Abstract
Purpose
This study aims to determine if intra-ovarian injection of bone marrow–derived mesenchymal stem cells (MSCs) improves or restores ovarian function in aged females.
Methods
Prospective randomized study of eight aged mares and six young mares receiving intra-ovarian injection of MSCs or vehicle. Main outcome measures were antral follicle count and serum anti-Müllerian hormone (AMH) (aged and young mares), and for aged mares, oocyte meiotic and developmental competence; gross and histological ovarian assessment; evaluation of presence of chimerism in recovered granulosa cells and in ovarian tissue samples; and gene expression in ovarian tissue as assessed by RNA sequencing.
Results
Injection of MSCs was not associated with significant changes in follicle number, oocyte recovery rate on follicle aspiration, oocyte maturation rate, or blastocyst rate after ICSI in aged mares, or in changes in follicle number in young mares. There were no significant changes in peripheral AMH concentrations, indicating a lack of effect on growing follicles. MSC donor DNA was not recovered in granulosa cells or in ovarian tissue, indicating lack of persistence of injected MSC. RNA sequencing revealed significant differences in gene expression between MSC- and vehicle-injected ovaries.
Conclusions
Intra-ovarian injection of bone marrow–derived MSCs altered gene expression but did not improve ovarian function in aged mares.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1371-6) contains supplementary material, which is available to authorized users.
Keywords: Aging, Anti-Müllerian hormone, Equids, Fertility, Follicle-stimulating hormone, Follicular development, Oocyte, Ovary, Stem cells
Introduction
Bone marrow–derived mesenchymal stem cells (MSCs) have emerged as a key element in regenerative medicine therapies in many organ systems, due to their ability to aid in repair of damaged tissue and their potential to treat degenerative disease [1–6]. The potential of MSCs to affect ovarian function has been suggested by reports showing an unexpected return of ovarian activity and fertility in chemotherapy-treated women who underwent autologous or allogeneic bone marrow transplantation as a component of their therapy [7–9]. In rats and mice administered chemotherapy to compromise the ovary, systemic or intra-ovarian injection of MSCs has repeatedly been shown to enhance ovarian function and restore fertility [10–13]. The means by which MSC injection improves ovarian function in damaged ovaries is not yet clear. While presence of transferred MSCs as oocyte-like structures within apparent immature follicles has been suggested [12], in other studies transplanted cells were not found within follicle-like structures, but did appear to persist in ovarian stromal tissue [10, 11]. Thus, it is likely that in these damaged ovaries, MSCs are affecting function via paracrine factors which help to alleviate the effects of the chemotherapeutic agents.
The success of MSC injection in restoring fertility after chemotherapy-induced damage has led to interest in applying MSC injection as a treatment for ovarian failure due to aging in women. If successful, this procedure would have a potential impact on millions of women undergoing assisted reproduction due to age-related infertility. In a case reported by Ali Farid et al. [14], intra-ovarian injection of peripheral blood mononuclear cells, which may have some stem-cell-like properties, in a 49-year-old woman was associated with an increase in anti-Müllerian hormone (AMH), pregnancy, and a live birth. In a recent report, Herraiz et al. [15] evaluated antral follicle count and AMH in poor-responder women after injection of autologous blood-derived MSCs into the ovarian artery. The authors reported that at 15 days after injection, the mean total antral follicle count of the population was significantly higher than that recorded for one baseline evaluation, whereas concentrations of AMH varied but were not significantly elevated. Little information is available on the effect of MSCs on the aging ovary in animal models. In one report in aging rats, intravenous injection of MSCs was associated with an increased number of follicles at all stages and an increase in serum AMH [16].
The horse mare offers a useful model to explore the effect of MSCs on the aging ovary, as ovarian dynamics and age-associated changes in fertility in the mare parallel those in women. In both mares and women, oocytes are maintained in meiotic arrest for decades, the cycle length is similar (22 days vs. 28 days, respectively, both with a 14-day luteal phase), only one follicle ovulates per cycle, and decreased oocyte quality has been identified in both species as the main factor in age-related infertility [17, 18]. In addition, considerable interest exists in the equine industry to establish pregnancies from older, valuable mares. Ultrasound-guided transvaginal ovarian puncture is used commonly to aspirate follicles for oocyte recovery in mares [19, 20], and this technique can be easily modified to deliver material to the ovary in the live mare without need for surgery.
To the best of our knowledge, intra-ovarian injections of MSCs have not been performed previously in a large-animal model. The objectives of our study were to determine if intra-ovarian injection of MSCs affects ovarian function or ovarian gene expression in mares experiencing ovarian failure associated with aging.
Materials and methods
Animals
Eight aged mares, 20–29 years old, that had not previously undergone follicle aspiration for oocyte recovery, and six young mares, 7–12 years old, members of the existing research herd and used previously for oocyte recovery, were housed in pens with herdmates and had ad libitum access to hay and fresh water throughout the study. Two yearling mares were used as MSC donors (donors A and B). All procedures were approved by the Texas A&M University Institutional Animal Care and Use Committee (AUP 2012-0237) and were performed using guidelines set forth by the United States Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training.
Experimental design—aged mares
The aged mares were stratified by age and divided randomly into three treatment groups. The groups were assigned to intra-ovarian injection with (1) MSCs from donor A (n = 3), (2) MSCs from donor B (n = 3), or (3) vehicle (V, vehicle injection, n = 3). The vehicle injected was the medium used for freezing of MSCs (95% serum, autologous to the mare to receive the injection, and 5% dimethyl sulfoxide). One mare in the V group was euthanized due to neoplasia of the urinary bladder before intra-ovarian injection was performed, leaving an n = 2 in the V group. The reproductive tracts of the aged mares were evaluated by ultrasonography per rectum three times weekly, with limited exceptions, starting 6 weeks before ovarian injection and continuing for 14 to 19 weeks after injection. The total number of follicles > 5 mm diameter in each ovary at each examination was determined from review of videotapes of the examinations. Once every 14 days, if five or more follicles were present, transvaginal ultrasound-guided follicle aspiration (TVA) was performed to recover oocytes. Recovered oocytes were subjected to in vitro maturation (IVM), intracytoplasmic sperm injection (ICSI), and in vitro culture for evaluation of meiotic and developmental competence.
Intra-ovarian injections were performed starting 6 weeks after the beginning of ovarian monitoring. Each mare had one ovary randomly assigned to be injected. The person performing the injections was blind as to treatment. In follicle aspirations performed after MSC injection, the aspirated fluid from each ovary was filtered separately, and the granulosa cells from both ovaries were flash-frozen for DNA microsatellite identification to detect chimerism.
The ovaries of all eight aged mares were scheduled to be removed surgically 14 to 19 weeks after intra-ovarian injection. Two weeks before ovariectomy, three MSC-injected mares were randomly selected to receive a second injection with MSCs, into the same ovary, from the alternate donor to that used in the first injection. After ovariectomy, the ovaries were examined for gross and histologic pathology, and five tissue samples from each MSC-injected ovary were separately analyzed for genomic and mitochondrial chimerism. Five additional tissue samples from each ovary were pooled for RNA extraction and sequencing.
Blood was collected from each aged mare weekly for evaluation of AMH concentration starting 4 weeks before the initial intra-ovarian injection and continuing until 2 weeks after ovariectomy.
Experimental design—young mares
To determine the effect of MSC injection on follicle number in mares with greater ovarian reserve, and to evaluate potential longer-term effects of MSC injection on follicle number, the six young mares were stratified by age and divided into (1) MSCs from donor A (n = 2), (2) MSCs from donor B (n = 2), or (3) vehicle (V, n = 2). The young mares received intra-ovarian injection, into one ovary, on one occasion. These mares had follicle counts obtained three times weekly, with limited exceptions, starting 7 to 8 weeks before ovarian injection, and continuing until 6 months after ovarian injection. Blood for serum AMH determination was collected from young mares weekly starting 5 weeks before ovarian injection and then twice weekly for 3 weeks after ovarian injection.
General methods
Bone marrow harvest and MSC characterization
Bone marrow was harvested from sternebral aspirates of the two 1-year-old donors as previously described [21]. The recovered nucleated cells were cultured in standard MSC culture medium consisting of Dulbecco’s modified Eagle’s medium (DMEM 1 g/l glucose; Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; HyClone Inc., Logan, UT), 2.5% HEPES buffer (Corning, St. Louis, MO), 1 ng/ml basic fibroblast growth factor (bFGF; Sigma-Aldrich), and 1% antibiotic–antimycotic (10,000 U penicillin, 10,000 μg/ml streptomycin, 25 μg/ml amphotericin B; GIBCO; Invitrogen, Carlsbad, CA) at 37 °C in a humidified atmosphere of 5% CO2 in air. The medium was refreshed three times per week, and cells were passaged at 70% confluence. For each aspirate, cells from passage 4 were trypsinized, washed in Dulbecco’s phosphate-buffered saline (DPBS; BioWhittaker; Lonza, Walkersville, MD), and resuspended in 95% serum, autologous to the mare to receive the injection, and 5% dimethyl sulfoxide, at a concentration of 10 × 106 cells/ml. The suspension was held for 24 h at − 80 °C, then transferred to liquid nitrogen.
The MSCs were characterized based on the criteria proposed by the International Society for Cellular Therapy [22] by their ability to adhere to plastic; immunophenotype for specific surface antigens, i.e., positive for CD44, CD29, and CD90, negative for CD45 [23], with variable expression of MHC II [24]; and trilineage differentiation potential. Antibodies used for immunophenotyping were mouse anti-horse antibodies to MHC class II (product no. MCS1085PE; Bio-Rad, Raleigh, NC), CD44 (product no. MCA1082F; Bio-Rad), CD29 (product no. 6603177; Beckman Coulter, Brea, CA), CD90 (product no. DG2011; VMRD Inc., Pullman, Washington), and CD45 (product no. HR-DG2009; VMRD). The antibodies against MHC II, CD44, and CD29 were fluorescently labeled with fluorescein isothiocyanate (FITC). The antibodies against CD90 and CD45 were not labeled with FITC and a secondary goat anti-mouse IgG antibody, labeled with phycoerythrin (PE), was used for visualization. Acquisition of the cell surface marker data was performed using a Becton Dickenson FACS Caliber flow cytometer using Cell Quest Version 3.3 (BD San Jose, CA). FITC, PE and 7-AAD were detected using a 488 nm laser with a 515/30 bandpass filter, 585/42 bandpass filter, and 650 Long Pass filter, respectively. At least 10,000 live events were collected. Data analysis was performed using FlowJo 9.8 Mac version (TreeStar Corp., Ashland, OR).
Chondrogenic differentiation was induced as previously described [25], using chondrogenesis-induction medium (DMEM with 4.5 g/l glucose, supplemented with 1% FBS, 2.5% HEPES buffer, 1% antibiotic-antimycotic (GIBCO), 10 ng/ml transforming growth factor beta (TGF-β3; Life Technologies), 0.6 μg/ml dexamethasone (Sigma-Aldrich), 50 μg/ml l-ascorbic acid, 40 μg/ml proline (Sigma-Aldrich), and 1% ITS premix (VWR)) and pellet formation. On day 21 of culture, pellets were fixed and paraffin-embedded and histological sections stained with Toluidine Blue to determine the presence of round chondrocytes embedded in extracellular matrix, which stains purple while fibrous tissue stains blue (Fig. 1a).
Fig. 1.
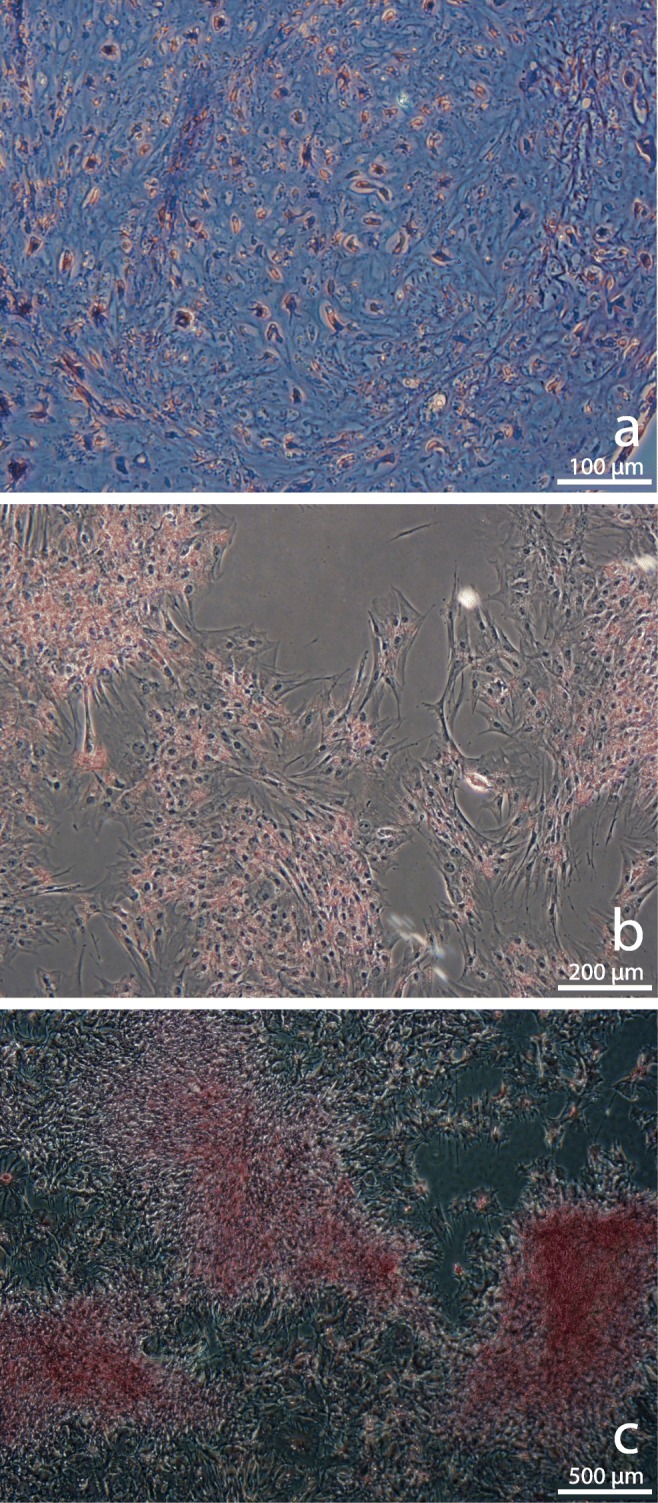
Trilineage differentiation of MSCs. a Chondrogenic pellet stained with Toluidine Blue showing cartilaginous extracellular matrix (purple) and fibrous tissue (blue), scale bar = 100 μm. b Adipogenic differentiation showing fat cells (red) stained with Oil Red O, scale bar = 200 μm. c Osteogenic differentiation showing calcified extracellular matrix and bone nodules (red) stained with 2% Alizarin Red, scale bar = 500 μm
Adipogenic differentiation was performed as previously described [25]. Briefly, once the MSCs reached 70% confluence, the medium was exchanged for adipogenic induction medium consisting of DMEM/F12 supplemented with 3% FBS, 1% antibiotic–antimycotic, 5% rabbit serum (Life Technologies), 33 μM biotin (Sigma-Aldrich), 17 μM/l pantothenate (Sigma-Aldrich), 1 μM/l insulin (Sigma-Aldrich), 1 μM/l dexamethasone, 225 μl isobutylmethylxanthine (Sigma-Aldrich), and 89 μl rosiglitazone, and cultured for 72 h. After 72 h, the medium was exchanged for adipogenic maintenance media (adipogenic induction medium without isobutylmethylxanthine and rosiglitazone) for an additional 72 h. Induced cells were stained with Oil Red O (Sigma-Aldrich) to detect lipid droplets within the cells, which stain red (Fig. 1b).
Osteogenic differentiation was performed as previously described [25]. Briefly, once MSCs reached 70% confluence, the medium was exchanged for osteogenic induction medium consisting of DMEM/F12 supplemented with 10% FBS, 1% antibiotic–antimycotic, 10 μM/l β-glycerophosphate (Sigma-Aldrich), 20 nM/l dexamethasone, and 50 μg/ml l-ascorbic acid. The plates were maintained in culture for 21 days. After 21 days, the plates were stained with 2% Alizarin Red (Sigma-Aldrich) to identify the presence of calcified extracellular matrix and bone nodules, which stain red (Fig. 1c).
Intra-ovarian injections
For intra-ovarian injection, 0.005–0.01 mg/kg detomidine, 10 mg butorphanol tartrate, and 120 mg n-butylscopolammonium bromide were administered to the mare i.v. MSCs previously cryopreserved in serum autologous to the mare being injected (10 × 106 cells in 1 ml) were thawed in a 37 °C water bath for 3 min and divided in four aliquots. Intra-ovarian injection was performed with the mare standing, using a transvaginal, ultrasound-guided technique as originally described for TVA by Brück et al. [26] and modified by Jacobson et al. [20]. Briefly, the perineum was washed three times with a povidone-iodine scrub and dried. A 5-mHz curvilinear transducer housed in a vaginal probe handle was introduced through the vulva and vestibule and placed with the transducer in the anterior fornix of the vagina. The ovary to be injected was grasped with the gloved hand per rectum and pulled toward the intravaginal transducer. An 18-G × 60-cm single-lumen transvaginal needle was passed through the vaginal wall into the ovarian stroma and one aliquot of cell suspension was gently expressed through tubing connected to the needle, followed by phosphate-buffered saline to flush the tubing, as the needle was slowly withdrawn from the ovary. This procedure was performed in four different, random locations per ovary, avoiding large follicles or visible luteal structures and passing the needle deeply (through more than half of the ovary thickness) into the ovarian stroma before infusing the cell suspension. After both ovaries were injected, the mares received 500 mg flunixin meglumine and 6.6 mg/kg gentamicin i.v., and 22,000 U/kg penicillin G procaine i.m. Temperature, pulse, and respiration rates were evaluated daily for 7 days after injection. The viability of cells remaining in the vial, as determined by propidium iodide and fluorescein diacetate staining, was > 80% for all samples.
Aspiration of follicles for oocyte recovery
Ultrasound-guided TVA of all immature follicles ≥ 8 mm in diameter was performed as described above for intra-ovarian injection, but with the modification that when the ovary was visualized on the ultrasound screen, a 12-G × 60-cm double-lumen oocyte aspiration needle (Cook Veterinary Products, New Buffalo, MI) was advanced through the cranial vaginal wall and into the ovary. Each visible follicle was aspirated and flushed six times with M199 with Hank’s salts and 25 mM HEPES (GIBCO) containing 0.4% FBS (GIBCO), 8 IU/ml heparin (Sigma-Aldrich), and 25 μg/ml gentamicin (GIBCO). After TVA was completed, each mare was administered 500 mg flunixin meglumine i.v. Recovered follicular aspirates were filtered through an embryo filter (EmCon filter; Immuno Systems, Inc., Spring Valley, WI) and cumulus–oocyte complexes were located using a dissection microscope. In aged mares after MSC injection, the aspirate from each ovary was filtered separately, and the granulosa cells from the aspirates were flash-frozen for genotyping of nuclear microsatellite markers and mitochondrial DNA sequencing as described below.
In vitro maturation and intracytoplasmic sperm injection of recovered oocytes
Recovered oocytes were matured in vitro as previously described [27]. Briefly, oocytes were held overnight [28] then transferred to maturation medium (M199 with Earle’s salts, 5 mU/ml FSH (Sioux Biochemicals, Sioux Center, IA), 10% FBS, and 25 μg/ml gentamicin) and cultured for 24–30 h at 38.2 °C in a humidified atmosphere of 5% CO2 in air. After the maturation period, oocytes were denuded of cumulus and oocytes with an intact membrane and a polar body underwent ICSI.
Intracytoplasmic sperm injection was performed as previously described [27] using a piezo drill. Presumptive zygotes were cultured in a commercial human embryo culture medium (GB; Global medium, LifeGlobal, Guilford, CT) supplemented with 10% FBS at 6% CO2, 5% O2, and 89% N2 at 38.2 °C. Medium was changed to GB with 20 mM added glucose at day 5 [27]. Embryos were evaluated on days 7 to 10 of culture for blastocyst development.
Ovariectomy and ovarian tissue processing
Ovariectomy via colpotomy was performed in the standing mare, under sedation with or without epidural analgesia, as previously described [29]. The recovered ovaries were examined for gross pathology both initially and after sagittal bisection. Tissue samples (four per ovary) were fixed for histological evaluation; sections were stained with periodic acid-Schiff (PAS) and hematoxylin (H&E) [30] and evaluated by a board-certified veterinary pathologist at the Texas A&M Veterinary Medical Diagnostic Laboratory. Additional 2-mm3 tissue fragments from random areas of the ovary, avoiding visible follicles or luteal structures, were obtained for DNA analysis (five per ovary, separate) and RNA extraction (five per ovary, pooled). Tissue fragments and granulosa cells from TVA aspirates were genotyped separately for microsatellite markers at the Veterinary Genetics Laboratory at the University of California using loci for one sex-associated gene (AME), one X-linked marker (LEX3), and 16 microsatellite identification markers [31]. Mitochondrial haplotype was evaluated by sequencing a 744-bp fragment of mtDNA spanning bases 15,382 through 16,125 of the hypervariable region of the D-loop between tRNAPro and the large conserved sequence block [32]. Findings were compared to genotyping results on analysis of hair root samples from the MSC donors, to detect genomic or mitochondrial chimerism indicating the presence of the transferred allogeneic cells.
RNA isolation and sequencing
Samples for RNA isolation were obtained by combining the five tissue sections from each ovary, providing one combined sample for each ovary. The ovarian tissues were pulverized with a biopulverizer (Cole-Parmer, Vernon Hills, IL) and RNA was extracted using a guanidinium thiocyanate–phenol–chloroform protocol (TRIzol Reagent; Life Technologies; Invitrogen) following the manufacturer’s instructions. Samples were processed with a final silica-gel spin-column cleanup. Quantitative and qualitative parameters of the RNA preparations were assessed using a Qubit 2.0 fluorometer (ThermoFisher; Invitrogen, Carlsbad, CA) and an Agilent RNA ScreenTape 2200 assay (Agilent Technologies, Santa Clara, CA). The qualitative parameter evaluated was RNA integrity as measured by the RNA integrity number (RIN) and the 28S/18S ribosomal RNA (rRNA) ratio. Samples with RNA integrity number ≥ 7 and a 28S/18S ratio ≥ 2 were considered of adequate quality for sequencing.
Isolated RNA samples were sequenced by the Texas A&M AgriLife Genomics and Bioinformatics core for generation of RNA-Sequencing (RNA-Seq) libraries and for RNA-Seq reactions. RNA-Seq libraries were generated using the TruSeq RNA preparation kit (Illumina) with a polyA selection step; polyA mRNA was fragmented and reverse-transcribed to cDNA for sequencing. The samples were sequenced (50-base-pair, single-end sequencing) on eight sequencing lanes of a HiSeq 2500 (Illumina). The raw data from the RNA-Seq reactions was processed by the Texas A&M Institute for Genome Sciences and Society (TIGSS). Briefly, a total of 1.36 billion reads were checked to trim any adapter sequences and low-quality bases using Trimmomatic [33], resulting in approximately 1.31 billion filtered reads (96%) out of which a total of 1.18 billion filtered reads (approximately 87%) mapped to the equCab2 genome assembly. Read mapping was performed using HISAT version 2.0.5 [34]. HTSeq [35] was used to generate raw read counts per gene using intersection-nonempty parameters to account for ambiguous read mappings. Differential gene expression tests were then performed using DESeq2 [36], following recommended guidelines. The differential expression between conditions was determined by the use of negative binomial generalized linear models.
Biological pathway analysis was performed using the Ingenuity Pathway Analysis toolkit (QIAGEN, Venlo, Netherlands; www.ingenuity.com Application Build 261,899, Content Version 18030641).
Serum AMH analysis
Collected serum was submitted to the Clinical Endocrinology Laboratory at the University of California for evaluation of AMH concentration.
Statistical analysis
A potential difference in follicle numbers in the injected ovary relative to the mare’s non-injected ovary was evaluated using Bayesian modeling in WinBUGS 1.4.3. Ovary-specific follicle counts were modeled separately as Poisson distributions; the Poisson parameters were modeled as a function of the expected follicle count, with a within-mare, ovary-specific random effect and a treatment effect. The 8-week period before injection (the initial injection, for those mares injected again before ovariectomy) was considered the “Pre-treatment” period, the 4-week period following injection was considered the “Treatment” period, and the 8-week period following the Treatment period was considered the “Post-treatment” period. The number of follicles on the injected ovary was then compared within mare group (MSC or V) among treatment periods. Bayesian models were also implemented to evaluate AMH concentrations in relation to treatment. The P value used for the Bayesian models was the posterior predictive P value [37]. A critical value of P < 0.05 was selected.
The oocyte recovery rate (oocytes recovered per follicle aspirated on TVA), proportion of oocytes reaching metaphase II in culture, and the proportion of oocytes developing to blastocyst after ICSI were compared among treatment periods within mare group using χ2 analysis, with Fisher’s exact test used when a value < 5 was expected for any parameter. Comparisons with a P < 0.05 were considered significant.
Results
Mare health and ovarian status
All 12 mares receiving intra-ovarian injections completed the study. Intra-ovarian injections of allogeneic MSCs (n = 10) or vehicle (n = 4) in aged or young mares were not associated with any observable signs of discomfort or systemic illness. Temperature, pulse, and respiration rates were within normal limits for the 7-day period in which they were evaluated after injection. All the mares continued to appear bright, alert, responsive, and in good health for the duration of the study.
Gross examination of the ovaries of the aged mares after ovariectomy revealed an abscess in the ovary of one of the mares. This mare had received two intra-ovarian MSC injections into the ovary in question and had undergone a total of six TVA procedures. The remainder of the ovaries showed no gross pathology. Histopathologic examination of the aged mare ovaries revealed small areas of granulation tissue in ovaries in all categories (MSC-injected, vehicle-injected, and non-injected), and this was considered by the pathologist to be of no clinical significance.
Characterization and phenotypic analysis of donor MSCs
The MSCs isolated and used for intra-ovarian injections showed evidence of differentiation to chondrogenic, adipogenic, and osteogenic cell lineages when cultured in appropriate conditions, as seen by the presence of cartilaginous extracellular matrix, intracytoplasmic lipid droplets, and calcified extracellular matrix, respectively (Fig. 1) [25]. Immunophenotypic analysis of MSCs at passage 3 by flow cytometry revealed that MSCs from both donors had a high expression of CD29 (> 99%) and CD90 (58.5–69.7%) markers, and low expression of CD44 (10.2–18.1%), CD45 (0.3–1.5%), and MHC II (1.7–5.2%) markers.
Antral follicle count
Of the eight aged mares, four mares (ages 20, 24, 20, and 20 years) consistently had follicular activity as defined by the presence of antral follicles visible on ultrasonographic examinations; the other four mares (ages 22, 23, 29, and 29 years, comprising three mares receiving MSC injection and one mare receiving vehicle injection) had no visible follicles throughout the study. TVA was performed on eight occasions (three mares) after injection of aged mares. At all other 14-day evaluations in aged mares, there were insufficient follicles present (fewer than five follicles with a diameter > 8 mm) and TVA was not performed.
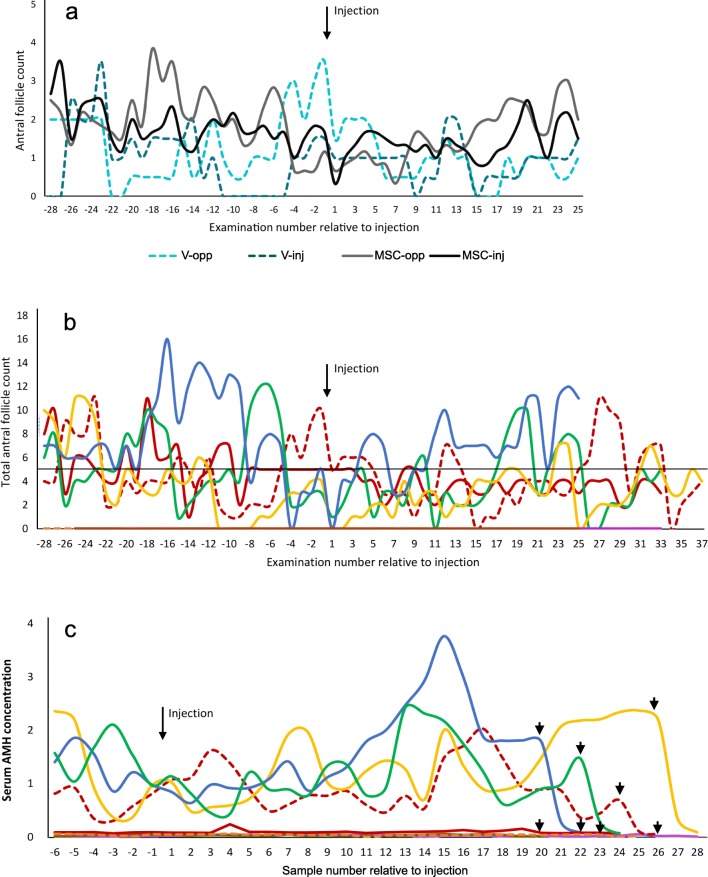
There were no significant differences in any parameter associated with the different MSC donors, thus mares injected with cells from either donor were grouped as “MSC mares.” For both old and young MSC mares, the number of follicles present did not differ significantly between MSC-injected and non-injected (MSC-opp) ovaries within mare throughout the study (Fig. 2a). There was also no difference in follicle count between injected and non-injected (V-opp) ovaries in the V mares. Within group, there were no significant differences in the total number of ovarian follicles among the Pre-treatment, Treatment, and Post-treatment periods for either aged or young mares (Table 1; Figs. 2b and 3). Aged mares tended to have fewer occasions on which they had five or more follicles, and thus had TVA performed on the scheduled day, after MSC injection (15.6% of scheduled TVA times vs. 30.6% of scheduled TVA times before injection; Fig. 2b). This was not evident in the V mares (27% vs. 22%, respectively).
Fig. 2.
Total antral follicle counts and serum AMH concentrations in aged mares undergoing intra-ovarian injection with MSCs (n = 6) or vehicle (V, n = 2). Follicle numbers were those recorded during videotaping of ultrasound examinations, performed three times weekly with limited exceptions. a Antral follicle counts in individual aged mares for the injected (inj) vs. non-injected (opp) ovaries, relative to date of intra-ovarian injection. Dashed lines represent values for V mares. There were no significant differences in follicle count between ovaries. b Total antral follicle counts for individual aged mares relative to date of intra-ovarian injection. Dashed lines represent values for V mares. There was no significant difference in antral follicle count among the Pre-treatment (8 weeks before injection), Treatment (4 weeks after injection), and Post-treatment (8 weeks after the Treatment period) periods. A line is drawn at the level of five follicles, the minimum required for conducting follicle aspiration. c AMH concentrations in aged mares relative to the date of injection; the same color line represents the same mare as in (b). Samples were taken once weekly. Dashed lines represent values for V mares. Arrowheads: dates when ovariectomies were performed. There were no significant differences in AMH concentration relative to Treatment period
Table 1.
Parameters for mares undergoing intra-ovarian injection of mesenchymal stem cells or vehicle during the Pre-treatment, Treatment, and Post-treatment periods
| Parameter | Mare group | Pre-treatment | Treatment | Post-treatment |
|---|---|---|---|---|
| Follicle number per mare | MSC | 2.8 (1, 4) | 1.7 (1, 3) | 2.3 (1, 4) |
| V | 1.9 (1, 2) | 1.8 (1, 3) | 2.0 | |
| MSC-Y | 4.1 (2, 7) | 4.8 (2, 7) | 4.1 (2, 6) | |
| V-Y | 2.8 (1, 4.25) | 1.8 (1, 3) | 2.4 (1, 4) | |
| Oocyte recovery | MSC | 56.1% (64/114) | 43.5% (10/23) | 59.5% (25/42) |
| V | 62.9% (17/27) | 45.5% (5/11) | 43.7% (7/16) | |
| Maturation to MII | MSC | 57.80% (37/64) | 50% (5/10) | 60% (15/25) |
| V | 58.80% (10/17) | 60% (3/5) | 85.7% (6/7) | |
| Blastocyst development | MSC | 29.7% (11/37) | 40%(2/5) | 13.3% (2/15) |
| V | 0% (0/10) | 0% (0/3) | 0% (0/6) |
Follicle numbers are for the injected ovary only and are expressed as mean (lower 2.5% and upper 97.5% limits). There were no significant differences in follicle number or in oocyte recovery, maturation, or blastocyst rates in either MSC-injected or vehicle-injected mares among periods, or between MSC-injected and vehicle-injected mares within period (P > 0.05)
MSC, aged mares receiving MSC injection (n = 6); V, aged mares receiving vehicle injection (n = 2); MSC-Y, young mares receiving MSC injection (n = 4); V-Y, young mares receiving vehicle injection (n = 2); Pre-treatment, the 8-week period before injection; Treatment, the 4-week period following injection; Post-treatment, the 8-week period following the Treatment period
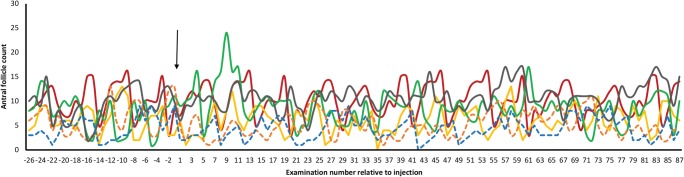
Fig. 3.
Total antral follicle counts in young mares undergoing intra-ovarian injection with MSCs (n = 4) or vehicle (V, n = 2). Follicle numbers were those recorded during videotaping of ultrasound examinations, performed three times weekly with limited exceptions. There was no significant difference in antral follicle count among the Pre-treatment (8 weeks before injection), Treatment (4 weeks after injection), and Post-treatment (8 weeks after the Treatment period) periods, nor over the remainder of the examination period (6 months from injection). Dashed lines represent values for V mares
Follicle numbers in the young mares continued to be assessed three times weekly for 4 months after the Post-treatment period (i.e., for 6 months after injection). The number of follicles did not change significantly during this time (Fig. 3). While this duration led to assessment during different seasons and the mare is a seasonal breeder, we have previously shown that there is no seasonal effect on the number of antral follicles > 5 mm diameter in mare ovaries [38].
Oocyte recovery, maturation, and blastocyst formation
In aged mares, there was no difference in oocyte recovery rate between the MSC-injected and vehicle-injected mares within any period (Table 1). The overall oocyte recovery rate was 55.3% (99/179) in aged MSC mares and 53.7% (29/54) in aged V mares. There were also no significant differences between periods within group in oocyte recovery, maturation, or blastocyst rates for either aged MSC or aged V mares (Table 1). Within the two aged V mares, no blastocysts were obtained from a total of 10 injected oocytes in the Pre-treatment period or from 9 injected oocytes in the periods after treatment, whereas in MSC mares the blastocyst development was 11/37 (29.7%) in the Pre-treatment and 4/20 (20%) in the Treatment and Post-treatment periods, combined. This different between groups was not significant.
Microsatellite analysis
The MSC donor fillies had distinguishable microsatellite and mtDNA sequences. No genomic chimerism and no mitochondrial chimerism was detected in the 16 TVA granulosa cell samples analyzed. Separate genetic analysis of the five fragments from each of the six ovaries of aged mares injected with MSCs and of the six contralateral (non-injected) ovaries of these same mares revealed no genomic or mitochondrial chimerism in any fragment.
Serum AMH
There was no significant difference in AMH among periods in either age group. The AMH values were reflective of follicle activity in the mares, in that the aged mares with no ovarian activity had basal AMH concentrations throughout the study, and AMH values for mares with follicle activity fell precipitously after ovariectomy (Fig. 2c).
Gene expression
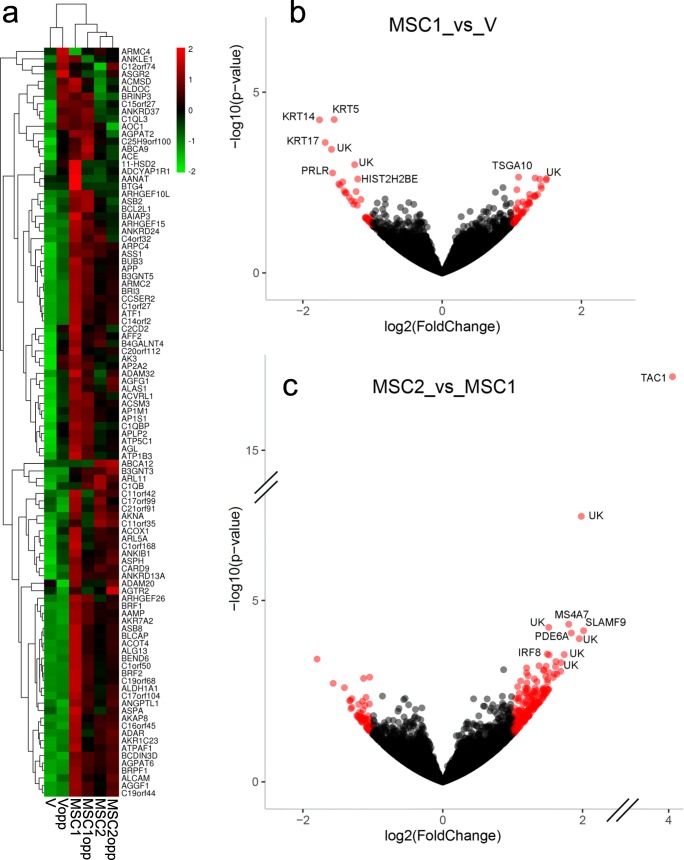
For gene expression analysis, MSC ovaries were grouped into those mares receiving only one MSC injection (MSC1) and mares which received a second MSC injection 2 weeks before ovariectomy (MSC2). MSC and V mare ovaries were also grouped by whether the ovary had received the injection, or was the contralateral ovary, which had not received injection(s) (opp). Heatmap analysis of ovarian gene expression in the six treatment conditions showed clear separation and clustering between V and MSC mares (Fig. 4a). There were 93 differentially-expressed genes, with 57 upregulated and 36 downregulated, in MSC1 ovaries compared to V ovaries; these two groups shared 1270 genes in common (Fig. 4b). The 20 most highly differentially regulated genes between MSC1 and V ovaries are presented individually in Table 2.
Fig. 4.
a Heat map of ovarian gene expression showing clustering of treatment groups. MSC1, ovaries of aged mares receiving intra-ovarian injection of MSC on one occasion 14 to 19 weeks before ovariectomy (n = 3); MSC2, ovaries of aged mares receiving an injection as for MSC1, then a second intra-ovarian injection of MSC 2 weeks before ovariectomy (n = 3); V, ovaries of aged mares receiving one intra-ovarian injection of vehicle 14 to 19 weeks before ovariectomy (n = 2); opp, the opposite (contralateral) ovaries of these mares. b Volcano plot of upregulated and downregulated genes in MSC1 ovaries compared to V ovaries. There were 57 upregulated genes and 36 downregulated genes. The 10 most differentially regulated genes are given in the figure: KRT (keratin 5, 14, and 17); PRLR (prolactin receptor); TSGA10 (testis specific 10); HIST2H2BE (histone cluster 2 H2B family member e). Unknown gene products are designated by “UK.” c Volcano plot of upregulated and downregulated genes in MSC2 ovaries compared to MSC1 ovaries. There were 200 upregulated genes and 61 downregulated genes. The 10 most differentially regulated genes are given in the figure: TAC1 (tachykinin precursor); MS4A7 (membrane spanning 4-domains A7); SLAMF9 (SLAM family member 9); PDE6A (phosphodiesterase 6A); IRF8 (interferon regulatory factor 8)
Table 2.
The 20 most differentially expressed genes identified by DESeq2 in MSC1 ovaries compared to vehicle-injected ovaries and in MSC2 ovaries compared to MSC1 ovaries
| Gene symbol | Log2FC | P value | Gene description |
|---|---|---|---|
| MSC1 vs. V | |||
| KRT5 | − 1.55 | 5.70E−05 | keratin 5 |
| KRT14 | − 1.76 | 5.80E−05 | keratin 14 |
| KRT17 | − 1.68 | 2.47E−04 | keratin 17 |
| PRLR | − 1.57 | 1.72E−03 | prolactin receptor |
| TSGA10 | 1.10 | 2.24E−03 | testis specific 10 |
| HIST2H2BE | − 1.21 | 2.53E−03 | histone cluster 2 H2B family member e |
| CNTN5 | 1.40 | 2.55E−03 | contactin 5 |
| CISH | − 1.43 | 3.03E−03 | cytokine inducible SH2 containing protein |
| ASB15 | − 1.48 | 3.42E−03 | Ankyrin repeat and SOCS box containing 15 |
| DCST2 | 1.42 | 4.59E−03 | DC-STAMP domain containing 2 |
| RASSF9 | 1.07 | 4.98E−03 | Ras association domain family member 9 |
| RNF17 | − 1.41 | 5.39E−03 | ring finger protein 17 |
| EPS8L3 | 1.26 | 6.71E−03 | EPS8 like 3 |
| MSLN | 1.37 | 6.76E−03 | mesothelin |
| ERBB4 | 1.34 | 7.10E−03 | erb-b2 receptor tyrosine kinase 4 |
| SLC4A9 | 1.32 | 7.66E−03 | solute carrier family 4 member 9 |
| ADCY1 | − 1.17 | 9.33E−03 | adenyl cyclase 1 |
| TLR1 | 1.09 | 1.14E−02 | toll like receptor 1 |
| IL33 | 1.25 | 1.16E−02 | interleukin 33 |
| ACVR1C | 1.26 | 1.20E−02 | activin A receptor type 1C |
| MSC2 vs. MSC1 | |||
| TAC1 | 4.36 | 7.79E−18 | tachykinin precursor |
| MS4A7 | 1.80 | 4.52E−05 | membrane spanning 4-domains A7 |
| SLAMF9 | 2.01 | 6.82E−05 | SLAM family member 9 |
| PDE6A | 1.84 | 7.89E−05 | phosphodiesterase 6A |
| IRF8 | 1.49 | 3.00E−04 | interferon regulatory factor 8 |
| LGALS4 | − 1.80 | 4.12E−04 | galectin 4 |
| SIGLEC1 | 1.38 | 6.34E−04 | sialic acid binding IG like lectin 1 |
| PLD4 | 1.48 | 6.53E−04 | phospholipase D family member 4 |
| LAT2 | 1.20 | 7.23E−04 | linker for activation of T-cells family member |
| GPR31 | 1.45 | 8.08E−04 | G protein-coupled receptor 31 |
| VAV1 | 1.44 | 8.60E−04 | vav guanine nucleotide exchange factor 1 |
| CD300LB | 1.69 | 8.95E−04 | CD300 molecule like family member b |
| CD163 | 1.52 | 9.80E−04 | CD163 molecule |
| RHBG | 1.63 | 1.08E−03 | Rh family B glycoprotein (gene/pseudogene) |
| GPR151 | 1.22 | 1.19E-03 | G protein-coupled receptor 151 |
| CD86 | 1.47 | 1.23E-03 | CD86 molecule |
| CRADD | −1.05 | 1.30E-03 | CASP2 and RIPK1 domain containing adaptor with death domain |
| SLAMF7 | 1.58 | 1.32E-03 | SLAM family member 7 |
| RASSF9 | −1.13 | 1.40E-03 | Ras association domain family member 9 |
| CYBB | 1.50 | 1.51E-03 | cytochrome b-245 beta chain |
FC, fold change; MSC1, aged mares receiving intra-ovarian injection of MSC on one occasion 14 to 19 weeks before ovariectomy (n = 3); MSC2, aged mares receiving an injection as for MSC1, then a second intra-ovarian injection of MSC 2 weeks before ovariectomy (n = 3); V, aged mares receiving one intra-ovarian injection of vehicle 14 to 19 weeks before ovariectomy
Interestingly, comparison of MSC1 versus MSC2 ovaries, reflecting ovaries injected only once, 14 to 19 weeks previously versus ovaries receiving both the initial injection and a second injection 2 weeks before ovariectomy, showed a greater number of differentially expressed genes (261) than did the above comparison, with 200 upregulated and 61 downregulated genes in MSC2 ovaries as compared to MSC1 (Fig. 4c). These two groups shared 1618 genes in common. The 20 most highly regulated genes between MSC2 and MSC1 ovaries are presented individually in Table 2.
All of the differentially expressed genes identified for each of the comparisons above, plus those for MSC1opp versus Vopp, are presented in Supplementary Tables S1–S3. Other comparisons were reviewed to detect any pronounced differences in gene expression; the most prominent was TAC1 (tachykinin precursor). This gene, which was the most highly upregulated gene in MSC2 ovaries versus MSC1 ovaries (Table 2), was also the most highly upregulated gene in MSC2 ovaries versus V ovaries (3.99, 1.81E−15; log2FC, P value, respectively), and in MSC2opp versus Vopp ovaries (3.70, 1.72E−13).
Discussion
To the best of our knowledge, this represents the first report of intra-ovarian injection of MSCs in a large animal model. Evaluation of the results from this study suggests that intra-ovarian injections of allogeneic MSCs are safe to perform in mares, as no systemic or ovarian effects were seen, with the exception that one mare developed an ovarian abscess. The direct cause of the abscess (whether from TVA or from MSC injection) could not be determined; a low incidence of ovarian abscess formation (~ 1 in 400 procedures) has been previously reported after TVA in mares [39]. Oocyte recovery, maturation, and blastocyst development rates remained unchanged in the treated mares following MSC injections, suggesting no detrimental effect on ovarian function.
Under the conditions of this study, there was no significant effect of MSC injection on follicle number in either aged or young mares. We followed follicle number in young mares to 6 months after injection to evaluate potential effects on primordial follicle development. It should be noted that follicle counts were obtained in mares undergoing follicle aspiration every 14 days; however, this interval has been previously shown not to affect antral follicle count at the time of TVA [40]. Follicle count may potentially have been affected by the fact that mares with fewer than five follicles at the scheduled time for follicle aspiration did not undergo TVA. The observed lack of effect of MSC on follicle number contrasts with the repeatable improvement in ovarian function after MSC treatment, by both intravenous and intra-ovarian injection, reported in laboratory animal species in females in which ovaries have been compromised by chemotherapy [10–13, 41, 42]. It is possible that rodent and rabbit ovaries react differently to MSCs than do equine ovaries [10, 13], or that MSCs have a different effect on chemotherapy-treated ovaries than on aged ovaries. This latter possibility could be related to the finding that it may be necessary to induce injury to tissues to clear the target tissue’s cell niches in order to enable engraftment of donor stem cells [43]. It is possible that chemotherapy causes injury to the ovarian tissue that allows MSC engraftment in functional niches, whereas injection of MSCs in an intact aged ovary does not support MSC engraftment. Lack of engraftment was demonstrated by the absence of detected chimerism in the ovaries of the MSC-injected aged mares, including ovaries undergoing a second MSC injection 2 weeks before ovariectomy. This lack of MSC persistence has been shown previously in other tissues [44, 45]. We evaluated both autosomal microsatellite markers and mitochondrial sequence to detect MSC chimerism, as it has been suggested that mitochondria from injected MSCs may be transferred to host cells [46].
The lack of follicular response to MSC treatment is supported by peripheral AMH concentrations, which were also not affected by MSC treatment. Concentrations of AMH are closely related to the total number of antral follicles present on the ovaries in mares, as in women [47]. Herraiz et al. [15] similarly reported no consistent pattern in total antral follicle count or AMH concentration after injection of autologous, blood-derived MSC in women. However, in that study, the authors reported that mean total antral follicle count was significantly increased at 15 days after injection, compared to a single baseline value taken before treatment. If valid, the rapid nature of this response suggests an effect of MSC injection on existing tertiary follicles, rather than stimulation of primordial or growing primary or secondary follicles. We did not find a similar effect on antral follicle count in either young or aged mares.
Injection of MSCs did significantly affect ovarian gene expression, in comparison to injection of vehicle, in our study. Because engraftment appeared not to have occurred, even in ovaries evaluated only 2 weeks after MSC injection (MSC2 mares), the most likely cause of this difference in gene expression is a paracrine/endocrine effect of the injected cells within the first 2 weeks after injection. One of the 20 most upregulated genes in MSC1 ovaries versus V ovaries was testis specific 10 (TSGA10). TSGA10 was once considered testis-restricted, but has since been shown to be expressed in normal tissues, including undifferentiated embryonic stem cells [48], and in a wide range of neoplasms, including ovarian cancer [49]. A search of the GEO Profiles database [50] in October 2018 revealed that TSGA10 has been identified in fetal and newborn ovaries, and in follicular cells from polycystic ovaries (GEO accessions GDS2719 [51]; GDS3841, [52]). In the testis, TSGA10 has been used as a marker of active spermatogenesis in vivo and in vitro [53]. In cancer cells, TSGA10 may be involved in angiogenesis, cell growth and division, motility, and migration [54]. The increased expression of TSGA10 seen in our study appears to be a paracrine effect of MSCs, as it was not seen in the non-injected contralateral ovaries. To the best of our knowledge, this is the first report of TSGA10 expression in the normal adult ovary.
RASSF9 (Ras-association domain family member 9) was upregulated in MSC1 ovaries compared to both V and MSC2 ovaries. RASSF genes encode proteins associated with membrane trafficking, apoptosis, and proliferation [55], and Rassf9-deficient mice exhibit signs of premature senescence [56]. Upregulation of RASSF9 in MSC1 ovaries suggests an anti-senescence effect of MSC injection.
One of the most striking findings on evaluation of gene expression in MSC2 and MSC2opp ovaries was the expression of tachykinin precursor (TAC1). Expression of this gene was highly significantly upregulated in MSC2 compared to both MSC1 (P = 7.79E−18) and V (P = 1.81E−15), and in MSC2opp compared to Vopp (P = 1.72E−13). TAC1 encodes four neurally active gene products, including substance P, a well-known mediator of response to inflammation and injury [57]. Substance P also stimulates cell growth, including proliferation of bone marrow cells, lymphocytes, and endothelial cells, and promotes angiogenesis [58]. The fact that TAC1 was upregulated in both the MSC2 and MSC2opp ovaries suggests that this is either a systemic effect or that MSCs migrated to the contralateral ovary and induced local paracrine effects. Interestingly, CRADD was significantly downregulated in MSC2 compared to MSC1. CRADD encodes a protein containing a death domain motif which recruits caspase 2/ICH1 to the cell death signal transduction complex to promote apoptosis [59], indicating an acute anti-apoptotic effect of MSC injection. In general, ovaries that had been injected a second time 2 weeks prior to ovariectomy (MSC2) had upregulation of genes involved in immune responses, and downregulation of genes involved in apoptosis, in comparison to ovaries that had been injected only once, 14 to 19 weeks before ovariectomy (MSC1; Supplementary Table S3). This reflects either an acute effect of MSC injection or an effect of a second exposure to MSCs. Inflammatory reaction to foreign antigens (associated with fetal bovine serum used during cell culture), and possibly to allogeneic cells, after a second intra-articular injection of MSCs has recently been reported [60]. Because of the potential for antigenic response, it is possible that use of allogeneic MSCs conveys less of a benefit than would use of autologous cells, or MSC from a less immunogenic source, such as neonatal cells. Most previous reports of successful reversal of ovarian damage have used MSC from one of these sources. However, rescue of ovarian activity has been reported after infusion of adult male-derived MSC, which are potentially immunogenic, in the rabbit [41].
In conclusion, intra-ovarian injection of allogeneic MSCs was easily performed and well-tolerated in the mare model. Under the conditions of this study, MSC injection altered ovarian gene expression, but did not significantly affect the ovarian functions assessed. The findings of this study do not support the use of intra-ovarian injection of MSCs as a treatment for age-related ovarian dysfunction in mares, which may serve as a model for women. This is in contrast to the established beneficial effects of MSCs in chemotherapy-damaged ovaries of rats, mice, and rabbits [10, 11, 41].
Electronic supplementary material
(DOCX 54 kb)
Acknowledgments
The authors thank Dr. Andrew Hillhouse for his help with RNA isolation; Dr. Gus Wright for his help with flow cytometry analyses; Dr. Young Ho Choi for performing ICSI on the recovered oocytes; Hsing Fann for her help with MSC culture, freezing, and thawing for intra-ovarian injections; and Angel del Valle for his help with mtDNA sequencing. The authors acknowledge Texas A&M Institute for Genome Sciences and Society (TIGSS) for providing computational resources for RNA-Seq data analysis and systems administration support for the TIGSS HPC Cluster.
Funding
Funded by the Clinical Equine ICSI Program at Texas A&M University, the Link Equine Research Fund at Texas A&M University, and a Postdoctoral Trainee Research Grant and a Graduate Student Core Facility Experiential Learning Program Grant from the College of Veterinary Medicine and Biomedical Sciences. S.T.G. was funded in part by a Texas A&M College of Veterinary Medicine & Biomedical Sciences Merit Scholars Fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Itay S, Abramovici A, Nevo Z. Use of cultured embryonal chick epiphyseal chondrocytes as grafts for defects in chick articular-cartilage. Clin Orthop. 1987;220:284–303. [PubMed] [Google Scholar]
- 2.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 3.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minguell JJ, Erices A. Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med (Maywood) 2006;231:39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 7.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, Lint MTV, Powles R, Jackson G, Hinterberger-Fischer M, Kolb HJ, Apperley JF. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/S0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 8.Sanders JE, Buckner CD, Amos D, Levy W, Appelbaum FR, Doney K, Storb R, Sullivan KM, Witherspoon RP, Thomas ED. Ovarian function following marrow transplantation for aplastic anemia or leukemia. J Clin Oncol. 1988;6:813–818. doi: 10.1200/JCO.1988.6.5.813. [DOI] [PubMed] [Google Scholar]
- 9.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. [PubMed] [Google Scholar]
- 10.Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, Murata N, Aida T, Nakama K, Aono F, Aoyama N, Kato K, Kato O. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Investig. 2013;93:181–193. doi: 10.1038/labinvest.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Yu L, Sun M, Mu S, Wang C, Wang D, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. doi: 10.1155/2013/690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H-J, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 13.Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10:353–363. doi: 10.1080/14653240802035926. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed Ali AF, et al. Fertility treatment of aged women by laparoscopic intra ovarian injection of peripheral blood mononuclear cell (PBMNC) a new modality. Fertil Mag. 2013;52–5.
- 15.Herraiz S, Romeu M, Buigues A, Martinez S, Diaz-Garcia C, Gomez-Segui I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110:496–505 el. doi: 10.1016/j.fertnstert.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Mao Q, He J, She H, Zhang Z, Yin C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8:55. doi: 10.1186/s13287-017-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnevale EM, Ginther OJ. Defective oocytes as a cause of subfertility in old mares. Biol Reprod. 1995;Monograph 1:209–214. doi: 10.1093/biolreprod/52.monograph_series1.209. [DOI] [Google Scholar]
- 18.Fitzgerald C, Zimon AE, Jones EE. Aging and reproductive potential in women. Yale J Biol Med. 1998;71:367–381. [PMC free article] [PubMed] [Google Scholar]
- 19.Colleoni S, Barbacini S, Necchi D, Duchi R, Lazzari G, Galli C. Application of ovum pick-up, intracytoplasmic sperm injection and embryo culture in equine practice. Proc Am Assoc Equine Pract. 2007;53:554–559. [Google Scholar]
- 20.Jacobson CC, Choi YH, Hayden SS, Hinrichs K. Recovery of mare oocytes on a fixed biweekly schedule, and resulting blastocyst formation after intracytoplasmic sperm injection. Theriogenology. 2010;73:1116–1126. doi: 10.1016/j.theriogenology.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Sellon DC. How to obtain a diagnostic bone marrow sample from the sternum of an adult horse. Proc Am Assoc Equine Pract. 2006;52:621–625. [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.De Schauwer C, Piepers S, Van de Walle GR, Demeyere K, Hoogewijs MK, Govaere JL, et al. In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytometry A. 2012;81:312–323. doi: 10.1002/cyto.a.22026. [DOI] [PubMed] [Google Scholar]
- 24.Schnabel LV, Pezzanite LM, Antczak DF, Felippe MJ, Fortier LA. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther. 2014;5:13. doi: 10.1186/scrt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell A, Rivas KA, Smith R, Watts AE. Cryopreservation of equine mesenchymal stem cells in 95% autologous serum and 5% DMSO does not alter post-thaw growth or morphology in vitro compared to fetal bovine serum or allogeneic serum at 20 or 95% and DMSO at 10 or 5% Stem Cell Res Ther. 2015;6:1–12. doi: 10.1186/s13287-015-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brück I, Raun K, Synnestvedt B, Greve T. Follicle aspiration in the mare using a transvaginal ultrasound-guided technique (short communication) Equine Vet J. 1992;24:58–59. doi: 10.1111/j.2042-3306.1992.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi YH, Ross P, Velez IC, Macias-Garcia B, Riera FL, Hinrichs K. Cell lineage allocation in equine blastocysts produced in vitro under varying glucose concentrations. Reproduction. 2015;150:31–41. doi: 10.1530/REP-14-0662. [DOI] [PubMed] [Google Scholar]
- 28.Choi YH, Love LB, Varner DD, Hinrichs K. Holding immature equine oocytes in the absence of meiotic inhibitors: effect on germinal vesicle chromatin and blastocyst development after intracytoplasmic sperm injection. Theriogenology. 2006;66:955–963. doi: 10.1016/j.theriogenology.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 29.Rowland AL, Glass KG, Grady ST, Cummings KJ, Hinrichs K, Watts AE. Influence of caudal epidural analgesia on cortisol concentrations and pain-related behavioral responses in mares during and after ovariectomy via colpotomy. Vet Surg. 2018;47:715–721. doi: 10.1111/vsu.12908. [DOI] [PubMed] [Google Scholar]
- 30.Alves KA, Alves BG, Rocha CD, Visonna M, Mohallem RF, Gastal MO, et al. Number and density of equine preantral follicles in different ovarian histological section thicknesses. Theriogenology. 2015;83:1048–1055. doi: 10.1016/j.theriogenology.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.van de Goor LH, Panneman H, van Haeringen WA. A proposal for standardization in forensic equine DNA typing: allele nomenclature for 17 equine-specific STR loci. Anim Genet. 2010;41:122–127. doi: 10.1111/j.1365-2052.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowling AT, Del Valle A, Bowling M. A pedigree-based study of mitochondrial D-loop DNA sequence variation among Arabian horses. Anim Genet. 2000;31:1–7. doi: 10.1046/j.1365-2052.2000.00558.x. [DOI] [PubMed] [Google Scholar]
- 33.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;btu170. [DOI] [PMC free article] [PubMed]
- 34.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Meth. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2014;btu638. [DOI] [PMC free article] [PubMed]
- 36.Love M, Anders S, Huber W. Differential analysis of count data—the DESeq2 package. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JL. Comparative investigation of three Bayesian p values. Comput Stat Data Anal. 2014;79:277–291. doi: 10.1016/j.csda.2014.05.012. [DOI] [Google Scholar]
- 38.Hinrichs K, Schmidt AL. Meiotic competence in horse oocytes: interactions among chromatin configuration, follicle size, cumulus morphology, and season. Biol Reprod. 2000;62:1402–1408. doi: 10.1095/biolreprod62.5.1402. [DOI] [PubMed] [Google Scholar]
- 39.Velez I, Arnold C, Jacobson C, Norris J, Choi Y, Edwards J, et al. Effects of repeated transvaginal aspiration of immature follicles on mare health and ovarian status. Equine Vet J. 2012;44:78–83. doi: 10.1111/j.2042-3306.2012.00606.x. [DOI] [PubMed] [Google Scholar]
- 40.Duchamp G, Bézard J, Palmer E. Oocyte yield and the consequences of puncture of all follicles larger than 8 millimetres in mares. Biol Reprod. 1995;Monograph 1:233–241. doi: 10.1093/biolreprod/52.monograph_series1.233. [DOI] [Google Scholar]
- 41.Abd-Allah SH, Shalaby SM, Pasha HF, El-Shal AS, Raafat N, Shabrawy SM, et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy. 2013;15:64–75. doi: 10.1016/j.jcyt.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Ghadami M, El-Demerdash E, Zhang D, Salama SA, Binhazim AA, Archibong AE, et al. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012;7:e32462. doi: 10.1371/journal.pone.0032462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, Biton IE, Tal O, Shakhar G, Ben-Hur H, Shneider D, Vaknin Z, Sadan O, Evron S, Freud E, Shoseyov D, Wilschanski M, Berkman N, Fibbe WE, Hagin D, Hillel-Karniel C, Krentsis IM, Bachar-Lustig E, Reisner Y. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med. 2015;21:869–879. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- 44.Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 45.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claes A, Ball BA, Scoggin KE, Esteller-Vico A, Kalmar JJ, Conley AJ et al. The interrelationship between anti-Mullerian hormone, ovarian follicular populations and age in mares. Equine Vet J. 2014. [DOI] [PubMed]
- 48.Behnam B, Modarressi MH, Conti V, Taylor KE, Puliti A, Wolfe J. Expression of Tsga10 sperm tail protein in embryogenesis and neural development: from cilium to cell division. Biochem Biophys Res Commun. 2006;344:1102–1110. doi: 10.1016/j.bbrc.2006.03.240. [DOI] [PubMed] [Google Scholar]
- 49.Mobasheri MB, Jahanzad I, Mohagheghi MA, Aarabi M, Farzan S, Modarressi MH. Expression of two testis-specific genes, TSGA10 and SYCP3, in different cancers regarding to their pathological features. Cancer Detect Prev. 2007;31:296–302. doi: 10.1016/j.cdp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41:D991–D9D5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15:89–103. doi: 10.1093/molehr/gan082. [DOI] [PubMed] [Google Scholar]
- 53.Miryounesi M, Nayernia K, Mobasheri MB, Dianatpour M, Oko R, Savad S, Modarressi MH. Evaluation of in vitro spermatogenesis system effectiveness to study genes behavior: monitoring the expression of the testis specific 10 (Tsga10) gene as a model. Arch Iran Med. 2014;17:692–697. [PubMed] [Google Scholar]
- 54.Tanaka R, Ono T, Sato S, Nakada T, Koizumi F, Hasegawa K, Nakagawa K, Okumura H, Yamashita T, Ohtsuka M, Asagoe K, Yamasaki O, Noguchi Y, Iwatsuki K, Nakayama E. Over-expression of the testis-specific gene TSGA10 in cancers and its immunogenicity. Microbiol Immunol. 2004;48:339–345. doi: 10.1111/j.1348-0421.2004.tb03515.x. [DOI] [PubMed] [Google Scholar]
- 55.Volodko N, Gordon M, Salla M, Ghazaleh HA, Baksh S. RASSF tumor suppressor gene family: biological functions and regulation. FEBS Lett. 2014;588:2671–2684. doi: 10.1016/j.febslet.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 56.Lee C-M, Yang P, Chen L-C, Chen C-C, Wu S-C, Cheng H-Y, Chang YS. A novel role of RASSF9 in maintaining epidermal homeostasis. PLoS One. 2011;6:e17867. doi: 10.1371/journal.pone.0017867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73:4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–278. doi: 10.1016/0026-2862(90)90024-L. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad M, Srinivasula SM, Wang L, Talanian RV, Litwack G, Fernandes-Alnemri T, Alnemri ES. CRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIP. Cancer Res. 1997;57:615–619. [PubMed] [Google Scholar]
- 60.Joswig AJ, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, 3rd, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. 2017;8:42. doi: 10.1186/s13287-017-0503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 54 kb)