Abstract
The mechanism of atrial fibrillation (AF) in patients with normal heart remains unclear. While exogenous adenosine can trigger AF, nothing is known about the behavior of endogenous adenosine plasma level (APL) at the onset of AF and during ablation procedure. Ninety-one patients (68 with paroxysmal AF: 40 males, 66 ± 16 years; 23 with persistent AF: 14 males, 69 ± 11 years) and 18 controls were included. Among paroxysmal patients: i) medical therapy alone was performed in 45 cases and ablation procedure in 23. AF was spontaneously resolutive in 6 cases; ii) 23 underwent ablation procedure and blood was collected simultaneously in a brachial vein and in the left atrium; 17 were spontaneously in sinus rhythm while 6 were in sinus rhythm after direct current cardioversion. Among persistent patients: i) in 17 patients, blood samples were collected in a brachial vein before and after direct current cardioversion; ii) in 6 patients, blood samples were collected simultaneously in a brachial vein and in left atrium before and after cardioversion during ablation procedure. CV-APL was higher in patients with persistent AF vs patients with paroxysmal AF (median [range]: 0.9[0.6–1.1] vs 0.7[0.4–1.1] μM; p < 0.001). In patients with paroxysmal AF, LA-APL increased during the AF episode (0.95[0.85–1.4] vs 2.7[1.5–7] μM; p = 0.03) and normalized in sinus rhythm after DCCV. In patients with persistent AF, LA-APL was higher than CV-APL (1.2[0.7–1.8] vs 0.9[0.6–1.1] μM; p < 0.001), and both normalized in sinus rhythm (CV-APL: 0.8[0.6–1.1] vs 0.75[0.4–1] μM; p = 0.03), (LA-APL: 1.95[1.3–3] vs 1[0.5–1.15] μM; p = 0.03). The occurrence of AF is associated with a strong increase of APL in the atrium. The cause of this increase needs further investigations.
Keywords: Adenosine, Atrial fibrillation, Ablation procedure
Introduction
Although atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, the precise mechanism that leads to the onset and persistence of AF in patients without structural heart disease has not been fully elucidated [1]. Several arguments for the involvement of adenosine are rising in the literature. Adenosine is partly generated via the ENTPD1-mediated hydrolysis of ATP. The nucleoside impacts the cardiovascular system via four membrane receptors namely A1R, A2AR, A2B R, and A3R receptors, pending on their pharmacological properties [2]. Exogenous adenosine administration can provoke spontaneous AF in up to 10% of susceptible patients [3, 4], and during AF ablation procedures, exogenous administration of adenosine induces transient reconnection of isolated pulmonary veins (PV) [5–7]. Moreover, exogenous adenosine can induce PV ectopic beats in electrically silent PV after isolation. [8] However, endogenous adenosine has not been addressed during AF. In particular, nothing is known about endogenous adenosine plasma level (APL) at the onset of AF and during ablation procedure and/or cardioversion in AF patients without structural heart disease. The aim of this study was to compare APL during AF vs sinus rhythm.
Methods
Population
We prospectively included patients referred in our center from November 2016 to November 2017 for specific management of AF. AF was characterized as paroxysmal or persistent according to recent ESC guidelines [9]. Paroxysmal AF patient (PAF) should have experienced at least one episode of AF within the last 12 months. According to the AF management strategy, patients were then classified in four groups: PAF with an indication of antiarrhythmic drugs only, PAF with an indication of catheter ablation, persistent AF patients (PeAF) with indication of direct current cardioversion, and PeAF with an indication of catheter ablation. Patients with valvular heart disease, previous acute coronary syndrome, previous cardiac surgery or coronary angioplasty, left ventricular dysfunction (LVEF < 50%), left ventricular hypertrophy > 13 mm, previous AF catheter ablation, previous pulmonary embolism, or younger than 18 years were excluded from the study. Other supra-ventricular tachycardia as atrial flutter or atrial tachycardia were not included. The control group was composed of healthy subjects matched for age and sex, recruited among the medical staff, without any compromised hemodynamic, underlying suspicion of cardiac abnormality, confirmed by a normal electrocardiogram, normal trans-thoracic echocardiogram. Those subjects did not have relevant medical history, cardiovascular risk factor, or medical treatment. Written informed consents were obtained from all the participants. This study complies with the declaration of Helsinki. The study was approved by the ethical committee of the Timone University hospital.
Cardiac investigation
Diagnosis of AF was made on 12 lead ECG. At least one ECG in AF should be documented for each patient. All patients had trans-thoracic echocardiogram. Left ventricular ejection fractions were calculated with modified Simpson’s method. According to modified Simpson’s biplane method, left atrium size was classified as normal, slightly or severely enlarged. Corresponding indexed left atrium volume were < 30 ml/m2, < 40 ml/m2, and > 40 ml/m2, respectively.
APL measurement
Blood samples (3 mL) were collected and processed as described previously, [10] using laboratory-prepared tubes containing 2 mL of stop solution under vacuum. Samples collection method allows whole blood to mix quickly with the stop solution in order to prevent red blood cell uptake and degradation of adenosine. APL was performed as previously described [10] by liquid chromatography-tandem mass spectrometry after extraction, using a Shimadzu UFLC XR system (Shimadzu, Marne la Vallée, France). The system was interfaced with an ABSciex 4500 triple quadrupole mass spectrometer (Les Ulis, France) operating with an electrospray ionization source using nitrogen. Liquid chromatography-mass spectrometry-grade methanol and water were purchased from VWR (Fontenay-sous-Bois, France). Formic acid, adenosine, and 2-chloroadenosine were obtained from Sigma Chemical (Saint-Quentin Fallavier, France). Whatman 903 protein saver cards™ for sample collection and preparation were acquired from GE Healthcare (Cardiff, UK).
Statistical analysis
Statistical analyses were performed using the SPSS software for Windows 13.1. Quantitative variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR), and qualitative variables as numbers and percentages. We used a Wilcoxon test for intra-individual comparison; A variance analysis (ANOVA two ways) was used for intergroup comparisons. Differences in the distribution of patient’s characteristics were tested using the Mann–Whitney non-parametric test (continuous variables) or using the χ2 or, where appropriate, the Fisher’s exact test (categorical variables). Two-sided tests were performed and p value < 0.05 was considered as significant.
Results
Among the 91 patients with AF, 68 were PAF and 23 PeAF. Their clinical characteristics are given in Table 1.
Table 1.
Characteristics of the patients
| Patients characteristics | Paroxysmal: n = 68 | Persistent: n = 23 | Controls: n = 18 |
|---|---|---|---|
| Age (years) | 66 ± 16 | 69 ± 11* | 61 ± 9 |
| Gender (M/W) | 40/28 | 14/9* | 12/6 |
| Weight (Kg) | 75 ± 12 | 84 ± 15* | 79 ± 11 |
| Size (cm) | 169[10] | 173[7.8]* | 167 ± 8 |
| Arterial hypertension (%) | 34 | 39* | – |
| Diabetes (%) | 18 | 21* | – |
| Time since the first AF episode (months) | 7.4 ± 4.3 | 6.4 ± 4.2* | – |
| Creatinemia (μmol/L) | 91 ± 17 μM | 94 ± 11* | – |
| Dyslipidemia (%) | 50 | 48* | – |
| Echocardiography | |||
| Left atrial diameter (mm) | 40.8 ± 2.6 | 41 ± 3.5* | 40.1 ± 1.9 |
| Left atrial volume index (ml/m2) | 29.6 ± 8.9 | 28.4 ± 9.8* | 29. 3 ± 8.7 |
| LVEF (%) | 61.4 ± 4* | 56 ± 13** | 59 ± 2 |
| Pharmacological treatment (%) | |||
| ACE inhibitors | 24 | 22* | – |
| Cordarone | 54 | 56* | – |
| Others antiarrhythmic drugs | 43 | 48* | – |
| Angiotensin receptor blocker | 12 | 11* | – |
| Beta-blockers | 26 | 21* | – |
| Aspirin | 16 | 18* | – |
| Statins | 50 | 48* | – |
| Diuretics | 11 | 13* | |
| Vitamin K antagonist | 24 | 22* | |
| NOAC | 64 | 77* | |
LVEF, left ventricular ejection fraction; NOAC, non-antivitamin K antagonist oral anticoagulants; M/W, men/women; ACE, angiotensin converting enzyme. Values are given as mean ± standard deviation, number of patients, or percentage (%). * p > 0.1 compared with paroxysmal; ** p = 0.03 compared with paroxysmal
No significant difference appeared between patients groups and controls concerning age and sex. No significant difference was found between PAF and PeAF concerning treatment (Table 1).
Blood samples collection timing
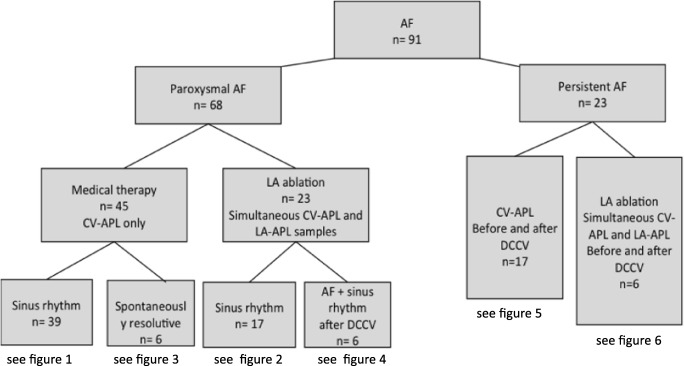
Blood samples were collected from a cubital vein (CV-APL) in all patients and controls. According to Fig. 1, all PAF had blood sample collection during sinus rhythm. Among those patients, 12 (6 medical therapies and 6 LA ablation strategies) had blood collection also during AF. All PeAF (n = 23) had blood collection during AF and after cardioversion. Finally, blood samples were collected simultaneously in CV and LA (LA-APL) in 29 patients (23 PAF and 6 PeAF) at the beginning of ablation procedure. Among PAF, six had AF documented during ablation, either induced by catheter movement or spontaneously induced. In these cases, blood samples were collected in sinus rhythm and during AF and after sinus rhythm restoration with direct current cardioversion (DCCV).
Fig. 1.
Flowchart of patients. AF: atrial fibrillation; DCCV: direct current cardioversion; CV-APL: adenosine plasma level (APL) in blood samples collected from cubital vein; LAAPL: APL in samples collected in left atrium. NB: in the medical therapy group, 6 patients among 45 had spontaneous resolutive AF during their hospitalization. The others (39 patients) were collected only in sinus rhythm.
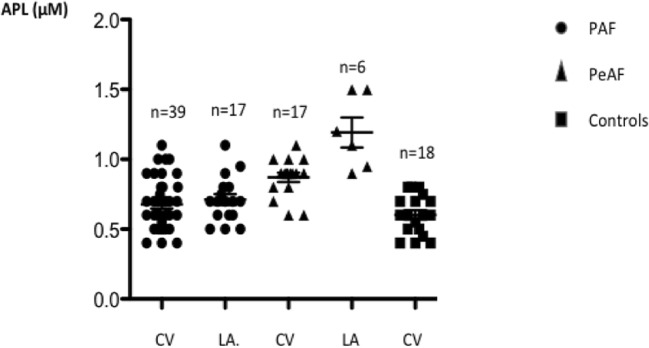
Basal APL
Basal APL (Fig. 2) was defined as APL evaluated at the time of hospital admission. CV-APL was higher in PeAF vs PAF (median [range]: 0.9[0.6–1.1] vs 0.7[0.4–1.1] μM; p < 0.001) and compared with controls (0.6[0.4–0.8] μM; p < 0.001). No significant difference exists between PAF and controls (p = 0.13). In PeAF, LA-APL was higher compared with CV-APL (1.15[0.9–1.5] vs 0.9[0.6–1.1] μM, p < 0.01). Among PAF patients, 34 (50%) and 12 (48%) were treated using statins. No significant difference in APL was observed between the two groups. Patients under statins vs others: PAF: 0.7[0.5–1] vs 0.65[0.4–1.1], p = 0.8; PeAF: 0.9[0.6–1.1] vs 0.9[0.6–1], p = 0.64.
Fig. 2.
Adenosine plasma level (APL) measured in blood samples collected from cubital vein (CV) or in left atrium (LA) in patients suffering from paroxysmal (PAF) or persistent atrial (PeAF) fibrillation
APL during spontaneous AF episode resolution (Fig. 3)
Fig. 3.
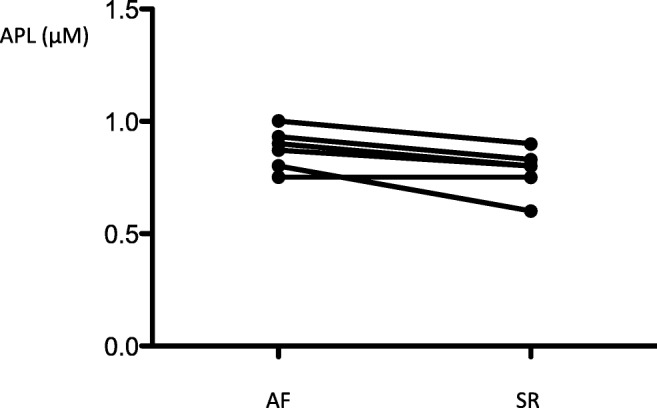
Adenosine plasma level (APL) measured in blood samples collected from a cubital vein during an atrial fibrillation (AF) episode in paroxysmal patients (n = 6) who spontaneously reduced (SR: sinus rhythm)
In 6 PAF, the AF episode occurred spontaneously during hospitalization and spontaneously resolved. For these patients, we observed a trend in decrease in CV-APL after spontaneous resolution (0.88[0.75–1] vs 0.8[0.6–0.9] μM; p = 0.057).
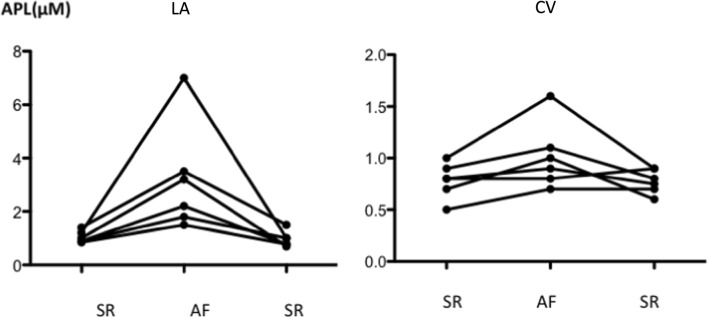
PAF APL during ablation procedure (Fig. 4)
Fig. 4.
Adenosine plasma level (APL) measured before (basal in sinus rhythm), during (AF) and after DCCV (recovery, in sinus rhythm (SR)) an atrial fibrillation episode occurring during an ablation procedure in paroxysmal patients (n = 6). Blood samples were collected both in left atrium (LA) and from a cubital vein (CV). AF episode duration was 10 min
In 6 PAF patients, LA-APL increased during AF episode (median [range]: 0.95[0.85–1.4] vs 2.7[1.5–7] μM; mean + 320%; range 76–420%; p = 0.03) and returned to basal values (0.95[0.85–1.4] vs 0.89[0.7–1.5] μM; p = 0.8) after DCCV (Fig. 4; left atrium (LA): left panel). A similar trend occurred for CV-APL (0.8[0.5–1] vs 0.95[0.7–1.6] μM; p = 0.06) (Fig. 4; cubital vein (CV): right panel). All those patients but one needed only one DCCV to return to sinus rhythm whereas AF lasted more than 5 min. The sixth one who presented the highest APL increase had monthly AF episodes lasting couple of hours in spite of anti-arrhythmic treatment before LA ablation. At the beginning of the procedure, an AF episode was mechanically induced during catheter positioning. It was subsequently not possible to restore sinus rhythm despite five DVVC. Sinus rhythm was finally recovered after the sixth DCCV delivered at the end of the procedure of pulmonary vein isolation. In this population, excluding this patient, the increase in LA-APL remains significant: median [range]: 0.9[0.85–1.4] vs 2.2[1.5–3.5] p = 0.04.
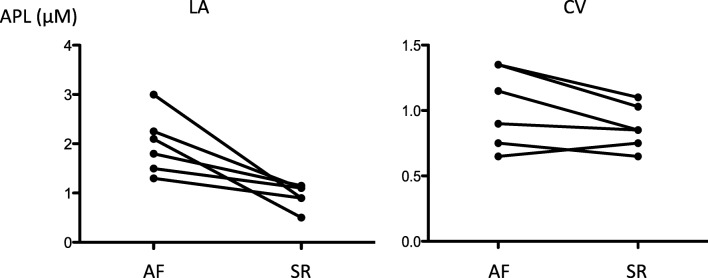
PeAF APL (Figs. 5 and 6)
Fig. 5.
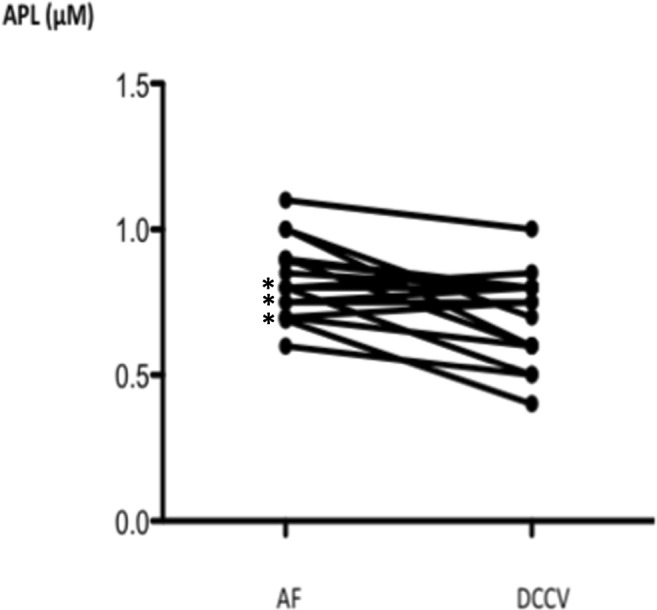
Adenosine plasma level (APL) was measured in samples collected from a cubital vein in patients (n = 17) suffering from persistent AF. Samples were collected during atrial fibrillation (AF) and 24 h after a direct current cardioversion (DCCV), in sinus rhythm (SR). *: weak APL increase (n = 3)
Fig. 6.
Adenosine plasma level (APL) measured in blood samples collected in left atrium (A) and from a cubital vein (B) in 6 patients suffering from persistent AF. Samples were collected during AF (AF) and 24 h after a direct current cardioversion (DCCV), in sinus rhythm (SR)
Samples were collected during AF and 24 h after DCCV in sinus rhythm. In 17 PeAF (Fig. 5), a decrease in CV-APL was observed during the DCCV procedure (0.8[0.6–1.1] vs 0.75[0.4–1] μM; p = 0.03). Of note, a weak increase of APL was observed in 3 patients (17%). In 6 cases (Fig. 6), LA-APL decreased strongly after LA ablation (1.95[1.3–3] vs 1[0.5–1.15] μM; p = 0.03; A) while no significant change was observed in CV-APL (1.02[0.65–1.35] vs 0.85[0.65–1.1] μM; p = 0.11; B).
Discussion
The main findings of this study are as follows: i) CV-APL was higher in PeAF compared with PAF and normalized in sinus rhythm after DCCV; ii) in PeAF, LA-APL was higher than CV-APL; iii) no significant difference in CV-APL was found between PAF and controls when blood samples were collected in sinus rhythm; iv) a strong increase in LA-APL was observed in PAF during the AF episode and LA-APL normalized in sinus rhythm; v) finally, high CV-APL was observed during spontaneous paroxysmal AF episode and normalized in sinus rhythm.
Several arguments already support a role of adenosine in AF onset: i) intravenous administration of adenosine can trigger AF in susceptible patients; [4] ii) while ectopic beats originating from PVs can initiate AF, explaining why PV isolation is the cornerstone of AF, [8] adenosine injection can transiently reveal PV reconnection in case of dormant conduction after catheter ablation; [5] iii) high APL was observed during AF in patients during cardiac surgery and this increase in APL, [11] which may trigger AF, results from hypoxia that was observed during the coronary bypass. Here, we included only patients without structural heart disease to avoid cardiovascular factors like chronic heart failure, coronary artery disease or other disorders that may interfere with the release of adenosine.
At the cellular level, both adenosine-dependent potassium and calcium currents have been implicated in the triggering of AF. By increasing potassium conductance through activation of inward rectifier channels (IKAdo), [12] adenosine shortens action potential duration and refractoriness, which favors AF [13, 14]. These potassium currents are strongly modulated by A1 R activation [12, 15]. In addition, it was recently found an heterogeneity in A1 R expression in human atrium and this may participate in the adenosine-induced AF episode [15]. An upregulation of A2AR associated with high basal level of sarcoplasmic reticulum calcium release has been also found in atrium of patients with AF [16], suggesting that A2A R is implicated in AF.
In a meta-analysis, it was shown that low doses of caffeine, a non-selective adenosine receptors antagonist, can protect against AF while theophylline administration [16], another non-selective adenosine antagonist, can reduce AF secondary to myocardial infarction via A1 R blockade [17]. Thus, both A1 and A2A R could be implicated in adenosine-induced AF.
Here we found high LA-APL in persistent AF (or in paroxysmal AF during the AF episode) and these levels normalized in sinus rhythm suggesting that adenosine release in atrium occurs during AF. We can rule out confounding factors such as drugs, DCCV, or ablation consequences in the increase of APL during AF because: i) this increase was strictly simultaneous with the AF episode; ii) APL returned to normal values in sinus rhythm even in the case of spontaneous resolution; iii) it is unlikely that drug concentration (sedative, anticoagulants, or antiarrhythmics) varies during the procedure. It was shown that statins enhance ENTPD1 expression and can therefore impact APL [18]. However we did not find significant difference in basal APL in patients under statins vs others.
We also found high APL in samples collected in CV in patients with persistent AF and a similar trend was found in PAF during the AF episode. It is unlikely that the higher APL measured at the peripheral level (CV-APL) resulted from atrium release, because the atrium volume is too small to influence the CV-APL. We hypothesized that the increase in CV-APL during the AF episode may result from the decrease in cardiac output that occurs during AF and generates tissue hypoxemia [19]. Yet, a decrease in cardiac output is associated with an increase in CV-APl [20]. We also observed a strong increase in APL mostly in left atrium during the triggering of AF in PAF and APL subsequently normalized in sinus rhythm. Thus, we concluded that high in situ, atrium, APL is associated with AF.
AF is the most frequently encountered arrhythmia and is associated with an increase in morbidity including heart failure, systemic embolism, and stroke [21]. Classical anti-arrhythmic drugs are often ineffective. Thus, new innovative, non-invasive treatments are needed. Among them, xanthine derivatives that act as antagonists of adenosine receptors may be promising, which is supported by the observation that the incidence of AF decreases in heavy coffee drinkers [22]. The use of more specific adenosine receptor antagonists could be useful in the treatment of AF.
Study limitation
The size of the panel we investigated was small because it is unusual to perform blood sample collection in atrium in the absence of indication of catheter ablation. Furthermore, the occurrence of AF followed by spontaneous resolution in paroxysmal patients is not frequent during a short period of hospitalization. These points participate in the weak size of subgroups. The reason why adenosine increased was not examined: adenosine is generated from the hydrolysis of ATP and ADP to AMP and the subsequent hydrolysis of AMP to adenosine [23]. We did not investigate the various components of the corresponding catalytic pathways. Finally, it was shown that clopidogrel—a P2Y12 antagonist used as antiplatelet drug in AF—inhibits ectonucleotidase and may interfere with APL [24] while ticagrelor, another P2Y12 receptor inhibitor, increases APL notably through inhibition of adenosine uptake by red blood cells [25]. It would be therefore interesting to address the influence of clopidogrel and ticagrelor on AF occurrence. Please note, however, that none of the patients we included was treated by clopidogrel or ticagrelor.
Conclusion
We found that a strong release of adenosine in left atrium is associated with AF in PeAF as well as in PAF during the occurrence of AF, while APL was normal in sinus rhythm. If this release is a consequence of AF or triggers AF episode on one hand and if this release is specific of this kind of arrhythmia needs further investigations. Finally, factors that promote the progression of paroxysmal to persistent AF remains not clearly understood [26]. That APL increase participates in this progression deserves further investigations.
Contributorship statement
BM, FF, and JCD: patient inclusion, study design, and writing the paper.
GM, RG, PM, AB, and RG: study design and writing the manuscript.
MM, DV, PD, MC, PM, and CG: critical review of the paper.
EM, PM, MG, and LK: patients selection and participating in study design.
MG, PM, and MC: biological analysis and statistical analysis.
EF, JR, and JLG: critical review of the paper.
Funding
Aix Marseille University and assistance Publique des Hopitaix de Marseille.
Conflict of interest
Baptiste Maille declares that he/she has no conflict of interest.
Marion Marlinge declares that he/she has no conflict of interest.
Donato Vairo declares that he/she has no conflict of interest.
Giovanna Mottola declares that he/she has no conflict of interest.
Linda Koutbi declares that he/she has no conflict of interest.
Pierre Deharo declares that he/she has no conflict of interest.
Marguerite Gastaldi declares that he/she has no conflict of interest.
Marine Gaudry declares that he/she has no conflict of interest.
Claire Guiol declares that he/she has no conflict of interest.
Sara Bottone declares that he/she has no conflict of interest.
Patrick Mace declares that he/she has no conflict of interest.
Rosita Gueant declares that he/she has no conflict of interest.
Mohamed Chefrour declares that he/she has no conflict of interest.
Elsa Martinez declares that he/she has no conflict of interest.
Pierre Michelet declares that he/she has no conflict of interest.
Jean Louis Gueant declares that he/she has no conflict of interest.
Alain Boussuges declares that he/she has no conflict of interest.
Jean Ruf declares that he/she has no conflict of interest.
Emmanuel Fenouillet declares that he/she has no conflict of interest.
Jean Claude Deharo declares that he/she has no conflict of interest.
Régis Guieu declares that he/she has no conflict of interest.
Frederic Franceschi declares that he/she has no conflict of interest.
Ethical approval
This study complies with the declaration of Helsinki. The study was approved by the ethical committee of the Timone University hospital. Written informed consents were obtained from all the participants.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baptiste Maille, Marion Marlinge, Régis Guieu and Frederic Franceschi contributed equally to this work.
References
- 1.Aldhoon B, Melenovský V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiol Res. 2010;59:1–12. doi: 10.33549/physiolres.931651. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120:207–228. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 3.Tebbenjohanns J, Schumacher B, Pfeiffer D, Jung W, Lüderitz B. Dose and rate-dependent effects of adenosine on atrial action potential duration in humans. J Interv Cardiol Electrophysiol. 1997;1:33–37. doi: 10.1023/A:1009758501195. [DOI] [PubMed] [Google Scholar]
- 4.Strickberger SA, Man KC, Daoud EG, Goyal R, Brinkman K, Knight BP, Weiss R, Bahu M, Morady F. Adenosine-induced atrial arrhythmia: a prospective analysis. Ann Intern Med. 1997;127:417–422. doi: 10.7326/0003-4819-127-6-199709150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, Haissaguerre M. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15:1041–1047. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal M, Jena A, Park HS, Baek YS, Lee KN, Roh SY, Shi JM, Choi JJ, Kim YH. Value of adenosine test to reveal dormant conduction or adenosine-induced atrial fibrillation after pulmonary vein isolation. J Arrhythm. 2017;33:602–607. doi: 10.1016/j.joa.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datino T, Macle L, Qi XY, Maguy A, Comtois P, Chartier D, Guerra PG, Arenal A, Fernandez-Aviles F, Nattel S. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010;121:963–972. doi: 10.1161/CIRCULATIONAHA.109.893107. [DOI] [PubMed] [Google Scholar]
- 8.Cheung JW, Ip JE, Chung JH, Markowitz SM, Liu CF, Thomas G, Lee JM, Lessner SJ, Lerman BB. Differential effects of adenosine on pulmonary vein ectopy after pulmonary vein isolation: implications for arrhythmogenesis. Circ Arrhythm Electrophysiol. 2012;5:659–666. doi: 10.1161/CIRCEP.112.971945. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. ESC scientific document group. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1616. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 10.MarlingeN M, Vairo D, Marolda V, Bruzzese L, Adjriou N, Guiol C, Kipson N, Bonnardel A, Gastaldi M, Kerbaul F, Michelet P, Deharo JC, Mottola G, Mace P, Chefrour M, Guieu R. Rapid measurement of adenosine in human blood using fixed potential amperometry: comparison with mass spectrometry and HPLC. J Anal Bioanal Tech. 2017;8:1000371–1000374. [Google Scholar]
- 11.Nee L, Franceschi F, Resseguier N, Gravier G, Giorgi R, Gariboldi V, Collart F, Michelet P, Deharo JC, Guieu R, Kerbaul F. High endogenous adenosine plasma concentration is associated with atrial fibrillation during cardiac surgery with cardiopulmonary bypass. Int J Cardiol. 2013;165:201–204. doi: 10.1016/j.ijcard.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Lerman BB, Belardinelli L. Cardiac electrophysiology of adenosine. Basic and clinical concepts. Circulation. 1991;83:1499–1509. doi: 10.1161/01.CIR.83.5.1499. [DOI] [PubMed] [Google Scholar]
- 13.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Brenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2334–2342. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 14.Kabell G, Buchanan LV, Gibson JK, Belardinelli L. Effects of adenosine on atrial refractoriness and arrhythmias. Cardiovasc Res. 1994;28:1385–1389. doi: 10.1093/cvr/28.9.1385. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Wagoner DR, Kilic A, Janssen PM, Biesiadecki BJ, Weiss JD, Fedorov VV. Adenosine-induced atrial fibrillation: localized reentrant drivers in lateral right atria due to heterogeneous expression of adenosine A1 receptors and GIRK4 subunits in the human heart. Circulation. 2016;134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casadó V, Ciruela F, Lluis C, Franco R, Cinca J, Hove-Madsen L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J. 2011;32:721–729. doi: 10.1093/eurheartj/ehq464. [DOI] [PubMed] [Google Scholar]
- 17.Bertolet BD, Hill JA, Kerensky RA, Belardinelli L. Myocardial infarction related atrial fibrillation: role of endogenous adenosine. Heart. 1997;78:88–90. doi: 10.1136/hrt.78.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hot A, Lavocat F, Lenief V, Miossec P. Simvastatin inhibits the pro-inflammatory and pro-thrombotic effects of IL-17 and TNF-α on endothelial cells. Ann Rheum Dis. 2013;72:754–760. doi: 10.1136/annrheumdis-2012-201887. [DOI] [PubMed] [Google Scholar]
- 19.Halmos PB, Patterson GC. Effects of atrial fibrillation on cardiac output. Br Heart J. 1965;27:719–722. doi: 10.1136/hrt.27.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi F, Deharo JC, Giorgi R, By Y, Monserrat C, Condo J, Ibrahim Z, Saadjian A, Guieu R. Peripheral plasma adenosine release in patients with chronic heart failure. Heart. 2009;95:651–655. doi: 10.1136/hrt.2008.155242. [DOI] [PubMed] [Google Scholar]
- 21.Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casiglia E, Tikhonoff V, Albertini F, Gasparotti F, Mazza A, Montagnana M, Danese E, Benati M, Spinella P, Palatini P. Caffeine intake reduces incident atrial fibrillation at a population level. Eur J Prev Cardiol. 2018;25:1055–1062. doi: 10.1177/2047487318772945. [DOI] [PubMed] [Google Scholar]
- 23.Yegutkin GG (2008) Nucleotide and nucleoside-converting ectoenzymes: important modulators of purinergic signaling cascade. Biochim Biophys Acta 1783:673–694 [DOI] [PubMed]
- 24.Lecka J, Rana MS, Sévigny J (2010) Inhibition of vascular ectonucleotidase activities by the pro-drugs ticlopidine and clopidogrel favours platelet aggregation. Br J Pharmacol 161:1150–1160 [DOI] [PMC free article] [PubMed]
- 25.Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, Camoin-Jau L, Paganelli F, Dignat-George F, Guieu R. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872–877. doi: 10.1016/j.jacc.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 26.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]