Abstract
In recent years, immunotherapy has produced many unexpected breakthroughs in oncological therapy; however, it still has many deficiencies. For example, the number of patients who are unresponsive to anti-programmed death-ligand 1 (PD-L1), anti-cytotoxic T-like antigen-4 (CTLA4), and anti-programmed death-1 (PD1) therapies cannot be ignored, and the search for an undiscovered immunosuppressive pathway is imminent. Five decades ago, researchers found that activation of the adenosinergic pathway was negatively correlated with prognosis in many cancers. This review describes the entire process of the adenosinergic pathway in the tumor microenvironment and the mechanism of immunosuppression, which promotes tumor metastasis and drug resistance. Additionally, the review explores factors that regulate this pathway, including signaling factors secreted by the tumor microenvironment and certain anti-tumor drugs. Additionally, the combination of adenosinergic pathway inhibitors with chemotherapy, checkpoint blockade therapy, and immune cell-based therapy is summarized. Finally, certain issues regarding treatment via inhibition of this pathway and the use of targeted nanoparticles to reduce adverse reactions in patients are put forward in this review.
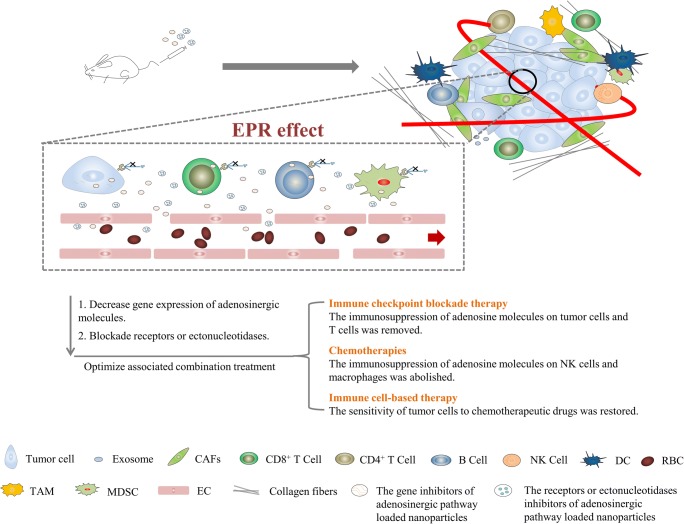
Graphical Abstract.
The inhibitors of adenosinergic pathway loaded nanoparticles enter tumor tissue through EPR effect, and inhibit adenosinergic pathway to enhance or restore the effect of immune checkpoint blockade therapy, chemotherapies and immune cell-based therapy. Note: EPR means enhanced penetration and retention, × means blockade
Keywords: Immunosuppression, Poor prognosis, Adenosine, Targeted nanoparticles
Introduction
To avoid being recognized by the immune system, tumor cells have developed mechanisms such as immune escape and immunosuppressive pathways that protect the tumor and continue to operate from the early stage to the advanced stage [1–3]. According to further research in this field, the immunosuppressive checkpoint molecules CTLA4 and PD1, which are expressed on CD8+ T lymphocytes, are targets to recover the immune response [4]. Currently, ipilimumab and nivolumab can successfully increase the survival of patients with various cancers, and the combination of ipilimumab and nivolumab has shown improved efficacy in patients with non-resectable metastatic melanoma [5, 6]. However, the adverse events caused by immunotherapy and the ineffectiveness of checkpoint inhibitors for certain patients should be seriously considered [7].
In recent years, in the adenosinergic pathway, the ADO (adenosine) generated by the ectonucleotidases CD39 and CD73 has been considered as a new “immune checkpoint mediator” that impairs the function of the immune system [8]. Interestingly, researchers found that regulatory T (Treg) cells are eliminated by checkpoint blockade therapy; however, they release a high amount of adenosine triphosphate (ATP), and CD39 and CD73 quickly transform ATP to ADO that targets T cells to hamper immune checkpoint blockade-mediated immune response [9]. This observation can explain why some patients relapse or experience worsened conditions after checkpoint blockade treatment. In addition, the adenosinergic pathway has an important effect on cancer cells and tumor microenvironment (TME); thus, it is worth considering it as a new target in clinical treatment [10].
Cancer patients have received great benefits from checkpoint blockade therapy, and how to optimize this treatment for more patients and less adverse reactions should be focused on in the next step [6, 7]. It has been shown that inhibitors of the adenosinergic pathway have great advantages of solving these difficulties, so we should explore how they can be combined with anti-PD1 and anti-CTLA4 therapies [9]. In this review, we propose to use nanoparticles for improving safety and efficacy of inhibitors of the adenosinergic pathway and also have shown an optimized approach of designing associated nanoparticles.
The adenosinergic pathway in cancer
What role the adenosinergic pathway plays in cancer?
High expression in malignant tumors
In humans, overexpression of CD73 has been shown in various cancers such as ovarian carcinoma, melanoma, breast cancer, colon cancer, and head and neck cancer, and these articles have reported a potential relationship between high CD39/CD73 expression and poor prognosis [11–19], high metastasis [20], and chemoresistance [21–23]. Similarly, this study analyzed publicly available gene expression data that correlated the expression of adenosine A2B receptor (A2BR) with prognosis and found that expression of A2BR was actually correlated with poor prognosis of triple-negative breast cancer (TNBC) patients [24], indicating that A2BR could be considered as a new target in some breast cancers. In addition, another study indicated that high expression levels of the adenosine A2A receptor (A2AR) gene and protein have same prognostic effects in non-small cell lung cancer (NSCLC) [17] (Table 1).
Table 1.
The clinical implication of adenosinergic molecules in cancers
| Biomarker | Cancer type | Clinical implication | Reference |
|---|---|---|---|
| CD39 | Chronic lymphocytic leukemia | High CD39 expression on T cells was associated with poor prognosis | [176] |
| High-grade serous ovarian cancer | High CD39 expression was associated with poor OS | [13] | |
| Gastric cancer | High CD39 expression associated with poor prognosis | [177] | |
| CD73 | Gastric cancer | High CD73 expression was associated with poor prognosis | [178] |
| Triple negative breast cancer | High CD73 expression was associated with a poor prognosis in TNBC patients but not in luminal or HER2+ breast cancer patients | [22] | |
| Non-small cell lung cancer | High CD73 expression was associated with poor prognosis | [16] | |
| Malignant melanoma | High CD73 expression was associated with metastatic site specificity in malignant melanoma | [179] | |
| Ovarian cancer | High CD73 expression was associated with poor prognosis in patients who have many CD73+CD8+ T cells in TME (meta-analysis) | [13] | |
| Rectal adenocarcinoma | High CD73 expression was associated with poor prognosis | [180] | |
| Colorectal cancer | High CD73 expression was associated with poor prognosis in human CRC | [18, 181] | |
| Gallbladder cancer | Patients with high NT5E (encoding CD73) expression was associated with poor prognosis | [182] | |
| Leukemia | High CD73 expression was associated with the development of leukemia subtype | [183] | |
| Chronic lymphoblastic leukemia | High CD73 expression was associated with poor prognosis | [23] | |
| Prostate cancer | High CD73 expression was associated with poor prognosis and more metastatic burden | [14, 184] | |
| A2AR | Non-small cell lung cancer | High A2AR gene and protein expression levels have same prognostic effects in non-small cell lung cancer | [17] |
| A2BR | Triple negative breast cancer | High A2BR expression was significantly associated with poorer survival in TNBC (meta-analysis) | [24] |
Several aspects of poor prognosis
Drug resistance
Drug resistance was involved in poor prognosis; the number of CD39+ Treg cells and level of ADO increased significantly and CD4+ T helper cells decreased after chemoradiotherapy, which could increase the tumor resistance to chemoradiotherapy [33]. Similarly, the EGF inhibitor cetuximab was found to upregulate the level of ADO to increase resistance in HNSCC [34]. Additionally, this study indicated that overexpression of CD73 would confer drug resistance to anthracyclines, such as doxorubicin [22]. Another study found that constant exposure to interferons derived from the adenosinergic pathway can confer an adaptive resistance to checkpoint blockade immunotherapy by inducing PD-L1 expression on tumor cells [35]. Similarly, in HER2/ErbB2 breast cancer, CD73 confers tumor cell resistance to anti-ErbB2 mAb has been reported [36].
Anti-apoptosis
Recently, it was observed that CD73 could downregulate the apoptosis of Jurkat leukemia cells mediated by tumor necrosis factor-related, apoptosis-inducing ligand (TRAIL) [37]. This effect is not associated with the enzymatic activity of CD73 but associated with the colocalization of CD73 with the TRAIL receptor DR5 [38]. Similarly, this article indicated that CD73 would downregulate the frequency of anti-apoptotic factors which always are BCL2 and BCLxL [39].
Tumor metastasis
CD73 has a significant effect on cancer cell growth and invasion [40]. In particular, high CD73 expression has been proposed as a biomarker for high metastasis in melanoma and breast cancer [41–43]. Similarly, this study found that CD73 is both expressed on MITFlow and a part of MITFhigh melanoma cells, and CD73 is considered a significant metastatic biomarker [44]. It was found that myeloid-derived suppressor cells (MDSCs) as source of VEGF which upregulated tumor cell metastasis in melanoma. Some A2BR inhibitors (PSB1115 and PBF-1129) delayed MDSC invasion and downregulated the VEGF concentration has been reported, which strongly reduced angiogenesis in these mice [45].
Immune suppression
CD39 has a significant effect on downregulating immune cell response [46]. These studies indicated that TGF-β-cultured Th17 cells expressed CD73 and exhibited the capability to inhibit the immunity function [47, 48]. Similarly, the ADO generated by CD39/CD73 that was expressed on Tregs exhibited strong immunosuppression in these studies [49, 50]. In another study, T cells and MDSCs could produce ADO that activated A2AR, which suppressed the immune response to tumor cells [51].
How does the adenosinergic pathway affect cancer?
Overview of the adenosinergic pathway
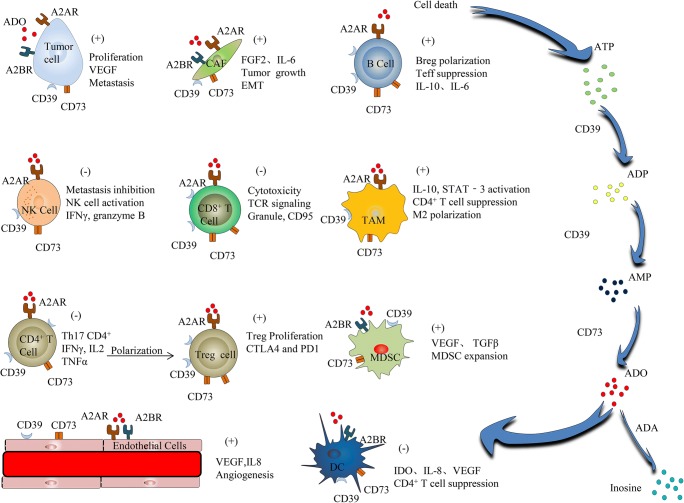
In normal physiological tissue, ATP was located in the intracellular compartment at concentrations greater than 1 mM but less than 10 mM and has a low concentration (10–100 nM) in TME [52]. Specially, extracellular ATP concentration rose strongly in response to homeostasis-interfered conditions, including inflammation, hypoxia, tissue stress, chemotherapy, radiotherapy, cell apoptosis, ischemia, and malignancy [11, 53]. Additionally, CD39 can catalyze extracellular ATP into adenosine diphosphate (ADP) and ADP into adenosine monophosphate (AMP). Next, CD73, which is downstream of CD39 in adenosinergic pathway, can convert AMP into ADO (Fig. 1). Importantly, the conversion of ATP into AMP via CD39 is reversible; however, the catalysis of AMP into ADO mediated by CD73 is not reversible [54]. Therefore, CD73 was regarded as the decisive enzyme in the progress of extracellular ADO production [55]. In addition, extracellular ADO can be catalyzed into inosine through ectoadenosine deaminase (ADA) (associated with CD26 in humans) [56] (Fig. 1).
Fig. 1.
The entire process of the adenosinergic pathway in the tumor microenvironment and the mechanism of immunosuppression, metastasis and drugs resistance. Note: Positive sign (+) means increasing, Negative sign (-) means decreasing
Ectonucleotidases
CD39 is widely expressed [57]. Consistent with all nucleoside triphosphate diphosphohydrolases (NTPDases), there are five conserved sequences that are also known as “apyrase conserved regions” on CD73, which participate in active site formation [58] and is important to ectoenzymatic activity [59, 60].
CD73 is a 70-kD, glycosylphosphatidylinositol (GPI) protein located on the cell membrane and is encoded by the NT5E gene [61]. The dissociative form of CD73 is similar to membrane-bound form of CD73 [62, 63]. CD73 has three domains, which contain an N-terminal domain, C-terminal domain, and a short alpha helix [64]. Importantly, functional CD73 must be a homodimer with a non-covalent interaction between adjacent C-terminal domains, which exist in two conformations: open and closed. Switching from the open to the closed conformation is required for ectoenzymatic activity by allowing substrate binding and product releasing [65]. Additionally, the zinc ions are important to the ectoenzymatic activity of CD73 [64, 65]. Additionally, functional glycosylation modifications occurred on CD39, which is also significant for the catalytic activity of CD39 [66]. In this research, it was found that the experiment for depleting membrane cholesterol through drugs exhibited a significant inhibition of the CD39 catalytic activity [57].
Receptors
ADO binds to P1 (type 1 purinergic receptors) receptors, a family of G protein-coupled receptors. This family includes four isoforms, A1R, A2AR, A2BR, and A3R [67]. A1R, A2AR, and A3R, which exhibit high activity in a low concentration of ADO (1–100 nM), are high-affinity receptors, but A2BR is a low-affinity receptor that only exhibits activity in surroundings with high levels of ADO, such as TME (25 μM) [39]. Therefore, the host A2BR is unlikely to be involved in metastasis [24]; however, the host A2AR contributes to tumor metastasis and immunosuppression [68]. Activated A2AR and A2BR promote adenylyl cyclase (AC) and the product cyclic AMP to suppress the function of immune cells [69–71]; however, the activated A1R and A3R inhibit cAMP generation to enhance the function of immune cells [72, 73]. A1R has a broad distribution and is particularly abundant in the nerves, heart, and kidneys. A2AR is expressed on cancer, NK cells, CD4+ T cells, B cells, Tregs, CAFs, CD8+ T cells, macrophages, the endothelium, and striatal area. A2BR is distributed in the endothelium, MDSCs, CAFs, DCs, and tumor cells. A3R is expressed in the nervous and cardiovascular systems, immune cell subsets, the liver, and tumor cells. [4].
The mechanism of the adenosinergic pathway
ADO catalyzed by CD39/CD73 acts on a wide range of cells and causes many cascades to contribute to tumor growth, anti-apoptosis, drug resistance, tumor metastasis, angiogenesis, and immune escape in TME [39].
Tumor cells
It has been observed that the inhibition of CD39 can improve development and immunosuppressive function of immune cells in melanoma, ovarian, and TNBC [74–76]. Similarly, the knockdown of A2BR in tumor cells decreased VEGF production and lung metastases and even reduced tumor cell proliferation and invasion [24]. Additionally, it activated A2BR in TNBC cells and enhanced their invasion and proliferation through the ERK signaling pathway. Another study indicated that A2BR as an activated form is important to growth and survival of tumor [71]. Interestingly, both host- and tumor-expressed CD73 are critical to immunosuppression. And knockdown or overexpression of CD73 on tumor cells can affect the proliferation and metastasis in cancer [75, 77]. Additionally, another study indicated that the overexpression of A2BR on tumor cells promotes metastasis [24].
CAFs
Activated A2BR on cancer-associated fibroblasts (CAFs) can upregulate the frequency of fibroblast activation protein (FAP)-positive fibroblasts. Additionally, activated A2BR results in the enhanced production of CXCL12, which is released by FAP-positive fibroblasts, and CXCL12 acts on tumor cells to release VEGF, which can increase the number of CD31+ endothelial cells [78]. Activated A2BR on CAFs can promote the protein kinase C (PKC) signaling to release interleukin-6 (IL-6), which is important for the epithelial-to-mesenchymal transition (EMT) [39]. In addition, this study has shown that fibroblasts can promote Tregs via ectoenzymatic activity of CD73. α,ß-Methylene adenosine-5′-diphosphate (APCP), a specific CD73 inhibitor, and an anti-CD73 mAb (AD2) can inhibit CD73 and are applied to promote CD8+ T cells [79, 80]; additionally, this study indicated that therapy via inhibiting CD73 can restore the function of immune cells.
CD4+ T cells/Treg cells
Several studies have shown that CD39 expressed on CD4+ T cells can downregulate IL-17 production by suppressing the function of T cells [81, 82] and can inhibit that T cells become Th17 cells [83, 84]. Additionally, in these studies, enhanced levels of CD39+ Treg cells, which impaired T cell immunity, were recognized in AT3 mammary carcinoma model [85, 86]. In another study, CD39+CD8+ T cells always exhibited the enhanced frequency of checkpoint molecules such as PD1 and CTLA4 [87]. Interestingly, it has been shown that CD4+FOXP3− T cells exhibited a high CD73 expression in mouse model [88]. The frequency balance between Treg cells and T helper cells could be mediated by adenosinergic pathway inhibitors in mice with malignant tumor [89]. In the TME, it had been shown that increased number of CD39+CD4+ T cells and the presence of CD73+CD4+ T cells expressing immune checkpoints were strongly associated with immunosuppression in breast and ovarian tumors [90].
B cells
Both CD39 and CD73 and adenosine receptors are co-expressed on human B cells. The CD39+CD73+ B cell subtype is associated with decreased immunity via CD4+/CD8+ effector T cells [91]. It has been observed that human regulatory B cells (Bregs) with high CD39 expression have a high proliferative capacity and produce a large amount of ADO and IL-10 [92]. It was found that ADO and agonists of B cell receptors (BCR) and toll-like receptors (TLR) can co-stimulate B cells and elevate the frequency of these cells to convert B cells to class-switched B cells. Additionally, high CD73 level was correlated with enhanced Breg [93]. In addition, it was illuminated that the ADO-producing B cells secreted IL-6, which contributed to tumor cell proliferation [9] and that the adenosinergic pathway was involved in inhibition of T cells [40].
CD8+ T cells
Recently, A2AR activated by ADO that regulated the intracellular Wnt-signaling has been reported, which led to the blockade of the conversion of immature CD8+ T cells into activated T cells [94, 95]. Another study found that CD73 expression influenced the reliability of the prognosis assessment of infiltrating CD8+ T cells in highgrade ovarian cancer in the clinic [13]. In addition, genetic ablation of A2AR and inhibition of A2AR via antagonists could enhance immune cell-based therapy that restored immunity in the TME [70]. Similarly, it has been indicated that A2AR located on tumor cells intrinsically regulated the frequency of CD8+ T cells in the TME [77, 96, 97].
NK cells
It has been indicated that the expression of CD73 on human NK cells increased after these cells contacted mesenchymal stromal cells (MSC) and that this could be involved in a follow-up immunosuppressive process [98]. On the other hand, only A2AR, and no A2BR, was expressed on NK cells, and its frequency was greatly enhanced under certain pathological stimuli such as sickle cell disease (SCD) [99]. Subsequently, upregulated A2AR contributed to the inhibition of activated NK cells and their cytotoxicity function [87]. Moreover, we have tested this idea by detecting the expression of granzyme B on NK cells isolated from A2AR-antagonist-treated mice, and it was found that the frequency of granzyme B was enhanced. Interestingly, it was indicated that the upregulation of A3R was correlated with enhanced NK cell activity [100]. In summary, A2AR signaling suppressed natural killer cell maturation and contributed to the metastasis of CD73+ tumors in the tumor microenvironment [101].
TAMs
It has been found that CD39 on monocytes could inhibit the chemotaxis, adhesion, and trans-migration capacity of these cells [102]. Compared with immunosuppressive M2 macrophages, M1 macrophages expressed lower frequency of CD39 and CD73 [103]. In associated studies, it was indicated that the CD73 inhibitor APCP increased the proportion of M1 macrophages between these macrophages [104]. Similarly, downregulated M2 macrophages have also been found in CD73-deficient mice [105]. In contrast, ADO binding to A2AR and A2BR contributed to impairment of macrophage function. For example, the increased level of M2 markers and IL-10-induced STAT-3 via the adenosinergic pathway has been shown to suppress CD4+ T cell proliferation [106–108].
MDSCs
The inhibition of A2BR could prevent the migration of CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs) and could downregulate VEGF levels, which are important for reducing angiogenesis [45]. In contrast, it was found that agonists of A2BR contributed to infiltration and expansion of MDSCs, and the potential mechanism could be phosphorylation of STAT-3 [109, 110].
Which factors regulate the adenosinergic pathway
Endogenous factors
Certain endogenous molecules have an important impact on the adenosinergic pathway. It was found that IL-27 contributed to the expression of CD39 on DCs via STAT3 and aryl hydrocarbon receptor (AhR) [46]. TNBC, ovarian cancer, and colorectal cancer often carried the TP53 mutation [111]. And TP53 mutation was positively associated with the epithelial-to-mesenchymal transition (EMT). Consequently, tumor cells underwent EMT, which exhibited enhanced expression of CD73 on the cell surface [36]. In addition, the expression of A2AR and A2BR was increased by tumor necrosis factor (TNF), due to activated NFκB pathway [112, 113]. Interestingly, HIF-2α but not HIF-1α in human lung endothelial cells promoted the expression of A2AR, and siRNA knockdown of HIF-2α completely downregulated the frequency of A2AR [114]. On the other hand, TLR4 (toll-like receptor 4)-dependent inflammation caused a significant improvement of A2AR and A2BR levels in tumor-associated macrophages (TAMs) [115]. Moreover, it was indicated that transforming growth factorβ (TGFβ) and hypoxia-inducible factor (HIF)-1α could profoundly upregulate the level of A2AR, CD39, and CD73, which is located on both CD8+ and CD4+ T cells [39].
Exogenous factors
In addition to endogenous molecules, exogenous treatments also took part in the progress of immunosuppression, metastasis, and drug resistance caused by the adenosinergic pathway. Metformin inhibits MDSC activity in patients with ovarian cancer by decreasing the levels of CD39 and CD73 [116]. In contrast, CD40 mAb, as an agonist, enhances CD73 and CD39 expression; however, the mechanism is not very clear [86]. Additionally, it has been shown that CD73 expression on tumor cells in a subset of patients with melanoma increases after receiving antiPD1 immunotherapy and that it impairs the treatment effect [44]. Similarly, another study has reported that carboplatin, doxorubicin, gemcitabine, and paclitaxel induce enrichment of CD47+/CD73+/PDL1+-immune-evasive tumor cells in TNBC [117]. Triiodothyronine (T3) treatment causes a significant enhancement of CD73 expression in a dose–response manner in C6 rat glioma cells and smooth muscle cells, and this effect may be caused by thyroid follicular cell hyperfunction [118, 119]. Interestingly, in rats receiving chronic stimulation via a low-iodine diet or treated with propylthiouracil, the enzymatic activity of CD73 is augmented [120]. Moreover, LEC (low-dose endotoxin conditioning) treatment of RPMФ (resident peritoneal macrophages) causes an ~ 3-fold increase in the A2AR level and an ~ 28-fold increase in the ADO level [121].
Current status of treatment of adenosinergic pathway
Combination therapy
Combination with checkpoint blockade treatment
Currently, a research indicated that antiPD1 therapy upregulated A2AR expression on CD8+ T cells in cancer. Similarly, it was indicated that CD73 expression increased in patients with acquired resistance to antiPD1 therapy, which showed that the adenosinergic pathway was involved in the impairment of immune checkpoint blockade therapies [47]. It has been shown that the simultaneous blockade of PD1 and A2AR upregulated the frequency of CD8+ T cells and NK cells, which could inhibit the metastasis of tumor cells [122]. In addition, the combination of CD73 mAb (MEDI9447) and antiPD1 therapy impaired the growth of mouse CT26 colon carcinoma [123]. Additionally, it has been shown that the CD73specific inhibitor APCP and antiCTLA4 profoundly reduced melanoma survival compared with monotherapy [124]. In another study, B7DC-Fc fusion protein could reduce checkpoint molecule-mediated immunosuppression, which improved the survival of mice [125]. It also indicated that combination therapy conferred a prolonged survival to mice [125].
Combination with chemotherapies
It has been reported that the blockade of CD73, A2AR, or A2BR enhances the effect of chemotherapy [22, 24, 126]. This indicates that inhibition of the adenosinergic pathway contributes to the enhancement of chemotherapy [22]. It has been shown that high CD73 level in patients is correlated with upregulated resistance to anthracycline [22]. It has also been elucidated that combination therapy with doxorubicin and anti-CD73 or A2AR antagonists contributes to prolonged survival in mice. Similar results have demonstrated that the blockade of CD39 on fibrosarcomas restores sensitivity to anthracyclines [127]. Moreover, a higher efficacy has been reported with the combination of A2BR inhibitors with chemotherapeutic drugs such as dacarbazine and doxorubicin, which showed similar results to those described above [24, 126].
Combination with immune cell-based therapy
In addition to conventional chemotherapies and checkpoint blockade treatment, the combination of the inhibition of the adenosinergic pathway and CD8+ T cell-based therapies is a significant strategy [128]. Antigenspecific T cells in combination with APCP enhanced the impairment of tumor growth compared with monotherapy [128]. It has been shown that the inhibition of A2AR significantly improved the treatment effect of chimeric antigen receptor (CAR) T cells on mammary tumor cells [129], which was explained by enhanced levels of CAR T cells and cytokines released by T cells [129]. In addition to T cell-based therapies, a high A2AR frequency has been reported on NK cells, and the suppression of A2AR could significantly enhance NK cells in malignant cancers [77, 122]. Thus, NK cell-based therapies were enhanced by blocking the activation of A2AR [39].
Drugs that have entered clinical studies
In recent years, many small molecule drugs or antibodies inhibiting the adenosinergic pathway are undergoing clinical trials. Their targets include CD73, A2AR, and A2BR but not CD39, and most of these inhibitors are applied to malignant tumors such as ovarian cancer, NSCLC, and other advanced cancers. Additionally, they will also be used in combination with checkpoint blockers such as anti-PDL1 and anti-PD1 to achieve better efficacy (Table 2). Many researchers and medical companies wait for the first clinical trial results, which would come from patients who are receiving inhibitors that reduce extracellular ADO generation or activity in tumors. These observations present a good opportunity to develop anti-CD73 therapy for the treatment of certain cancer patients. Based on this knowledge, although there is still a long way to go, future studies can be expected that aim at translating anti-CD73 therapy for cancer patients in the clinic.
Table 2.
Clinical trials of antagonists of the adenosinergic pathway
| Target | Drug | Clinical trial number | Cancer type | Combination partner | Study phase | References |
|---|---|---|---|---|---|---|
| CD73 | MEDI9447 | NCT02503774 | Solid tumors | Anti-PDL1 (MEDI4736) | Phase 1 | [25] |
| NCT03267589 | Ovarian cancer | Anti-PDL1 (durvalumab) | Phase 2 | [26] | ||
| CPI-006 | NCT03454451 | Advanced cancers | Anti-PDL1 (pembrolizumab) A2ARi(CPI-444) | Phase 1/1b | [27] | |
| A2AR | NIR178 | NCT03207867 | Solid tumors and non-Hodgkin lymphoma | Anti-PD1 (PDR001) | Phase 2 | [28] |
| PBF-509 | NCT02403193 | NSCLC | Anti-PD1 (PDR001) | Phase 1, phase 2 | [29] | |
| CPI-444 | NCT02655822 | Advanced cancers | Anti-PDL1 (durvalumab) | Phase 1/1b | [30] | |
| AZD4635 | NCT02740985 | Advanced cancers | Anti-PDL1 (durvalumab) | Phase 1 | [31] | |
| A2BR | PBF-1129 | NCT03274479 | NSCLC | N/A | Phase 1 | [32] |
What should we do for a better therapeutic effect in the future?
Because A2AR/A2BR and CD39/CD73 are important to maintain the health and homeostasis in normal tissues, the ubiquitous effects of drugs in the body will destroy these intact systems and cause unnecessary or even fatal toxic side effects. For example, a study has shown that some cells have a high expression of efflux pump P-glycoprotein (P-gp), which pumps out drugs to promote drug resistance; activating A2AR via an FDA-approved A2AR agonist (Lexiscan) significantly decreased P-gp expression and function to enhance the drug efficacy in a time-dependent manner. This suggests that medication without delivery systems targeting tumor sites may cause some serious adverse reactions [130]. Similarly, the ubiquitous expression of CD73 has a significant effect in adjusting the dynamic balance between ATP and ADO to maintain physiological homeostasis; therefore, drug delivery without tumor-targeting may decrease effective metabolic activity of CD73 [131]. Another article has reviewed that CD73-KO mice exhibits functional defects in multiple aspects [132]; for example, intestinal epithelium motility and permeability, reducing damage associated with ischemia, hypoxia-induced vascular leakage, renal function, leukocyte trafficking, immunity and endothelial barrier function, are downregulated compared with wild-type mice [133–143]. Therefore, care should be taken during treatment, considering that an antagonist of CD73 could cause potential damage to patients who receive adenosinergic pathway blockade treatment in the clinic [132]. On the other hand, formulations with no carrier are unstable for drug delivery to tumor sites. Many cells, such as macrophages, can clear the drug, and other molecules can neutralize the active ingredients via processes such as opsonization. In addition, drugs without nanoencapsulation will be rapidly metabolized by the liver and excreted by the kidneys, which will result in a very low half-life and will greatly reduce the efficacy of the drug [144]. Overall, to enhance the efficacy and reduce the side effects of adenosinergic pathway blockade drugs, the use of tissue- and cell-targeting systems has become the focus of future drug development in this area.
The advantages of nanoparticles for delivering inhibitors of the adenosinergic pathway
In short, the original intention of developing drug delivery systems was to improve the therapeutic effect by altering the transport process for drug molecules in the body. The optimization of drug delivery systems focused on aspects including formulation, controlled release, delivery routes, and half-life, which could significantly enhance treatment efficacy [145–147]. In recent years, drug delivery systems have been developed quickly and efficiently, and nanoparticles (NPs) gradually have become the most popular drug-carrier [147]. Scientists highly recommend nanoparticles because of multiple aspects. There are many efficient and convenient manufacturing methods (solvent displacement, salting out, emulsion diffusion, emulsion–solvent evaporation, in situ polymerization) to form a wide variety of NPs such as micelles, liposomes, magnetic nanoparticles, gold nanoshells, carbon nanotubes (CNTs), nanohydrogels, and dendrimers, which can effectively cope with various diseases [148–150]. They possess the capacity to carry multiple types of insoluble drugs [149]. For example, NP formulations of hydrophobic drugs such as paclitaxel can overcome the difficulties of drug insolubility in water [151]. Efficient delivery systems could be applied in diagnostics in a multitude of diseases [152]. NPs exhibit increased drug-loading capacity due to their large surface area to volume ratio [153]. NPs exhibit a longer systemic circulation than conventional systems and reduce clearance by the kidneys. NPs can protect drug molecules from attack by the host immune system and enzymatic degradation, and certain immune-modifications on NPs can inhibit the suppressive TME, which is associated with tumors, for enhancing cancer chemotherapy and reducing drug resistance. NPs extravasate into tissues from leaky blood vessels more easily compared with drug molecules. Combining this factor with the enhanced permeation and retention (EPR) effect reduces the challenges of off-target effects caused by increasing the drug concentration [154, 155]. In addition, NPs, such as liposomes, can avoid drug efflux pumps to weaken multidrug resistance (MDR), which will enhance the anti-tumor drug accumulation in tumor cells. Additionally, safer and less expensive nanocarriers are constantly being developed and innovated to achieve more effective drug delivery and improved efficacy [156]. Targeted delivery systems have been studied for delivering adenosine pathway antagonists [157], and it has been found that CD73-specific siRNA-loaded chitosan-lactate nanoparticles (ChLa NPs) can lead to reduced expression of CD73 in tumor cells. This decreases tumor growth and metastasis and improves mice survival, thus enhancing the efficacy of DC-based cancer immunotherapy compared with naked CD73-specific siRNA and eventually achieving the downregulation of Tregs, MDSCs, and TAMs in the TME. Therefore, the potential of NPs has been identified to improve the efficiency of drug delivery and to decrease the side effects associated with present therapeutics.
Applying nanoparticles in combination therapy
In the earlier part of this review, the combination of adenosinergic pathway blockade and other therapies (checkpoint blockade treatment, chemotherapy and immune cell-based therapy) has been elucidated in detail, and combination therapy is expected to show great prospects. However, it is worth exploring a strategy to achieve an effective cooperation of two or even more drugs. The emergence of NPs has solved this problem because NPs can effectively combine the effects of multiple drugs and serve as a multilayered, synchronous, and collaborative drug delivery system. In addition, theoretically, the NPs can be used in evaluating the efficacy of combinatorial therapy via designing multiple drug-loaded particles. Compared with separated drug administration, co-delivery of two or more drugs in a carrier has some significant merits, including (1) reduce drug dosage, (2) significantly improves patient compliance, and (3) controls the individual dosage [158]. In this study, the application of nanoparticle for combination therapy confers several potential advantages such as good biodistribution, enhanced blood stability, controllable drug release, high carrier capacity, prolonged systemic circulation lifetime, and multidrug encapsulation for combination therapy [159]. Some researches indicated that co-delivery of two therapeutic agents has a synergistic effect, and it could enhance the efficiency of delivering two drugs to the same cell population by at least one order of magnitude when compared with delivering them in two separate carriers. It is important to recognize the relationship between the mechanisms of each agent, pharmacological activity, and the mode of delivery. Conversely, the propound understanding could contribute to the better design of co-delivery vectors with proper timing and sequence of delivery of individual drugs of the combination [160].
The design of active targeted nanoparticles
Compared with passive targeting, active targeting (also known as ligand-mediated targeting) involves modification of the surface of NPs with affinity ligands for specific retention and uptake by the targeted cells. Therefore, it is urgent to seek appropriate modifiers to be applied in NPs for more effective adenosinergic pathway blockade therapy. Representative ligands include antibodies, proteins, peptides, nucleic acids, sugars, and small molecules such as vitamins [161]. Thus, using antagonists of CD39, CD73, A2AR, and A2BR as modifiers on NPs could be a good choice according to the targeted therapy theory [161]. At present, multiple inorganic analogues have been shown to effectively bind to CD73 and potently inhibit CD73 enzymatic activity, including non-hydrolyzable AMP analogs such as APCP, flavonoid-based compounds such as quercetin, and purine nucleotide analogs such as tenofovir and sulfonic acid compounds [162–164]. In addition, several proteins have been identified such as concanavalin A and tenascin C, which can potentially inhibit CD73 enzymatic activity [165, 166]. It is worth studying intrinsic protein inhibitors, including various monoclonal antibodies targeting CD73 and TY/23, which specifically targets murine CD73 [167–169]; other monoclonal antibodies targeting human CD73 include IE9, 7G2, 4G4, and AD2 [63, 80, 167, 168]. Similarly, antagonists of CD39 such as POM1 [8], inhibitors of A2AR such as ZM241385, and antagonists of A2BR such as PSB1115 should also be considered as modifiers on NPs for active targeting [39]. The targeted therapy theory put forward by Paul Ehrlich in 1906 conceived of a selective delivery of effective drugs. For example, chemotherapy is ubiquitously toxic to tumor and normal tissues. There would be excellence in that these toxic agents are only delivered to the cancer tissues and cancer-related tissues to kill cancer cells with minimal adverse reactions. Therefore, it is significant that active targeting drug delivery enables the targeted delivery of agents to specified tissues via active ligands [170].
Conclusion and prospect
This review has addressed the whole process of the adenosinergic pathway in the TME. The pathway-associated enzymes (CD39 and CD73) and activated receptors (A2AR and A2BR) play roles in promoting tumor growth, metastasis, immunosuppression, and drug resistance through multiple mechanisms. Endogenous factors (IL-27, TGFβ, HIF-1/2α, TP53 mutation, EMT transcription factor, TNF, and TLR4) and exogenous factors (metformin, CD40 mAb, antiPD1, carboplatin, doxorubicin, gemcitabine, paclitaxel, triiodothyronine, low-iodine, propylthiouracil and low-dose endotoxin), which can regulate adenosinergic pathway-associated molecules, have also been summarized. Next, the three major treatments that inhibit this pathway in combination with other therapies such as checkpoint blockade, chemotherapy, and immune cell-based therapy will greatly improve the efficiency of treatment, and this approach will be the focus of future treatments. Most importantly, because many enzymes and receptors in the pathway are widely distributed throughout the body, using the drug by itself may result in many unavoidable side effects. Therefore, the best choice is to encapsulate these antagonists via nanocarrier systems to reduce unnecessary drug distribution. Passive and active targeted nanocarrier systems is significant in cancer therapy, and finding suitable targeting modifications according to the targeted therapy theory could prove to be highly efficient. This review additionally clarifies the direction of future treatments in this area.
To apply the inhibitors of the adenosinergic pathway via a more effective and safe approach in the future, certain problems should be focused on and solved. It is important to investigate whether the suppression of A2AR can enhance the susceptibility of A2BR to ADO, and conversely, whether the suppression of A2BR can enhance the susceptibility of A2AR to ADO. The low A2AR level would downregulate A2BR expression in splenocytes [171], which needs further study to clearly explain the intrinsic mechanism. It remains unknown whether the therapeutic effect of CD73 inhibition is similar to that of A2A/A2B inhibition. It may be possible that blocking CD73 will be more efficient than blocking A2A/A2B receptors due to the combined inhibition of enzymatic and non-enzymatic functions of CD73. It may be more effective to induce activation of the CD26 pathway plus inhibition of the CD73 pathway for adenosinergic pathway blockade than inhibition of the CD73 pathway alone. (CD26-associated ADA can degrade ADO, while CD73 generates ADO.) When selecting patients for clinical trials or selecting experimental tumor models for animal experiments, we should know how to select appropriate subjects for a better experimental outcome. High expression levels of relevant biomarkers, including CD39, CD73, A2AR, and A2BR, should be the standard for better choice. NECA, 5′-(N-ethylcarboxamido) adenosine, a non-specific ADO receptor agonist, was identified to upregulate caspase-3 and cause tumor cells apoptosis in cancers which have a high A2BR expression [172]. Additionally, the NECA was also identified to upregulate caspase-dependent apoptosis caused by chemotherapeutics in osteosarcoma [173]. Reversely, the activation of A2BR was identified to improve growth of prostate cancer [174] and glioblastoma [175]. We should clearly define the different mechanisms that are involved. Overall, we believe that the inhibition of the adenosinergic pathway will result in a good outcome in clinical applications and will become an indispensable part of oncological therapy in the future.
Funding information
This work was supported by The Fundamental Research Funds of Shandong University (No. 2018JC006) and Jiangxi Province Outstanding Young Talents Program (20162BCB23034).
Conflict of interest
Yi Huang declares that he/she has no competing interest.
Zili Gu declares that he/she has no competing interest.
Yang Fan declares that he/she has no competing interest.
Guangxi Zhai declares that he/she has no competing interest.
Xiaogang Zhao declares that he/she has no competing interest.
Qifeng Sun declares that he/she has no competing interest.
Yanbin Shi declares that he/she has no competing interest.
Guimei Lin declares that he/she has no competing interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barrett J, Blazar B. Genetic trickery—escape of leukemia from immune attack. N Engl J Med. 2009;361:524–525. doi: 10.1056/NEJMe0903177. [DOI] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, Zi X, Kwak M, Bergholtz H, Su Y, Ding L, Russnes HG, Richardson AL, Babski K, Min Hui Kim E, McDonnell CH, III, Wagner J, Rowberry R, Freeman GJ, Dillon D, Sorlie T, Coussens LM, Garber JE, Fan R, Bobolis K, Allred DC, Jeong J, Park SY, Michor F, Polyak K. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7:1098–1115. doi: 10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Smith DC, Brahmer JR, Gettinger SN. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36:293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Young A, Ngiow SF, Madore J, Reinhardt J, Landsberg J, Chitsazan A, Rautela J, Bald T, Barkauskas DS, Ahern E, Huntington ND, Schadendorf D, Long GV, Boyle GM, Holzel M, Scolyer RA, Smyth MJ. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017;77:4684–4696. doi: 10.1158/0008-5472.CAN-17-0393. [DOI] [PubMed] [Google Scholar]
- 13.Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, Rahimi K, Le Page C, Provencher D, Mes-Masson AM, Stagg J. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75:4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J. CD73 expression is an independent prognostic factor in prostate cancer. Clin Cancer Res. 2016;22:158–166. doi: 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- 15.Gaudreau PO, Allard B, Turcotte M, Stagg J. CD73-adenosine reduces immune responses and survival in ovarian cancer patients. Oncoimmunology. 2016;5:1–10. doi: 10.1080/2162402X.2015.1127496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue Y, Yoshimura K, Kurabe N, Kahyo T, Kawase A, Tanahashi M, Ogawa H, Inui N, Funai K, Shinmura K. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017;8:8738–8751. doi: 10.18632/oncotarget.14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, Ghiringhelli F. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–5252. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 18.Wu XR, He XS, Chen YF, Yuan RX, Zeng Y, Lian L, Zou YF, Lan N, Wu XJ, Lan P. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 19.Allard B, Turcotte M, Stagg J. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol. 2012;2012:1–8. doi: 10.1155/2012/485156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leth-Larsen R, Lund R, Hansen H, Laenkholm A, Tarin D, Jensen O, Ditzel H. Metastasis-related plasma membrane proteins of human breast cancer cells identified by comparative quantitative mass spectrometry. Mol Cell Proteomics. 2009;8:1436–1449. doi: 10.1074/mcp.M800061-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra S, Horenstein AL, Vaisitti T, Brusa D, Rossi D, Laurenti L, D’Arena G, Coscia M, Tripodo C, Inghirami G, Robson SC, Gaidano G, Malavasi F, Deaglio S. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 24.Mittal D, Sinha D, Barkauskas D, Young A, Kalimutho M, Stannard K, Caramia F, Haibe-Kains B, Stagg J, Khanna KK, Loi S, Smyth MJ. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res. 2016;76:4372–4382. doi: 10.1158/0008-5472.CAN-16-0544. [DOI] [PubMed] [Google Scholar]
- 25.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT02503774
- 26.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT03267589
- 27.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT03454451
- 28.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT03207867
- 29.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT02403193
- 30.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT02655822
- 31.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT02740985
- 32.June 15, 2018. https://www.clinicaltrials.gov/ct2/show/NCT03274479
- 33.Schuler PJ, Harasymczuk M, Schilling B, Saze Z, Strauss L, Lang S, Johnson JT, Whiteside TL. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res. 2013;19:6585–6596. doi: 10.1158/1078-0432.CCR-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4(+) regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75:2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167:1540–1554. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, Pommey S, Delisle V, Loi S, Joensuu H, Kellokumpu-Lehtinen PL, Sotiriou C, Smyth MJ, Stagg J. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017;77:5652–5663. doi: 10.1158/0008-5472.CAN-17-0707. [DOI] [PubMed] [Google Scholar]
- 37.Mikhailov A, Sokolovskaya A, Yegutkin GG, Amdahl H, West A, Yagita H, Lahesmaa R, Thompson LF, Jalkanen S, Blokhin D, Eriksson JE. CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J Immunol. 2008;181:464–475. doi: 10.4049/jimmunol.181.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 40.Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannons JL, Lu KT, Schwartzberg PL. Lymph node choreography: B cells take the lead. Nat Immunol. 2012;13:630–632. doi: 10.1038/ni.2349. [DOI] [PubMed] [Google Scholar]
- 42.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwata Y, Matsushita T, Horikawa M, DiLillo D, Yanaba K, Venturi G, Szabolcs P, Bernstein S, Magro C, Williams A. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinhardt J, Landsberg J, Schmid-Burgk JL, Ramis BB, Bald T, Glodde N, Lopez-Ramos D, Young A, Ngiow SF, Nettersheim D, Schorle H, Quast T, Kolanus W, Schadendorf D, Long GV, Madore J, Scolyer RA, Ribas A, Smyth MJ, Tumeh PC, Tuting T, Holzel M. MAPK signaling and inflammation link melanoma phenotype switching to induction of CD73 during immunotherapy. Cancer Res. 2017;77:4697–4709. doi: 10.1158/0008-5472.CAN-17-0395. [DOI] [PubMed] [Google Scholar]
- 45.Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget. 2015;6:27478–27489. doi: 10.18632/oncotarget.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol. 2016;37:427–439. doi: 10.1016/j.it.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC, Eberl G, Pallandre JR, Borg C, Ryffel B, Apetoh L, Rebe C, Ghiringhelli F. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 49.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee S, Thyagarajan K, Kesarwani P, Song JH, Soloshchenko M, Fu J, Bailey SR, Vasu C, Kraft AS, Paulos CM, Yu XZ, Mehrotra S. Reducing CD73 expression by IL1beta-programmed Th17 cells improves immunotherapeutic control of tumors. Cancer Res. 2014;74:6048–6059. doi: 10.1158/0008-5472.CAN-14-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:1–3. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 53.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 54.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 55.Resta R, Yamashita Y, Thompson L. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 56.Luca Antonioli RC, Federico Da Settimo CB, C.L.M., Fornai M, Tuccori M, Awwad O. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr Drug Targets. 2012;13:842–862. doi: 10.2174/138945012800564095. [DOI] [PubMed] [Google Scholar]
- 57.Papanikolaou A, Papafotika A, Murphy C, Papamarcaki T, Tsolas O, Drab M, Kurzchalia TV, Kasper M, Christoforidis S. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280:26406–26414. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- 58.Kirley TL, Crawford PA, Smith TM. The structure of the nucleoside triphosphate diphosphohydrolases (NTPDases) as revealed by mutagenic and computational modeling analyses. Purinergic Signal. 2006;2:379–389. doi: 10.1007/s11302-005-5301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grinthal A, Guidotti G. CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why? Purinergic Signal. 2006;2:391–398. doi: 10.1007/s11302-005-5907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grinthal A, Guidotti G. Transmembrane domains confer different substrate specificities and adenosine diphosphate hydrolysis mechanisms on CD39, CD39L1, and chimeras. Biochemistry. 2002;41:1947–1956. doi: 10.1021/bi015563h. [DOI] [PubMed] [Google Scholar]
- 61.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 62.Laura Airas JN, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gennady Yegutkin PB, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Brit J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heuts DP, Weissenborn MJ, Olkhov RV, Shaw AM, Gummadova J, Levy C, Scrutton NS. Crystal structure of a soluble form of human CD73 with ecto-5′-nucleotidase activity. Chembiochem. 2012;13:2384–2391. doi: 10.1002/cbic.201200426. [DOI] [PubMed] [Google Scholar]
- 65.Knapp K, Zebisch M, Pippel J, El-Tayeb A, Muller CE, Strater N. Crystal structure of the human ecto-5′-nucleotidase (CD73): insights into the regulation of purinergic signaling. Structure. 2012;20:2161–2173. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Zhong X, Malhotra R, Woodruff R, Guidotti G. Mammalian plasma membrane ecto-nucleoside triphosphate diphosphohydrolase 1, CD39, is not active intracellularly. The N-glycosylation state of CD39 correlates with surface activity and localization. J Biol Chem. 2001;276:41518–41525. doi: 10.1074/jbc.M104415200. [DOI] [PubMed] [Google Scholar]
- 67.Faas MM, Saez T, de Vos P. Extracellular ATP and adenosine: the Yin and Yang in immune responses? Mol Asp Med. 2017;55:9–19. doi: 10.1016/j.mam.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohta ASM. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 70.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vecchio EA, Tan CY, Gregory KJ, Christopoulos A, White PJ, May LT. Ligand-independent adenosine A2B receptor constitutive activity as a promoter of prostate cancer cell proliferation. J Pharmacol Exp Ther. 2016;357:36–44. doi: 10.1124/jpet.115.230003. [DOI] [PubMed] [Google Scholar]
- 72.Cronstein BNLR, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 73.Butler M, Sanmugalingam D, Burton VJ, Wilson T, Pearson R, Watson RP, Smith P, Parkinson SJ. Impairment of adenosine A3 receptor activity disrupts neutrophil migratory capacity and impacts innate immune function in vivo. Eur J Immunol. 2012;42:3358–3368. doi: 10.1002/eji.201242655. [DOI] [PubMed] [Google Scholar]
- 74.Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, Cochaud S, Laprevotte E, Funck-Brentano E, Hemon P, Gros L, Bec N, Larroque C, Alberici G, Bensussan A, Eliaou JF. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3:254–265. doi: 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 75.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 77.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 78.Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression. Oncotarget. 2016;7:64274–64288. doi: 10.18632/oncotarget.11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou X, Zhi X, Zhou P, Chen S, Zhao F, Shao Z, Ou Z, Yin L. Effects of ecto-5′-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 80.Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol. 2013;191:4165–4173. doi: 10.4049/jimmunol.1301274. [DOI] [PubMed] [Google Scholar]
- 81.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 82.Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, Vergani D, Longhi MS. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–1015. doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ngiow SF, Young A, Blake SJ, Hill GR, Yagita H, Teng MW, Korman AJ, Smyth MJ. Agonistic CD40 mAb-driven IL12 reverses resistance to anti-PD1 in a T-cell-rich tumor. Cancer Res. 2016;76:6266–6277. doi: 10.1158/0008-5472.CAN-16-2141. [DOI] [PubMed] [Google Scholar]
- 87.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells. Immunol Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- 88.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, Stritesky GL, Malhotra D, Pauken KE, Linehan JL, O’Sullivan MG, Fife BT, Hogquist KA, Jenkins MK, Mueller DL. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol. 2016;17:304–314. doi: 10.1038/ni.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K (2016) Two FOXP3 + CD4 + T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 22:679–684 [DOI] [PubMed]
- 90.Gourdin N, Bossennec M, Rodriguez C, Vigano S, Machon C, Jandus C, Bauche D, Faget J, Durand I, Chopin N, Tredan O, Marie JC, Dubois B, Guitton J, Romero P, Caux C, Menetrier-Caux C (2018) Autocrine adenosine regulates tumor polyfunctional CD73+CD4+ effector T cells devoid of immune checkpoints. Cancer Res 78(13):3604–3618 [DOI] [PubMed]
- 91.Michael Romio BR, Bongardt S, Hüls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol Cell Physiol. 2011;301:530–539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 92.Figueiro F, Muller L, Funk S, Jackson EK, Battastini AM, Whiteside TL. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg) Oncoimmunology. 2016;5:e1082703. doi: 10.1080/2162402X.2015.1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol. 2014;193:5904–5913. doi: 10.4049/jimmunol.1400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flores-Santibanez F, Fernandez D, Meza D, Tejon G, Vargas L, Varela-Nallar L, Arredondo S, Guixe V, Rosemblatt M, Bono MR, Sauma D. CD73-mediated adenosine production promotes stem cell-like properties in mouse Tc17 cells. Immunology. 2015;146:582–594. doi: 10.1111/imm.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bono MR, Fernandez D, Flores-Santibanez F, Rosemblatt M, Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 96.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 97.Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res. 2014;74:7239–7249. doi: 10.1158/0008-5472.CAN-13-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123:594–595. doi: 10.1182/blood-2013-09-524827. [DOI] [PubMed] [Google Scholar]
- 99.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morello S, Sorrentino R, Montinaro A, Luciano A, Maiolino P, Ngkelo A, Arra C, Adcock IM, Pinto A. NK1.1+ cells and CD8+ T cells mediate the antitumor activity of Cl-IB-MECA in a mouse melanoma model. Neoplasia. 2011;13:365–IN320. doi: 10.1593/neo.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, Degli-Esposti MA, Vivier E, Waddell N, Linden J, Huntington ND, Souza-Fonseca-Guimaraes F, Smyth MJ. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 102.Hyman MC, Petrovic-Djergovic D, Visovatti SH, Liao H, Yanamadala S, Bouis D, Su EJ, Lawrence DA, Broekman MJ, Marcus AJ, Pinsky DJ. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zanin RF, Braganhol E, Bergamin LS, Campesato LF, Filho AZ, Moreira JC, Morrone FB, Sevigny J, Schetinger MR, de Souza Wyse AT, Battastini AM. Differential macrophage activation alters the expression profile of NTPDase and ecto-5′-nucleotidase. PLoS One. 2012;7:e31205. doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ponce NE, Sanmarco LM, Eberhardt N, Garcia MC, Rivarola HW, Cano RC, Aoki MP. CD73 inhibition shifts cardiac macrophage polarization toward a microbicidal phenotype and ameliorates the outcome of experimental Chagas cardiomyopathy. J Immunol. 2016;197:814–823. doi: 10.4049/jimmunol.1600371. [DOI] [PubMed] [Google Scholar]
- 105.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, Jalkanen S, Salmi M. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 106.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, Virag L, Leibovich SJ, Hasko G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J, Hausler SFM. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages—a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. doi: 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morello S, Ito K, Yamamura S, Lee KY, Jazrawi E, DeSouza P, Barnes P, Cicala C, Adcock IM (2006) IL-1 beta and TNF-alpha regulation of the adenosine receptor (A2A) expression: differential requirement for NF-kappa B binding to the proximal promoter. J Immunol 177:7173–7183 [DOI] [PubMed]
- 113.Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106:10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chavez-Valdez R, Ahlawat R, Wills-Karp M, Gauda EB. Mechanisms of modulation of cytokine release by human cord blood monocytes exposed to high concentrations of caffeine. Pediatr Res. 2016;80:101–109. doi: 10.1038/pr.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, Li F, Chen X, Ping Y, Wang D, Gao Q, He Q, Huang L, Li H, Huang J, Zhao X, Xue W, Sun Z, Lu J, Yu JJ, Zhao J, Zhang B, Zhang Y. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78:1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A. 2018;115:E1239–E1248. doi: 10.1073/pnas.1718197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wink MR, Tamajusuku ASK, Braganhol E, Casali EA, Barreto-Chaves MLM, Sarkis JJF, Battastini AMO. Thyroid hormone upregulates ecto-5′-nucleotidase/CD73 in C6 rat glioma cells. Mol Cell Endocrinol. 2003;205:107–114. doi: 10.1016/s0303-7207(03)00197-7. [DOI] [PubMed] [Google Scholar]
- 119.Tamajusuku AS, Carrillo-Sepulveda MA, Braganhol E, Wink MR, Sarkis JJ, Barreto-Chaves ML, Battastini AM. Activity and expression of ecto-5′-nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Mol Cell Biochem. 2006;289:65–72. doi: 10.1007/s11010-006-9148-0. [DOI] [PubMed] [Google Scholar]
- 120.Bastomsky C, Zakarija M, Mckenzie J. Thyroid hydrolysis of cyclic amp as influenced by thyroid gland activity. Biochim Biophys Acta. 1971;230:286. doi: 10.1016/0304-4165(71)90215-7. [DOI] [PubMed] [Google Scholar]
- 121.Murphy PS, Wang J, Bhagwat SP, Munger JC, Janssen WJ, Wright TW, Elliott MR. CD73 regulates anti-inflammatory signaling between apoptotic cells and endotoxin-conditioned tissue macrophages. Cell Death Differ. 2017;24:559–570. doi: 10.1038/cdd.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 123.Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, Hammond SA, Rothstein R, Rios-Doria J, Poon E, Holoweckyj N, Durham NM, Leow CC, Diedrich G, Damschroder M, Herbst R, Hollingsworth RE, Sachsenmeier KF. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5:e1208875. doi: 10.1080/2162402X.2016.1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raffaella Iannone LM, Maiolino P, Pinto A, Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am J Cancer Res. 2014;4:172–181. [PMC free article] [PubMed] [Google Scholar]
- 125.Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother. 2012;61:917–926. doi: 10.1007/s00262-011-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–IN1410. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 128.Long Wang JF, Thompson LF, Yi Z, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Investig. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, Davenport AJ, John LB, Mardiana S, Slaney CY, Johnstone RW, Trapani JA, Stagg J, Loi S, Kats L, Gyorki D, Kershaw MH, Darcy PK. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–941. doi: 10.1172/JCI89455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim DG, Bynoe MS. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J Clin Invest. 2016;126:1717–1733. doi: 10.1172/JCI76207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao ZW, Dong K, Zhang HZ. The roles of CD73 in cancer. Biomed Res Int. 2014;2014:460654. doi: 10.1155/2014/460654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology. 2008;134:1116–1126. doi: 10.1053/j.gastro.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 134.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Investig. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 136.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 137.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73–deficient mice. J Clin Investig. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Annika Ålgars MK, Yegutkin GG, Stoitzner P, Niemelä J, Salmi M, Jalkanen S. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood. 2011;117:4387–4393. doi: 10.1182/blood-2010-11-321646. [DOI] [PubMed] [Google Scholar]
- 140.Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S, Niemela J, Airas L, Bynoe MS. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 143.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A(2B) receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 145.Lim SB, Banerjee A, Onyuksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release. 2012;163:34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Ibrahim BM, Park S, Han B, Yeo Y. A strategy to deliver genes to cystic fibrosis lungs: a battle with environment. J Control Release. 2011;155:289–295. doi: 10.1016/j.jconrel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 147.Omid C, Farokhzad RL. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 148.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 149.Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv. 2014;32:778–788. doi: 10.1016/j.biotechadv.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 151.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 152.Di Bei JM, Youan B-BC. Engineering nanomedicines for improved melanoma therapy: progress and promises. Nanomedicine. 2010;5:1385–1399. doi: 10.2217/nnm.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830–3852. doi: 10.1039/c6cs00592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J, Lu Z, Yeung BZ, Wientjes MG, Cole DJ, Au JL. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors. J Control Release. 2014;178:79–85. doi: 10.1016/j.jconrel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jadidi-Niaragh F, Atyabi F, Rastegari A, Kheshtchin N, Arab S, Hassannia H, Ajami M, Mirsanei Z, Habibi S, Masoumi F, Noorbakhsh F, Shokri F, Hadjati J. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J Control Release. 2017;246:46–59. doi: 10.1016/j.jconrel.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 158.Zhang L, Radovic-Moreno AF, Alexis F, Gu FX, Basto PA, Bagalkot V, Jon S, Langer RS, Farokhzad OC. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. ChemMedChem. 2007;2:1268–1271. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 159.Jack C-M, Hu LZ. Therapeutic nanoparticles to combat cancer drug resistance. Curr Drug Metab. 2009;10:836–841. doi: 10.2174/138920009790274540. [DOI] [PubMed] [Google Scholar]
- 160.Li J, Wang Y, Zhu Y, Oupicky D. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J Control Release. 2013;172:589–600. doi: 10.1016/j.jconrel.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Braganhol E, Tamajusuku AS, Bernardi A, Wink MR, Battastini AM. Ecto-5′-nucleotidase/CD73 inhibition by quercetin in the human U138MG glioma cell line. Biochim Biophys Acta. 2007;1770:1352–1359. doi: 10.1016/j.bbagen.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 163.Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets. 2014;18:863–881. doi: 10.1517/14728222.2014.915315. [DOI] [PubMed] [Google Scholar]
- 164.Iqbal J, Saeed A, Raza R, Matin A, Hameed A, Furtmann N, Lecka J, Sevigny J, Bajorath J. Identification of sulfonic acids as efficient ecto-5′-nucleotidase inhibitors. Eur J Med Chem. 2013;70:685–691. doi: 10.1016/j.ejmech.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 165.Rafal Sadej JS, Andrzej C, Skladanowski Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 166.Sadej R, Inai K, Rajfur Z, Ostapkowicz A, Kohler J, Skladanowski AC, Mitchell BS, Spychala J. Tenascin C interacts with ecto-5′-nucleotidase (eN) and regulates adenosine generation in cancer cells. Biochim Biophys Acta. 2008;1782:35–40. doi: 10.1016/j.bbadis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 167.Thomson LF, Ruedi JM, Glass A, Moldenhauer G, Moller P, Low MG, Klemens MR, Massaia M, Lucas AH. Production and characterization of monoclonal antibodies to the glycosyl phosphatidylinositol-anchored lymphocyte differentiation antigen ecto-5′-nucleotidase (CD73) HLA. 1990;35:9–19. doi: 10.1111/j.1399-0039.1990.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 168.L Airas MS, Jalkanen S. Lymphocyte-vascular adhesion protein-2 is a novel 70-KDA molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol. 1993;151:4228–4238. [PubMed] [Google Scholar]
- 169.Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 170.Zhan C, Li C, Wei X, Lu W, Lu W. Toxins and derivatives in molecular pharmaceutics: drug delivery and targeted therapy. Adv Drug Deliv Rev. 2015;90:101–118. doi: 10.1016/j.addr.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 171.Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–39288. doi: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hajiahmadi S, Panjehpour M, Aghaei M, Shabani M. Activation of A2b adenosine receptor regulates ovarian cancer cell growth: involvement of Bax/Bcl-2 and caspase-3. Biochem Cell Biol. 2015;93:321–329. doi: 10.1139/bcb-2014-0117. [DOI] [PubMed] [Google Scholar]
- 173.Long JS, Schoonen PM, Graczyk D, O’Prey J, Ryan KM. p73 engages A2B receptor signalling to prime cancer cells to chemotherapy-induced death. Oncogene. 2015;34:5152–5162. doi: 10.1038/onc.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wei Q, Costanzi S, Balasubramanian R, Gao ZG, Jacobson KA. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013;9:271–280. doi: 10.1007/s11302-012-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu TZ, Wang X, Bai YF, Liao HZ, Qiu SC, Yang YQ, Yan XH, Chen J, Guo HB, Zhang SZ. The HIF-2alpha dependent induction of PAP and adenosine synthesis regulates glioblastoma stem cell function through the A2B adenosine receptor. Int J Biochem Cell Biol. 2014;49:8–16. doi: 10.1016/j.biocel.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 176.Abousamra NK, Salah El-Din M, Hamza Elzahaf E, Esmael ME (2015) Ectonucleoside triphosphate diphosphohydrolase-1 (E-NTPDase1/CD39) as a new prognostic marker in chronic lymphocytic leukemia. Leuk Lymphoma 56:113–119 [DOI] [PubMed]
- 177.Cai XY, Wang XF, Li J, Dong JN, Liu JQ, Li NP, Yun B, Xia RL (2015) Overexpression of CD39 and high tumoral CD39(+)/CD8(+) ratio are associated with adverse prognosis in resectable gastric cancer. Int J Clin Exp Pathol 8:14757–14764 [PMC free article] [PubMed]
- 178.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G (2016) Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends in Cancer 2(2):95–109 [DOI] [PMC free article] [PubMed]
- 179.Wang H, Lee S, Nigro CL, Lattanzio L, Merlano M, Monteverde M, Matin R, Purdie K, Mladkova N, Bergamaschi D, Harwood C, Syed N, Szlosarek P, Briasoulis E, McHugh A, Thompson A, Evans A, Leigh I, Fleming C, Inman GJ, Hatzimichael E, Proby C, Crook T (2012) NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer 106:1446–1452 [DOI] [PMC free article] [PubMed]
- 180.Zhang B, Song B, Wang X, Chang XS, Pang T, Zhang X, Yin K, Fang GE (2015) The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol 36:5459–5466 [DOI] [PubMed]
- 181.Liu N, Fang XD, Vadis Q (2012) CD73 as a novel prognostic biomarker for human colorectal cancer. J Surg Oncol 106:918–919 [DOI] [PubMed]
- 182.Xiong L, Wen Y, Miao X, Yang Z (2014) NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res 355:365–374 [DOI] [PMC free article] [PubMed]
- 183.Ren ZH, Yuan YX, Ji T, Zhang CP (2016) CD73 as a novel marker for poor prognosis of oral squamous cell carcinoma. Oncol Lett 12:556–562 [DOI] [PMC free article] [PubMed]
- 184.Yang Q, Du J, Zu L (2013) Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res 19:811–814 [DOI] [PubMed]