinoma, MicroRNAs
INTRODUCTION
Breast cancer is the most common cancer among all newly diagnosed female patients, and accounts for 15% of all new cases in China [1]. Among all breast cancer patients, approximately 15% to 20% of women are diagnosed as ductal carcinoma in situ (DCIS), and the prevalence of DCIS continues to increase due to the wide use of mammography screening in China [2,3].
DCIS is a type of preinvasive carcinoma with a long overall survival (OS). The 10-year survival rate exceeds 95% [4], and few cases relapsed or progressed to invasive cancer, showing a poor prognosis. The molecular mechanism of DCIS progression is unclear, reflecting the limited data on patients with local or distant recurrence. Certain clinical and pathological factors, such as larger tumor size, necrosis, and positive margin status have been implicated as high-risk factors for disease progression [5]. A better understanding of the mechanism of DCIS progression would assist in the identification of high-risk DCIS at the time of diagnosis and help in decision-making concerning potential therapeutic strategies.
MicroRNAs (miRNAs) have essential roles in aspects of the malignancy of breast epithelial cells, including progression, cell invasion, and cell differentiation [6]. The regulation of miRNAs has been implicated in many cancer processes, in both tumor suppression and oncogenic progression [7], including metastasis, metabolism, stem cell division, cell growth, differentiation and apoptosis. Approximately 30% of the human genome is regulated by miRNAs [8]. miRNAs contribute to invasive ductal carcinoma (IDC) formation and progression [9,10]. However, the role of miRNA for DCIS is reported.
The objective of this study was to screen out and identify high-risk miRNAs that contributed to local or distant recurrence of DCIS. Moreover, we analyzed the functional role of miRNA-654-5p in promoting cell invasion in DCIS.
METHODS
Patients and tissue samples
Approval for this study was granted by the Ethics Committee of West China Hospital of Sichuan University (IRB number: 2013-191). DCIS was pathologically diagnosed by continuous section of tissue every 0.2 cm after examining the whole tumor area. Good prognosis was defined as no local nor distant recurrence during a follow-up of at least 60 months. Poor prognosis was defined as either local or distant recurrence during the follow-up period. The 316 DCIS patients were retrospectively reviewed from the medical records of Fourth Hospital of Hebei Medical University and West China Hospital of Sichuan University between 2000 and 2016, and between 1996 and 2015. Disease-free survival was defined as the time from the date of the diagnosis to the earliest occurrence of all local, regional, or distant recurrences. OS was defined as the time from the date of the diagnosis of DCIS to death.
Three pairs of formalin-fixed and paraffin-embedded (FFPE) tissue obtained from good and poor prognosis cases of DCIS with or without microinvasion were used for microarray profiling. The FFPE samples for real-time quantitative polymerase chain reaction (RT-qPCR) were from 16 female DCIS patients (4 poor prognosis, 12 good prognosis) and 22 female DCIS with microinvasion (DCIS-Mi) patients (5 poor prognosis, 17 good prognosis) who underwent surgical resection for breast cancer from 2010 to 2017 at the Fourth Hospital of Hebei Medical University and the West China Hospital of Sichuan University. A flow chart of the screening design is shown in Figure 1.
Figure 1. Flow chart of our study design. miRNA profiling was performed using a microRNA array analysis of three good prognoses and three metastatic cases from different patients. The selected metastasis related miRNAs (n = 5) in DCIS and DCIS-Mi in 2 independent sets of good and metastatic formalin-fixed and paraffin-embedded samples (4 metastatic and 12 nonmetastatic DCIS patients; 5 metastatic and 17 nonmetastatic DCIS-Mi patients) using qPCR.
miRNA = MicroRNA; DCIS = ductal carcinoma in situ; DCIS-Mi = ductal carcinoma in situ with microinvasion; qPCR = quantitative polymerase chain reaction.
Microarray analysis
The microarray analysis for the miRNA profiling was performed at Kangcheng Technology (Shanghai, China) using the miRCURY LNA™ microRNA Array (v.19.0; Exiqon, Vedbaek, Denmark). Total RNA for the miRNA microarray was isolated using TRIzol (Invitrogen, Carlsbad, USA) and purified with an RNeasy mini kit (QIAGEN, Duesseldorf, Germany) according to the manufacturers’ instructions. RNA quality and quantity were measured using a NanodropND-1000 nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, USA) and the RNA integrity was determined by gel electrophoresis. After miRNA labeling, the samples were hybridized on the miRCURY LNA™ array. The slides were scanned using a GenePix 4000B microarray scanner (Axon Instruments, Foster City, USA).
Extraction of microRNA and real-time quantitative polymerase chain reaction
Total miRNA was isolated from the patient samples using the miRNeasy FFPE Kit (QIAGEN) and stored at −80°C or immediately used for subsequent reverse transcription. Reverse transcription was carried out using the All-in-One™ miRNA RT-qPCR Detection Kit (GeneCopoeia, Guangzhou, China) according to the manufacturer's instructions. Primers for all the miRNAs, U6, U44, and U47 were purchased from GeneCopoeia. Total miRNA was extracted from the cells using TRIzol reagents (Life Technologies, Carlsbad, USA) and reverse-transcribed to cDNA using a reverse transcription reagents kit (Kangwei, Beijing, China). Primers for cell proliferation, migration and apoptosis were from Invitrogen. β-Actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used for normalization. Each sample was analyzed in triplicate, and negative controls were included. Each sample was analyzed in triplicate.
Cell culture and transfection
MDA-MB-231 human breast cancer cells were maintained in Dulbecco's modified Eagle's medium (Hyclone, Logan, USA) containing 10% fetal bovine serum (FBS; PAN-Bio-tech, Adenbach, Germany) and 1× antibiotic antimycotic solution (Hyclone). Cells were incubated in a humidified atmosphere with 5% CO2 at 37°C. Cell digestion with trypsin and subculture were conducted after the cells growth was 85% confluent upon microscopy examination.
Chemically synthesized of miRNA-654-5p analog mimics (GenePharma, Shanghai, China) were used to overexpress miRNA-654-5p. A negative control was included. MDA-MB-231 cells were transfected using Lipofectamine 3000 with the miRNA-654-5p mimic and negative control following the manufacturer's instructions when growth in the wells of a 6-well plate was 80% confluent. Successful transfection was ensured before the cells were used for RT-qPCR.
Wound healing and metastasis assays
A scratch was made at the axis of the well using a pipette tip after the transfected cells reached 60% to 80% confluency after 24 hours and were treated again with 2% FBS in dimethylsulfoxide. Images of MDA-MB-231 cells migration into the wound were captured at 0, 24, and 48 hours using an inverted microscope (40×). Each test was repeated in triplicate.
The metastasis assay was performed using Transwell chambers (Corning, Corning, USA). Briefly, the cells were trypsinized and resuspended in serum-free medium. Thereafter, 8 × 104 cells were added to the upper chamber, while the lower chamber was filled with medium containing 10% FBS. After incubation for 48 hours, the cells that traversed the coated membrane into the lower surface were fixed, stained, and counted using a microscope (40× and 100×). Each test was repeated in triplicate.
Western blotting analysis
All cells were seeded in 100-mm tissue culture dishes and harvested after transfection for 72 hours. Thirty micrograms of protein per lane were loaded and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%) and the resolved proteins were transferred to a polyvinylidene fluoride membrane. The membrane was blocked by 5% nonfat milk and probed with primary antibody overnight at 4°C. The antibodies and their dilution included mouse anti-Vimentin (1:2,000; ZENBIO, Chengdu, China), rabbit anti-Snail (1:500; Abcam, Cambridge, UK), and mouse anti-cadherin-1 (CDH1; 1:2,000; ZENBIO). Membranes were washed three times in Tris-buffered saline-Tween buffer and incubated for 60 minutes at room temperature with secondary antibodies. Each blots was washed again and developed using an enhanced chemiluminescence system (KeyGEN Biology Co., Ltd., Nanjing, China). Protein loading was normalized by blots with rabbit anti-GAPDH antibody (1:1,000; ZENBIO).
Immunofluorescence staining for F-actin
MDA-MB-231 cells were transfected with miRNA-654-5p mimics or negative controls after 24 hours and plated onto fibronectin-coated chamber slides and stained on day 3. Cells were fixed in 4% paraformaldehyde and permeabilized in 0.05% Triton X-100 for 20 minutes. For F-actin staining, the cells were incubated with rhodamine-phalloidin (Invitrogen) for 10 minutes and stained with 4′-6-diamidino-2-phenylindole to detect the nuclei. Cells were observed and photographed using a light microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analyses
SPSS version 23.0 software (IBM Corp., Armonk, USA) was used for the statistical analyses. The statistical significance of the studies was analyzed by independent-sample U tests in the miRNA expression levels between the poor or good prognosis samples or those with different expression in RT-qPCR. A p-value < 0.05 was considered significant. The square of the receiver operating characteristic (ROC) curve was calculated to make sure that the test was accurate and steady.
RESULTS
Clinicopathological features of DCIS and relationship with poor prognosis
The base line clinicopathological features of 316 patients with DCIS are presented in Supplementary Table 1. During the 33–112-month follow-up (median, 64 months), 12 DCIS cases displayed local or distant recurrence. All these cases were middle or high nuclear grade. A higher grade of DCIS was associated with higher levels of invasion and higher recurrence risk than the low grade of DCIS. Tumor size was significantly larger in the high-grade group (p = 0.001). Detailed information is provided in Supplementary Table 2.
Poor prognosis cases were significantly associated with larger tumor size and lymph node metastasis (both p < 0.001) (Table 1). Univariate analysis revealed that tumor size, lymph node metastasis, and estrogen receptor-positive rate were associated with poor prognosis. Tumor size and lymph node metastasis were independent factors for poor prognosis (Supplementary Table 3).
Table 1. Clinicopathological features of DCIS patients with poor and good prognosis.
| Characteristic | Poor prognosis (n = 12) | Good prognosis (n = 304) | p-value | |
|---|---|---|---|---|
| Age (yr) | 1.000 | |||
| < 50 | 7 (58.3) | 181 (59.5) | ||
| ≥ 50 | 5 (41.7) | 123 (40.5) | ||
| Size (cm) | 0.034 | |||
| ≤ 2 | 3 (25.0) | 144 (47.4) | ||
| > 2, ≤ 5 | 6 (50.0) | 141 (46.4) | ||
| > 5 | 3 (25.0) | 19 (6.2) | ||
| Nuclear grade | 0.546 | |||
| Low | 0 | 16 (5.3) | ||
| Middle | 4 (33.3) | 124 (40.8) | ||
| High | 8 (66.7) | 164 (53.9) | ||
| Necrosis | 0.252 | |||
| Yes | 4 (33.3) | 156 (51.3) | ||
| No | 8 (66.7) | 148 (48.7) | ||
| Lymph node metastasis | 0.000 | |||
| Yes | 4 (33.3) | 3 (1.0) | ||
| No | 8 (66.7) | 301 (99.0) | ||
| ER | 0.130 | |||
| Negative | 7 (58.3) | 108 (35.5) | ||
| Positive | 5 (41.7) | 196 (64.5) | ||
| PR | 0.372 | |||
| Negative | 7 (58.3) | 127 (41.8) | ||
| Positive | 5 (41.7) | 177 (58.2) | ||
| HER2 | 0.348 | |||
| Negative/1+ | 6 (50.0) | 137 (45.1) | ||
| 2+ | 4 (33.3) | 61 (20.0) | ||
| 3+ | 2 (16.7) | 106 (34.9) | ||
| Ki-67 (%) | 1.000 | |||
| ≤ 14 | 5 (41.7) | 114 (37.5) | ||
| > 14 | 7 (58.3) | 190 (62.5) | ||
Values are presented as number (%).
DCIS = ductal carcinoma in situ; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.
Screening of progression-related microRNAs in DCIS and DCIS with microinvasion
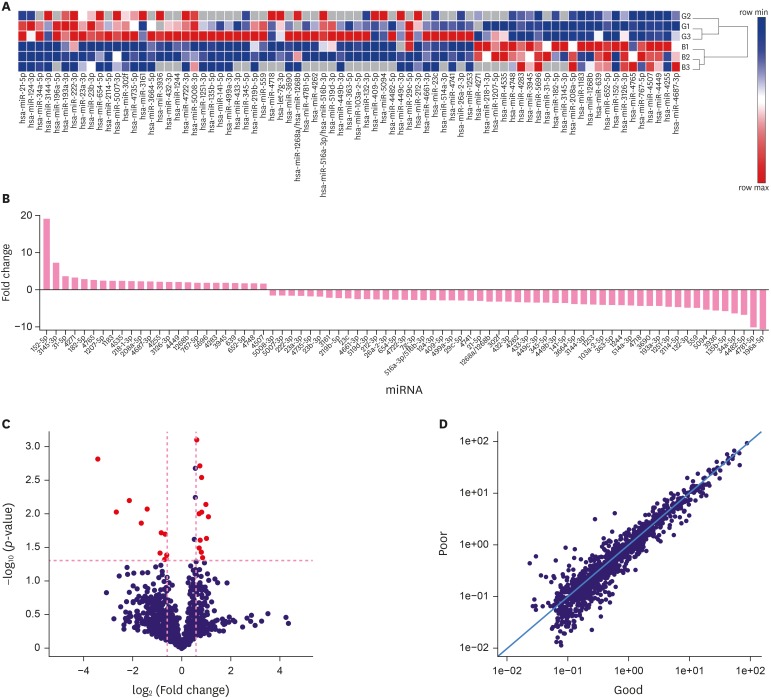
To screen high-risk miRNAs associated with poor prognosis for DCIS patients, the miRCURY LNA™ microRNA array was used to examine FFPE samples from DCIS and DCIS-Mi patients. For the primary screening, the disease progressed at 65, 100, 91, and 81 months respectively after the initial surgery, with patients with good prognosis for control (Supplementary Table 4). From the miRNA array analysis, 77 miRNAs were upregulated or downregulated by more than 1.5-fold (Figure 2). Figure 2C shows a Volcano plot of the data. A scatter plot (Figure 2D) showed that the result of the miRNA array was reproducible. A total of 66 miRNAs were screened out as primary candidate molecules for further validation.
Figure 2. A total of 77 differentially expressed miRNAs in metastatic versus good prognosis patients. (A) 77 miRNAs in three pairs of poor and good prognosis patients with or without microinvasion by miRNA microarray analysis (the red part represents the upregulated miRNAs, while the green part represents the downregulated miRNAs). (B) The 77 miRNAs had a greater than 1.5-fold upregulated or downregulated fold-change value. (C) Volcano plot; red pots represented the miRNA consistently overexpressed or under expressed more than 1.5-fold at p < 0.05. (D) The better the repeatability, the closer the plots were to the diagonal line in the scatter plot.
miRNA = MicroRNA.
Diverse prognostic microRNA patterns between DCIS and DCIS with microinvasion
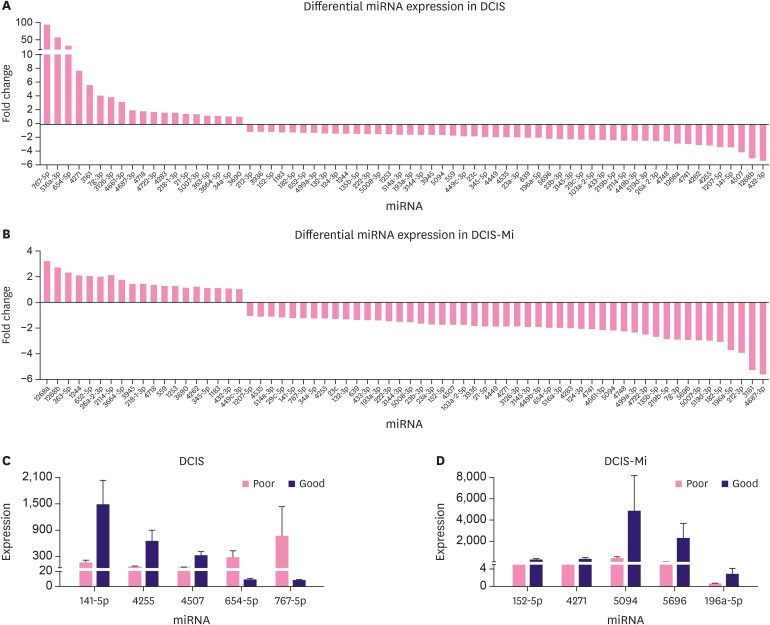
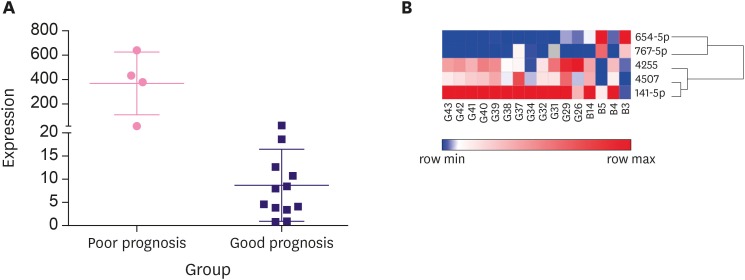
To further confirm the potential candidates that promote DCIS or DCIS-Mi progression, a total of 16 pure DCIS and 22 DCIS-Mi samples were used for validation. After screening the initial 66 (only 66 of 77 candidates were commercially available) miRNAs, 5 differentially expressed miRNAs in patients with good or poor prognosis were selected from the DCIS samples by qPCR verification. The same procedure was performed on DCIS-Mi samples (Supplementary Table 5). Another 5 differentially expressed miRNAs were screened out, and were considered potential biomarkers of the promotion of progression. The identified miRNAs in the DCIS and DCIS-Mi groups were remarkably and totally different (Table 4, Figure 3A and B). Five candidate miRNAs that displayed statistical differences are listed Table 2. The 5 were miRNA-654-5p (p = 0.048), miRNA-141-5P (p = 0.037), miRNA-767-5p (p = 0.027), miRNA-4507 (p = 0.042), and miRNA-7g-3p (p = 0.048). Preliminary data revealed that the miRNA-654-5p levels were consistently upregulated in all metastasized samples compared with the non-metastasized samples (Figure 4A). A cluster analysis dendrogram classified the expression of miRNA-654-5p in patients with different prognoses (Figure 4B). The area under the ROC curve revealed the high sensitivity and specificity of the qPCR analysis (Supplementary Figure 1).
Figure 3. The screening of the distinct prognostic miRNAs by quantitative polymerase chain reaction. (A, B) The 66 miRNAs had a greater than 1.5-fold upregulated or downregulated fold-change value in DCIS and DCIS-Mi, respectively. (C, D) Five differentially expressed miRNAs were screened in DCIS and DCIS-Mi.
miRNA = MicroRNA; DCIS = ductal carcinoma in situ; DCIS-Mi = ductal carcinoma in situ with microinvasion.
Table 2. List of miRNA related to the metastasis in DCIS.
| miRNA | Expression | Expression changes | p-value | ||
|---|---|---|---|---|---|
| In poor prognosis (n = 4) | In good prognosis (n = 12) | Fold change | |||
| miR-654-5P | 274.25 ± 313.53 | 8.59 ± 7.79 | 32.430 | Upregulated | 0.048 |
| miR-141-5P | 152.93 ± 130.50 | 1,479.46 ± 1,928.93 | 0.297 | Downregulated | 0.037 |
| miR-767-5p | 751.95 ± 1,367.12 | 7.33 ± 6.40 | 93.909 | Upregulated | 0.027 |
| miR-4507 | 36.87 ± 35.75 | 312.87 ± 364.83 | 0.246 | Downregulated | 0.042 |
| miR-7g-3p | 83.28 ± 56.03 | 25.59 ± 22.75 | 0.323 | Downregulated | 0.048 |
Data are presented as mean ± standard deviation.
miRNA = microRNA; DCIS = ductal carcinoma in situ.
Figure 4. The expression of miRNA-654-5p in different prognosis. (A) The expression of miRNA-654-5p was verified by quantitative polymerase chain reaction in different prognosis and consistently high in poor prognosis. (B) According to the variation of relative distance in cluster analysis dendrogram, the expression of miRNA-654-5p in different prognosis can be classified alone.
miRNA-654-5p promotes motility in breast epithelial cells
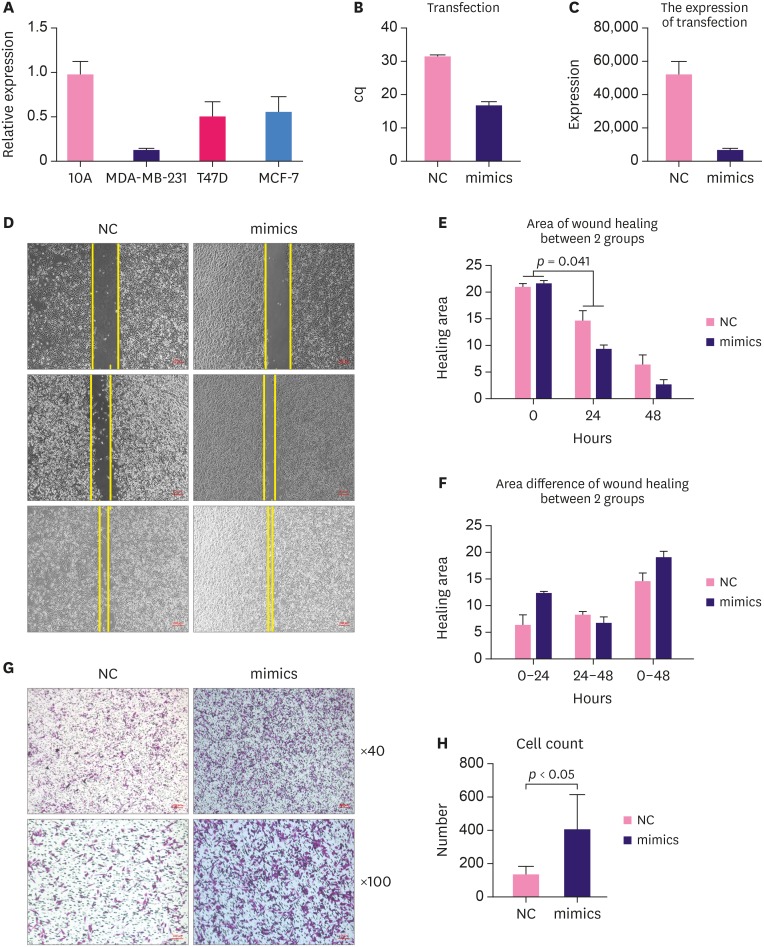
Before a functional study, the endogenous expression of the candidate miRNA was tested in different breast cell lines including MCF-7 cells and T47D cells (human epidermal growth factor receptor 2-negative, estrogen receptor-positive, progesterone receptor-positive), MAD-MB-231 triple-negative breast cancer cells. MCF-10A non-tumorigenic epithelial cells were used as a normal cell control. miRNA-654-5p was downregulated in MCF-7, MAD-MB-231, and T47D cells (Figure 5A).
Figure 5. High expression of miRNA-654-5p promoted motility in breast cancer cells. (A) The basic expression of miRNA-654-5p in 3 different breast cancer cell lines. (B, C) The efficiency after transfection of miRNA-654-5p mimics in MDA-MB-231 cells. (D) Wound healing indicated that the miRNA-654-5p mimic group migrated significantly faster than the control group (×40). (E, F) The decreased area in the treatment group was apparently more than that in the negative control group after 24 hours (p < 0.05). (G, H) The number of cells migrated to the lower wells in the treatment group were more than the negative group (×40, ×100, p < 0.05).
cq = cycle quantification; NC = negative control.
miRNA-654-5p expression was relatively extremely low in MAD-MB-231 cells, We transfected miRNA-654-5p mimics into MAD-MB-231 cell using Lipofectamine 3000 to explore their function in the breast cancer cells. The transfection efficiency and target gene expression data are presented in Figure 5B and C, respectively. A wound healing assay was used to examine the effects of miRNA-654-5p on the migration of MDA-MB-231 cells. miRNA-654-5p significantly increased cell migration in the treatment group compared with the control. The decreased area in the treatment group was significantly greater than that in the negative control group after 24 hours (p < 0.05) (Figure 5D). After 48 hours, the area in the treatment group was obviously decreased (Figure 5E). Wound healing assays showed that treatment with miRNA-654-5p for 24 hours markedly increased the migration ability of MDA-MB-231 cells.
To further confirm the function of miRNA-654-5p in migration, a Transwell assay was performed (Figure 5G). MAD-MB-231 cells transfected with miRNA-654-5p mimics or control plasmid were cultured in the upper wells. After 48 hours, more cells had migrated to the lower wells in the treatment group than in the negative group, which indicated that miRNA-654-5p obviously increased the migration ability of the MDA-MB-231 cells. Breast cancer cells transfected with miRNA-654-5p mimics displayed a significantly higher rate of metastasis than control cells (p < 0.05) (Figure 5H).
miRNA-654-5p promotes tumor invasion and expression of metastasis-related genes
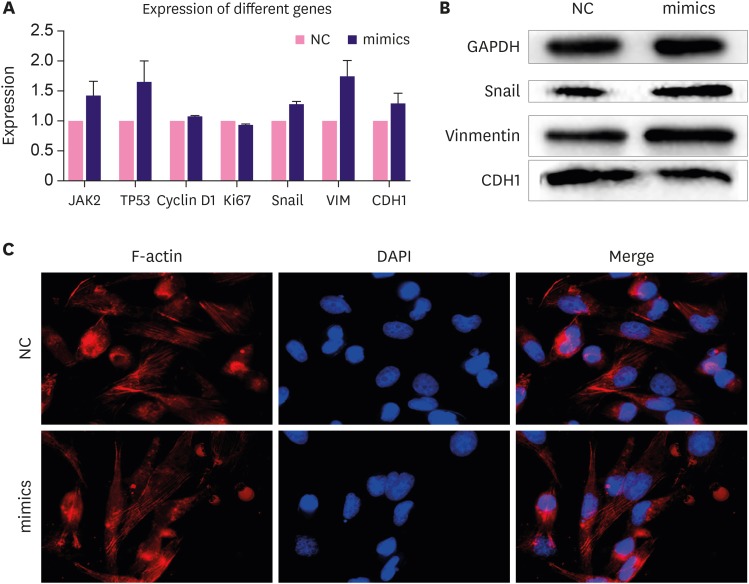
To test the molecular effect of miRNA-654-5p on downstream genes in the breast cancer cells, we tested the expression of invasion- and metastasis-related genes in cells transfected with miRNA-654-5p. The expression of metastasis-associated genes was generally higher in the miRNA-654-5p overexpression group than in the negative control group (Figure 6A). Snail and other invasion and metastasis-related genes, such as Vimentin, were upregulated at least 1.5-fold compared to the control group. At the same time, apoptosis-inhibiting genes and proliferation-promoting genes were also upregulated. These data were consistent with previous observations that miRNA-654-5p could promote metastasis and proliferation as well as inhibit apoptosis [11].
Figure 6. Upregulated miRNA-654-5p changed the expression of the protein related to the metastasis verified by quantitative polymerase chain reaction and Western blotting. (A) For the expression in the negative control and the standard, the expression of the gene involved in migration was upregulated, especially the expression of snail (p < 0.05). (B) Cellular lysates from the experimental group cells were used to measure epithelial-mesenchymal transition-related protein expression by western blot. (C) F-actin staining (left panels) of MDA-MB-231 cells transfected with synthetic miRNA-654-5p for 4 days. DAPI staining was used to detect the nuclei. The merged panels showed different cellular morphology.
DAPI = 4′,6-diamidino-2-phenylindole; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; NC = negative control.
Upregulation of miRNA-654-5p alters expression of metastasis-related proteins
We used western blotting to quantify the total proteins of MAD-MB-231 cells extracted after transfection of the miRNA-654-5p mimics after 72 hours. Expression of proteins related to metastases was altered. In particular, the expressions of Snail and Vimentin were upregulated and that of CDH1 was downregulated (Figure 6B). Snail, Vimentin, and CDH1 are marker proteins for the epithelial-mesenchymal transition (EMT), and are indicative of the migration of cells. Phalloidin staining was used to observe the cytoskeleton morphology; reduced cell polarity was observed in the experimental group with more looser mesenchymal structures (Figure 6C).
DISCUSSION
DCIS is a preinvasive lesion that does not break through the basement membrane. Thus, distant metastasis or local recurrence is uncommon. However, these vents can occur and carry a poor prognosis. A recent investigative interests in patients with DCIS has been the cells' potential to invade and metastasize. DCIS might be a precursor of IDC. Furthermore, experimental data have shown that IDC precursor cells exist in DCIS lesions [12].
miRNAs have good stability and long-term effects in vivo and are potential biomarkers that predicting different outcomes in DCIS. This predictive capability is partly due to their small size, abundance, stability, and easy detection. Previous studies identified several miRNAs associated with the poor survival of patients with breast cancer. These miRNAs promote proliferation, metastasis, and stemness or inhibit apoptosis. Early studies demonstrated that the loss of tumor suppressor miRNA-125b and the gain of the oncogenic miRNAs miRNA-182 and miRNA-183 might relate to early breast cancer development [13]. Chen et al. [14] studied miRNAs in eight normal, four atypical ductal hyperplasia, six DCIS and seven IDC samples; miRNA-21, miRNA-200b/c, miRNA-141, and miRNA-183 were consistently overexpressed in ADH, DCIS, and IDC compared to normal patients, while miRNA-557 also had uniquely low expression in DCIS. Li et al. [15] showed, compared with the normal tissues, miRNA-10b, miRNA-125b, miRNA-132, miRNA-145, miRNA-154-3p, miRNA-382-5p, and miRNA-409-3p were significantly deregulated in DCIS. The miRNAs we screened in DCIS and DCIS-Mi patients having a poor prognosis were different, which indicated that miRNA may be expressed differently in different stages of the development and progression of breast cancer.
Some other miRNAs we screened were actually related to neoplasia or malignancy. miRNA-767-5p was previously reported to be expressed at a 15-fold higher rate in a prostate cancer cell line than in the normal prostate epithelial cell [16]. The cells would have a greater migratory ability in SK-HEP-1 cells with highly expressed miRNA-767-5p [17]. Previous studies have reported that the miRNAs we screened are related to cell oncogenesis or deleterious behavior. miRNA-141 is a member of the miRNA-200 family, whose downregulation leads to increased EMT by targeting ZEB1 and SIP1 [18]. Downregulation of miRNA-141 expression promotes breast cancer cell proliferation, migration, and invasion [19,20]. No related data have been published concerning miRNA-7g-3p or miRNA-4507. As well, no report has addressed the effect of miRNA on the prognosis of DCIS patients. The expression of miRNA-654-5p is consistently upregulated in poor prognosis DCIS cases. It has been reported that miRNA-654-5p can be an inhibitor in prostate cancer that features decreased androgen-induced proliferation [21]. Downregulation miRNA-654-5p was also reportedly related with the classical Hodgkin lymphoma [22]. Upregulated miRNA-654-5p in late-stage oral squamous cell carcinoma has also been reportedly correlated with poor prognosis [11]. In that study, miRNA-654-5p activated Ras/mitogen-activated protein kinase signaling by targeting Grb-2-related adaptor protein and in the further promotion of the EMT process [11]. Another study demonstrated that miR-654-5p is a tumor suppressor in breast cancer by targeting EPSTI1. This gene is related to tumor cell proliferation, invasion, apoptosis and EMT [23]. Other genes have been predicted as the direct target of miRNA-654-5p by five computational tools (RDB, DIANA, TargetScan, miRTarbase, and microRNA.org). We found that the function of FGF19, Wnt11, CAMK2A, and TGFβ1 mainly focused on cancer promotion and cell adhesion. The present and previous findings support the suggestion that miRNA-654-5p is a key biomarker for DCIS and progression of other tumors.
The present findings differ from those of a previous study [23]. The previous study involved MB-468 and BT-549 cells, while we used MDA-MB-231 cells as our experimental cell line, which may explain the different results. Although the patients had already been diagnosed as having breast cancer, it was not clear that what kind of breast cancer they had, as different heterogeneous breast carcinomas display totally different signatures. The expression level of different miRNAs is distinct in invasive carcinoma and carcinoma in situ. Specifically, let-7d, miRNA-210, and miRNA-221 are downregulated in carcinoma in situ and upregulated in the invasive transition [24]. The collective results obtained to date indicate that the aberrant expression of miRNA-654-5p may differ in the different stages of breast cancer and may have various effects on breast cancer progression.
We had intended to explore the function of miRNA-654-5p in DCIS progression. The expression level of miRNA-654-5p was quite low in the MDA-MB-231 invasive carcinoma cell line, which is the reason we chose this cell line for the in vitro test. The cells in the experimental group had stronger migration ability than the control group, which proved that miRNA-654-5p could increase the capacity to metastasize.
Metastasis is the main cause for the poor prognosis in breast cancer, including DCIS. During metastasis, epithelial cells display decreased expression of epithelial markers (loss of epithelial keratins, CDH1) and acquired mesenchymal traits (upregulation of Vimentin and Snail) [25]. Snail and Vimentin were verified as being closely related to EMT and may be involved in the potential mechanism to promote metastasis [26,27,28,29]. Therefore, we quantified the EMT protein and identified that proteins were upregulated in miRNA-654-5p overexpressed cell lines. miRNA-654-5p may promote metastasis by targeting EMT-related proteins. The high expression level of Snail followed the upregulation of miRNA-654-5p, which suggests that this miRNA might promote metastasis by promoting EMT.
In a few words, the expression of miRNA-654-5p is significantly upregulated in DCIS patients having poor prognosis with the probable mechanism of EMT.
ACKNOWLEDGMENTS
At the point of finishing this paper, I would like to express my gratitude to all those who helped me during the writing of this article. First of all, I would like to extend my sincere gratitude to my supervisor, Prof. Liu. I am deeply grateful for her instructive advice and useful suggestions on my manuscript. I am also deeply indebted to Prof. Ye and all the other teachers for their direct and indirect help to me of this paper. Secondly, I'd like to express my gratitude to my friends and family who gave me their help and time in listening to me and helping me work out my problems during the difficult course of the thesis. Last but not the least, I'd like to thank editors and reviewers for helping me figure out my mistakes and help me correct them. Without their help, it would be much harder for me to finish my study and this paper.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Clinicopathological features of DCIS
Clinicopathological features of high and low grade DCIS
Correlation between DCIS clinicopathological features and poor prognosis
Clinicopathological features of poor prognosis DCIS cases in primary screening
List of miRNA related to the recurrence in DCIS-Mi
The area under the curve in (A) was 0.817, which verified that the quantitative polymerase chain reaction result was accurate and stable. The area in the (B-E) curves respectively were 0.833, 0.855, 0.854, 0.833 which were all greater than 0.8 showed that the experiment was dependable.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu JS, et al. Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China. Breast Cancer Res Treat. 2009;117:409–416. doi: 10.1007/s10549-008-0303-z. [DOI] [PubMed] [Google Scholar]
- 3.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 4.Rakovitch E, Mihai A, Pignol JP, Hanna W, Kwinter J, Chartier C, et al. Is expert breast pathology assessment necessary for the management of ductal carcinoma in situ? Breast Cancer Res Treat. 2004;87:265–272. doi: 10.1007/s10549-004-9454-8. [DOI] [PubMed] [Google Scholar]
- 5.Mardekian SK, Bombonati A, Palazzo JP. Ductal carcinoma in situ of the breast: the importance of morphologic and molecular interactions. Hum Pathol. 2016;49:114–123. doi: 10.1016/j.humpath.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 7.O'Bryan S, Dong S, Mathis JM, Alahari SK. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur J Cancer. 2017;72:1–11. doi: 10.1016/j.ejca.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Hannafon BN, Sebastiani P, de las Morenas A, Lu J, Rosenberg CL. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011;13:R24. doi: 10.1186/bcr2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, et al. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–4453. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Wang C, Chen W, Mao C, Wang J. miR-654-5p targets GRAP to promote proliferation, metastasis, and chemoresistance of oral squamous cell carcinoma through Ras/MAPK signaling. DNA Cell Biol. 2018;37:381–388. doi: 10.1089/dna.2017.4095. [DOI] [PubMed] [Google Scholar]
- 12.Mardekian SK, Bombonati A, Palazzo JP. Ductal carcinoma in situ of the breast: the importance of morphologic and molecular interactions. Hum Pathol. 2016;49:114–123. doi: 10.1016/j.humpath.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Hannafon BN, Sebastiani P, de las Morenas A, Lu J, Rosenberg CL. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011;13:R24. doi: 10.1186/bcr2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Li Y, Fu Y, Peng J, Mo MH, Stamatakos M, et al. Role of deregulated microRNAs in breast cancer progression using FFPE tissue. PLoS One. 2013;8:e54213. doi: 10.1371/journal.pone.0054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Meng H, Zhou F, Zhai L, Zhang L, Gu F, et al. MicroRNA-132 is frequently down-regulated in ductal carcinoma in situ (DCIS) of breast and acts as a tumor suppressor by inhibiting cell proliferation. Pathol Res Pract. 2013;209:179–183. doi: 10.1016/j.prp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Ueno K, Hirata H, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, et al. microRNA-183 is an oncogene targeting Dkk-3 and SMAD4 in prostate cancer. Br J Cancer. 2013;108:1659–1667. doi: 10.1038/bjc.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zha R, Guo W, Zhang Z, Qiu Z, Wang Q, Ding J, et al. Genome-wide screening identified that miR-134 acts as a metastasis suppressor by targeting integrin beta1 in hepatocellular carcinoma. PLoS One. 2014;9:e87665. doi: 10.1371/journal.pone.0087665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Xu T, Zhou X, Liao L, Pang G, Luo W, et al. Downregulation of miRNA-141 in breast cancer cells is associated with cell migration and invasion: involvement of ANP32E targeting. Cancer Med. 2017;6:662–672. doi: 10.1002/cam4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy SS, Gonugunta VK, Bandyopadhyay A, Rao MK, Goodall GJ, Sun LZ, et al. Significance of PELP1/HDAC2/miR-200 regulatory network in EMT and metastasis of breast cancer. Oncogene. 2014;33:3707–3716. doi: 10.1038/onc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Östling P, Leivonen SK, Aakula A, Kohonen P, Mäkelä R, Hagman Z, et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71:1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 22.Paydas S, Acikalin A, Ergin M, Celik H, Yavuz B, Tanriverdi K. Micro-RNA (miRNA) profile in Hodgkin lymphoma: association between clinical and pathological variables. Med Oncol. 2016;33:34. doi: 10.1007/s12032-016-0749-5. [DOI] [PubMed] [Google Scholar]
- 23.Tan YY, Xu XY, Wang JF, Zhang CW, Zhang SC. MiR-654-5p attenuates breast cancer progression by targeting EPSTI1. Am J Cancer Res. 2016;6:522–532. [PMC free article] [PubMed] [Google Scholar]
- 24.Volinia S, Galasso M, Sana ME, Wise TF, Palatini J, Huebner K, et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci U S A. 2012;109:3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–5688. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szynglarewicz B, Kasprzak P, Donizy P, Biecek P, Halon A, Matkowski R. Epithelial-mesenchymal transition inducer Snail1 and invasive potential of intraductal breast cancer. J Surg Oncol. 2017;116:696–705. doi: 10.1002/jso.24708. [DOI] [PubMed] [Google Scholar]
- 27.Scribner KC, Behbod F, Porter WW. Regulation of DCIS to invasive breast cancer progression by Singleminded-2s (SIM2s) Oncogene. 2013;32:2631–2639. doi: 10.1038/onc.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A, et al. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer. 2015;15:399. doi: 10.1186/s12885-015-1386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szynglarewicz B, Kasprzak P, Donizy P, Biecek P, Halon A, Matkowski R. Epithelial-mesenchymal transition inducer Snail1 and invasive potential of intraductal breast cancer. J Surg Oncol. 2017;116:696–705. doi: 10.1002/jso.24708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathological features of DCIS
Clinicopathological features of high and low grade DCIS
Correlation between DCIS clinicopathological features and poor prognosis
Clinicopathological features of poor prognosis DCIS cases in primary screening
List of miRNA related to the recurrence in DCIS-Mi
The area under the curve in (A) was 0.817, which verified that the quantitative polymerase chain reaction result was accurate and stable. The area in the (B-E) curves respectively were 0.833, 0.855, 0.854, 0.833 which were all greater than 0.8 showed that the experiment was dependable.