Abstract
This study analyzed the interaction of commercial monoclonal anti-methylglyoxal antibodies that predominantly recognize argpyrimidine with unmodified and modified model proteins and small heat shock proteins. These antibodies specifically recognize methylglyoxal (MG)-modified bovine serum albumin and lysozyme, but they react equally well with both unmodified and MG-modified HspB1. Mutation R188W decreased the interaction of these antibodies with unmodified HspB1, thus indicating that this residue participates in the formation of antigenic determinant. However, these antibodies did not recognize either short (ESRAQ) or long (IPVTFESRAQLGGP) peptides with primary structure identical to that at Arg188 of HspB1. Neither of the peptides obtained after the cleavage of HspB1 at Met or Cys residues were recognized by anti-argpyrimidine antibodies. This means that unmodified HspB1 contains a discontinuous epitope that includes the sequence around Arg188 and that this epitope is recognized by anti-argpyrimidine antibodies in unmodified HspB1. Incubation of HspB1 with MG is accompanied by the accumulation of hydroimidazolones, but not argpyrimidines. Therefore, conclusions based on utilization of anti-argpyrimidine antibodies and indicating that HspB1 is the predominant and preferential target of MG modification in the cell require revision.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-00975-3) contains supplementary material, which is available to authorized users.
Keywords: Small heat shock proteins, Methylglyoxal, HspB1, Hsp27
Introduction
Methylglyoxal (MG) is a natural low-molecular-weight dicarbonyl compound that is formed both enzymatically and non-enzymatically during the metabolism of carbohydrates, certain amino acids, and ketone bodies (Chakraborty et al. 2014; Rabbani and Thornalley 2012). The MG concentration is strictly regulated by glyoxalases, but in highly glycolytic proliferating cells (i.e., tumor cells) or in cases of diabetes, its concentration is increased, and it can modify DNA and certain proteins combined in the dicarbonyl proteome (Rabbani et al. 2016). Chemical modifications of DNA and proteins by MG induce changes in their structure and properties and lead to the development of various diseases (Chakraborty et al. 2014; Rabbani et al. 2016). Therefore, it is greatly important to identify the main MG targets and to analyze the effects induced by MG modifications.
To analyze the dicarbonyl proteome and to detect proteins modified by MG, it is desirable to obtain antibodies that recognize modified proteins. Modification by MG results in the formation of a number of different products (Rabbani and Thornalley 2012), and therefore, the production of specific anti-MG antibodies is a very complicated problem. Upon searching the available antibodies, we found three types of monoclonal anti-MG antibodies. Antibodies of 9E7 clone provided by Biorbyt, Novus Biologicals, StressMarq Biosciences, and Arigo Biolaboratories are claimed to recognize proteins modified by MG at Arg residues. Antibodies of the 3D11 clone (Cell Biolabs, Merck) predominantly recognize hydroimidazolones that are formed after arginine modification by MG. Finally, antibodies of clone 3C provided by Abcam, the Genox Corporation, JaICA, and AdipoGen Life Sciences are claimed to predominantly interact with the argpyrimidine that forms after the modification of arginine by MG. These antibodies were developed, well characterized, and have been widely used to analyze MG-modified proteins (Oya et al. 1999).
By using this type of antibodies, which we refer to as anti-argpyrimidine antibodies (AArgPyrAb), Sakamoto et al. found that the small heat shock protein HspB1 (Hsp27) is the main target of MG modification in a number of cancer cells (Sakamoto et al. 2002). It was also found that modification by MG led to the formation of argpyrimidine, that Arg188 was the predominant site of modification, and that modification by MG was accompanied by an increase of the antiapoptotic activity of HspB1 (Sakamoto et al. 2002). AArgPyrAb detected HspB1 modified by MG in glomerular mesangial cells (Padival et al. 2003). This effect was especially pronounced in diabetic rats and was accompanied by a decrease of its affinity to cytochrome c (i.e., a decrease of its antiapoptotic activity) (Padival et al. 2003).
By using AArgPyrAb, HspB1 (Hsp27) was determined as the main target of MG modification in endothelial cells (Schalkwijk et al. 2006) and in human non-small cell lung cancer (van Heijst et al. 2006). HspB1 was the major argpyrimidine-containing protein in lens epithelial cells with brown cataracts, and MG modification increased the chaperone-like and antiapoptotic activity of HspB1 (Oya-Ito et al. 2006). HspB1 was revealed as the major MG-modified and argpyrimidine-containing protein in human hearts, and diabetes was accompanied by an increase of its modification and a decrease of its antiapoptotic activity (Gawlowski et al. 2009). Thus, the literature indicates that in different cells and tissues, HspB1 is the major target of MG modification, although the physiological effect of this modification remains controversial. Certain publications indicate an MG-induced increase of antiapoptotic activity (Oya-Ito et al. 2006; Sakamoto et al. 2002; van Heijst et al. 2006), whereas other publications indicate a decrease of this activity (Antognelli et al. 2014; Padival et al. 2003; Wang et al. 2014).
An analysis of the literature data (Gawlowski et al. 2009; Oya-Ito et al. 2006, 1999; Padival et al. 2003; Sakamoto et al. 2002; Schalkwijk et al. 2006; van Heijst et al. 2006) raises certain questions. The first concerns why HspB1 is the major or even the single target of MG modification among many cellular proteins, and why MG does not modify other small heat shock proteins that are expressed in large quantities and very similar to HspB1. The second concerns why and how MG selectively modifies only one Arg188 of HspB1 in the cell. The final question concerns why modification by MG is accompanied by the specific accumulation of only the argpyrimidine derivative of HspB1 although this is a rather rare product of MG modification, and these products are usually predominantly presented in the form of different hydroimidazolones (Ahmed et al. 2005; Gao and Wang 2006). In order to answer these questions, we analyzed the interaction of HspB1 and other small heat shock proteins with MG in vitro. Furthermore, we investigated the interaction of commercial anti-methylglyoxal antibodies with various MG-modified proteins.
Materials and methods
Proteins
Recombinant human HspB1, its point mutants associated with congenital diseases, and human HspB5 (αB-crystallin), HspB6 (Hsp20), and HspB8 (Hsp22) were purified as described previously and stored in buffer B (20 mM Tris/acetate pH 7.6, containing 10 mM NaCl, 0.1 mM EDTA, 0.1 mM PMSF and 2 mM DTT) at − 20 °C (Chalova et al. 2014; Gerasimovich et al. 2017; Muranova et al. 2015; Nefedova et al. 2015; Weeks et al. 2018). All proteins were homogeneous according to SDS-gel electrophoresis (Laemmli 1970).
MG modification
In order to remove DTT and to change the buffer, all proteins were passed through a NAP column equilibrated with 10 mM phosphate (pH 7.4) containing 150 mM NaCl and 0.01% sodium azide. The protein concentration was determined spectrophotometrically using A2800.1% values of 1.775, 0.694, 0.582, and 1.225 for HspB1, HspB5, HspB6, and HspB8, respectively. All proteins (1.0–1.5 mg/ml) were incubated with MG at concentrations in the range of 10 μM–3 mM at 37 °C for 24–48 h. The reaction was stopped by the addition of excess β-mercaptoethanol, and if required, the mixture was desalted on a NAP column. A similar procedure (except for the addition of β-mercaptoethanol) was used for the MG modification of bovine serum albumin and chicken egg lysozyme.
Chemical cleavage of HspB1
CNBr cleavage of HspB1 was performed either in 70% trifluoroacetic or 70% formic acid at a molar ratio CNBr/HspB1 of 100/1 for 24 h at 37 °C. HspB1 contains only two Met residues (Met 1 and Met 169), which means that CNBr cleavage leads to the formation of large (residues 2–169) and small (residues 170–205) peptides. Cleavage at a single Cys137 was performed as follows. HspB1 (4.5 mg/ml) was incubated in 6 M urea containing 150 mM Tris/acetate pH 8.0 and 1.5 mM EDTA with 0.5 mM DTT for 30 min at 37 °C. An equal volume of 10 mM 5,5′-dithiobis(2-nitrobenzoic acid) dissolved in urea buffer was added to the protein solution, and the incubation at 37 °C was continued for another 30 min. Solid KCN was added up to the final concentration of 50 mM, and the resulting mixture was incubated for 24 h at 37 °C.
Crosslinking of synthetic peptide to bovine serum albumin
The peptide IPVTFESRAQLGGP containing C-terminal cysteine and corresponding to the sequence restricted by residues 181–194 and containing Arg188 of HspB1 was synthesized by Peptide 2.0 (USA). This peptide was crosslinked to bovine serum albumin by sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Pierce) by using the procedure recommended by the manufacturer.
HeLa cell culture
HeLa cells were obtained from the cell culture collection of the Institute of Cytology Russian Academy of Sciences, St. Petersburg. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1 mM sodium pyruvate, 10 μg/ml gentamycin, and 10% fetal bovine serum. The medium was renewed 2 to 3 times per week. The cells were lysed by sonification in a Branson S250D ultrasonic disintegrator in lysis buffer at pH 7.4 and containing 20 mM HEPES, 10 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 14 mM mercaptoethanol, and 0.1 mM PMSF. The protein concentration of lysates was estimated using a Bradford microassay (Bradford 1976).
Electrophoresis and blotting
Electrophoresis was performed in 12.5–15% polyacrylamide gel in the presence of SDS (Laemmli 1970). Proteins and peptides were transferred to nitrocellulose or PVDF membrane in Towbin buffer (Towbin et al. 1979) (25 mM Tris, 193 mM glycine, 10–20% ethanol) in the presence or absence of 0.1% SDS. The membrane was blocked by TBST buffer (10 mM Tris/HCl, 150 mM NaCl pH 7.5, 0.1% Tween-20) containing 5% nonfat dry milk. Afterwards, blots were incubated with primary mouse anti-methylglyoxal antibodies 3C ab194226 Abcam predominantly interacting with argpyrimidine or 3D11 mouse anti-methylglyoxal monoclonal antibodies STA-11 (Cell BioLabs), or with 9E7 methylglyoxal antibodies conjugated with HRP orb396689 (Biorbyt). After washing, the blots were incubated (if needed) with secondary antimouse antibodies conjugated with horseradish peroxidase and developed in TBS buffer (50 mM Tris/HCl, pH 7.5) containing 0.06% diaminobenzidine and 0.003% hydrogen peroxide.
Results
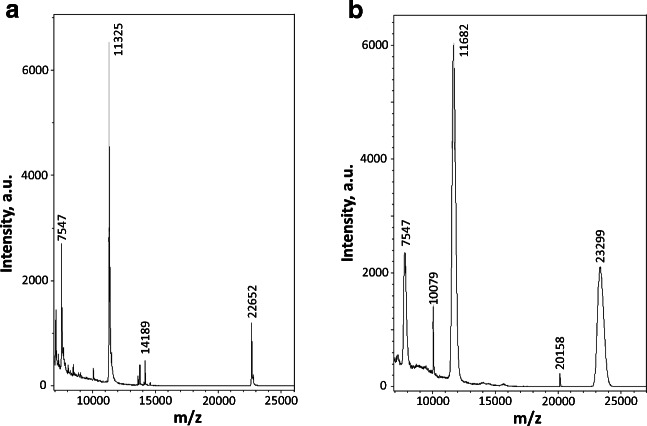
All recombinant proteins used in this investigation were homogeneous according to SDS-gel electrophoresis. However, in order to exclude any posttranslational modifications, we determined the molecular weight of all proteins by means of mass spectroscopy (MS). According to the MS data, the human recombinant HspB1 used in this investigation had a molecular weight of 22,652, which corresponds to the molecular weight of human HspB1 (22,651.33) lacking the first Met residue (Fig. 1a). The data indicate that the analyzed protein does not contain any posttranslational modifications.
Fig. 1.
MALDI mass spectroscopy of unmodified human HspB1 (a) and of HspB1 incubated at 37 °C with 1 mM MG for 24 h (b). Representative data of two independent experiments are presented
When starting our investigation, we analyzed the specificity of AArgPyrAb obtained from Abcam (Fig. 2). As expected, the antibodies recognized bovine serum albumin (BSA) and lysozyme modified by MG and did not stain unmodified proteins (Fig. 2a, b). These antibodies recognized MG-modified monomers (apparent molecular weight ~ 25 kDa) and MG-crosslinked dimers (apparent molecular weight ~ 50 kDa) of HspB1 (Fig. 2c). However, even unmodified HspB1 immunoreacted with these antibodies (Fig. 2c). This unexpected result could be explained if our sample of HspB1 had already been subjected to posttranslational modifications. However, based on the MS data, we can exclude this possibility.
Fig. 2.
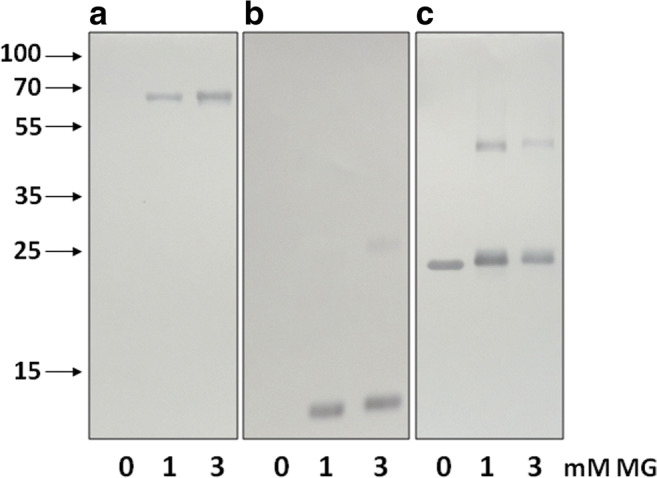
Western blotting of bovine serum albumin (a), lysozyme (b), and HspB1 (c) with anti-argpyrimidine antibodies. Analyzed proteins were incubated for 24 h in the absence (0 mM) or presence of 1 or 3 mM MG. Arrows indicate positions of molecular weight standards (in kDa). Representative data of five experiments are demonstrated
Another possible explanation is based on the suggestion that all small heat shock proteins with a highly homologous α-crystalline domain are non-specifically detected by AArgPyrAb. We checked this suggestion by immunostaining five unmodified human small heat shock proteins with these antibodies (Fig. 3). Among all proteins analyzed, only unmodified HspB1 was recognized by anti-methylglyoxal antibodies. These data indirectly indicate that the antigenic determinant recognized by these antibodies is located outside of the conservative α-crystalline domain of HspB1. To check this suggestion, we analyzed the interaction of AArgPyrAb with a panel of point mutants of HspB1 associated with Charcot-Marie-Tooth disease (Fig. 4). All point mutants analyzed except for R188W were equally recognized by the antibodies. The data could indirectly indicate that the antigenic determinant recognized by these antibodies is located near Arg188 and outside of the α-crystalline domain of HspB1.
Fig. 3.
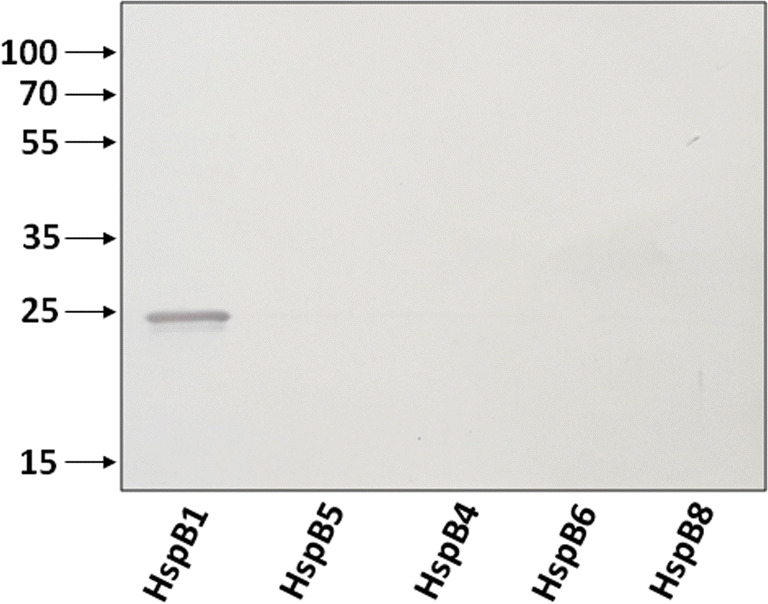
Western blotting of unmodified HspB1, HspB5, HspB4, HspB6, and HspB8 with anti-argpyrimidine antibodies. About 100 ng of each protein was subjected to SDS-gel electrophoresis followed by Western blotting. Arrows indicate position of molecular weight markers. Representative results of three experiments are presented
Fig. 4.
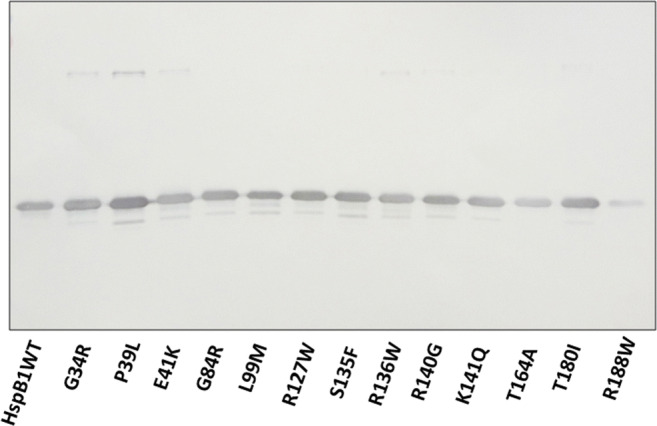
Interaction of anti-argpyrimidine antibodies with unmodified different point mutants of HspB1. Equal quantities (100 ng) of the wild-type protein (HspB1 WT) or its point mutants were loaded on the gel followed by western blotting. The representative results of two independent experiments are demonstrated
We tried to find proteins with primary structure identical to that around Arg188 of HspB1. We found that human and rabbit tropomyosins TMP-1 contain pentapeptide ESRAQ exactly corresponding to the pentapeptide at Arg188 of HspB1. We compared the staining of unmodified HspB1, its unmodified R188W mutant, and human and rabbit tropomyosins with anti-methylglyoxal antibodies (Supplement 1). As expected, the R188W mutant was poorly immunostained, whereas neither of the two tropomyosins was recognized by anti-methylglyoxal antibodies. We supposed that the epitope recognized by the analyzed antibodies is larger than five residues, and therefore, tropomyosins containing only short pentapeptide ESRAQ identical to that of HspB1 were not stained by these antibodies. Thus, we synthesized a larger peptide, IPVTFESRAQLGGP, with primary structure identical to that at Arg188 of HspB1 and analyzed its antigenic properties. This peptide was not recognized by anti-methylglyoxal antibodies in an isolated state or when crosslinked to bovine serum albumin (Supplement 2).
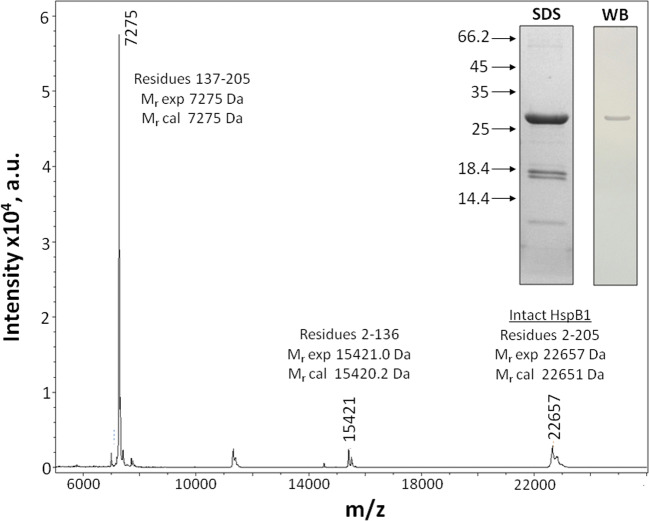
Since we were unable to exclude the possibility that the antigenic determinant is very large, we decided to obtain very large fragments of HspB1 and analyze their interaction with AArgPyrAb. HspB1 contains only one Cys residue Cys137. Therefore, cleavage at this residue leads to the formation of two peptides, 2-136 and 137-205.The cleavage at Cys137 was incomplete, but we detected two expected peptides in SDS electrophoresis and confirmed their presence by MS (Fig. 5). Western blotting indicated that only uncleaved HspB1 was recognized by AArgPyrAb, whereas the N- or the C-terminal peptides remained unstained (Fig. 5 inset). Similar results were obtained after CNBr cleavage of HspB1 at Met residues. In this case, we expected to obtain two peptides restricted by residues 2-169 and 170-205. Unfortunately, according to the MS data, we obtained a mixture of peptides restricted by residues 2-48, 2-161, 49-205, 162-205, and 170-205 (data not shown), which was due to the non-specific acid hydrolysis in addition to the two aforementioned peptides. Neither of these peptides was recognized by AArgPyrAb. Thus, the data indicate that AArgPyrAb recognizes and interacts with a highly stable discontinuous structural determinant in HspB1.
Fig. 5.
MALDI mass spectroscopy of peptide mixture obtained after cleavage of unmodified HspB1 at Cys137 and SDS electrophoresis of this mixture (stained by Coomassie Blue) (SDS) and western blotting of this gel stained by anti-argpyrimidine antibodies (WB). Arrows indicate position of molecular weight markers (in kDa). The data of two independent experiments are presented
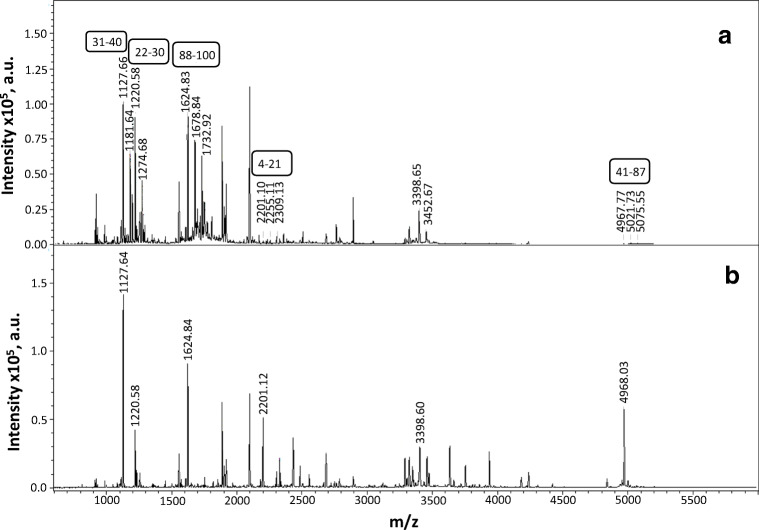
Argpyrimidine is a rather rare product of MG modification since the formation of this product requires 2 mol of MG per mole of arginine. Therefore, we decided to analyze the nature of the products that accumulate after the incubation of isolated HspB1 with methylglyoxal. HspB1 (1 mg/ml) was incubated with 1 mM MG at 37 °C for 24 h. MG modification was accompanied by an increase of the molecular weight of HspB1 from 22,652 up to 23,299 and broadening of the protein peak (Fig. 1b). Unmodified and modified HspB1 were subjected to hydrolysis by GluC endopeptidase, and the mass spectra of the peptides obtained were compared (Fig. 6). Six peptides containing extra mass of 54 were detected. This extra mass exactly corresponds to hydroimidazolones that formed after the reaction of MG with Arg. At the same time, we were unable to detect any peptide with extra molecular mass of 80 corresponding to the formation of argpyrimidine.
Fig. 6.
Comparison of MALDI mass spectra of peptides obtained after GluC cleavage of HspB1 incubated at 37 °C with 1 mM of MG for 24 h (a) and peptides obtained after GluC cleavage of unmodified HspB1 (b). Extra molecular weight (Δ54) corresponds to peptides containing Arg residues converted to hydroimidazolones. The corresponding peptides with residues numbering are indicated in frames
We identified five peptides containing one or two Arg residues modified by MG (Table 1). The modified Arg residues were located in either the N-terminal (residues 2–40) or in the beginning of the α-crystalline domain (residues 41–100). We failed to detect modified Arg residues located in the C-terminal half of HspB1 and converted to argpyrimidine. The data indicated that incubation even with a very high concentration (1 mM) of MG was not accompanied by the accumulation of argpyrimidine in the structure of HspB1 and that the main modification products were hydroimidazolones.
Table 1.
Composition of HspB1 peptides containing hydroimidazolones formed by modification of Arg by methylglyoxal
| Mr exp | Mr cal | Residues | Composition |
|---|---|---|---|
| 1127.66 | 1126.63 | 31–40 | D.QAFGLPRLPE.E |
| 1220.58 | 1219.57 | 22–30 | D.WYPHSRLFD.Q |
| 1624.84 | 1623.83 | 88–100 | E.IRHTADRWRVSLD.V |
| 2201.10 | 2200.12 | 4–21 | E.RRVPFSLLRGPSWDPFRD.W |
| 4967.77 | 4967.02 | 41–87 | E.EWSQWLGGSSWPGYVRPLPPAAIESPAVAAPAYSRALSRQLSSGVSE.I |
Potentially modified Arg residues are marked red
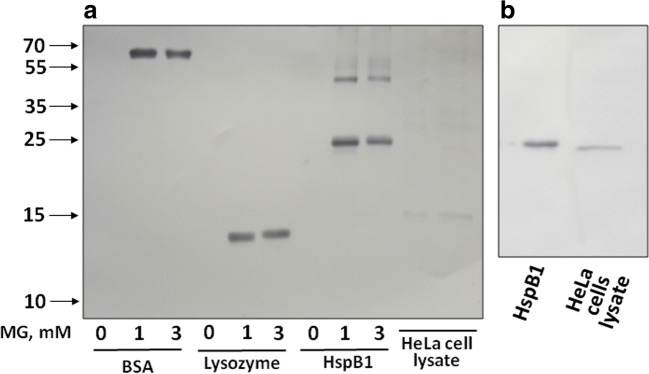
We tested three types of commercial antibodies that have been declared to recognize predominantly argpyrimidine (Abcam), hydroimidazolones (Cell Biolabs), or different products of MG-modified Arg (Biorbyt) for their ability to interact with modified and unmodified HspB1, bovine serum albumin, lysozyme, and HeLa cell lysate. As mentioned, anti-argpyrimidine antibodies (AArgPyrAb) specifically recognized MG-modified bovine serum albumin and lysozyme and non-specifically interacted with unmodified HspB1 (Fig. 2). These antibodies detected only one band with electrophoretic mobility corresponding to HspB1 in HeLa cell lysate (Fig. 7b). The western blot data (Fig. 7a) indicate that the Biorbyt anti-MG antibodies did not interact with unmodified bovine serum albumin, lysozyme, and HspB1 and effectively stained these proteins modified by MG. Similar results were obtained with Cell Biolabs antibodies. Again, these antibodies recognized only MG-modified bovine serum albumin, lysozyme, and HspB1 and did not cross-react with unmodified proteins (Supplement 3). None of the protein with apparent molecular weight ~ 25 kDa was detected in total HeLa cell lysates by these antibodies.
Fig. 7.
Western blotting of HspB1, model protein substrates, and HeLa cell lysates. a Western blotting of unmodified (0 mM) and MG-modified (1 or 3 mM of MG) model proteins (bovine serum albumin (BSA) and lysozyme), HspB1, and HeLa cell lysates stained by Biorbyt anti-methylglyoxal antibodies (HRP). b Western blotting of unmodified HspB1 and HeLa cell lysates stained by anti-argpyrimidine antibodies of Abcam. About 500 ng of individual protein or 100 μg of total protein lysate was loaded on the gel. Representative results of three independent experiments are presented
Discussion
Analysis of dicarbonyl proteome is impossible without using antibodies that recognize MG-modified proteins. At the same time, incubation with MG leads to the formation of a large number of different conjugates, and therefore, it is desirable to have antibodies that recognize only specific products. Such highly specific anti-argpyrimidine antibodies were obtained by Oya et al. (Oya et al. 1999). To our knowledge, these are the only highly specific antibodies that recognize argpyrimidine, and therefore, they have been produced commercially and are widely used for the detection of MG-modified proteins. All investigations dealing with the MG modification of HspB1 have actually been performed using these antibodies (Bair 3rd et al. 2010; Gawlowski et al. 2009; Oya-Ito et al. 2006, 2011; Padival et al. 2003; Sakamoto et al. 2002; Schalkwijk et al. 2006; van Heijst et al. 2006).
To our surprise, we found that these antibodies (AArgPyrAb) effectively interacted with unmodified HspB1 (Fig. 2). Similar results were reported by Oya-Ito et al. (2011) (see Fig. 3 of this paper). However, this fact was overlooked. We tried to localize the epitope detected by these antibodies in HspB1. By using a panel of point mutants, we found that the R188W mutant of HspB1 was less effectively stained by AArgPyrAb than any other mutant or the wild-type protein (Fig. 4). These data agree with the results of Sakamoto et al. (Sakamoto et al. 2002), which indicate that the mutation R188G decreased or completely prevented the interaction of these antibodies with HspB1. These data indicate that Arg188 is somehow involved in the formation of an epitope recognized by AArgPyrAb.
To our surprise, AArgPyrAb recognized neither tropomyosin containing pentapeptide with a primary structure identical to that at Arg188 of HspB1 nor a longer peptide containing residues 181-194 of HspB1 (Supplements 1, 2). Moreover, even larger peptides containing residues 2-136 and 137-205 were not stained by these antibodies (Fig. 5). Therefore, we have to conclude that these antibodies recognize a stable discontinuous structural epitope of HspB1.
Amazingly, this structural epitope was recognized by monoclonal antibodies even after SDS-gel electrophoresis and blotting. Although this is not very common, there are several examples of such stable epitopes in the literature. For instance, four monoclonal antibodies against hepatitis E virus reacted with a corresponding antigen on western blotting and also recognized discontinuous epitopes in the protein structure (Zhou et al. 2007). Similar results were obtained with monoclonal antibodies to vaccinia virus B5 protein (Chen et al. 2006) and monoclonal antibodies recognizing receptor-induced binding sites in Glu-plasminogen (Han et al. 2011).
The utilization of AArgPyrAb was based on assumption that modification by MG is accompanied by the formation of argpyrimidine in the structure of HspB1. However, this assumption is questionable. The formation of argpyrimidine requires the participation of two molecules of MG for arginine modification, and therefore, argpyrimidine is accumulated later than the primary product of MG modification (i.e., hydroimidazolone). Moreover, the formation of argpyrimidine is much rarer than the formation of hydroimidazolones (Ahmed et al. 2005; Brock et al. 2007; Oya-Ito et al. 2011). Indeed, according to Oya-Ito et al. (2011), the incubation of HspB1 with 5 mM of MG results in the modification of 22 Arg residues, among which only two were presented in the form of argpyrimidine. In our case, the incubation of isolated HspB1 with 1 mM MG was accompanied by the formation of at least nine different hydroimidazolones and not argpyrimidine.
The intracellular concentration of MG is in the range of 1–4 μM (Rabbani et al. 2016). Therefore, the specific and exclusive modification of Arg188 of HspB1 with the formation of argpyrimidine postulated earlier (Sakamoto et al. 2002) seems to be highly improbable. Indeed, the incubation of HspB1 with a very high concentration of MG (1 mM) was accompanied by the accumulation of only hydroimidazolones, which were detected in only the N-terminal half of the HspB1 molecule (Fig. 6; Table 1). Under the conditions used, we were unable to detect any argpyrimidine in the HspB1 structure. Therefore, the utilization of AArgPyrAb predominantly interacting with argpyrimidine for the investigation of MG-induced modification of HspB1 seems to be very questionable.
The interaction of MG with proteins leads to the formation of a number of different products (Rabbani and Thornalley 2012). Therefore, it is impossible to obtain monoclonal anti-MG antibodies that effectively recognize all products of MG modification. In any case, these antibodies will preferentially interact with concrete products and will not recognize other products of MG modification. We compared three types of commercially available anti-MG antibodies. The anti-argpyrimidine antibodies developed in laboratory of Dr. Uchida (Oya et al. 1999) and provided by Abcam are widely used in HspB1 investigations and cross-react with unmodified HspB1. These antibodies detected only one protein band in HeLa cell lysates (Fig. 7b). This is the reason why HspB1 was postulated to be the major target of MG modification. The antibodies provided by Biorbyt preferentially interact with MG-modified Arg residues, and the antibodies provided by Cell Biolabs preferentially interact with hydroimidazolones. These antibodies recognized only MG-modified HspB1 and failed to stain any protein bands in HeLa cell lysates (Fig. 7a, Supplement 3). Summing up, we conclude that HspB1 cannot be considered to be the major target of MG modification and that earlier published data concerning this problem require revision.
Electronic supplementary material
(PNG 213 kb)
(TIF 379 kb)
(PNG 216 kb)
(TIF 361 kb)
(PNG 165 kb)
(TIF 294 kb)
Acknowledgements
The authors are grateful to Dr. Marina V. Serebryakova (A.N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow 119991, Russian Federation) for performing mass spectroscopy experiments. The MALDI MS facility became available in the framework of the Moscow State University Development Program PNG 5.13.
Funding information
This investigation was supported by the Russian Foundation for Basic Science (16-04-00016, 19-04-00038).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed N, Dobler D, Dean M, Thornalley PJ (2005) Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem 280:5724–5732. 10.1074/jbc.M410973200 [DOI] [PubMed]
- Antognelli C, Palumbo I, Aristei C, Talesa VN. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-kappaB. Br J Cancer. 2014;111:395–406. doi: 10.1038/bjc.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bair WB, 3rd, Cabello CM, Uchida K, Bause AS, Wondrak GT. GLO1 overexpression in human malignant melanoma. Melanoma Res. 2010;20:85–96. doi: 10.1097/CMR.0b013e3283364903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock JW, Cotham WE, Thorpe SR, Baynes JW, Ames JM. Detection and identification of arginine modifications on methylglyoxal-modified ribonuclease by mass spectrometric analysis. J Mass Spectrom. 2007;42:89–100. doi: 10.1002/jms.1144. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Karmakar K, Chakravortty D. Cells producing their own nemesis: understanding methylglyoxal metabolism. IUBMB Life. 2014;66:667–678. doi: 10.1002/iub.1324. [DOI] [PubMed] [Google Scholar]
- Chalova AS, Sudnitsyna MV, Strelkov SV, Gusev NB. Characterization of human small heat shock protein HspB1 that carries C-terminal domain mutations associated with hereditary motor neuron diseases. Biochim Biophys Acta. 2014;1844:2116–2126. doi: 10.1016/j.bbapap.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Chen Z, Earl P, Americo J, Damon I, Smith SK, Zhou YH, Yu F, Sebrell A, Emerson S, Cohen G, Eisenberg RJ, Svitel J, Schuck P, Satterfield W, Moss B, Purcell R. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci U S A. 2006;103:1882–1887. doi: 10.1073/pnas.0510598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wang Y. Site-selective modifications of arginine residues in human hemoglobin induced by methylglyoxal. Biochemistry. 2006;45:15654–15660. doi: 10.1021/bi061410o. [DOI] [PubMed] [Google Scholar]
- Gawlowski T, Stratmann B, Stork I, Engelbrecht B, Brodehl A, Niehaus K, Körfer R, Tschoepe D, Milting H. Heat shock protein 27 modification is increased in the human diabetic failing heart. Horm Metab Res. 2009;41:594–599. doi: 10.1055/s-0029-1216374. [DOI] [PubMed] [Google Scholar]
- Gerasimovich ES, Strelkov SV, Gusev NB. Some properties of three alphaB-crystallin mutants carrying point substitutions in the C-terminal domain and associated with congenital diseases. Biochimie. 2017;142:168–178. doi: 10.1016/j.biochi.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Han J, Baik N, Kim KH, Yang JM, Han GW, Gong Y, Jardi M, Castellino FJ, Felez J, Parmer RJ, Miles LA. Monoclonal antibodies detect receptor-induced binding sites in Glu-plasminogen. Blood. 2011;118:1653–1662. doi: 10.1182/blood-2010-11-316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muranova LK, Weeks SD, Strelkov SV, Gusev NB. Characterization of mutants of human small heat shock protein HspB1 carrying replacements in the N-terminal domain and associated with hereditary motor neuron diseases. PLoS One. 2015;10:e0126248. doi: 10.1371/journal.pone.0126248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefedova VV, Muranova LK, Sudnitsyna MV, Ryzhavskaya AS, Gusev NB. Small heat shock proteins and distal hereditary neuropathies. Biochemistry. 2015;80:1734–1747. doi: 10.1134/S000629791513009X. [DOI] [PubMed] [Google Scholar]
- Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, Uchida K. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem. 1999;274:18492–18502. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- Oya-Ito T, Liu BF, Nagaraj RH. Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem. 2006;99:279–291. doi: 10.1002/jcb.20781. [DOI] [PubMed] [Google Scholar]
- Oya-Ito T, Naito Y, Takagi T, Handa O, Matsui H, Yamada M, Shima K, Yoshikawa T. Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim Biophys Acta. 2011;1812:769–781. doi: 10.1016/j.bbadis.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Padival AK, Crabb JW, Nagaraj RH. Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS Lett. 2003;551:113–118. doi: 10.1016/S0014-5793(03)00874-3. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Xue M, Thornalley PJ. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J. 2016;33:513–525. doi: 10.1007/s10719-016-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Yamamoto K, Tsuruo T (2002) Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem 277:45770–45775. 10.1074/jbc.M207485200 [DOI] [PubMed]
- Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006;580:1565–1570. doi: 10.1016/j.febslet.2006.01.086. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijst JW, Niessen HW, Musters RJ, van Hinsbergh VW, Hoekman K, Schalkwijk CG. Argpyrimidine-modified heat shock protein 27 in human non-small cell lung cancer: a possible mechanism for evasion of apoptosis. Cancer Lett. 2006;241:309–319. doi: 10.1016/j.canlet.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kuramitsu Y, Tokuda K, Okada F, Baron B, Akada J, Kitagawa T, Nakamura K. Proteomic analysis indicates that overexpression and nuclear translocation of lactoylglutathione lyase (GLO1) is associated with tumor progression in murine fibrosarcoma. Electrophoresis. 2014;35:2195–2202. doi: 10.1002/elps.201300497. [DOI] [PubMed] [Google Scholar]
- Weeks SD, Muranova LK, Heirbaut M, Beelen S, Strelkov SV, Gusev NB. Characterization of human small heat shock protein HSPB1 alpha-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci Rep. 2018;8:688. doi: 10.1038/s41598-017-18874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Chen Z, Purcell RH, Emerson SU. Positive reactions on Western blots do not necessarily indicate the epitopes on antigens are continuous. Immunol Cell Biol. 2007;85:73–78. doi: 10.1038/sj.icb.7100004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 213 kb)
(TIF 379 kb)
(PNG 216 kb)
(TIF 361 kb)
(PNG 165 kb)
(TIF 294 kb)