Abstract
Mechanisms controlling ureter lenght and the position of the kidney are poorly understood. Glial cell-line derived neurotrophic factor (GDNF) induced RET signaling is critical for ureteric bud outgrowth, but the function of endogenous GDNF in further renal differentiation and urogenital system development remains discursive. Here we analyzed mice where 3′ untranslated region (UTR) of GDNF is replaced with sequence less responsive to microRNA-mediated regulation, leading to increased GDNF expression specifically in cells naturally transcribing Gdnf. We demonstrate that increased Gdnf leads to short ureters in kidneys located in an abnormally caudal position thus resembling human pelvic kidneys. High GDNF levels expand collecting ductal progenitors at the expense of ureteric trunk elongation and result in expanded tip and short trunk phenotype due to changes in cell cycle length and progenitor motility. MEK-inhibition rescues these defects suggesting that MAPK-activity mediates GDNF’s effects on progenitors. Moreover, Gdnf hyper mice are infertile likely due to effects of excess GDNF on distal ureter remodeling. Our findings suggest that dysregulation of GDNF levels, for example via alterations in 3′UTR, may account for a subset of congenital anomalies of the kidney and urinary tract (CAKUT) and/or congenital infertility cases in humans and pave way to future studies.
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) are common birth defects affecting around 1% of live births, and causing most cases of the chronic kidney disease in children1. CAKUT covers a wide range of malformations that derive from deficiencies in embryonic kidney and lower urinary tract development, including obstruction of ureteropelvic junction, renal dysplasia, hydro-, ectopic and short ureters. Genetic causes for these malformations remain largely unknown despite the extensive sequencing efforts in gene coding regions2.
The morphogenesis of urogenital system is influenced by a common nominator, the nephric duct (ND), also known as Wolffian duct3. The ND extends caudally towards the posterior end of the embryo to connect to the cloaca through an endoderm-derived structure called the urogenital sinus. Proper positioning and timing of ND connection to the cloaca are important steps for the later development and function of the kidney and rest of the urogenital system. At least 1–2% of male infertility associates with ND defects4 and the development of Müllerian ducts, which give rise to oviducts (uterine tubes), the uterus and the upper part of vagina, depends on the normal caudal extension of ND5. An imperforate hymen is seen in 0.1% of newborn girls6 and 43% of vaginal and/or hymen agenesis patients co-present malformations in the kidney indicating common etiology between urinary and genital tract development7.
Development of the kidney begins by budding of the ND towards renal mesenchyme to create a ureteric bud (UB), which then undergoes extensive branching morphogenesis to generate the renal collecting duct system8. According to the “Ureteral Bud Theory” of Mackie and Stephens (1975) and supported by recent transgenic studies9, the site where the UB forms greatly influences ureter maturation and determines the final position of the connection to the bladder. Specifically, cranial budding results in failure to segregate the ureter from the ND, resulting in obstruction, while caudal budding results in the ureter connection to more lateral or anterior sites on bladder, leading to reflux10.
Concomitantly with UB branching begins the distal ureter maturation, which remodels the most caudal segment of ND, also called the common ND. The vertical displacement process moves the ureter upwards and separates it from the ND, which in males differentiates into the vas deferens and epididymal ducts, and in females plays an important function in Müllerian duct guidance9,11,12. The urogenital sinus is an important signaling center expressing several hormones and receptor tyrosine kinase ligands such as GDNF13,14 that may influence ND to cloaca attachment and distal ureter remodeling. The regulation of distal ureter remodeling is beginning to emerge15, but how ureter length and anatomical positioning of urogenital organs occur are less well understood.
It is well established that kidney development critically depends on GDNF induced RET tyrosine kinase signaling16 (see also Table 1). Mice lacking Gdnf, receptor Ret or co-receptor Gfra1 show renal aplasia while those with specific mutations in Ret have additional impairment in ND differentiation12,17–19 and display phenotypes that are not reported in Gdnf knockouts20–22. Ectopic transgenic misexpression of Gdnf in ND and derivatives suggested that GDNF is not chemoattractive signal for ureteric bud but stimulates excessive ectopic budding and frequent defects in ureter to bladder connection23. Similar excess budding is seen in mouse models lacking SLIT2-ROBO2 signaling which is required to restrict Gdnf expression to its normal domain, and Sprouty1, a protein needed to negatively regulate activation of receptor tyrosine kinase signaling24,25. These results suggest that normal nephrogenesis necessitates critically regulated Gdnf expression. However, what other functions endogenous GDNF has in developing kidney e.g. in regulating ureteric bud morphogenesis and differentiation, or differentiation of ND associated reproductive organs remains to be studied.
Table 1.
Summary of GDNF functions identified using GDNFhyper mice.
| Published overexpression/functional inactivation studies | Endogenous GDNF elevation (this study) | ||
|---|---|---|---|
| Phenotype | Strategy | Phenotype | Novelty |
| ND: extra budding rostrally | Exogenous GDNF protein in kidney explant cultures57 | ND: normal until common ND remodelling | New |
| UB formation: not affected | UB formation: abnormally wide UB | New | |
| Kidney proper: expanded UB tips | Kidney proper: expanded UB tips | In-line | |
| ND: extra budding along the entire ND & ureter connecting to sex ducts | Inducible, ectopic GDNF over-expression in ND23 | ND: normal until common ND remodelling, | New |
| & ureter connecting to sex ducts | In-line | ||
| Mesonephros: extra budding | Mesonephros: normal | New | |
| Kidney proper: expanded UB tips | Kidney proper: expanded UB tips | In-line | |
| ND: normal | Conventional Gdnf deletion20–22 | ND: normal until common ND remodelling | In-line |
| UB formation: not affected | UB formation: abnormally wide | New | |
| Kidney proper: no kidney | Kidney proper: short UB trunk & pelvic kidney | New | |
| ND: extra budding rostrally | Conventional deletion of Robo2/Slit2 (needed to restrict Gdnf expression)24,58 | ND: normal until common ND remodelling | New |
| UB formation: not reported | UB formation: abnormally wide UB | New | |
| Kidney proper: expanded UB tips, several ureters, hydroureters | Kidney proper: expanded UB tips, occational hydroureters | New & In-line | |
| ND: extra budding rostrally | Conventional deletion of Spry1 (negative inhibitor of Ret signaling)24,25,59,60 | ND: normal until common ND remodelling | New |
| UB formation: abnormally wide UB | UB formation: abnormally wide UB | In-line | |
| Kidney proper: expanded UB tips, several ureters, hydroureters, blind ended ureters | Kidney proper: expanded UB tips, occational hydroureters | In-line | |
| RPO: ureter connecting to vas deference, abnormally located testes | RPO: Failure to separate ureter | New | |
| from common ND, vaginal imperforation, abnormal connection between vas deference and seminal vesicle, sperm in | New & In-line | ||
| seminal vesicle, infertility | New | ||
| RPO: Proliferative response & increase in urethral mesenchyme thickness | Exogenous GDNF protein in urogenital sinus explant cultures45 | RPO: Failure to separate ureter from common ND, ureter and sex duct misconnections | New |
| RPO: Increase in ovarian primordial follicle development | Exogenouse GDNF protein in ovary explant cultures61 | RPO: Vaginal imperforation, infertility | New |
| RPO: Patchy loss of spermatogenesis -> normal fertility | GDNF haploinsufficiency, Gdnf+/−43 | RPO: Sperm in the of seminal vesicle, abnormal connection between vas deference and seminal vesicle, | New |
| RPO: Spermatogonial differentiation failure, accumulation of stem cells -> infertility | Ectopic GDNF overexpression with EF1 promoter43 | infertility without spematogonial differentiation defect | New & In-line |
The main findings of this study are reported in comparison with published reports from ectopic GDNF applications and GDNF knock-out mice. The forth column indicates whether the observations made in GDNFhyper mice are consistent with function predicted from ectopic GDNF applications and from Gdnf gene deletion studies or provide novel information. Abbreviations: ND; nephric duct, RPO; reproductive organ, UB; ureteric bud.
Previously, we showed that microRNAs miR-9, miR-96, miR-133b and miR-146a suppress GDNF expression by interaction with Gdnf 3′UTR, and that in vivo replacement of Gdnf 3′UTR with a sequence less responsive to regulation by microRNAs and RNA-binding proteins leads to increased endogenous GDNF expression26 (Gdnfhyper). Our previous analysis focused on brain dopamine system development and function26, mainly because of high hopes for using GDNF in Parkinson’s disease treatment and ongoing clinical trials. We also reported that quite unexpectedly for a kidney morphogen, Gdnf hyper/hyper mice have small and malformed kidneys. Importantly, endogenous Gdnf expression pattern is maintained in Gdnf hyper/hyper mice, which have kidneys and are rescued from the early postnatal lethality caused by Gdnf deletion26. This allows examination of GDNF functions beyond ureteric bud induction and in postnatal pups.
Here we found that GDNF regulates ureteric bud trunk length through the control of collecting ductal progenitors and by this way defines the position of kidneys in the abdominal cavity. GDNF coding region aberrations are rare in CAKUT patients and occur mostly together with mutations in RET (Table 2). However, our results illustrate the pathogenic potential of dysregulation in GDNF levels and suggest that such analysis, though technically challenging in patients, may reveal new pathogenesis of CAKUT.
Table 2.
Overview of mutational analysis in GDNF/RET signaling components in patients with congenital anomalies of kidney and urinary track.
| Publications analyzing GDNF/RET/ | Identified variantion | Cohort & association with kidney | Notes | ||
|---|---|---|---|---|---|
| GFRa1 aberrations in humans | RET | GDNF | GFRa1 | ||
| Skinner et al.63 | yes | only concurrently with RET variant (1) | no | 33 stillborn fetuses/aplasia or severe dysplasia | A GDNF variant detected in only one fetus with unilateral agenesis and additional mutation in RET |
| Yang et al.64 | yes | NA | NA | 118 Canadian pVUR patients | 70% of the patients carry SNP in RET potential phosphorylation site |
| Zhang et al.62 | yes | NA | NA | 136 full-term healthy infants | A common RET variant associates with reduced renal size & function |
| Jeanpierre et al.53 | yes | NO | NA | 105 fetuses with bilateral renal defects (agenesis, hypodysplasia, multicystic dysplasia) | Analyzed coding, promoter & 3′UTR regions + copy number variations to identify low frequency of potential RET mutations |
| Chatterjee et al.52 | yes | yes (2)+ concurrent- ly with RET variant (1) | yes (1) | 122 unrelated CAKUT patients | A GDNF variant detected concurrently with RET variant in one VUR, and alone in one VUR+ ectopia&hydronephro− tis and one VUR + unilateral agenesis&ectopia patient |
| Kaczmarczyk et al.65 | yes | NA | no | 188 full-term healthy infants | Confirms the association of common RET variant with reduced renal size & function reported by Zhang et al.62 |
Targeted and whole genome sequencing approaches have revealed genetic aberrations in GDNF receptor Ret but mutations in Gdnf itself are largely either missing or in combination with Ret variations. Abbreviations: NA; not analyzed, pVUR; primary vesicoureteral reflux, VUR; vesicoureteral reflux.
Results
Genetically enhanced endogenous GDNF expression shifts expansion of primary ureteric bud rostrally and distorts ureter morphogenesis
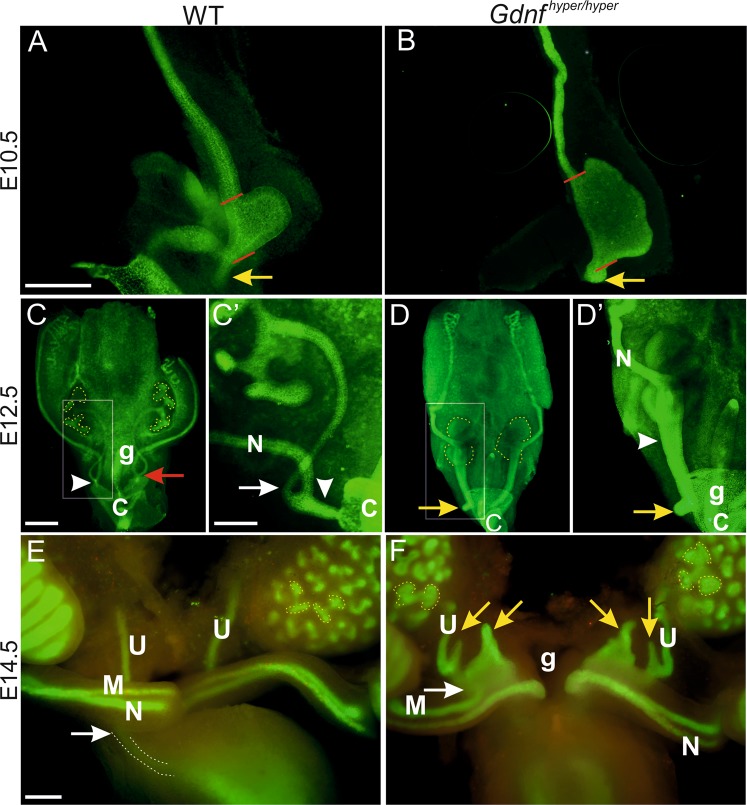
We have previously reported that GDNF protein levels are increased 3-fold in Gdnf wt/hyper and 6-fold in homozygous kidneys of Gdnf hyper/hyper mice at embryonic day 18.5 (E18.5) and result in reduced kidney size at birth26. Here we investigated how GDNF 3′UTR regulation, which does not affect endogenous expression pattern, influences the development of the whole urogenital system, where excess Gdnf expression is confined in the cells endogenously expressing it (Fig. S1A–E). We first analyzed nephric duct (ND) morphogenesis and UB morphology at early developmental stages. After its formation, the ND extends caudally towards the posterior end of the embryo to connect to the cloaca (Figs. 1A, S1E,F). At E10.5, the ND was connected to the cloaca in both control and Gdnf hyper/hyper embryos but the connecting segment of ND itself was dilated in the Gdnf hyper/hyper embryos (Fig. 1A,B). As previously described, the UBs were also wider in Gdnf hyper/hyper than those in wild type (WT) embryos (Fig. 1A,B). Moreover, the primary bud in Gdnf hyper/hyper embryos was formed more rostrally than in the WT embryos, placing the ureteric budding site to an abnormal location. According to the budding theory, this would predict ureter obstruction during later development10.
Figure 1.
Ureteric bud and distal ureter morphogenesis are severely disturbed in embryos with enhanced GDNF levels. Whole-mount calbindin staining of E10.5 (A) wild type (WT) and (B) Gdnf hyper/hyper urogenital blocks. Red lines show the width of the primary bud, yellow arrow points to normal (WT) and expanded (Gdnf hyper/hyper) end of nephric duct. Whole mount E-cadherin staining of E12.5 (C) WT and (D) Gdnf hyper/hyper urogenital blocks. Ureteric bud tips are depicted by yellow, dotted lines, arrowheads point to common nephric duct, which failed to start remodeling in Gdnf hyper/hyper urogenital system, white arrow shows the distinction of ureter from nephric duct in WT control (C’). Red arrow in C indicates the side where vertical displacement has completely occurred, yellow arrows show extra ureteric budding near to cloaca in the posterior nephric duct (D, D’). (E) E14.5 WT sample stained with E-cadherin (green) and cleaved-Caspase3 (red) shows normal ureteric buds (yellow, dotted lines) and ureters connecting to the lateral sides of upper bladder (white dotted line pointed by white arrow), while (F) in Gdnf hyper/hyper embryos ureters show large extension-like extra buds (yellow arrows) that connect to sex ducts (white arrow) and dilatated ureteric bud tips (yellow, dotted lines). Abbreviations: C; cloaca, g; gut, M; mesonephric duct, N; nephric duct, U; ureter. Scale bars: 200 µm.
We next studied further nephric duct morphogenesis and UB branching at specific developmental stages known to be critical for ND remodeling, distal ureter remodeling and ureter maturation9. At the onset of UB branching morphogenesis, distal ND showed distinct segment of connecting piece and separation of ureter from ND in WT embryos, while Gdnf hyper/hyper and fraction of Gdnf wt/hyper embryos (45%, unilateral) showed abnormal UB morphology and failure to segregate distal ureter from ND (Fig. S2A–C). After branching morphogenesis initiation, WT kidneys typically showed several UB tips (8–9/kidney at E12.5) (Fig. 1C). Vertical displacement was either initiated (left side) or completed (right side) in the WT at E12.5 (Fig. 1C-C’). At this point, ureteric branching was severely retarded in the Gdnf hyper/hyper embryos (Fig. 1D). Also extra budding was seen at the rostral end of the Gdnf hyper/hyper common ND, which failed to start normal vertical displacement and exhibited a dilated common ND (Fig. 1D-D’). The common ND remodelling was completed in WT embryos by E13.5 as seen by separated ureter and nephric duct (Fig. S2D). The common ND in Gdnf hyper/hyper embryos though remained aberrantly long and showed a widely dilated caudal end (Fig. S2E). Similar ND abnormalities were still present in Gdnf hyper/hyper embryos at E14.5 and interfered with Müllerian duct development as seen by abnormal extensions in its caudal end, something which was not observed in WT samples (Fig. 1E,F).
High GDNF levels result in short and misplaced ureters
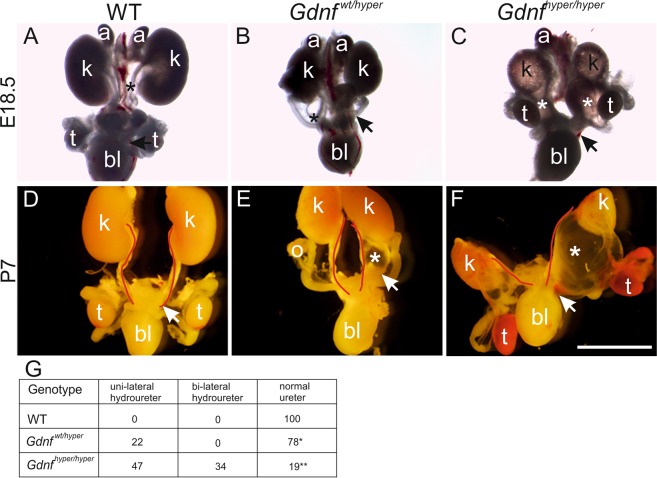
The finding that the most caudal ureteric bud that gives rise to the future ureter fails to separate from the nephric duct in Gdnf hyper/hyper embryos predicts potential defects in later development of the ureter and other nephric duct derivatives. Examination of the urogenital system at late-embryonic and early-postnatal stages indeed revealed unilateral hydroureters in Gdnf wt/hyper kidneys, which were often also unilaterally hypoplastic and irregularly shaped (Fig. 2A,B,E,G). In Gdnf hyper/hyper animals the entire urogenital system was severely malformed and kidneys were small and multicystic as previously described26 (Fig. 2A–F). Almost half of the Gdnf hyper/hyper and 1/5 of the Gdnf wt/hyper mice also develop hydroureters (Fig. 2G), which is a hallmark of abnormal ureter-to-bladder connection. Accordingly, ureteric bud and collecting duct cysts were regularly seen in postnatal kidneys (Fig. S3C–E)26. Strikingly, the ureter length in Gdnf hyper/hyper kidneys at E18.5 (56.6 ± 12.9%, n = 3) was markedly shorter than that seen in WT controls (100 ± 7.7% n = 4, p = 0.028), and therefore kidneys were postnatally positioned low in the abdominal cavity (Fig. S3A,B, see also Fig. 2C,F). This indicates that GDNF regulates ureter length and thereby indirectly the final localization of the kidneys in the adult body.
Figure 2.
Kidney and lower urogenital tract defects in Gdnf hypermorphic mice. (A) Representative images of wild type (WT), (B) Gdnf wt/hyper and (C) Gdnf hyper/hyper urogenital system at E18.5 and (D–F) at P7, demonstrating renal hypoplasia, unilateral hydroureters (black & white asterisks) and severe renal hypodysplasia accompanied with short ureters. Red lines depict medial edge of ureter. Note hemorrhaging in testes. (G) Distribution of distinct ureter phenotype percentages in different genotypes at late embryonic and early postnatal stages (n = 38, 41, 32 for WT, Gdnf wt/hyper Gdnf hyper/hyper, respectively). In the table, one asterisk indicates short ureter while two is severely shorter. Abbreviations: a; adrenal gland, bl; bladder k; kidney, o; ovary, t; testis. Scale bar: 250 µm.
Abnormally wide primary ureteric bud in Gdnfhyper/hyper embryos associates with a proliferation burst in the early nephric duct
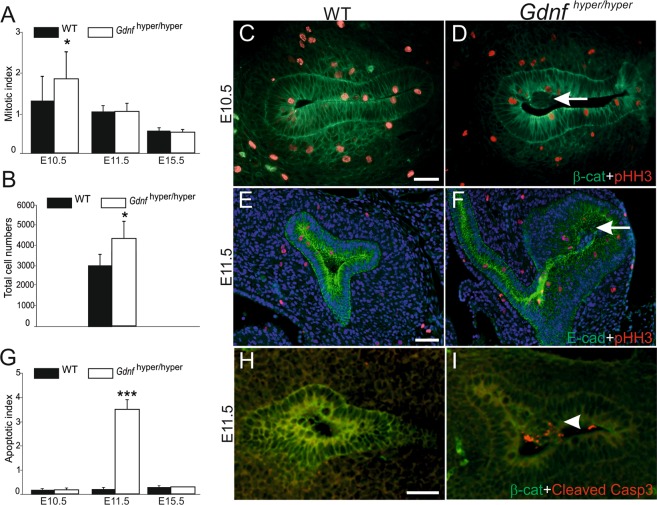
Next we sought to characterize the cellular causes for the abnormalities in primary UB and distal ureter morphogenesis leading to short and misconnected ureter in Gdnf hyper/hyper animals. We first examined proliferation and apoptosis in control and hypermorphic kidneys and found that at the time of primary ureteric bud formation (E10.5), the mitotic index in the caudal ND and ureteric epithelium of Gdnf hyper/hyper was 40% higher than in the controls (Fig. 3A). This excess proliferation at the time of primary UB formation likely explains the observed increase in primary ureteric bud size in early Gdnf hyper/hyper kidneys (Fig. 1). Surprisingly, the mitotic index was normalized in tips of Gdnf hyper/hyper UBs soon after the onset of branching, despite the significant difference in the total ureteric epithelial cell numbers (Fig. 3A,B). Interestingly, accumulation of cells negative for mitosis marker pHH3 were observed in the luminal cavity of UB both at E10.5 and E11.5 in Gdnf hyper/hyper kidneys, while these were not seen in WT kidneys (Fig. 3C–F). Simultaneously, apoptosis was significantly increased in Gdnf hyper/hyper ureteric bud epithelium (Fig. 3G–I). This data show that excess GDNF increases cell proliferation in the caudal nephric duct, specifically at the emergence of the ureteric bud, and suggest that later in UB epithelium excess GDNF may interfere with luminal mitosis mechanism27 as accumulation of cell mass and increased apoptosis were observed in the lumen of Gdnf hyper/hyper UB.
Figure 3.
GNDF augments mitosis in caudal nephric duct at the time of ureteric bud outgrowth. (A) Mitotic indices were determined as the percentage of pHH3-positive cells within b-catenin or E-cadherin positive epithelium. Graphs show indices of wild type (WT, black bars) and Gdnf hyper/hyper embryos (white bars) in caudal nephric duct (E10.5), ureteric bud epithelium (E11.5) and ureteric bud tips (E15.5). A statistically significant increase in mitotic indices was observed at E10.5 samples (7–9 vibratome sections per embryo, n = 3/genotype, p < 0.05, Student’s t-test). (B) Total cell counts in E11.5 WT and Gdnf hyper/hyper ureteric bud tips (p < 0.05). (C) Representative E10.5 WT and (D) Gdnf hyper/hyper kidney primordia shown after staining with pHH3 (red) and b-catenin (green). (E) E11.5 WT kidney shows typical T-shaped ureteric bud (E-cadherin, green), but (F) Gdnf hyper/hyper epithelium is abnormally enlarged without normal branching pattern. Arrows in (D) and (F) point to cell mass accumulated in the luminal side of the epithelium. (G) Regardless of the genotype, labeling for cleaved Caspase3 does not reveal apoptosis in the kidney primordia of E10.5 embryos (n = 7) while a statistically significant increase is seen at E11.5 in Gdnf hyper/hyper epithelium (white bar, n = 5 for WT and 4 for Gdnf hyper/hyper, Student’s t-test, p < 0.001). At E15.5 cleaved caspase 3 labeling reveals similar amount of apoptotic cells in the cortex of WT (n = 3) and Gdnf hyper/hyper (n = 2) kidneys. (H) An example of WT kidney stained with cleaved Caspase3 (red, n = 5) shows virtually no apoptosis, while (I) numerous apoptotic cells (arrowhead) are detected in the ureteric bud lumen of Gdnf hyper/hyper embryos at E11.5 (n = 4). Scale bars 50 µm. Error bars on all graphs represent standard deviation.
Previously, it has been indicated that apoptosis is an essential process in common nephric duct remodelling18,28,29. We stained common nephric ducts with apoptosis marker cleaved Caspase3 and found reduced apoptosis in subset of Gdnf hyper/hyper samples at E11.5 representing the stage for the onset of remodelling (Fig. S4A,B). Despite the clear defect in nephric duct morphology at later stages, the amount of apoptotic cells in all Gdnf hyper/hyper samples was comparable to WT NDs at at E12.5 (Fig. S4C,D). Staining of the lower urinary tract samples at E14.5 showed that timing of apoptosis was not changed in Gdnf hyper/hyper samples as again they were comparable to WTs (Fig. S4E,F). This suggests that common nephric duct remodelling utilizes additional mechanisms than apoptosis to achieve normal morphological outcome.
High GDNF levels block ureteric bud and renal growth by restricting emigration of progenitors from ureteric bud tips
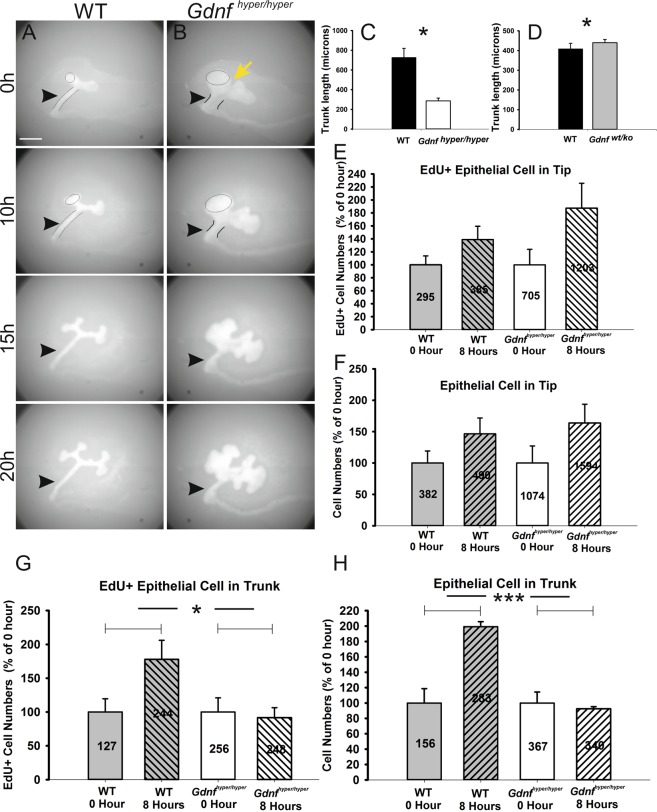
To analyze the effect of excess GDNF on further UB morphogenesis we crossed Gdnf wt/hyper mice with HoxB7CreGFP mice expressing green fluorescent protein (GFP) in the ND and its derivatives30. Normally, new ureteric bud tips are generated from the existing tips, which first expand to form the ampulla that then bifurcate to generate two new tips8,31. Bifurcation is followed by trunk elongation and some cells initially found in the tips are later found in the trunk region where the collecting duct differentiation takes place32. Indeed, time-lapse imaging of control kidneys showed typical, long and elongated trunks between the nephric duct and tip epithelia (Fig. 4A, movie 1). Control kidneys produced new branches from the ampulla, and also showed obvious trunk elongation. The UB tips in Gdnf hyper/hyper kidneys, however, were enlarged and exhibited a cleft-like furrow in the middle and bud-like structures all over the tips (Fig. 4B, movie 2). The Gdnf hyper/hyper tip expanded considerably more than the trunk - the future ureter, elongated, and remained significantly shorter than in WT controls (arrowheads in Fig. 4A,B; trunk lengths at E12.0 kidneys exemplified in Fig. S5A,B: 1135.2 ± 284.4 µm in WT (n = 5), 446.3 ± 87.3 µm in Gdnf hyper/hyper (n = 5), p = 0.0018). Thus, the ureteric bud tip growth occurred at the expense of trunk elongation in Gdnf hyper/hyper embryos.
Figure 4.
In vitro analysis of ureteric bud and trunk growth. Time-lapse imaging of 24 h cultured E11.5 (A) wild type (WT) and (B) Gdnf hyper/hyper kidneys where nephric ducts and ureteric bud epithelia are visualized by transgenic Hoxb7CreGFP expression. Yellow arrow marks cleft-like furrow between the distinct ends of ureteric bud tips, arrowhead points to ureteric trunk. (C) Measurement of trunk lengths, presented as the average primary trunk length ± SEM, in WT and Gdnf hyper/hyper kidneys at E12 reveals that UB trunks are significantly shorter in Gdnf hyper/hyper kidneys (728.6 ± 91.3 µm in WT, 286.5 ± 28.0 µm in Gdnf hyper/hyper, n = 4/genotype, p = 0.013, unpaired two-tailed Student’s t-test). (D) Trunk measurements in WT and Gdnf wt/ko kidneys show that trunk length in Gdnf wt/ko kidneys is increased (408.9 ± 27.9 µm in WT (n = 13), 439.8 ± 16.0 µm in Gdnf wt/ko (n = 16), p = 0.019, unpaired two-tailed Student’s t-test). Analysis of (E) EdU-positive cell counts and (F) total epithelial cell counts (average cell numbers depicted inside the bars on (E–H) in ureteric bud tips (marked by black circles in A and B at 0 and 10 h images) are shown as percentage of growth rates (WT, n = 5; Gdnf hyper/hyper, n = 4). Growth rate at 0 h was set to 100% in both genotypes. The increase ratio in wild type cell numbers was approximately the same in EdU+ (38.9 ± 20.5%, n = 5) and total cell counts (46.6 ± 25.2%, n = 4), while both EdU+ cells (87.5 ± 38.3%, n = 3) and total cells (63.9 ± 30%, n = 3) increased remarkably more in Gdnf hyper/hyper tips. (G) Corresponding analysis of growth rates in the ureter trunks of E11.5 WT and (H) Gdnf hyper/hyper kidneys. Dramatic increase in both EdU+ cells (77.9 ± 28.1%, n = 4) and total cell numbers (99.2 ± 6.6%, n = 3) were observed in WT kidney trunks. Gdnf hyper/hyper trunk cells failed to increase either EdU+ (91.6 ± 14.7%, n = 3) or total cell numbers (92.6 ± 2.6%, n = 3). The area of measurements is depicted in A and B images of 0 and10h as black lines. Scale bar: 200 µm.
Next we investigated how tips in Gdnf hyper/hyper kidneys remain enlarged despite a normal mitotic index after primary budding and why trunks in Gdnf hyper/hyper kidneys fail to elongate at a similar pace as in the WT. Highly proliferative tips host progenitor cells, which are capable of populating the renal collecting duct system32–35. Normal RET and its downstream activities appear critical for maintaining epithelial progenitor cells in the tip domain32,36. On the other hand, cells with low or no RET activity are left behind from the tip domain (the niche) and are later found in trunks where they differentiate into collecting duct cells. We hypothesized that a high GDNF level positively regulates progenitor expansion and has a negative effect on trunk elongation by restricting emigration of progenitors from the niche. If this is correct, low GDNF levels should result in longer trunks. The analysis of trunk lengths in Gdnfwt/ko kidneys, which have only one functional allele expressing Gdnf37 (Fig. S5C,D), indeed revealed a small but statistical increase in trunk length (Fig. 4D, 408.93 ± 27.86 µm in WT (n = 13), 439.84 ± 16.02 µm in Gdnf wt/ko (n = 16), p = 0.019). This suggests that low GDNF dosage allows more cells to leave the tips to populate the trunks for elongation and differentiation.
Next, tip and trunk segments (Fig. S5E) were analyzed separately in Gdnf hyper/hyper kidneys. Previous characterization of cell cycle lengths in the developing kidney (14.5 h in tips and 22.4 h in trunks35) was utilized in designing the pulse-labelling experiments (5-ethynyl-2′-deoxyuridine (EdU), 30 min). Similar to mitotic index (Fig. 3A,B), EdU+ (295 ± 40 in WT (n = 5); 705 ± 168 in Gdnf hyper/hyper (n = 3)) and total cell (382 ± 73 in WT (n = 4); 1074 ± 293 in Gdnf hyper/hyper (n = 3)) counts were higher in Gdnf hyper/hyper tips than in WT tips (Fig. 4E,F). On average, 77% (75.59 ± 3.05%) of the WT (n = 4) tip cells and 66% (67.62 ± 3.63%) of the tip cells in Gdnf hyper/hyper kidneys (n = 3) were in S-phase (EdU+/total cells) at the initiation of the culture period (0 h). The proportion of EdU+ cells increased to 79% (78.78 ± 2.80%) in WT (n = 6) and to 75% (76.70 ± 5.79%) in Gdnf hyper/hyper tips at 8 h, showing a greater proportional increase with excess GDNF (Fig. S5F). This indicates that tip cells cycle faster in UBs of Gdnf hyper/hyper kidneys. This also suggests that shorter cell cycle time contributes to increased total tip cell numbers and ureteric bud size in Gdnf hyper/hyper kidneys.
Control ureteric bud trunks are longer than those in Gdnf hyper/hyper kidneys (Fig. 4A–C). Indeed, while EdU+ and total cell number almost doubled in the trunks of WT kidneys at 8 h, in sharp contrast neither parameter increased in the Gdnf hyper/hype trunks during this time period (Fig. 4G,H). These results imply that high GDNF levels result in large tips by the combination of two mechanisms: by restricting emigration from the tip niche to the trunks and by shortening the cell-cycle time in the tips. Alternatively, it is also possible that an excess of GDNF may have a direct negative effect on trunk cell proliferation, but this is unlikely as ureteric trunk cells do not express any known GDNF receptors13.
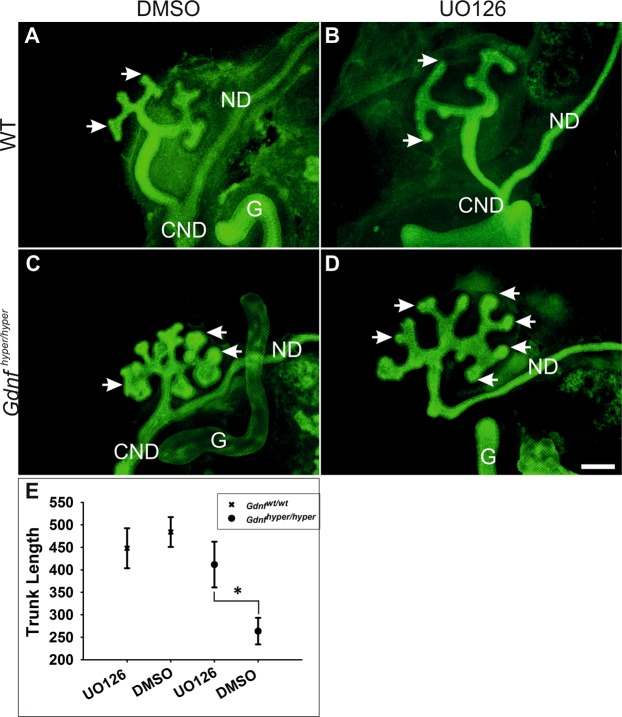
MEK inhibition normalizes ureteric bud morphology and branching in Gdnfhyper/hyper kidneys
Several intracellular cascades are activated downstream of RET receptor upon GDNF binding38. In order to assess which of these intracellular cascades could meditate GDNF’s effect on collecting ductal progenitors and ureter lenght we next performed chemical inhibition experiments in cultured kidney explants to examine potential predominant intracellular pathway in Gdnf hyper/hyper kidneys. Consistent with previous reports, inhibitor-specific responses were observed in WT kidneys where PI3K/AKT or MAPK cascades were inhibited39–41. Interestingly, only MAPK pathway suppression with MEK inhibitor UO126 normalized UB tip morphology and improved trunk length in Gdnf hyper/hyper kidneys (Fig. 5). Chemical MEK inhibition, similarly to its genetic abrogation specifically in UB and derivatives, results in elongation only phenotype, where UB tips fail to expand into an ampullae and rarely produce new tips41 (Fig. 5A,B). The UB tips in Gdnf hyper/hyper kidneys are wide and expanded but showed significant normalization upon 48 h culture with MEK inhibitor (Fig. 5C–E). Our results thus indicate that a MAPK dependent imbalance in collecting ductal progenitor self-renewal vs. differentiation drives the ureteric bud phenotype in Gdnf hyper/hyper kidneys. It remais to be seen whether MAPK pathway is responsible for mediating GDNF functions also in distal ureter remodeling.
Figure 5.
MEK inhibition rescues ureteric bud morphology in Gdnf hyper/hyper kidneys. (A) Wild type (WT) kidney cultured in mock medium for 48 h shows normal morphology of ureteric bud tips (arrows). (B) Similarly to published results39,41, inactivation of MAPK pathway by MEK inhibitor UO126 results in failure to expand ureteric bud tips (arrow) in WT kidneys. (C) Gdnf hyper/hyper kidney cultured in mock medium shows expanded ureteric bud tips and very short trunks. (D) MAPK pathway inhibition significantly improves both ureteric bud tip morphology and trunk length in Gdnf hyper/hyper kidneys. (E) Statistical analysis of UO126 treatment effects on trunk lengths in WT and Gdnf hyper/hyper kidneys shows that MEK inhibition significantly promotes trunk length in Gdnf hyper/hyper kidneys (411.69 ± 50.93 µm in Gdnf hyper/hyper kidneys cultured with UO126, 263.59 ± 29.55 µm in Gdnf hyper/hyper kidney cultured with DMSO, p = 0.047, n = 9, paired sample t-test; 448.01 ± 44.49 µm in WT kidney cultured with UO126, 484.03 ± 33.16 µm in WT kidney cultured with DMSO, p = 0.146, n = 3, paired sample t-test). Data presented as average trunk length ± the standard error of mean (SEM), *denotes P < 0.05 in paired sample t-test. Abbreviations: CND; common nephric duct, G; gut, ND; nephric duct. Scale bar: 200 µm.
Endogenous overexpression of GDNF causes infertility in both genders
The “Ureteral Bud Theory” of Mackie and Stephens predicts that changes in the location where primary UB forms affect further ureter development10. Accordingly, rostrally expanded budding site in Gdnf hyper/hyper embryos results in failure to segregate the ureter from the ND ureter (Figs 1 and S2). In addition to giving rise to ureteric bud, segments of nephric duct also differentiate into the vas deferens and epididymal ducts in males, while in females nephric duct guides Müllerian duct development9,11. We hypothesized that defects in ND and distal ureter remodelling may lead to impaired development of reproductive organs whose differentiation also depends on nephric duct. Indeed, the hypothesis is supported by the finding that Gdnf hyper/hyper mice showed occasionally attachment of the ureter to the testis (Fig. 2F).
Gdnf hyper/hyper mice survive less than three weeks26 and therefore we could not test their fertility potential. However, we noticed that Gdnf wt/hyper mice became infertile after back-crossing original mixed C57Bl/6NCrl/129SvOla background strain to the isogenic C57Bl/6NCrl strain. As an example, 9/22 Gdnf wt/hyper males in F1 generation and all Gdnf wt/hyper males in F2 (n = 9) failed to impregnate WT females despite clear vaginal plugs. Likewise, 10/22 Gdnf wt/hyper females in F1, and all Gdnf wt/hyper females in F2 were infertile, setting a limit on the number of possible backcrosses. As a consequence, the Gdnf wt/hyper mice had to be maintained in mixed background (C57BL/6NCrl/ICR/and129SvOla) to avoid infertility and produce the mouse colony for the experiments.
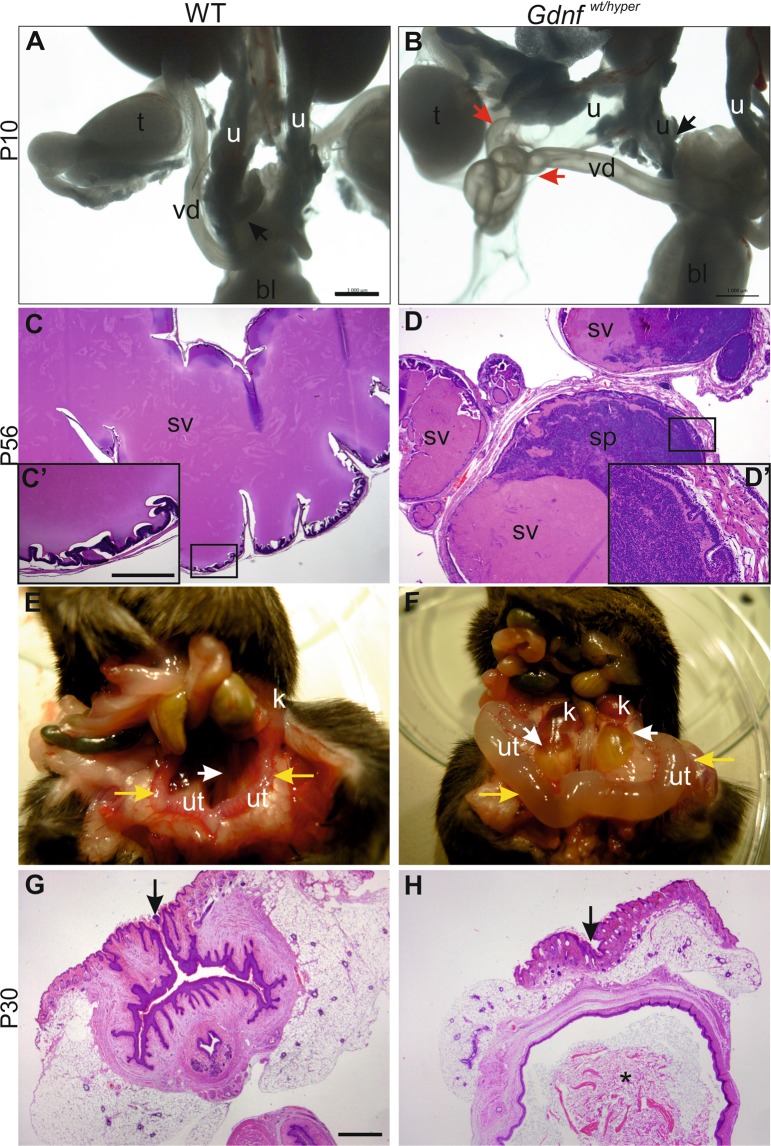
We next sought to understand the causes of infertility in sub-isogenic Gdnf wt/hyper mice. Anatomical examination of the F1 and F2 Gdnf wt/hyper males in the C57BL/6NCrl backcross revealed defects in the connections between the ureter, vas deferens, and seminal vesicles (Fig. 6A,B). For example, vas deferens was directly connected to seminal vesicles, which caused abnormal accumulation of sperm in the seminal vesicles of Gdnf wt/hyper male mice (Fig. 6C-D’). However, sperm collected from the epididymis appeared normal both in morphology and motility (Fig. S6A,B). Infertility in females was likely caused by an imperforate vagina (Figs 6E–H, S6C,D), which prevents penetrance of the sperm to the female oviduct. These data show that enhanced GDNF levels cause defects not only in ureter maturation but have also functional consequences on fertility through the control of reproductive organ development in both sexes.
Figure 6.
Reproductive organ defects in Gdnf wt/hyper mice. (A) Male wild type (WT) lower urinary tract and reproductive organs at P10. Black arrow denotes ureter (u) connection to bladder (bl). (B) Gdnf wt/hyper male in F2 generation exhibits split ureter with one (u, black arrow) connecting to bladder (bl) and the other, hydroureter (u) connecting to vas deferens (vd, red arrow). (C) Hematoxylin-eosin staining of P56 wild type seminal vesicle. Squared area is enlarged in C’. (D) Histology of P56 Gdnf wt/hyper seminal vesicle shows sperm inside the lumen and loss of typical lobular shape of seminal vesicles. Squared area is enlarged in D’. (E) Normal female mouse abdomen at P35. Yellow arrows point to uterus, white arrows indicate ureter. (F) Corresponding abdomen of Gdnf wt/hyper female shows hydrotic ureters and badly swollen uteri (yellow arrows). Hematoxylin-eosin staining of frontal sections of (G) WT and (H) Gdnf wt/hyper vagina at the surface level. Asterisk in H shows accumulation of keratinized mass inside the vagina, which is indicative of fluid blockage. Black arrows point to vaginal opening in wild type mice, and to the site where vaginal opening should be in Gdnf wt/hyper mice. All images are examples of F2 generation of backcrossing to isogenic C57BL/6NCrl background. Abbreviations: bl; bladder, sp; sperm, sv; seminal vesicle, t; testis, u; ureter, ut; uterus, vd; vas deferens. Scale bars: 1 mm and C’: 250 µm.
Discussion
The functional importance of the 3′UTR on executing gene function is currently unknown for most genes. Very little is known about how the kidneys are positioned in the abdominal cavity and how ureter length is specified. Here we report that GDNF levels, as regulated by its functional 3′UTR, define ureter length and thereby delineate the position of the kidneys in abdominal cavity. Furthermore, we find that correct GDNF levels are important for normal reproductive organ development in both genders.
Our results also highlight that genetic studies on 3′UTRs can provide information, which complements and enhances existing knowledge from gene deletion or mutational analysis. Gene deletion studies have shown that GDNF is required for the initiation of kidney development, but no defects in reproductive organ development has been reported8,20,22,42–44. Furthermore, knowledge on endogenous GDNF’s function beyond the induction of ureteric bud outgrowth and bifurcate branching is largely lacking. We found that GDNF regulates the expansion of collecting ductal progenitors, a cell population located in the ureteric bud tips capable of populating the entire collecting duct system35. Specifically, we revealed that an enhanced GDNF level increases the number of collecting ductal progenitors at the expense of the elongation of the trunk, the future ureter, thereby resulting in short ureters. Our results suggest that GDNF may participate in regulation of a novel form of cell divisions called luminal mitosis. It was shown that dividing cells of ureteric bud delaminate from the epithelial sheet to enter the luminal space of the epithelium27. This occurs just prior the cell division, after which the two cells re-enter the epithelial sheet couple of cells apart. Our observation of abnormal cellular mass in the lumen of Gdnf hyper/hyper UB tips together with accelerated cell cycle and increased apoptosis implies defects in re-entry process of luminal mitosis, but further experiments are needed to verify this hypothesis.
We also found that excess GDNF can result in infertility in adults likely due to defects in common nephric duct remodeling, a developmental process necessary for segregation of ureters from reproductive organs and known to depend on GDNF receptor RET12,17,18. The precise mechanism how GDNF regulates female reproductive tract development and specifically vaginal perforation remains to be determined. Previously GDNF was shown to induce proliferation in the urogenital sinus45, which functions as a precursor for the bladder and lower urogenital system (prostatic and penile urethrae in males; urethra and lower vagina in females). Changes in this process may contribute to development-derived infertility in Gdnf hyper/hyper mice and make an important aspect for future studies.
Interestingly, activating and inactivating mutations reported in Gdnf receptor RET do not phenocopy urogenital system malformations we found in Gdnf hyper/hyper mice. Activating RET mutations, which cause constitutively active signaling, result in pediatric cancer syndromes such as multiple endocrine neoplasia (MEN) type 2A and 2B46,47 but except for the rare occasions (total of five patients in three distinct kindreds48–50 of MEN2A), the majority of MEN patients display normal kidney morphology. Indeed, while urogenital system development in MEN2B mice is normal47, we found that Gdnf hyper mice display multiple defects not only in kidney but also in reproductive organs. Further, manipulation of specific docking sites responsible for activation of distinct intracellular cascades downstream of RET have resulted in varying degrees of changes in morphology of the kidney, nephric duct and its derivatives8,12,17,18,51. This, together with our results, suggests that a precisely tuned signaling cascade, where regulation of expression levels of the ligand, GDNF, is essential for defining the activation amplitude and cell-type specific responses in GDNF-RET regulated tissues.
Finally, our finding that increased GDNF levels due to disruption of its 3′UTR function - not mutations in GDNF or in its receptor protein encoding sequences20,22,52,53 - result in phenotype homologous to human CAKUT and infertility, may be clinically informative because despite of the advances in sequencing techniques, the disease causing mutation remains unknown in most patients suffering from CAKUT and infertility2,54. It is becoming increasingly clear that mutations in coding sequence of DNA alone do not account for many human disorders and epigenetic as well as other mechanisms controlling gene expression levels may explain many congenital diseases.
Methods
Mouse models, genotyping and ethics statement. Generation of Gdnf hyper/hyper and knockout mouse lines used in the study was described by Kumar et al.26. Both lines were maintained on triple mixed background (C57BL/6NCrl/ICR/and129SvOla) for all experiments except those examining reproductive organs (indicated in the text). Hoxb7CreGFP30 line was bred to Gdnf hyper/hyper mice to enable visualization of ND and its derivatives. The genotyping was performed by PCR as described previously20,26.
Embryonic staging was determined as reported previously55 and all experiments were approved by the Finnish National Animal Care and Use Committee. The experiments were designed according 3R principles and followed European Union directives (Directive 2010/EU/63) in compliance with the Code of Ethical Conduct for Animal Experimentation.
Tissue collection, processing, hematoxylin-eosin (HE) and immunofluorescent staining
Embryos and tissues of indicated stages were dissected in Dulbecco’s medium supplemented with 0.2% bovine serum albumin and fixed with 4% PFA. Further processing for paraffin embedding was done according to standard procedure with an automatic tissue processor (Leica ASP 200).
HE, whole-mount, and immunostaining on paraffin sections were performed as previously described55. More details in supporting information file.
Organ culture, live imaging and EdU-labelling
Kidneys were cultured on a Trowell-type system in medium of F12:DMEM/10% fetal bovine serum/Glutamax/penicillin-streptomycin, and either processed for whole-mount immunofluorescent staining (see above) or imaged with an epifluorescent microscope (see below). The rudimentary lower urogenital blocks were dissected from Gdnf hyper/hyper and Hoxb7CreGFP; Gdnf hyper/hyper embryos at E10-11.5 and halved along the linea mediana ventralis before placing for culture on permeable support (Transwell®; Costar).
The kidneys used for ureteric bud trunk length measurements were either cultured for 1 h and followed by whole-mount immunofluorescent staining (n = 5 for WT and Gdnf hyper/hyper at E12, and n = 11 in WT and n = 7 in Gdnf wt/ko at E11.5), or measured directly from stereomicroscopy images of E18.5 kidneys (n = 4 in WT, n = 3 in Gdnf hyper/hyper) by using ImageJ software. See the details of live imaging with time-lapse microscopy and EdU pulse labelling experiments in supplemental information. Statistical analyses were performed with unpaired two-tailed Student’s t-test or paired-samples t-test, when appropriate. The level of statistical significance was set to p < 0.05. The calculations were carried out with SPSS (IBM; Version 22).
Quantification of mitotic indices and apoptosis
Quantification of mitotic index at indicated stages was performed on vibratome sections (three embryos per genotype and 7–9 sections per kidneys were studied for pHH3+ nephric duct/ureteric bud cells) similarly as previously described56. Proliferation significances were tested with Independent samples t-test (1-tailed, equal variances).
Apoptosis was analyzed on vibratome sections by double staining with cleaved-Caspase3 and β-catenin. Samples were collected at E10.5 (n = 7 for both genotypes), E11.5 (wt n = 5, Gdnf hyper/hyper n = 4) and E15.5 (wt n = 3, Gdnf hyper/hyper n = 2), and cleaved-Caspase3 positive cells were counted per section in each genotype for statistical testing with Independent samples t-test (1-tailed, equal variances).
Sperm motility test
For motility testing, sperm from three F1 and two F2 Gdnf wt/hyper male mice backcrossed to C57BL/6NCrl isogenic background for one or two generations, respectively, were collected. Cauda was dissected out and two small incisions were made to allow sperm to swim out into HTF medium containing BSA (K-RVFE-50, William A. Cook Australia Pty. Ltd) for 15 min at 37 °C (N2/6% CO2/5% O2), after which sperm were counted according to standard categorization criteria (a-b-c-d) using a Bürker hemocytometer chamber (Hawksley). After motility assessment, a fraction of the sperm was pipetted onto a microscope slide for morphological analysis followed by HE staining.
Supplementary information
Movie 2: GDNF hypermorphic kidney branching.
Acknowledgements
Authors thank Mart Saarma for initiating in vivo studies with GDNF and for providing lab space. We thank Cristina Cebrian, Cathy Mendelsohn and Maxime Bouchard for valuable discussions, Saara Ollila for critical reading of the manuscript and Susanna Wiss and Iiro Uotila for technical help. Imaging was performed at the Light Microscopy Unit, Institute of Biotechnology, and animals were housed at Laboratory Animal Centre of University of Helsinki. This work was supported by grants to K.S. from the Academy of Finland (grants 138283 and 294243), Jane and Aatos Erkko, Sigrid Juselius, and Maud Kuistila Foundations, to A.J.O. from Academy of Finland grant 297727, Sigrid Juselius Foundation, Faculty of Medicine at the University of Helsinki, Helsinki Institute of Life Science Fellow grant and by European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, grant agreement nr 724922, and to S.H. from EVO funding TYH2013351 by the University Central Hospital of Helsinki.
Author Contributions
L.H. and J.M. have performed majority of the experiments and actively participated in preparing the figures and writing the manuscript. O.R. did in situ hybridizations and G.Y. immunofluorescent stainings, K.A. participated in phenotype characterization and S.P did perform the sperm analysis. S.H. planned the experiments together with K.S. and A.J.-O., who also analyzed and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Li and Madis Jakobson contributed equally.
Satu Kuure and Jaan-Olle Andressoo jointly supervised this work.
Contributor Information
Satu Kuure, Email: satu.kuure@helsinki.fi.
Jaan-Olle Andressoo, Email: jaan-olle.andressoo@ki.se.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40457-1.
References
- 1.Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol. 2015;11:720–731. doi: 10.1038/nrneph.2015.140. [DOI] [PubMed] [Google Scholar]
- 2.Heidet, L. et al. Targeted Exome Sequencing Identifies PBX1 as Involved in Monogenic Congenital Anomalies of the Kidney and Urinary Tract. J Am Soc Nephrol, 10.1681/ASN.2017010043 (2017). [DOI] [PMC free article] [PubMed]
- 3.Georgas KM, et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015;142:1893–1908. doi: 10.1242/dev.117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–151. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- 5.Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation. 2011;82:117–126. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakalkale R, Samarakkody U. Familial occurrence of imperforate hymen. J Pediatr Adolesc Gynecol. 2005;18:427–429. doi: 10.1016/j.jpag.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Kimberley N, Hutson JM, Southwell BR, Grover SR. Vaginal agenesis, the hymen, and associated anomalies. J Pediatr Adolesc Gynecol. 2012;25:54–58. doi: 10.1016/j.jpag.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Costantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley interdisciplinary reviews. Developmental biology. 2012;1:693–713. doi: 10.1002/wdev.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uetani N, Bouchard M. Plumbing in the embryo: developmental defects of the urinary tracts. Clin Genet. 2009;75:307–317. doi: 10.1111/j.1399-0004.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 10.Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114:274–280. doi: 10.1016/S0022-5347(17)67007-1. [DOI] [PubMed] [Google Scholar]
- 11.Mullen RD, Behringer RR. Molecular genetics of Mullerian duct formation, regression and differentiation. Sex Dev. 2014;8:281–296. doi: 10.1159/000364935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batourina E, et al. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32:109–115. doi: 10.1038/ng952. [DOI] [PubMed] [Google Scholar]
- 13.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- 14.Weiss AC, et al. Nephric duct insertion requires EphA4/EphA7 signaling from the pericloacal mesenchyme. Development. 2014;141:3420–3430. doi: 10.1242/dev.113928. [DOI] [PubMed] [Google Scholar]
- 15.Stewart K, Bouchard M. Coordinated cell behaviours in early urogenital system morphogenesis. Semin Cell Dev Biol. 2014;36:13–20. doi: 10.1016/j.semcdb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Costantini F. GDNF/Ret signaling and renal branching morphogenesis: From mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6:252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia I, et al. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development. 2011;138:2089–2097. doi: 10.1242/dev.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshi M, Batourina E, Mendelsohn C, Jain S. Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development. 2012;139:2405–2415. doi: 10.1242/dev.078667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short KM, Smyth IM. The contribution of branching morphogenesis to kidney development and disease. Nat Rev Nephrol. 2016;12:754–767. doi: 10.1038/nrneph.2016.157. [DOI] [PubMed] [Google Scholar]
- 20.Moore MW, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez MP, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 22.Pichel JG, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 23.Shakya R, et al. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Grieshammer U, et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/S1534-5807(04)00108-X. [DOI] [PubMed] [Google Scholar]
- 25.Basson MA, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, et al. GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function. PLoS Genet. 2015;11:e1005710. doi: 10.1371/journal.pgen.1005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packard A, et al. Luminal Mitosis Drives Epithelial Cell Dispersal within the Branching Ureteric Bud. Dev Cell. 2013;27:319–330. doi: 10.1016/j.devcel.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uetani N, et al. Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J Clin Invest. 2009;119:924–935. doi: 10.1172/JCI37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batourina E, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat. 2004;204:241–255. doi: 10.1111/j.0021-8782.2004.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney D, Lindstrom N, Davies JA. Developmental plasticity and regenerative capacity in the renal ureteric bud/collecting duct system. Development. 2008;135:2505–2510. doi: 10.1242/dev.022145. [DOI] [PubMed] [Google Scholar]
- 35.Riccio P, Cebrian C, Zong H, Hippenmeyer S, Costantini F. Ret and Etv4 Promote Directed Movements of Progenitor Cells during Renal Branching Morphogenesis. PLoS Biol. 2016;14:e1002382. doi: 10.1371/journal.pbio.1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuure S, Chi X, Lu B, Costantini F. The transcription factors Etv4 and Etv5 mediate formation of the ureteric bud tip domain during kidney development. Development. 2010;137:1975–1979. doi: 10.1242/dev.051656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopra J, et al. GDNF is not required for catecholaminergic neuron survival in vivo. Nature neuroscience. 2015;18:319–322. doi: 10.1038/nn.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurtzeborn, K., Cebrian, C. & Kuure, S. Regulation of renal differentiation by trophic factors. Frontiers in Physiology, 10.3389/fphys.2018.01588 (2018). [DOI] [PMC free article] [PubMed]
- 39.Fisher CE, Michael L, Barnett MW, Davies JA. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development. 2001;128:4329–4338. doi: 10.1242/dev.128.21.4329. [DOI] [PubMed] [Google Scholar]
- 40.Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- 41.Ihermann-Hella A, et al. Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion. PLoS Genet. 2014;10:e1004193. doi: 10.1371/journal.pgen.1004193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cullen-McEwen LA, Drago J, Bertram JF. Nephron endowment in glial cell line-derived neurotrophic factor (GDNF) heterozygous mice. Kidney Int. 2001;60:31–36. doi: 10.1046/j.1523-1755.2001.00767.x. [DOI] [PubMed] [Google Scholar]
- 43.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 44.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park HJ, Bolton EC. Glial cell line-derived neurotrophic factor induces cell proliferation in the mouse urogenital sinus. Mol Endocrinol. 2015;29:289–306. doi: 10.1210/me.2014-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Runeberg-Roos P, Virtanen H, Saarma M. RET(MEN 2B) is active in the endoplasmic reticulum before reaching the cell surface. Oncogene. 2007;26:7909–7915. doi: 10.1038/sj.onc.1210591. [DOI] [PubMed] [Google Scholar]
- 47.Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. Embo J. 2000;19:612–622. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hibi Y, et al. A MEN2A family with two asymptomatic carriers affected by unilateral renal agenesis. Endocr J. 2014;61:19–23. doi: 10.1507/endocrj.EJ13-0335. [DOI] [PubMed] [Google Scholar]
- 49.Lore F, Di Cairano G, Talidis F. Unilateral renal agenesis in a family with medullary thyroid carcinoma. N Engl J Med. 2000;342:1218–1219. doi: 10.1056/NEJM200004203421615. [DOI] [PubMed] [Google Scholar]
- 50.McIntyre E, et al. Multiple endocrine neoplasia type 2A: an unusual clinical presentation and association with renal dysplasia. Cancer Genet Cytogenet. 2003;141:157–159. doi: 10.1016/S0165-4608(02)00663-5. [DOI] [PubMed] [Google Scholar]
- 51.Jain S, Encinas M, Johnson EM, Jr., Milbrandt J. Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev. 2006;20:321–333. doi: 10.1101/gad.1387206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatterjee R, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet. 2012;131:1725–1738. doi: 10.1007/s00439-012-1181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeanpierre C, et al. RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet. 2011;48:497–504. doi: 10.1136/jmg.2010.088526. [DOI] [PubMed] [Google Scholar]
- 54.Capone, V. P., Morello, W., Taroni, F. & Montini, G. Genetics of Congenital Anomalies of the Kidney and Urinary Tract: The Current State of Play. Int J Mol Sci18, 10.3390/ijms18040796 (2017). [DOI] [PMC free article] [PubMed]
- 55.Kuure S, et al. Crosstalk between Jagged1 and GDNF/Ret/GFRalpha1 signalling regulates ureteric budding and branching. Mech Dev. 2005;122:765–780. doi: 10.1016/j.mod.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Chi X, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sainio K, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- 58.Wainwright EN, Wilhelm D, Combes AN, Little MH, Koopman P. ROBO2 restricts the nephrogenic field and regulates Wolffian duct-nephrogenic cord separation. Dev Biol. 2015;404:88–102. doi: 10.1016/j.ydbio.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Basson MA, et al. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 60.Michos O, et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 2010;6:e1000809. doi: 10.1371/journal.pgen.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dole G, Nilsson EE, Skinner MK. Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction. 2008;135:671–682. doi: 10.1530/REP-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19:2027–2034. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet. 2008;82:344–351. doi: 10.1016/j.ajhg.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Houle AM, Letendre J, Richter A. RET Gly691Ser mutation is associated with primary vesicoureteral reflux in the French-Canadian population from Quebec. Hum Mutat. 2008;29:695–702. doi: 10.1002/humu.20705. [DOI] [PubMed] [Google Scholar]
- 65.Kaczmarczyk M, et al. Association Between RET (rs1800860) and GFRA1 (rs45568534, rs8192663, rs181595401, rs7090693, and rs2694770) Variants and Kidney Size in Healthy Newborns. Genet Test Mol Biomarkers. 2016;20:624–628. doi: 10.1089/gtmb.2016.0079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 2: GDNF hypermorphic kidney branching.