Abstract
Purpose
To explore whether it is possible to predict the number and quality of the embryo using a few particular hTERT SNPs.
Methods
We included 997 Han Chinese women who were genetically unrelated and underwent assisted reproduction using IVF from September 2014 to December 2015. DNA was genotyped by using TaqMan real-time quantitative PCR.
Results
Among the 997 patients, individuals with the CC genotype of rs2075786 had a significantly lower number of good-quality embryos than those with the TT+TC genotypes. Compared with the CT+CC genotype carriers, patients carrying the TT genotype of rs2853677 had a significantly lower number of oocytes retrieved, mature oocytes and available embryos. Among the 750 patients aged ≤ 35 years, individuals with the AA+AG genotypes of rs2853691 had a significantly higher number of good-quality embryos than those with the GG genotype. The haplotype analysis showed that the TTTG (rs2853672/rs2853669/rs2735940/rs2736108) haplotype was more likely to lead to more than three good-quality embryos in patients aged ≤ 35 years.
Conclusions
Our study suggests that the hTERT SNP is associated with IVF outcomes.
Keywords: Human telomerase reverse transcriptase (hTERT), Single nucleotide polymorphisms (SNP), In vitro fertilisation (IVF), Good-quality embryo
Introduction
The outcome of controlled ovarian hyperstimulation is closely associated with ovarian reserve function. Ovarian reserve refers to the ability of follicles to grow, develop and form fertilisable oocytes in the ovarian cortex, reflecting female fertility [1]. Female fertility declines with increasing age [2]. Our previous study showed that a large difference exists in the rate of fertility decline in the same age group of women; some patients exhibit significantly lower ovarian response to drug stimulation and lower fertility potential than do other women of the same age group [3]. Thus, ovarian function decline occurs faster in some women, and these women should give birth at a younger age.
With regard to the process of oocyte formation and growth, the oocyte quality is associated with the functional status of ovarian granulosa cells and the growth factors they secrete. However, the growth and ageing of granulosa cells are tightly associated with telomerase activity [4]. Telomerase is a ribonucleoprotein reverse transcriptase that elongates telomere ends in eukaryotic chromosomes to maintain cell division and passage [5, 6]. Our previous study indicated that telomerase activity is detectable in ovarian granulosa cells; for women of childbearing age, telomerase activity is a better predictor than telomere length for the outcome of in vitro fertilisation (IVF) [7], and patients with high telomerase activity are more likely to have a clinical pregnancy [8].
The synthesis of telomerase is controlled by its catalytic ubunit-human telomerase reverse transcriptase (hTERT).The hTERT, which determines the major biological function of telomerase, is the unique rate-limiting enzyme in telomeresynthesis [6, 9]. Transcription of hTERT can facilitate growth of granulosa cells [10]. Transfecting the hTERT gene into cells can induce telomerase activity and extend telomere structure [11]. The hTERT gene not only maintains telomere length but also directly promotes cell proliferation [12]. Activation of an oestrogen-responsive element may occur in the region of the TERT [13].
Single nucleotide polymorphisms (SNPs) in the hTERT gene are associated with telomerase activity or telomere length. A study found that hTERT rs2736100, rs2853672 and rs2853677 are associated with telomere length [14]. In particular, rs2736100 participates in the regulation of hTERT expression and telomere length [15]. Another study showed that Chinese patients with the hTERT rs2735940 and rs2853669 CC genotype have a shorter telomere length than do those with the TT genotype; the former group has a higher chance of peripheral arterial disease [16]. Moreover, Yan et al. [17] presented the first evidence that genetic variations of TERT rs2736100 and telomerase-associated protein 1 rs1713449 increase the risk of male infertility. Thus far, no work has been reported concerning the effect of hTERT SNP on female fertility. Nonetheless, research on the hTERT SNPs in other diseases suggests that from the perspective of reproductive medicine or fertility, it is worth studying whether hTERT SNPs affect ovarian reserve, ovulation induction outcomes and embryo quality. Here, we raise the following question: Is it possible to predict the occurrence of ovarian function decline or the quality of oocytes and embryos through a few particular hTERT SNPs?
Materials and methods
Study population
We included 997 genetically unrelated Han Chinese women who underwent the first cycle of IVF and transferred fresh embryo cycles at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University from September 2014 to December 2015. The inclusion criteria included tubal factor infertility with a regular menstrual cycle (24–38 days) [18], normal ovulation and ovulation induction treatment using the conventional mid-luteal long protocol. The exclusion criteria included male infertility, a history of ovarian surgeries that might affect ovarian function within the 3 months prior to the study, endometriosis or endometrial cysts, hyperprolactinemia, functional ovarian cysts, uterine fibroids, uterine anatomical abnormalities, congenital malformations, endocrine diseases in other organs of the body, and other diseases that were not suitable for enrolment in the study.
The baseline and clinical data of the patients, including age, duration of infertility, body mass index (BMI), serum anti-Mullerian hormone (AMH) level and antral follicle count (AFC), were retrieved from the central database. All patients signed an informed consent form and voluntarily donated 2 mL peripheral blood for the study on the day of oocyte retrieval.
Treatment procedure
A mid-luteal long protocol was used for ovulation induction. Gonadotropin-releasing hormone agonist (GnRH-a) was injected for pituitary down-regulation in the mid-luteal phase of the menstrual cycle (days 20–22 in the conventional cycle) 1 month prior to ovulation induction. Measurements of hormone levels and vaginal ultrasound were performed 10–14 days after the GnRH-a injection. Patients were given recombinant follicle-stimulating hormone (FSH) and/or human menopause gonadotropin (HMG) to start ovulation induction if oestradiol (E2) < 50 ng/L, FSH < 5 IU/L, luteinising hormone (LH) < 5 IU/L, endometrial thickness < 5 mm and bilateral ovarian follicular diameter < 10 mm. The follicular development and the serum E2, progesterone and LH levels were monitored during ovulation induction. Human chorionic gonadotropin (HCG) was injected when an ultrasound showed more than three follicles with diameters ≥ 16 mm, more than two follicles with diameters ≥ 17 mm or at least one follicle with a diameter ≥ 18 mm in the bilateral ovaries and the serum E2 level was consistent with the number of large follicles. Oocytes were retrieved 34–36 h after HCG injection. The method of fertilisation was conventional IVF. Embryo transfer was performed under transabdominal ultrasound on day 3 after oocyte retrieval. One or two embryos were transferred according to the patient’s age, the embryo quality and the previous pregnancy. Patients were given 60 mg of progesterone by intramuscular injection on a daily basis after oocyte retrieval, and the urine HCG/serum ß-HCG was tested on day 14 after embryo transfer. Cases with positive results were diagnosed as biochemical pregnancy, for which luteal support was continued to 10–12 weeks of pregnancy. A transvaginal ultrasound was performed 35 days after embryo transfer. Cases with a gestational sac and foetal bud in the uterus and a foetal heartbeat were diagnosed as clinical pregnancy.
Main outcome measure: number of good-quality embryos; secondary outcome measures: number of oocytes retrieved, number of mature oocytes, two pronuclear (2PN) fertilisation rate, cleavage rate and number of available embryos.
Definitions
Embryo assessment was performed according to the Istanbul consensus [19]. Mature oocytes: corona radiate cells extended radially from the surrounding of oocytes, granulosa cells extended into translucent uncertain body and visible first polar bodies.
Fertilisation: pronuclei were formed, and second polar bodies appeared.
Good-quality embryos: on day 2, the number of embryonic blastomeres ranged between 2 and 4 cells, with a uniform size, no cell debris, or < 5% debris. On day 3, the number of embryonic blastomeres reached 7–9 cells, with a uniform size, < 5–20% cell debris and grade 1–2 embryos.
SNP selection
We searched telomerase-related hTERT SNPs according to the literature and referred to the gene information in the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/) and Hap Map database (http://hapmap.ncbi.nlm.nih.gov/) for CHB (Han Chinese in Beijing, China). Eight polymorphisms with a minor allele frequency ≥ 5% were selected: rs2075786 T>C, rs2853677 C>T, rs2736108 G>A, rs2735940 T>C, rs2853669 T>C, rs2736100 G>T, rs2853672 G>T and rs2853691 A>G.
Genotyping
Granulosa cells were isolated and extracted from the follicular fluid according to previously described methods [19]. DNA was extracted from the peripheral blood and granulosa cells of each patient using the TIANamp Genomic DNA Kit (TIANGEN Biotech, Beijing, China) following the manufacturer’s instructions and was stored at − 20 °C until further analyses. The DNA purity and concentration were tested by measuring the light absorption at 260 nm and 280 nm using a UV spectrophotometer (Nano Drop Technologies, Inc., Wilmington, DE, USA).
The peripheral blood DNA was genotyped in 997 patients, and the genotype was consistent in both the peripheral blood DNA and granulosa cell DNA in 249 of the patients. All specimens were genotyped by the TaqMan real-time quantitative polymerase chain reaction (qPCR) using a 7500 sequence detector system (Applied Biosystems, Foster City, CA, USA). The TaqMan universal qPCR kits were purchased from KAPA Biosystems (Boston, MA, USA). The probes and primers used for SNP genotyping were purchased from CHBT (Shanghai, China). The sequences of the probes and primers used are shown in Table 1.
Table 1.
Primers and probes for eight hTERT SNPs
| SNP | Primers | Probes |
|---|---|---|
| rs2075786 T>C | Forward 5′-GACGCACCCAGGTTACACAC-3′ Reverse 5′-GGAGCCGCCACTCTTGAC-3′ |
FAM-CAGGAGCCAGGTCAC-MGB HEX-CAGGAGCCGGGTCAC-MGB |
| rs2853677 C>T | Forward 5′-CCAATCCAGTCTGACAGTCGT-3′ Reverse 5′-ACATGCAACAGGCAAGTGG-3′ |
FAM-TGCACCAGACGGGTC-MGB HEX-TGCACCAGGCGGGTC-MGB |
| rs2736108 G>A | Forward 5′-GCCATCTGCCAGTAGAAACCT-3′ Reverse 5′-ATGTTTCAGCACACACTGAGACA-3′ |
FAM-TCAGGGCGCAAGTGT-MGB HEX-TCAGGGCGCGAGTGT-MGB |
| rs2735940 T>C | Forward 5′-CCCTTTAAAAAGGCTTAGGGA-3′ Reverse 5′-TGGAGGTTAGCCTCGTCTTG-3′ |
FAM-TTAGGATTACAGGTCG-MGB HEX-TTAGGATTGCAGGTCG-MGB |
| rs2853669 T>C | Forward 5′-GCTCCCAGTGGATTCGC-3′ Reverse 5′-TGGAAGGTGAAGGGGCA-3′ |
FAM-ACCGCGCTCCCCAC-MGB HEX-ACCGCGCTTCCCAC-MGB |
| rs2736100 G>T | Forward 5′-CCCCACAAGCTAAGCATTATTA-3′ Reverse 5′-AGAAGACAGACGGGGAACAA-3′ |
FAM-CAAAGCTACAGAAA-MGB HEX-CAAAGCTAAAGAAA-MGB |
| rs2853672 G>T | Forward 5′-ATGAGAGGTGGGGACGAGA-3′ Reverse 5′-CCGTCAACACACAATTAAATCTTA-3′ |
FAM-CACGTGCACCTGCA-MGB HEX-CACGTGCAACTGCA-MGB |
| rs2853691 A>G | Forward 5′-CGAAGGGCAGCAGGGAT-3′ Reverse 5′-CTTCCCCATCTTCCCCG-3′ |
FAM-CTGCCACCCCGGCC-MGB HEX-CTGCCACCCTGGCC-MGB |
The PCR reaction (20 μL) contained 50 ng genomic DNA, 10 μM each primer, 10 μM probe, 10 μL 2× buffer and PCR-grade water. The PCR conditions were a single step of 3 min at 95 °C to activate the polymerase, followed by 3 s of denaturation at 95 °C and 35 s of annealing and extension at 60 °C, for a total of 40 cycles.
For quality control, one positive control and one negative control were set in each 96-well plate. The success rate of SNP detection reached more than 99%. Duplicate detection was performed with 5% of the samples randomly selected. The results showed a concordance rate of 100%.
Statistical methods
Statistical analysis was performed using the IBM SPSS 20.0 statistics package (IBM SPSS, Somers, NY, USA). For different genotypes, clinical indices, e.g. BMI, bFSH, bLH, AFC, AMH, total dose of gonadotropin (Gn), number of oocytes retrieved, 2PN fertilisation rate and number of good-quality embryos, were expressed as the mean ± standard deviation and compared using analysis of variance. Given that the multiple testing was included, the tests were two-sided at α = 0.01, with P < 0.01 defined as statistically significant. Pairwise comparisons of the indices between every two genotypes were conducted using the Bonferroni test.
Linkage disequilibrium analysis was conducted on the eight hTERT SNPs using HAPLOVIEW software. The linkage disequilibrium coefficient between different SNPs was denoted as D′. Additionally, haplotypes were constructed and analysed to predict the number of good-quality embryos.
Results
Characteristics of the subjects
For the 997 patients, the mean age was 32.46 ± 4.79 years and the mean BMI was 21.39 ± 3.10 kg/m2. The mean duration of infertility was 4.76 ± 3.46 years, and the mean AFC was 15.47 ± 9.61. The mean basal FSH, LH and E2 levels were 8.34 ± 4.89 IU/L, 5.40 ± 3.75 IU/L and 46.73 ± 31.61 ng/L, respectively. Women aged ≤ 35 years accounted for 75.23% (750/997) and those aged > 35 years accounted for 24.77% (247/997) of the patients.
For the eight hTERT SNPs, three genotypes of each polymorphism showed no significant differences in terms of woman’s age, duration of infertility, BMI, AFC, basal FSH, basal LH, basal E2, AMH or total dose of Gn (P > 0.05) (Table 2).
Table 2.
Characteristics of the subjects
| SNP | Genotypes (no.) | Age (years) | BMI (kg/m2) | Infertility duration (years) | AFC (n) | bFSH (IU/L) | bLH (IU/L) | AMH (ng/mL) | Total Gn dose (IU) |
|---|---|---|---|---|---|---|---|---|---|
| rs2853691 | AA (n = 590) | 32.31 ± 4.87 | 21.59 ± 2.97 | 4.75 ± 3.65 | 15.30 ± 9.58 | 8.44 ± 5.77 | 5.45 ± 4.04 | 4.79 ± 3.68 | 2118.75 ± 909.86 |
| AG (n = 327) | 32.74 ± 4.66 | 21.12 ± 3.06 | 4.75 ± 3.18 | 15.21 ± 9.43 | 8.29 ± 3.31 | 5.33 ± 3.35 | 5.19 ± 7.20 | 2107.91 ± 818.95 | |
| GG (n = 32) | 32.34 ± 4.76 | 21.13 ± 3.14 | 5.25 ± 3.82 | 16.67 ± 11.83 | 8.05 ± 3.40 | 5.72 ± 2.78 | 5.05 ± 4.13 | 2078.91 ± 809.12 | |
| F | 0.867 | 2.819 | 0.317 | 0.316 | 0.167 | 0.216 | 0.531 | 0.042 | |
| P | 0.420 | 0.060 | 0.728 | 0.729 | 0.846 | 0.806 | 0.588 | 0.959 | |
| rs2075786 | TT (n = 643) | 32.41 ± 4.75 | 21.31 ± 3.03 | 4.69 ± 3.32 | 15.55 ± 9.52 | 8.35 ± 5.40 | 5.45 ± 3.84 | 5.03 ± 5.69 | 2113.16 ± 870.60 |
| TC (n = 315) | 32.50 ± 4.82 | 21.54 ± 3.25 | 4.90 ± 3.58 | 15.50 ± 9.71 | 8.24 ± 3.79 | 5.33 ± 3.68 | 4.86 ± 3.68 | 2115.52 ± 883.38 | |
| CC (n = 27) | 33.26 ± 5.10 | 21.60 ± 2.25 | 5.00 ± 4.16 | 13.12 ± 11.47 | 8.38 ± 3.49 | 4.74 ± 2.49 | 3.77 ± 3.81 | 2159.61 ± 830.81 | |
| F | 0.417 | 0.674 | 0.471 | 0.797 | 0.059 | 0.511 | 0.679 | 0.035 | |
| P | 0.859 | 0.510 | 0.625 | 0.451 | 0.943 | 0.600 | 0.507 | 0.965 | |
| rs2736100 | GG (n = 186) | 32.44 ± 4.66 | 21.67 ± 3.30 | 4.83 ± 3.30 | 15.65 ± 9.42 | 7.82 ± 2.68 | 5.33 ± 3.58 | 4.94 ± 3.84 | 2101.08 ± 860.37 |
| GT (n = 459) | 32.39 ± 4.81 | 21.20 ± 2.78 | 4.87 ± 3.71 | 15.20 ± 9.66 | 8.63 ± 6.28 | 5.54 ± 4.02 | 4.87 ± 3.82 | 2140.34 ± 889.22 | |
| TT (n = 306) | 32.61 ± 4.82 | 21.56 ± 3.17 | 4.66 ± 3.24 | 15.42 ± 9.69 | 8.27 ± 3.59 | 5.24 ± 3.50 | 5.06 ± 7.26 | 2072.44 ± 862.03 | |
| F | 0.209 | 2.198 | 0.337 | 0.141 | 1.782 | 0.600 | 0.107 | 0.561 | |
| P | 0.812 | 0.112 | 0.714 | 0.868 | 0.169 | 0.549 | 0.899 | 0.571 | |
| rs2853677 | CC (n = 161) | 32.41 ± 4.70 | 21.47 ± 3.23 | 4.83 ± 3.32 | 15.46 ± 9.47 | 7.88 ± 2.15 | 4.99 ± 3.19 | 4.59 ± 3.77 | 2146.56 ± 870.70 |
| CT (n = 596) | 32.43 ± 4.77 | 21.29 ± 3.17 | 4.86 ± 3.63 | 15.72 ± 9.99 | 8.36 ± 5.71 | 5.60 ± 4.16 | 4.96 ± 3.75 | 2090.66 ± 851.70 | |
| TT (n = 234) | 32.49 ± 4.86 | 21.61 ± 2.83 | 4.47 ± 2.96 | 14.89 ± 8.74 | 8.52 ± 3.85 | 5.14 ± 2.93 | 5.11 ± 8.06 | 2145.92 ± 920.03 | |
| F | 0.019 | 0.978 | 1.154 | 0.591 | 0.867 | 2.353 | 0.446 | 0.478 | |
| P | 0.982 | 0.376 | 0.316 | 0.554 | 0.421 | 0.096 | 0.641 | 0.620 | |
| rs2853672 | GG (n = 271) | 32.30 ± 4.77 | 21.67 ± 3.21 | 4.91 ± 3.27 | 15.50 ± 9.94 | 8.31 ± 4.69 | 5.09 ± 3.12 | 4.86 ± 3.94 | 2080.22 ± 912.65 |
| GT (n = 468) | 32.60 ± 4.79 | 21.28 ± 2.73 | 4.77 ± 3.68 | 15.42 ± 9.55 | 8.41 ± 5.66 | 5.66 ± 4.14 | 4.84 ± 3.72 | 2132.99 ± 841.71 | |
| TT (n = 215) | 32.43 ± 4.88 | 21.42 ± 3.33 | 4.63 ± 3.30 | 14.93 ± 9.38 | 8.27 ± 3.43 | 5.17 ± 3.40 | 5.25 ± 8.26 | 2103.99 ± 874.18 | |
| F | 0.367 | 1.396 | 0.407 | 0.235 | 0.069 | 2.504 | 0.451 | 0.323 | |
| P | 0.693 | 0.248 | 0.666 | 0.790 | 0.933 | 0.082 | 0.637 | 0.724 | |
| rs2853669 | TT (n = 296) | 32.18 ± 4.62 | 21.17 ± 3.11 | 4.64 ± 3.23 | 15.64 ± 9.23 | 8.36 ± 6.45 | 5.26 ± 3.71 | 4.94 ± 3.53 | 2062.50 ± 811.08 |
| TC (n = 459) | 32.60 ± 4.88 | 21.53 ± 2.88 | 4.90 ± 3.71 | 15.38 ± 9.77 | 8.30 ± 3.40 | 5.48 ± 3.72 | 5.04 ± 6.38 | 2166.46 ± 895.31 | |
| CC (n = 148) | 32.33 ± 4.89 | 21.45 ± 3.27 | 4.67 ± 3.00 | 15.22 ± 10.34 | 8.33 ± 5.86 | 5.13 ± 3.44 | 4.80 ± 4.03 | 2027.74 ± 863.49 | |
| F | 0.735 | 1.290 | 0.583 | 0.105 | 0.013 | 0.648 | 0.106 | 2.079 | |
| P | 0.480 | 0.276 | 0.558 | 0.901 | 0.987 | 0.524 | 0.900 | 0.126 | |
| rs2735940 | TT (n = 264) | 32.39 ± 4.81 | 21.58 ± 3.15 | 4.77 ± 3.31 | 15.34 ± 9.51 | 8.27 ± 4.72 | 5.25 ± 3.64 | 4.77 ± 3.83 | 2059.43 ± 908.66 |
| TC (n = 477) | 32.48 ± 4.78 | 21.25 ± 2.97 | 4.86 ± 3.64 | 15.58 ± 9.62 | 8.44 ± 5.62 | 5.59 ± 3.99 | 4.88 ± 3.77 | 2140.59 ± 859.11 | |
| CC (n = 220) | 32.37 ± 4.72 | 21.42 ± 3.31 | 4.55 ± 3.22 | 15.03 ± 9.75 | 8.24 ± 3.42 | 5.21 ± 3.38 | 5.28 ± 8.23 | 2110.45 ± 873.34 | |
| F | 0.049 | 1.002 | 0.637 | 0.230 | 0.176 | 1.053 | 0.588 | 0.726 | |
| P | 0.952 | 0.368 | 0.529 | 0.795 | 0.839 | 0.349 | 0.556 | 0.484 | |
| rs2736108 | GG (n = 444) | 32.25 ± 4.67 | 21.26 ± 3.04 | 4.66 ± 3.35 | 15.86 ± 9.67 | 8.33 ± 5.84 | 5.34 ± 3.67 | 5.29 ± 6.34 | 2050.23 ± 844.89 |
| GA (n = 434) | 32.62 ± 4.77 | 21.51 ± 3.16 | 4.93 ± 3.62 | 15.53 ± 9.91 | 8.42 ± 4.08 | 5.61 ± 3.99 | 4.77 ± 3.82 | 2171.63 ± 906.17 | |
| AA (n = 108) | 32.31 ± 5.17 | 21.48 ± 3.13 | 4.43 ± 2.92 | 13.88 ± 8.03 | 7.56 ± 1.96 | 4.71 ± 3.13 | 4.23 ± 3.40 | 2146.23 ± 820.42 | |
| F | 0.695 | 0.717 | 1.190 | 1.714 | 1.418 | 2.495 | 2.090 | 2.201 | |
| P | 0.499 | 0.488 | 0.305 | 0.181 | 0.243 | 0.083 | 0.124 | 0.111 |
Clinical indices were expressed as the mean ± standard deviation and compared using analysis of variance. Three genotypes of each polymorphism showed no significant differences in terms of woman’s age, duration of infertility, BMI, AFC, basal FSH, basal LH, basal E2, AMH or total dose of Gn
BMI body mass index, AFC antral follicle count, bFSH basal follicle-stimulating hormone, bLH basal luteinizing hormone, AMH anti-Mullerian hormone, F statistics for the analysis of variance
P <0.01 was considered statistically significant
Association between hTERT SNPs and embryo quality
In the 997 patients, carriers of the CC genotype of rs2075786 T>C had a significantly lower number of good-quality embryos (1.48 ± 1.57) than did those with the TT+TC genotypes (2.41 ± 2.78, P = 0.006).
Among the 750 patients aged ≤ 35 years, carriers of the AA+AG genotypes of rs2853691 A>G had a significantly higher number of good-quality embryos (2.61 ± 2.88) than did those with the GG genotype (1.58 ± 1.47, P = 0.003). Additionally, the number of good-quality embryos was significantly higher for the TT+TC genotypes (2.62 ± 2.83) than for the CC genotype of rs2075786 (1.47 ± 1.42, P = 0.003).
Association between hTERT SNPs and other laboratory parameters
For each of the eight selected hTERT SNPs, three genotypes were compared to examine the differences regarding the number of oocytes retrieved, the number of mature oocytes, the 2PN fertilisation rate, the cleavage rate and the number of available embryos.
For the polymorphism rs2853677 C>T, the numbers of oocytes retrieved for the CC, CT and TT genotypes were 12.05 ± 7.92, 12.03 ± 6.91 and 10.76 ± 6.07, respectively (P = 0.048). Nonetheless, in the case of the comparable Gn dose, the number of oocytes retrieved was significantly lower for the TT genotype than for the CC and CT genotypes (12.03 ± 7.13, P = 0.008). The numbers of mature oocytes for the three genotypes were 10.27 ± 7.16, 10.31 ± 5.87 and 9.08 ± 5.39, respectively (P = 0.025). Moreover, compared with the combined CC+CT genotypes, the TT genotype also exhibited a significantly lower number of mature oocytes (P = 0.004). The 2PN fertilisation rate for the CC, CT and TT genotypes were 62.78 ± 21.55, 66.49 ± 21.01 and 62.50 ± 22.24, respectively (P = 0.022). The number of available embryos for the CC, CT and TT genotypes were 5.35 ± 4.21, 5.71 ± 4.04 and 4.91 ± 3.57, respectively (P = 0.037). Similarly, when the CC and CT genotypes were combined, the number of available embryos was significantly lower for the TT genotype than for the CC+CT genotype (P = 0.011). No significant differences were found in the other indices between the three genotypes of rs2853677 C>T. In addition, the cleavage rate was significantly lower for the GG+GT genotype of rs2853672 G>T than for the TT genotype (P = 0.002). The above laboratory indices showed no significant differences between the various genotypes for the other polymorphisms tested (Table 3).
Table 3.
Laboratory parameters of three genotypes in every hTERT SNP
| SNP | Genotypes (no.) | Oocytes (n) | Mature oocytes (n) | 2PN fertilisation rate (%) | Cleavage rate (%) | Embryos available (n) | Good-quality embryos (n) |
|---|---|---|---|---|---|---|---|
| rs2853691 | AA (n = 590) | 11.57 ± 6.99 | 9.86 ± 6.04 | 64.72 ± 22.25 | 95.72 ± 14.13 | 5.31 ± 3.82 | 2.28 ± 2.65 |
| AG (n = 327) | 11.55 ± 6.68 | 9.86 ± 5.83 | 64.57 ± 20.83 | 95.97 ± 13.93 | 5.53 ± 4.30 | 2.58 ± 3.09 | |
| GG (n = 32) | 10.91 ± 6.57 | 9.34 ± 5.20 | 69.90 ± 16.35 | 96.53 ± 13.43 | 5.22 ± 2.87 | 1.59 ± 1.48 | |
| AA+AG (n = 917) | 11.56 ± 6.89 | 9.86 ± 5.96 | 64.67 ± 21.74 | 95.81 ± 14.04 | 5.39 ± 4.00 | 2.39 ± 2.82 | |
| GG+AG (n = 359) | 11.49 ± 6.67 | 9.81 ± 5.78 | 64.59 ± 19.73 | 96.02 ± 13.86 | 5.50 ± 4.19 | 2.50 ± 3.00 | |
| F | 0.141 | 0.116 | 0.914 | 0.074 | 0.335 | 2.494 | |
| P | 0.869 | 0.890 | 0.401 | 0.929 | 0.716 | 0.083 | |
| P’ (AA+AG vs GG) | 0.597 | 0.629 | 0.178 | 0.774 | 0.805 | 0.112 | |
| P” (GG+AG vs AA) | 0.863 | 0.887 | 0.215 | 0.75 | 0.484 | 0.238 | |
| rs2075786 | TT (n = 643) | 11.73 ± 6.86 | 10.02 ± 6.00 | 64.97 ± 21.68 | 95.81 ± 14.98 | 5.42 ± 3.96 | 2.35 ± 2.75 |
| TC (n = 315) | 11.77 ± 6.95 | 10.04 ± 6.05 | 65.23 ± 20.98 | 96.08 ± 11.57 | 5.62 ± 4.08 | 2.52 ± 2.81 | |
| CC (n = 27) | 10.74 ± 7.74 | 9.30 ± 6.12 | 63.63 ± 22.36 | 97.92 ± 4.76 | 4.69 ± 3.11 | 1.48 ± 1.57 | |
| TT+TC (n = 958) | 11.74 ± 6.89 | 10.03 ± 6.01 | 65.05 ± 21.44 | 95.90 ± 13.95 | 5.49 ± 4.00 | 2.41 ± 2.78 | |
| CC+TC (n = 342) | 11.69 ± 7.02 | 9.99 ± 6.05 | 65.10 ± 21.67 | 96.23 ± 11.2 | 5.55 ± 4.02 | 2.44 ± 2.75 | |
| F | 0.278 | 0.196 | 0.071 | 0.314 | 0.768 | 0.023 | |
| P | 0.757 | 0.822 | 0.932 | 0.731 | 0.464 | 0.977 | |
| P’ (TT+TC vs CC) | 0.458 | 0.532 | 0.738 | 0.460 | 0.316 | 0.006 | |
| P” (CC+TC vs TT) | 0.927 | 0.925 | 0.433 | 0.654 | 0.631 | 0.609 | |
| rs2736100 | GG (n = 186) | 11.98 ± 7.34 | 10.07 ± 6.45 | 62.35 ± 22.31 | 96.88 ± 9.92 | 5.44 ± 4.07 | 2.41 ± 2.86 |
| GT (n = 459) | 11.36 ± 6.85 | 9.76 ± 6.05 | 65.69 ± 21.67 | 94.82 ± 16.67 | 5.24 ± 3.83 | 2.20 ± 2.62 | |
| TT (n = 306) | 11.66 ± 6.69 | 9.89 ± 5.53 | 64.71 ± 20.97 | 96.87 ± 11.37 | 5.63 ± 4.14 | 2.59 ± 2.96 | |
| GG+GT (n = 645) | 11.54 ± 6.99 | 9.85 ± 6.17 | 64.72 ± 21.39 | 95.41 ± 15.07 | 5.30 ± 3.90 | 2.26 ± 2.69 | |
| TT+GT (n = 765) | 11.48 ± 6.79 | 9.81 ± 5.84 | 65.30 ± 21.39 | 95.64 ± 14.82 | 5.39 ± 3.96 | 2.36 ± 2.76 | |
| F | 0.559 | 0.182 | 1.567 | 2.541 | 0.894 | 1.871 | |
| P | 0.572 | 0.834 | 0.209 | 0.079 | 0.409 | 0.155 | |
| P’ (GG+GT vs TT) | 0.807 | 0.94 | 0.264 | 0.135 | 0.23 | 0.084 | |
| P” (TT+GT vs GG) | 0.377 | 0.591 | 0.097 | 0.281 | 0.880 | 0.825 | |
| rs2853677 | CC (n = 161) | 12.05 ± 7.92 | 10.27 ± 7.16 | 62.78 ± 21.55 | 94.93 ± 15.08 | 5.35 ± 4.21 | 2.32 ± 2.86 |
| CT (n = 596) | 12.03 ± 6.91 | 10.31 ± 5.87 | 66.49 ± 21.01 | 96.35 ± 13.56 | 5.71 ± 4.04 | 2.48 ± 2.80 | |
| TT (n = 234) | 10.76 ± 6.07 | 9.08 ± 5.39 | 62.50 ± 22.24 | 95.65 ± 13.29 | 4.91 ± 3.57 | 2.21 ± 2.57 | |
| CC+CT (n = 757) | 12.03 ± 7.13 | 10.30 ± 6.16 | 65.71 ± 21.16 | 96.05 ± 13.90 | 5.63 ± 4.08 | 2.45 ± 2.81 | |
| TT+CT (n = 830) | 11.67 ± 6.70 | 9.96 ± 5.76 | 65.36 ± 21.63 | 96.16 ± 13.48 | 5.48 ± 3.93 | 2.39 ± 2.76 | |
| F | 3.041 | 3.702 | 3.850 | 0.741 | 3.304 | 0.898 | |
| P | 0.048 | 0.025 | 0.022 | 0.477 | 0.037 | 0.408 | |
| P’ (CC+CT vs TT) | 0.008 | 0.004 | 0.047 | 0.699 | 0.011 | 0.248 | |
| P” (TT+CT vs CC) | 0.525 | 0.547 | 0.669 | 0.305 | 0.711 | 0.707 | |
| rs2853672 | GG (n = 271) | 11.68 ± 7.48 | 9.82 ± 6.55 | 63.58 ± 23.07 | 95.16 ± 15.03 | 5.31 ± 4.14 | 2.27 ± 2.80 |
| GT (n = 468) | 11.52 ± 6.71 | 10.00 ± 5.88 | 65.80 ± 21.17 | 95.49 ± 15.76 | 5.40 ± 3.83 | 2.34 ± 2.63 | |
| TT (n = 215) | 11.57 ± 6.53 | 9.71 ± 5.36 | 64.59 ± 20.44 | 97.61 ± 6.26 | 5.55 ± 4.09 | 2.55 ± 3.06 | |
| GG+GT (n = 739) | 11.58 ± 7.00 | 9.93 ± 6.13 | 64.99 ± 21.89 | 95.37 ± 15.49 | 5.37 ± 3.94 | 2.31 ± 2.69 | |
| TT+GT (n = 683) | 11.54 ± 6.65 | 9.91 ± 5.72 | 65.42 ± 20.81 | 96.15 ± 13.55 | 5.45 ± 3.91 | 2.41 ± 2.78 | |
| F | 0.046 | 0.204 | 0.930 | 2.176 | 0.219 | 0.685 | |
| P | 0.955 | 0.815 | 0.395 | 0.114 | 0.803 | 0.505 | |
| P’ (GG+GT vs TT) | 0.978 | 0.624 | 0.812 | 0.002 | 0.552 | 0.264 | |
| P” (TT+GT vs GG) | 0.769 | 0.827 | 0.889 | 0.324 | 0.634 | 0.479 | |
| rs2853669 | TT (n = 296) | 11.92 ± 6.43 | 10.15 ± 5.50 | 65.22 ± 21.18 | 97.25 ± 8.44 | 5.59 ± 3.94 | 2.56 ± 2.90 |
| TC (n = 459) | 11.61 ± 7.25 | 9.99 ± 6.29 | 65.03 ± 21.17 | 95.83 ± 14.44 | 5.42 ± 4.05 | 2.27 ± 2.71 | |
| CC (n = 148) | 11.42 ± 6.76 | 9.47 ± 5.98 | 63.23 ± 24.28 | 94.23 ± 18.83 | 5.27 ± 3.89 | 2.45 ± 2.87 | |
| TT+TC (n = 755) | 11.73 ± 6.93 | 10.05 ± 5.99 | 65.11 ± 21.16 | 96.39 ± 12.44 | 5.49 ± 4.01 | 2.38 ± 2.79 | |
| CC+TC (n = 607) | 11.57 ± 7.13 | 9.86 ± 6.21 | 64.59 ± 22.73 | 95.44 ± 15.62 | 5.39 ± 4.01 | 2.31 ± 2.75 | |
| F | 0.303 | 0.657 | 0.463 | 2.491 | 0.326 | 1.017 | |
| P | 0.739 | 0.519 | 0.630 | 0.083 | 0.722 | 0.362 | |
| P’ (TT+TC vs CC) | 0.612 | 0.275 | 0.385 | 0.185 | 0.556 | 0.773 | |
| P” (CC+TC vs TT) | 0.472 | 0.504 | 0.99 | 0.063 | 0.479 | 0.215 | |
| rs2735940 | TT (n = 264) | 11.52 ± 7.30 | 9.89 ± 6.41 | 63.56 ± 22.86 | 95.73 ± 13.85 | 5.26 ± 4.13 | 2.30 ± 2.83 |
| TC (n = 477) | 11.68 ± 6.79 | 10.09 ± 5.92 | 65.56 ± 21.11 | 95.16 ± 16.31 | 5.46 ± 3.86 | 2.34 ± 2.61 | |
| CC (n = 220) | 11.65 ± 6.66 | 9.84 ± 5.53 | 64.92 ± 20.81 | 97.66 ± 6.20 | 5.57 ± 4.10 | 2.58 ± 3.06 | |
| TT+TC (n = 741) | 11.62 ± 6.97 | 9.94 ± 6.10 | 64.85 ± 21.75 | 95.36 ± 15.48 | 5.39 ± 3.95 | 2.32 ± 2.69 | |
| CC+TC (n = 697) | 11.67 ± 6.75 | 10.01 ± 5.80 | 65.36 ± 20.97 | 95.95 ± 13.98 | 5.49 ± 3.94 | 2.41 ± 2.76 | |
| F | 0.044 | 0.408 | 0.722 | 2.433 | 0.374 | 0.746 | |
| P | 0.957 | 0.665 | 0.486 | 0.088 | 0.688 | 0.475 | |
| P’ (TT+TC vs CC) | 0.950 | 0.816 | 0.964 | 0.032 | 0.556 | 0.227 | |
| P” (CC+TC vs TT) | 0.768 | 0.457 | 0.471 | 0.827 | 0.427 | 0.566 | |
| rs2736108 | GG (n = 444) | 11.78 ± 6.41 | 10.09 ± 5.48 | 65.72 ± 20.47 | 96.89 ± 10.98 | 5.63 ± 3.84 | 2.54 ± 2.84 |
| GA (n = 434) | 11.83 ± 7.33 | 10.09 ± 6.39 | 64.91 ± 21.68 | 95.48 ± 15.23 | 5.40 ± 4.14 | 2.23 ± 2.60 | |
| AA (n = 108) | 11.26 ± 7.05 | 9.46 ± 6.39 | 61.55 ± 24.05 | 93.98 ± 17.48 | 5.17 ± 3.89 | 2.50 ± 2.99 | |
| GG+GA (n = 878) | 11.80 ± 6.88 | 10.09 ± 5.94 | 65.32 ± 21.07 | 96.19 ± 13.26 | 5.52 ± 3.99 | 2.39 ± 2.73 | |
| AA+GA (n = 542) | 11.71 ± 7.27 | 9.97 ± 6.39 | 64.24 ± 22.87 | 95.18 ± 15.70 | 5.36 ± 4.09 | 2.28 ± 2.69 | |
| F | 0.303 | 0.530 | 1.616 | 2.367 | 0.712 | 1.487 | |
| P | 0.738 | 0.589 | 0.199 | 0.094 | 0.491 | 0.227 | |
| P’ (GG+GA vs AA) | 0.440 | 0.303 | 0.125 | 0.211 | 0.398 | 0.686 | |
| P” (GG+GA vs GG) | 0.887 | 0.752 | 0.86 | 0.054 | 0.286 | 0.144 |
2PN fertilisation rate was calculated by number of two-pronuclei/number of oocytes retrieved when IVF cycles were performed or number of two-pronuclei/number of metaphase II oocytes when ICSI cycles were performed. Cleavage rate was calculated by number of cleavages/number of oocytes retrieved when IVF cycles were performed or number of cleavages/number of metaphase II oocytes when ICSI cycles were performed. Laboratory parameters were expressed as the mean ± standard deviation and compared using analysis of variance. Statistically significant differences are shown in italics
F statistics for the analysis of variance
P < 0.01 was considered statistically significant
Among the 750 patients aged ≤ 35 years, the patients with the CC+CT genotype of the polymorphism rs2853677 C>T had a higher number of oocytes retrieved (P = 0.012) and mature oocytes (P = 0.011) than those with the TT genotype. Those with the TT or GT genotype of rs2853672 G>T showed higher cleavage rate than the GG genotype carriers, but the difference was not significant (P = 0.122). The TT, TC and CC genotypes of rs2853669 T>C showed significant differences in terms of the cleavage rate (P = 0.001). Specifically, the patients with the CC genotype exhibited a lower cleavage rate than those with the TT (P = 0.001) or TC (P = 0.002) genotypes. Those with the CC or TC genotype of rs2735940 T>C showed higher cleavage rate than the TT genotype carriers, but the difference was not significant. The cleavage rates for the GG, GA and AA genotypes of rs2736108 G>A were 97.29 ± 9.29%, 96.20 ± 13.11% and 92.58 ± 19.83%, respectively (P = 0.010). The pairwise comparisons showed that patients with the AA genotype had a significantly lower cleavage rate than those with the GG genotype (P = 0.008) (Table 4).
Table 4.
Laboratory parameters of three genotypes in every hTERT SNP in patients ≤ 35 years
| SNP | Genotypes (no.) | Oocytes (n) | Mature oocytes (n) | 2PN fertilisation rate (%) | Cleavage rate (%) | Embryos available (n) | Good-quality embryos (n) |
|---|---|---|---|---|---|---|---|
| rs2853691 | AA (n = 449) | 12.69 ± 6.87 | 10.80 ± 5.97 | 64.48 ± 21.11 | 95.82 ± 14.13 | 5.73 ± 3.93 | 2.51 ± 2.76 |
| AG (n = 241) | 12.58 ± 6.56 | 10.72 ± 5.64 | 64.45 ± 18.95 | 96.99 ± 9.49 | 5.92 ± 4.36 | 2.79 ± 3.09 | |
| GG (n = 24) | 12.25 ± 6.80 | 10.42 ± 5.22 | 67.32 ± 13.25 | 95.38 ± 15.41 | 5.67 ± 3.06 | 1.58 ± 1.47 | |
| AA+AG (n = 690) | 12.65 ± 6.76 | 10.77 ± 5.85 | 64.47 ± 20.37 | 96.23 ± 12.71 | 5.80 ± 4.08 | 2.61 ± 2.88 | |
| GG+AG (n = 265) | 12.55 ± 6.57 | 10.69 ± 5.60 | 64.71 ± 16.20 | 96.84 ± 10.14 | 5.89 ± 4.25 | 2.68 ± 3 | |
| F | 0.060 | 0.058 | 0.230 | 0.698 | 0.176 | 2.296 | |
| P | 0.941 | 0.944 | 0.794 | 0.498 | 0.839 | 0.101 | |
| P’ (AA+AG vs GG) | 0.775 | 0.770 | 0.321 | 0.749 | 0.842 | 0.003 | |
| P” (GG+AG vs AA) | 0.818 | 0.822 | 0.105 | 0.308 | 0.589 | 0.39 | |
| rs2075786 | TT (n = 482) | 12.68 ± 6.79 | 10.76 ± 5.96 | 64.20 ± 20.62 | 96.12 ± 13.79 | 5.78 ± 4.06 | 2.52 ± 2.78 |
| TC (n = 240) | 13.15 ± 6.91 | 11.26 ± 5.90 | 66.08 ± 19.33 | 96.60 ± 10.25 | 6.14 ± 4.15 | 2.82 ± 2.92 | |
| CC (n = 19) | 11.32 ± 6.05 | 10.21 ± 5.69 | 63.09 ± 17.65 | 97.40 ± 5.46 | 5.39 ± 3.24 | 1.47 ± 1.42 | |
| TT+TC (n = 722) | 12.84 ± 6.83 | 10.93 ± 5.94 | 64.83 ± 20.21 | 96.28 ± 12.72 | 5.90 ± 4.09 | 2.62 ± 2.83 | |
| CC+TC (n = 259) | 13.02 ± 6.86 | 11.19 ± 5.88 | 65.86 ± 18.49 | 96.65 ± 9.99 | 6.09 ± 4.09 | 2.72 ± 2.87 | |
| F | 0.841 | 0.715 | 0.765 | 0.187 | 0.783 | 2.453 | |
| P | 0.432 | 0.489 | 0.466 | 0.830 | 0.457 | 0.087 | |
| P’ (TT+TC vs CC) | 0.337 | 0.604 | 0.717 | 0.709 | 0.601 | 0.003 | |
| P” (CC+TC vs TT) | 0.524 | 0.349 | 0.69 | 0.58 | 0.319 | 0.362 | |
| rs2736100 | GG (n = 142) | 13.06 ± 7.18 | 10.85 ± 6.41 | 60.64 ± 20.72 | 96.32 ± 10.98 | 5.66 ± 4.11 | 2.55 ± 2.89 |
| GT (n = 349) | 12.56 ± 6.74 | 10.84 ± 6.01 | 65.77 ± 20.10 | 95.67 ± 14.53 | 5.68 ± 3.97 | 2.43 ± 2.76 | |
| TT (n = 227) | 12.66 ± 6.57 | 10.68 ± 5.25 | 64.94 ± 19.80 | 97.17 ± 10.68 | 6.14 ± 4.19 | 2.82 ± 2.90 | |
| GG+GT (n = 491) | 12.71 ± 6.87 | 10.85 ± 6.12 | 64.29 ± 19.97 | 95.85 ± 13.59 | 5.67 ± 4.00 | 2.47 ± 2.80 | |
| GT+TT (n = 576) | 12.60 ± 6.67 | 10.78 ± 5.72 | 65.44 ± 19.97 | 96.26 ± 13.16 | 5.86 ± 4.06 | 2.59 ± 2.83 | |
| F | 0.281 | 0.060 | 3.347 | 0.948 | 1.035 | 1.319 | |
| P | 0.755 | 0.942 | 0.036 | 0.388 | 0.356 | 0.268 | |
| P’ (GG+GT vs TT) | 0.926 | 0.73 | 0.329 | 0.201 | 0.15 | 0.117 | |
| P” (GT+TT vs GG) | 0.464 | 0.895 | 0.011 | 0.961 | 0.602 | 0.888 | |
| rs2853677 | CC (n = 124) | 13.37 ± 8.02 | 11.30 ± 7.32 | 62.14 ± 20.35 | 94.88 ± 14.33 | 5.65 ± 4.36 | 2.47 ± 2.92 |
| CT (n = 447) | 13.07 ± 6.74 | 11.16 ± 5.69 | 65.99 ± 19.90 | 96.64 ± 12.78 | 6.14 ± 4.11 | 2.68 ± 2.81 | |
| TT (n = 177) | 11.79 ± 5.93 | 9.99 ± 5.26 | 63.28 ± 20.41 | 96.48 ± 10.40 | 5.36 ± 3.68 | 2.48 ± 2.71 | |
| CC+CT (n = 571) | 13.13 ± 7.03 | 11.19 ± 6.08 | 65.17 ± 20.04 | 96.26 ± 13.14 | 6.04 ± 4.17 | 2.64 ± 2.84 | |
| TT+CT (n = 624) | 12.71 ± 6.54 | 10.83 ± 5.6 | 65.22 ± 20.16 | 96.60 ± 12.15 | 5.92 ± 4.01 | 2.63 ± 2.79 | |
| F | 2.736 | 2.829 | 2.369 | 0.972 | 2.515 | 0.475 | |
| P | 0.065 | 0.060 | 0.094 | 0.379 | 0.082 | 0.622 | |
| P’ (CC+CT vs TT) | 0.012 | 0.011 | 0.277 | 0.840 | 0.056 | 0.536 | |
| P” (TT+CT vs CC) | 0.32 | 0.42 | 0.578 | 0.166 | 0.515 | 0.565 | |
| rs2853672 | GG (n = 204) | 12.81 ± 7.48 | 10.66 ± 6.62 | 62.38 ± 22.16 | 95.00 ± 15.27 | 5.58 ± 4.26 | 2.40 ± 2.87 |
| GT (n = 355) | 12.76 ± 6.56 | 11.13 ± 5.73 | 66.36 ± 18.95 | 96.28 ± 13.38 | 5.95 ± 3.92 | 2.63 ± 2.75 | |
| TT (n = 159) | 12.36 ± 6.33 | 10.27 ± 5.04 | 63.52 ± 19.72 | 97.78 ± 5.73 | 5.83 ± 4.11 | 2.70 ± 2.98 | |
| GG+GT (n = 559) | 12.78 ± 6.90 | 10.96 ± 6.07 | 64.91 ± 20.25 | 95.81 ± 14.09 | 5.82 ± 4.05 | 2.55 ± 2.80 | |
| TT+GT (n = 514) | 12.63 ± 6.49 | 10.87 ± 5.53 | 65.48 ± 19.34 | 96.74 ± 11.59 | 5.92 ± 3.98 | 2.65 ± 2.83 | |
| F | 0.241 | 1.282 | 2.828 | 2.114 | 0.562 | 0.589 | |
| P | 0.786 | 0.278 | 0.060 | 0.122 | 0.570 | 0.555 | |
| P’ (GG+GT vs TT) | 0.491 | 0.191 | 0.444 | 0.087 | 0.960 | 0.551 | |
| P” (TT+GT vs GG) | 0.749 | 0.667 | 0.929 | 0.10 | 0.31 | 0.292 | |
| rs2853669 | TT (n = 228) | 12.68 ± 6.31 | 10.75 ± 5.33 | 64.77 ± 20.03 | 97.53 ± 6.10 | 5.87 ± 3.93 | 2.66 ± 2.83 |
| TC (n = 344) | 13.00 ± 7.14 | 11.22 ± 6.20 | 65.18 ± 19.02 | 97.02 ± 10.79 | 5.98 ± 4.21 | 2.57 ± 2.88 | |
| CC (n = 111) | 12.28 ± 6.52 | 10.00 ± 5.78 | 61.73 ± 23.49 | 92.51 ± 21.31 | 5.37 ± 3.83 | 2.50 ± 2.81 | |
| TT+TC (n = 572) | 12.87 ± 6.82 | 11.04 ± 5.87 | 65.01 ± 19.41 | 97.22 ± 9.21 | 5.94 ± 4.10 | 2.60 ± 2.86 | |
| CC+TC (n = 455) | 12.82 ± 6.99 | 10.92 ± 6.12 | 64.34 ± 21.26 | 95.92 ± 14.22 | 5.83 ± 4.13 | 2.55 ± 2.86 | |
| F | 0.508 | 1.887 | 1.260 | 7.236 | 0.947 | 0.123 | |
| P | 0.602 | 0.152 | 0.284 | 0.001 a | 0.389 | 0.884 | |
| P’ (TT+TC vs CC) | 0.397 | 0.089 | 0.169 | 0.001 | 0.180 | 0.734 | |
| P” (CC+TC vs TT) | 0.799 | 0.723 | 0.689 | 0.104 | 0.894 | 0.653 | |
| rs2735940 | TT (n = 197) | 12.68 ± 7.39 | 10.53 ± 6.54 | 62.01 ± 21.86 | 95.24 ± 15.34 | 5.56 ± 4.30 | 2.47 ± 2.91 |
| TC (n = 364) | 12.90 ± 6.56 | 11.20 ± 5.73 | 66.36 ± 18.99 | 96.16 ± 13.36 | 5.99 ± 3.93 | 2.62 ± 2.73 | |
| CC (n = 166) | 12.34 ± 6.55 | 10.34 ± 5.30 | 64.14 ± 20.28 | 97.80 ± 5.67 | 5.81 ± 4.11 | 2.68 ± 2.96 | |
| TT+TC (n = 561) | 12.82 ± 6.86 | 10.97 ± 6.03 | 64.83 ± 20.13 | 95.84 ± 14.08 | 5.84 ± 4.07 | 2.57 ± 2.79 | |
| CC+TC (n = 530) | 12.73 ± 6.55 | 10.93 ± 5.61 | 65.67 ± 19.64 | 96.67 ± 11.55 | 5.93 ± 3.99 | 2.64 ± 2.8 | |
| F | 0.388 | 1.549 | 3.063 | 1.853 | 0.704 | 0.276 | |
| P | 0.679 | 0.213 | 0.047 | 0.157 | 0.495 | 0.759 | |
| P’ (TT+TC vs CC) | 0.424 | 0.230 | 0.697 | 0.087 | 0.940 | 0.654 | |
| P” (CC+TC vs TT) | 0.935 | 0.416 | 0.523 | 0.179 | 0.275 | 0.479 | |
| rs2736108 | GG (n = 342) | 12.58 ± 6.31 | 10.73 ± 5.31 | 65.38 ± 19.74 | 97.29 ± 9.29 | 5.96 ± 3.86 | 2.68 ± 2.80 |
| GA (n = 323) | 13.23 ± 7.26 | 11.30 ± 6.40 | 65.01 ± 19.60 | 96.20 ± 13.11 | 5.96 ± 4.35 | 2.56 ± 2.81 | |
| AA (n = 80) | 12.05 ± 6.84 | 10.01 ± 6.20 | 61.05 ± 23.63 | 92.58 ± 19.83 | 5.29 ± 3.74 | 2.50 ± 2.86 | |
| GG+GA (n = 665) | 12.90 ± 6.79 | 11.01 ± 5.87 | 65.20 ± 19.66 | 96.76 ± 11.32 | 5.96 ± 4.10 | 2.62 ± 2.81 | |
| AA+GA (n = 403) | 13.00 ± 7.19 | 11.04 ± 6.38 | 64.22 ± 21.62 | 95.48 ± 14.74 | 5.83 ± 4.24 | 2.55 ± 2.82 | |
| F | 1.345 | 1.770 | 1.541 | 4.615 | 0.986 | 0.227 | |
| P | 0.261 | 0.171 | 0.215 | 0.010 d | 0.374 | 0.797 | |
| P’ (GG+GA vs AA) | 0.292 | 0.155 | 0.135 | 0.005 | 0.160 | 0.716 | |
| P” (AA+GA vs GG) | 0.394 | 0.478 | 0.534 | 0.051 | 0.649 | 0.513 |
Laboratory parameters were expressed as the mean ± standard deviation and compared using analysis of variance. Pairwise comparisons of the indices between every two genotypes were conducted using the Bonferroni test
F statistics for the analysis of variance
P < 0.01 was considered statistically significant
aFor the polymorphism rs2853669 T>C, the patients with the CC genotype exhibited a lower cleavage rate than those with the TT (P = 0.001) or TC (P = 0.002) genotypes
bFor the polymorphism rs2736108 G>A, the patients with the AA genotype had a significantly lower cleavage rate than those with the GG genotype (P = 0.008)
Among the 247 patients aged > 35 years, no significant differences were found in the above indices between the various genotypes of each tested polymorphism.
Linkage disequilibrium and haplotype analysis
According to their physical location on the chromosome, the eight hTERT SNPs from the 3′ to 5′ end were rs2853691, rs2075786, rs2736100, rs2853677, rs2853672, rs2853669, rs2735940 and rs2736108, and they were sequentially named polymorphisms 1, 2, 3, 4, 5, 6, 7 and 8, respectively (Fig. 1). We calculated the linkage disequilibrium coefficient D′ between every two hTERT SNPs sequentially (Table 5).
Fig. 1.
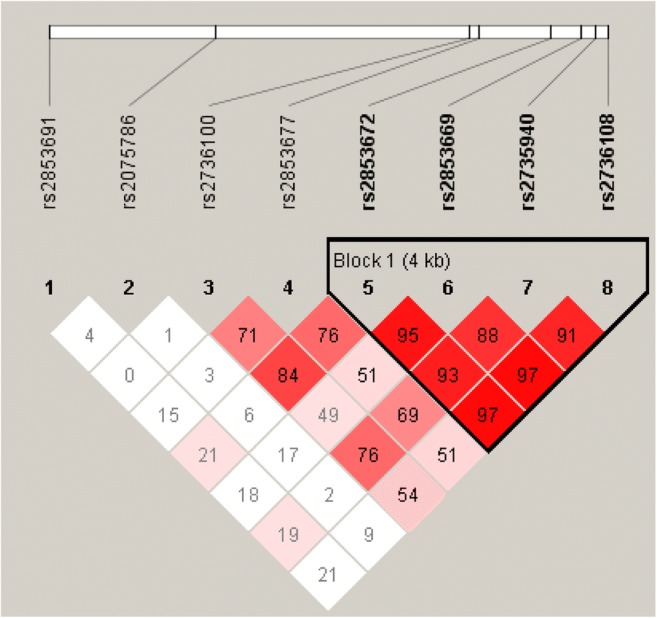
HaploBlock analysis of rs2853691, rs2075786, rs2736100, rs2853677, rs2853672, rs2853669, rs2735940 and rs2736108. The numbers in each box represent the linkage disequilibrium coefficient between the two hTERT SNPs in the upper left and upper right of the box. Dark colour boxes are representative of high linkage disequilibrium (LD), and light colour boxes indicate low LD. According to the results of the linkage disequilibrium analysis, we constructed haplotypes using rs2853672, rs2853669, rs2735940 and rs2736108 (polymorphisms 5, 6, 7 and 8, respectively) jointly
Table 5.
Linkage disequilibrium analysis among eight hTERT SNPs
| rs2853691 | rs2075786 | rs2736100 | rs2853677 | rs2853672 | rs2853669 | rs2735940 | rs2736108 | |
|---|---|---|---|---|---|---|---|---|
| rs2853691 | 0.047 | 0.008 | 0.148 | 0.209 | 0.182 | 0.196 | 0.216 | |
| rs2075786 | 0.014 | 0.031 | 0.059 | 0.175 | 0.024 | 0.086 | ||
| rs2736100 | 0.716 | 0.846 | 0.490 | 0.764 | 0.545 | |||
| rs2853677 | 0.760 | 0.511 | 0.689 | 0.517 | ||||
| rs2853672 | 0.955 | 0.928 | 0.979 | |||||
| rs2853669 | 0.886 | 0.979 | ||||||
| rs2735940 | 0.919 | |||||||
| rs2736108 |
According to the results of the linkage disequilibrium analysis, we constructed haplotypes using rs2853672, rs2853669, rs2735940 and rs2736108 (polymorphisms 5, 6, 7 and 8, respectively) jointly. After adjusting for age, we analysed the predictive value of each haplotype for the number of good-quality embryos according to whether the number of good-quality embryos was more than 3. The frequency of haplotypes constructed using polymorphisms 5, 6, 7 and 8 ranked from high to low as follows: TTCG, GCTA, GTTG, GCTC, TTTG, GTCG and GCCA. We found that the TTTG haplotype (polymorphisms 5, 6, 7 and 8) had a higher likelihood of leading to three or more good-quality embryos than to fewer than three good-quality embryos for the 750 patients aged ≤ 35 years (0.030 vs. 0.014, χ2 = 3.971, P = 0.046). However, no haplotype was found to have predictive value for the outcome regarding the number of good-quality embryos in the 247 patients aged > 35 years (P > 0.05).
Discussion
This study is the first to demonstrate the effect of hTERT SNPs on female fertility. Telomerase consists of two basic subunits: an RNA component, which is the template for telomeric DNA synthesis, and a catalytic subunit, hTERT, which exhibits telomerase reverse transcriptase activity [6]. As the only rate-limiting enzyme in telomerase synthesis, hTERT controls the major biological function of telomerase. The hTERT gene is located on chromosome 5p15.33, containing 18 exons. Transcription of hTERT can facilitate the growth of granulosa cells. We found that the genotypes of the peripheral blood DNA were consistent with those in the granulosa cells; thus, we detected the patients’ peripheral blood samples instead of their granulosa cells.
Because it is the unique rate-limiting enzyme for telomerases, TERT maintains telomerase activity and thereby maintains telomere length. Additionally, TERT can maintain the integrity of the genome by preventing the degradation of the chromosome ends [20]. Han-Woong, using mice as the study subject, observed a spermatogenesis defect in male telomerase knockout mice, with increased apoptosis and decreased proliferation of cells in the testes. That is, a lack of the telomerase gene can lead to infertility in mice [21]. Moreover, telomere erosion was found in human cell lines with suppressed TERT expression, ultimately leading to cell ageing [22]. In contrast, TERT overexpression in cells without telomerase activity could sustainably induce telomerase activity and induce cell immortalisation [23]. Moreover, various tumour cells have been shown to have high telomerase activity and sustained TERT expression [24].
Telomerase is expressed in cells and tissues with high proliferative potential in the human body. Varying degrees of telomerase activity are found in the peripheral blood, umbilical cord blood and bone marrow stem cells; however, there is no telomerase activity in mature human oocytes [25, 26]. Nevertheless, Lee et al. [12] found that even without telomerase activity, TERT can independently promote cell proliferation and can inhibit apoptosis by suppressing the mitochondrial apoptosis pathway. Therefore, changes in TERT expression may occur earlier than changes in telomerase activity.
We found that when two genotypes were combined, the homozygotes for particular hTERT SNPs had a suggestive value for the laboratory indices. For example, patients with the CC genotype of rs2075786 T>C had a significantly lower number of good-quality embryos than did those with the TT+TC genotypes containing the T allele. Patients with the TT genotype of rs2853677 C>T had lower number of oocytes retrieved, number of mature oocytes, and number of available embryos than did those with the C allele. The younger patients with the GG genotype of rs2853691 A>G had a significantly lower number of good-quality embryos than did those with the AA+AG genotypes containing the A allele. These results suggest the possibility of using hTERT SNP homozygotes to predict the development of oocytes and embryos. A study showed that the Lynch syndrome patients with the T allele of rs2075786 are at high risk to develop a Lynch syndrome-related tumour at an early age. The SNP probably causes early telomerase activation [27]. And a study found that the rs2853677 associated with lung cancer occurrence. The 1-kb region around rs2853677 exists an enhancer which regulates the expression of TERT. The SNAI1 can bind to SNP regions while T allele on rs2853677, when SNAI1 bind to the SNP, can inhibit the activity of enhancer, thereby reducing the expression level of TERT gene [28]. This leads us to suspect that the SNP might play the potential negative or positive effects on the oocyte numbers and the quality of oocytes and early embryos by changes in TERT expression or telomerase activity.
Our haplotype analysis showed that the TTTG haplotype constructed using rs2853672/rs2853669/rs2735940/rs2736108 had a higher likelihood of leading to more than three good-quality embryos in younger patients. The frequency of this haplotype was only 0.019, which is lower than the frequencies of TTCG, GCTA, GTTG and GCTG. This small population may obtain a higher number of good-quality embryos at a single time and may obtain more cumulative good-quality embryos in clinics, which is important for reducing the number of IVF cycles during cycle therapy.
The limitation of this study lies in the fact that no significant differences were found in patients aged > 35 years, perhaps due to an insufficient sample size or the possibility that age has a more important effect on fertility. And there existed limitation in regard to generalizability, which only the patients with tubal factor infertility using the conventional mid-luteal long protocol.
Conclusions
The hTERT SNP is associated with IVF outcomes. The TTTG haplotype of rs2853672, rs2853669, rs2735940 and rs2736108 has a higher likelihood of leading to more than three good-quality embryos.
Acknowledgements
The authors would like to thank all of the research workers who contributed to this study. We thank Professor Yiming Wang, a senior geneticist, for the genetics advice and Professor Caixia Li, an advanced statistician, for the statistical support.
Authors’ role
All authors contributed significantly to this work. Wenjun Wang designed the research study and wrote the paper; Kailing Dai, Hongmei Xu and Xiaomiao Zhao performed the experiments, analysed the data and wrote the manuscript; Nengyong Ouyang and Ping Yuan performed the experiments; Liangan Wang followed up the patients and collected the data; Ying Li collected the data and prepared figures and tables. All authors reviewed the manuscript. In addition, all authors approved the final draft.
Funding
Merck Serono China Research Fund for Fertility Experts; Guangdong Medical Science and Technology Research Fund (Grant No. A2015143); Guangdong Provincial Natural Science Foundation (Grant No. 2015A030313086); Special Fund for Public Welfare Research and Capacity Building in Guangdong Province (social development areas) (Grant No. 2014A020213014). These funding sources provided financial support for the conduct of the research.
Compliance with ethical standards
The project was approved by the reproductive ethics committee of the Sun Yat-Sen Memorial Hospital and has been registered in the Chinese Clinical Trial Registry under registration number ChiCTR-OCC-14005259.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kailing Dai and Hongmei Xu contributed equally to this work.
References
- 1.Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. 2016;9:63–69. doi: 10.4103/0974-1208.183514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amer Coll Obstetricians; Practice Comm Amer Soc Reprod Med Female age-related fertility decline. Fertil Steril. 2014;101(3):633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Fang T, Su Z, Wang L, Yuan P, Li R, Ouyang N, Zheng L, Wang W. Predictive value of age-specific FSH levels for IVF-ET outcome in women with normal ovarian function. Reprod Biol Endocrinol. 2015;13:63. doi: 10.1186/s12958-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chronowska E. Regulation of telomerase activity in ovarian granulosa cells. Indian J Exp Biol. 2012;50:595–601. [PubMed] [Google Scholar]
- 5.Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, Yao J, Jones E, Gochuico BR, Heller T, Wu CO, Calado RT, Scheinberg P, Young NS. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015;29:1095–1105. doi: 10.1101/gad.263863.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Chen H, Li R, Ouyang N, Chen J, Huang L, Mai M, Zhang N, Zhang Q, Yang D. Telomerase activity is more significant for predicting the outcome of IVF treatment than telomere length in granulosa cells. Reproduction. 2014;147:649–657. doi: 10.1530/REP-13-0223. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Wang W, Mo Y, Ma Y, Ouyang N, Li R, Mai M, He Y, Bodombossou-Djobo MMA, Yang D. Women with high telomerase activity in luteinised granulosa cells have a higher pregnancy rate during in vitro fertilisation treatment. J Assist Reprod Genet. 2011;28:797–807. doi: 10.1007/s10815-011-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu JP, Li H. Telomerase in the ovary. Reproduction. 2010;140(2):215–222. doi: 10.1530/REP-10-0008. [DOI] [PubMed] [Google Scholar]
- 11.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Sung YH, Cheong C, Choi YS, Jeon HK, Sun W, Hahn WC, Ishikawa F, Lee HW. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene. 2008;27:3754–3760. doi: 10.1038/sj.onc.1211037. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann M. Danazol treatment for telomere diseases. N Engl J Med. 2016;375:1095–1096. doi: 10.1056/NEJMp1607591. [DOI] [PubMed] [Google Scholar]
- 14.Melin BS, Nordfjall K, Andersson U, Roos G. hTERT cancer risk genotypes are associated with telomere length. Genet Epidemiol. 2012;36:368–372. doi: 10.1002/gepi.21630. [DOI] [PubMed] [Google Scholar]
- 15.Choi BJ, Yoon JH, Kim O, Choi WS, Nam SW, Lee JY, Park WS. Influence of the hTERT rs2736100 polymorphism on telomere length in gastric cancer. World J Gastroenterol. 2015;21:9328–9336. doi: 10.3748/wjg.v21.i31.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Chen Y, Yang X, Fan J, Mi X, Wang J, Zhang C, Hu FB, Hui R. Functional haplotypes of the hTERT gene, leukocyte telomere length shortening, and the risk of peripheral arterial disease. PLoS One. 2012;7:e47029. doi: 10.1371/journal.pone.0047029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan L, Wu S, Zhang S, Ji G, Gu A. Genetic variants in telomerase reverse transcriptase (TERT) and telomerase-associated protein 1 (TEP1) and the risk of male infertility. Gene. 2014;534:139–143. doi: 10.1016/j.gene.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker L, Critchley HO. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016;34:54–65. doi: 10.1016/j.bpobgyn.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaban B, Brison D, Calderon G, Catt J, Conaghan J, Cowan L, et al. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 20.Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S, Farsetti A. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20:3764–3771. doi: 10.1128/MCB.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 22.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Nakanishi E, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 24.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello G, Wright W, Weinrich S, Shay J. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 25.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20(1):15–30. doi: 10.1093/molehr/gat055. [DOI] [PubMed] [Google Scholar]
- 27.Bellido F, Guinó E, Jagmohanchangur S, Seguí N, Pineda M, Navarro M, et al. Genetic variant in the telomerase gene modifies cancer risk in Lynch syndrome. Eur J Hum Genet. 2013;21(5):511–516. doi: 10.1038/ejhg.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Xu X, Fang J, Wang L, Mu Y, Zhang P, Yao Z, Ma Z, Liu Z. Rs2853677 modulates Snail1 binding to the TERT enhancer and affects lung adenocarcinoma susceptibility[J] Oncotarget. 2016;7(25):37825–37838. doi: 10.18632/oncotarget.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]


