Abstract
Purpose
To evaluate the effect of medical or surgical treatment prior to embryo transfer in women with elevated endometrial BCL6 expression and suspected endometriosis in a prospective, cohort study design at a university-associated infertility clinic.
Methods
All subjects had at least 1 year of unexplained infertility (UI) and each prospectively underwent endometrial biopsy and immunostaining for the oncogene BCL6, prior to embryo transfer during an assisted reproductive technology (ART) cycle. To be included, subjects had to have an abnormal BCL6 result, defined by elevated HSCORE ≥ 1.4. Women that were pre-treated with laparoscopy or medical suppression with GnRH agonist (depot leuprolide acetate; Lupron®, Abbvie, Inc., Chicago, IL) for 2 months were compared to a group that went untreated (controls). Endpoints included implantation rate (IR), clinical pregnancy rate (CPR), and live birth rate (LBR), and as well as cycle characteristics. Miscarriage rate were also compared between treatment and control group.
Results
Women in each group had similar characteristics. Those treated by medical suppression and those undergoing laparoscopy for endometriosis had a significantly higher LBR, (5/10; 50%; 95%CI 23.7 to 76.3%) and (11/21; 52.4%; 95%CI 32.4 to 71.7), respectively, compared to controls (4/54; 7.4%; 95%CI 2.9 to 17.6). An absolute benefit of 44.2% (16/31; 95%CI 24.6 to 61.2) and a number need to treat of 3 for those that received treatment (medical suppression and laparoscopy), compared to no treatment. Miscarriages were significantly more common in the control group.
Conclusions
Women with suspected endometriosis and aberrant endometrial BCL6 expression have worse reproductive outcomes following embryo transfer, including a high miscarriage rate, poor IR, and low LBR and CPR compared to cycles pre-treated with medical and surgical management.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1388-x) contains supplementary material, which is available to authorized users.
Keywords: Endometrium; BCL6; Prognosis; IVF; Pregnancy, endometriosis, miscarriage
Introduction
Endometriosis is an inflammatory, estrogen-stimulated disease affecting 190 million women worldwide. It is estimated that up to 60% to 80% of unexplained infertility is associated with undiagnosed endometriosis [1–3]. While endometriosis has been shown to negatively impact fertility and treatment has been shown to improve pregnancy outcomes [1, 2, 4, 5], the association with infertility, the role of endometriosis in the setting of in vitro fertilization (IVF), remains controversial [6–8].
Less than 10% of women in the SART database carry the diagnosis of endometriosis (www.sart.org) [9], which is unexpectedly close to the general population [10]. Indeed, efforts to diagnose and treat endometriosis prior to IVF have steadily decreased over the past 20 years, according to SART statistics (see Fig. 1). Further, women with a diagnosis of endometriosis that undergo ART cycles have previously undergone surgery to document and possibly treat this condition. Surgery has been shown to improve subsequent IVF outcomes [1, 2, 4]. Given this conundrum and the dramatic under-reporting of endometriosis in the population of women undertaking assisted reproductive technology (ART) cycles, meta-analyses to ascertain an effect of endometriosis based on SART data are unlikely to provide accurate predictions about pregnancy outcomes in women with this disease [11].
Fig. 1.
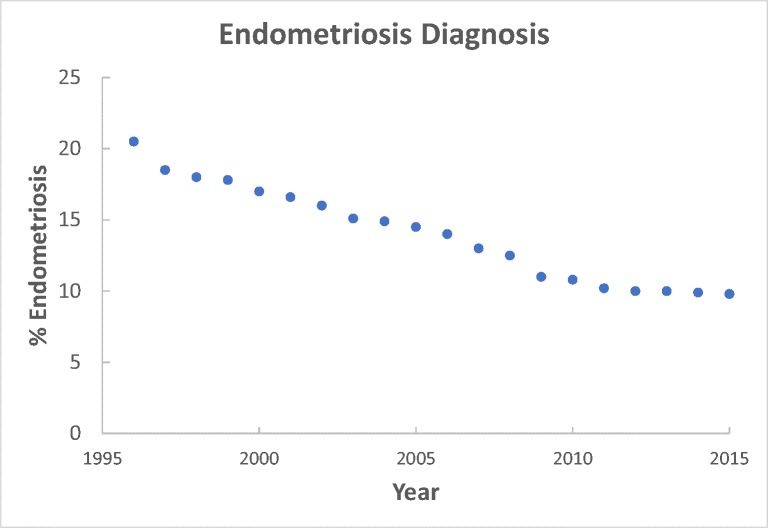
According to SART statistics, the prevalence of endometriosis as a diagnosis in women undergoing IVF has steadily declined. This is likely due to a decrease in laparoscopy and greater reliance on IVF as a primary treatment for unexplained infertility. (Source: provided by ART Surveillance and Research Team, CDC, Atlanta, GA)
The endometrium, like embryo quality, has been shown to be a limiting factor for successful pregnancy in the setting of ART [6]. On average, clinical pregnancy rates for women less than 38 years old remain near or below 50% in most centers (www.sart.org), even when normal PGS–defined euploid embryos are transferred [12]. Endometriosis has been shown to double the miscarriage rate in recent studies during IVF in women with known endometriosis [13]. Given the declining use of laparoscopy before IVF, most women with endometriosis entering ART programs do not know they have this disease. Clearly, more attention needs to be focused on the role of defective endometrium and endometrial receptivity defects as barriers to ART success, especially related to the effect of undiagnosed endometriosis on implantation and pregnancy loss.
We recently reported that many women with UI and failed IVF attempts highly express an endometrial oncogene, BCL6 [14]. BCL6 is a gene repressor that is regulated by progesterone in the endometrium but dramatically over-expressed in conditions associated with systemic inflammation such as endometriosis [15]. BCL6 colocalizes with a histone deacetylase, Sirtuin 1 (SIRT1); together, this complex targets specific gene promoters and is capable of epigenetic modification of gene expression. In the endometrium, BCL6 and SIRT1 have been shown to target progesterone-regulated gene pathways [16]. A diminished response to progesterone likely directly relates to previously published findings that aberrant endometrial BCL6 expression is associated with worse ART outcomes [14]. The objective of this study was to extend those findings in a second dataset and examine women with high endometrial BCL6 expression who underwent surgical or medical treatment for suspected endometriosis prior to embryo transfer, compared to a control group who underwent embryo transfer without intervention.
Materials and methods
Patients and controls
This prospective cohort study was conducted (recruitment, exposure, follow-up, and data collection) between 2011 and 2018, at the Fertility Center of the Carolinas of the Greenville Health System, Greenville, South Carolina, USA. The Institutional Review Board approval was obtained from the Committee for the Protection of Human Subjects (GHS #00013885).
Women, aged 27 to 42 with unexplained infertility (UI), were recruited to undergo LH-timed endometrial biopsy and immunostaining for BCL6 between January 1, 2011, and January 1, 2018. All subjects had elevated BCL6 expression (HSCORE ≥ 1.4) and each underwent an embryo transfer (fresh or frozen). To be included, each woman was required to have regular cyclic menses (25 to 32 days apart), partners with normal sperm parameters according to the World Health Organization [17], and at least one patent fallopian tube. An endometrial biopsy was performed 6 to 10 days after ovulation in ovulatory cycles, at least 1 month prior to embryo transfer. Exclusion included the discovery of significant fibroids (any submucosal or intramural ≥3 cm), male factor infertility, endometritis on endometrial biopsy, or lack of adequate tissue for analysis on the biopsy result. Patients were excluded if complete cycle data was not available related to the fresh or frozen embryo transfer cycle.
Control cycles included those in women with no prior surgical or medical treatment for suspected endometriosis. Decisions regarding treatment were based on physician and patient preferences and subjects were not randomized into any of the three groups. In all cases, embryo transfer occurred immediately following medical suppression, while transfer occurred 2 to 10 months after surgery (mean 6.3 months).
Endometrial biopsies
Endometrial biopsies were performed in all participants using a Pipelle device (Cooper Surgical, Trumbull, CT), 6 to 10 days after a positive urinary LH surge. Endometrial biopsies were placed in 10% buffered formalin and transported to the pathology laboratory of the Greenville Health System for paraffin embedding, sectioning, and immunostaining. Menstrual dating was determined histologically, according to Noyes et al. [18].
Immunohistochemistry
All immunohistochemical staining was performed on an automated system using the Bond immunostainer platform (Leica Biosystems, Buffalo Grove, IL) and read by a certified pathologist (Pathology Associates, GHS, Greenville, South Carolina). Sections of endometrium were stained for BCL6, using an automated system, with clone LN22 as primary antibody (Leica Biosystems), as previously described [15]. Lymph nodes served as positive external controls.
Semi-quantitative assessment of BCL6 expression was assigned using a histological score (HSCORE), which ranged from 0 to 4. HSCORE was calculated using the following equation: HSCORE = ∑ Pi (i + 1)/100, where i = intensity of staining with a value of 1, 2, or 3, (weak, moderate or strong, respectively) and Pi is the percentage of stained epithelial cells at each intensity, varying from 0 to 100% as previously described [19]. All HSCOREs were assigned in a blinded fashion without knowledge of the clinical history or outcome. The HSCORE was assigned by a gynecologic pathologist with over 20 years of experience (DPS) who currently reports results for ReceptivaDx testing. The establishment of a cutoff of ≥ 1.4 HSCORE was based on prior validation using the same pathologist [15]. To reduce any risk of bias, related to HSCORE reading, the pathologist was unaware of any of the clinical data related to the subjects ART cycles or outcomes.
Variables
The following variables were analyzed: age, body mass index (BMI), gonadotropin dose (IUs), number of oocytes retrieved, fertilization rate, number of embryos transferred, clinical pregnancy rate (CPR), live birth rate (LBR), and implantation rate (IR). CPR was defined as a pregnancy documented by ultrasound that shows a gestational sac in the uterus with a yolk sac or fetal pole. A positive and rising hCG serum level, without evidence of an intrauterine sac on ultrasound (biochemical pregnancy), was not counted as a pregnancy, but designated a biochemical pregnancy. Miscarriages were defined as the complete loss of a pregnancy after visualization of a gestational sac with fetal pole and/or yolk sac. Anti-Mullerian hormone (AMH) was also compared between groups.
We used the HSCORE results with a threshold level of BCL6 immunostaining ≥ 1.4, based on previous receiver operating characteristic (ROC) analysis [15]. Errors in reading HSCOREs, while of low probability, would be expected to be equally distributed between treatment groups.
Treatment protocols
Treatment groups were not randomly selected and based on discussions between physician and patient. Two treatment strategies were compared, including depot leuprolide acetate (Lupron®, Abbvie, Inc., Chicago, IL) for 2 months followed by frozen embryo transfer, or fresh IVF initiated at the time of next menses. Hormonal add-back therapy during medical suppression consist of norethindrone acetate (Abbvie by Glenmark Pharmaceuticals, Ltd., Goa, India). In the surgically treated group, diagnostic laparoscopy with excision or ablation of suspected endometriosis was performed followed by expectant management or advancement to IVF or FET. The majority of subjects in this study with high endometrial BCL6 expression remained untreated and served as the control group.
Embryo transfer occurred 5 to 6 days following oocyte retrieval (in fresh cycles) or during programmed estrogen/progesterone uterine replacement cycles (in frozen embryo transfer—FET cycles). The protocol used for FET cycles was standardized and included downregulation with daily subcutaneous leuprolide acetate followed by series of estrogen patches and progesterone supplementation. The protocols were similar for both fresh and frozen cycles between treatment and control groups. Fresh cycles varied between long luteal and antagonist protocols based on individual patient characteristics. Women receiving 2 months of depot leuprolide acetate all proceeded directly to IVF or FET, while those women treated with laparoscopy underwent embryo transfer within 2 to 10 months of surgery (mean 6.2 months). A histogram showing the distribution of time from surgery to transfer is shown in supplemental Fig. 1. Cycle characteristics (Table 1) were based on the IVF cycle in which embryos were created, in cases where FET cycles were included.
Table 1.
Patient characteristics of the sample population based on treatment
| Characteristics | GnRHa | Laparoscopy | No treatment | p |
|---|---|---|---|---|
| n = 10 | n = 21 | n = 54 | ||
| Age—years (mean ± SD) | 34.3 ± 3.2 | 34.3 ± 4.3 | 34.4 ± 3.7 | 0.9a |
| BMI—median (range) | 24.3 (18.5–32.4) | 22.8 (19.2–38.4) | 23.3 (17.9–44.6) | 0.9b |
| Anti-Müllerian hormone (AMH)—mean (range) | 2.5 (1.5–4.8) | 2.2 (0.3–5.4) | 1.9 (0.3–8.6) | 0.4b |
| Previous IVF failure n (%) | 4 (40) | 6 (28.6) | 13 (23.2) | 0.6 |
| Parity | ||||
| 0 | 10 | 15 | 48 | |
| 1 | 0 | 4 | 5 | 0.1c |
| 2 + | 0 | 2 | 1 | |
aANOVA test
bKruskal-Wallis test
cChi-square for trend test
dNumber of sacs/number of embryo transferred
Data sources/measurement
Data were obtained from the SART database and institutional medical records. Data were analyzed as mean (± standard deviation) or as median (range), depending if they were normally distributed.
Bias
Two researches (BAL, LJC) collectively verified the electronic records (SART database) to reduce bias. The biopsy HSCOREs were read by a blinded experienced pathologist without knowledge of patient characteristics or ART outcome. The HSCORE consistency was previously validated by two independent readers (unpublished validation results). The pregnancy tests and pregnancy ultrasound results were performed without direct knowledge of BCL6 results, although a history of prior treatment was not intentionally blinded.
Study size
Calculation of sample size was performed as a superior trial for binary outcome according to the literature [20]. All calculations considered an alpha error of 5% and a power of 80%. Based on similar studies in the literature, we expect a 20% pregnancy rate for those without treatment and a 76% in those who underwent treatment [4]. With these assumptions, we would need at least 9 cases in each group.
Quantitative variables
Age, BMI, and gonadotropin use were counted as continuous variables. Parity and number of embryos transferred were analyzed as nominal variables. Prior IVF failure was compared between groups. The cycle type (fresh or FET) was analyzed as categorical variables. Treatment was analyzed as a categorical variable and was compared to the outcome of CPR (pregnant, non-pregnant), live birth rate (LBR), implantation rate (IR), fertilization rate (FR), and miscarriage rate (MR). Three groups were compared: medical suppression (depot leuprolide acetate for 2 months; GnRHa), laparoscopy (L/S), or no-treatment (control group) categories.
Statistical methods
Chi-square for trend, chi-square, Fisher’s exact test, relative risk, and 95% confidence intervals were used for comparisons of categorical data. Parametric data were compared between groups using ANOVA if data had a Gaussian distribution. Gaussian distribution was verified by D’Agostino and Pearson omnibus normality test. A Kruskal-Wallis test was used if a Gaussian distribution was not present. Statistical analysis was performed with GraphPad Prism version 6.00 for Mac, (GraphPad Software, La Jolla California, USA).
Results
Participants and descriptive data
A total of 85 cycles met the inclusion criteria and were analyzed. As shown in Table 1, there were 10 cycles in women who completed 2 months of medical suppression with GnRH agonist (GnRHa), 21 cycles followed laparoscopic surgery (L/S), and 54 cycles with no additional treatment before embryo transfer (controls). The proportion of fresh versus frozen transfer was unequally distributed between groups; one cycle in the surgical group was an FET (5%), compared to 3 of 10 (30%) of the GnRHa group and 10 of 54 (18.5%) of controls. Overall, success rates in our program for IVF and FET cycles are similar (~ 50%); therefore, a difference in cycle type would not be expected to impact pregnancy outcomes. Age, BMI, parity, number of oocytes obtained, gonadotropin use, number of embryos transferred fertilization rate, and AMH were similar between each treatment groups (Tables 1 and 2). While there were more subjects with prior IVF failure in the GnRHa treatment group, this difference did not reach significance (Table 1). Fertilization rates, CPR, LBR, and IR as well as miscarriage rate were significantly different between treatment and no-treatment groups (Table 2).
Table 2.
Cycle characteristics in the sample population by treatment type
| Cycle type (n) | GnRHa n = 10 |
Laparoscopy n = 21 |
No treatment n = 54 |
p |
|---|---|---|---|---|
| In vitro fertilization (IVF) | 7 | 20 | 44 | 0.9a |
| Frozen embryo transfer (FET) | 3 | 1 | 10 | |
| Gonadotropin used (IU) median (range) | 2006 (750–3375) | 2400 (1163–4875) | 2100 (750–6300) | 0.1b |
| Number of oocytes median (range) | 13 (5–56) | 10 (2–23) | 13 (2–48) | 0.5b |
| PGD (n; %) | 0(0) | 0 (0) | 2 (3.9) | n.a. |
| Fertilization rate (%) Fert oocytes/oocytes retrieved |
77/172 (44.7) |
113/250 (45.2) |
207/840 (24.6) |
< 0.0001a |
| Embryos transferred median (range) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.7b |
| Implantation rate (IR; %) | 42.9(9/21) | 40(18/45) | 11.7(12/103) | < 0.0001a |
| Clinical pregnancy rate (%)c Viable pregnancies/transfer |
6/10 (60) |
13/21 (61.9) |
8/54 (14.8) |
0.002a |
| Live birth rate, n (%) Live births/transfer |
5/10 (50) |
11/21 (52.4) |
4/54 (7.4) |
0.1a |
| Relative risk (95%CI) | 16/31 | 4/54 | ||
| 6.9 (2.5 to 18.9) | ||||
| Singleton (n) | 3 | 9 | 6 | 0.5a |
| Twins (n) | 3 | 3 | 2 | |
| Triplets (n) | 0 | 1 | 0 | |
| Miscarriage rate (%) | 3/19 (15.7%) | 4/8 (50%) | 0.001d | |
aChi-square for trend
bKruskal-Wallis test
cNo. intrauterine pregnancy/no. transferred
dFisher’s exact test
Outcome data and main results
All of the subjects that underwent surgery were found to have endometriosis, consistent with previous reports in similar populations with UI [15]. The cycle characteristics were similar between groups including number of oocytes retrieved, gonadotropin use, fertilization rates, and numbers of embryos transferred (Table 2). Based on treatment data, women who underwent medical suppression or surgery had a significantly higher CPR (6/10; 60%; 95%CI 31.3 to 83.2) and (13/21; 61.9%; 95%CI 40.9 to 79.2), respectively, compared to cycles in untreated women (8/54; 14.8; 95%CI 7.7 to 26.6). Life birth rate was similarly better in both GnRHa and surgery treatment groups (50% and 52%, respectively) (Table 2); pooled treatment group data on LBR compared to controls was strikingly better as well (GnRHa and L/S—16/31; 51.6%; 95%CI 34.8 to 68), compared to controls (4/54; 7.4%; 95%CI 2.9 to 17.6). These results yield a relative risk of achieving a live birth rate of 6.9 (95%CI = 2.5 to 18.9; i.e., 16 out of 31 in both treatment groups vs. 4 out of 54 in the no treatment (control group). An absolute benefit of 44.2% (95% CI 24.6 to 61.2) and a number need to treat of 3.
A high miscarriage rate was seen in the control cycles compared to pre-treated cycles. Based on overall clinical pregnancies, the miscarriage rate for untreated cycles was 50% (4/8; 95%CI 21.5 to 78.5), compared to 16.7% (1/6; 95%CI 3 to 56.4) and 15.4% (2/13; 95%CI 4.3 to 42.2) in the GnRHa and L/S groups, respectively. When treated groups are combined (3/19; 15.8% - 95%CI 5.5 to 37.6) compared to untreated cycles combined, the relative risk reduction of miscarriage is 68.4% (95%CI − 10 to 90.9).
These findings raise serious questions related to the role of undiagnosed endometriosis on ART outcomes following fresh or frozen embryo transfer. One reason this effect of endometriosis on ART success may have been ignored or underappreciated in the past is our dependence on previously diagnosed endometriosis. Large contemporaneous studies of women previously diagnosed with endometriosis show little effect of this diagnosis on ART outcomes [24]. It should be remembered that most women who carry the diagnosis of endometriosis have likely already been surgically treated and therefore may not be the same as women that have not yet been diagnosed or treated. Our data, and the data from Linda Giudice (6) support this concern regarding prior surgical treatment.
Recent studies have documented an increased risk for miscarriage in women with endometriosis, especially mild disease [8, 21, 22], though not all studies agree [23]. In the current analysis of our data, we demonstrate that miscarriages are common in the control group and that treatment can increase pregnancy rates and reduce the risk of miscarriage. No study, to date, has performed this type of investigation where endometriosis is detected using endometrial biomarker expression. When subjects with suspected endometriosis are untreated, pregnancy rates are low and miscarriage rates are high. LBR and CPR and miscarriage rate were each improved by treatment prior to the next transfer. Recently, Mohmed and colleagues reported that pretreatment with GnRH agonist therapy in women with known endometriosis resulted in significantly higher LBR and CPR, in the next embryo transfer [5, 22]. Our results were of a greater magnitude, possibly because our GnRHa subjects were not preselected using surgical intervention.
Conclusions
Abnormal expression of endometrial BCL6 (> 1.4 HSCORE) was associated with poor reproductive outcomes in IVF cycles similar to previously reports [14]. High BCL6 expression is a validated biomarker for detection of endometrial inflammation and it is commonly associated with the finding of endometriosis in women with UI [15] and associated with inflammatory proteins including STAT3, IL-6, Kras, and SIRT1 [16].
This is the first paper to examine the effect of treatment on ART outcomes based on abnormal endometrial BCL6 expression. Studies are underway to perform RCT studies to validate these findings at independent sites. While preliminary, these data provide a proof of concept, and suggest that an endometriosis-associated phenotype (high BCL6 expression) is associated with reduced ART success after both fresh and frozen transfers. These studies also show that endometrial receptivity defects can be rescued using both medical and surgical treatment prior to embryo transfer in the setting of ART. The benefit of both medical and surgical approaches appears similar. Others have shown that unexplained IVF failure is associated with endometriosis and that surgical therapy improves outcomes in both IVF and natural cycles after treatment [4, 5]. The use of BCL6 to identify suspected endometrial receptivity defects is similar to prior studies finding aromatase overexpression in women who fail in IVF, by Brosens et al. [25]. Analogous to our current study using GnRHa, we previously demonstrated the usefulness of aromatase inhibitors in women with endometrial receptivity defects related to abnormal integrin expression [26]. Since BCL6 is associated with the same inflammatory pathways as aromase expression [27], we now suggest that elevated BCL6 and aromatase are common defects related to progesterone resistance and estrogen dominance that exist in women with endometriosis.
Inflammation is associated with progesterone (P)-resistance that has an immune-regulated impact on endometrial function [28–30]. Abnormal endometrial BCL6 expression appears to participate in the development of P-resistance in the endometrium, which might account for poor reproductive outcomes reported in this and prior studies [14]. As progesterone is essential for pregnancy, P-resistance would logically be associated with downstream changes in gene expression in the endometrium, as previously shown [31]. BCL6 pairs with the histone deacetylase SIRT1, which is stimulated by the oncogene, Kras. Both are intimately associated with epigenetic alterations in endometrial gene expression associated with P-regulated pathways [16]. This phenomenon of P-resistance might account for the unexpectedly high miscarriage rate that we observed in the untreated women suspected endometriosis in this study (Table 2). An appreciation of endometriosis and its effect on miscarriage is limited and deserves more attention [13]. This inflammatory pathway may also account for the poor fertilization rate seen in the untreated controls, compared to the two other treatment groups. The results of improved pregnancy outcomes were observed in subjects with occult (hithertofore undiagnosed) endometriosis. Whether similar results would apply to known cases of endometriosis remains to be tested.
The mean CPR published by SART in women with unknown factors, for all ages, is 27.1% [32], similar to the overall CPR of 35.9% (95%CI 27.3 to 45.5%) found in our study. Thus, a majority of untreated subjects will not achieve pregnancy based on community standards. Even as overall IVF success rates improved in the past 10 years, they remain below 50% per cycle for most centers. The use of laparoscopy in the diagnostic workup for infertile women has dramatically decreased [33], and the proportion of women in the SART database with endometriosis as their diagnosis has steadily declined (Fig. 1). Based on our findings, one can ponder whether most unexplained cycle failures in the setting of ART could be due to unrecognized or untreated endometriosis.
Medical suppression using GnRH agonist therapy has been studied in the context of ART cycles [34]. Long, but not short suppression protocols were found to be associated with some improvements in pregnancy rates [35]. The choice of 2 months of GnRH agonist was arbitrary and deserves further study. In addition, the use of new generation, orally active GnRH antagonists, including elagolix, may prove useful for suppression of endometriosis in the setting of IVF as well. One question to be addressed in future studies is whether surgical or medical treatment of elevated endometrial BCL6 would reduce these levels to normal BCL6 on repeat biopsy. The use of biomarkers such as BCL6 could be useful for comparisons between treatments, such as oral contraceptives, aromatase inhibitors, or newer generation GnRH antagonists, and for evaluation of length of medical suppression.
This study has some limitations. The subjects were neither randomized nor was their treatment blinded to the physicians involved in their embryo transfers. The effect of endometrial scratching may have had a benefit on implantation rates before IVF, although data on this topic are controversial and still being investigated [36, 37]. Since biopsy was done closer to the time of embryo transfer in the non-treatment group, this seems unlikely to be a confounding factor. More FET cycles were performed in the non-treatment group. When analyzed separately, the effect of surgical or medical therapy persisted even when only fresh IVF cycles were compared. Finally, we had low power to study the effect of treatment on miscarriage prevention.
There are clear strengths in this study. In this cohort study, subjects were prospectively biopsied and evaluated at a common point in their infertility evaluation. We used clinically relevant outcomes, including IR, CPR, and LBR. The follow-up for all patients was uniform and complete, using non-biased assessment for pregnancy outcome. The pregnancy tests and ultrasound results were performed without knowledge of the biopsy results. All treatment cycles for each group were included (not selectively omitted). The biopsies were read by a single blinded pathologist without knowledge of IVF outcome. The pathologist and primary researcher (BAL) had independently verified the HSCORE concordance in 30 test cases (data not shown). The number of subjects with elevated BCL6 in our study was large enough and allowed us to perform a post hoc power analysis for the association of treatment category and pregnancy outcome (> 99% power), showing we had adequate power to show benefit. We expect our data will have external validity since our results are similar to those published in the SART database.
In conclusion, the aberrant expression of endometrial BCL6 is associated with worse reproductive outcomes after embryo transfer, compared to cycles pre-treated with GnRH agonist or surgery for endometriosis. Women with suspected endometrial receptivity defects based on BCL6 testing may benefit from medical or surgical therapy, prior to embryo transfer, compared to traditional ART protocols. Elevated expression of BCL6 expression was associated with the finding of endometriosis at laparoscopy, and endometriosis should be considered as the most likely confounding diagnosis prior to undergoing embryo transfer in this setting [14]. Downregulation with GnRH agonist can be helpful in restoring near-normal implantation rates. In addition, a new, non-peptide, orally active GnRH antagonists, now available [38], may provide further treatment options for women with IVF failure and suspected defects in endometrial receptivity. Randomized controlled trials (RCT) are needed to confirm the benefit of directed therapy for suspected endometrial receptivity defects in women undergoing ART cycles.
Electronic supplementary material
(DOCX 160 kb)
Funding
This study was supported by NICHD/NIH R01 HD067721 Grant 99999.003035/2015-08 (BAL) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior: CAPES/PROAP (RFS).
Compliance with ethical standards
The Institutional Review Board approval was obtained from the Committee for the Protection of Human Subjects (GHS #00013885).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsuji I, Ami K, Miyazaki A, Hujinami N, Hoshiai H. Benefit of diagnostic laparoscopy for patients with unexplained infertility and normal hysterosalpingography findings. Tohoku J Exp Med. 2009;219:39–42. doi: 10.1620/tjem.219.39. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa K, Ohgi S, Horikawa T, Kojima R, Ito M, Saito H. Laparoscopy should be strongly considered for women with unexplained infertility. J Obstet Gynaecol Res. 2007;33:665–670. doi: 10.1111/j.1447-0756.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 3.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 4.Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84:1574–1578. doi: 10.1016/j.fertnstert.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed AMF, Chouliaras S, Jones CJP, Nardo LG. Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;156:177–180. doi: 10.1016/j.ejogrb.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Prapas Y, Goudakou M, Matalliotakis I, Kalogeraki A, Matalliotaki C, Panagiotidis Y, Ravanos K, Prapas N. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod BioMed Online. 2012;25:543–548. doi: 10.1016/j.rbmo.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Díaz I, Navarro J, Blasco L, Simón C, Pellicer A, Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril. 2000;74:31–34. doi: 10.1016/S0015-0282(00)00570-7. [DOI] [PubMed] [Google Scholar]
- 8.Zullo F, Spagnolo E, Saccone G, Acunzo M, Xodo S, Ceccaroni M, et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil Steril 2017;108:667–72.e5. [DOI] [PubMed]
- 9.National Summary Report [Internet] [cited 2017 Jul 21];Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2014.
- 10.D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330. [DOI] [PubMed] [Google Scholar]
- 11.Senapati S, Sammel MD, Boudhar S, Morse CB, Barnhart KT. The impact of endometriosis on IVF: an evaluation using the society of assisted reproductive technologies (SART) database. Fertil Steril. 2014;102:e48–e49. doi: 10.1016/j.fertnstert.2014.07.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–1703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 13.Pallacks C, Hirchenhain J, Krüssel J-S, Fehm TN, Fehr D. Endometriosis doubles odds for miscarriage in patients undergoing IVF or ICSI. Eur J Obstet Gynecol Reprod Biol. 2017;213:33–38. doi: 10.1016/j.ejogrb.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Almquist LD, Likes CE, Stone B, Brown KR, Savaris R, Forstein DA, Miller PB, Lessey BA. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: a cohort study. Fertil Steril. 2017;108:1063–1069. doi: 10.1016/j.fertnstert.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, Schammel DP, Young SL. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci. 2016;23:1234–1241. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo J-Y, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW, Lessey BA. KRAS activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep. 2017;7:6765. doi: 10.1038/s41598-017-04577-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 2010.
- 18.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/S0015-0282(16)30062-0. [DOI] [PubMed] [Google Scholar]
- 19.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 20.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. doi: 10.2307/2531021. [DOI] [PubMed] [Google Scholar]
- 21.Kohl Schwartz AS, Wölfler MM, Mitter V, Rauchfuss M, Haeberlin F, Eberhard M, et al. Endometriosis, especially mild disease: a risk factor for miscarriages. Fertil Steril. 2017;108:806–14.e2. doi: 10.1016/j.fertnstert.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Santulli P, Marcellin L, Menard S, Thubert T, Khoshnood B, Gayet V, Goffinet F, Ancel PY, Chapron C. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum Reprod. 2016;31:1014–1023. doi: 10.1093/humrep/dew035. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Huang R, Cai M, Liang X. Endometriosis has no negative impact on outcomes of in vitro fertilisation in women with poor ovarian response. BJOG. 2016;123 Suppl 3:76–81. doi: 10.1111/1471-0528.14018. [DOI] [PubMed] [Google Scholar]
- 24.González-Comadran M, Schwarze JE, Zegers-Hochschild F, Souza M do CB, Carreras R, Checa MÁ. The impact of endometriosis on the outcome of assisted reproductive technology. Reprod Biol Endocrinol. 2017;15:8. doi: 10.1186/s12958-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, Hägglund L, Mardesic T, Varila E, Zech J, Brosens I. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19:352–356. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- 26.Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, 3rd, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27:881–888. doi: 10.1093/humrep/der452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox C, Morin S, Jeong J-W, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril 2016;105:873–884. [DOI] [PMC free article] [PubMed]
- 28.Bulun SE, Cheng Y-H, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 30.Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31:109–124. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- 31.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 32.Website [Internet] . [cited 2018 Apr 19]. Available from: National Summary Report [Internet][cited 2017 Jul 21];Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2014.
- 33.Feinberg EC, Levens ED, DeCherney AH. Infertility surgery is dead: only the obituary remains? Fertil Steril. 2008;89:232–236. doi: 10.1016/j.fertnstert.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 34.Yu LP, Liu N, Liu Y. Effect of luteal-phase gonadotropin-releasing hormone agonist administration on pregnancy outcome in IVF/ICSI cycles: a systematic review and meta-analysis. Zhonghua Fu Chan Ke Za Zhi. 2016;51:850–858. doi: 10.3760/cma.j.issn.0529-567X.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Siristatidis CS, Gibreel A, Basios G, Maheshwari A, Bhattacharya S. Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database Syst Rev 2015;CD006919. [DOI] [PMC free article] [PubMed]
- 36.Lensen S, Sadler L, Farquhar C. Endometrial scratching for subfertility: everyone’s doing it. Hum Reprod. 2016;31:1241–1244. doi: 10.1093/humrep/dew053. [DOI] [PubMed] [Google Scholar]
- 37.van Hoogenhuijze NE, Torrance HL, Mol F, Laven JSE, Scheenjes E, Traas MAF, Janssen C, Cohlen B, Teklenburg G, de Bruin JP, van Oppenraaij R, Maas JWM, Moll E, Fleischer K, van Hooff MH, de Koning C, Cantineau A, Lambalk CB, Verberg M, Nijs M, Manger AP, van Rumste M, van der Voet LF, Preys-Bosman A, Visser J, Brinkhuis E, den Hartog JE, Sluijmer A, Jansen FW, Hermes W, Bandell ML, Pelinck MJ, van Disseldorp J, van Wely M, Smeenk J, Pieterse QD, Boxmeer JC, Groenewoud ER, Eijkemans MJC, Kasius JC, Broekmans FJM. Endometrial scratching in women with implantation failure after a first IVF/ICSI cycle; does it lead to a higher live birth rate? The SCRaTCH study: a randomized controlled trial (NTR 5342) BMC Womens Health. 2017;17:47. doi: 10.1186/s12905-017-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Rowan JP, Duan WR, Ng J, Schwefel B, Thomas JW, Jain RI, Chwalisz K. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28–40. doi: 10.1056/NEJMoa1700089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 160 kb)


