Abstract
Background:
Recent studies have shown that induced pluripotent stem cells (iPSCs) could be differentiated into mesenchymal stem cells (MSCs) with notable advantages over iPSCs per se. In order to promote the application of iPSC-MSCs for osteoregenerative medicine, the present study aimed to assess the ability of murine iPSC-MSCs to differentiate into osteoblast phenotype.
Methods:
Osteogenic differentiation medium, blending mouse osteoblast-conditioned medium (CM) with basic medium (BM) at ratio 3:7, 5:5 and 7:3, were administered to iPSC-MSCs, respectively. After 14 days, differentiation was evaluated by lineage-specific morphology, histological stain, quantitative reverse transcription-polymerase chain reaction and immunostaining.
Results:
The osteogenesis-related genes, alp, runx2, col1 and ocn expressions suggest that culture medium consisting of CM:BM at the ratio of 3:7 enhanced the osteogenic differentiation more than other concentrations that were tested. In addition, the alkaline phosphatase activity and osteogenic marker Runx2 expression demonstrate that the combination of CM and BM significantly enhanced the osteogenic differentiation of iPSC-MSCs.
Conclusion:
In summary, this study has shown that osteoblast-derived CM can dramatically enhance osteogenic differentiation of iPSC-MSCs toward osteoblasts. Results from this work will contribute to optimize the osteogenic induction conditions of iPSC-MSCs and will assist in the potential application of iPSC-MSCs for bone tissue engineering.
Keywords: Induced pluripotent stem cells, Mesenchymal stem cells, Osteoblasts, Conditioned medium, Osteogenic differentiation
Introduction
Bone grafts such as autografts and allografts are commonly used to treat critical size bone defects. However, they are frequently associated with the major limitations of donor-site morbidity, risk of disease transmission and immune-rejection [1, 2]. As an alternative, stem cells-based bone tissue engineering (BTE) approach has become very promising to fulfil the dream of regenerative repair of bone defects. In this context, one of the key tasks in the application of stem cells for BTE is to regulate their differentiation into osteogenic lineage [3, 4]. Bone mesenchymal stem cells (BMSCs) isolated from the bone marrow are so far the most widely used stem cell source candidate for replacing or regenerating damaged bone tissue [5]. However, the number of BMSCs that can be obtained from a single donor is limited, and the capacity of these cells for long-term proliferation is poor, which hamper their clinical application [6]. Alternatively, pluripotent stem cells, such as embryonic stem cells (ESCs), have enormous potential in cell therapy for the regeneration of bone or cartilage [7–9]. However, one of the major hurdles for further clinical application of ESCs was the potential of immunogenicity and ethical issues [7].
iPSCs are a new type of pluripotent stem cell that can be generated from somatic cells. With a wealth of merits such as high proliferation and differentiation ability, without the burden of immunological rejection and ethical controversy, they are now considered a promising alternative cell sources for personalized cell therapy [10, 11] and bone tissue engineering [7]. However, clinical applications of iPSCs have been criticized because of the possibility to form tumors by integrated oncogenes, consequently hampering the clinic application of iPSC-line for cell therapies and bone regeneration [12]. To enable iPSCs for practical applications, safety precaution needs to be taken. Recently, MSCs derived from mouse or human iPSCs (iPSC-MSCs) have been established and utilized for osteogenesis both in vitro and in vivo [13]. These iPSC-MSCs resembled primary MSCs in terms of immunophenotype (CD90, CD73 and CD105) and were capable of differentiating into mesenchymal lineages (osteoblasts, adipocytes, and chondrocytes), offering a scalable, ethically unencumbered cell source for bone tissue engineering [14].
Although iPSC-MSCs could be an advantageous cell source for bone regeneration, attempts to derive osteogenic lineage from iPSC-MSCs have required cumbersome or untranslatable processes, such as 3D scaffolds culture [13], or cytokines/growth factors manipulation [14]. Till now, it is still lack of well-defined and high-efficiency protocols for directing their differentiation into the osteogenic lineage [15]. An alternative strategy for directing and enhancing osteogenic differentiation is the use of conditioned media (CM) [16]. In recent years, the use of CM has been applied to direct ESCs [17] or MSCs [18] osteogenic differentiation in vitro. These studies provided a highly efficient way to induce osteoblasts formation and reduce formation of other contaminating lineages [17]. However, there are no studies to elucidate the potential of osteoblasts CM in directing iPSC-MSC osteogenic differentiation. Since these iPSC-MSCs recapitulated similar biological properties of MSCs, it is possible that CM from osteobalsts can stimulate osteogenic differentiation of iPSC-MSCs.
The objectives of this study were to derive iPSC-MSCs from mouse iPSCs and to determine the effects of osteobalst CM on the differentiation of mouse iPSC-MSCs to an osteobalst phenotype. To this end, we derived a mouse iPSC-MSC lineage from induced pluripotent stem cells and investigated the potential of osteobalst CM for osteogenic differentiation of iPSC-MSCs. We hope that our research can provide theoretical support for the development of effective methods to induce bone remodeling in bone tissue engineering.
Materials and methods
Isolation and culture of osteoblasts
Osteoblast cells were isolated from the calvarial bones of postnatal 1 day (P1) ICR mice (Slac laboratory animal, Shanghai, China) using adhere culture and enzyme-digestion techniques described by Jonason and O’Keefe and Wu et al. [19, 20]. Briefly, calvarial bones were cut into approximately 1 mm2 fragments and flushed with sterile D-PBS (Invitrogen, Carlsbad, CA, USA). The bone fragments were attached and cultured in 60 mm tissue culture petri dishes (Corning, Corning, NY, USA) in Dulbecco’s modified eagle medium (DMEM) (Gibco, Carlsbad, CA, USA) containing 10% heat inactivated fetal bovine serum {Fetal bovine serum (FBS), Gibco}, supplemented with 100 U/ml penicillin and streptomycin (Gibco, Carlsbad, CA, USA), and incubated at 37 °C in a humidified 5% CO2 incubator. The fragments were incubated for 7 days to allow cell outgrowth, with medium replenished every other day. Then, the calvarial bones were removed, and the outgrowth osteoblast cells from bone fragments were subsequently digested by 0.125% Trypsin–EDTA solution (Gibco, Carlsbad, CA, USA). The harvested cells were seeded at 105 cells/mL in T25 tissue culture flasks (Corning, Corning, NY, USA) containing a basic medium (BM) (DMEM supplemented with 10% FBS, l-glutamine and antibiotics, all from Gibco, Carlsbad, CA, USA) at 37 °C in humidified air containing 5% CO2.
Preparation of conditioned medium
Osteobalsts were cultured in a humidified incubator until reaching 80% confluence as the culture medium was changed every 48 h. After the last change of the medium, the cells were further cultured for 48 h, and the medium designated as “conditioned medium (CM)” was collected and passed through 0.22 μm filters (Nalgene, Rochester, NY, USA) to remove any cells. This procedure was strictly followed in 3 independent experiments performed to obtain conditioned medium. Then, different combinational ratios of CM with BM, e.g., 3:7, 5:5, and 7:3 were prepared as osteogenic induction medium for further experiments [21], and the basic medium was used as control.
Generation of iPSC-MSCs
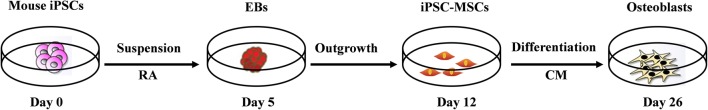
Mouse iPSCs (MiPS-01, Sidansai Biotechnology, Shanghai, China) were cultured in T25 tissue culture flasks (Corning, Corning, NY, USA) with a feeder layer of mitomycin-C (1 mg/100 mL; Sigma-Aldrich, St. Louis, MO, USA) treated mouse embryonic fibroblasts (MEFs). The MEFs were grown in medium composed of DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA), and 100 U/mL penicillin/streptomycin (Gibco, Carlsbad, CA, USA). The iPSCs were maintained in knockout DMEM (Gibco, Carlsbad, CA, USA) supplemented with 15% FBS (Gibco, Carlsbad, CA, USA), 100 U/mL penicillin/streptomycin (Gibco, Carlsbad, CA, USA), 1% non-essential amino acid (NEAA, Gibco, Carlsbad, CA, USA), 1% glutamine and 0.1 mM of 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). ESCs medium was changed every day. A stepwise osteogenic differentiation process of the murine iPSCs was schematically shown in Fig. 1.
Fig. 1.
Induction procedure of osteogenic differentiation from mouse iPSCs in vitro. * RA: retina acid; EBs: embryoid bodies; CM: conditioned medium
A standard procedure based on our previous studies to generate iPSC-MSCs was utilized [22]. In brief, after dissociation using 0.125% Trypsin–EDTA solution (Gibco, Carlsbad, CA, USA) at 37 °C for 3–5 min, 4 × 106 iPSCs were resuspended in 10 mL embryonic bodies (EBs) formation medium (ESCs medium) for suspension culture to form EBs after 3 days of culture. On day 3, EBs were treated with 10−7 M all-trans retinoic acid (RA, Sigma-Aldrich, St. Louis, MO, USA) for 48 h. On day 5, 20–30 clones of EBs were mechanically picked up using 10 μL pipettes, and re-seeded on 6-well culture dishes (Corning, Corning, NY, USA). EBs were grown in MSCs culture medium composed of DMEM/F12 1:1 (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 100 U/mL penicillin/streptomycin (all from Gibco, Carlsbad, CA, USA), and incubated at 37 °C in a humidified 5% CO2. The culture medium was changed every 2 days. After 7 days of culture, the outgrowth cells from the edge of the EB clones were treated with 0.125% Trypsin–EDTA solution (Invitrogen, Carlsbad, CA, USA) for 3–5 min at 37 °C. After the trypsin neutralization with a basic medium, a single-cell suspension was acquired by repeated pipetting. Subsequently, 1 × 105 cell aliquots were inoculated in 6-well plates in MSCs medium. Culture medium was changed every other day until reaching 80% confluence. After that, the cells were propagated for subculture to be used in further experimentation.
For cell proliferation assays, iPSC-MSCs were cultured in 96-well plates (Corning, Corning, NY, USA) at 1 × 103 cells per well with MSCs culture medium. On day 1–6, 20 μL of CCK-8 solution reagent (Dojindo Laboratories, Kumamoto, Japan) were switched to the culture media and cultured for additional 4 h. Cell proliferation was assayed by measuring the colorimetric CCK-8 on a microplate reader (MK3, Thermo, Waltham, MA, USA) at 450 nm. The assay was performed in triplicate.
Osteogenic induction
iPSC-MSCs at propagated 3 were incubated in 0.125% Trypsin–EDTA solution (Gibco, Carlsbad, CA, USA) for 3–5 min at 37 °C. After the trypsin neutralization with a basic medium, a single-cell suspension was acquired by repeated pipetting. Subsequently, 1 × 105 cells/mL aliquots were inoculated in 24-well plates or 6-well plates (Corning, Corning, NY, USA). For osteogenic differentiation, iPSC-MSCs were incubated in different combinations of CM:BM, i.e., 3:7, 5:5 and 7:3, respectively. The culture medium was changed every 2 days. After 14 days of culture, the derived cells from osteogenic induction were collected for gene analysis and immunocytochemistry.
ALP staining and quantitative analysis
After 2 weeks of culture, ALP activity was assayed by using a BCIP/NBT alkaline phosphatase colour development kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. For quantitative analysis, each sample was transferred into 96-well plates to determine the optical density (OD) value at 405 nm using a microplate reader (MK3, Thermo, Waltham, MA, USA).
qRT-PCR assay
qRT-PCR was used to evaluate the osteogenic differentiation capacity of iPSC-MSCs after 14 days culture with different combinational media. Total RNA was purified using Trizol (Tiangen Biotech, Beijing, China) following the protocol of the manufacturer. Extracted RNA was then treated with DNase I (Takara, Otsu, Japan) to remove DNA. After inactivation of DNase I, first-strand cDNA was synthesized from 1 μg of total RNA using PrimeScript® RT reagent Kit (Takara, Otsu, Japan). Osteogenesis-related genes, alkaline phosphatase activity (alp), runt-related transcription factor 2 (runx2), collagen I (col1) and osteocalcin (ocn) were assessed with primer sequences as provided in Table 1 (Sangon Biotech, Shanghai, China). qRT-PCR was performed using SuperReal PreMix Plus (SYBR Green) reagent Kit (Tiangen Biotech, Beijing, China) with an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The relative mRNA quantification of each gene was analyzed by the comparative 2−ΔΔCt method where the target is normalized to reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All reactions were carried out in triplicate.
Table 1.
Primers for qRT-PCR analyses
| Genes | Forward | Reverse |
|---|---|---|
| ocn | AGG GCA GCG AGG TAG TGA AGA | TAG ACC GGG CCG TAG AAG C |
| runx2 | GTG ATA AAT TCA GAA GGG AGG | CTT TTG CTA ATG CTT CGT GT |
| alp | ATG TCT GGA ACC GCA CTG AA | CGC CTG GTA GTT GTT GTG AGC ATA G |
| col1 | ATG GAT TCC AGT TCG AGT AGG C | CAT CGA CAG TGA CGC TGT AGG |
| GAPDH | GGG TGT GAA CCA CGA GAA AT | ACA GTC TTC TGG GTG GCA GT |
Immunocytochemistry
Immunocytochemistry analysis was carried out to explore the osteogenic differentiation of iPSC-MSCs after 2 weeks culture. Briefly, cells were fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich, St. Louis, MO, USA), and then cellular membranes were permeabilized with 0.1% Triton X-100 in PBS containing 1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the nonspecific binding sites were blocked in 10% goat serum (Invitrogen, Carlsbad, CA, USA) for 30 min. Cells were then incubated with mouse anti-Runx2 (1:200, Abcam, Cambridge, MA, USA) overnight at 4 °C, followed by incubation with rabbit anti-mouse IgG-FITC as second antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 60 min at room temperature. All nuclei were stained with DAPI (Invitrogen, Carlsbad, CA, USA). Fluorescence was examined with fluorescence microscope (Nikon Eclipse TE 2000-U, Nikon, Tokyo, Japan). Negative control experiments were performed as above by omitting the primary antibody.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (n = 4). Statistical analysis was determined using the one-way analysis of variance (ANOVA) with Tukey post hoc test (Origin 8.0, OriginLab, Northampton, MA, USA). A value of p < 0.05 was considered to be statistically significant.
Results
Culture of osteoblasts
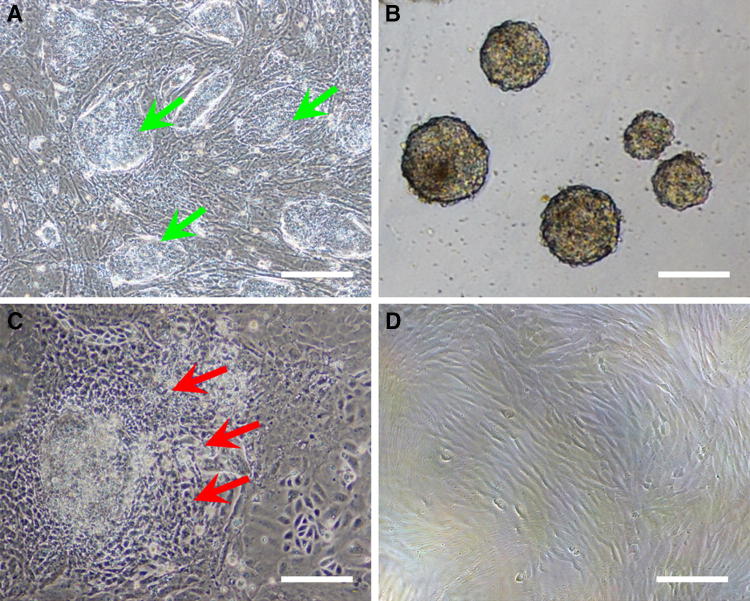
Osteoblasts were isolated from the calvariae of 1-day-old ICR mice using enzyme-digestion technique modified from a previous one [23]. In the present study, 15–20 calvarial bones were incubated in a 60 mm dish (Fig. 2A). After 4–7 days, osteoblast cells grew out from the edge of the calvarial bones (Fig. 2B). The outgrowth osteoblast cells from bone fragments were subsequently digested by Trypsin–EDTA and re-seeded in T25 tissue culture flasks (Fig. 2C). Our isolated osteoblasts are similar with previous findings in morphology and growth performance [19, 23].
Fig. 2.
The outgrowth of osteoblasts isolated from 1-day-old mice calvarial bone. A The attached culture of mice calvariae. B Osteoblasts (yellow arrows) grew out from the cultured cranial bone. C Morphologies of passaged 1 osteoblasts. Scale bar: 100 μm. (Color figure online)
Formation of iPSC-MSCs
To generate mouse iPSC-MSCs population, we dispersed undifferentiated mouse iPSC colonies (Fig. 3A) and cultured the cells under feeder-free suspension condition in ESC medium. Such a culture condition promoted the cell differentiation, and led to the initial generation of a population of EBs within 3 days (Fig. 3B). Then, EBs were cultured with ESC medium supplemented with 10−7 M RA during the following 2 days. It was previously reported that this kind of culture condition promoted the mesoderm cells differentiation [24–26]. Morphology of the iPSC-MSC-like cells (iPSC-MSCs) grew out from the EBs by attaching culture as shown in Fig. 3C. After a few passages, the cells exhibited elongated spindle shape (Fig. 3D), which was differed significantly from the undifferentiated iPSCs.
Fig. 3.
Derivation of an iPSC-MSCs population from mouse iPSCs. A Typical morphology of undifferentiated mouse iPSCs colonies (green arrows). B Obtained EBs in suspension culture condition. C Outgrowth of iPSC-MSCs from the attached EB (red arrows). D Rpresentative images of iPSC-MSCs at passaged 3. Scale bar: 100 μm. (Color figure online)
Thereafter, self-renewal ability of the above-derived iPSC-MSCs was examined. It was observed that first confluence could occur after approximately 5–7 days culture, which formed a more uniform population of spindle-shaped cells (Fig. 4A) with subsequent passage 2–3 (P2–P3). These cells grew at high density in a fingerprint whorl pattern and phenotypically resembled human BMSCs [27] (Fig. 4B). Representative image is shown for the iPSC-MSCs (P3) in high density at day 6 (Fig. 4C). Quantitatively, the property of strong self-renewal capability is also evidenced that the iPSC-MSCs derived in the present study possessed mesenchymal cell characters (Fig. 4D).
Fig. 4.
Self-renewal ability of the iPSC-MSCs. A The Morphologies of iPSC-MSCs at 1 day after culture was observed under an inverted microscope. B Low density of the iPSC-MSCs in uniform spindle shapes at 3 days after culture. C High density of the derived iPSC-MSCs fused into a spiral shape at day 6. D Representative the growth curve of the derived iPSC-MSCs. Scale bar: 50 μm
Directed osteogenic differentiation of iPSC-MSCs by CM
Gene expressions of the osteoblast-related markers alp, runx2, ocn, and col1 were analyzed using a quantitative RT-PCR method after 2-week cultivation of iPSC-MSCs with various concentrations of conditioned media, and cells cultured in basic medium were used as control (Fig. 5). qRT-PCR analysis of mRNA transcripts showed clear upregulation in expressing the representative osteobalst markers alp, runx2, ocn, and col1 from the iPSC-MSCs cultured in the medium consisting of CM:BM at the ratio of 3:7 (p < 0.01). Significant upregulation of alp and col1 was found in the group of CM:BM = 5:5 (p < 0.01). However, no regulating effect was found in the group of CM:BM = 7:3 except col1. These results preliminarily indicated that the effects of osteoblast-conditioned medium on osteogenic differentiation of iPSC-MSCs are related to conditioned media concentration.
Fig. 5.
qRT-PCR analysis of mRNA transcript expression levels with respect to representative osteobalst markers of Aalp; Brunx2; Cocn; and Dcol1. CM: conditioned medium, BM: basic medium. **Indicate p < 0.01 in all comparison
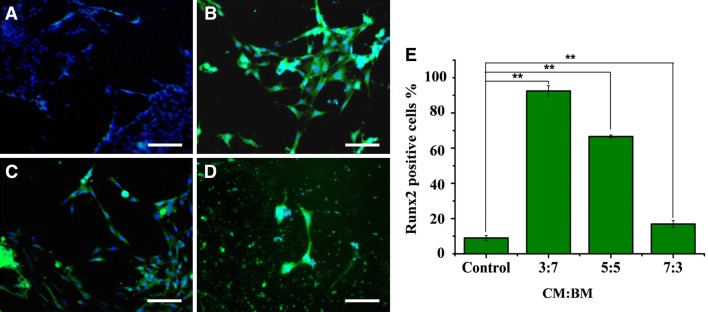
Immunocytochemical analysis of specific markers for osteobalsts
After 2 weeks of culture in various CM, osteogenic differentiation capacity of the iPSC-MSCs was also analyzed for osteoblast-related protein marker (Runx2) using an immunohistochemical method (Fig. 6). Positive immunostaining of Runx2 in the cells was seen at very high frequencies under the CM:BM ratios of 3:7 (Fig. 6B), 5:5 (Fig. 6C) and 7:3 (Fig. 6D) compared with control (Fig. 6A). The percentages of immuno-positive cells for the investigated Runx2 were quantified as follows: 92 ± 3.2% (3:7), 75 ± 4.1% (5:5); and 30 ± 3.5% (7:3) (Fig. 6E). In accordance with the above gene expression results (Fig. 5), the combinational medium with CM:BM ratio of 3:7 again demonstrated a dramatically promoting effect on the osteogenic differentiation of iPSC-MSCs.
Fig. 6.
Immunostaining for osteogenic marker Runx2. A Cells were cultured in basic medium. B Combinational media with the CM:BM ratio 3:7. C Combinational media with the CM:BM ratio 5:5. D Combinational media with the CM:BM ratio 7:3, respectively. The nuclei were stained with DAPI. E Positively stained cells for Runx2 were quantified. CM: conditioned medium, BM: basic medium. Scale bar: 50 μm. **Indicate p < 0.01 in all comparison
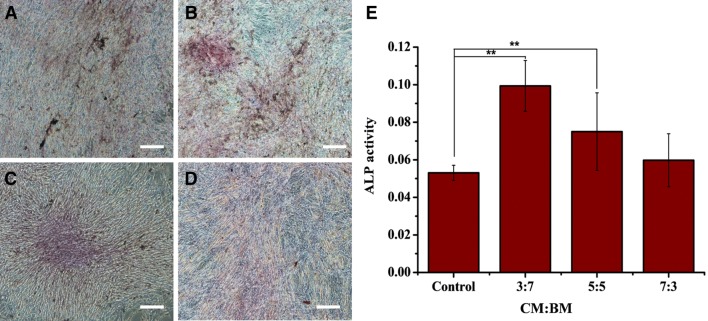
ALP staining
Results of ALP staining of iPSC-MSCs cultured under various conditions of CM for 2 weeks were shown in Fig. 7. Coincidentally, iPSC-MSCs cultured under CM:BM ratio of 3:7 exhibited distinctive ALP activity than other 3 groups. Quantitative measurements (Fig. 7E) indicated that significantly higher ALP activity was produced by the cells cultured under 3:7 and 5:5 conditions compared with control (p < 0.01). In particular, the cells cultured under 3:7 condition was nearly 2 times higher than that of the control.
Fig. 7.
ALP staining of iPSC-MSCs cultured in various combinational media for 2 weeks. A Basic medium as control. B Under combinational medium CM:BM = 3:7. C Under combinational medium CM:BM = 5:5. D Under combinational medium CM:BM = 7:3. E ALP activity at 14 days after culture. CM: conditioned medium, BM: basic medium. Scale bar: 100 μm. **Indicate p < 0.01 is from comparison to control
Discussion
iPSCs can differentiate into various cell types upon being appropriately induced, thus holding tremendous potential for personalized cell-based repair strategies to treat bone damage or defect [28–30]. However, one of the major problems in the clinical application of the pluripotent cells is that the final product for transplantation will often differentiate into teratoma in vivo [31]. Therefore, developing reliable and reproducible standard protocols to differentiate and select iPSC-derived cellular products is a pressing issue. Several studies have shown evidence that the derivation of iPSC-MSCs is necessary to reduce the risk of tumorigenicity as well as beneficial for osteoblast maturation in the differentiated process [13, 28]. The results seen in our current study suggest that the iPSC-MSCs generated closely recapitulated the characteristics of mouse MSCs and are comparable to the human iPSC-MSCs generated by other groups [29].
The essential prerequisite of iPSC-MSCs for bone regeneration application is to develop well defined and efficient protocols for directing cell differentiation into the osteogenic lineage in vitro. This is necessary to reduce the likelihood of spontaneous differentiation of iPSC-MSCs into divergent lineages in vivo [32]. For inducing iPSC-MSCs to differentiate into the osteogenic lineage, there are various protocols available. For example, it is well-established that a chemical cocktail comprising dexamethasone, vitamin C and beta-glycerol phosphate promoted the osteogenesis of iPSCs [33, 34]. Previous studies have also demonstrated that 3D scaffolds stimulated the proliferation and osteogenic differentiation of iPSC-MSCs, resulting in enhanced osteogenesis [13]. Other than those traditional strategies used for osteogenic differentiation in stem cells, several studies have showed that CM plays an important role in inducing osteogenic differentiation of both ESCs and MSCs [21, 35]. However, the effect of osteoblast CM on the differentiation of iPSC-MSCs to an osteogenic lineage has not been elucidated yet. In present study, various combinational media, e.g., conditioned medium versus basic medium at 3:7, 5:5 or 7:3, were selected to induce the osteogenic differentiation of iPSC-MSCs. After 14 days induction, all combinational medium groups showed positive results of ALP staining and Runx2 positive immunostaining, among which the CM:BM (3:7) group demonstrated the best induction effects. Real-time PCR assay also showed enhanced expression of osteogenic genes, ocn, runx2, alp, col, especially in the group of 3:7. The concentration of CM administered was based on results of a previous study [36], which indicated that 25–50% CM appears to provide a good balance between the amount of soluble factors and the pH of the medium. The osteogenic differentiation cocktail containing 30% CM in this study gave rise to higher enhancement in the osteogenic differentiation than other concentrations that were tested, which produced a similar fashion as previously described (yielding a 33% dilution) [37]. Whereas other previous works indicated 50% of osteoblasts CM significantly stimulated osteogenic differentiation of MSCs [21]. These results suggest that the MSCs could respond to soluble factors released by the osteogenic cells in a proportional manner. Future work is needed for a more in-depth evaluation of different concentrations of CM, including their effects in culture systems.
The underlying mechanism of osteogenic induction could be associated with the contained biological factors in the osteoblast-CM. Osteoblasts have been shown to produce and release several growth factors and cytokines, including bone morphogenetic protein-2 (BMP-2) and transforming growth factor-β (TGF-β), fibroblast growth factor-2 (FGF-2), IGF-1, and consequently they are likely present in osteoblast CM [21, 38]. BMP-2 is generally associated with an increase in osteogenesis as studies have shown its strong ability to increase osteogenic differentiation [39]. Growth factors, such as those from the TGF-β family, are known to provide the stimuli for bone formation [40]. Fibroblast growth factor-2 (FGF-2) has been proven to be effective at promoting the expression of osteogenic markers in MSCs [41]. Insulin-like growth factors (IGFs) are important local regulators during fracture healing. As osteoblasts are known to express high levels of IGF1R as they differentiate into osteocytes, it is probable this family of factors play a role in osteoblast differentiation [42]. In addition, a recent study reported CM-derived microvesicles were transferred to osteoclast precursors, which resulted in the stimulation of osteoclast formation [43]. Further investigation should elucidate which growth factors, cytokines or microvesicles in CM definitely contribute to osteogenic differentiation, as well as explore the mechanisms of CM-mediated osteogenic differentiation of iPSC-MSCs.
These findings strongly suggest that conditioned medium derived from mouse osteoblasts could significantly enhance the differentiation of iPSC-MSCs into osteobasts if formulated in optimal ratio. This work advances our understanding of the commitment of stem cells to osteogenic differentiation, providing new insights to bone modeling and remodeling, which may lead to the development of new strategies for bone tissue engineering.
Acknowledgements
This work was supported by the Foundation of Shanghai Municipal Natural Science (15ZR1400500) and the Fundamental Research Funds for the Central Universities (2232016A3-04).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the Scientific Advisory Board and Ethical Review Committee at Donghua University (IRB No. 2013-20).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harris JS, Bemenderfer TB, Wessel AR, Kacena MA. A review of mouse critical size defect models in weight bearing bones. Bone. 2013;55:241–247. doi: 10.1016/j.bone.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keefe RJ, Mao J. Bone tissue engineering and regeneration: from discovery to the clinic-an overview introduction. Tissue Eng Part B Rev. 2011;17:389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grässel S, Stöckl S, Jenei-Lanzl Z. Isolation, culture, and osteogenic/chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Methods Mol Biol. 2012;879:203–267. doi: 10.1007/978-1-61779-815-3_14. [DOI] [PubMed] [Google Scholar]
- 4.Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther. 2006;1:345–364. doi: 10.2174/157488806778226803. [DOI] [PubMed] [Google Scholar]
- 5.Havasi P, Nabioni M, Soleimani M, Bakhshandeh B, Parivar K. Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells. Mol Biol Rep. 2013;40:3023–3031. doi: 10.1007/s11033-012-2376-3. [DOI] [PubMed] [Google Scholar]
- 6.Gadkari R, Zhao L, Teklemariam T, Hantash BM. Human embryonic stem cell derived-mesenchymal stem cells: an alternative mesenchymal stem cell source for regenerative medicine therapy. Regen Med. 2014;9:453–465. doi: 10.2217/rme.14.13. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Im GI. Embryonic stem cells and induced pluripotent stem cells for skeletal regeneration. Tissue Eng Part B Rev. 2014;20:381–391. doi: 10.1089/ten.teb.2013.0530. [DOI] [PubMed] [Google Scholar]
- 8.zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 9.Karp JM, Ferreira LS, Khademhosseini A, Kwon AH, Yeh J, Langer RS. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 12.Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 13.Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC, et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci Rep. 2013;3:2243. doi: 10.1038/srep02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung Y, Bauer G, Nolta JA. Concise review: induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang LF, Qi J, Zuo G, Jia P, Shen X, Shao J, et al. Osteoblast-secreted factors promote proliferation and osteogenic differentiation of bone marrow stromal cells via VEGF/heme-oxygenase-1 pathway. PLoS One. 2014;9:e99946. doi: 10.1371/journal.pone.0099946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang YS, Randle WL, Bielby RC, Polak JM, Mantalaris A. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng. 2006;12:1381–1392. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 18.Heino TJ, Hentunen TA, Väänänen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into ostcoblasts. Exp Cell Res. 2004;294:458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Jonason JH, O’Keefe RJ. Isolation and culture of neonatal mouse calvarial osteoblasts. Methods Mol Biol. 2014;1130:295–305. doi: 10.1007/978-1-62703-989-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Qin C, Yu Z, Wang X, Zhang Y, Lou X. Nanofibrous scaffold containing osteoblast-derived extracellular matrix for the proliferation of bone marrow mesenchymal stem cells. J Donghua Univ. 2017;34:756–760. [Google Scholar]
- 21.Maxson S, Burg KJ. Conditioned media cause increases in select osteogenic and adipogenic differentiation markers in mesenchymal stem cell cultures. J Tissue Eng Regen Med. 2008;2:147–154. doi: 10.1002/term.76. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Lou X, Wang X, Yang L, Zhang Y. Electrospun nanofibers of hydroxyapatite/collagen/chitosan promote osteogenic differentiation of the induced pluripotent stem cell-derived mesenchymal stem cells. J Control Release. 2015;213:e53. doi: 10.1016/j.jconrel.2015.05.087. [DOI] [PubMed] [Google Scholar]
- 23.Granholm S, Henning P, Lindholm C, Lerner UH. Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone. 2013;52:83–92. doi: 10.1016/j.bone.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Teramura T, Onodera Y, Mihara T, Hosoi Y, Hamanishi C, Fukuda K. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell Reprogram. 2010;12:249–261. doi: 10.1089/cell.2009.0086. [DOI] [PubMed] [Google Scholar]
- 25.Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Yu D, Zhu H. Optimization of culture condition of human bone marrow stromal cells in terms of purification, proliferation, and pluripotency. In Vitro Cell Dev Biol Anim. 2014;50:822–830. doi: 10.1007/s11626-014-9778-6. [DOI] [PubMed] [Google Scholar]
- 28.Bilousova G, Jun du DH, King KB, De Langhe S, Chick WS, Torchia EC, et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011;29:206–216. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Niyibizi C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. BMC Cell Biol. 2012;13:35. doi: 10.1186/1471-2121-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 32.Ye CP, Heng BC, Liu H, Toh WS, Cao T. Culture media conditioned by heat-shocked osteoblasts enhances the osteogenesis of bone marrow-derived mesenchymal stromal cells. Cell Biochem Funct. 2007;25:267–276. doi: 10.1002/cbf.1330. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi T, Misawa H, Nakahara H, Noguchi H, Yoshida A, Kobayashi N, et al. Transplantation of osteogenically differentiated mouse iPS cells for bone repair. Cell Transplant. 2012;21:591–600. doi: 10.3727/096368911X605529. [DOI] [PubMed] [Google Scholar]
- 34.Ko JY, Park S, Im GI. Osteogenesis from human induced pluripotent stem cells: an in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014;23:1788–1797. doi: 10.1089/scd.2014.0043. [DOI] [PubMed] [Google Scholar]
- 35.Maxson S, Burg KJ. Conditioned media enhance osteogenic differentiation on poly(L-lactide-co-epsilon-caprolactone)/hydroxyapatite scaffolds and chondrogenic differentiation in alginate. J Biomater Sci Polym Ed. 2010;21:1441–1458. doi: 10.1163/092050609X12518804794703. [DOI] [PubMed] [Google Scholar]
- 36.Maxson S, Burg KJ. Synergistic effects of conditioned media and hydrostatic pressure on the differentiation of mesenchymal stem cells. Cell Mol Bioeng. 2012;5:414–426. doi: 10.1007/s12195-012-0248-5. [DOI] [Google Scholar]
- 37.Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by ostroblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. doi: 10.22203/eCM.v023a02. [DOI] [PubMed] [Google Scholar]
- 38.Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23:183–188. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- 39.Ji XH, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- 40.Rawadi G, Vayssière B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 41.Gupta RR, Yoo DJ, Hebert C, Niger C, Stains JP. Induction of an osteocyte-like phenotype by fibroblast growth factor-2. Biochem Biophys Res Commun. 2010;402:258–264. doi: 10.1016/j.bbrc.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, Wang Y, Menendez A, Fong C, Babey M, Tahimic CG, et al. Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res. 2015;30:1572–1584. doi: 10.1002/jbmr.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, et al. Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res. 2016;95:673–679. doi: 10.1177/0022034516633189. [DOI] [PMC free article] [PubMed] [Google Scholar]