Abstract
Extracellular ATP and nicotinamide adenine dinucleotide (β-NAD) demonstrate properties of neurotransmitters and neuromodulators in peripheral and central nervous system. It has been shown previously that ATP and β-NAD affect cardiac functioning in adult mammals. Nevertheless, the modulation of cardiac activity by purine compounds in the early postnatal development is still not elucidated. Also, the potential influence of ATP and β-NAD on cholinergic neurotransmission in the heart has not been investigated previously. Age-dependence of electrophysiological effects produced by extracellular ATP and β-NAD was studied in the rat myocardium using sharp microelectrode technique. ATP and β-NAD could affect ventricular and supraventricular myocardium independent from autonomic influences. Both purines induced reduction of action potentials (APs) duration in tissue preparations of atrial, ventricular myocardium, and myocardial sleeves of pulmonary veins from early postnatal rats similarly to myocardium of adult animals. Both purine compounds demonstrated weak age-dependence of the effect. We have estimated the ability of ATP and β-NAD to alter cholinergic effects in the heart. Both purines suppressed inhibitory effects produced by stimulation of intracardiac parasympathetic nerve in right atria from adult animals, but not in preparations from neonates. Also, ATP and β-NAD suppressed rest and evoked release of acetylcholine (ACh) in adult animals. β-NAD suppressed effects of parasympathetic stimulation and ACh release stronger than ATP. In conclusion, ATP and β-NAD control the heart at the postsynaptic and presynaptic levels via affecting the cardiac myocytes APs and ACh release. Postsynaptic and presynaptic effects of purines may be antagonistic and the latter demonstrates age-dependence.
Keywords: Heart, Cholinergic effects, Postnatal development, β-NAD, ATP, Purine cotransmitters
Introduction
It is widely accepted that extracellular ATP acts as neurotransmitter and neuromodulator in neuromuscular junctions and autonomic nerves within various tissues [1–4]. Similarly, neurotransmitter properties of another well-known purine compound, the nicotinamide adenine dinucleotide (β-NAD), were shown recently [5–7]. It has been demonstrated that β-NAD is released from nerve terminals together with classical neurotransmitters. In autonomic nervous system, extracellular β-NAD acts as inhibitory neurotransmitter and participates in neural control of vascular or nonvascular smooth musculature [6, 8].
It is well known that ATP is endogenous agonist of P2 purine receptors, although P1 receptors are also partly involved in mediation of ATP effects [2, 3]. In addition, the activation of both P2 and P1 purine receptors by β-NAD has been demonstrated [6, 9].
While the role of β-NAD in regulation of smooth muscle has been extensively studied, the physiological significance and mechanisms of action of this compound remain poorly investigated in cardiac tissue. However, we have recently reported that exogenous β-NAD affects action potential (AP) waveform in a pacemaker and working myocardium from adult rats [10]. In contrast to β-NAD, the effects of ATP on electrical activity and contractility of the heart were widely investigated in numerous studies. Nevertheless, in the developing heart, the electrophysiological effects of ATP have not been elucidated completely and effects of β-NAD have never been described. Therefore, the first aim of the present study was to reveal the effects of exogenous β-NAD in early postnatal ontogenesis in supraventricular and ventricular working myocardium and compare it with effects of ATP.
The neuromodulatory role of purine compounds in noncardiac tissues has been investigated in detail. It has been shown that ATP and β-NAD are costored in vesicles with noradrenaline. The activation of sympathetic nerve terminals leads to release of vesicles containing both noradrenaline and purines. Upon release, ATP and β-NAD modulate the effects of noradrenaline in vascular and nonvascular smooth musculature at postjunctional and prejunctional levels [2, 8, 11]. ATP and β-NAD are also costored in cholinergic nerve terminals. Nerve-derived ATP modulates release of acetylcholine (ACh) in neuromuscular junctions [12, 13]. ATP also modulates cholinergic effects and release of ACh from autonomic parasympathetic nerves in various tissues [2, 14]. Modulation of parasympathetic effects by β-NAD has been demonstrated only in the urinary bladder [15], although the mechanisms of such modulation have never been investigated. Purinergic modulation of ACh release and its effects in the heart are still poorly investigated.
Various purine compounds may act as morphogens during pre-and postnatal development. P1 and P2 receptors which are differently expressed at various stages of ontogenesis were shown to mediate morphogenetic effects of purines [16–18]. Thus, high concentrations of extracellular purine compounds may be present in tissues even at early stages of ontogenesis, prior to maturation of autonomic regulation. Moreover, effects of purine neurotransmitters and their neuromodulatory properties may vary at different developmental stages due to the difference in expression of purine receptor subtypes. Therefore, modulation of cardiac parasympathetic effects by ATP and β-NAD was investigated in the present study in the myocardium of rats at various stages of postnatal ontogenesis. We have demonstrated that both ATP and β-NAD modulate cardiac parasympathetic effects in an age-dependent manner.
Materials and methods
Animals
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experimental protocol was approved by Bioethics Committee of Moscow State University. The Wistar rats of both sexes weighing 210–300 g (6–8 weeks old) were held in the animal house for 2 weeks under a 12:12 h light to dark photoperiod at 20–24 °C and 40–70% humidity in standard T4 cages and fed ad libitum. Male adult animals were used for experiments; also, adult animals were mated and used for production of offspring.
Pregnant female rats were housed throughout gestation in standard individual T3 cages. After delivery, the rat offspring were kept in the same cages with mothers until the age of 3 weeks. Before the experiments, the pups were sorted by gender and weighed. Neonatal and juvenile male pups were used in the experiments at the 1st, the 14th, or the 21st day of life with 5 ± 0.7, 36 ± 3.8, and 62 ± 5.2 g of weight, respectively. The broods were reduced proportionally by removing of the female pups throughout investigation.
Isolation of cardiac atrial and ventricular multicellular preparations
Rat pups and juveniles were subjected to euthanasia by cervical dislocation. Adult rats were anesthetized with intraperitoneal injection of 80 mg/kg ketamine and 10 mg/kg xylazine. Heparin (1000 U/kg) was added to the anesthetics solution to prevent blood coagulation in the coronary vessels of the excised heart. The hearts were excised and rinsed as described earlier [10]. Preparations of right atria (RA), left atria (LA), right ventricular wall (RV), or pulmonary vein (PV) myocardial sleeves were isolated and pinned endocardial side up to the bottom of experimental chamber (3 ml) with continuous flow of physiological solution (10 ml min−1, 37.5 °C). The composition of solution was as follows (mM): NaCl 118.0, KCl 2.7, NaH2PO4 2.2, MgCl2 1.2, CaCl2 1.2, NaHCO3 25.0, glucose 11.0, bubbled with carbogen (95% O2 + 5% CO2), pH 7.4 ± 0.1. Since all the types of preparations except RA lacked intrinsic pacemaker activity, they were paced with a pair of silver Teflon-coated electrodes (pacing rate, 3 Hz; pulse duration, 2 ms: pulse amplitude, 2 times threshold) throughout the experiment.
Isolation of multicellular pulmonary veins preparations
Rats were anesthetized as described in “Isolation of cardiac atrial and ventricular multicellular preparations.” The chest was opened; the heart with lung lobes was rapidly excised and rinsed with physiological solution. To allow outflow of the solution, the outer edges of the lung lobes were trimmed. The left atrium was incised at the atrioventricular border and cannulated. Blood from the left atria and pulmonary veins was flushed out by injection of physiological solution. Fascia and pulmonary arteries were removed, and preparation of isolated supraventricular region including pulmonary veins and lung lobes was pinned in a preparation bowl. Finally, tubular PV preparations were isolated from one or two lung lobes. Isolated PVs were cut along the axis and pinned in experimental chamber with inner side up. During the following experiments, PV preparations were perfused and paced as described in “Isolation of cardiac atrial and ventricular multicellular preparations.”
Intracellular recording of action potentials
Action potentials were recorded from all types of multicellular preparations with sharp glass microelectrodes (30–45 MΩ) filled with 3 M KCl and connected to a high input impedance amplifier Model 1600 (A-MSystems, Sequim, WA, USA). The signal was digitized and analyzed using specific hardware (E-154 analog-to-digital converter, L-card, Moscow, Russia, www.lcard.ru) and software (PowerGraph 3.3, DISoft, Russia, www.powergraph.ru/en). Stable impalements were maintained during the entire period of drugs application. Changes in the AP duration at 90% of repolarization (APD90) were determined in RA, LA, RV, and PV preparations; in addition, the frequency of APs was estimated in spontaneously active right atrial preparations. The AP duration was calculated with MiniAnalysis 6.0.7 (Synaptosoft, Fort Lee, NJ, USA, www.synaptosoft.com).
Stimulation of intramural nerves
The excitation of intracardiac autonomic nerves was elicited by 100 Hz trains of rectangular pulses (0.1 ms 0.1 mA) of 3–5 s duration which were delivered to RA preparations surface via silver bipolar Teflon-coated electrodes. Intramural nerve stimulation episodes were separated at least by 5 min periods of quiescence. Not more than 2–3 pulse trains were applied per each experiment in RA preparations from neonatal rats.
ACh assay
Isolated left atria of adult (60-day old) rats were used in experiments. The concentration of ACh released in atria was estimated optically using an Amplex Red Acetylcholine Assay Kit (Molecular Probes, USA). This approach is based on the detection of choline released from the hydrolysis of ACh by ACh-esterase. Choline oxidase decomposes choline to H2O2 and betaine, and H2O2 in the presence of HRP reacts with Amplex Red reagent to produce a stable fluorescent molecule, resorufin, which is monitored [19, 20].
A freshly isolated atria were incubated in a bath (volume 0.4 ml) with physiological saline containing choline oxidase (0.2 U/ml), HRP (2 U/ml), acetylcholine esterase (1 U/ml), and 400 μM Amplex Red at 37.5 C. The solution was constantly mixed during incubation by recirculating mechanism (total volume 1.2 ml).
We used four protocols for the detection of changes in ACh release (n = 6 atria for each group). (1) Rest production of ACh by the atria was determined at incubation of the samples for 10 min in the kit-containing physiological solution. Then preparation was exposed to next 10 min into a new solution also containing all chemicals for the detection of ACh. (2) For stimulation of ACh release, we used prolonged high-frequency stimulation (100 Hz, 3 min; suprathreshold 0.1-ms voltage pulses) via filed platinum electrodes using DS3 Stimulator. Atria were also exposed to the kit-containing solution for 10 min twice, but between 5 and 8 min of each 10 min-period atria were simulated to produce evoked ACh release. Protocols (3) and (4) are the same as (1) and (2), but during the second 10-min exposition 10 μM NAD or 10 μM ATP was present in the working solution.
Fluorescence images were captured using a BX51WI Olympus microscope equipped with FluoLED illuminators (Fraen) and CCD-camera DP72 (Olympus). Image Pro software (Media Cybernetics) was used for image analysis. Fluorescence of resorufin was measured after incubation of the collected solution at room temperature for 45 min using excitation at 535 ± 10 nm and emission detection at 590 ± 20 nm. Measurements of the red fluorescence were used to estimate the amount of ACh (calculated in a.u. of fluorescence per gram of atria tissue). The ACh concentration was estimated by comparing resorufin fluorescence to a stand curve of resorufin fluorescence versus ACh concentration. For each measurement, we made a correction for background fluorescence caused by the release of endogenous H2O2 [21] from reactions other than choline oxidation. Samples of 10 μl were taken for analysis.
Experimental protocols
All tissue myocardial preparations were allowed to equilibrate for 1 h before the start of electrophysiological recordings. Action potentials were recorded in RA, LA, RV, and PV preparations from rats at postnatal day 1 (P1), 14 (P14), 21 (Р21), and 60 (which were considered as adult) during 5-min administration of β-NAD (10 μM) or ATP (10 μM) proceeded with 5-min control recording. These days of postnatal development were selected for experiments as days that represent the different stages of the maturation of autonomic nervous control in rats. Only one purine compound and only once was applied in one tissue preparation to prevent artifacts derived from purine receptors desensitization. In total, 53 tissue preparations were used from 35 animals in this series of the experiments.
In the second series of experiments, the ability of ATP and β-NAD to alter the response to evoked ACh release from parasympathetic intracardiac nerves was tested. APs in RA preparations of adult and P1 rats were recorded during the stimulation of intracardiac postganglionic nerves in control conditions and after 5-min application of 10 μM ATP or β–NAD. Adrenoblockers propranolol (1 μM) and prazosin (1 μM) were continuously present in extracellular solution to exclude putative sympathetic effects. In this series of experiments, the tissue preparation was consecutively subjected to the administration of both ATP and β-NAD but with 30-min interval. This protocol was used to prevent variation in “bradicardic” response to PNS. In total, 11 tissue preparations from 11 animals were used.
The experimental protocol used to assay ACh release in the presence of ATP or β-NAD is described in previous section. In these experiments, 24 preparations from 24 animals were used.
Finally, the ability of ATP and β-NAD to alter the response to spontaneously released ACh was checked. APs were recorded in RA preparations from neonatal rats during 5-min application of ATP or β-NAD (10 μM) after 5-min pretreatment with 5 μΜ neostigmine. Particular tissue preparation was treated only by ATP or β-NAD, and in total, 10 tissue preparations from 10 animals were utilized.
Drugs
5’-ATP, β-NAD, ACh, atropine sulfate, propranolol, prazosin, and neostigmine methylsulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Heparin sodium solution (5000 units/mL) was purchased from Moscow Endocrine Plant (Moscow, Russia); xilazine HCl (Rometar) solution (20 mg/mL) was purchased from Bioveta (Ivanovice na Hane, Czech Republic).
Statistical analysis
All data in the text and figures except the original recordings are presented as means ± SD (except experiments with resorufin where data expressed as means ± SEM) for n experiments. GraphPad Prism 7 (GraphPad Software, USA) was used for statistical analysis of the data. The normality of the groups was tested using the Shapiro–Wilk test. Hypothesis testing was carried out using an one- or two-way ANOVA (with further Dunnett and Sidak correction based posthoc test for multiple comparisons in groups with repeated or independent measurements) where it was appropriate. A value p < 0.05 was considered as statistically significant.
Results
Effects of ATP and β-NAD on AP waveform in the myocardial preparations from rats of various ages
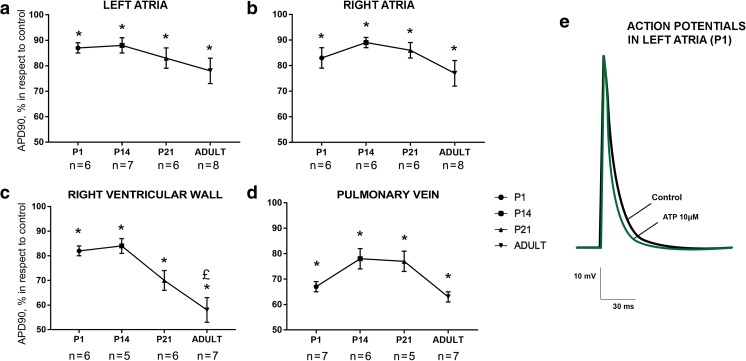
Exogenous ATP significantly affected AP waveform in all types of myocardial preparations (RA, LA, PV, and RV, the control values of AP durations are represented in Table 1) from rats of all studied ages. The decrease of AP duration in response to 10-μM ATP application was observed both in the supraventricular and ventricle myocardium (Fig. 1). The effect of ATP was age-independent from 1st until 21st day of the postnatal development either in supraventricular or ventricular preparations. The inhibitory effect of ATP was significantly greater only in RV preparations from adult animals relative to other ages (Fig. 1c).
Table 1.
The absolute values of the action potentials in the different tissue preparations from the rats at various ages under control conditions. Total number of animals used is 35
| Day of a postnatal development | APD90, ms | |||||||
|---|---|---|---|---|---|---|---|---|
| Left atria | Right atria | Right ventricle | Pulmonary vein | |||||
| P1 | 63.2 ± 3 | n = 6 | 61 ± 3 | n = 6 | 53.7 ± 4 | n = 7 | 37.3 ± 2 | n = 6 |
| P14 | 41 ± 3 | n = 6 | 39 ± 3 | n = 7 | 23.5 ± 3 | n = 6 | 21.6 ± 3 | n = 6 |
| P21 | 33 ± 2 | n = 6 | 30 ± 4 | n = 6 | 33 ± 2 | n = 6 | 27.2 ± 2 | n = 6 |
| P60 | 42 ± 2 | n = 8 | 41 ± 2 | n = 8 | 42 ± 2 | n = 7 | 55 ± 2 | n = 8 |
Fig. 1.
Age-dependence of ATP-induced (10 μM) AP shortening in the left (a) and right (b) atrial, ventricular (c), and pulmonary vein (d) myocardial preparations from rats of various ages. Asterisk indicates significant difference of the parameter from the control value. Pound sign indicates significant differences of the parameter between age groups, p < 0.05 (two-way ANOVA). P1, 1st day of postnatal development; P14, 14th day of postnatal development; P21, 21st day of postnatal development (e). Representative example of action potentials recorded in control conditions (black trace) and in the presence of 10 μM ATP (green trace) in the left atrial myocardial preparations from rats at 1st day of a postnatal development. Different marks in a–d are used to display preparations obtained from distinct animals
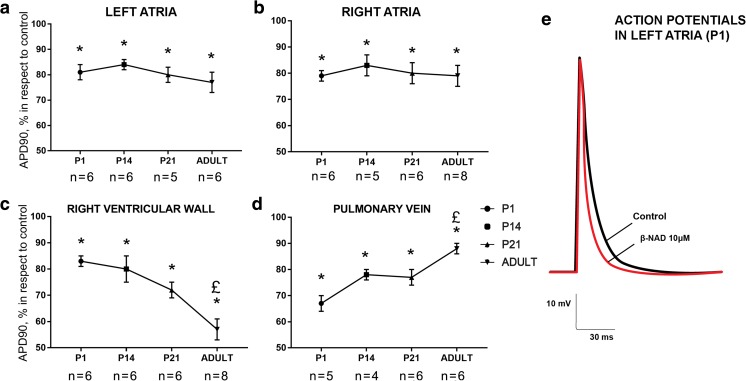
Similar to ATP, the application of 10 μM β-NAD resulted in a significant reduction of AP duration both in the supraventricular and ventricular myocardial preparations from rats of all studied ages (Fig. 2). AP shortening induced by β-NAD was similar in all types of preparations except ventricular myocardium and pulmonary veins. In RV preparations, β-NAD elicited more prominent effect in adult rats, while in PV, β-NAD was less effective in comparison with its effect in preparations from rats at P1–P21 day of development (Fig. 2c, d).
Fig. 2.
Age-dependence of β-NAD-induced (10 μM) AP shortening in the left (a) and right (b) atrial, ventricular (c), and pulmonary vein (d) myocardial preparations from rats of various ages. Asterisk indicates significant difference of the parameter from the control value. Pound sign indicates significant differences of the parameter between age groups, p < 0.05 (two-way ANOVA). P1, 1st day of postnatal development; P14, 14th day of postnatal development; P21, 21st day of postnatal development (e). Representative example of action potentials recorded in control conditions (black trace) and in the presence of 10 μM β-NAD (red trace) in the left atrial myocardial preparations from rats at 1st day of a postnatal development. Different marks in a–d are used to display preparations obtained from distinct animals
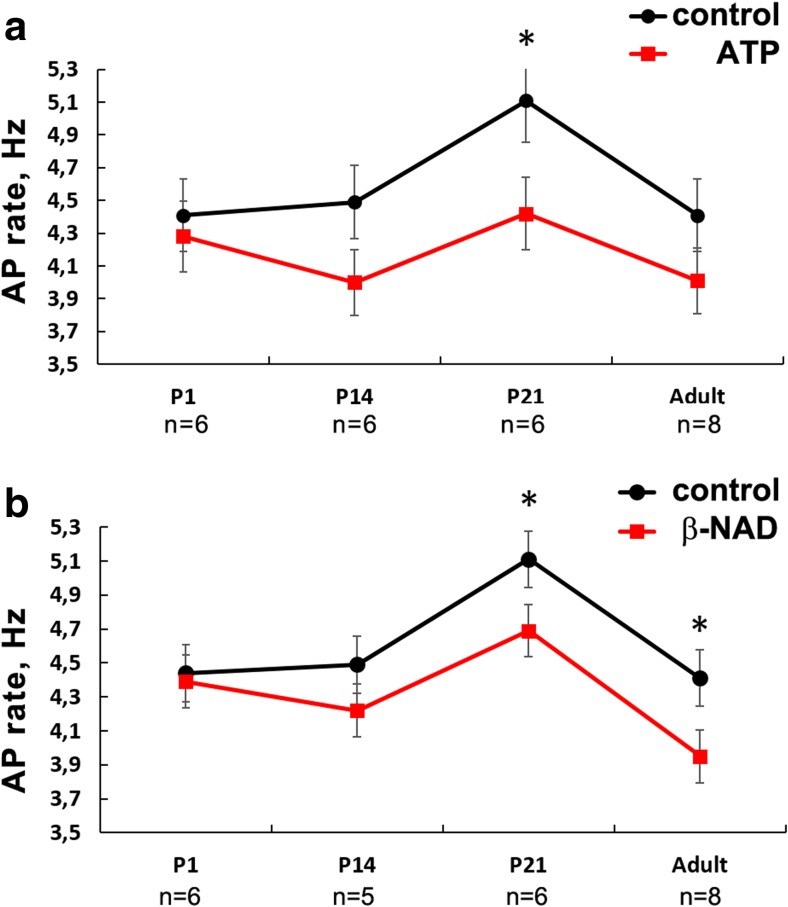
The ability of ATP to affect the pacemaking at various ages was tested using spontaneously active RA preparations. The highest rhythm in isolated spontaneously active RA preparations was observed in P21 rats (Fig. 3). ATP failed to alter AP frequency in neonatal rats, weakly changed rhythm in the older animals with only significant decreasing of AP frequency in P21 rats (Fig. 3a).
Fig. 3.
Age-dependence of ATP (a) and β-NAD (b) effect on rhythm of spontaneously active rat right atrial myocardial preparations. ATP and β-NAD were used in 10-μM concentration. Both purines caused weak alteration of intrinsic rhythm of RA preparations. SAP, spontaneous AP. Asterisk indicates significant difference of the parameter from the control value, p < 0.05 (one-way ANOVA). P1, 1st day of postnatal development; P14, 14th day of postnatal development; P21, 21st day of postnatal development. Different marks in a–d are used to display preparations obtained from distinct animals
In addition, extracellular β-NAD caused significant decrease of AP frequency in preparations from either P21 or adult rats (Fig. 3b); however, β-NAD completely failed to affect sinus rhythm in neonatal animals.
Effects of ATP and β-NAD on negative chronotropy produced by evoked ACh release
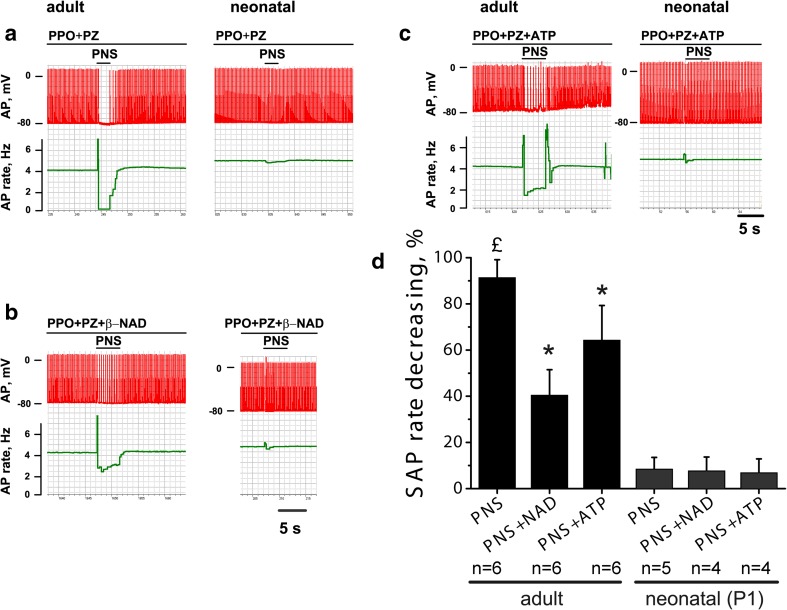
In the presence of adrenoblockers, the stimulation of intramural postganglionic nerves caused a transient termination of spontaneous firing (n = 4) or profound rhythm slowing (n = 2) in RA preparation from adult rats, but failed to produce similar effects in neonatal myocardium (n = 5) (Fig. 4a). The mean relative reduction of spontaneous AP rate was 91.4 ± 7.7% in adult rats, but only 8.5 ± 5% in RA preparations from neonates. The effect of intramural nerve stimulation was completely abolished by 1 μM of atropine (data not shown). ATP (10 μM) and β-NAD (10 μM) significantly suppressed the effect of intramural nerve stimulation (p < 0.05) in adult animals (Fig. 4b, c). The relative rhythm slowing was only 40.5 ± 11% (n = 6) in the presence of ATP, while 64.3 ± 15% (n = 6) in the presence of β-NAD. In contrast to adult animals, either ATP or β-NAD failed to alter effect of intramural nerve stimulation in preparations from neonatal rats (Fig. 4d).
Fig. 4.
Effect of ATP and β-NAD on the reduction of AP frequency caused by the stimulation of the postganglionic nerves in the atrial preparations from neonatal and adult rats. a–c Representative examples of the original records that show spontaneous AP (red traces) and changes of AP frequency (green traces) in the atrial preparations from neonatal (1st day of postnatal development) and adult rats under control conditions and under electrical stimulation of the postganglionic nerves (PNS) in the presence of ATP (10 μΜ) or β-NAD (10 μΜ). d Changes of the spontaneous AP (SAP) in response to the PNS alone or in response to PNS in ATP or β-NAD pretreated preparations in preparations from neonatal and adult animals. PPO, propranolol (1 μΜ); PZ, prazosin (1 μΜ). P1, 1st day of postnatal development. Pound sign indicates significant difference of the parameter from the control AP rate. Asterisk indicates significant differences from the PNS-group. p < 0.05 (two-way ANOVA)
Effects of ATP and β-NAD on ACh release
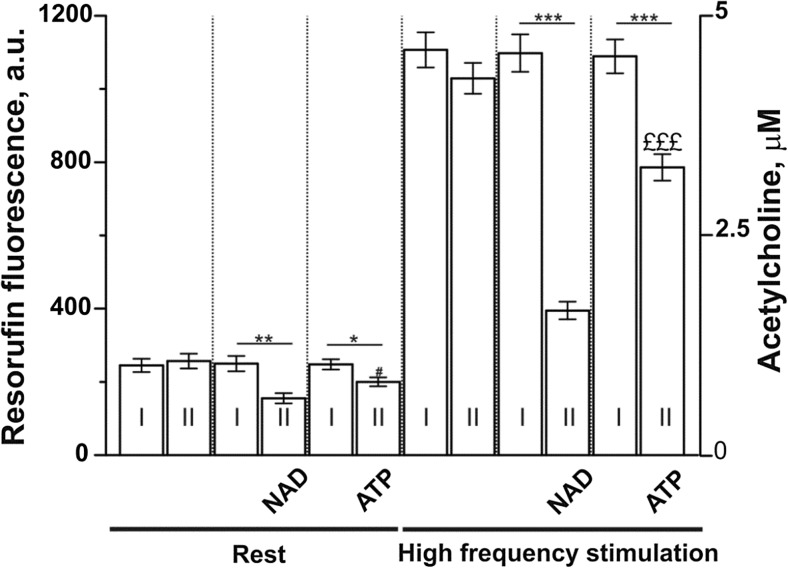
It is possible that β-NAD and ATP might lower the level of endogenous ACh release. To test this possibility, we estimated the level of extracellular Ach released from left atria at rest and in response to high-frequency stimulation (Fig. 5). These experiments showed that, at the rest conditions, ACh fluorescence decreased by 38.0 ± 5.6% (n = 6; p < 0.01) or 19.4 ± 4.8% (n = 6; p < 0.05) in the presence of β-NAD or ATP, respectively. Additionally, β-NAD and ATP markedly suppressed high frequency stimulation-induced acetylcholine release by 64.0 ± 2.2% (n = 6; p < 0.001) and 27.8 ± 3.3% (n = 6; p < 0.001), respectively. Note that β-NAD had a more profound effect on the acetylcholine levels compared with ATP.
Fig. 5.
Extracellular ACh levels at rest conditions and after high frequency stimulation of the intramural nerves in the rat left atrial tissue preparations. Shown resorufin fluorescence (a.u.) in the bath solution after two sequential incubations (I and II) of the atria. Separate incubation lasted 10 min. Rest, atria were not stimulated during the incubations. High-frequency stimulation, atria were stimulated at 100 Hz for 3 min during the each of incubations. In the controls, the atria were exposed to the kit containing physiological solution without β-NAD or ATP. 10 μM β-NAD or 10 μM ATP were added to the bath during the second incubations. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the corresponding control (I) bar. £££p < 0.001 is β-NAD versus ATP (two-way ANOVA). The resorufin fluorescence was matched with the ACh standard curve to estimate the concentration of ACh (in μM) accumulated in 1.2 ml of bathing solution normalized per gram of atria (right Y-axis). n = 6 animals for each couple (I + II) of bars
Effects of ATP and β-NAD on negative chronotropy produced by spontaneous ACh release
Since ATP and β-NAD failed to affect spontaneous rhythm of RA preparations from neonatal rats, it was possible to test their influence on modest effects produced by spontaneous ACh release. Carbamate acetylcholinesterase inhibitor neostigmine unmasked the effects of ACh, which is continuously released from parasympathetic nerve terminals via nonvesicular mechanism [22] and quickly hydrolyzed by acetylcholinesterase. Neostigmine (5 μΜ) induced accumulation of ACh in myocardial preparations leading to progressive AP shortening and rhythm slowing in RA preparations. The latter effect reached 12 ± 6% in 5 min and 42 ± 10% of control sinus rhythm rate (n = 8, p < 0.001) in 15 min. Both ATP and β-NAD failed to alter neostigmine-induced rhythm slowing (data not shown). The neostigmine-induced slowing was 11 ± 4.5% in the presence of ATP (5 min, 10 μΜ, n = 5) and 14 ± 5.2% in the presence of β-NAD (5 min, 10 μΜ, n = 5).
Discussion
In the present study, the cardiotropic effects induced by extracellular ATP and β-NAD at various stages of postnatal development were investigated for the first time. In addition, we have found that extracellular ATP and β-NAD could suppress the effects of postganglionic parasympathetic nerve stimulation.
Age-specific effects of ATP and β-NAD in the rat heart
The results of the present study indicate that purines cause AP shortening both in ventricular and supraventricular myocardium of the rat neonatal and adult heart. The inhibitory effects produced by ATP and β-NAD were weakly age-dependent and were almost similar at all studied stages of postnatal ontogenesis in all types of myocardial preparations except adult ventricular myocardium. Several studies showed that extracellular ATP affects cardiac function in juvenile and adult mammals via purine P1 and P2 receptors [23]. Here, we show that β-NAD induces direct postsynaptic nerve-independent cardiac effects in early postnatal life similarly to ATP. The effects of β-NAD in early postnatal development are likely to be mediated via purine receptors as in adult animals [24, 25]. Our results also show that postsynaptic effects of ATP and β-NAD have similar pattern in various departments of the heart.
It should be noted that among AP shortening, β-NAD can produce AP prolongation, but only in atrial myocardium [25]. This effect was observed in adult animals, it was weak and transient and always was followed by sustained decrease in APD. We did not consider aforementioned effect in the present study because it was impossible to estimate and prove the significance of the “positive” β-NAD effect in tissue preparations from nonadult animals.
We have also demonstrated that ATP and β-NAD induce shortening of AP in pulmonary veins from rats of all tested ages, including neonatal and adult animals. It is known that PV myocardium demonstrates specific electrophysiology that differs from those in the atrial myocardium [26]. A lot of attention is paid to the regulation of PV myocardium since pioneering studies of Haissaguerre and coworkers [27] that suggested this tissue as the main source of ectopic activity facilitating the initiation of atrial arrhythmias. The present study demonstrates the contribution of ATP and β-NAD to the control of PV bioelectrical activity, although the effect of purines is generally the same as in working atrial myocardium. The AP shortening induced by purines can be both pro- or antiarrhythmic in PV, depending on many interfering factors like autonomic nerves activation, cytoplasmic Ca2+ changes [28]. It is also possible to speculate that the (patho)physiological relevance of ATP and β-NAD in PV can be associated not only with nerve-independent effects in myocardium but with action of purines as sympathetic cotransmitters or with alteration of ACh release.
In our experiments, both ATP and β-NAD failed to alter spontaneous rhythm in RA preparations from rats at early postnatal development, but slowed it down in 21-day animals. Only β-NAD reduced the rhythm rate in adults. The systemic administration of ATP was shown to produce heart rate reduction both in human and in laboratory animals [29, 30]. It has been decisively demonstrated in vivo that central vagal reflex activation is involved in the effects of extracellular nerve- or cardiomyocyte-derived ATP [31, 32]. However, a direct chronotropic effect of extracellular ATP and β-NAD independent on activation of vagus or sensomotor intracardiac nerves was not described until the present study. This additional mechanism of cardiac rhythm regulation may involve activation of purine receptors in pacemaking cardiomyocytes at the late stages of postnatal development.
Potential targets of ATP and β-NAD in the rat myocardium at various ages
The resemblance of inhibitory effect caused by ATP and β-NAD in nonadult animals supports the hypothesis that effects of both purines might be mediated by the same type of purinergic receptors. Nevertheless, literature analysis allows to suggest that purines can activate various receptors. As has been suggested in previous studies, the extracellular β-NAD can affect P2Y receptors [6, 25] besides other targets such as P1 receptors, CD38, ATRs [33], and P2X7 [34]. Four subtypes of P2Y receptors (P2Y1, P2Y2, P2Y6, P2Y11) may be substantially expressed in mammalian myocardium [35]. It was proposed that P2Y1 [6, 36] and P2Y11 are the primary targets for extracellular β-NAD [37]. However, Gs-coupled P2Y11 receptors lack in rats [38, 39]. Thus, Gq-coupled P2Y1 is the main P2 candidate for the role of β-NAD receptor that mediate inhibitory effects in the rat myocardium. It has been shown that direct nonvagal postsynaptic effects of ATP in the myocardium are mediated by P2Y receptors [24], in particular by P2Y2, P2Y4, and P2Y11 (absent in the rat) subtypes [35, 40].
Gq-coupled signaling is involved in many pathways of heart regulation, including inhibitory effect of M3 cholinoreceptors stimulation by ACh [41, 42], which is more prominent in neonatal than adult rat myocardium [43]. Also, it has been demonstrated in cardiomyocytes from neonate rats that P2Y1 receptors are expressed at higher level than P2Y2 and P2Y4 [18]. Thus, the expression of different P2Y receptors takes place in rat neonatal and adult myocardium, and this phenomenon might underlie the age-dependence of response to ATP or β-NAD. However, this study has revealed weak age-dependence of the purinergic effects with only more prominent AP duration reduction in the ventricle myocardium of adult animals. Accordingly, the expression of differential purine receptors may provide substantial changes in response to purines only at very late stages of the rat heart development and only in particular tissue regions.
In addition, extracellular β-NAD can display the properties of so-called “biased agonist” [44] for the particular purine receptors and probably can unproduced nonsimilar effects even if it activates the same P2Y as ATP. The biased agonism along with different receptor expression may underlie the difference in effects of ATP and β-NAD on rhythm in RA preparations.
Modulation of parasympathetic effects by ATP and β-NAD in neonatal and adult rat heart
High frequency electrical stimulation causes activation of intracardiac nerve fibers and release of neurotransmitters in the myocardium [45, 46]. The slowing of rhythm or even transient pacemaker arrest is the well-known ACh-induced response to the stimulation of cholinergic autonomic nerves [47], which we have observed in RA preparations in the presence of α and β-adrenoblockers.
We found a strong suppression of negative chronotropy induced by nerve stimulation with either ATP or β-NAD in RA preparations from adults but not neonates. On the other hand, ATP and β-NAD exert own direct negative chronotropic effect in RA. Therefore, it is possible to hypothesize that inhibition of stimulation-induced negative chronotropy results from suppression of ACh released from cholinergic nerve terminals. The experiments with resorufin show the decrease of ACh fluorescence in the presence of β-NAD and ATP both in resting conditions and under postganglionic nerves stimulation. Therefore, these experiments clearly confirm the ability of purines to suppress endogenous quantal ACh release from presynaptic terminals. Thus, besides weak direct postsynaptic effect on cardiac myocytes, ATP and β-NAD regulate cardiac function via more profound presynaptic influence leading to suppression of ACh release in the heart.
It is well established that ATP suppresses evoked ACh release in neuromuscular junctions and therefore controls excitatory neurotransmission [48, 49]. This effect is mediated by presynaptic P2Y and P1 receptors (due to degradation of ATP to adenosine) [50, 51]. Thus, the effect of ATP, which is likely to be attributed to inhibition of ACh release in the heart, is not surprising. Our results allow to suggest that ATP restricts inhibitory effects of ACh in the heart.
The ability of β-NAD to affect cholinergic neurotransmission in the heart has not been studied earlier. However, β-NAD can play a role of autonomic inhibitory neurotransmitter or cotransmitter in various tissues [11, 15]. Noteworthy, β-NAD was more potent than ATP in suppression of rhythm slowing produced by intramural nerve stimulation. The reasons of that difference remain unclear and need further study. It could be speculated that β-NAD acts as more potent agonist of its receptors in comparison with ATP in the heart probably due to “biased agonism”. However, the activation of different receptors in vagal nerve terminals by β-NAD and ATP, similarly to cardiac tissue, cannot be excluded. Besides, pre- and postsynaptic effects of both β-NAD and ATP may be mediated by distinct inhibitory purinergic receptor subtypes expressed in cardiomyocytes and parasympathetic nerve terminals.
The higher extracellular level of nerve-derived β-NAD may underlie more prominent effects of the purine compound in vivo. It has been reported that extracellular concentration of β-NAD exceeded the concentration of ATP 30-fold after autonomic nerves stimulation in visceral smooth muscles [6]. The higher content of β-NAD in presynaptic vesicles of autonomic nerves in the rat heart also has been demonstrated.
As mentioned above, extracellular ATP triggers vagal reflexes in the heart and, therefore, central mechanisms are involved in the purinergic heart rhythm control [30, 52]. Our results indicate that ATP attenuates parasympathetic activity via reduction of ACh release from intracardiac nerves. Thus, peripheral presynaptic action of purines may be considered as a novel mechanism antagonistic to ATP-mediated reflectory regulation.
In addition, the inhibitory influence of ATP and β-NAD on ACh release may be considered as antiarrhythmic effect in atrial myocardium, since it is well known that excessive parasympathetic postganglionic fiber activation leads to AF initiation (so called vagal-induced AF) via AP shortening and reentry facilitation. Purine compounds can attenuate refractoriness shortening and reentry induction by means of ACh release reduction. More profound effect of β-NAD than ATP on ACh release allows to suggest that the former purine is a neurotransmitter with more potent antiarrhythmic properties. It should be noted that β-NAD effects and mechanism of action may be different in case of atrial and ventricular arrhythmias. Undoubtedly, additional in vivo experiments are needed to confirm the proposed effects of β-NAD.
In RA preparations from neonatal rats, ATP and β-NAD failed to attenuate the effect of cholinergic nerve stimulation, which by itself was very modest in comparison to adult rats. This could be partially explained by the immaturity of cardiac cholinergic innervation in neonates. Cholinergic neuroeffector transmission first appears in the rat heart on embryonic day 21, the day of birth. On earlier developmental stages, the stimulation of intramural nerves does not induce release of ACh from parasympathetic endings [53]. Therefore, evoked ACh release is likely to be very weak in the rat heart on the first day of life.
Effect of ATP and β-NAD on spontaneous release of ACh in the neonatal rat heart
It is now accepted that ACh can be released from cholinergic nerve endings via nonquantal (nonvesicular) mechanism in addition to the main quantal mechanism. Nonquantal ACh secretion is independent on excitation of nerve terminals and occurs spontaneously in neuromuscular junctions [54], myocardium [12, 22], and airway smooth muscle [55]. In neuromuscular junction, ATP strongly inhibits nonquantal ACh release via presynaptic P2Y receptors, while adenosine fails to modulate this process [56, 57].
We have chosen neonatal rat heart to check possible involvement of purines in regulation of nonquantal ACh release in cardiac tissue, since postsynaptic effects of ATP and β-NAD on heart rate, which represent an obstacle for nonquantal release investigation, are the least intensive at this age. Moreover, immatureness of quantal-evoked ACh release in neonates [53] makes nonquantal ACh more important at this particular stage of development. Similar to earlier studies [12, 22], we used the value of cholinergic effect which appears spontaneously in the absence of acetylcholinesterase activity, as a measure of nonquantal ACh release intensity. In our experiments, both purine compounds failed to alter effect of spontaneously released and accumulated ACh. We suggest that ATP and β-NAD do not participate in modulation of nonquantal ACh release at least in neonatal supraventricular myocardium.
In conclusion, both ATP and β-NAD affect rat myocardium at postsynaptic level in adults and during early postnatal ontogenesis practically in similar extent. In adult rats, both purines suppress the effects attributed to stimulation of intramural cholinergic nerves and decrease ACh release, thereby acting at presynaptic level. In the contrast to adult rats, in neonates, ATP and β-NAD do not modulate either evoked ACh release or spontaneous nonquantal ACh secretion. Thus, we suggest that ATP and β-NAD are involved in the regulation of heart function at postsynaptic and presynaptic levels. Postsynaptic and presynaptic effects are antagonistic and the latter are age-dependent.
Abbreviations
- ACh
Acetylcholine
- AF
Atrial fibrillation
- AP
Action potential
- APD
Action potential duration
- APD90
Action potential duration at 90% of repolarization
- LA
Left atrium
- NST
Neostigmine
- P1
1st day of postnatal development
- P14
14st day of postnatal development
- P21
21st day of postnatal development
- PV
Pulmonary vein
- RA
Right atrium
- RV
Right ventricular wall
- SAP
Spontaneous action potential
- β-NAD
β-nicotinamide adenine dinucleotide
Author contributions
Participated in the study planning: Kuzmin VS.
Performed experiments and data analysis: Pustovit KB, Potekhina VM, Ivanova AD.
Contributed to the discussion and reviewed/edited the manuscript: Petrov AM.
Wrote the manuscript: Abramochkin DV, Kuzmin VS.
Funding information
This study was supported by Russian Science Foundation grant 14-15-00268.
Conflicts of interest
Pustovit KB declares that she has no conflict of interest.
Potekhina VM declares that she has no conflict of interest.
Ivanova AD declares that she has no conflict of interest.
Petrov AM declares that he has no conflict of interest.
Abramochkin DV declares that he has no conflict of interest.
Kuzmin VS declares that he has no conflict of interest.
Ethical approval
This study was approved by Bioethics Committee of Moscow State University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoyle CH, Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986;124:285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic cotransmission. F1000 Biol Rep. 2009;1:46. doi: 10.3410/B1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Cotransmission in the autonomic nervous system. Handb Clin Neurol. 2013;117:23–35. doi: 10.1016/B978-0-444-53491-0.00003-1. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy C. ATP as a cotransmitter in the autonomic nervous system. Auton Neurosci. 2015;191:2–15. doi: 10.1016/j.autneu.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Alefishat E, Alexander SPH, Ralevic V. Effects of NAD at purine receptors in isolated blood vessels. Purinergic Signal. 2015;11:47–57. doi: 10.1007/s11302-014-9428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutafova-Yambolieva VN, Durnin L. The purinergic neurotransmitter revisited: a single substance or multiple players. Pharmacol Ther. 2014;144:162–191. doi: 10.1016/j.pharmthera.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G, Hoyle CH. Actions of adenine dinucleotides in the Guinea-pig taenia coli: NAD acts indirectly on P1-purinoceptors; NADP acts like a P2-purinoceptor agonist. Br J Pharmacol. 1985;84:825–831. doi: 10.1111/j.1476-5381.1985.tb17376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pustovit KB, Kuzmin VS, Sukhova GS. Effect of exogenous extracellular nicotinamide adenine dinucleotide (nad+) on bioelectric activity of the pacemaker and conduction system of the heart. Bull Exp Biol Med. 2015;159(2):188–191. doi: 10.1007/s10517-015-2919-4. [DOI] [PubMed] [Google Scholar]
- 11.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of beta-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J Biol Chem. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro JA, Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975;54:213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro JA, Cunha RA, Correia-de-Sa P, Sebastiao AM. Purinergic regulation of acetylcholine release. Prog Brain Res. 1996;109:231–241. doi: 10.1016/S0079-6123(08)62107-X. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G, Cocks T, Crowe R, Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. Beta-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- 16.Cheung K, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228(2):254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci. 1998;62(8):697–703. doi: 10.1016/S0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]
- 18.Webb TE, Boluyt MO, Barnard EA. Molecular biology of P2Y purinoceptors: expression in rat heart. J Auton Pharmacol. 1996;16:303–307. doi: 10.1111/j.1474-8673.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai KJ, Tsai YC, Shen CK. G-CSF rescues the memory impairment of animal models of Alzheimer's disease. J Exp Med. 2007;204(6):1273–1280. doi: 10.1084/jem.20062481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrov AM, Naumenko NV, Uzinskaya KV, Giniatullin AR, Urazaev AK, Zefirov AL. Increased non-quantal release of acetylcholine after inhibition of endocytosis by methyl-β-cyclodextrin: the role of vesicular acetylcholine transporter. Neuroscience. 2011;186:1–12. doi: 10.1016/j.neuroscience.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Odnoshivkina UG, Sytchev VI, Nurullin LF, Giniatullin AR, Zefirov AL, Petrov AM. β2-adrenoceptor agonist-evoked reactive oxygen species generation in mouse atria: implication in delayed inotropic effect. Eur J Pharmacol. 2015;765:140–153. doi: 10.1016/j.ejphar.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Abramochkin DV, Nurullin LF, Borodinova AA, Tarasova NV, Sukhova GS, Nikolsky EE, Rosenshtraukh LV (2010) Non-quantal release of acetylcholine from parasympathetic nerve terminals in the right atrium of rats. 95(2):265–273. 10.1113/expphysiol.2009.050302 [DOI] [PubMed]
- 23.Anikina TA, Bilalova GA, Zverev AA, Sitdikov FG. Effect of ATP and its analogs on contractility of rat myocardium during ontogeny. Bull Exp Biol Med. 2007;144:4–7. doi: 10.1007/s10517-007-0239-z. [DOI] [PubMed] [Google Scholar]
- 24.Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 25.Kuzmin VS, Pustovit KB, Abramochkin DV. Effects of exogenous nicotinamide adenine dinucleotide (NAD+) in the rat heart are mediated by P2 purine receptors. J Biomed Sci. 2016;23(50):50. doi: 10.1186/s12929-016-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doisne N, Maupoil V, Cosnay P, Findlay I. Catecholaminergic automatic activity in the rat pulmonary vein: electrophysiological differences between cardiac muscle in the left atrium and pulmonary vein. Am J Physiol Heart Circ Physiol. 2009;297(1):H102–H108. doi: 10.1152/ajpheart.00256.2009. [DOI] [PubMed] [Google Scholar]
- 27.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 28.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91(1):265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 29.Sharma AD, Klein GJ. Comparative quantitative electrophysiologic effects of adenosine triphosphate on the sinus node and atrioventricular node. Am J Cardiol. 1988;61:330–335. doi: 10.1016/0002-9149(88)90939-3. [DOI] [PubMed] [Google Scholar]
- 30.Pelleg A, Belhassen B. The mechanism of the negative chronotropic and dromotropic actions of adenosine 5′-triphosphate in the heart: an update. J Cardiovasc Pharmacol. 2010;56:106–109. doi: 10.1097/FJC.0b013e3181e0f8b2. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelleg A. Electrophysiologic effects of adenosine versus ATP. J Cardiovasc Electrophysiol. 2017;28:E5. doi: 10.1111/jce.13243. [DOI] [PubMed] [Google Scholar]
- 33.Billington RA, Bruzzone S, De Flora A, Genazzani AA, Koch-Nolte F, Ziegler M, Zocchi E. Emerging functions of extracellular pyridine nucleotides. Mol Med. 2006;12:324–327. doi: 10.2119/2006-00075.Billington. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz N, Fliegert R, Adriouch S, Seman M, Guse AH, Haag F, Koch-Nolte F. Activation of the P2X7 ion channel by soluble and covalently bound ligands. Purinergic Signal. 2009;5:139–149. doi: 10.1007/s11302-009-9135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang S, Blair P, Durnin L, Mutafova-Yambolieva V, Sanders K, Ward S. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590(8):1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De Flora A. Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem. 2006;281:31419–31429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- 38.Dreisig K, Kornum BR. A critical look at the function of the P2Y11 receptor. Purinergic Signal. 2016;12(3):427–437. doi: 10.1007/s11302-016-9514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy C. P2Y(11) receptors: properties, distribution and functions. Adv Exp Med Biol. 2017;1051:107–122. doi: 10.1007/5584_2017_89. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura A, Sunggip C, Oda S, Numaga-Tomita T, Tsuda M, Nishida M. Purinergic P2Y receptors: molecular diversity and implications for treatment of cardiovascular diseases. Pharmacol Ther. 2017;180:113–128. doi: 10.1016/j.pharmthera.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Abramochkin DV, Tapilina SV, Sukhova GS, Nikolsky EE, Nurullin LF. Functional M3 cholinoreceptors are present in pacemaker and working myocardium of murine heart. Pflugers Arch. 2012;463(4):523–529. doi: 10.1007/s00424-012-1075-1. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Lu Y, Wang Z. Function of cardiac M3 receptors. Auton Autocoid Pharmacol. 2007;27:1–11. doi: 10.1111/j.1474-8673.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 43.Tapilina SV, Abramochkin DV. Decrease in the sensitivity of myocardium to M3 muscarinic receptor stimulation during postnatal ontogenisis. Acta Nat. 2016;8(2):127–131. [PMC free article] [PubMed] [Google Scholar]
- 44.Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol. 2018;19:638–653. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 45.Glitsch HG, Pott L. Effects of acetylcholine and parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol. 1978;279:655–668. doi: 10.1113/jphysiol.1978.sp012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takei M, Furukawa Y, Narita M, Ren LM, Karasawa Y, Murakami M, Chiba S. Synergistic nonuniform shortening of atrial refractory period induced by autonomic stimulation. Am J Phys. 1991;261:H1988–H1993. doi: 10.1152/ajpheart.1991.261.6.H1988. [DOI] [PubMed] [Google Scholar]
- 47.Chiba S. Selective stimulation of intracardiac pre-ganglionic vagal fibres of the dog atrium. Clin Exp Pharmacol Physiol. 1978;5:465–469. doi: 10.1111/j.1440-1681.1978.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 48.Ribeiro JA, Walker J. Action of adenosine triphosphate on endplate potentials recorded from muscle fibres of the rat-diaphragm and frog sartorius. Br J Pharmacol. 1973;49:724–725. doi: 10.1111/j.1476-5381.1973.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro J, Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975;54(2):213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guarracino JF, Cinalli AR, Fernandez V, Roquel LI, Losavio AS. P2Y13 receptors mediate presynaptic inhibition of acetylcholine release induced by adenine nucleotides at the mouse neuromuscular junction. Neuroscience. 2016;326:31–44. doi: 10.1016/j.neuroscience.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 51.Ribeiro JA, Dominguez ML. Mechanisms of depression of neuromuscular transmission by ATP and adenosine. J Physiol. 1978;74:491–496. [PubMed] [Google Scholar]
- 52.Pelleg A, Hurt CM, Hewlett EL. ATP shortens atrial action potential duration in the dog: role of adenosine, the vagus nerve, and G protein. Can J Physiol Pharmacol. 1996;74:15–22. doi: 10.1139/y95-220. [DOI] [PubMed] [Google Scholar]
- 53.Marvin WJ, Jr, Hermsmeyer K, McDonald RI, Roskoski LM, Roskoski R., Jr Ontogenesis of cholingergic innervation in the rat heart. Circ Res. 1980;46(5):690–695. doi: 10.1161/01.RES.46.5.690. [DOI] [PubMed] [Google Scholar]
- 54.Vyskocil F, Malomouzh AI, Nikolsky EE. Non-quantal acetylcholine release at the neuromuscular junction. Physiol Res. 2009;58(6):763–784. doi: 10.33549/physiolres.931865. [DOI] [PubMed] [Google Scholar]
- 55.Nassenstein C, Wiegand S, Lips KS, G4 L, Klein J, Kummer W (2015) Cholinergic activation of the murine trachealis muscle via non-vesicular acetylcholine release involving low-affinity choline transporters. 29(1):173–180. 10.1016/j.intimp.2015.08.007 [DOI] [PubMed]
- 56.Malomouzh AI, Nikolsky EE, Vyskocil F. Purine P2Y receptors in ATP-mediated regulation of non-quantal acetylcholine release from motor nerve endings of rat diaphragm. Neurosci Res. 2011;71:219–225. doi: 10.1016/j.neures.2011.07.1829. [DOI] [PubMed] [Google Scholar]
- 57.Galkin AV, Giniatullin RA, Mukhtarov MR, Svandova I, Grishin SN, Vyskocil F. ATP but not adenosine inhibits nonquantal acetylcholine release at the mouse neuromuscular junction. Eur J Neurosci. 2001;13:2047–2053. doi: 10.1046/j.0953-816x.2001.01582.x. [DOI] [PubMed] [Google Scholar]