Abstract
There is paucity of data from developing countries on the clinical outcomes in myeloma post-autologous transplantation. In this retrospective study, we used hospital records to retrieve data of patients with multiple myeloma undergoing autologous stem cell transplantation (ASCT) from January 1995 to December 2014 at our centre. During the study period, 245 patients underwent ASCT for myeloma. Of these, 19%, 37% and 37% were in complete response, very good partial response and partial response respectively at the time of ASCT. Only in 14 (5.7%) patients, the stem cells were cryopreserved. The transplant related mortality was 2.86%. The median follow up was 40.7 months (range 0–237.4 months). The 5-year overall survival (OS) and progression-free survival (PFS) for the entire cohort was 61.6% ± 3.8% and 37.2% ± 3.9% respectively. Independent predictors of OS included mononuclear cell dose infused, pre- and post-transplant response; and the use of maintenance therapy. Independent predictors of PFS included age at diagnosis, pre- and post-transplant response; and the use of maintenance therapy. In a resource limited setting, ASCT for myeloma is associated with low transplant related mortality. Pre- and post-transplant response and maintenance therapy are predictors of survival.
Keywords: Autologous transplantation, Multiple myeloma, Developing countries, Non-cryopreserved grafts
Introduction
Multiple myeloma is characterized by clonal proliferation of malignant plasma cells in the bone marrow, monoclonal protein in blood or urine and associated organ dysfunction [1]. It accounts for 13% of hematologic malignancies with an annual age-adjusted incidence of 5.6 cases per 100,000 persons [2].
The current standard of care for transplant eligible patients with myeloma is 4–6 cycles of bortezomib based induction with three drugs which induces response in 80–90% patients, followed by autologous stem cell transplantation (ASCT). The survival in patients with myeloma has at least doubled over the last decade however most patients eventually relapse [3].
A meta-analysis comparing ASCT with conventional therapy showed significant benefit with ASCT in terms of event-free survival but no benefit in terms of overall survival [4]. Hence ASCT constitutes the present standard of care for eligible patients.
The challenges in developing countries like access to novel agents, access to facilities for stem cell cryopreservation, disparities in economy and health care infrastructure necessitate a region specific approach to treatment [5].
There is limited data from developing countries on the outcome of ASCT in myeloma to guide such an approach. From India, data is available from three large centres [6–8] showing promising long-term outcomes in patients with myeloma undergoing ASCT with pre- and post-transplant responses being predictive of survival. Hence we decided to undertake a retrospective analysis of patients with myeloma who underwent autologous transplantation at our institution over a 20 year period.
Methods
We conducted a retrospective analysis of patients undergoing ASCT for myeloma at our centre from January 1995 to December 2014. This study was approved by the institutional review board. Hospital records were used to retrieve the data.
Mobilization strategy used was GCSF 5 microgram per kg per dose twice daily for 4 days following which on the 5th day after the morning dose of GCSF, patients underwent peripheral blood stem cell (PBSC) apheresis using COBE spectra auto PBSC system (Terumo BCT, USA). The target cell dose was 2 × 106/kg. Patients not achieving the target cell dose were continued on GCSF and underwent apheresis on the subsequent day as well. After apheresis, the PBSC were stored at 4 °C in a blood bank refrigerator up to 72 h. Whenever adequate cell dose was not achieved with 2 days of apheresis, the PBSC product was cryopreserved and patients underwent another session of mobilization.
The conditioning regimen used was single agent melphalan. In patients with normal renal function, 12 h post melphalan infusion, the entire PBSC product was infused using infusion sets without filters (JMS, Singapore) or regular blood transfusion sets (Terumo Penpol, India). In patients with renal dysfunction, the infusion of PBSC was done 24 h after melphalan. GCSF was routinely started post-transplant to hasten engraftment as per physician discretion. No antibacterial, antifungal or antiviral prophylaxis was used during the study period.
Neutrophil engraftment was defined as the 1st of 3 consecutive days with absolute neutrophil count (ANC) > 0.5 × 109/L. Platelet engraftment was defined as the 1st of 3 consecutive days with platelet count ≥ 20 × 109/L without platelet transfusions for at least 7 days.
Patients who had progressive disease (defined as per the International Myeloma Working group uniform response criteria 2009) [9] at last follow up and subsequently had no follow up for the last 1 year were considered as expired at the time of last contact. Patients with complete response (CR) or very good partial response (VGPR) at last follow up and having no follow up for the last 1 year were censored as alive at the time of last contact. Survival analysis and Cox regression analysis were used to analyze the data. Analysis was performed using IBM SPSS software.
Results
Between January 1995 and December 2014, 245 patients underwent ASCT for myeloma at our centre. Table 1 describes the transplant characteristics. The median age was 51 years (range 23–68); 69% were males. The median International staging system (ISS) stage at diagnosis was II (range I–III). The median time from diagnosis to ASCT was 10.5 months (range 3.9–113.4). Ninety-six (39.2%) patients underwent ASCT beyond 12 months of diagnosis. Prior to ASCT, about 52%, 35% and 10% patients had received one, two and three lines of chemotherapies respectively. About 19%, 37% and 37% were in complete response (CR), very good partial response (VGPR) and partial response (PR) respectively at the time of ASCT.
Table 1.
Transplant characteristics
| Characteristic | Median (range) or Number (%) |
|---|---|
| Age (in years) | 51 (23–68) |
| Male (sex) | 169 (69) |
| ISS (International Staging System) stage (n = 134) | 2 (1–3) |
| 1 | 41 (30.6) |
| 2 | 47 (35.07) |
| 3 | 46 (34.33) |
| Number of chemotherapy regimen before autologous transplantation (n = 244) | 1 (1–5) |
| 1 | 127 (52.05) |
| 2 | 85 (34.84) |
| 3 | 24 (9.84) |
| 4 | 7 (2.86) |
| 5 | 1 (0.4) |
| Drugs used during induction (n = 244) | |
| Novel agents (bortezomib or lenalidomide) | 128 (52.46) |
| Time from diagnosis to transplantation (in months) | 10.5 (3.9–113.4) |
| Response post induction chemotherapy (n = 239) | |
| Complete response (CR) | 46 (19.25) |
| Very good partial response (VGPR) | 88 (36.82) |
| Partial response (PR) | 89 (37.24) |
| Stable disease (SD) | 10 (4.18) |
| Progressive disease (PD) | 6 (2.51) |
| Mononuclear cell dose (× 108/kg) | 4.99 (0.96–19.64) |
| CD34 cell dose (× 106/kg) | 4.57 (1.15–23.7) |
| Platelet rich concentrate transfusions | 10 (0–78) |
| Packed red cell concentrate transfusions | 1 (0–11) |
| Fresh frozen plasma transfusions | 0 (0–20) |
| Primary engraftment failure | 1 (0.44) |
| Death during peri-transplantation period | 7 (2.86) |
| Cause of death | |
| Invasive fungal infection | 4 |
| Intracranial bleed | 2 |
| Sepsis | 1 |
| Post transplantation response at day 100 (n = 216) | |
| Complete response (CR) | 94 (43.52) |
| Very good partial response (VGPR) | 86 (39.81) |
| Partial response (PR) | 24 (11.11) |
| Stable disease (SD) | 3 (1.39) |
| Progressive disease (PD) | 9 (4.17) |
| Maintenance therapy (n = 216) | 152 (70.37) |
| Interferon (± melphalan) | 19 |
| Thalidomide | 66 |
| Lenalidomide | 59 |
| Bortezomib | 6 |
| Cyclophosphamide and dexamethasone | 2 |
| Duration of maintenance (in months) | 14.5 (1–120) |
The initial mobilization strategy was GCSF alone in 243 patients (99.2%) and upfront cyclophosphamide mobilization was done for 2 patients (0.8%). Both patients who underwent upfront cyclophosphamide mobilization had an adequate cell dose collection and underwent transplant with a non-cryopreserved graft.
Among the remaining 243 patients who underwent mobilization with GCSF alone, adequate cell dose was collected in 228 patients. Among these 228 patients with adequate cell dose collection, 224 patients underwent transplant using non-cryopreserved grafts. The stem cell product was cryopreserved for 4 patients despite adequate cell dose collection (1—developed hypoxemia during melphalan infusion, 1—patient with seizure disorder developed breakthrough seizure on 2nd day of stem cell harvest, 1—developed fever with left lower lobe consolidation after the stem cell harvest, 1—reason not mentioned in hospital records).
Of the 15 patients who failed initial GCSF mobilization, 5 underwent another session of GCSF mobilization, 1 patient underwent mobilization with GCSF and plerixafor, 8 patients underwent mobilization with cyclophosphamide and 1 required cyclophosphamide along with plerixafor. Amongst these patients, only the freshly collected stem cell product from the second session of mobilization was used for the transplant in 5 patients while for the remaining 10 patients both the fresh product from second session of mobilization along with the cryopreserved product from the first session of mobilization was infused.
Cryopreserved graft was infused in a total of 14 (5.7%) patients. The number of days of the harvest was 1 in 94 patients (38.4%), 2 in 141 patients (57.6%), 3 in 3 patients (1.2%) and 4 in 7 patients (2.8%).
The dose of melphalan used in mg/m2 was 200, 180 and 140 in 171 (69.8%), 47 (19.2%) and 23 (9.4%) patients respectively. Patients were started on GCSF after ASCT. The day of starting GCSF was commonly between day 5 and 7 (day 7 in 148 patients, 60.4%; day 6 in 35 patients, 14.3%; day 5 in 37 patients; 15.1%). The median time to neutrophil engraftment was 12 days (range 9–22) while the median time to platelet engraftment was 17 days (range 10–44).
Mucositis (data available for 216 patients) was grade 2 or less in 77 (35.65%) patients while 139 (64.35%) patients had grade 3 or 4 mucositis. Six (2.45%) patients required dialysis, 6 (2.45%) had seizures, 5 (2.04%) had metabolic encephalopathy, 15 (6.12%) required admission into intensive care unit, 13 (5.31%) required inotropic support, 5 (2.04%) had cardiac arrhythmias and 7 (2.86%) required mechanical ventilation.
Infectious complications: Twenty-eight (11.43%) patients had positive blood cultures, of which 13 were positive for gram positive organisms (2—Staphylococcus aureus, 11—coagulase negative Staphylococcus), 9 for gram negative organisms (1—carbapenem resistant Klebsiella, 2—Klebsiella, 4—non-fermenting gram negative bacillus, 2—Pseudomonas) while 6 for Candida. Fourteen (5.71%) patients had positive cultures from samples other than blood, of which 7 were positive for gram negative organisms, 5 for gram positive organisms and 2 for fungi. Eleven (4.49%) patients had invasive fungal infection (7— fungal infection of which 6 had candidemia and 1 had pneumocystis jiroveci pneumonia, 1—probable fungal pneumonia, 3—possible fungal pneumonia). Four had cytomegalovirus (CMV) disease and another 5 had CMV reactivation requiring treatment.
Day 100 response: Of the 245 patients who underwent ASCT, 7 expired early post transplant at a median of 20 days (range 0–53). The cause of death was intracranial bleed in 2 patients, sepsis in 1 patient and invasive fungal infection in 4 patients of which 2 had candidal sepsis and 2 had fungal pneumonia. One patient had primary engraftment failure (ANC < 0.5 × 109/L on day 28 with a hypocellular marrow) and was salvaged with an allogeneic stem cell transplant using a HLA identical sibling donor. Fourteen patients had no follow up after ASCT while 7 patients did not have response assessment done post ASCT. Of the remaining 216 patients who were assessable at day 100, 43.5%, 39.8% and 11.1% were in CR, VGPR and PR respectively. Maintenance therapy was given to 70.3% patients for a median duration of 14 months (range 1–120).
OS and PFS: Table 2 shows the follow-up data of the cohort. At a median follow up of 40.7 months, 81 patients had died; of which 7 were attributed to TRM while another 5 died without disease progression (2—late infections, 1—secondary myelodysplasia with sepsis, 1—ischemic heart disease and 1—died in the immediate post-transplant period of an allogeneic transplant planned due to presence of del17p). The remaining 69 deaths were due to progressive disease. Additionally, 17 patients had progressive disease at last follow up.
Table 2.
Survival and progression data on follow up
| Characteristic | Median (range) or Number (%) |
|---|---|
| Status at last follow up (n = 225) | |
| Death | 81 (36) |
| Complete response (CR) | 83 (36.89) |
| Very good partial response (VGPR) | 22 (9.78) |
| Partial response (PR) | 9 (4) |
| Stable disease (SD) | 13 (5.78) |
| Progressive disease (PD) | 17 (7.56) |
| Cause of death | |
| Transplant related mortality (TRM) | 7 |
| Death in absence of disease progression | |
| Late infectious complications | 2 |
| Myelodysplastic syndrome with sepsis | 1 |
| TRM during planned allotransplant | 1 |
| Ischemic heart disease | 1 |
| Progressive disease causing death | 69 |
| Progression at any time point (n = 225) | 114 (50.67) |
| Salvaged with chemotherapy | 28 |
| Death | 69 |
| Alive with disease progression at last follow up | 17 |
| Type of progression (n = 114) | |
| Biochemical | 29 (25.44) |
| Biochemical followed by clinical | 35 (30.7) |
| Clinical relapse | 50 (43.86) |
| Duration of follow up (in months) | 40.7 (0–237.4) |
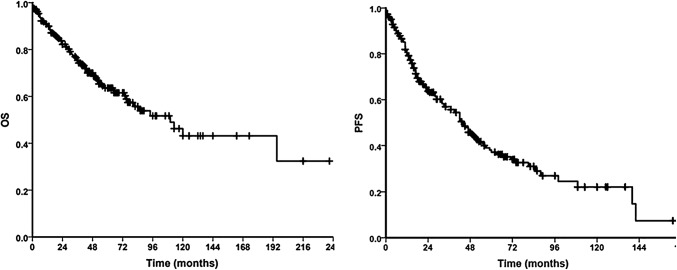
Figure 1 show the OS and PFS of the cohort. The 5-year OS and PFS for the entire cohort was 61.6% ± 3.8% and 37.2% ± 3.9% respectively.
Fig. 1.
Overall and progression free survival for the entire cohort (n = 245)
Predictors of OS: The factors independently predicting the overall survival were remission status post-induction [HR of 3.35; p 0.031 for any response vs. stable disease (SD)/progressive disease (PD)], mononuclear cell dose infused (HR 0.85; p 0.008), remission status post-transplant (HR 29.59; p 0.000 for any response vs. PD) and maintenance therapy (HR 1.75; p 0.037). (See Table 3).
Table 3.
Predictors of overall and progression free survival
| Predictors of overall survival | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Univariate analysis (Cox regression hazards ratio with 95% confidence interval and p value) | Multivariate analysis (Cox regression hazards ratio with 95% confidence interval and p value) | ||||
| HR | 95%CI | p value | HR | 95%CI | p value | |
| Remission status post-induction therapy (any response vs. SD/PD) | 3.66 | 1.52–8.84 | 0.004 | 3.35 | 1.12–9.99 | 0.031 |
| Mononuclear cell dose | 0.90 | 0.82–0.99 | 0.028 | 0.85 | 0.76–0.96 | 0.008 |
| Remission status post-transplant (any response vs. PD) | 15.07 | 6.55–34.66 | 0.000 | 29.59 | 9.79–89.45 | 0.000 |
| Maintenance therapy (maintenance vs. no maintenance) | 1.86 | 1.19–2.93 | 0.007 | 1.75 | 1.04–2.96 | 0.037 |
| Predictors of progression free survival | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Univariate analysis (Cox regression hazards ratio with 95% confidence interval and p value) | Multivariate analysis (Cox regression hazards ratio with 95% confidence interval and p value) | ||||
| HR | 95%CI | p value | HR | 95%CI | p value | |
| Age at diagnosis | 1.03 | 1.01–1.05 | 0.019 | 1.03 | 1.00–1.06 | 0.027 |
| Number of pre-transplant chemotherapy regimen | 1.34 | 1.07–1.66 | 0.01 | 1.18 | 0.91–1.52 | 0.216 |
| Remission status post-induction therapy (any response vs. PR) | 1.73 | 1.01–2.97 | 0.046 | 1.81 | 1.01–3.27 | 0.047 |
| Remission status post-induction therapy (any response vs. SD/PD) | 3.22 | 1.55–6.71 | 0.002 | 3.90 | 1.55–9.78 | 0.004 |
| Remission status post-transplant (any response vs. PR) | 1.89 | 1.07–3.34 | 0.028 | 1.08 | 0.55–2.13 | 0.825 |
| Remission status post-transplant (any response vs. PD) | 54.74 | 21.79–137.48 | 0.000 | 59.21 | 21.20–165.38 | 0.000 |
| Maintenance therapy (maintenance vs. no maintenance) | 1.83 | 1.27–2.65 | 0.001 | 1.93 | 1.27–2.93 | 0.003 |
Predictors of PFS: The factors independently predicting progression free survival were age at diagnosis (HR 1.03; p 0.027), remission status post-induction (HR 1.81; p 0.047 for any response vs. PR) (HR 3.90; p 0.004 for any response vs. SD/PD), remission status post-transplant (HR 59.21; p 0.000 for any response vs. PD) and maintenance therapy (HR 1.93; p 0.002) (see Table 3).
Discussion
In this study, we present retrospective data of patients who underwent ASCT for myeloma at our centre over the last 20 years. Almost half of the patients did not receive novel agents during induction therapy. About 39% patients were transplanted beyond 1 year of diagnosis. Despite these challenges, the transplant related mortality (TRM) was 2.86%. At a median follow up of 40 months, 50% patients did not have disease progression. Also in 95% patients, the stem cell product was infused after storage at 4 °C up to 3 days without the need for cryopreservation. The 5-year OS and PFS for the entire cohort was 61.6% ± 3.8% and 37.2% ± 3.9% respectively.
We noted that refractory disease post induction therapy (stable disease or progressive disease pre-transplant), a low mononuclear cell dose, progressive disease post transplant and non-use of maintenance therapy were the factors associated with worse overall survival on multivariate analysis. Factors associated with worse progression free survival were greater age at diagnosis, partial or lesser response post induction therapy, progressive disease post transplant and non-use of maintenance therapy.
Table 4 shows transplant data of patients with myeloma from the present study along with other studies from India. Previous reports from India [6–8] have shown that ASCT is a safe and feasible option for eligible patients with myeloma in developing countries. Also, most of these transplants have been done with freshly apheresed stem cell grafts without cryopreservation; however unlike at our centre, most have used prophylactic antifungals, antibiotics and antivirals. The proportion of patients with positive blood cultures (11.43%) and the transplant related mortality was similar to studies wherein antimicrobial prophylaxis was used [7]. Thus, routine antibacterial prophylaxis does not seem to be necessary.
Table 4.
Comparison of results of the present study with previous studies
| Characteristic | Present study | Kumar et al. [6] | Malhotra et al. [7] | Gokarn et al. [8] |
|---|---|---|---|---|
| N | 245 | 225 | 94 | 85 |
| Median age (years) | 51 | 53 | 53 | 49 |
| Males (%) | 69 | 69 | 73.7 | NR |
| Use of novel agents (%) | 52.5 | 56.9 | > 80 | > 75 |
| Median time to transplant (in months) | 10.5 | 10 | 10.5 | 10.5 |
| Pretransplant CR+VGPR (%) | 56 | 44 | 81 | 72 |
| Posttransplant CR+VGPR (%) | 83 | 74 | NR | 73 |
| TRM (%) | 2.86 | 7.2 | 3.19 | NR |
| OS | 5 year–61.6 ± 3.8% | 5 year–63.2% | 76.7% at 6.5 years | 3 year–91% |
| PFS | 5 year–37.2 ± 3.9% | 5 year–38.5% | 55.8% at 6.5 years | 3 year–58% |
| Factors associated with OS/PFS | Pre-ASCTR; Post-ASCTR; M; | Post-ASCTR; Alb; extramedullary disease; ISS; Chemo; ALC; DSS | NR | Pre-ASCTR; extramedullary disease; negative PET-CT pre-transplant |
N number of patients, Pre-ASCTR remission status pretransplant, Post-ASCTR remission status post-transplant, Chemo number of lines of pretransplant chemotherapies, M maintenance therapy, Alb serum albumin, ISS ISS stage, DSS Durie–Salmon stage, ALC absolute lymphocyte count at diagnosis, NR not reported
However, in the present study, the incidence of invasive fungal infections was high (4.5%). We also noted that 9 patients required treatment of cytomegalovirus of which 7 had received bortezomib during induction. The rate of fungal infections post autologous transplant varies from 1.2 to 10% [10–12]. Also it is known that prior use of bortezomib is a risk factor for post-transplant symptomatic CMV reactivation [13].
Based on this data, we are now using antifungal prophylaxis with fluconazole 400 mg per day till day 30 in all patients undergoing autologous transplantation and antiviral prophylaxis with acyclovir 400 mg twice daily till day 90 in all patients previously treated with bortezomib.
The predictors of survival in previous studies [6–8] have been the pre-transplant remission status, pre-transplant PET-CT response, presence of extramedullary disease, ISS stage, Durie–Salmon stage, absolute lymphocyte count at diagnosis, serum albumin less than 3.5 g/dL, more than 2 lines of induction therapy pre-transplant and post-transplant complete response. Consistent with these findings, we noted that pre-transplant remission status and post-transplant remission status seem to be important predictors of survival.
Data from the European society for blood and marrow transplantation registry shows that age, sex, calendar year of transplantation, the disease duration prior to ASCT and the remission status prior to ASCT are independent predictors of survival in patients with myeloma undergoing ASCT [14]. Poor survival has also been reported with poor performance status, IgA subtype, need for more than 1 pretransplant induction chemotherapy and resistant disease prior to ASCT [15]. Similarly, we noted that age at diagnosis and remission status pre-transplant were predictors of survival.
In our cohort, the use of maintenance therapy was associated with improved overall and progression free survival post-transplant. At present, maintenance therapy post-transplant is considered as the standard of care [16].
We also noted that a low mononuclear cell dose was associated with worse overall survival. This has been reported previously and is postulated to be due to aggressive biology of the disease leading to poor stem cell mobilization and also due to its association with greater number of lines of chemotherapies used pre-transplant [17].
Based on the present and previous studies (Table 4), the pre-transplant response seems to be an important potentially modifiable risk factor for survival in patients with myeloma. However retrospective analysis suggests that, although additional lines of therapy before autologous stem cell transplantation may improve the response depth in patients with less than a partial response, this does not impact long-term survival [18].
Thus in patients with less than partial response post-induction therapy, approaches like additional chemotherapy pre-transplant or consolidation therapy post-transplant need to be evaluated prospectively.
Conclusion
In a resource-limited setting, autologous stem cell transplantation for myeloma is associated with low transplant related mortality and good survival. Pre- and post-transplant response and maintenance therapy are predictors of survival after autologous transplantation for myeloma.
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Since this was a retrospective study, the institutional review board granted a waiver of consent.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, et al. SEER cancer statistics review. National Cancer Institute: Bethesda; 2007. [Google Scholar]
- 3.Miguel JFS. Introduction to a series of reviews on multiple myeloma. Blood. 2015;125:3039–3040. doi: 10.1182/blood-2015-01-613596. [DOI] [PubMed] [Google Scholar]
- 4.Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, Richardson PG, Anderson KC, Soiffer RJ, Alyea EP., 3rd High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transpl. 2007;13(2):183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Tan D, Chng WJ, Chou T, Nawarawong W, Hwang SY, Chim CS, Chen W, Durie BG, Lee JH. Management of multiple myeloma in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e571–e581. doi: 10.1016/S1470-2045(13)70404-2. [DOI] [PubMed] [Google Scholar]
- 6.Kumar L, Boya RR, Pai R, Harish P, Mookerjee A, Sainath B, Patekar MB, Sahoo RK, Malik PS, Sharma OD, Gupta R. Autologous stem cell transplantation for multiple myeloma: long-term results. Natl Med J India. 2016;29(4):192–199. [PubMed] [Google Scholar]
- 7.Malhotra P, Yanamandra U, Khadwal A, Prakash G, Lad D, Law AD, Khurana H, Sachdeva MUS, Bose P, Das R, Varma N, Varma S. Autologous stem cell transplantation for multiple myeloma: single centre experience from North India. Indian J Hematol Blood Transfus. 2018;34(2):261–267. doi: 10.1007/s12288-017-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokarn A, Bonda A, Mathew L, Bagal B, Philip D, Kannan S, Khattry N (2017) High dose chemotherapy with autologous stem cell transplantation for multiple myeloma: outcomes at Tata Memorial Centre. In: Abstract presented at the 16th international myeloma workshop 2017, New Delhi
- 9.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Post MJ, Lass-Floerl C, Gastl G, Nachbaur D. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl Infect Dis. 2007;9(3):189–195. doi: 10.1111/j.1399-3062.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T, Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D, Melillo L, de Waure C, Reddiconto G, Fianchi L, Valentini CG, Girmenia C, Leone G, Aversa F. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study—sorveglianza epidemiologica infezioni fungine nelle emopatie maligne. Clin Infect Dis. 2007;45(9):1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 12.Teh BW, Teng JC, Urbancic K, Grigg A, Harrison SJ, Worth LJ, Slavin MA, Thursky KA (2015) Invasive fungal infections in patients with multiple myeloma: a multi-center study in the era of novel myeloma therapies. Haematologica. 100(1):e28–e31. 10.3324/haematol.2014.114025. Epub 10 Oct 2014 PubMed PMID: 25304609; PubMed Central PMCID: PMC4281332 [DOI] [PMC free article] [PubMed]
- 13.Marchesi F, Mengarelli A, Giannotti F, Tendas A, Anaclerico B, Porrini R, Picardi A, Cerchiara E, Dentamaro T, Chierichini A, Romeo A, Cudillo L, Montefusco E, Tirindelli MC, De Fabritiis P, Annino L, Petti MC, Monarca B, Arcese W, Avvisati G. Rome Transplant Network. High incidence of post-transplant cytomegalovirus reactivations in myeloma patients undergoing autologous stem cell transplantation after treatment with bortezomib-based regimens: a survey from the Rome transplant network. Transpl Infect Dis. 2014;16(1):158–164. doi: 10.1111/tid.12162. [DOI] [PubMed] [Google Scholar]
- 14.Auner HW, Szydlo R, Hoek J, Goldschmidt H, Stoppa AM, Morgan GJ, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transpl. 2015;50(2):209–215. doi: 10.1038/bmt.2014.255. [DOI] [PubMed] [Google Scholar]
- 15.Saad A, Mahindra A, Zhang MJ, Zhong X, Costa LJ, Dispenzieri A, et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transpl. 2014;20(3):402–408.e1. doi: 10.1016/j.bbmt.2013.12.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, Sengsayadeth S, Abidi M, Hari P, Mohty M, Chen YB, Koreth J, Landau H, Lazarus H, Leather H, Majhail N, Nath R, Osman K, Perales MA, Schriber J, Shaughnessy P, Vesole D, Vij R, Wingard J, Giralt S, Savani BN. American society for blood and marrow transplantation. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American society for blood and marrow transplantation. Biol Blood Marrow Transpl. 2015;21(7):1155–1166. doi: 10.1016/j.bbmt.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Moreb JS, Byrne M, Shugarman I, Zou F, Xiong S, May WS, Norkin M, Hiemenz J, Brown R, Cogle C, Wingard JR, Hsu JW. Poor peripheral blood stem cell mobilization affects long-term outcomes in multiple myeloma patients undergoing autologous stem cell transplantation. J Clin Apher. 2018;33(1):29–37. doi: 10.1002/jca.21556. [DOI] [PubMed] [Google Scholar]
- 18.Vij R, Kumar S, Zhang MJ, Zhong X, Huang J, Dispenzieri A, Abidi MH, Bird JM, Freytes CO, Gale RP, Kindwall-Keller TL, Kyle RA, Landsburg DJ, Lazarus HM, Munker R, Roy V, Sharma M, Vogl DT, Wirk B, Hari PN. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transpl. 2015;21(2):335–341. doi: 10.1016/j.bbmt.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]