Abstract
As chronic lymphocytic leukemia (CLL) has a variable disease course, novel prognostic markers and risk assessment models are being developed in order to identify high-risk patients who may need early treatment. The two tumor necrosis factor family proteins BAFF and APRIL and their receptors BAFF-R, TACI and BCMA are considered to play a critical role in the survival of normal B cells. In order to highlight the pathophysiological role of this complicated biological network, we aimed to analyze the potential prognostic effects of BAFF, APRIL, TACI and BCMA in CLL patients. We investigated the prognostic impact of serum BCMA, TACI, BAFF and APRIL levels in 129 newly diagnosed CLL patients [median age: 64 (39–88) years; male/female: 85/44]. Serum BAFF, TACI and BCMA levels were significantly lower in the patient group compared to the control group (p < 0.001), while serum APRIL level did not differ significantly between two groups (p > 0.05). Serum BCMA [(p = 0.029; r = 0.208)] and TACI levels [(p = 0.011; r = 0.241)] were positively correlated with serum free light chain ratio. Serum BAFF [(p = 0.008; r = − 0.236)] and BCMA [(p = 0.042; r = − 0.183)] levels were negatively correlated with Rai stage. Overall survival (OS) was relatively better in patients with low serum BAFF levels [60 (1–187) months vs 39.5 (0–256) months; p = 0.063]. Probability of OS was higher in patients with low BAFF levels when compared to patients with normal levels, without statistical significance (53.6% vs 23.6%; p > 0.05). Large prospective studies are needed to validate the prognostic role of this essential biological pathway in CLL.
Keywords: Chronic lymphocytic leukemia, BAFF, APRIL, BCMA, TACI, Prognosis
Introduction
Chronic lymphocytic leukemia (CLL), which is considered to be one of the most common leukemias in Western countries, has a variable clinical course based on several prognostic parameters. Novel prognostic markers and risk assessment models are being developed in order to identify high-risk patients who may need early treatment [1–3]. Clinical stage, expression of zeta chain related protein 70 (ZAP70) and CD38, immunoglobulin variable region heavy chain (IGHV) mutational status and several cytogenetic/molecular abnormalities, particularly del11q12, del17p, del13q14 and trisomy 12, are the most pronounced adverse prognostic factors [2, 4].
B cell activator factor (BAFF; B-Lys; TNFSF13B), a tumor necrosis factor (TNF) family protein, has been shown to play a critical role in B cell survival, proliferation and differentiation. BAFF activates B cells through a group of receptors which are defined as B-cell maturation antigen (BCMA; TNFRSF17), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI; TNFRSF13B) and BAFF receptor (BAFF-R; TNFRSF13C). BAFF-R is mainly expressed on B cells and BAFF/BAFF-R signaling plays a central role in the survival and growth of normal and neoplastic B lymphocytes [3, 5]. High serum levels of BAFF were found to be associated with poor prognosis in follicular lymphoma (FL) and multiple myeloma (MM) patients [6–8]. On the other hand, there is substantial data regarding the potential role of BCMA expression in lymphoma, MM, autoimmune and infectious diseases and TACI expression in immune deficiency syndromes [9–14]. Furthermore, APRIL, a proliferation-inducing ligand, also functions along with this cytokine network. APRIL and BAFF support CLL cell survival through the up-regulation of CD40 ligands. Patients with lower APRIL/BAFF expression were shown to have longer survival when compared to patients who had high expression of APRIL/BAFF [1]. Activation of BAFF/APRIL receptors was reported to be associated with cell survival and growth in B-cell lymphomas [5, 15–19]. High APRIL expression was also indicated to be a predictor of aggressive disease in T-cell leukemia/lymphoma 1A CLL mice models [20]. Solid tumors including ovarian, bladder, head and neck, lung, colorectal, renal cell and pancreas cancer represented a higher expression of APRIL which was associated with worse prognosis [21–27].
In order to highlight the pathophysiological role of this complicated biological network, we aimed to analyze the potential prognostic effects of BAFF, APRIL, TACI and BCMA in CLL patients [16, 18, 28–36].
Materials and Methods
A total of 129 newly diagnosed CLL patients [median age: 64 (39–88) years; male/female: 85/44] with a median follow-up of 48 (0–256) months and 26 healthy volunteers were included in this study.
Serum BAFF (Human BAFF ELISA Kit 96 wells Boster/USA-EK0063), TACI (Human TNFRSF13B/TACI ELISA Kit 96 wells Boster/USA-EK0986), BCMA (Human TNFRSF17/BCMA ELISA Kit 96 wells Boster/USA-EK0661) and APRIL (Human TNFSF13/APRIL ELISA Kit 96 wells Boster/USA-EK0921) levels were measured at diagnosis by Enzyme Linked Immunosorbent Assay (ELISA) method.
Diagnosis of CLL was based on the updated criteria of International Workshop on CLL (IWCLL) [2]. Rai and modified Rai staging systems were used for clinical staging and risk assessment. Expressions of ZAP70 and CD38, with a threshold of 20% for positivity, were quantified by flow cytometry. Cytogenetic/molecular risk profile, including del11q, del13q, trisomy 12 and del17p, was determined by intephase fluorescence in situ hybridization (FISH) on peripheral blood. Clinical and laboratory data was obtained from medical records of the patients.
Statistical Analysis
Continuous variables were compared using Mann–Whitney U and Kruskal–Wallis tests while Chi square test was used for categorical variables. Correlation analysis was performed using Pearson and Spearman tests. Kaplan–Meier test was used for survival analysis. Risk factors for survival were determined by Cox regression and log rank tests. SPSS 16.0 (SPSS Inc, Chicago, IL, USA) programme was used for statistical analysis and p < 0.05 was considered as statistically significant.
The study was approved by the Ethical Commitee of Gazi Medical School.
Results
Patient and Disease Characteristics
A total of 124 patients were staged clinically at diagnosis according to Rai and modified Rai systems. Sixty patients (48.4%) were evaluated as stage 0, 25 patients (20.2%) stage I, 26 patients (21%) stage II, 7 patients (5.6%) stage III and 6 patients (4.8%) stage IV disease according to Rai staging system. A total of 60 patients (48.4%) were low-risk, 51 patients (41.2%) intermediate-risk and 13 patients (10.4%) high-risk according to modified Rai risk stratification. Cytogenetic/molecular risk assessment was performed by FISH in 91 patients. A total of 28 patients (30.8%) had low-risk, 13 patients (14.3%) intermediate-risk and 21 patients (23.1%) high-risk profile. Patient and disease characteristics are represented in Table 1.
Table 1.
Patient and disease characteristics; demographics, baseline laboratory parameters and CLL risk profile
| Age (year) [median (range)] | 64 (39–88) |
| Gender (male/female) (n) | 85/44 |
| Performance status (Eastern Cooperative Oncology Group) [median (range)] | 1 (0–2) |
| White blood cell (/µL) [median (range)] | 25,000 (3600–404,000) |
| Hemoglobin (g/dL) [median (range)] | 13.5 (6.3–17.9) |
| Platelet (/μL) [median (range)] | 188,100 (7440–429,000) |
| Lymphocyte (%) [median (range)] | 76.5 (23.6–97.4) |
| Stage (Rai) (n = 124) [n (%)] | |
| Rai 0 | 60 (48.4%) |
| Rai I | 25 (20.2%) |
| Rai II | 26 (21%) |
| Rai III | 7 (5.6%) |
| Rai IV | 6 (4.8%) |
| Stage (Modified Rai) (n = 124) [n (%)] | |
| Low risk | 60 (48.4%) |
| Intermediate risk | 51 (41.1%) |
| High risk | 13 (10.5%) |
| Immunoglobulin G (mg/dL) [median (range)] | 1030 (288–2880) |
| Immunoglobulin A (mg/dL) [median (range)] | 126 (17.6–551) |
| Immunoglobulin M (mg/dL) [median (range)] | 44.9 (4.17–600) |
| Free light chain ratio (kappa/lambda) [median (range)] | 1.395 (0.18–35) |
| Bone marrow infiltration (n = 105) [n (%)] | |
| Nodular | 33 (31.4%) |
| Diffuse | 47 (44.8%) |
| Interstitial | 25 (23.8%) |
| Concomittant autoimmune disorder (n = 125) [n (%)] | |
| None | 111 (88.8%) |
| Immune thrombocytopenia | 6 (4.8%) |
| Autoimmune hemolytic anemia | 6 (4.8%) |
| Autoimmune hemolytic anemia + thrombocytopenia | 2 (1.6%) |
| Lactate dehydrogenase (U/L) [median (range)] | 119.5 (115–679) |
| β2 microglobulin (mg/L) [median (range)] | 1.98 (0.1–17.4) |
| CD38 expression (n = 123) (%) [median (range)] | 24 (0–92) |
| ZAP70 expression (n = 104) (%) [median (range)] | 19.44 (0–92) |
| Fluorescence in situ hybridization (FISH) (n = 91) [n (%)] | |
| Negative | 29 (31.8%) |
| 13q14 deletion | 28 (30.8%) |
| Trisomy 12 | 13 (14.3%) |
| 17p13 deletion | 11 (12.1%) |
| 11q23 deletion | 10 (11%) |
| Molecular risk profile (FISH) (n = 62) [n (%)] | |
| Low risk | 28 (30.8%) |
| Intermediate risk | 13 (14.3%) |
| High risk | 21 (23.1%) |
Study Parameters
Normal levels of serum BCMA, TACI and APRIL were reported in all patients. Nevertheless, serum BAFF levels were below the normal range in a total of 40 patients, whereas 89 CLL patients represented normal levels. Besides, median serum levels of BAFF [0.08 (0.05–2.08) ng/ml vs 1.46 (0.24–2.95) ng/ml, p < 0.001], BCMA [0.16 (0.12–1.93) ng/ml vs 1.43 (0.87–3.03) ng/ml, p < 0.001] and TACI [0.14 (0.1–1.07) ng/ml vs 0.28 (0.24–0.87) ng/ml, p < 0.001] were significantly lower in the patient group compared to controls. However, median APRIL levels were not statistically different between patient and control groups [0.28 (0.2–5.44) ng/ml vs 0.26 (0.24–0.65) ng/ml, p > 0.05] (Table 2).
Table 2.
Comparative analysis of median BAFF, BCMA, TACI ve APRIL levels between patient and control groups
| Patient group | Control group | p value | |
|---|---|---|---|
| BAFF (ng/mL) | 0.08 (0.05–2.08) | 1.46 (0.24–2.95) | < 0.001 |
| BCMA (ng/mL) | 0.16 (0.12–1.93) | 1.43 (0.87–3.03) | < 0.001 |
| TACI (ng/mL) | 0.14 (0.1–1.07) | 0.28 (0.24–0.87) | < 0.001 |
| APRIL (ng/mL) | 0.28 (0.2–0.65) | 0.26 (0.24–0.65) | 0.089 |
Serum TACI levels were relatively elevated in CD38+ patients (p = 0.06), while serum BAFF levels were higher in low-risk patients based on modified Rai staging system (p = 0.059), without statistical significance. Serum levels of BAFF, BCMA, TACI and APRIL did not display a significant difference based on lactate dehydrogenase (LDH) levels, ZAP70 expression, Rai stage and cytogenetic/molecular risk profile (p > 0.05).
A positive correlation was demonstrated between BAFF and BCMA (p < 0.001; r = 0.936), TACI and BAFF (p < 0.001; r = 0.627), BAFF and APRIL (p < 0.001; r = 0.433), BCMA and TACI (p < 0.001; r = 0.734) and BCMA and APRIL (p = 0.028; r = 0.282) levels. Furthermore, there was also a positive correlation between TACI and BAFF (p = 0.009; r = 0.502) and TACI and BCMA levels (p = 0.008; r = 0.509) in healthy individuals. Serum BCMA [(p = 0.029; r = 0.208)] and TACI levels [(p = 0.011; r = 0.241)] were positively correlated with serum free light chain (FLC) ratio. Additionally, serum BAFF [(p = 0.008; r = − 0.236)] and BCMA [(p = 0.042; r = − 0.183)] levels were negatively correlated with Rai stage.
When the study cohort was divided into two subgroups based on “low” or “normal” serum BAFF levels, conventional prognostic parameters including white blood cell count, Rai stage, modified Rai stage, LDH, β2 microglobulin, CD38 and ZAP70 expression and cytogenetic/molecular risk groups were found to be similar between two groups. However, overall survival (OS) was relatively better in patients with low serum BAFF levels, without statistical significance [60 (1–187) months vs 39.5 (0–256) months; p = 0.063] (Fig. 1). Probability of OS was higher in patients with low BAFF levels when compared to patients with normal BAFF levels, without statistical significance (53.6% vs 23.6%; p > 0.05) (Fig. 2).
Fig. 1.
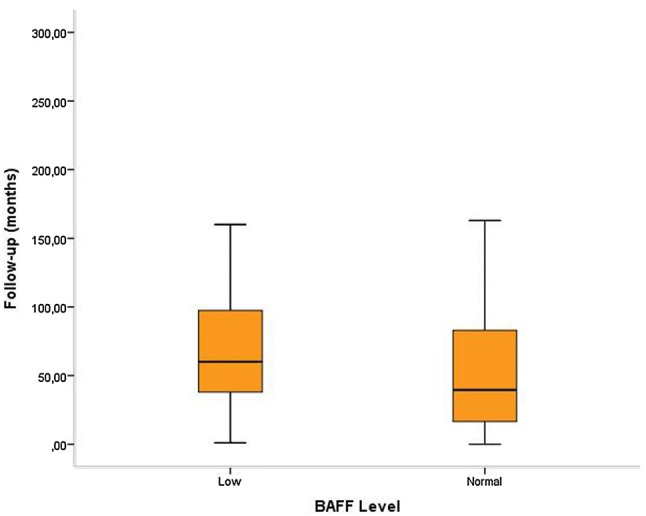
Overall survival was better in CLL patients with low serum BAFF levels [60 (1–187) months vs 39.5 (0–256) months; p = 0.063]
Fig. 2.
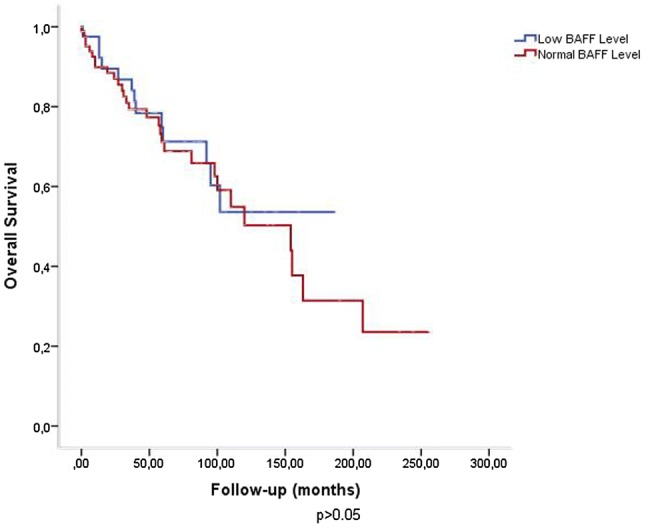
Probability of overall survival was higher in patients with low BAFF levels when compared to patients with normal levels (53.6% vs 23.6%; p > 0.05)
In univariate analysis; age (p = 0.001), Rai stage (p = 0.001), modified Rai stage (p = 0.015) and performance status (p = 0.008) were shown to have significant impact on OS. In multivariate analysis, age (p = 0.002), Rai stage (p = 0.005) ve modified Rai stage (p = 0.051) represented prognostic significance.
Discussion
The current study was planned to indicate the prognostic role of BAFF, BCMA, TACI and APRIL in the clinical course of CLL. Although serum levels of BAFF, BCMA and TACI at diagnosis were found to be significantly lower with respect to healthy controls, any significant association with classical prognostic parameters or survival was not observed.
In recent years, microenvironment has drawn attention in CLL pathophysiology and various signalling pathways were considered to play an essential role in the biological behaviour of the disease which effect the clinical course indirectly via certain key points. Chronic lymphocytic leukemia cells interact with different types of stromal cells, such as mesenchymal stromal cells, nurse like cells (NLC) and T cells, which are collectively defined as the “microenvironment”. Nurse like cells protect CLL cells from spontaneous or drug-induced apoptosis through CXCL12, BAFF and APRIL and activation of the BCR signaling cascade. By this way, BAFF and APRIL were shown to protect CLL cells from spontaneous apoptosis in vitro and prolong cell survival in vivo, whereas their cellular receptors BCMA, TACI and BAFF-R play essential roles in B cell development and microenvironmental balance [5]. Nevertheless, Andersen et al. [1] reported that BAFF and APRIL levels did not have an impact on physiological stress in relapsed/refractory CLL patients which was considered to be secondary to prior treatment load. Future attempts dealing with this issue may give rise to new treatment approaches including novel targeted therapy modalities.
There are several studies which investigate the prognostic role of these molecules in hematological malignancies, particularly in CLL. In a study by Mamara et al. [28], BAFF and TACI levels were found to be lower in 94 CLL patients, however a significant association with clinical or laboratory risk factors was not demonstrated in concordance with our results. Similar results were reported in two studies by Planelles et al. and Haiat et al. [30, 31]. In the latter, APRIL levels were not significantly different between the patient and control groups [31]. Kreuzaler et al. [36] showed significantly lower BAFF levels in CLL patients among a heterogeneous study cohort including immune deficiency syndromes and autoimmune diseases. In a study by Jasek et al. [3], single nucleotide polymorphisms (SNP) in BAFF and BAFF-R genes were indicated to be potential risk factors for CLL prognosis, however when they compared the genotype distribution of SNPs of BAFF and BAFF-R between CD38+ and CD38− and ZAP70+ and ZAP70− CLL patients, they did not find any significant difference in their genetic profiles.
Bojarska-Junak et al. [34] found that intracellular BAFF levels were elevated in CLL patients despite low serum levels in the same population and intracellular levels were positively correlated with CD38 and ZAP70 expressions. Besides, intracellular BAFF levels represented a significant impact on survival although serum BAFF levels did not. Nevertheless, BCMA levels were found to be significantly lower in CLL patients which indicated no clear correlation with prognostic factors and survival. Diverse effects of BAFF molecule in different body compartments with distinctive microenvironmental features may be an appropriate explanation for these contradictory results. As BAFF is internalized via its receptor on the cell membrane and shows its main biological effect in the intracellular compartment, intracellular protein levels may represent a better predictor profile with respect to serum levels. A study by Cols et al. [19] which indicates an activation process of BAFF and APRIL by an enzymatic reaction induced by CD40L supports the issue.
In another study by Bojarska-Junak et al. [37], TACI levels were found to be lower in CLL patients. Furthermore, an adverse correlation was demonstrated between TACI expression and leukocyte count, Rai stage, CD38 and ZAP70 expressions. However, in the current study, we observed relatively higher TACI levels in CD38+ patients which was statistically insignificant. As CD38 is considered to be a marker of proliferation in CLL, elevated levels of TACI may indicate increased apoptosis which may be secondary to relatively higher cell proliferation in CD38+ patients [38, 39]. Lack of statistical significance may be explained by small sample size and needs further confirmation.
Although several studies have represented a higher expression of TACI in CLL patients [16, 18]. Kyrtsonis et al. [29] were the first to show elevated serum levels of TACI in the same population. They reported a significant impact of TACI levels on survival. Furthermore, serum TACI levels were positively correlated with β2-microglobulin levels, while a negative correlation was demonstrated with anemia, thrombocytopenia, Binet stage and serum FLC ratio. In contrast, TACI levels were positively correlated with serum FLC ratio in our study. As a matter of fact, the potential physiopathological connection between TACI levels and FLCs should be elucidated with future attempts.
Ferrer et al. [40] reported increased BCMA levels in CLL patients which represented a negative correlation with cytogenetic features and PFS. In concordance, an adverse relationship was demonstrated between BAFF and BCMA levels and clinical stage in our study. Nevertheless, BAFF levels were higher in low-risk patients according to modified Rai which also requires further confirmation based on statistical insignificance.
The limitations of the current study may include small size of the study population and sample preference for analysis. Intracellular concentrations of the molecules rather than serum would be more appropriate for the assessment of their impact on prognosis and survival. As BAFF has been demonstrated to play an essential role in CLL cell survival, the contradictory results of the presented study which represents low serum levels of BAFF in CLL patients may be explained by its wide and distinct spectrum of activity in different biological compartments. Therefore, analysis of intracellular BAFF concentration may be more feasible to evaluate the biological function of this molecule. This strict dependence to microenvironmental conditions emphasizes the significance of molecular relationships in this network in order to sustain CLL cell survival. Statistically insignificant association of low BAFF levels with better OS, therefore, needs to be confirmed with intracellular levels in larger populations.
Based on its heterogeneous biological and clinical landscape, prognosis of CLL is highly variable which requires more standardized risk assessment strategies. In spite of intensive efforts for the discovery of new clinical and molecular predictors, treatment decision still remains to be an enigma from different points of view. This study aimed to comprehend the complicated biological steps of B cell development in order to evoke targeted therapy modalities which may improve survival and quality of life, particularly in high-risk CLL patients. Larger prospective studies, which mainly focus on the biologically active form of these molecules, are essential to acquire a proper microenvironmental aspect for future projects.
Acknowledgements
None.
Author’s Contributions
İBM: Hypothesis and design, data collection, manuscript writing; ZAY: Hypothesis and design, manuscript writing, statistical analysis, manuscript review; SG: Laboratory analysis, method design; ZNÖ: Data collection, manuscript review; MY: Data collection, manuscript review.
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and Animal Rights
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Andersen BL, Goyal NG, Weiss DM, Westbrook TD, Maddocks KJ, Byrd JC, Johnson AJ. Cells, cytokines, chemokines, and cancer stress: a biobehavioral study of patients with chronic lymphocytic leukemia. Cancer. 2018;124(15):3240–3248. doi: 10.1002/cncr.31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallek M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90(5):446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 3.Jasek M, Bojarska-Junak A, Wagner M, Sobczyński M, Wołowiec D, Roliński J, Karabon L, Kuśnierczyk P. Association of variants in BAFF (rs9514828 and rs1041569) and BAFF-R (rs61756766) genes with the risk of chronic lymphocytic leukemia. Tumour Biol. 2016;37(10):13617–13626. doi: 10.1007/s13277-016-5182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer P, Hallek M. Prognostic factors in chronic lymphocytic leukemia-what do we need to know? Nat Rev Clin Oncol. 2011;8(1):38–47. doi: 10.1038/nrclinonc.2010.167. [DOI] [PubMed] [Google Scholar]
- 5.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti AM, Kenny CH, Khalil AM, Pan Q, Ralph KL, Ritchie J, Venkataramani S, Presky DH, DeWire SM, Brodeur SR. Unexpected potency differences between B-cell-activating factor (BAFF) antagonist antibodies against various forms of BAFF: trimer, 60-Mer, and membrane-bound. J Pharmacol Exp Ther. 2016;359(1):37–44. doi: 10.1124/jpet.116.236075. [DOI] [PubMed] [Google Scholar]
- 7.Li YJ, Li ZM, Xia ZJ, Li S, Xia Y, Huang HQ, Huang JJ, Yi PY, Jiang WQ. High APRIL but not BAFF serum levels are associated with poor outcome in patients with follicular lymphoma. Ann Hematol. 2015;94(1):79–88. doi: 10.1007/s00277-014-2173-2. [DOI] [PubMed] [Google Scholar]
- 8.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C, Moine P, Rossi JF, Klein B, Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103(8):3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu A, Xu W, He B, Dillon SR, Gross JA, Sievers E, Qiao X, Santini P, Hyjek E, Lee JW, Cesarman E, Chadburn A, Knowles DM, Cerutti A. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109(2):729–739. doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oden F, Marino SF, Brand J, Scheu S, Kriegel C, Olal D, Takvorian A, Westermann J, Yilmaz B, Hinz M, Daumke O, Höpken UE, Müller G, Lipp M. Potent anti-tumor response by targeting B cell maturation antigen (BCMA) in a mouse model of multiple myeloma. Mol Oncol. 2015;9(7):1348–1358. doi: 10.1016/j.molonc.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A, Oregon-Romero E, Machado-Contreras JR, Muñoz-Valle JF, Orozco-López M, Marín-Rosales M, Palafox-Sánchez CA. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus. 2015;25(6):582–592. doi: 10.1177/0961203315608254. [DOI] [PubMed] [Google Scholar]
- 12.Nagatani K, Itoh K, Nakajima K, Kuroki H, Katsuragawa Y, Mochizuki M, Aotsuka S, Mimori A. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis Rheum. 2007;56(11):3554–3563. doi: 10.1002/art.22929. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji S, Cortesão C, Bram RJ, Platt JL, Cascalho M. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. 2011;118(22):5832–5839. doi: 10.1182/blood-2011-05-353961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou H, Yan Z, Zhang M, Xu X. APRIL, BCMA and TACI proteins are abnormally expressed in non-small cell lung cancer. Oncol Lett. 2016;12(5):3351–3355. doi: 10.3892/ol.2016.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172(5):3268–3279. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 16.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Simonin PY, Feldblum S, Kolb JP. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 17.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, Zvaifler NJ, Kipps TJ. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106(3):1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo T, Nishio M, Enzler T, Cottam HB, Fukuda T, James DF, Karin M, Kipps TJ. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109(2):703–710. doi: 10.1182/blood-2006-06-027755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cols M, Barra CM, He B, Puga I, Xu W, Chiu A, Tam W, Knowles DM, Dillon SR, Leonard JP, Furman RR, Chen K, Cerutti A. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. J Immunol. 2012;188(12):6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lascano V, Guadagnoli M, Schot JG, Luijks DM, Guikema JE, Cameron K, Hahne M, Pals S, Slinger E, Kipps TJ, van Oers MH, Eldering E, Medema JP, Kater AP. Chronic lymphocytic leukemia disease progression is accelerated by APRIL-TACI interaction in the TCL1 transgenic mouse model. Blood. 2013;122(24):3960–3963. doi: 10.1182/blood-2013-04-497693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mhawech-Fauceglia P, Allal A, Odunsi K, Andrews C, Herrmann FR, Huard B. Role of the tumour necrosis family ligand APRIL in solid tumour development: retrospective studies in bladder, ovarian and head and neck carcinomas. Eur J Cancer. 2008;44(15):2097–2100. doi: 10.1016/j.ejca.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188(6):1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Park JW, Suh JH, Moon KC. High expression of APRIL correlates with poor prognosis in clear cell renal cell carcinoma. Pathol Res Pract. 2015;211(11):824–828. doi: 10.1016/j.prp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Qian Z, Qingshan C, Chun J, Huijun Z, Feng L, Qiang W, Qiang X, Min Z. High expression of TNFSF13 in tumor cells and fibroblasts is associated with poor prognosis in non–small cell lung cancer. Am J Clin Pathol. 2014;141(2):226–233. doi: 10.1309/AJCP4JP8BZOMHEAW. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Wang F, Ding W, Wang J, Jing R, Li H, Wang X, Wang Y, Ju S, Wang H. APRIL induces tumorigenesis and metastasis of colorectal cancer cells via activation of the PI3K/Akt pathway. PLoS ONE. 2013;8(1):e55298. doi: 10.1371/journal.pone.0055298. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.García-Castro A, Zonca M, Florindo-Pinheiro D, Carvalho-Pinto CE, Cordero A, Gutiérrez del Fernando B, García-Grande A, Mañes S, Hahne M, González-Suárez E, Planelles L. APRIL promotes breast tumor growth and metastasis and is associated with aggressive basal breast cancer. Carcinogenesis. 2015;36(5):574–584. doi: 10.1093/carcin/bgv020. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Chen L, Ding W, Wang G, Wu Y, Wang J, Luo L, Cong H, Wang Y, Ju S, Shao J, Wang H. Serum APRIL a potential tumor marker in pancreatic cancer. Clin Chem Lab Med. 2011;49(10):1715–1719. doi: 10.1515/CCLM.2011.608. [DOI] [PubMed] [Google Scholar]
- 28.Mamara A, Germenis AE, Kompoti M, Palassopoulou M, Mandala E, Banti A, Giannakoulas N, Speletas M (2015) TACI expression and signaling in chronic lymphocytic leukemia. J Immunol Res 478753 [DOI] [PMC free article] [PubMed]
- 29.Kyrtsonis MC, Sarris K, Koulieris E, Maltezas D, Nikolaou E, Angelopoulou MK, Bartzis V, Tzenou T, Dimou M, Siakandaris MP, Viniou NA, Sachanas S, Kalpadakis C, Sfikakis PP, Pangalis GA, Panayiotidis P (2014) Serum soluble TACI, a BLyS receptor is a powerful prognostic marker of outcome in chronic lymphocytic leukemia. Biomed Res Int 159632 [DOI] [PMC free article] [PubMed]
- 30.Planelles L, Castillo-Gutiérrez S, Medema JP, Morales-Luque A, Merle-Béral H, Hahne M. APRIL but not BLyS serum levels are increased in chronic lymphocytic leukemia: prognostic relevance of APRIL for survival. Haematologica. 2017;92(9):1284–1285. doi: 10.3324/haematol.10317. [DOI] [PubMed] [Google Scholar]
- 31.Haiat S, Billard C, Quiney C, Ajchenbaum-Cymbalista F, Kolb JP. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118(3):281–292. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molica S, Digiesi G, Battaglia C, Cutrona G, Antenucci A, Molica M, Giannarelli D, Sperduti I, Gentile M, Morabito F, Ferrarini M. BAFF serum level predicts time to first treatment in early chronic lymphocytic leukemia. Eur J Haematol. 2010;85(4):314–320. doi: 10.1111/j.1600-0609.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 33.Saulep-Easton D, Vincent FB, Quah PS, Wei A, Ting SB, Croce CM, Tam C, Mackay F. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia. 2016;30(1):163–172. doi: 10.1038/leu.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bojarska-Junak A, Hus I, Chocholska S, Wasik-Szczepanek E, Sieklucka M, Dmoszyńska A, Roliński J. BAFF and APRIL expression in B-cell chronic lymphocytic leukemia: correlation with biological and clinical features. Leuk Res. 2009;33(10):1319–1327. doi: 10.1016/j.leukres.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer G, Hodgson K, Pereira A, Juan M, Elena M, Colomer D, Roué G, Aymerich M, Baumann T, Montserrat E, Moreno C. Combined analysis of levels of serum B-cell activating factor and a proliferation-inducing ligand as predictor of disease progression in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52(11):2064–2068. doi: 10.3109/10428194.2011.591008. [DOI] [PubMed] [Google Scholar]
- 36.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, Plebani A, Lougaris V, Quinti I, Thon V, Litzman J, Schlesier M, Warnatz K, Thiel J, Rolink AG, Eibel H. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188(1):497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 37.Bojarska-Junak A, Hus I, Sieklucka M, Surdacka A, Kusz ML, Wasik-Szczepanek E, Dmoszynska A, Rolinski J. The role of TACI expression in chronic lymphocytic leukemia. Cent Eur J Immunol. 2011;36(1):46–50. [Google Scholar]
- 38.Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood. 2011;118(13):3470–3478. doi: 10.1182/blood-2011-06-275610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgler S. Role of CD38 expression in diagnosis and pathogenesis of chronic lymphocytic leukemia and its potential as therapeutic target. Crit Rev Immunol. 2015;35(5):417–432. doi: 10.1615/CritRevImmunol.v35.i5.50. [DOI] [PubMed] [Google Scholar]
- 40.Ferrer G, Bosch R, Hodgson K, Tejero R, Roué G, Colomer D, Montserrat E, Moreno C. B cell activation through CD40 and IL4R ligation modulates the response of chronic lymphocytic leukaemia cells to BAFF and APRIL. Br J Haematol. 2014;164(4):570–578. doi: 10.1111/bjh.12645. [DOI] [PubMed] [Google Scholar]


