Abstract
Biotin is an indispensable adipogenic agent, and its ability to coordinate carbohydrate, lipid, and amino acid metabolism sensitizes insulin signaling in adipocytes. This enables the organism to adapt and survive under nutrient stress by synthesis and storage of lipids. Biotin deficiency mimics insulin resistance with alterations in cellular intermediary metabolism. Though the mechanism of lipogenesis is well established across cell types, considering its predisposition to accumulate only lipids, it is necessary to elucidate the mechanism that minimizes the effects of biotin on adipocyte protein synthesis. In order to determine the differential metabolic phenotype by biotin, the primary cultures of adipocytes were induced to differentiate in the presence and absence of excess biotin. Serum pre-incubated with avidin was used to limit biotin availability in cultured cells. Biotin restricts cellular signaling associated with protein synthesis without altering total protein content. The decline in autophagy elicits endoplasmic reticulum stress to inhibit protein synthesis by eIF2α phosphorylation possibly via accumulation of misfolded/long-lived proteins. Furthermore, the compensatory increase in Unc51 like autophagy activating kinase 1 possibly competes with eukaryotic initiation factor 4E-binding protein 1 and ribosomal p70 S6kinase phosphorylation by mechanistic targets of rapamycin complex 1 to uncouple its effect on protein synthesis. In conclusion, autophagy inhibition by biotin uncouples protein synthesis to promote lipogenesis by eliciting endoplasmic reticulum stress and differential phosphorylation of mechanistic targets of rapamycin complex 1 substrates.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-00967-9) contains supplementary material, which is available to authorized users.
Keywords: Biotin, Autophagy, ER stress, mTORC1, Amino acids, Protein synthesis
Introduction
Adipogenesis enables organisms to survive under nutrient stress by predominant synthesis and storage of lipids. Biotin determines cellular lipid synthesis by regulating acetyl-CoA carboxylase (ACC), the rate-limiting enzyme of fatty acids biosynthesis. Biotin deficiency mimics insulin resistance with attenuation of insulin-induced adipogenesis (Dakshinamurti et al. 1968; Kuri-Harcuch et al. 1978). Additionally, the regulation of tricarboxylic acid cycle anaplerosis by biotin establishes metabolic crossroads to demonstrate the interconversion between carbohydrates, lipids, and amino acids. For example, the amino acids augment gluconeogenesis and lipogenesis by replenishing tricarboxylic acid cycle intermediates via pyruvate carboxylase (PC), β-methylcrotonyl-CoA carboxylase (MCC), and propionyl-CoA carboxylase (PCC) (Lynen 1967; Dakshinamurti and Desjardins 1968; Boeckx and Dakshinamurti 1974). Likewise, pyruvate carboxylase also minimizes amino acid anaplerosis by utilizing pyruvate from glucose metabolism (Cheng et al. 2011) and increases the incorporation of amino acids into proteins of various organs (Boeckx and Dakshinamurti 1974). Nevertheless, the evolution of adipocyte-specific lipogenic phenotype by biotin questions its role in protein synthesis.
The intracellular levels of amino acids are sensed by general control nonderepressible 2 (GCN2) and mechanistic targets of rapamycin (mTORC1) to determine protein synthesis (Eleftheriadis et al. 2016). The interaction of GCN2 with uncharged tRNA under the deprivation of amino acids inhibits protein synthesis by phosphorylating eukaryotic initiation factor 2α (eIF2α). In contrast, amino acids activate mTORC1 at the lysosomal surface to phosphorylate eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and ribosomal p70 S6Kinase (p70S6K) to induce protein synthesis. Furthermore, like biotin, mTORC1 sensitizes insulin signaling and demonstrates tissue-specific metabolic phenotype by coordinating carbohydrate, lipid, and protein synthesis (Dibble and Manning 2013).
The anabolic effect of mTORC1 is also demonstrated by its ability to inhibit catabolism. Notably, mTORC1 inhibits the lysosomal-dependent bulk degradation process called autophagy (Wong et al. 2015). Besides dietary amino acids uptake, autophagy recycles endogenous organelles and macromolecules, including misfolded/long-lived proteins, to replenish cellular amino acids (Onodera and Ohsumi 2005; Yu et al. 2010). However, the existing lysosomal centered regulatory loop between autophagy and mTORC1 allows the cells to appropriately sense and respond to nutrient-rich and -deficient conditions by determining the nature and extent of macromolecular turnover in cells.
Unlike starvation, the nutrient- and insulin-enriched conditions favor adipogenesis (Zhang et al. 2009) and the same inhibit autophagy (Meijer and Codogno 2008). The inhibition of autophagy augments cellular storage. However, unlike lipid droplets, the prolonged accumulation of proteins elicits endoplasmic reticulum (ER) stress to inhibit protein synthesis via protein kinase R-like endoplasmic reticulum kinase (PERK)-mediated phosphorylation of eIF2α (Harding et al. 2000). Since amino acid-mTORC1 signaling is indispensable for adipogenesis (Zhang et al. 2009) and the GCN2 activation negatively regulates mTORC1 signaling by phosphorylating regulatory subunit of mTORC1 (Averous et al. 2016; Yuan et al. 2017), the supplementation of biotin is anticipated to spare dietary amino acids to sensitize insulin signaling and to sustain both lipid and protein synthesis via mTORC1 activation. In contrast, biotin accelerates insulin-induced adipogenesis by inhibiting autophagy. This uncouples adipocyte protein synthesis from lipogenesis via differential phosphorylation of mTORC1 substrates and ER stress without significant alterations in intracellular levels of amino acids.
Materials and methods
Cell culture and experimental design
The adipocyte primary culture was established as described by Negrel and Dani (2001). Briefly, retroperitoneal adipose tissue from male Wistar albino rats was digested with 2 mg/mL Type IV collagenase (3 mL/g tissue) in DMEM for 1 h at 37 °C. The filtered digest was centrifuged at 400×g for 10 min to pellet the stromal vascular fraction that was further re-suspended in DMEM containing 20% newborn calf serum and 1% penicillin-streptomycin-amphotericin mix. The re-suspended fraction was seeded in 12-well plates and incubated at 37 °C under 5% CO2. After 24 h of incubation, the cells were rinsed and maintained in DMEM containing 10% newborn calf serum and 1% penicillin-streptomycin-amphotericin mix until confluence. The confluent cultures were differentiated by adding 4 ng/mL of insulin and divided into four groups viz. control (C), biotin (B), avidin plus biotin (A + B), and avidin (A) with medium replacements every 48 h. The final concentrations of biotin and avidin were 2 μM and 0.2 μM, respectively. On the sixth day after differentiation, the cells were washed twice with ice-cold phosphate-buffered saline (PBS) to harvest samples for subsequent experiments. The protocols for animal maintenance and usage were approved by the Institutional Animal Ethics Committee.
Immunoblot analysis
The PBS-rinsed cells were lysed using cell lysis buffer containing protease and phosphatase inhibitors under ice-cold conditions. Protein concentrations of the lysates were determined based on Lowry’s protocol (Lowry et al. 1951), and equal amounts of proteins were resolved by SDS-PAGE. The proteins were transferred to 0.2 μm supported nitrocellulose membrane. The total protein on the membrane was stained with Ponceau S and imaged. The destained membrane was blocked with 5% skimmed milk powder (or fatty acid free-bovine serum albumin to detect phospho proteins) for 1 h at room temperature and probed overnight with 1:1000 diluted primary antibodies for the corresponding proteins of interest at 4 °C. Following this, the membranes were washed and incubated with the respective HRP-conjugated secondary antibodies for 45 min at room temperature. The bands were visualized using Super Signal West Femto Chemiluminescent Substrate. The signals were recorded by LI-COR Odyssey Fc imager and analyzed using Image studio software version 5.2. The signals were normalized against the total proteins across the lanes stained with Ponceau S.
Amino acids analysis using reverse-phase HPLC
Reverse-phase liquid chromatography was used to separate and quantify phenyl isothiocyanate (PITC) derivatized amino acids as per Okayasu et al. (1997) and Hariharan et al. (1993) with slight modifications. To ensure a uniform DNA concentration, the volumes of cell lysates were adjusted with cell lysis buffer containing norleucine as the internal standard. Briefly, 250 μl of total cell lysates were deproteinized by mixing with 166.6 μL of acetonitrile (60:40, v/v) and centrifuged at 9000 rpm for 1 min. Following centrifugation, 300 μl of the supernatant was dried in a vacuum centrifuge and treated with 10 μl of methanol/water/triethylamine (2:1:1, v/v) with subsequent mixing and vacuum drying. The vacuum-evaporated samples were further subjected to PITC derivitization by treatment with 20 μl of ethanol/water/triethylamine/PITC (7:1:1:1, v/v), mixing, incubation for 10 min, and vacuum drying. The samples were then re-suspended in 750 μl of sodium acetate (pH 7.5)-acetonitrile (98:2, v/v) and filtered through 0.2-μm filter before HPLC injection. HPLC analysis was performed using Agilent 1200 infinity HPLC system with auto-sample injection. The column for the HPLC separation was a C18 column (150 × 4.6 mm I.D., 3 μm) (Inertsil-ODS-2) thermostated at 41 °C with a simple multistep linear gradient of two solvents at a flow rate of 1.2 mL/min. Solvent A was 0.05 M sodium acetate (pH 5.1)-acetonitrile (98:2, v/v), and solvent B was water-acetonitrile (40:60, v/v). The detection of the separated PITC amino acid derivatives was done using UV detector at 254 nm. The efficacy of the protocol was validated by running an amino acid standard (Agilent 1200 infinity series, USA) following which random samples were spiked with 50 μl of the same standard to validate the peaks. The area under curve (AUC) values of the respective amino acid peaks were normalized against norleucine and used for relative quantification.
Oil Red O staining and estimation of triglycerides in adipocytes
The cellular lipid droplet content was determined by Oil Red O staining. Briefly, the PBS-rinsed cells were fixed using 4% para-formaldehyde at room temperature for 30 min. After fixation, the cells were rinsed thrice in PBS and stained with freshly diluted Oil Red O solution. The stained cells were washed with distilled water and imaged on a phase-contrast inverted microscope. Hematoxylin counterstain was used to discern the nucleus. The concentration of triglycerides in total cell lysates was determined based on a coupled enzymatic assay using ERBA kit, Germany. The DNA content of the samples was evaluated by using Quant-iT™ PicoGreen® dsDNA assay kit for normalization against the cell number.
Statistical analysis
The values are reported as average ± SD and differences across groups were determined using t test and one-way ANOVA followed by Tukey’s test for post hoc analysis.
Results and discussion
Biotin accelerates adipogenesis
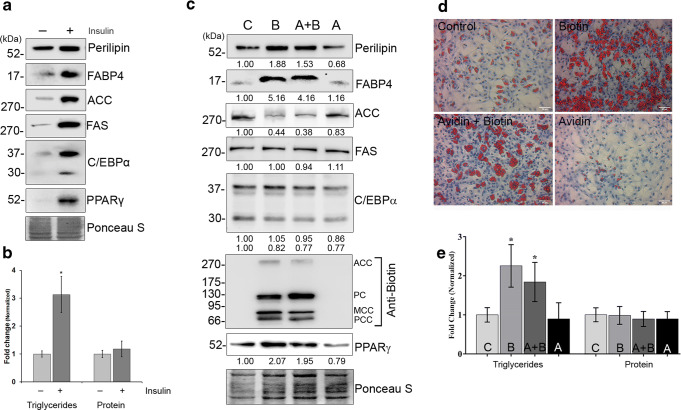
The increase in cellular triglyceride content and levels of adipogenic markers by insulin (Fig. 1a, b) supports its well-established role in adipogenesis (Sarjeant and Stephens 2012). However, the additional increase in lipogenesis by exogenous biotin demonstrates its ability to accelerate insulin-induced adipogenesis (Fig. 1c–e). The protein levels of ACC were decreased by biotin without altering fatty acid synthase (FAS) levels across treatments (Fig. 1c). To correlate this decrease, the biotinylated status of ACC was evaluated by immunoblot using anti-biotin antibody. Notably, the biotinylated carboxylases were detectable only in biotin-added groups (Fig. 1c) irrespective of decreased levels of ACC apoenzyme. This is in agreement with the earlier reports that the rats fed with biotin deficient diet for 2 weeks accumulate catalytically inactive apoenzyme of ACC in adipose tissue (Jacobs et al. 1970). Avidin treatment reduced the expression of perilipin and CCAAT-enhancer-binding proteinα (C/EBPα) without significant changes in triglyceride content as well as other markers of adipocyte differentiation compared to control. The endogenous biotin cycling (Hymes and Wolf 1999) possibly restricts biotin depletion to a significant extent during the experimental period.
Fig. 1.
Biotin accelerates insulin induced adipogenesis. [a] Differentiation potential under basal and insulin-stimulated conditions of cultured primary cells was determined using immunoblot by targeting the markers of adipocyte differentiation. [b] Accumulation of triglycerides during adipocyte differentiation. The values were normalized against cellular DNA content and those with p < 0.05 were considered significant. [c] Effects of biotin on lipogenesis were determined by immunoblotting against the markers of adipocyte differentiation. Total proteins were stained using Ponceau S to normalize protein loading. The values were denoted as fold changes of three independent experiments. [d] The phase contrast microscopic images of ρ-formaldehyde fixed cells were stained with oil red O (red) and hematoxylin (blue) to detect lipid droplets and nucleus, respectively (bar 50 μm). [e] Total cell lysates from day 6 of adipocyte differentiation were used to determine the cellular triglyceride and protein content. The values were normalized against their respective DNA content to minimize the variations in cell lysis or loading and represented as fold changes with respect to control (n = 5). *p < 0.05 versus control groups
Biotin inhibits autophagy to accumulate lipid droplets
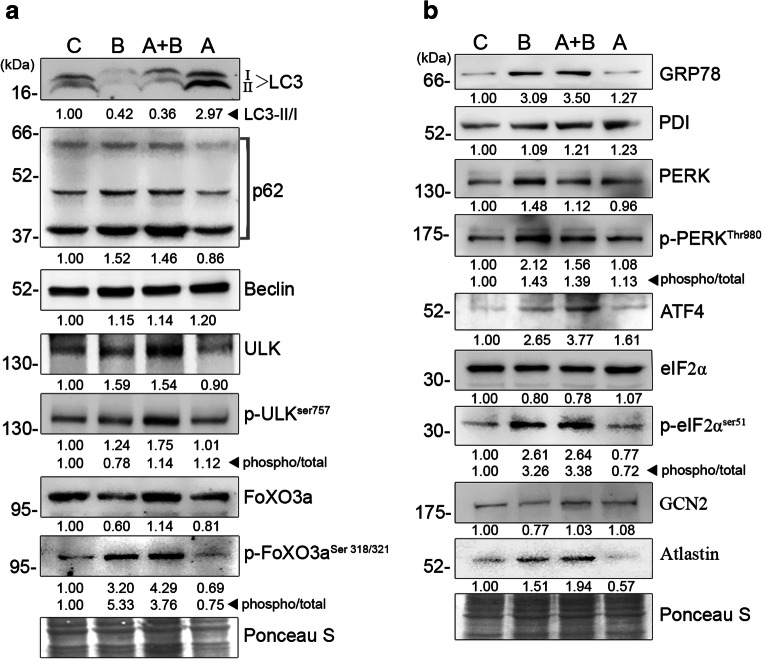
Autophagy is involved in organelle and macromolecular turnover by regulating their trafficking for lysosomal degradation. Since lipid droplets are considered as intracellular organelles (Martin and Parton 2006), the autophagic events were monitored in order to correlate the observed increase in lipid content by biotin. The Unc-51 like autophagy activating kinase 1 (Ulk-1) plays a critical role in initiation of autophagosome formation, and its phosphorylation at serine 757 by mTORC1 inhibits autophagy (Kim et al. 2011). However, the observed increase in total and phosphorylated levels of Ulk-1 by biotin maintains its active form similar to other groups (Fig. 2a). This necessitates the determination of the maturation of autophagosomes by targeting lipidation of cytosolic microtubule-associated protein 1A/1B-light chain 3 (LC3-I) to form LC3-II. The ratio of LC3-II to LC3-I as well as the total levels of LC3-I was reduced by biotin (Fig. 2a). Since Forkhead box O3 (FoXO3) regulates the gene expression of LC3-I and its phosphorylation by Akt decreases its transcriptional activity due to nuclear exit (Zhao et al. 2007), the status of Akt and FoXO3 was assessed to monitor the upstream events of autophagy. The increase in total Akt and its phosphorylation (Fig. 3) is associated with a 5.3-fold elevation in the phosphorylation of FoXO3 in biotin-treated groups (Fig. 2a). This observed reduction in autophagosome content by biotin is correlated with reduced autophagic activity as evidenced by the accumulation of a complex reporter of autophagic flux, p62 (Fig. 2a). Furthermore, the inhibition of lysosomal activity using lysosomtropic agents stimulates lipogenesis (Chen et al. 1986), and the turnover of biotinylated carboxylases also occurs predominantly via autophagy (Chandler and Ballard 1985). Therefore, the observed decrease in autophagy is also attributed to elevated levels of lipid accumulation by biotin.
Fig. 2.
Biotin elicits ER stress via autophagy inhibition in adipocytes: Effect of biotin on ER stress and autophagy were determined using immunoblot against markers and transcriptional regulators of [a] autophagy and [b] ER stress. On the sixth day of differentiation, cell lysates for immunoblot were prepared using cell lysis buffer containing protease and phosphatase inhibitors cocktail. Nitrocellulose membranes were stained with Ponceau S for total protein to normalize protein loading. The values were expressed as average fold changes of three independent immunoblot experiments. The ratio of phosphorylated versus total levels of respective proteins indicates their phosphorylation status
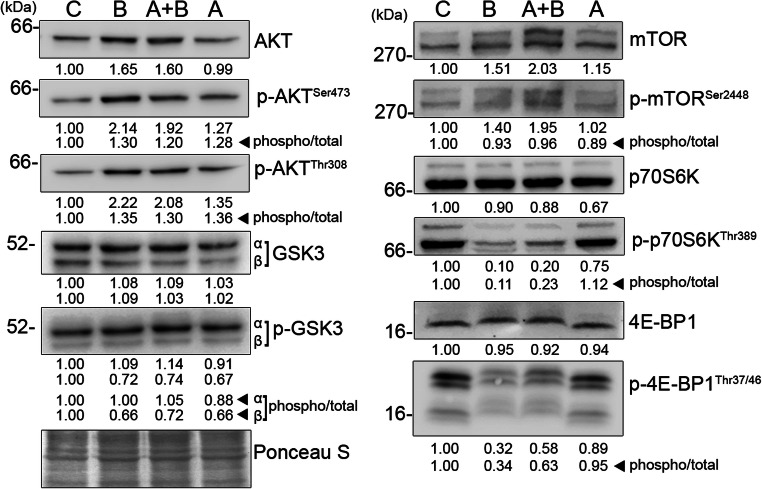
Fig. 3.
Biotin uncouples mTORC1-dependent protein synthesis. Effect of biotin on upstream and downstream candidates of mTORC1 signaling were determined using immunoblot. Total cell lysates for immunoblot were prepared on the sixth day of differentiation using cell lysis buffer containing protease and phosphatase inhibitors cocktail. Equal amount of protein were resolved by SDS-PAGE and transferred to nitrocellulose membrane. The Ponceau S-stained membranes were used to normalize protein loading. The values were expressed as average fold changes of three independent experiments. The ratio of phosphorylated versus total levels of respective proteins indicates their phosphorylation status
Biotin uncouples mTORC1-dependent protein synthesis
The inhibition of autophagy necessarily accumulates not only lipids but also proteins (Zhao et al. 2015). However, unlike triglyceride levels, the total protein content was not altered by biotin (Fig. 1e). This demands the elucidation of the signaling pathways upstream of protein synthesis, in particular mTORC1 due to its effect on both autophagy and protein synthesis. The addition of biotin decreased the phosphorylation of 4E-BP1 and p70S6K irrespective of total increase in mTOR and its phosphorylation at serine 2448 residue (Fig. 3). In contrast, the maintenance of mTORC1-dependent Ulk-1ser757 phosphorylation (Fig. 2a) suggests that the mTORC1 substrates undergo differential regulation in adipocytes. This differential phosphorylation can be envisaged by a progressive competition among mTORC1 substrates due to a total increase in Ulk-1 levels (Fig. 2a). mTORC1-dependent phosphorylation of 4E-BP1 and p70S6K is decreased in the cells overexpressing Ulk-1 by manipulating the ability of Raptor to interact with its substrate, 4E-BP1 (Dunlop et al. 2011). The dephosphorylated 4E-BP1 inhibits cap-dependent protein synthesis by sequestering eukaryotic initiation factor 4E by direct interaction (Matsuo et al. 1997). Furthermore, the manipulation of protein synthesis during adipogenesis is exemplified by higher levels of 4E-BP1 in white adipose tissue as well as reduced adiposity in 4E-BP1 knockout mice (Tsukiyama-Kohara et al. 2013).
Biotin manipulates ER stress to regulate protein synthesis
Autophagy regulates endogenous amino acid availability as well as ER stress by recycling damaged/long-lived proteins (Ogata et al. 2006). GCN2 and PERK downregulate protein synthesis via eIF2α phosphorylation in response to amino acid deprivation and ER stress, respectively (Wek et al. 2006). Biotin contributed towards the increase in eIF2αser51 phosphorylation and elevated levels of activating transcription factor 4 (Fig. 2b) that is a preferential translational target for phosphorylated eIF2α (Harding et al. 2003). To determine the role of ER stress on eIF2α phosphorylation, PERK and its associated ER chaperone glucose-regulated protein 78 (GRP78) levels were determined. The levels of GRP78 were found to be higher in biotin-treated cells without major changes in total PERK levels. However, PERK undergoes dimerization and autophosphorylation at serine 980 residue once dissociated from GRP78 (Ma et al. 2002). Therefore, the increase in phosphorylation of PERKser980 by biotin confirms the existence of ER stress signaling during adipocyte differentiation. Previous studies have also shown that ER stress activates fatty acid synthesis via 5′-cap-independent translation of sterol regulatory element-binding protein 1c mRNA by heterogenous nuclear ribonucleoprotein A1 (Damiano et al. 2010; Damiano et al. 2013; Fang et al. 2013). On the contrary, the knockdown of GRP78 attenuates adipogenesis (Zhu et al. 2013). Together, the adipocyte differentiation elicits ER stress to uncouple adipocyte protein synthesis.
Since ER plays a critical role in protein and lipid synthesis, the observed selective inhibition of protein synthesis suggests the possibility of ER modifications to maintain the adipocyte lipogenic phenotype. It is demonstrated that expansion of ER membrane by promoting lipid synthesis alleviates ER stress independent of unfolded protein response (Schuck et al. 2009). The dynamin-related membrane GTPase, Atlastin, regulates ER network formation by carrying out homotypic ER fusion, and its loss limits ER expansion by undergoing fragmentation (Orso et al. 2009). Furthermore, Atlastins influence lipid droplet size and total triglyceride content of an organism (Klemm et al. 2013), possibly, due to their effect on ER fusion and the close association between ER and lipid droplets (Blanchette-Mackie et al. 1995). Therefore, the observed increase in Atlastin levels by biotin (Fig. 2b) supports the possibility of promoting lipogenesis by manipulating ER stress. Nevertheless, the ultrastructural analysis of ER is warranted for further validation.
Biotin alters intracellular amino acid profile
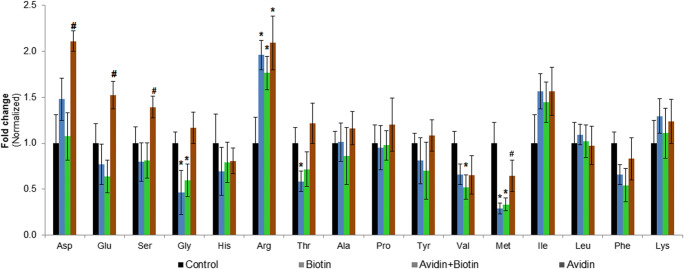
Though GCN2 targets eIF2α phosphorylation, the requirement of GCN2 dimerization in an anti-parallel manner during amino acid scarcity (Dey et al. 2007) restricts the discussion of the role of GCN2 solely based on the available immunoblot data (Fig. 2b). Alternatively, the intracellular amino acid profile was determined using HPLC to indirectly evaluate the role of GCN2 on protein synthesis. The total amino acid levels did not vary across treatments (Supplementary Fig. 1); however, the relative decrease in at least one amino acid per group (Fig. 4) restricts us to discuss the role of GCN2 as an upstream regulator of eIF2α phosphorylation.
Fig. 4.
Biotin alters the intracellular levels of certain amino acids. Reverse-phase HPLC was carried out determine the intracellular levels of amino acids. The cells were lysed on the sixth day of differentiation, and the lysate volumes across samples were adjusted to a uniform DNA concentration using cell lysis buffer containing norleucine as internal standard. Equal volumes of lysate were delipidated, deproteinized, and PITC-derivatized for amino acid analysis using HPLC. The AUC value of each analyte was normalized against norleucine and subjected to relative quantification. Symbols * and # indicate a p value < 0.05 significance compared with control and biotin-treated group (n = 4), respectively
In summary, autophagy inhibition by biotin accelerates insulin-induced adipogenesis. The compensatory increase in Ulk-1 possibly interferes with 4E-BP1 and p70S6K phosphorylation by mTORC1. Furthermore, the decline in autophagy elicits ER stress to inhibit protein synthesis via eIF2α phosphorylation. Though the levels of certain amino acids are decreased, its association with differential regulation of mTORC1 and GCN2 is complex. Since autophagosomes deliver substrates to lysosomes, we envisage that the altered lysosomal flux manipulates mTORC1 localization in order to differentially recognize its phosphorylation substrates.
Electronic supplementary material
(PNG 1417 kb)
Acknowledgements
We would also like to acknowledge DBT-BUILDER program (BT/PR12153/INF/22/200/2014) for providing HPLC instrumentation and Dr. S. Meenakshisundaram’s research group for their assistance with HPLC analysis. The authors SRS and ARS acknowledge ICMR, Government of India for Senior Research Fellowship award.
Funding information
This study was supported in part by grants-in-aid for research from DST-SERB (SR/S0/HS/0051/2012 and EMR/2016/003276).
Compliance with ethical standards
The protocols for animal maintenance and usage were approved by the Institutional Animal Ethics Committee.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Averous J, Lambert-Langlais S, Mesclon F, Carraro V, Parry L, Jousse C, Bruhat A, Maurin AC, Pierre P, Proud CG, Fafournoux P. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci Rep. 2016;6:1–10. doi: 10.1038/srep27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. doi: 10.1016/S0022-2275(20)41129-0. [DOI] [PubMed] [Google Scholar]
- Boeckx RL, Dakshinamurti K. Biotin-mediated protein biosynthesis. Biochem J. 1974;140:549–556. doi: 10.1042/bj1400549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CS, Ballard FJ. Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem J. 1985;232:385–393. doi: 10.1042/bj2320385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GL, Sutrina SL, Frayer KL, Chen WW. Effects of lysosomotropic agents on lipogenesis. Arch Biochem Biophys. 1986;245:66–75. doi: 10.1016/0003-9861(86)90190-6. [DOI] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamurti K, Desjardins PR. Lipogenesis in biotin deficiency. Can J Biochem. 1968;46:1261–1267. doi: 10.1139/o68-189. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Modi VV, Mistry SP. Some aspects of carbohydrates metabolism in biotin-deficient rats. Proc Soc Exp Biol Med. 1968;127:396–400. doi: 10.3181/00379727-127-32699. [DOI] [PubMed] [Google Scholar]
- Damiano F, Alemanno S, Gnoni GV, Siculella L. Translational control of the sterol-regulatory transcription factor SREBP-1 mRNA in response to serum starvation or ER stress is mediated by an internal ribosome entry site. Biochem J. 2010;429:603–612. doi: 10.1042/BJ20091827. [DOI] [PubMed] [Google Scholar]
- Damiano F, Rochira A, Tocci R, Alemanno S, Gnoni A, Siculella L. hnRNP A1 mediates the activation of the IRES-dependent SREBP-1a mRNA translation in response to endoplasmic reticulum stress. Biochem J. 2013;449:543–553. doi: 10.1042/BJ20120906. [DOI] [PubMed] [Google Scholar]
- Dey M, Cao C, Sicheri F, Dever TE. Conserved intermolecular salt bridge required for activation of protein kinases PKR, GCN2, and PERK. J Biol Chem. 2007;282:6653–6660. doi: 10.1074/jbc.M607897200. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop EA, Hunt DK, Acosta-Jaquez HA, Fingar DC, Tee AR. ULK1 inhibits mTORC1 signaling, promotes multisite raptor phosphorylation and hinders substrate binding. Autophagy. 2011;7:737–747. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis T, Pissas G, Antoniadi G, et al. Differential effects of the two amino acid sensing systems, the GCN2 kinase and the mTOR complex 1, on primary human alloreactive CD4+T-cells. Int J Mol Med. 2016;37:1412–1420. doi: 10.3892/ijmm.2016.2547. [DOI] [PubMed] [Google Scholar]
- Fang DL, Wan Y, Shen W, Cao J, Sun ZX, Yu HH, Zhang Q, Cheng WH, Chen J, Ning B. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol Cell Biochem. 2013;381:127–137. doi: 10.1007/s11010-013-1694-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hariharan M, Naga S, VanNoord T. Systematic approach to the development of plasma amino acid analysis by high-performance liquid chromatography with ultraviolet detection with precolumn derivatization using phenyl isothiocyanate. J Chromatogr. 1993;621:15–22. doi: 10.1016/0378-4347(93)80071-B. [DOI] [PubMed] [Google Scholar]
- Hymes J, Wolf B. Human biotinidase isn’t just for recycling biotin. J Nutr. 1999;129:485S–489S. doi: 10.1093/jn/129.2.485S. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Kilburn E, Majerus PW. Acetyl coenzyme a carboxylase. The effects of biotin deficiency on enzyme in rat liver and adipose tissue. J Biol Chem. 1970;245:6462–6467. doi: 10.1016/S0021-9258(18)62631-6. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, Norton JP, Cole RA, Li CS, Park SH, Crane MM, Li L, Jin D, Boye-Doe A, Liu TY, Shibata Y, Lu H, Rapoport TA, Farese RV, Jr, Blackstone C, Guo Y, Mak HY. A conserved role for Atlastin GTPases in regulating lipid droplet size. Cell Rep. 2013;3:1465–1475. doi: 10.1016/j.celrep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W, Wise LS, Green H. Interruption of the adipose conversion of 3T3 cells by biotin deficiency: differentiation without triglyceride accumulation. Cell. 1978;14:53–59. doi: 10.1016/0092-8674(78)90300-8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Lynen F. The role of biotin-dependent carboxylations in biosynthetic reactions. Biochem J. 1967;102:381–400. doi: 10.1042/bj1020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277:18728–18735. doi: 10.1074/jbc.M200903200. [DOI] [PubMed] [Google Scholar]
- Martin S, Parton RG. Lipid droplets: a unified view of dynamic organelle. Mol Cell. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Li H, McGuire AM, et al. Structure of translation factor elF4E bound to m7GDP and interaction with 4E-binding protein. Nat Struct Biol. 1997;4:717–724. doi: 10.1038/nsb0997-717. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Autophagy: a sweet process in diabetes. Cell Metab. 2008;8:275–276. doi: 10.1016/j.cmet.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Negrel R, Dani C. Cultures of adipose precursor cells and cells of clonal lines from animal white adipose tissue. Methods Mol Biol. 2001;155:225–237. doi: 10.1385/1-59259-231-7:225. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu T, Ikeda M, Akimoto K, Sorimachi K. The amino acid composition of mammalian and bacterial cells. Amino Acids. 1997;13:379–391. doi: 10.1007/BF01372601. [DOI] [Google Scholar]
- Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem. 2005;280:31582–31586. doi: 10.1074/jbc.M506736200. [DOI] [PubMed] [Google Scholar]
- Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A. Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature. 2009;460:978–983. doi: 10.1038/nature08280. [DOI] [PubMed] [Google Scholar]
- Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Katsume A, Kimura K, Saito M, Kohara M. 4E-BP1 regulates the differentiation of white adipose tissue. Genes Cells. 2013;18:602–607. doi: 10.1111/gtc.12059. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang H-Y, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wong PM, Feng Y, Wang J, Shi R, Jiang X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat Commun. 2015;6:8048. doi: 10.1038/ncomms9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Guo S, Gao J, Zhong M, Yan G, Wu W, Chao Y, Jiang Y. General control nonderepressible 2 (GCN2) kinase inhibits target of rapamycin complex 1 in response to amino acid starvation in saccharomyces cerevisiae. J Biol Chem. 2017;292:2660–2669. doi: 10.1074/jbc.M116.772194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Düvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the Autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhai B, Gygi SP, Goldberg AL. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci U S A. 2015;112:15790–15797. doi: 10.1073/pnas.1521919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ye R, Jung DY, Barron E, Friedline RH, Benoit VM, Hinton DR, Kim JK, Lee AS. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013;27:955–964. doi: 10.1096/fj.12-213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 1417 kb)