Abstract
BACKGROUND:
Nano-hydroxyapatite/polyamide 66 (nHA/PA66) is a composite used widely in the repair of bone defects. However, this material is insufficient bioactivity. In contrast, D-RADA16-RGD self-assembling peptide (D-RADA16-RGD sequence containing all D-amino acids is Ac-RADARADARADARADARGDS-CONH2) shows admirable bioactivity for both cell culture and bone regeneration. Here, we describe the fabrication of a favorable biomaterial material (nHA/PA66/D-RADA16-RGD).
METHODS:
Proteinase K and circular dichroism spectroscopy were employed to test the stability and secondary structural properties of peptide D-RADA16-RGD respectively. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to characterize the surface of these materials. Confocal laser scanning (CLS), cell counting kit-8 tests (CCK-8), alizarin red S staining, cell immunofluorescence analysis and Western blotting were involved in vitro. Also biosafety and bioactivity of them have been evaluated in vivo.
RESULTS:
Proteinase K and circular dichroism spectroscopy demonstrated that D-RADA16-RGD in nHA/PA66 was able to form stable-sheet secondary structure. SEM and TEM showed that the D-RADA16-RGD material was 7–33 nm in width and 130–600 nm in length, and the interwoven pore size ranged from 40 to 200 nm. CLS suggests that cells in nHA/PA66/D-RADA16-RGD group were linked to adjacent cells with more actin filaments. CCK-8 analysis showed that nHA/PA66/D-RADA16-RGD revealed good biocompatibility. The results of Alizarin-red S staining and Western blotting as well as vivo osteogenesis suggest nHA/PA66/D-RADA16-RGD exhibits better bioactivity.
CONCLUSION:
This study demonstrates that our nHA/PA66/D-RADA16-RGD composite exhibits reasonable mechanical properties, biocompatibility and bioactivity with promotion of bone formation.
Electronic supplementary material
The online version of this article (10.1007/s13770-018-0171-5) contains supplementary material, which is available to authorized users.
Keywords: nHA/PA66/D-RADA16-RGD, Bone defect, Bone regeneration, Peptide hydrogel
Introduction
It is difficult to repair some bone defects, particularly load-bearing or large bone defects, which may cause non-union and thus cause effect upon a patient’s quality of life [1]. Bones do have the potential to regenerate; however, bone induction and conduction for the recovery of large or load-bearing bone defects require bone grafts, which are supported mechanically [2, 3]. Indeed, autologous grafts represent biologically compatible materials for bone transplantation hey have some limitations, including the limited availability, pain, donor site morbidity, and the mismatch between harvested bone grafts as well as defect sites [4]. Allogeneic bone grafts serve as an alternative to autologous grafts but may trigger immune rejection and disease transmission [5].
Current synthetic materials are limited by a number of factors, including insufficient bioactivity or biocompatibility, as well as unsatisfactory degradation or non-degradation properties [6]. Favorable biomaterials are associated with bioactivity and ductility provided by both inorganic and macromolecular compounds [7, 8]. Nano-hydroxyapatite/polyamide 66 (nHA/PA66) consists of nHA and PA66, and exhibits the features of good biocompatibility, osteo-conductivity and mechanical behavior. Furthermore, many experiments have shown that nHA/PA66 not only satisfies the requirements to act as a substitute for natural bone, but also has similar biomechanics to human bones [9, 10]. As a consequence, this composite has been widely used to repair bone, and various derivative products consisting of nHA/PA66 have also been increasingly used in clinical work [11–13]. Research has shown that nHA/PA66 is able to directly integrate with human bone and does not appear to be associated with negative effects after implantation [14, 15]. A new biomaterial demands both preferable biomechanics and biological activity; however, nHA/PA66 composite has insufficient bioactivity compared with other biomaterials. As a result, our research aimed to enhance the bioactivity of nHA/PA66 composite. For the its ionic self-complementary characteristic, D-RADA16-RGD (D-RADA16-RGD sequence containing all D-amino acids is Ac-RADARADARADARADARGDS-CONH2) is able to spontaneously assemble into nanofibers.
A previous study showed that D-RADA16-RGD peptides can be made into three-dimensional (3D) scaffolds that resemble the microstructures of the natural extracellular matrix (ECM) [16]. The D-RADA16-RGD peptide amphiphiles, including the hydrophobic domain, a short peptide sequence capable of forming intermolecular hydrogen bonding, charged amino acids for the design of pH and salt-responsive nanostructures, as well as the hydrophobic alkyl tail for the interactions with cells or proteins could have favorable influence on cell function such as cell adhesion, proliferation and differentiation [17, 18]. Additionally, these 3D scaffolds were subsequently found to significantly facilitate not only the delivery of oxygen and soluble signaling molecules, but also the metabolism of waste product [19, 20]. A recent study reported that D-RADA16-RGD materials also help to promote cell proliferation, or repair processes following injury [21].
The present study evaluates D-RADA16-RGD peptides as a newly designed material for bone repair with a particular emphasis on bioactivity. The RGD sequence found in fibronectin, as well as other ECM proteins, could promote cell differentiation and migration, and are designed for peptide materials modifying [22]. We specifically aimed to investigate the bioactivity of nHAPA66/D-RADA16-RGD composite. In order to do this, composite biomaterials were fabricated by incorporating D-RADA16-RGD nano-fibers into nHA/PA66. Next, we investigated the biomechanics and surface of nHAPA66/D-RADA16-RGD composite. Finally, MC-3T3 cells and animal experiment were used to test the biocompatibility and bioactivity of nHAPA66/D-RADA16-RGD composite.
Materials and methods
Peptide purification
Solid-phase peptides of D-RADA16-RGD were synthesized by Shanghai Biotech Bioscience and Technology Co., Ltd. (Shanghai, People’s Republic of China) with a purity of 98.58%.
Circular dichroism spectroscopy and Rheology test
A circular dichroism (CD) spectrometer (J-810, JASCO Corporation, Tokyo, Japan) was employed to measure the peptide samples, which consisting of 1.0 mg/ml peptide aqueous stock were dissolved and adjusted to 100 M using 20 mM CaCl2, at 25 °C, 37 °C, and 60 °C respectively. The analysis was done using a CD cuvette with a 5 mm path length and measured in the 190–290 nm range. Rheology test on 1° stainless steel cone-controlled rheometer with a diameter of 20 mm (Thermo Fisher Science, Waltham, MA, USA) Samples were dissolved in PBS (pH 7.4) at a concentration of 20.0 mg/ml. The peptide solution was diluted with PBS (pH 7.4) to 10 mg/ml, 5 mg/ml and 2.5 mg/ml respectively and stored at 4 °C in a refrigerator over night, and 150–200 μl of samples were used for analysis at 25 °C.
Preparation of the nHA/PA66 composite
nHA [14], the main inorganic component of human and animal bones, and PA66 materials were purchased from Nanotechnology Co., Ltd (Chengdu, People’s Republic of China). The synthesis of materials was completed at the Nanotechnology company.
nHA/PA66 composite slurry was then prepared in accordance with methodology described previously [23]. Foam agent, which would turn to a gaseous phase at high temperature (1:50) was added to the composite PA66 slurry at 70 °C. Then, the PA66/foam was added to the nHA slurry and stirred constantly for 2 h. Throughout this paper, the nHA/PA66 composites used contained 40% (by weight) of nHA. The composite slurry was then allowed to set for 4 h at room temperature, and the bubbles were completely removed. The slurry was then cast onto a plate and the composites heated to 300 °C for 1 h. The resultant materials were then washed repeatedly with deionized water.
The preparation of several specifications of porous nHA/PA66 is completed.(Φ 2.5 mm × 3 mm for animal experiment),(Φ 32 mm × 4 mm and ect for vitro experiment).
Preparation of the nHA/PA66/D-RADA16-RGD composite
The prepared porous nHA/PA66 composites (including Φ 2.5 mm × 3 mm, Φ 32 mm × 4 mm and ect) were completely immersed in D-RADA16-RGD solution (5 mg/ml) (5 mg/ml peptides is the optimum coating concentration, which can form a stable three-dimensional structure without agglomeration as supplement showed) at room temperature by dissolving lyophilized peptide powder in PBS (pH 7.4) for 48 h to make the nHA/PA66/D-RADA16-RGD composite for further studies.
Characterization of D-RADA16-RGD, nHA/PA66 and nHA/PA66/D-RADA16-RGD composite
Scanning electron microscopy (SEM) (Nova NanoSEM 400, FEI, Hillsboro, OR, USA) was used to image the surface morphology of nHA/PA66(with or without D-RADA16-RGD) composites. The nHA/PA66 composite was created as described in the article; the D-RADA16-RGD composite used in our study was created as follows. D-RADA16-RGD peptide powder was dissolved in PBS for 48 h at room temperature at a concentration of 5 mg/ml. Next, 10 ml of peptide solution was loaded onto a copper grid. Redundant solution was removed, and 10 ml of uranyl acetate was used to dye the peptide solution for 30 s; the solution was then dried for 12 h in a desiccator. Finally, transmission electron microscopy (TEM) (Philips Tecnai G2 F20, FEI Company, Hillsboro, OR, USA) was used to take images. Mechanical tests were also carried out on the two composites using 15 × 10 × 10 mm samples. These tests showed that pressing speed was 5 mm/min and compression height ratio was 50%.
Peptide degradation by proteinase K
0.25 mg proteinase K was added to the solution (1 mg of peptide was dissolved in 5 ml 0.001 M PBS with or not with nHA/PA66 scaffolds), resulting in a 0.2 and 0.05 mg/ml concentration of peptide and proteinase K, respectively. After incubating at 37 °C on shaking tables for 1–24 h, 400 μl aliquots of samples, consisting of hydrogels and proteinase K, were taken after 7 time points (1, 2, 4, 6, 8, 12 and 24 h). phenylmethanesulfonylfluoride (PMSF) was employed to terminated the enzymatic reaction and the concentration of the intact peptide was analyzed by HPLC (LCMS-2020; Shimadzu, Kyoto, Japan) using following conditions: column: Venusil XBP C18, 5 μm, 4.6 × 50 mm (Agela, Tianjin, People’s Republic of China); sample injection volume: 100 μl; gradient elution: A, 0.035% tri uoroacetic acid (TFA) in water; and B, 0.035% TFA in acetonitrile/water (80:20, vol/vol). A gradient of 10–100% B in 10 min was conducted. The experiment was conducted in 3 replicates.
Cell culture
MC-3T3 cells (Cell Bank of Chinese Academy of Sciences, Shanghai, People’s Republic of China,) were used for cell culture and were maintained in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12; Hyclone, Logan, UT, USA) with fetal bovine serum at 10%. MC-3T3 cells were purchased from Procell Life Science and Technology Co., Ltd. from the Cell Bank of Chinese Academy of Sciences, Shanghai, People’s Republic of China. (MC-3T3 Subclone 14, Cat.No: CL-0378, organize: mus musculus, mouse, age: newborn, tissue: bone/calcaria, morphology: fibroblast).
Cytotoxicity assays
Cells were cultured according to the manufacturer’s instructions, and a cell counting kit-8 (CCK-8 kit; Dojindo, Kumamoto, Japan) was used to evaluate cell proliferation in each sample at 1, 3 and 7 days. CCK-8 was added to the medium at a concentration of 10 μl/well, and then samples were incubated at 37 °C for 2 h. The absorbance at 450 nm was then read with a microplate reader (Thermo Fisher Scientific Co Ltd, Waltham, MA, USA). Each assay was repeated three times.
Determination of Osteogenic capacity
Blank control group (no materials), nHA/PA66 group and nHA/PA66/D-RADA16-RGD group were synthesized for Western blotting in 6-well plates (for samples that were 32 mm in diameter) and alizarin red staining in 24-well plates (for 1 × 1 × 0.1 cm samples). After co-culturing for 24 h, culture medium (DMEM/F12) was replaced by osteogenic induction medium (Cyagen Biosciences Co, Ltd Guangzhou, People’s Republic of China). The density of cells in each well was 3 × 104 viable cells per cm2. Every 3 days, the osteogenic induction medium was replaced.
Cell attachment and spreading
The nHA/PA66/D-RADA16-RGD and nHA/PA66 materials were been prepared as 1 × 1 × 0.1 cm samples for this experiment. MC-3T3 were co-cultured with different samples in 24-well plates and cell counting plate was employed to calculate the cell density of each well at about 2 × 104 cells per cm2. After two time points (12 h and 24 h) of co-culture, cells were washed with PBS three times and then fixed in 4% paraformaldehyde for 15 min. Then, Triton X-100, a detergent, was added at a concentration of 0.1% for 5 min. Cells were then re-washed with PBS three times. Rhodamine-phalloidin (50 μg/ml; Cyagen, Guangzhou, People’s Republic of China) was then added into each sample for 30 min for microfilament cytoskeleton dyeing and then removed. Next, cells were stained with diamidino-phenylindole (DAPI; 0.1 mg/ml; Cyagen, Guangzhou, People’s Republic of China) for 20 min for nuclears staining. Finally, confocal laser microscopy (Nikon, Tokyo, Japan) was used to analyze the microfilament cytoskeleton and nucleus to observe cell adhesion, proliferation, differentiation, etc. in each sample.
Alizarin red S staining
Osteogenic differentiation in MC-3T3 cells over a period of 21 days was determined by Alizarin red S staining, which was used to identify calcium deposits in cell samples (nHA/PA66 discs: 10 × 10 × 1 mm and nHA/PA66/D-RADA16-RGD discs: 4 × 105 cells/cm2). In brief, cells were first fixed with 4% paraformaldehyde and then stained with 1% Alizarin red S (1%; Sigma-Aldrich, St. Louis, MO, USA) for 1 h at room temperature. Samples were then analyzed using a microscope (Carl Zeiss, Jena, Germany). In order to quantitatively analyze calcium nodules in each sample, we used cetylpyridinium chloride (10%; Cyagen, Guangzhou, People’s Republic of China) solution to desorb the stain on the samples. The absorbance of the dissolving solution collected from each sample was then tested at a wavelength of 620 nm using a Thermo Scientific microplate reader (Multiskan, MA, USA).
Western blotting
Two types of composite disc (nHA/PA66 and nHA/PA66/D-RADA16-RGD; 32 mm in diameter) were co-cultured with MC-3T3 cells at a density of 3 × 104 per cm2. At three time-points of incubation (14, 21 and 28 days), cells were sampled and mixed with RIPA lysis buffer (Cyagen, Guangzhou, People’s Republic of China) to create cell lysates. We then used the bicinchoninic acid method (Cyagen, Guangzhou, People’s Republic of China) to determine the total protein concentration of whole cell lysates. For each sample, 40 µg of protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with blocking buffer (Abcam, Massachusetts, UK) for 1 h at 37 °C, membranes were incubated for 12 h with primary antibodies against three osteogenic related proteins: collagen-1 (COL-1), osteopontin (OP), osteocalcin (OC) (1:1000; Sigma, USA) and β-actin (1:5000; Beyotime, Jiangsu, People’s Republic of China) as internal control. Finally, a secondary anti-rabbit IgG antibody (Beyotime, Jiangsu, People’s Republic of China) was added to the membranes and incubated for 4 h. Bound antibodies were visualized using a chemiluminescence system.
Animal experiment
All animal research was conducted in accordance with the Declaration of Helsinki and in accordance with the Guide for Care and Use of Laboratory Animals. All experimental protocols were approved by the Ethics Committee of Chongqing Medical University (Reference Number: IACUC.NO: 2016-059). The Laboratory Animal Center provided 24 female Sprague–Dawley rats weighing 200–240 g.
An intravenous administration of chloral hydrate (10%, 5.0–7.5 ml/kg) was used to anesthetize animals. First, we exposed the distal femoral condyle of each rat. Next we created a bone defect that was 2.5 mm in diameter and 3 mm in depth in two hind legs of each animal. Rats were randomly divided into 2 groups (each group at each time points has 6 rats), and the defects in each leg from each group were filled with respective materials (nHA/PA66 and of nHA/PA66/D-RADA16-RGD). After defects in the distal femoral condyle were filled, the soft tissues were closed. Penicillin was administered by intramuscular injection in order to prevent potential infection. After surgery at two time-points (8 and 12 weeks), rats were culled by an overdose of anesthesia. Three groups were culled at the 8-week time-point and six groups were culled at the 12-week time-point. Micro-computed tomography (CT) was then used to analyze harvested femoral condyles.
Micro-CT analysis
Following surgery, two groups of rats were culled at 8 weeks and 12 weeks. The femoral condyle was harvested and imaged by micro-CT (Viva CT40, Scanco Medical AG, Bassersdorf, Switzerland). Computer software was used to remove materials by different sitethreos (the sitethreos of materials are less than 220). We then determined the trabecular structure index on bone samples using a three-dimensional technique. We also determined relative bone volume of the trabecular bone using a three-dimensional mesh structure diagram of bone volume density (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular spacing (Tb.Sp). The micro-CT settings were 70 kVp and 114 uA, with an image voxel size of 7.0 um and a 0.01 mm slice thickness. The integration time was 350 ms. The average scanning time was approximately 45 min per rat per time point, which produced approximately 600 images. Three-dimensional images of the new bone structure in the defective areas were then rebuilt by computer. Tb.N and Tb.Th are two key indicators that describe the trabecular bone in terms of number and thickness; Tb.Sp can also be used to measure separation between trabecular bones.
Statistical analysis
Data were expressed as means ± standard deviation (SD), and differences between groups were analyzed by analysis of variance (ANOVA) or the independent t test using SPSS software (version 20.0) (SPSS Inc, Chicago, IL, USA). All experiments were carried out in triplicate, and values p < 0.05 were considered to be statistically significant.
Results
Microstructural analysis of different composite materials
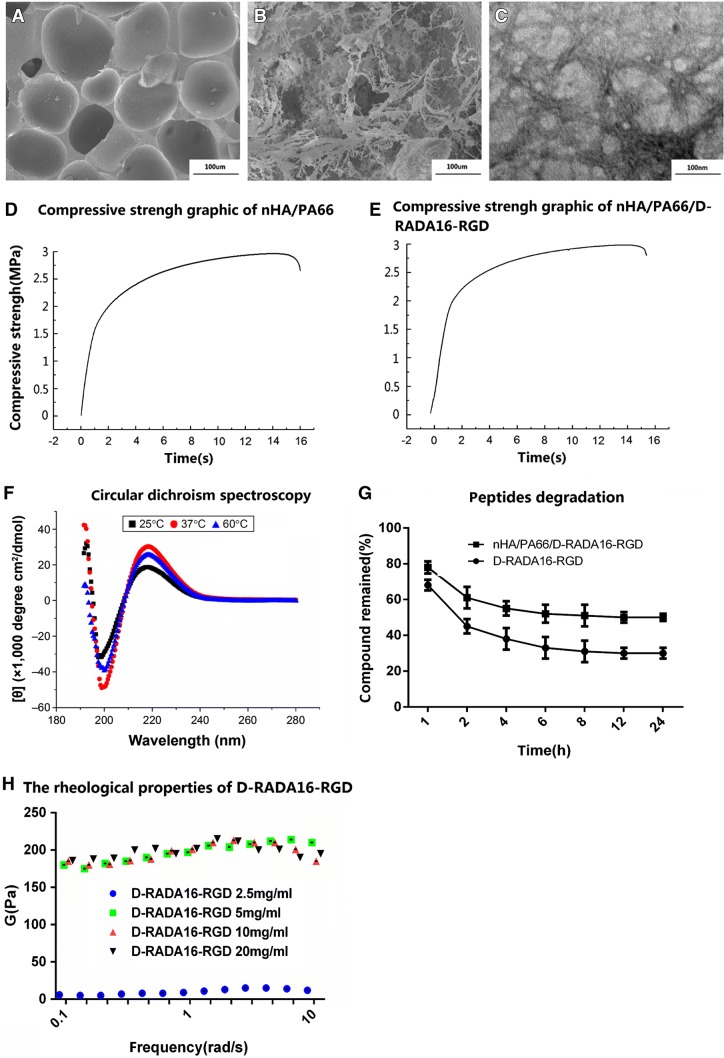
The D-RADA16-RGD material was 7–33 nm in width and 130–600 nm in length, and the interwoven pore size ranged from 40 to 200 nm (Figure 1A, B). demonstrate the surface morphology of the two materials, respectively. The pore size of nHA/PA66 ranged from 150 to 250 µm. D-RADA16-RGD was homogeneously distributed on the surface and in the porous of the materials. TEM measurements of the D-RADA16-RGD material demonstrated that nano-fibers were self-assembled at room temperature, and a network structure was created by interwoven nano-fibers (Fig. 1C). The mean macro-pore size of the nHAPA66 composite was 628.91 ± 121.94 μm, the porosity was 79.88 ± 6.33% and the compressive strength was 3.01 ± 1.07 MPa (Table 1, Fig. 1D, E). None of the physical parameters of nHA/PA66/D-RADA16-RGD were significantly different to those of the nHA/PA66 scaffolds (p > 0.05).
Fig. 1.
Characterization of materials: A SEM: nHA/PA66 B SEM: nHA/PA66/D-RADA16-RGD, and TEM images of microstructure of C L-RADA16-RGD (100 nm). D Compressive strength graphic of nHA/PA66. E Compressive strength graphic of nHA/PA66/D-RADA6-RGD. F Result of circular dichroism spectroscopy. G D-RADA16-RGD peptide degradation by proteinase K. H The rheological properties of D-RADA16-RGD. TEM transmission electron microscopy, SEM scanning electron microscope
Table 1.
Mechanical results of nHA/PA66/D-RADA16 and nHA/PA66
| Material | Average macropore size (um) | Porosity (%) | Compressive strength (Mpa) |
|---|---|---|---|
| nHA/PA66 | 628.91 ± 121.94 | 79.88 ± 6.33 | 3.01 ± 1.07 |
| nHA/PA66/D-RADA16-RGD | 607.66 ± 101.37 | 76.88 ± 7.21 | 3.11 ± 0.09 |
No Significantly difference (p > 0.05)
Secondary structural properties of peptide D-RADA16-RGD
Compared to ellipticity from 198.5 to 218 nm at 25 °C, the CD spectrum of peptide D-RADA16-RGD has a maximum and increased ellipticity at 218.5 nm and a minimum ellipticity at 199 nm at 37 °C. However, at 60 °C, its CD spectrum showed a maximum but relatively reduced ellipticity at 218.5 nm and a minimum ellipticity at 200 nm (Fig. 1F). As a result, the peptide D-RADA16-RGD was able to form stable-sheet secondary structure when the temperature ranged from 37 to 60 °C.
Cell attachment and spreading
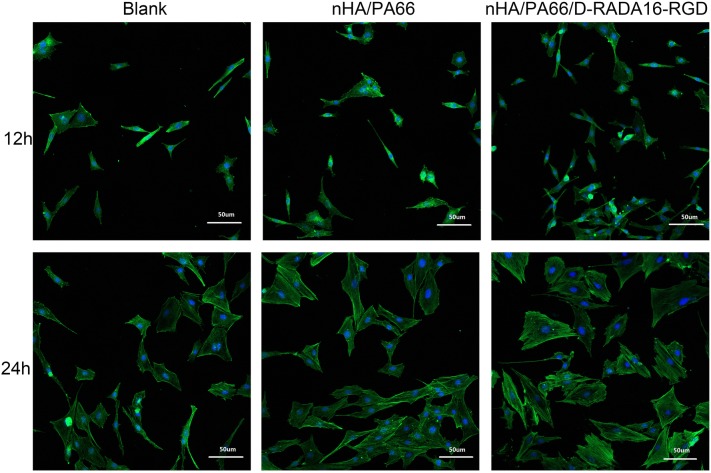
Confocal laser scanning (CLS) microscopy was used to image cell immunofluorescence and identify the cell microfilaments of MC-3T3 cells, as shown in Fig. 2. We can find that, at time point 12 h, the number of MC-3T3 cells in the D-RADA16-RGD group was significantly higher than in the other groups (p < 0.05), which indicated that the nHA/PA66/D-RADA16-RGD material may have better cell adhesion induction properties or facilitate early cells proliferation, which consistent with the result of CCK-8. At the time point 24 h, cells exhibited a long and spindle-shaped morphology in both the nHA/PA66/D-RADA16-RGD group and nHA/PA66 group compared to a polygonal shape in the blank control group (6-well plate without biomaterials). Cells, in D-RADA16-RGD group, were linked to adjacent cells with more actin filaments compared with other groups. In the blank control group, there were fewer actin filaments in one monolayer and cells are dispersive morphology. This result may be influence by the bioactivity of RGD and the 3D structure of D-RADA16-RGD structure may play an important role.
Fig. 2.
The spreading of MC-3T3 cells (× 400) on the surface of a 24-well cell plate (blank control), nHA/PA66 and nHA/PA66/D-RADA16-RGD after 12 and 24 h
Stability of peptides
To avoid the effects of resistance due to hydrogelation, the concentration of D-RADA16-RGD was set at 0.2 mg/ml, which is markedly lower than the critical gelation concentration of the hydrogel. After 7 time points of incubation with proteinase K respectively, the compound remained was employed to test the stability of peptides of different materials (D-RADA16, D-RADA16-RGD and nHA/PA66/D-RADA16-RGD) and the results demonstrated that compared to D-RADA16-RGD > 35% remained after 24 h, the nHA/PA66/D-RADA16-RGD remained more than 50% after 24 h which may because of the peptides protected by porous structure of the nHA/PA66 (Fig. 1G).
We chose typical ions (Na/K) in the cell media to investigate the gelation nanomechanical properties. Rheological experiments measure a storage modulus by evaluating elastic response (rigidity) and viscous response of material, respectively, by varying frequencies of applied oscillatory stress. Upon comparing the rheological property of D-RADA16-RGD self-assembly in water or salt solution (0.01 M PBS, Na/K) to compare the effect of varying concentration of peptide.
Obviously, the mechanical properties of 2.5 mg/ml concentration of peptides was lower than others (5 mg/ml, 10 mg/ml and 20 mg/ml). However, as shown in Fig. 1H, there was no significant difference in the mechanical properties of the other three materials in the experiment. High concentration (5 mg/ml, 10 mg/ml and 20 mg/ml) of D-RADA16-RGD materials demonstrated similar mechanical properties which may means that all of them could form a stable nanofibers and three-dimensional scaffolds. As a result, this experiment is useful in a process of selection of peptide concentration and we believe that 2.5 mg/ml D-RADA16-RGD peptides may not form an effective three-dimensional scaffolds compared to higher concentration peptides (5 mg/ml, 10 mg/ml and 20 mg/ml).
Cytotoxicity assays
As shown in Fig. 3, the number of MC-3T3 cells increased as incubation time increased. There was no significant difference between each group at most time points of the time-points (except 6 h), thus indicating similar proliferation capacity in each group (p > 0.05) As for 6 h, we believe that 3D structure will facilitate early cell proliferation, which means it may be affected by 3D structure of D-RADA16-RGD structure as well as the bioactivity of RGD to accelerate cell adhesion (p < 0.05).CCK-8 analysis showed that nHA/PA66/D-RADA16-RGD revealed good biocompatibility.
Fig. 3.
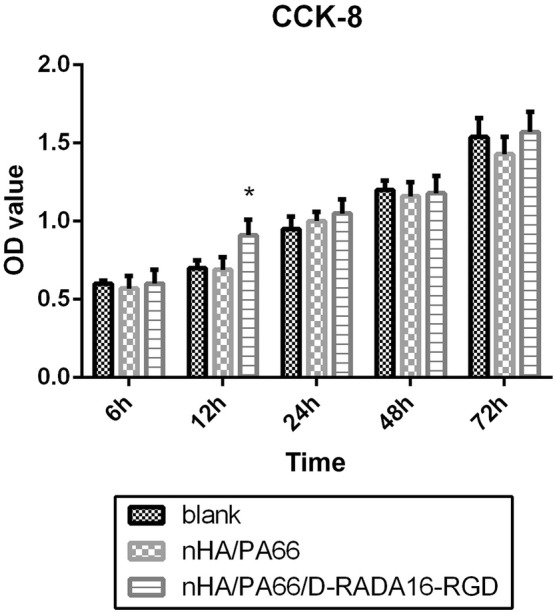
CCK-8 assays for proliferation of MC-3T3 cells co-cultured with DMEM/F12 (blank control), extracts of nHA/PA66 composite and extracts of nHA/PA66/D-RADA16-RGD composite for 6, 12, 24, 48, 72 h
Alizarin-red S staining
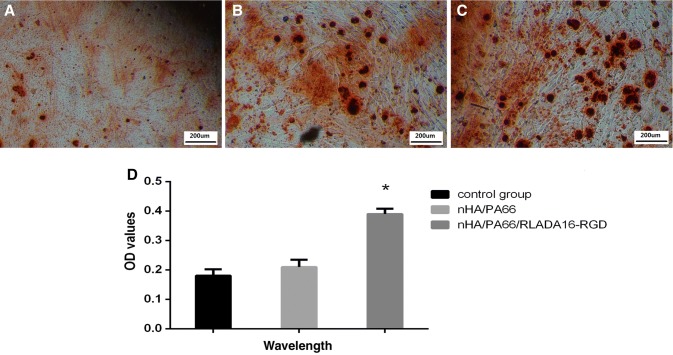
After 21 days of co-culture with osteogenic induction, MC-3T3 cells were stained by alizarin-red S (Fig. 4). Alizarin red staining allowed us to visualize calcium nodule formation; analysis showed that there were more calcium nodules on the nHA/PA66/D-RADA16-RGD composite group than the other groups (p < 0.01).
Fig. 4.
Optical microscopy images of alizarin red S staining × 100 (A–C) and quantitative analysis of calcium nodules on the composite at wavelength: 540 nm (D). *Significant difference compared with blank control or nHA/PA66 groups (p < 0.01). Notes: Control group(A), nHA/PA66(B), nHA/PA66/D-RADA16-RGD(C). OD optical density
Osteogenic differentiation-related protein
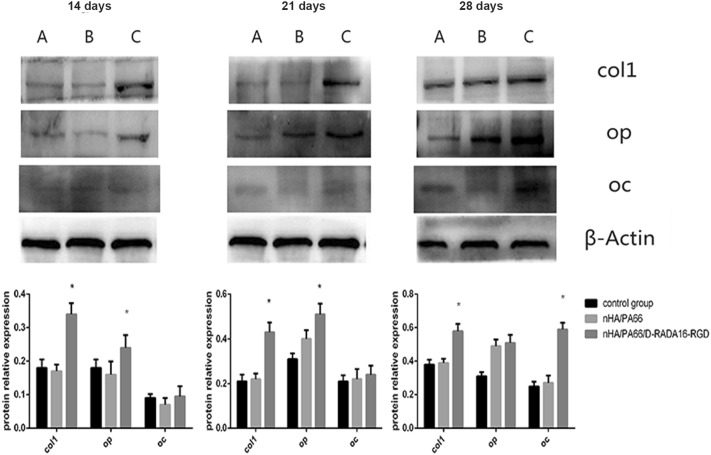
To evaluate the influence of the combination of D-RADA16-RGD and nHA/PA66 material on osteoblast differentiation in MC-3T3 cells, we used Western blotting to investigate COL-1, OP and OC; these are all important proteins in osteogenic differentiation. Figure 5 shows the expression of these three proteins in MC-3T3 cells. nHA/PA66/D-RADA16-RGD do not reduce the expression of these proteins. The level of COL1 was significantly higher in the nHA/PA66/D-RADA16-RGD group compared with the other two groups (p < 0.01). However, the expression of OP was significantly higher in the nHA/PA66/D-RADA16-RGD group at both the 14- and 21-day time-points (p < 0.01) but showed no significant difference on day 21. Only after 28 days of culture did the expression of OC show a significantly higher level of expression compared to the control group and the nHA/PA66 group (p < 0.01); these results suggest that nHA/PA66/D-RADA16-RGD exhibits better bioactivity.
Fig. 5.
The expression of osteogenic differentiation-related proteins (collagen 1, osteopontin, osteocalcin) of MC-3T3 cells co-cultured with different composites in osteogenic inductive medium after different days. * Significant difference compared with blank control or nHA/PA66 groups (p < 0.01). Notes: Control group(A), nHA/PA66(B), nHA/PA66/D-RADA16-RGD(C). COL1 collagen 1, OP osteopontin, OC osteocalcin
In vivo osteogenesis
The 3D reconstructions of the femoral condyles at 8 and 12 weeks and imaged by micro-CT analysis of the explants are shown in Fig. 6. Compared to nHA/PA66 treatment, the nHA/PA66/D-RADA16-RGD composites group showed better bone repair in the defective bones. Micro-CT was used to acquire the following quantitative parameters in each group: BV/TV; Tb.N; Tb, Th and Tb.Sp. Compared to the nHA/PA66 group, the nHA/PA66/D-RADA16-RGD composite group demonstrated a significantly greater volume of regenerating bone in the femoral condyles (p < 0.05). Furthermore, there was a significantly higher ratio of BV/TV in the new bone in the nHA/PA66/D-RADA16-RGD group (p < 0.05). The nHA/PA66/D-RADA16-RGD treatment also showed a significantly higher Tb.N in new bone at the defect sites (Table 2; p < 0.05). The Tb.Sp of new bone also significantly increased in the defective bones of the nHA/PA66 treatment group (Table 2), when compared with the nHA/PA66/D-RADA16-RGD group (p < 0.05). However, there were no significant differences in Tb.N at the 12-week time-point (p = 0.114, > 0.05), and one reason may be the value of them reach to normal. It can be seen that the progress of bone repair in the nHA/PA66/D-RADA16-RGD group is better than that in the nHA/PA66 group.
Fig. 6.

Osteogenic observation of various materials 8 and 12 weeks after implantation. Notes: The morphology of the explanted femoral condyles undergoing 3D reconstructed micro-CT images, A nHA/PA66 8 weeks, B nHA/PA66/D-RADA16-RGD 8 weeks C nHA/PA66 12 weeks D nHA/PA66/D-RADA16-RGD 12 weeks. PBS phosphate-buffered saline, 3D three-dimensional, micro-CT micro computed tomography
Table 2.
Osteogenic observation of various materials 8 weeks and 12 weeks after implantation
| Trabecular bone parameters | 8 Weeks | 12 Weeks | Normal bone | ||||
|---|---|---|---|---|---|---|---|
| nHA/PA66 | nHA/PA66/D-RADA16-RGD | p | nHA/PA66 | nHA/PA66/D-RADA16-RGD | p | ||
| TV(mm3) | 27.27 | 27.27 | 27.27 | 27.27 | |||
| BV(mm3) | 5.27 ± 0.4 | 9.17 ± 0.6 | 0.001 | 9.83 ± 0.82 | 12.39 ± 0.71 | 0.015 | 13.31 ± 0.61 |
| BV/TV(%) | 0.19 ± 0.01 | 0.33 ± 0.02 | 0.002 | 3.39 ± 0.27 | 0.45 ± 0.03 | 0.021 | 0.49 ± 0.03 |
| Tb.N(1/mm) | 2.42 ± 0.01 | 4.17 ± 0.21 | 0.001 | 3.39 ± 0.27 | 6.01 ± 0.51 | 0.001 | 5.97 ± 0.37 |
| TB.TH(mm) | 0.13 ± 0.02 | 0.27 ± 0.03 | 0.003 | 0.27 ± 0.05 | 0.39 ± 0.09 | 0.114 | 0.41 ± 0.08 |
| TB.sp(mm) | 0.49 ± 0.03 | 0.29 ± 0.04 | 0.004 | 0.39 ± 0.01 | 0.30 ± 0.03 | 0.008 | 0.31 ± 0.03 |
Discussion
Bone consists of both organic (e.g. collagen I) and inorganic components (e.g. bony salts) [24]. Porous nHA/PA66 composites mimic a bony structure in the sense that nHA acts as a bony salt and PA66 acts as the porous structure to cancellous bone; it is these features that have made this material become more and more popular over recent years [8]. In China, this kind of porous nHA/PA66 materials are used as an artificial bone for the filling of large bone defects in the clinic. However, The material needs to be improved in osteogenesis. On the other hand, compared to L-forms amino acids, peptide bonds formed by d-amino acids have higher stability than that of l-amino acids, as evidenced by the proteases being able to degrade L-form peptide bonds but unable to degrade D-form peptide bonds [25, 26]. Indeed, many D-form self-assembling peptide hydrogel scaffolds have been found to hold significant promise in cell culture and tissue regeneration. RADA16 peptide material has been used to improve the outgrowth of nerites, cardiac repair and wound healing [6, 27–29]. D-RADA16-RGD peptides would have demonstrated extraordinary propensity to assemble into b-sheet structures and 3D nanostructures by means of intermolecular hydrogen bonding and others [20]. The two most common peptide secondary structures are b-sheet and a-helix as well as in the b-sheet and a-helix system, many ionic self-complementary peptides are designed and fabricated, including L-RAD16, and D-RAD16. They readily undergo self-assembly into nano-fiber and 3D biomaterial scaffolds by means of intermolecular hydrogen bonding and others [1]. Short peptide epitope RGD is found in bronectin and other ECM proteins, and can assist in inducing cell differentiation and migration through binding to integrin receptor [30]. Furthermore, peptide RGD can be combined with PHSRN to fabricate peptide PHSRNG6RGD which results in the improvement of cell binding, possibly due to its better similarity to functional structures of bronectin [31]. The D-RADA16-RGD peptide has also been shown to be beneficial for both chondrocyte culture and cartilage regeneration [32, 33]. Other studies have demonstrated that D-RADA16-RGD has been of great benefit to hemostasis and cell culture [1, 34]. In the present study, we designed a new material to enhance the bioactivity of porous HA/PA66 in bone repair, notably in osteogenesis, by combination with by D-RADA16-RGD.
In the present study, we wished to determine whether D-RADA16-RGD would trigger self-assembly process and form well-ordered nano-fiber. TEM results showed that D-RADA16-RGD material was the same width as D-RADA16-RGD nano-fibers, and ranged from 40 to 200 nm; these dimensions had positive effects on cell adhesion and infiltration. In our cytology experiments, the nHA/PA66/D-RADA16-RGD composite substantially improved the bioactivity of MC-3T3 cells, suggesting that our new composite has favorable cell spreading ability and biocompatibility. Adhesion and spreading of cells plays an important role in influencing bone repair and improving cell–material interaction [34, 35]. Confocal laser microscopy was used to observe initial cell attachment and subsequent proliferation on the surface of the two materials; this allowed us to compare cell adhesion and biocompatibility.
A previous study demonstrated that cell microfilaments had a significant influence on several cell bioactivities [36]. For example, stretching pseudopodia are vital in cell connection, and would therefore be important in cell attachment. A greater extent of microfilaments and pseudopodia were found in the nHA/PA66/D-RADA16-RGD group compared with other groups, implying that cells spread faster on the surface of our nHA/PA66/D-RADA16-RGD composite. Spindle-shaped cells were more commonly found on the surface of the nHA/PA66/D-RADA16-RGD composite, indicating that cells were more prone to adhere to the surface of the nHA/PA66/D-RADA16-RGD composite. The increased number of adhesion cells and spindle-shaped cells on the nHA/PA66/D-RADA16-RGD composite indicated a more favorable capacity for osteogenic differentiation [37, 38]. A greater number of adhesion and spindle-shaped cells may be beneficial in the repair of defective bones and increasing the success rate of implantation.
We believe that the effects of these peptides are mainly due to their self-assembling ability, which can form scaffolds to mimic the extracellular environment for cell growth, and the transport of oxygen, nutrients and waste products to take place in a 3D environment, while the amino acids of these peptides themselves do not interact with the cell [39, 40]. The result of Peptide degradation by proteinase K showed that the porous nHA/PA66 would reinforce the stability of D-RADA16-RGD peptides significantly (p < 0.01) which means that these results indicate that the chiral hydrogel scaffolds promote cell–cell interactions and provide a long-term effective 3D microstructure, basing on the stability of peptides, for cell migration, which is vital to facilitate tissue regeneration in the field of angiogenesis, wound closure and repairing of bone [32].
Pervious study showed that some peptide nano-fiber scaffolds demonstrate potential abilities for controlled release of some therapeutic agents, such as some drugs or growth factors. The integrity of peptide nano-fiber scaffolds is critical for sustained release, which means that nHA/PA66/D-RADA16-RGD is much more stable than D-RADA16-RGD demonstrate the nHA/PA66/D-RADA16-RGD would revise more potential in sustained release. Previous study showed that the sustained release is beneficial to constrain side effects of nerve injury and stimulate nerve regeneration. Controlled release of the drug dexamethasone has been feasible within nano-fiber hydrogels, and it can effectively reduce the occurrence of inflammation and prevent the secondary injury to nerve tissue [41].
The osteogenic abilities of MC-3T3 cells were determined by alizarin red staining and Western blotting of COL-1, OP, and OC; the expression of these proteins would reflect osteogenic differentiation of MC-3T3 cells during bone repair. Compared to other groups, Western blotting showed that the expression of these proteins significantly increased in the nHA/PA66/D-RADA16-RGD group at most time-points. Furthermore, calcium nodules, the final differentiation phase secreted by MC-3T3 cells, were quantified by Alizarin red staining and showed the same experimental outcome. Meanwhile, positive effects on both the expression of these three osteogenesis-related proteins, and the secretion of calcium nodules, indicated that the nHA/PA66/D-RADA16-RGD composite showed significantly better biocompatibility and bioactivity. In comparison with other composites already used in clinical treatments, such as polylactic acids, the advantages of the nHA/PA66/D-RADA16-RGD composite would not have opposing influences on the differentiation of cells by not releasing lactic acid and therefore preventing a drop in pH [38]. This indicates that the nHA/PA66/D-RADA16-RGD composite could be used in clinical surgery as a safe implant. Owing to the fact that nHA/PA66/D-RADA16-RGD is similar to human bone in terms of its biomechanical behavior because of the nHA/PA66 materials and better bioactivity by D-RADA16-RGD self-assembling peptide.
In order to investigate the osteogenic capacity of different composite materials, nHA/PA66/D-RADA16-RGD and nHA/PA66 were used to repair defective femoral condyles in rats. Bone morphology change, and the analysis of BV/TV, Tb.Th, Tb. NTb.Sp and BMD, in new bones from harvested femoral condyles by micro-CT showed that nHA/PA66/D-RADA16-RGD composites exhibit a significant influence on bone defect repair (Fig. 6 and Table 2). Compared with the nHA/PA66 material group, the bone defect of the nHA/PA66/D-RADA16-RGD material group recovered better, and we believe that D-RADA16-RGD plays an important role in it.
We believe that compared the nHA/PA66 scaffolds, which were macroscopically filled with the defects of bone, the D-RADA16-RGD peptide can form a three-dimensional mesh scaffold structure on the surface of the material and the pores, which can effectively facilitate cells proliferation and adhesion on the material to promote the progress of bone repairing. What is more, RGD, a type of heparin-binding protein, as a functional group on the D-RADA16 scarf folds is highly beneficial for proliferation and differentiation too. nHA/PA66/D-RADA16-RGD material as a ternary composite exhibits better bone repair performance than nHA/PA66.
Modifying our peptide scaffold composite with RGD significantly enhanced its effects on bone repair; this probably occurred because of the increasing concentration of RGD or electrostatic interactions between the peptide and the RGD [42]. The nHA/PA66/D-RADA16-RGD group described herein showed significant potential for osteogenesis. Furthermore, the D-ADA16-RGD hydrogel used in a previous study presented with significant potential ability for the controlled release of TGF-beta in order to promote cell proliferation [43].
We believe that the biological activity of D-RADA16-RGD peptide on the surface of new materials and in porous plays an important role in bone healing. The reason may because that the D-RADA16-RGD peptide scaffolds might act as a support for the RGD domain could be the explanation for the effects on MSCs observed after incubation with the D-RADA16-RGD peptide in soluble form, which induced a significant enhancement of matrix mineralization by these cells [42]. During the first days of incubation, when the cells have still not synthesized an ECM, the soluble D-RADA16-RGD peptide might be able to trigger integrin-dependent signaling pathways to direct cell differentiation, as it has been shown that soluble RGD peptides can bind to integrins.
Another reason may be related to the activation of RGD-cell receptor-associated signaling pathways and the biological activity of peptide-forming bioscaffolds [44]. Ho-Wook Jun et al. found that the three-dimensional scaffold formed by the RGD-modified amphiphilic peptide (PA) has a certain effect on the differentiation of mesenchymal stem cells into osteoblasts, especially when the RGD-modified amphiphilic peptide is mixed with hydroxyapatite. Significantly increase the differentiation potential of mesenchymal stem cells, thereby enhancing bone formation and accelerating bone healing [45]. However, its specific osteoinduction mechanism is unclear, which may be due to the RGD functional motif-mediated osteoinduction [46], which may be related to the signaling pathway involved in integrin-activated ERK gene [47].
There are still some shortcomings in our experiments. Fist of all, to evaluate the osteogenic suitability of new materials, more kinds of cells (such as BMSC’s) should be involved to increase credibility. The resorption activity, which would form an integral component of bone turnover, on the new composite materials should been studied in further studies and the reaction of osteoclasts on such a material also needs to be involved as well.
In conclusion, the addition of D-RADA16-RGD scaffold into nHA/PA66 materials significantly improved the bioactivity of nHA/PA66/D-RADA16-RGD material. Cytology experiments demonstrated that nHA/PA66/D-RADA16-RGD could facilitate cell proliferation, differentiation and adhesion, indicating that this nHA/PA66/D-RADA16-RGD composite exhibits good bioactive properties and biocompatibility. On the basis of bone morphology change, the quantification of micro-CT analyses showed that nHA/PA66/D-RADA16-RGD composites could significantly enhance the bioactivity of bone repair. In summary, we developed a novel composite of D-RADA16-RGD-combined nHA/PA66 that exhibited good biological properties, and appears to have good prospects for broad application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of Young Scientists of China (81501876) and the National Natural Science Foundation of China (81472057).
Compliance with ethical standards
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethical statement
All experimental protocols were approved by the Ethics Committee of Chongqing Medical University (Reference Number: IACUC.NO:2016-059). The experimental scheme is demonstrated by the ethics committee of Chongqing Medical University.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Qiao, Email: qiaobo1985@163.com.
Dianming Jiang, Email: jdm571026@vip.163.com.
References
- 1.He B, Ou Y, Zhou A, Chen S, Zhao W, Zhao J, et al. Functionalized d-form self-assembling peptide hydrogels for bone regeneration. Drug Des Devel Ther. 2016;10:1379–1388. doi: 10.2147/DDDT.S97530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long T, Zhu Z, Awad HA, Schwarz EM, Hilton MJ, Dong Y. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials. 2014;35:2752–2759. doi: 10.1016/j.biomaterials.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi M, Hu J, Li J, Li J, Dong W, Feng X, et al. Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone. 2012;50:119–127. doi: 10.1016/j.bone.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Russell TA, Insley G. Bone substitute materials and minimally invasive surgery: a convergence of fracture treatment for compromised bone. Orthop Clin North Am. 2017;48:289–300. doi: 10.1016/j.ocl.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abedi G, Jahanshahi A, Fathi MH, Haghdost IS, Veshkini A. Study of nano-hydroxyapatite/zirconia stabilized with yttria in bone healing: histopathological study in rabbit model. Pol J Pathol. 2014;65:40–47. doi: 10.5114/pjp.2014.42668. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–3348. doi: 10.1016/j.biomaterials.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Zhang Y, Zhang X, Wang Y, Wang J, Lu M, et al. Mechanical properties and cytocompatibility of carbon fibre reinforced nano-hydroxyapatite/polyamide66 ternary biocomposite. J Mech Behav Biomed Mater. 2015;42:267–273. doi: 10.1016/j.jmbbm.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Qiao B, Li J, Zhu Q, Guo S, Qi X, Li W, et al. Bone plate composed of a ternary nano-hydroxyapatite/polyamide 66/glass fiber composite: biomechanical properties and biocompatibility. Int J Nanomedicine. 2014;9:1423–1432. doi: 10.2147/IJN.S57353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su B, Peng X, Jiang D, Wu J, Qiao B, Li W, et al. In vitro and in vivo evaluations of nano-hydroxyapatite/polyamide 66/glass fibre (n-HA/PA66/GF) as a novel bioactive bone screw. PLoS One. 2013;8:e68342. doi: 10.1371/journal.pone.0068342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang P, Bian C, Huang X, Shi A, Wang C, Wang K. Core decompression in combination with nano-hydroxyapatite/polyamide 66 rod for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2014;134:103–112. doi: 10.1007/s00402-013-1885-4. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Song Y, Liu L, Liu H, Zeng J, Pei F. Anterior reconstruction with nano-hydroxyapatite/polyamide-66 cage after thoracic and lumbar corpectomy. Orthopedics. 2012;35:e66–e73. doi: 10.3928/01477447-20111122-10. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Deng X, Jiang D, Luo X, Tang K, Zhao Z, et al. Long-term results of anterior cervical corpectomy and fusion with nano-hydroxyapatite/polyamide 66 strut for cervical spondylotic myelopathy. Sci Rep. 2016;6:26751. doi: 10.1038/srep26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Man Y, Zuo Y, Zhang L, Huang C, Liu M, et al. In vitro and in vivo evaluation of a nHA/PA66 composite membrane for guided bone regeneration. J Biomater Sci Polym Ed. 2011;22:263–275. doi: 10.1163/092050609X12602753096279. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, Ren C, Zhang B, Yang H, Lang Y, Min L, et al. Analyzing the behavior of a porous nano-hydroxyapatite/polyamide 66 (n-HA/PA66) composite for healing of bone defects. Int J Nanomedicine. 2014;9:485–494. doi: 10.2147/IJN.S52990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He B, Yuan X, Zhang H, Jiang D. Research progress of self-assembling peptide nanofiber scaffold for bone repair. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28:1303–1306. [PubMed] [Google Scholar]
- 17.Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ. Magnetic resonance imaging of self-assembled biomaterial scaffolds. Bioconjug Chem. 2005;16:1343–1348. doi: 10.1021/bc050153h. [DOI] [PubMed] [Google Scholar]
- 18.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 2005;5:1–4. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 19.He B, Yuan X, Zhou A, Zhang H, Jiang D. Designer functionalised self-assembling peptide nanofibre scaffolds for cartilage tissue engineering. Expert Rev Mol Med. 2014;16:e12. doi: 10.1017/erm.2014.13. [DOI] [PubMed] [Google Scholar]
- 20.Luo Z, Wang S, Zhang S. Fabrication of self-assembling D-form peptide nanofiber scaffold d-EAK16 for rapid hemostasis. Biomaterials. 2011;32:2013–2020. doi: 10.1016/j.biomaterials.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, et al. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- 22.Woerly S, Pinet E, de Robertis L, Van Diep D, Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel) Biomaterials. 2001;22:1095–1111. doi: 10.1016/S0142-9612(00)00354-9. [DOI] [PubMed] [Google Scholar]
- 23.Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, et al. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008;24:357–361. doi: 10.1016/j.dental.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Barber FA, Dockery WD, Hrnack SA. Long-term degradation of a poly-lactide co-glycolide/β-tricalcium phosphate biocomposite interference screw. Arthroscopy. 2011;27:637–643. doi: 10.1016/j.arthro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci U S A. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Z, Zhao X, Zhang S. Structural dynamic of a self-assembling peptide d-EAK16 made of only d-amino acids. PLoS One. 2008;3:e2364. doi: 10.1371/journal.pone.0002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Aza AH, Chevalier J, Fantozzi G, Schehl M, Torrecillas R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials. 2002;23:937–945. doi: 10.1016/S0142-9612(01)00206-X. [DOI] [PubMed] [Google Scholar]
- 28.Elpers M, Nam D, Boydston-White S, Ast MP, Wright TM, Padgett DE. Zirconia phase transformation, metal transfer, and surface roughness in retrieved ceramic composite femoral heads in total hip arthroplasty. J Arthroplasty. 2014;29:2219–2223. doi: 10.1016/j.arth.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Kohal RJ, Wolkewitz M, Mueller C. Alumina-reinforced zirconia implants: survival rate and fracture strength in a masticatory simulation trial. Clin Oral Implants Res. 2010;21:1345–1352. doi: 10.1111/j.1600-0501.2010.01954.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-X. [DOI] [PubMed] [Google Scholar]
- 31.Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–821. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- 32.Gelain F, Unsworth LD, Zhang S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J Control Release. 2010;145:231–239. doi: 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Györgyey Á, Ungvári K, Kecskeméti G, Kopniczky J, Hopp B, Oszkó A, et al. Attachment and proliferation of human osteoblast-like cells (MG-63) on laser-ablated titanium implant material. Mater Sci Eng C Mater Biol Appl. 2013;33:4251–4259. doi: 10.1016/j.msec.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Tan H, Guo S, Yang S, Xu X, Tang T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012;8:2166–2174. doi: 10.1016/j.actbio.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Ma R, Tang S, Tan H, Lin W, Wang Y, Wei J, et al. Preparation, characterization, and in vitro osteoblast functions of a nano-hydroxyapatite/polyetheretherketone biocomposite as orthopedic implant material. Int J Nanomedicine. 2014;9:3949–3961. doi: 10.2147/IJN.S67358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer U, Buchter A, Wiesmann HP, Joos U, Jones DB. Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater. 2005;9:39–49. doi: 10.22203/eCM.v009a06. [DOI] [PubMed] [Google Scholar]
- 39.Höke A. Neuroprotection in the peripheral nervous system: rationale for more effective therapies. Arch Neurol. 2006;63:1681–1685. doi: 10.1001/archneur.63.12.1681. [DOI] [PubMed] [Google Scholar]
- 40.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/S0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 41.Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou A, Chen S, He B, Zhao W, Chen X, Jiang D. Controlled release of TGF-beta 1 from RADA self-assembling peptide hydrogel scaffolds. Drug Des Devel Ther. 2016;10:3043–3051. doi: 10.2147/DDDT.S109545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Auria G, Vacatello M, Falcigno L, Paduano L, Mangiapia G, Calvanese L, et al. Self-assembling properties of ionic-complementary peptides. J Pept Sci. 2009;15:210–219. doi: 10.1002/psc.1083. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JM, Patterson JL, Vines JB, Javed A, Gilbert SR, Jun HW. Biphasic peptide amphiphile nanomatrix embedded with hydroxyapatite nanoparticles for stimulated osteoinductive response. ACS Nano. 2011;5:9463–9479. doi: 10.1021/nn203247m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satija NK, Gurudutta GU, Sharma S, Afrin F, Gupta P, Verma YK, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 47.Song JH, Kim JH, Park S, Kang W, Kim HW, Kim HE, et al. Signaling responses of osteoblast cells to hydroxyapatite: the activation of ERK and SOX9. J Bone Miner Metab. 2008;26:138–142. doi: 10.1007/s00774-007-0804-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.