Abstract
With the intensive development of the sheep industry and increasing global temperatures, heat stress in sheep has become an increasingly severe and important issue in recent years. The level of N6-methyladenosine (m6A) RNA methylation changes in response to stress plays important roles in stress responses. However, the role of m6A in the heat stress response of sheep remains unclear. To explore this issue, we measured heat stress protein (HSP) expression, liver function indexes, m6A on RNA, m6A-related enzyme expression, and tissue damage in sheep that had been subjected to heat stress. At the transcriptome level, our results showed significant increases in m6A on RNA and increased mRNA levels of HSPs (HSP70, HSP90, and HSP110) and m6A-related enzymes [METTL3 (methyltransferase-like 3), METTL14 (methyltransferase-like 14), WTAP (wilms tumor 1-associated protein), FTO (fat mass and obesity-associated protein), ALKBH5 (alkB homologue 5), YTHDF1-3 (YTH domain family proteins), and YTHDC1-2 (YTH domain-containing proteins)] following heat stress. At the protein level, the expression of METTL3, YTHDF1-2, and YTHDC2 showed no significant differences following heat stress. However, in contrast to its mRNA level after heat stress, the protein expression of YTHDF3 was reduced, while the expression of HSPs (HSP70, HSP90, and HSP110), METTL14, WTAP, FTO, ALKBH5, YTHDF3, and YTHDC1 increased in line with their measured mRNA levels. Histological experiments revealed that heat stress caused varying degrees of damage to sheep liver tissue. Moreover, immunohistochemical staining indicated that the m6A-related enzymes were expressed in sheep hepatocytes, and differences in expression patterns were observed between the control and heat stress groups. In summary, differences in the level of m6A and the expression of m6A-related enzymes in the liver of sheep were observed after heat stress. This indicates that m6A is involved in the regulation of heat stress in sheep. Our findings provide a new avenue for studying the responses to heat stress in sheep.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-00965-x) contains supplementary material, which is available to authorized users.
Keywords: Heat stress, Hu sheep, N6-methyladenosine, RNA methylation, m6A-related enzymes
Introduction
The heat stress response describes the physiological responses that occur when a cell or organism is exposed to a high-temperature and high-humidity environment. Under these conditions, the ambient temperature is higher than the organism’s surface temperature. Animals absorb heat from the environment via conduction and radiation and dissipate heat through breathing and sweating (Banerjee et al. 2014b). Recent large-scale intensive development of the sheep industry and widespread increases in atmospheric temperatures meant that heat stress is an increasing problem for animals. Heat stress has both direct and indirect impacts on the physiological functions, productivity, and health of animals (Pantoja et al. 2017; Vilas Boas Ribeiro et al. 2018; Cui et al. 2016).
Growing evidence indicates that DNA methylation plays a key role in the regulation of heat stress (Hao et al. 2016; Vinoth et al. 2018; Zhu et al. 2017). Epigenetic modifications have effects not only at the genomic level but also at the level of the transcriptome. It is necessary for mRNA levels to change rapidly to adapt to environmental changes. Research has shown that m6A on mRNA changes in response to stress, connecting external stress to a complex network of transcriptional, post-transcriptional, and translational processes. In mouse embryonic fibroblasts, certain adenosines within the 5′ untranslated region (5′ UTR) of newly transcribed mRNAs are preferentially methylated in response to heat stress (Zhou et al. 2015). The 5′ UTR methylation pattern of stress-induced transcripts of nuclear YTH domain family protein 2 (YTHDF2) remains unchanged after heat stress through limitation of the activity of fat mass and obesity-associated protein (FTO). In addition, the expression levels of heat stress proteins (HSPs) are regulated by m6A on RNA (Yu et al. 2018). Moreover, the translation of heat stress-induced HSP70 is mediated by 5′ UTR m6A at a single site, which increases following heat stress (Meyer et al. 2015; Dominissini et al. 2012). Many studies have focused on combinations of hypoxia stress and m6A modification, and m6A methylase ALKBH5 (alkB homologue 5) is a direct target of hypoxia-inducible factor 1α (Fry et al. 2017; Thalhammer et al. 2011). A hypoxic environment increases ALKBH5 expression so as to promote m6A demethylation. RNA m6A methylation can regulate the ultraviolet-induced DNA damage response. UV-induced DNA damage causes an increase of m6A level, through which METTL3 (methyltransferase-like 3) and METTL14 become localized in the regions suffering DNA damage (Xiang et al. 2017). Therefore, m6A-related enzymes should play important roles in the RNA methylation process. Methyltransferases identified to data include METTL3, METTL14, WTAP (wilms tumor 1-associated protein), KIAA1429, RBM15 (RNA-binding motif protein 15), RBM15B, METTL16, and Zc3h13 (zinc finger CCCH domain-containing protein 13); the main demethylases are FTO and ALKBH5; while the main m6A-binding proteins are YTHDF1-3, YTHDC1-2 (YTH domain-containing proteins), elF3 (eukaryotic initiation factor 3), HNRNPA2B1 (heterogeneous nuclear ribonucleoprotein A1B1), HNRNPC, and IGF2BP (insulin-like growth factor-2 mRNA-biding proteins). These m6A-related enzymes participate in a series of metabolic processes of mRNA stabilization, splicing, transportation, and translation, among others, and can exert important effects on RNA fate (Supplementary Table S1). Therefore, studying the expression of these m6A-related enzymes in response to stress is one of the focuses of in-depth studies of m6A functions.
As one of the major animal species in farms and pastoral areas, sheep are particularly important for the incomes of farmers and herdsmen. Although significant development of the sheep industry has been achieved in recent years, numerous problems have also emerged. For example, global temperature increases have resulted in sheep being increasingly exposed to severe heat stress. The modification of RNA methylation patterns, including m6A, is key to regulating the heat stress reactions of organisms and cells, although the mechanism of action is poorly understood. This study sought to investigate the effects of heat stress in sheep and the importance of m6A in response to such stress. This was achieved through histological studies, analysis of m6A, and measurement of mRNA and protein expression levels of m6A-related enzymes.
Materials and methods
Animal and sample collection
All of the experimental procedures mentioned in the present study were approved by the Science Research Department (in charge of animal welfare issues) of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS) (Beijing, China). Ethical approval on animal survival was granted by the animal ethics committee of IAS-CAAS (No. IAS-CAAS-AE-03, 12 December 2016). In August 2017 (heat stress group) and in January 2018 (control group), 36 Hu sheep of similar weight and health (5 months old) were selected from Jiangsu Qianbao Sheep Industry Co. Ltd. (Yancheng, China, N33° 28′ 37.72″, E120° 16′ 29.65″, subtropical climate) to be raised in the same sheep house, with free access to the same feed and water. Five temperature and humidity meters were hung around the sheep house and in its middle, at a height of 1.5 m above the ground. The temperature (T) and relative humidity (RH) were recorded every 2 h from 08:00 to 20:00 every day. According to Marai et al., the temperature-humidity index (THI) can be used to assess the degree of heat stress in sheep, and is calculated as follows: THI = T – [(0.31–0.31RH) (T – 14.4)] (Marai et al. 2007). A THI value of < 22.2 was considered to represent no heat stress, 22.2 ≤ THI < 23.3 was considered moderate heat stress, 23.3 ≤ THI < 25.6 was considered severe heat stress and THI ≥ 25.6 was considered extreme severe heat stress. When THI was found to be consistently greater than 23.3 or less than 22.2 for 1 week for the August 2017 or January 2018 group, respectively, four sheep were randomly selected for slaughter. Samples of liver tissue were dissected and cleaned with physiological saline solution. The tissues were then either stored in liquid nitrogen to be used for RNA and protein extraction, or stored in 4% paraformaldehyde (Solarbio, Beijing, China) for sectioning.
RNA extraction and quantitative real-time PCR
TRlzol Reagent (Invitrogen, MA, USA) was used for the extraction of RNA, in accordance with the manufacturer’s instructions. Subsequently, a cDNA Synthesis Kit (Takara, Dalian, China) was used for RNA reverse transcription (oligo dT Primer was used in cDNA synthesis). TransStart Green qPCR SuperMix (TransGen Biotech, Beijing, China) and LightCycler 480IIapparatus (Roche, Basel, Switzerland) were used for qRT-PCR. cDNA samples in a series of twofold dilutions were prepared (1, 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, and 1/128). The eight cDNA samples were taken as a template, qRT-PCR was carried out for the target genes and reference genes, and then, a standard curve was drawn to evaluate the primer amplification efficiency. Each sample was measured in triplicate to ensure the accuracy of the quantification, and amplification curves and melting curves were observed after the reaction ended. The 2−ΔΔCt method was used to calculate the relative changes in gene expression, using ACTB (Beta-actin) as an internal reference gene. Details of the primers used are presented in Table S2.
Detection of m6A patterns in total RNA
The extracted RNA was prepared in accordance with the qRT-PCR protocol. The m6A RNA Methylation Quantification Kit (Epigentek, NY, USA) was then used to assess m6A on RNA. Briefly, 200 ng RNA samples were placed into the wells of a 96-well plate, and capture and detection antibody solutions were added in accordance with the manufacturer’s instructions. The absorbance at 450 nm was measured and a standard curve was used to quantify m6A.
Index-based determination of sheep liver function
Blood was collected from the jugular vein on an empty stomach on the day of slaughtering the sheep. Five sheep were selected for this at random in heat stress and control groups, for which non-anticoagulant vacutainer tubes were used to collect 5 ml blood. Serum was then separated from it by centrifugation for 15 min at 3000 r/min. A full automated biochemical analyzer (TOSHIBA Accute 40, Tokyo, Japan) was used to determine the levels of total bilirubin (T-BIL), direct bilirubin (D-BIL), total protein (TP), albumin (ALB), globulin (GLB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and glutamyl transpeptidase (GGT).
Histology
Liver tissue samples preserved in 4% paraformaldehyde were dehydrated and then embedded in paraffin and sectioned (5 μm in thickness, at least three sections per sample). These sections were stained with hematoxylin-eosin, and then observed and photographed with a bright-field microscope (Olympus Dp71, Tokyo, Japan).
Protein extraction and western blot analysis
RIPA protein lysis buffer (Beyotime Biotechnology, Shanghai China) was used to extract protein as per the manufacturer’s instructions, and the BCA (Solarbio, Beijing, China) method was used to determine the total protein content in the liver samples. Following extraction, 25-μg protein samples were separated using electrophoresis on 12% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). The PVDF membranes were blocked using 5% skimmed milk powder solution (Millipore, MA, USA) at room temperature for 2 h. The membranes were then incubated with the primary antibody overnight at 4 °C. Membranes subsequently were washed four times for 8 min each time in Tris-buffered saline with 0.5% Tween-20 (TBST, Solarbio, Beijing, China). The membranes were incubated with a secondary antibody (goat anti-rabbit/mouse IgG-HRP, 1:8000, Bioss, Beijing, China) at room temperature for 1.5 h. Finally, the membranes were washed four times for 8 min each time with TBST. SuperSignal™ West Pico Chemiluminescent Substrate Kit (Thermo, MA, USA) and Tanon 2500 gel imaging system (Tanon, Shanghai, China) were used to obtain images. Negative control samples were processed in an identical manner, but PBS was used instead of a primary antibody. The integral optical densities of the bands were calculated using Image-Pro Plus software (Media Cybernetics, MD, USA). The level of ACTB protein was used as an internal reference to calculate the relative expression of each protein. Information on the antibodies used in this study is listed in Table S3.
Immunohistochemical staining
The paraffin sections were dewaxed and hydrated, and then boiled in the antigen repair solution (0.01 mol/L sodium citrate buffer, pH 6.0, Solarbio, Beijing, China) for 10 min. After allowing them to cool to room temperature, samples were washed with phosphate-buffered solution (PBS) three times for 5 min each time. Sections were permeated for 10 min in 3% H2O2 (Bioss, Beijing, China) to quench endogenous peroxidase activity, after which they were blocked with 3% bovine serum albumin (Bioss, Beijing, China) at room temperature for 30 min. The blocking solution on the sections was removed gently. After adding the appropriate amount of primary antibody (Table S3), sections were placed in a wet box and incubated at 4 °C overnight. After incubation, the sections were washed three times in PBS for 5 min each time, and then incubated with HRP-conjugated anti-rabbit/mouse IgG secondary antibodies (Bioss, Beijing, China) for 30 min at 37 °C, followed by 3,3′-diaminobenzidine (Bioss, Beijing, China) coloration. Negative control samples were processed in an identical manner, but PBS was used instead of a primary antibody. A microscope (Olympus Dp71, Tokyo, Japan) and CaseViewer software (3DHISTECH, Budapest, Hungary) were used to scan and analyze the images of the sections. All analyses were performed in triplicate.
Statistical analysis
All data reported are expressed as mean ± SD. Student’s t test was carried out using SPSS software (IBM, NY, USA) for statistical analysis of the data. A p value of < 0.05 was considered to be statistically significant.
Results
Determination of m6A in sheep liver samples
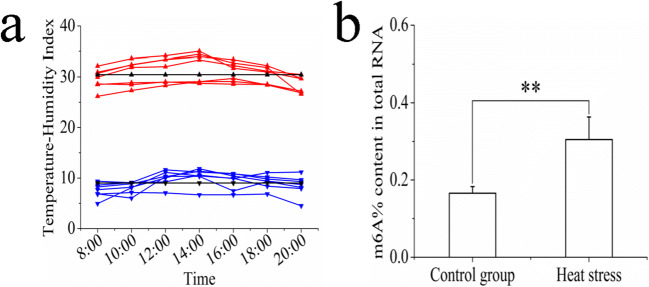
We calculated THI values according to the temperature and relative humidity of the sheep house 1 week before slaughter (Fig. 1a). During the control group period, THI was calculated to be ≤ 11.82 at each time point, with an average of 9.01, indicating that the sheep were not under heat stress. During the heat stress period, THI was ≥ 26.18 at each time point, with an average of 30.44, a level at which the sheep would have been under extremely severe heat stress. The level of m6A on total RNA was also found to be significantly higher in the heat stress group than in the control group (Fig. 1b). These results strongly implicate m6A in the heat stress response of sheep.
Fig. 1.
Determination of m6A level in sheep liver. a The THI of the sheep house 1 week before slaughter. Red line indicates THI during the heat stress period, the blue line indicates THI during the control group period, and the black line indicates the average THI. b The m6A level in sheep liver. Data are presented as the mean ± SD of three independent experiments for each sample. *p < 0.05, **p < 0.01, NS: no significant difference
Influence of heat stress on HSP expression
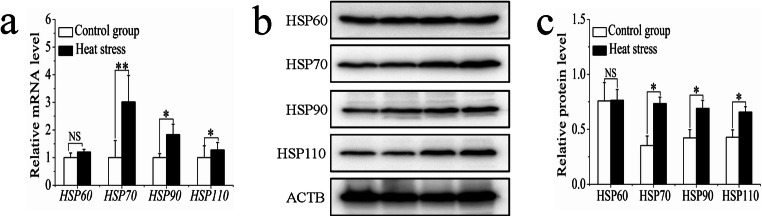
We determined the influence of heat stress on HSP60, HSP70, HSP90, and HSP110 expressions in sheep. At the transcriptional level, the mRNA expression of HSP70, HSP90, and HSP110 in heat-stressed sheep was significantly higher than that in the control group, while no significant difference was identified for HSP60 between the two groups (Fig. 2a). The same results were obtained in the analyses at the protein level (Fig. 2b, c).
Fig. 2.
The influence of sheep heat stress on HSP expression. a The mRNA expression of HSPs. b The HSPs detected by western blotting. c The protein expression of HSPs. Data are presented as the mean ± SD of three independent experiments for each sample. *p < 0.05, **p < 0.01, NS: no significant difference
Influence of heat stress on sheep liver function indexes and organization structure
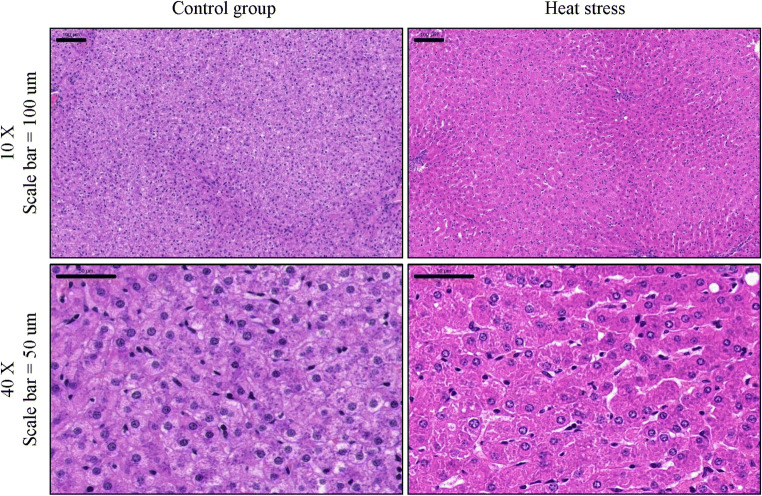
Table 1 shows the influence of heat stress on sheep liver function indexes, with ALT, ALP, and GGT being significantly higher than those in the control group; other indexes show no significant difference between the two groups. In addition, heat stress caused varying degrees of damage to sheep liver tissues (Fig. 3). The control group showed a clear cell structure and transparent endochylema. Heat stress disrupted the cell structure, resulting in an increased amount of cell debris being observed. Some nuclei exhibited migration after heat stress, showing displacement within the cell and a concentrated nucleoplasm. In addition, cell vacuoles and hydropic degeneration were observed after heat stress.
Table 1.
Influence of heat stress on sheep liver function indexes
| Item | Number | Control group | Heat stress group |
|---|---|---|---|
| T-BIL (umol/L) | 5 | 5.08 ± 0.46 | 4.90 ± 1.04 |
| D-BIL (umol/L) | 5 | 0.81 ± 0.16 | 1.72 ± 0.31 |
| IB (umol/L) | 5 | 4.28 ± 0.36 | 2.58 ± 0.68 |
| TP (g/L) | 5 | 67.60 ± 3.21 | 54.60 ± 1.10 |
| ALB (g/L) | 5 | 32.30 ± 0.38 | 37.40 ± 2.51 |
| GLB (g/L) | 5 | 22.30 ± 1.20 | 30.20 ± 2.59 |
| ALT (U/L) | 5 | 9.20 ± 3.49 | 17.40 ± 3.21 |
| AST (U/L) | 5 | 12.50 ± 3.59 B | 76.00 ± 6.86 A |
| ALP (U/L) | 5 | 140.20 ± 20.96B | 278.40 ± 61.92A |
| GGT (U/L) | 5 | 13.60 ± 1.34b | 63.80 ± 5.89a |
In the same line, values with different uppercase letters are highly significantly different (p < 0.01), while those with different lowercase letters are significantly different (p < 0.05)
Fig. 3.
HE staining of sheep liver tissue
Influence of heat stress on m6A methyltransferase expression
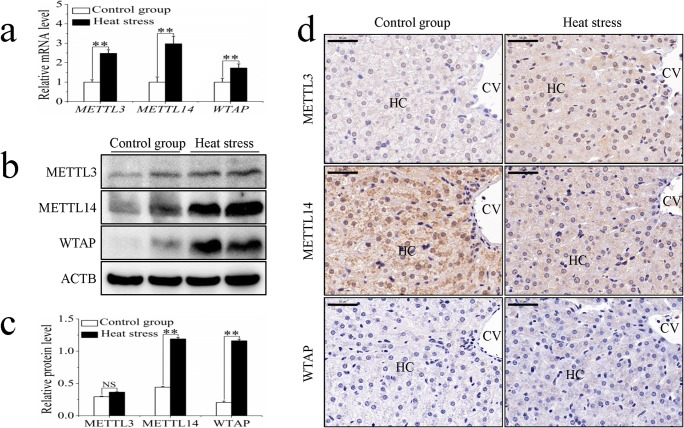
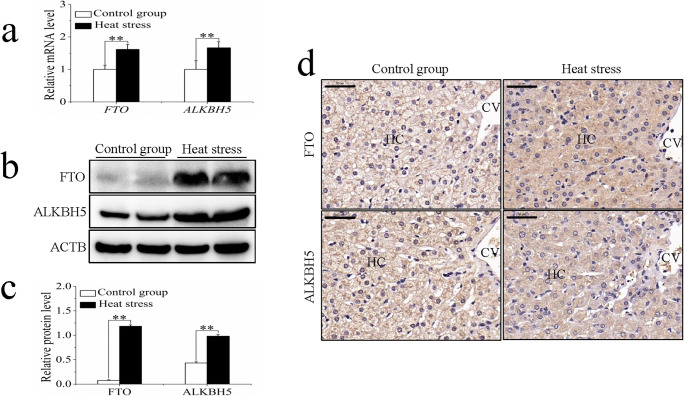
The m6A on RNA is mediated by a methyltransferase complex. The study of this complex is crucial for advancing research on m6A. To this end, we analyzed the mRNA levels and protein expression of METTL3, METTL14, and WTAP in sheep in response to heat stress. Compared with those in the control group, the mRNA levels of METTL3, METTL14, and WTAP of the heat stress group increased significantly (Fig. 4a). There was no significant difference in the protein expression of METTL3 between the control and heat stress groups, while METTL14 and WTAP protein expression levels increased significantly following heat stress (Fig. 4b, c). Immunohistochemistry revealed that METTL3 and METTL14 were present in the hepatocytes of both control and heat stress groups. However, the staining of METTL3 was stronger in the heat stress group, whereas the staining of METTL14 was stronger in the control group (Fig. 4d). In addition, in the cytoplasm of hepatocytes from the heat stress group, METTL14 was seen to be distributed in discrete clusters, whereas the control group showed a more uniform distribution (Fig. 4d). Almost no expression of WTAP was observed in the hepatocytes of either group (Fig. 4d).
Fig. 4.
The influence of sheep heat stress on m6A methyltransferase expression. (a) The mRNA expression of m6A methyltransferase. (b) The m6A methyltransferase detected by western blotting. (c) The protein expression of m6A methyltransferase. (d) The immunohistochemical staining analysis of m6A methyltransferase. Data are presented as the mean ± SD of three independent experiments for each sample. *p < 0.05, **p < 0.01, NS: no significant difference. HC: hepatocyte, CV: central vein, scale bar = 100 μm
Influence of heat stress on m6A demethylase expression
The discovery of m6A demethylases promoted research into RNA methylation and made it possible for m6A to be investigated during the dynamic regulation of cells. The mRNA levels of the m6A demethylases FTO and ALKBH5 were significantly higher in the heat stress group than in the control group (Fig. 5a). Western blot analysis revealed that the expression levels of FTO and ALKBH5 were both significantly increased in the heat stress group (Fig. 5b, c). Immunohistochemical analysis indicated that FTO and ALKBH5 were expressed in the hepatocytes (Fig. 5d), with increased staining seen in the cytoplasm compared with that in the nucleus.
Fig. 5.
The influence of sheep heat stress on m6A demethylase expression. (a) The mRNA expression of m6A demethylase. (b) The m6A demethylase detected by western blotting. (c) The protein expression of m6A demethylase. (d) The immunohistochemical staining analysis of m6A demethylase. Data are presented as the mean ± SD of three independent experiments for each sample. *p < 0.05, **p < 0.01, NS: no significant difference. HC: hepatocyte, CV: central vein, scale bar = 100 μm
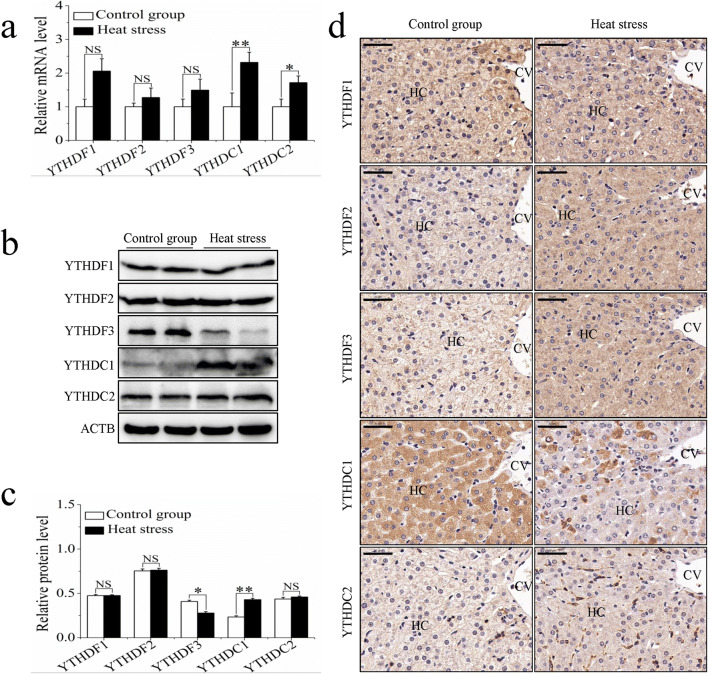
Influence of heat stress on m6A-binding protein expression
The recognition of m6A sites by m6A-binding proteins can occur through both direct and indirect mechanisms and may affect RNA metabolism. We analyzed the expression of five m6A-binding proteins in the liver tissues of the control and heat stress groups. The mRNA levels of YTHDC1 and YTHDC2 were significantly higher in the heat stress group, while the mRNA levels of YTHF1-3 were not significantly different between the two groups (Fig. 6a). Significant differences were seen between the groups in the protein expression levels of YTHDF3 and YTHDC1, which showed decreased and increased expression respectively (Fig. 6b, c). Immunohistochemistry revealed that the five m6A-binding proteins were expressed in the hepatocytes. They were found to be strongly expressed in the cytoplasm, but minimally expressed in the nucleus (Fig. 6d). The distribution of YTHDC1 within the cytoplasm appeared to be punctate (Fig. 6d).
Fig. 6.
The influence of sheep heat stress on m6A-binding protein expression. (a) The mRNA expression of m6A-binding protein. (b) The m6A-binding protein detected by western blotting. (c) The protein expression of m6A-binding protein. (d) The immunohistochemical staining analysis of m6A-binding protein. Data are presented as the mean ± SD of three independent experiments for each sample. *p < 0.05, **p < 0.01, NS: no significant difference. HC: hepatocyte, CV: central vein, scale bar = 100 μm
Discussion
Sheep are homeothermic animals, meaning that their body temperature must remain within a narrow physiological range to maintain homeostasis. The regulation of heat stress in sheep is controlled by the hypothalamus, which induces endocrine and behavioral responses to such stress via various neural pathways (Keim et al. 2002). A heat stress response occurs when the ambient temperature is higher than the optimum physiological range, and the thermoregulatory system is activated to dissipate excessive heat. If the excessive heat energy cannot be dissipated, the animal’s internal temperature rises, resulting in decreases in feed intake, rumination, and reproductive capacity, and potentially the loss of the sheep production chain, with serious implications for the industry (Das et al. 2016; De et al. 2017; Narayan et al. 2018). Study of the molecular mechanisms of the sheep heat stress response is therefore critical to production performance.
In response to heat stress, certain adenosine residues within the 5′ UTR are preferentially methylated. Furthermore, UV-induced DNA damage promotes m6A methylation (Zhou et al. 2015; Xiang et al. 2017). We have shown that the m6A on RNA of sheep subjected to heat stress was significantly higher than that of the control group. These findings support published results that have shown that the methyl donor betaine affects m6A levels, and its inclusion in the diet of sheep and goats can relieve heat stress (DiGiacomo et al. 2016; Dangi et al. 2016). However, it has been reported that hypoxic stress promotes m6A demethylation and reduces m6A levels, which contradicts other studies (Zhang et al. 2016). Although these studies confirm that m6A is involved in various stress responses, they highlight the possibility that its physiological roles may vary, and the specific mechanisms are yet to be determined. This further emphasizes the importance of m6A in regulating the heat stress response of sheep and the need to study the mechanisms involved.
The HSPs are highly conservative proteins that protect organs such as the liver, heart, and kidney from injuries caused by stress (Li et al. 2018; Sun et al. 2018; Wang et al. 2018). Under stress conditions, the expression of HSPs increases, which prevents the accumulation of intracellular denatured proteins and facilitates their repair, thus increasing the resistance of cells. We found in our research that the expression of all of HSP70, HSP90, and HSP110 in heat-stressed sheep was significantly higher than that in the control group, whereas HSP60 showed no significant difference between the two groups. Research has shown that HPS60 expression in goat presented a trend of first increasing and then decreasing during heat stress, which is related to the negative feedback regulation mechanism of the HSPs (Dangi et al. 2014; Dangi et al. 2015). To resist stress, cells need to use a large amount of HSP60, which results in its consumption. When a certain amount of HSP60 has been used up, its expression increases via a negative feedback regulatory mechanism. In this research, the heat-stressed sheep were in a state of extremely severe heat stress, so large amounts of HSP60 had already been consumed by cells, causing less change in its expression. Among all of the HSPs, HSP70 is the most sensitive to temperature and is regarded as an important marker gene (Bhargav et al. 2008; Yang et al. 2007; Zhong et al. 2012). In our study, we found that the expression of HSP70 in heat-stressed sheep was almost three times that in the control group; its sharp increase in expression is thus a reflection of heat stress. Similarly, HSP70 expression has also been found to increase in species such as goats, pigs, and chickens after heat stress (Archana et al. 2018; Pearce et al. 2014; Roushdy et al. 2018). In the case of HSP90, the protective mechanisms conferred by it are currently unclear. A study showed that goat HSP90 expression is higher in summer than in winter, the same as our result (Dangi et al. 2012). HSP110 is an important branch of HSP70 and regulates the partner system, so that there is a necessary connection between HSP110 expression change with HSP70.
The liver is one of the most metabolically active organs, so the liver function index is useful to reflect the level of damage to liver cells and metabolism, excretion, and immune function, among others. Compared with that in the control group, the serum TP in heat-stressed sheep exhibited a decreasing trend, which may have been caused by the reduction of food intake by the sheep during the heat stress period (Indu et al. 2015). ALT and AST are indexes reflecting liver parenchymal damage (Banerjee et al. 2014a). When liver cells are damaged, the ALT level in serum rises one time, and a continuous rise of AST can occur, which indicates the aggravation of damage. In our study, we found that the ALT level in heat-stressed sheep showed an increasing trend. The significant increase of AST level showed that heat stress caused liver damage; this was confirmed by the results of HE staining of sheep liver, which revealed nuclear migration, chromatin condensation, and cell vacuole and hydropic degeneration. These results basically match those from research on rats (Zhang et al. 2003).
It had already been indicated that m6A modification in mRNA plays an important role in the stress response process, but little was known about the expression of m6A-related enzymes for the exertion of m6A function before and after stress. Therefore, we analyzed the impact of heat stress on the expression of these enzymes in sheep through qRT-PCR and western blotting. At the transcriptome level, mRNA levels of YTHDF1-3 were slightly higher in the heat-stressed group than in the control group, whereas mRNA levels of all other enzymes studied increased significantly. However, there were no significant differences in the expression levels of METTL3, YTHDF1-2, and YTHDC2 between the two groups. In contrast to the mRNA levels, the protein expression of YTHDF3 was reduced in the heat stress group, but the expression levels of the other proteins correlated with their mRNA levels. Methyltransferases and demethylases play opposite roles in the regulation of m6A. However, both the methyltransferase and demethylase enzymes that were analyzed in this study were found to be upregulated during heat stress. It is well known that m6A is a key factor in the post-transcriptional regulation of genes as it regulates RNA splicing, stability, decay, and translation (Fu et al. 2014; Yang et al. 2018). The enzymes in this study may have roles in post-transcriptional regulation, and their increased expression potentially indicates that an m6A-mediated stress response has been initiated. Studies on human HepG2 cells have reported that METTL3, METTL14, and FTO were downregulated 6 h after heat stress, METTL3 and FTO were downregulated and METTL14 was upregulated 12 h later, and all proteins were upregulated 24 h later (Yu et al. 2018). These findings suggest that the m6A heat stress response varies over time, but further research is needed to reveal the fundamental mechanisms behind these changes. METTL3, METTL14, WTAP, FTO, ALKBH5, and YTHDC1 are generally expressed in the nucleus, while YTHDF1-3 and YTHDC2 are expressed in the cytoplasm (Batista 2017). However, immunohistochemistry carried out in this study revealed that these enzymes were expressed in both the nucleus and the cytoplasm of sheep hepatocytes. Differences in the expression levels between the control and heat stress groups implicated m6A in regulating the heat stress response of sheep. Moreover, studies using mouse embryonic fibroblasts and human HepG2 cells have reported that YTHDF2 relocates from the cytoplasm to the nucleus in response to heat stress (Zhou et al. 2015; Yu et al. 2018). This phenomenon was not observed in the present study, although the expression of YTHDF2 was significantly increased following heat stress. Tissues are much more complex than cells in terms of structure and are therefore affected by more factors that could explain the discrepancies.
In conclusion, our results indicate that m6A on RNA plays a key role in regulating the heat stress response of sheep. This finding provides important information for managing this important issue and opens a new avenue into studying the heat stress response in sheep.
Electronic supplementary material
qRT-PCR standard curves. Amplification efficiencies of all primers range between 95% and 105%, which meets the experimental requirements of qRT-PCR. (PNG 742 kb)
qRT-PCR amplification curves. Amplification curves of all primers are smooth, without amplification in the beginning. The starting moment of peak is normal. (PNG 1277 kb)
qRT-PCR melting curves. Melting curves of all primers present a single narrow peak, which indicates the absence of redundant products and primer dimers. (PNG 939 kb)
m6A-related enzymes and their roles in RNA metabolism. (DOCX 31 kb)
The primers used in this study for qRT-PCR. (DOCX 22 kb)
The antibodies used in this study for western blotting and immunohistochemistry. (DOCX 20 kb)
Author contributions
Caihong Wei, Liping Zhang, and Zengkui Lu conceived and designed the experiments; Zengkui Lu, Youji Ma, Enmin Liu, Qing Li, and Meilin Jin collected samples; and Zengkui Lu performed the experiments and wrote the paper.
Funding information
This work was supported by the National Modern Agricultural Industry Technology Fund for Scientists in Sheep Industry System (No. CARS-38) and the National Natural Science Foundation of China (No.31672380).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liping Zhang, Email: zhangliping@gsau.edu.cn.
Caihong Wei, Email: weicaihong@caas.cn.
References
- Archana PR, Sejian V, Ruban W, Bagath M, Krishnan G, Aleena J, Manjunathareddy GB, Beena V, Bhatta R. Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Sci. 2018;141:66–80. doi: 10.1016/j.meatsci.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh DTK, De S. Seasonal variations in physio-biochemical profiles of Indian goats in the paradigm of hot and cold climate. Biol Rhythm Res. 2014;46:221–236. doi: 10.1080/09291016.2014.984999. [DOI] [Google Scholar]
- Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh GJM, Polley S, Mukherjee A, Das TK, De S. Seasonal variation in expression pattern of genes under HSP70 family in heat-and cold-adapted goats (Capra hircus) Cell Stress Chaperones. 2014;19:401–408. doi: 10.1007/s12192-013-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ. The RNA modification N(6)-methyladenosine and its implications in human disease. Genomics Proteomics Bioinformatics. 2017;15:154–163. doi: 10.1016/j.gpb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargav D, Pratap SM, Murthy RC, Mathur N, Misra D, Saxena DK, Kar CD. Toxic potential of municipal solid waste leachates in transgenic Drosophila melanogaster (hsp70-lacZ): hsp70 as a marker of cellular damage. Ecotoxicol Environ Saf. 2008;69:233–245. doi: 10.1016/j.ecoenv.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Cui YJ, Hao Y, Li JL, Bao WG, Li G, Gao YL, Gu XH. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. Int J Mol Sci. 2016;17:E393. doi: 10.3390/ijms17050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi SS, Gupta M, Maurya D, Yadav VP, Panda RP, Singh G, Mohan NH, Bhure SK, Das BC, Bag S, Mahapatra R, Taru Sharma G, Sarkar M. Expression profile of HSP genes during different seasons in goats (Capra hircus) Trop Anim Health Prod. 2012;44:1905–1912. doi: 10.1007/s11250-012-0155-8. [DOI] [PubMed] [Google Scholar]
- Dangi SS, Gupta M, Nagar V, Yadav VP, Dangi SK, Shankar O, Chouhan VS, Kumar P, Singh G, Sarkar M. Impact of short-term heat stress on physiological responses and expression profile of HSPs in Barbari goats. Int J Biometeorol. 2014;58:2085–2093. doi: 10.1007/s00484-014-0809-5. [DOI] [PubMed] [Google Scholar]
- Dangi SS, Gupta M, Dangi SK, Chouhan VS, Maurya VP, Kumar P, Singh G, Sarkar M. Expression of HSPs: an adaptive mechanism during long-term heat stress in goats (Capra hircus) Int J Biometeorol. 2015;59:1095–1106. doi: 10.1007/s00484-014-0922-5. [DOI] [PubMed] [Google Scholar]
- Dangi SS, Dangi SK, Chouhan VS, Verma MR, Kumar P, Singh G, Sarkar M. Modulatory effect of betaine on expression dynamics of HSPs during heat stress acclimation in goat (Capra hircus) Gene. 2016;575:543–550. doi: 10.1016/j.gene.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Das R, Sailo L, Verma N, Bharti P, Saikia J, Imtiwati KR. Impact of heat stress on health and performance of dairy animals: a review. Vet World. 2016;9:260–268. doi: 10.14202/vetworld.2016.260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De K, Kumar D, Saxena VK, Thirumurugan P, Naqvi SMK. Effect of high ambient temperature on behavior of sheep under semi-arid tropical environment. Int J Biometeorol. 2017;61:1269–1277. doi: 10.1007/s00484-016-1304-y. [DOI] [PubMed] [Google Scholar]
- DiGiacomo K, Simpson S, Leury BJ, Dunshea FR. Dietary betaine impacts the physiological responses to moderate heat conditions in a dose dependent manner in sheep. Animals (Basel) 2016;6:E51. doi: 10.3390/ani6090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Fry NJ, Law BA, Ilkayeva OR, Holley CL, Mansfield KD. N6-methyladenosine is required for the hypoxic stabilization of specific mRNAs. RNA. 2017;23:1444–1455. doi: 10.1261/rna.061044.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- Hao Y, Cui YJ, Gu XH. Genome-wide DNA methylation profiles changes associated with constant heat stress in pigs as measured by bisulfite sequencing. Sci Rep. 2016;6:27507. doi: 10.1038/srep27507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indu S, Sejian V, Naqvi SMK. Impact of simulated semiarid tropical environmental conditions on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes. Anim Prod Sci. 2015;55:243–252. doi: 10.1071/AN14085. [DOI] [Google Scholar]
- Keim SM, Guisto JA, Sullivan JB. Environmental thermal stress. Ann Agric Environ Med. 2002;9:1–15. [PubMed] [Google Scholar]
- Li PC, Li XN, Du ZH, Wang H, Yu ZR, Li JL. Di (2-ethyl hexyl) phthalate (DEHP)-induced kidney injury in quail (Coturnix japonica) via inhibiting HSF1/HSF3-dependent heat shock response. Chemosphere. 2018;209:981–988. doi: 10.1016/j.chemosphere.2018.06.158. [DOI] [PubMed] [Google Scholar]
- Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM. Physiological traits as affected by heat stress in sheep—a review. Small Rumin Res. 2007;71:1–12. doi: 10.1016/j.smallrumres.2006.10.003. [DOI] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5' UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan E, Sawyer G, Parisella S. Faecal glucocorticoid metabolites and body temperature in Australian merino ewes (Ovis aries) during summer artificial insemination (AI) program. PLoS One. 2018;13:e0191961. doi: 10.1371/journal.pone.0191961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja MHA, Esteves SN, Jacinto MAC, Pezzopane JRM, Paz CCP, Silva J, Lourenco JJB, Brandao FZ, Moura ABB, Romanello N, Botta D, Garcia AR. Thermoregulation of male sheep of indigenous or exotic breeds in a tropical environment. J Therm Biol. 2017;69:302–310. doi: 10.1016/j.jtherbio.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Pearce SC, Sanz-Fernandez MV, Hollis JH, Baumgard LH, Gabler NK. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J Anim Sci. 2014;92:5444–5454. doi: 10.2527/jas.2014-8407. [DOI] [PubMed] [Google Scholar]
- Roushdy EM, Zaglool AW, El-Tarabany MS. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J Therm Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Sun F, Zuo YZ, Ge J, Xia J, Li XN, Lin J, Zhang C, Xu HL, Li JL. Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production. Poult Sci. 2018;97:2638–2646. doi: 10.3382/ps/pey146. [DOI] [PubMed] [Google Scholar]
- Thalhammer A, Bencokova Z, Poole R, Loenarz C, Adam J, O'Flaherty L, Schödel J, Mole D, Giaslakiotis K, Schofield CJ, Hammond EM, Ratcliffe PJ, Pollard PJ. Human AlkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1α (HIF-1α) PLoS One. 2011;6:e16210. doi: 10.1371/journal.pone.0016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas Boas Ribeiro BP, Lanferdini E, Palencia JYP, Lemes MAG, Teixeira de Abreu ML, Souza Cantarelli V, Ferreira RA. Heat negatively affects lactating swine: a meta-analysis. J Therm Biol. 2018;74:325–330. doi: 10.1016/j.jtherbio.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Vinoth A, Thirunalasundari T, Shanmugam M, Uthrakumar A, Suji S, Rajkumar U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones. 2018;23:235–252. doi: 10.1007/s12192-017-0837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li XN, Li PC, Liu W, Du ZH, Li JL (2018) Modulation of heat-shock response is associated with Di (2-ethyl hexyl) phthalate (DEHP)-induced cardiotoxicity in quail (Coturnix japonica). Chemosphere [DOI] [PubMed]
- Xiang Y, Laurent B, Hsu CH, Nachtergaele S, Lu ZK, Sheng WQ, Xu CY, Chen H, Ouyang J, Wang SQ, Ling D, Hsu PH, Zou L, Jambhekar A, He C, Shi Y. RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543:573–576. doi: 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XB, Zheng JP, Bai Y, Tian FJ, Yuan J, Sun JY, Liang HS, Guo L, Tan H, Chen WH, Tanguay RM, Wu TC. Using lymphocyte and plasma Hsp70 as biomarkers for assessing coke oven exposure among steel workers. Environ Health Perspect. 2007;115:1573–1577. doi: 10.1289/ehp.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Li Y, Wang T, Zhong X. Modification of N6-methyladenosine RNA methylation on heat shock protein expression. PLoS One. 2018;13:e0198604. doi: 10.1371/journal.pone.0198604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Xu L, Drake VJ, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J. 2003;17:2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- Zhang CZ, Samanta D, Lu HQ, Bullen JW, Zhang HM, Chen I, He XS, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:2047–2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Li W, Huang XX, Zhang LL, Yimamu M, Raiput N, Zhou YM, Wang T. Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones. 2012;17:495–505. doi: 10.1007/s12192-012-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao XW, Zhang XQ, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YW, Lu L, Liao XD, Li WX, Zhang LY, Ji C, Lin X, Liu HC, Odle J, Luo XG. Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and anti-apoptotic abilities. Oncotarget. 2017;8:89665–89680. doi: 10.18632/oncotarget.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qRT-PCR standard curves. Amplification efficiencies of all primers range between 95% and 105%, which meets the experimental requirements of qRT-PCR. (PNG 742 kb)
qRT-PCR amplification curves. Amplification curves of all primers are smooth, without amplification in the beginning. The starting moment of peak is normal. (PNG 1277 kb)
qRT-PCR melting curves. Melting curves of all primers present a single narrow peak, which indicates the absence of redundant products and primer dimers. (PNG 939 kb)
m6A-related enzymes and their roles in RNA metabolism. (DOCX 31 kb)
The primers used in this study for qRT-PCR. (DOCX 22 kb)
The antibodies used in this study for western blotting and immunohistochemistry. (DOCX 20 kb)