Abstract
Purpose
To describe the clinical and electrophysiological features of an unusual retinopathy in a patient with a novel genotype of CNGB1, mutations in which are implicated in autosomal recessive retinitis pigmentosa (rod-cone dystrophy).
Observations
A 61-year old asymptomatic woman was referred to the inherited retinal disorders clinic because of peripheral retinal pigmentary changes. She had normal visual acuity and color vision. Clinical examination and detailed imaging of the macula were normal, but there was atrophy of the outer retina in the periphery with sparse intra-retinal pigmentation. Electroretinography (ERG) revealed undetectable rod responses, with normal cone-mediated responses. The pattern ERG was normal. Genetic analysis identified two previously unreported variants in CNGB1: (c.2258T > A, p.[Leu753*] and c.807G > C, p.[Gln269His]), shown to be in trans.
Conclusions and importance
This report describes a functionally cone-isolated retina in an adult, apparently hemizygous for a novel missense mutation in CNGB1, a novel phenotype for this gene. The p.[Gln269His] allele is the first missense change, within the glutamic acid-rich protein (GARP) domain of CNGB1, to be associated with retinal disease in humans.
Keywords: Cone-isolated retina, Cyclic nucleotide-gated channels, Glutamic-acid rich protein (GARP), Night blindness, Retinitis pigmentosa, Rod dysfunction
1. Introduction
Light absorption at the photoreceptor outer segment activates the phototransduction cascade, which results in hydrolysis of the 3′-5′-cyclic GMP (cGMP) by the activated phosphodiesterase. This leads to closure of the cyclic nucleotide-gated (CNG) channels in the photoreceptor outer segment and hyperpolarization of the photoreceptors.1 The CNG channels conduct the photoreceptor ion currents and are modulated by cGMP.1 The rod CNG channels are heterotetramers composed of one β1-subunit and three α1-subunits.2 The α1-subunits control the gating of the CNG channel, and the β1-subunits modulate the kinetics of the gating in response to cGMP.2 CNGB1 encodes the β1-subunits as well as two soluble glutamic-acid rich proteins (GARPs).3 The function of the GARP domains has not been fully elucidated, but in vitro studies implicated these proteins in the modulation of the rod CNG channels.3,4
Biallelic mutations in CNGB1, associated with autosomal recessive rod-cone dystrophy (retinitis pigmentosa, RP, MIM 613767), are commonly presumed ‘null’ (nonsense, frameshifting, splice-site mutations) but missense mutations have also been reported.5,6 Expression of CNGB1 in the retina is restricted to the rod photoreceptors, and the observed cone dysfunction evident in cases with RP is likely consequent upon outer retinal degeneration.7 A recent report of ten patients with autosomal recessive retinitis pigmentosa due to CNGB1 mutations showed that despite childhood-onset nyctalopia, peripheral visual field constriction could occur no later than the third decade of life, with all patients showing subnormal photopic ERG responses in the absence of rod responses.8
This report describes a case with a novel phenotype associated with biallelic mutations in CNGB1.
2. Case report
A 61-year-old woman, of South Asian ancestry, was referred to the inherited retinal disorders clinic with bilateral peripheral retinal pigmentary changes detected on routine diabetic retinopathy screening. The patient had controlled non-insulin dependent diabetes mellitus but was otherwise well. There was no family history of consanguinity, nyctalopia, or poor vision; and both parents had normal vision into their seventh decade of life (Fig. 1).
Fig. 1.
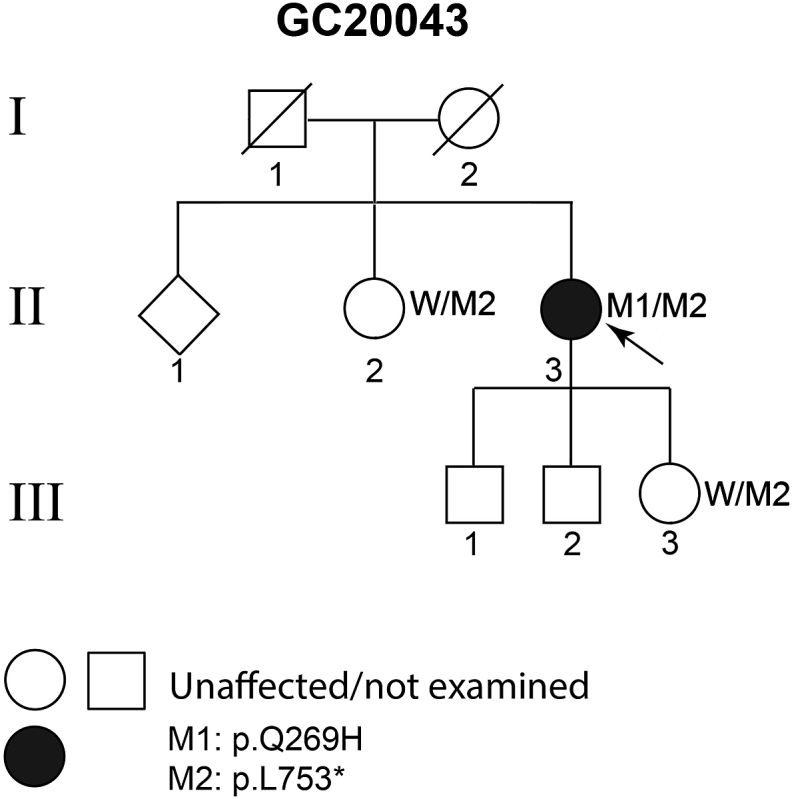
Pedigree GC20043: both parents (I:1, I:2) were asymptomatic in their seventies. The proband II:3 has two asymptomatic older siblings, one of whom (female, aged 71 years) is heterozygous for the nonsense mutation M2: p.[Leu753*] but not the missense change M1: p.[Gln269His]; and three asymptomatic children one of whom is heterozygous for the nonsense mutation M2, but did not harbour the missense change M1; indicating that the two mutations are in trans in II:3 and segregate at least in two clinically discordant siblings, as expected for a recessive disorder. W: wild type allele.
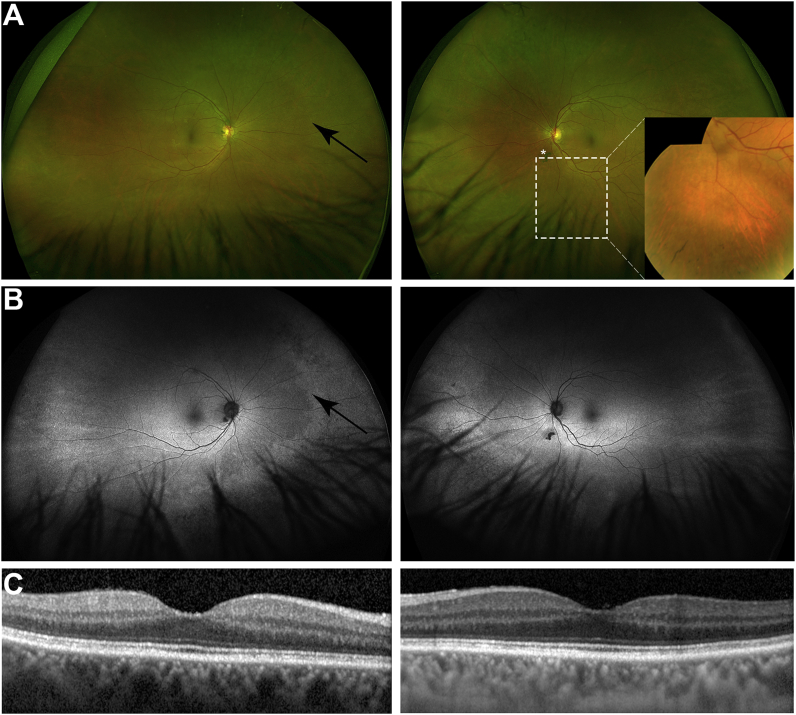
The patient reported no night blindness initially but on direct questioning, mentioned slightly prolonged adaptation to dim lighting conditions. Visual acuity was 20/20 in the right eye, 20/30 left, with normal color vision tested using the Hardy, Rand, and Rittler plates. Although formal perimetry was not performed, as the patient did not wish to undergo further testing, the visual fields to confrontation were normal. There were mild cataracts in both eyes. Fundus examination showed normal retinal appearance in the posterior pole, with peripheral retinal atrophic changes and sparse intra-retinal pigment in both eyes. There were no signs of diabetic retinopathy (Fig. 2). Spectral-domain optical coherence tomography (OCT) of the central macula was normal bilaterally. Wide-field fundus autofluorescence (FAF) showed peripheral hyperautofluorescence in the nasal and inferior quadrants.
Fig. 2.
Fundus imaging: (A) wide-field retinal images, showing the right and left fundi. The posterior pole shows no abnormalities, but the mid-peripheral retina shows mild pallor (arrow) suggesting outer retinal degeneration. The left fundus shows sparse intra-retinal pigment (inset); an incidental vitreous opacity is marked with the asterisk (*). (B) fundus autofluorescence (AF) showing bilaterally normal signal in the posterior pole, however, the AF signal intensity is increased in the mid-peripheral retina (arrow) with an edge that almost co-localizes with that seen on the pseudo-color image. The AF signal is reduced in the far-peripheral retina. (C) optical coherence tomography of the right and left maculae, showing normal structure.
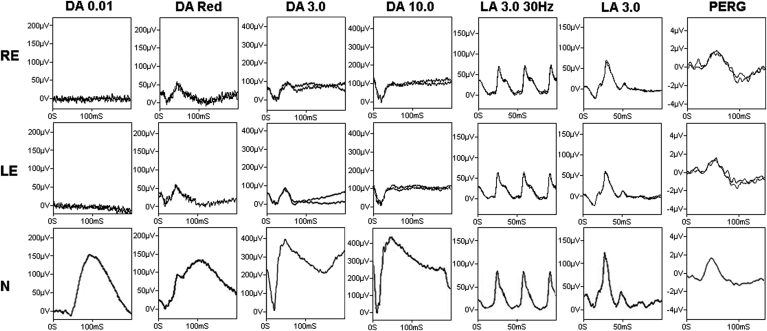
Full field and pattern electroretinography (ERG; PERG) were performed, using extended protocols that incorporated those of the International Society for Clinical Electrophysiology of Vision.9, 10, 11 The results are shown in Fig. 3. The PERG P50 and N95 components were normal and revealed no evidence of macular or retinal ganglion cell dysfunction. The dark-adapted (DA) dim flash rod (DA 0.01) ERG was undetectable bilaterally. The DA red flash ERG showed preservation of the x-wave, derived from DA cones, but the rod-mediated b-wave was undetectable bilaterally. The DA 3.0 ERG a-wave was reduced, and the waveform had a low b:a ratio, with a b-wave of similar peak time and shape to the DA red flash ERG x-wave. The DA 10.0 (strong flash) ERG a-wave was subnormal and the b-wave was of lower amplitude than the a-wave. Light-adapted (LA 3.0) 30 Hz flicker and single flash cone (LA 3.0) ERG were normal for age. The ERG findings are consistent with a severe and selective loss of rod photoreceptor function, with DA 3.0 and DA 10.0 ERGs (mixed rod and cone system responses) likely representing contributions from the dark-adapted cone system only (the photopic hill phenomenon). Vitamin A (retinol) serum level was subsequently measured and was normal (2.09 μmol/l; normal range: 0.66–4.87μmol/l).12
Fig. 3.
Full field electroretinogram (ERG) for the right (RE) and left eye (LE) of the proband, and a 72- year-old normal control (N). The dark-adapted, rod-specific ERG response (DA 0.01) is undetectable bilaterally; using a red flash, under dark adaptation (0.3 cd.s m−2), shows a clear response from the dark-adapted cones (x-wave, peak time 47 ms (RE) and 46 ms (LE)) but no rod component. The dark-adapted response to the 3.0 cd.s m−2 shows subnormal a-wave and b-wave with a b-wave peak time of 49 ms (RE), and 46 ms (LE). The similarity between the peak times of the x-wave and the b-wave of the DA 3.0 suggests that both responses are driven by the dark-adapted cones. The bright flash dark-adapted ERG (DA 10.0) shows a delayed and subnormal a-wave, with reduced b:a ratio in keeping with a dark-adapted cone system origin. Photopic flicker (LA 30 Hz) and single flash ERGs (LA 3.0) show normal amplitudes and peak times. The pattern ERG (PERG) was within the normal range for age.
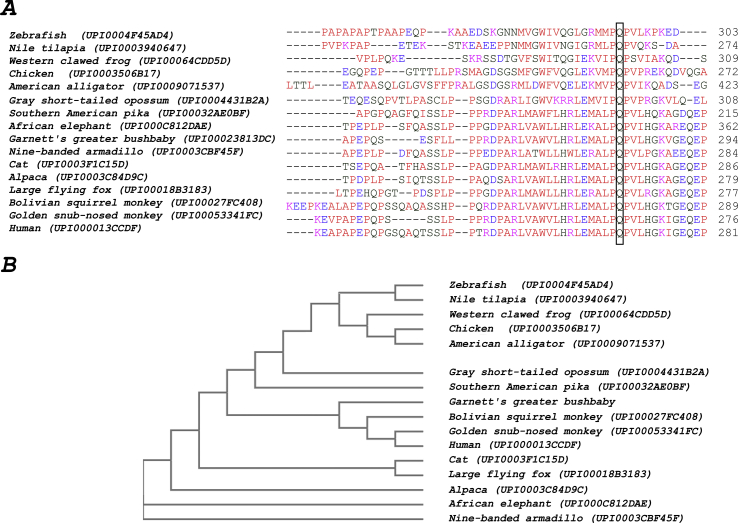
After obtaining informed consent, a blood sample was collected for genetic testing and the DNA was extracted. Because of the mild peripheral retinal changes, initial screening for mutations in RHO, by Sanger sequencing of all coding exons and intron-exon boundaries, identified no pathogenic variants. Subsequently, whole exome sequencing (WES) and deletion and duplication analysis were undertaken as described previously.13 Filtering of the variants, including deletions and duplications, was performed as follows: variants that met the following criteria were identified, using the Ingenuity Variant Analysis tool (QIAGEN Bioinformatics, USA), and prioritized: i) variants in genes that have been associated with retinal disease (https://sph.uth.edu/retnet/, accessed 12 January 2018); ii) labelled as high or moderate impact (splice-site, mutations that cause loss of the start codon, nonsense, non-synonymous and frameshifting mutations); iii) those with an allele frequency in the gnomAD dataset (http://gnomad.broadinstitute.org/) of less than 0.001. Additionally, the WES data including a separate analysis for deletions and insertions were manually interrogated for known mutations in genes associated with retinal disease, with allele frequencies greater than 0.001. The variants that met these criteria are given in Table S1. The CNGB1 gene harboured two rare variants that survived filtering, in keeping with the expected inheritance for the gene: c.2258T > A, p.[Leu753*], and c.807G > C, p.[Gln269His] (transcript NM_001297.4). In silico prediction tools showed the p.[Gln269His] to be deleterious with a SIFT score of 0, and probably damaging with a PolyPhen-2 score of 0.99. Multiple alignments of the amino acid sequences of CNGB1 showed the glutamine residue at position 269 to be conserved in diverse vertebrate species (Fig. S1). Neither variants had been reported in the literature, nor seen in 246,080 (p.[Leu753*]) and 246,190 (p.[Gln269His]) alleles in the gnomAD database. The two alleles are most likely to be in trans and segregated with retinal dysfunction. This was confirmed by DNA sequencing of her offspring who harbored only the p.[Leu753*] variant (Fig. 1). The patient's sibling (age 71 years) had a normal clinical examination and ERG and was shown to harbour only the p.[Leu753*] variant. No other family members were available for genetic analysis.
3. Discussion
This report describes an unusual retinal functional phenotype characterised by full-field ERG evidence of severe and selective loss of rod photoreceptor function, associated with novel missense and nonsense mutations in CNGB1. There may be localized areas of atrophy and intra-retinal pigment in the periphery, but the fundus abnormalities are mild compared with the severity of rod dysfunction and are too focal to affect either the cone-mediated full-field ERGs or macular function. The ERG features are consistent with a functionally cone-isolated retina (see Fig. 3) and resemble those in fundus albipunctatus due to mutations in RDH5 and Oguchi disease caused by mutations in either SAG or GRK1. Both these forms of congenital stationary night blindness (CSNB) are usually characterised by distinctive fundus abnormalities and recovery of rod-mediated ERGs after extended dark adaptation,14 but no such genetic variants were present on WES. A normal retinal appearance and irreversible absence of rod function with preservation of cone function (“Riggs-type CSNB”), may also be caused by dominant alleles of GNAT1, PDE6B, RHO, and recessive alleles of SLC24A1.14
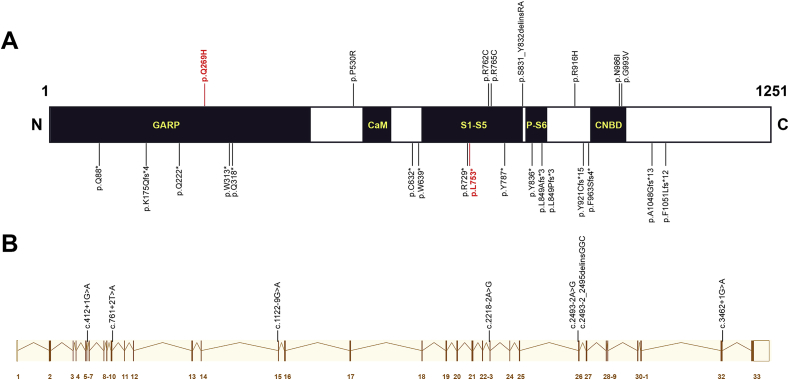
To date, 30 likely disease-causing CNGB1 mutations have been reported in patients with RP (Fig. 4). Only 7 of these were missense changes, mainly clustering within the trans-membrane domains or the cyclic nucleotide binding domain6. In this case, the p.[Leu753*] variant is likely to be subjected to nonsense-mediated decay, but if translated the resulting protein would lack the carboxyl 468 amino acid residues, and be still non-functional. It is probable then, that the missense mutation, p.Gln269His, confers the unusual retinal pathology. This variant exchanges a glutamine residue for histidine within a conserved recurring motif, a Pro-Gln-Pro triplet, in the GARP domain of the protein (Fig. 4). Interestingly, no disease-causing missense mutations within the GARP domain have been previously reported to our knowledge.15 The precise function of this domain has not been established.3,4,15 However, conservation of this domain in vertebrates suggests that it has an important function. It is reasonable to speculate that the missense mutation p.Gln269His, allows processing and assembly of the rod CNG channel but renders it insensitive to cGMP modulation. This might still allow flow of Ca2+ ions to an extent that achieves a non-toxic intracellular concentration, but somehow abrogate the timely closure to light abolishing the rod response. Further biochemical analysis of this mutant and its function in response to changes in cGMP concentrations would be required to understand the precise pathophysiology.
Fig. 4.
Schematic representation of the CNGB1 protein (A) from the canonical transcript ENST00000251102 (B) modified from: http://www.ensembl.org/Homo_sapiens/Transcript/Summary?db=core;g=ENSG00000070729;r=16:57882340-57971116;t=ENST00000251102). The CNGB1 gene product has a glutamic-acid rich protein (GARP) domain, a calmodulin-binding domain (CaM), and six trans-membrane domains (S1-S6) including the pore-forming domain (P), and a cyclic nucleotide binding domain (CNBD).16 The previously reported disease-causing mutations are shown on A; top: missense changes including the variant p.[Gln269His] (red); bottom: nonsense and frameshifting mutations including the p.[Leu753*] (red) mutation. (B): the previously reported splice-site mutations are aligned with the CNGB1 exons. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusion
This report describes an unusual phenotype of a functionally cone-isolated retina associated with a novel missense mutation, in the GARP domain of CNGB1 in trans with a predicted loss-of-function variant. The phenotype is rare and indistinguishable from documented cases of “Riggs-type CSNB”, with relatively mild peripheral retinal changes suggesting possible slow degeneration. The case highlights the importance of an intact GARP domain of the CNGβ1 subunit in the function of the rod photoreceptors.
Patient consent
Written consent to publish this case has been obtained from the patient as part of the ongoing genotype-phenotype correlation study approved by the local ethics committees.
Acknowledgements and disclosures
Funding
Funding: Diana Davis Spencer Clinical Fellowship from the Foundation Fighting Blindness–USA (RB); Foundation Fighting Blindness –USA (GEH). Early Career Investigator Award, Fight for Sight UK (GA); NIHR Biomedical Research Centre at Moorfields Eye Hospital, and UCL Institute of Ophthalmology (GEH, GA, RB, ARW, AGR).
Proprietary interest
None.
Conflicts of interest
All the authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.03.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig S1.
Multiple alignments of the amino acid sequences of the protein product of CNGB1 in various vertebrate species. (A) The alignment was done using the Clustal Omega algorithm and the amino acid sequences from the UniParc database (https://www.uniprot.org/uniparc/). The P-Q-P triplet is a conserved repeat in several vertebrate species from zebrafish to the nine-banded armadillo and the degree of similarity is shown in the cladogram (B).
References
- 1.Lamb T.D., Pugh E.N., Jr. Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Investig Ophthalmol Vis Sci. 2006;47:5137–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 2.Shuart N.G., Haitin Y., Camp S.S. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun. 2011;2:457. doi: 10.1038/ncomms1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Körschen H.G., Beyermann M., Müller F. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature. 1999;400:761–766. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]
- 4.Michalakis S., Zong X., Becirovic E. The glutamic acid-rich protein is a gating inhibitor of cyclic nucleotide-gated channels. J Neurosci. 2011;31:133–141. doi: 10.1523/JNEUROSCI.4735-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Körschen H.G., Illing M., Seifert R. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Bareil C., Hamel C.P., Delague V. Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet. 2001;108:328–334. doi: 10.1007/s004390100496. [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro P.A., Mir T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog Retin Eye Res. 2018;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Hull S., Attanasio M., Arno G. Clinical characterization of CNGB1-related autosomal recessive retinitis pigmentosa. JAMA Ophthalmol. 2017;135:137–144. doi: 10.1001/jamaophthalmol.2016.5213. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch D.L., Marmor M.F., Brigell M.G. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 10.Bach M., Brigell M.G., Hawlina M. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126:1–7. doi: 10.1007/s10633-012-9353-y. [DOI] [PubMed] [Google Scholar]
- 11.Thompson D.A., Fujinami K., Perlman I. ISCEV extended protocol for the dark-adapted red flash ERG. Doc Ophthalmol. 2018;136:191–197. doi: 10.1007/s10633-018-9644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBain V.A., Egan C.A., Pieris S.J. Functional observations in vitamin A deficiency: diagnosis and time course of recovery. Eye (Lond) 2007;21:367–376. doi: 10.1038/sj.eye.6702212. [DOI] [PubMed] [Google Scholar]
- 13.Arno G., Holder G.E., Chakarova C. Recessive retinopathy consequent on mutant G-protein β subunit 3 (GNB3) JAMA Ophthalmol. 2016;134:924–927. doi: 10.1001/jamaophthalmol.2016.1543. [DOI] [PubMed] [Google Scholar]
- 14.Zeitz C., Robson A.G., Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015;45:58–110. doi: 10.1016/j.preteyeres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Molday L.L., Molday R.S. Knockout of GARPs and the β-subunit of the rod cGMP-gated channel disrupts disk morphogenesis and rod outer segment structural integrity. J Cell Sci. 2009;122:1192–1200. doi: 10.1242/jcs.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardell M.D., Bedsole D.L., Schoborg R.V., Pittler S.J. Genomic organization of the human rod photoreceptor cGMP-gated cation channel beta-subunit gene. Gene. 2000;245(2):311–318. doi: 10.1016/s0378-1119(00)00023-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.