Abstract
Purpose
The aim of this study was to evaluate the incidence of an inter-chromosomal effect (ICE) in blastocyst-stage embryos from carriers of balanced chromosome inversions.
Methods
Infertility patients (n = 52) with balanced inversions (n = 66 cycles), and maternal age-matched controls that concurrently cycled (n = 66), consented to an IVF cycle with preimplantation genetic testing for aneuploidy (PGT-A). Blastocyst-stage embryos underwent trophectoderm biopsy for PGT-A with only euploid blastocysts transferred in a subsequent frozen embryo transfer. Subtypes of inversions were included in aggregate: paracentric/pericentric, polymorphic/non-polymorphic, male/female carriers, and varying inversion sizes.
Results
The incidence of aneuploidy was not significantly higher for the inversion patients compared to the controls (inversion = 48.8% vs. control = 47.2% ns). Following euploid blastocyst transfer, there were excellent live birth outcomes.
Conclusions
Carriers of balanced chromosome inversions did not exhibit higher aneuploidy rates for chromosomes that were not involved in the inversion compared to maternal age-matched controls, signifying the absence of an inter-chromosomal effect for this data set. These results provide the largest investigation of blastocyst embryos regarding the debated existence of an ICE resulting from the presence of an inversion during meiosis. However, further studies are warranted to investigate an ICE among inversions subtypes that were outside the scope of this study.
Keywords: Inter-chromosomal effect, Balanced inversion carriers, Preimplantation genetic testing-aneuploidy, Structural chromosome rearrangement
Introduction
A frequent indication for preimplantation genetic testing (PGT) is the presence of a chromosome rearrangement. The incidence of chromosome rearrangements, including inversions, is approximately 5% higher for couples with infertility that have experienced recurrent IVF failure or recurrent pregnancy loss than that found in the general population [1, 2]. Heterozygous carriers of balanced chromosome inversions frequently seek assisted reproductive technologies (ART) with PGD to achieve a healthy pregnancy as their infertility may be influenced by a high frequency of unbalanced gametes as a result of the chromosomal rearrangement.
An inversion is an intrachromosomal structural rearrangement whereby a two-break event occurs, the intercalary segment rotates 180 degrees, reinserts, and the breaks unite. The majority of carriers of chromosomal inversions are phenotypically normal as the reorientation of the genetic material within the breakpoints does not appear to influence its function, assuming no gain or loss of DNA has occurred. There are a few recognized polymorphic inversions in the human population that are suspected to be clinically non-relevant due to breakpoint locations that reside within heterochromatic regions of the chromosome [3, 4]. However, in individuals who are balanced inversion carriers, decreased fertility, higher rates of miscarriage, and children born with multiple congenital anomalies have also been observed [4]. For example, 33% of parents of individuals diagnosed with Williams-Beuren syndrome, often caused by a 1.5 Mb microdeletion at 7q11, were found to have an inversion variant present in approximately 5% of the general population [5].
During meiosis, the method of homosynapsis of homologous chromosomes for inversion carriers depends largely on the length of the inversion. Short inversions may remain unpaired at the site of the inversion to maximize synapsis along the remaining lengths of the bivalent. Long inverted regions form a hair-pin loop to maximize nucleotide homology. The inversion loop configuration aligns all of the homologous regions by twisting and folding the inverted segment. In cases where the inversion is very long, synapsis of the inversion with its non-inverted homolog prevails at the expense of pairing at the terminal ends of the bivalent; these terminal segments can remain unpaired. When recombination occurs within the inverted segment, unbalanced recombinant chromatids can result in the gametes: duplications/deletions can occur in pericentric inversion carriers while dicentric and acentric fragments (involving duplications/deletions) can result from paracentric inversions. Furthermore, disruption to bivalent and chiasmata formation can lead to recombination failure and meiotic arrest [6, 7]. As a result of these meiotic challenges, individuals who are heterozygous inversion carriers are at risk of gamete production failure and/or the production of unbalanced gametes which could lead to compromised fertility [7, 8].
The clinical outcome for inversion carrier patients will largely depend on the degree of imbalance in the recombinant chromosomes. Inversion heterozygotes in which one or both distal segments are small have a risk for an ongoing pregnancy or live birth that is chromosomally unbalanced and phenotypically abnormal. Carriers with large distal segments can only produce recombinants with a large degree of genetic imbalance leading to implantation failure or lethality in utero [9].
It has been described that structural chromosome reorganizations can influence disjunction and segregation of other chromosome pairs leading to full chromosomal aneuploidy in the resulting gametes. This phenomenon of interference during meiosis is referred to as an inter-chromosomal effect (ICE) and was first described in 1963 [10]. The mechanism for ICE is related to the formation of heterosynapsis in the meiotic configurations of the chromosome involved in the inversion [8]. It is postulated that this effect could significantly increase the risk for numerical chromosomal abnormalities in the gametes of inversion carriers.
Multiple studies utilizing different approaches have been used to evaluate the segregation products of inversion carriers. Fluorescence in situ hybridization (FISH)-based tests analyzed the segregation outcome of single sperm from male pericentric, non-polymorphic inversion carriers. Fluorescent DNA probes labeled with different fluorochromes were used to differentiate the segregation products through color-spot patterns. The frequency of unbalanced spermatozoa ranged from 0 to 37.85% [11–13]. The studies also concluded that the size of an inverted segment and its proportion in the entire chromosome impacts its pairing ability such that an inversion size of at least 100 Mbp and 50% of the chromosome is required to result in a segregation error leading to a recombinant chromosome in the mature sperm [3, 7].
Several studies aimed to assess the occurrence of an ICE in inversion heterozygotes analyzed the frequencies of numerical abnormalities in sperm nuclei with conflicting results. These studies utilized FISH technologies to detect aneuploidies in a handful of chromosomes unrelated to the one containing the inversion. Chromosomes X and Y were universally used as they are more susceptible to heterosynapsis due to their singular characteristics. Autosomes 8, 9, 13, 18, and 21 were also included in some of these investigations. Two small studies describe an increase in aneuploid sperm [11, 14] for both a polymorphic and non-polymorphic inversion carrier, whereas others were unable to identify ICE with the limited number of chromosomes analyzed [15–19]. A recent review of the literature only reported ICE in 7.7% of the male inversion carrier cases analyzed [8]. This corresponded to a single individual with an inversion in the polymorphic heterochromatic region of chromosome 9 in a population size of just 13 individuals. Estimating the effect of inversions on the risk for ICE requires not only a larger patient population than reported in these studies but also a PGT platform that is comprehensive and allows for detection of aneuploidy caused by inversions that are both maternally and paternally derived.
With technological advances, it is now possible to screen for all 23 chromosome pairs with PGT. An initial case study, utilizing the clinical application of PGT-A, reported errors in multiple chromosomes unrelated to the inverted chromosome from two pericentric, non-polymorphic heterozygous inversion carriers [20]. However, this study included patients with a variety of chromosome rearrangements including reciprocal/Robertsonian translocations and inversions. No conclusion regarding the existence of an ICE was drawn. To date, there has been no large study solely focused on inversion carriers investigating the occurrence of ICE with PGT-A.
The aim of this study was to evaluate the chromosomal ploidy status in IVF blastocysts from a consecutive series of 66 inversion carrier IVF cycles. It was determined that the presence of an inversion does not contribute to an inter-chromosomal effect as the rate of aneuploidy for chromosomes not involved in the inversion was not significantly higher when compared to maternal age-matched controls that concurrently cycled in the IVF laboratory.
Methods
In vitro fertilization cycle
Fifty-two inversion carrier patients participated in 66 concurrent IVF cycles, without any omissions or exclusions. Fifty percent of the cycles analyzed in this data set (n = 33) resulted from male inversion carriers and 50% of the cycles (n = 33) involved female carriers. The control group was maternal age-matched infertility patients who cycled concurrently with the inversion group patients in the IVF laboratory for the purpose of eliminating any potential laboratory variables. The average maternal age for both groups was 34.6 years.
Ovarian reserve parameters (described by AMH, FSH, and antral follicle count) were comparable for the two groups. Standard regimens for controlled ovarian hyperstimulation were employed using recombinant FSH and LH along with GnRH agonist or antagonist. Oocyte maturation was stimulated with recombinant hCG alone or in combination with GnRH agonist when the leading follicle exceeded 18 mm. Routine transvaginal ultrasound-guided oocyte aspiration was performed under anesthesia 35 h later. Following oocyte retrieval, cumulus cells were mechanically and enzymatically removed prior to fertilization using standard intracytoplasmic sperm injection procedures. Embryos were routinely cultured in sequential media until the blastocyst stage. On day 3 of embryonic development, a 5–10-μm channel was opened in the zona pellucida with a series of five pulses at 200 microseconds each at 100% power (Hamilton-Thorne Research). At the blastocyst stage, a trophectoderm (TE) biopsy was performed using laser dissection for PGT-A when an inner cell mass and blastocele expansion of grade 3 or above (defined by the standard Gardner and Schoolcraft grading system) was identified. The biopsied blastocyst was then vitrified using the Cryotop method, as previously published [21].
The inversion test group produced a total of 410 blastocyst-stage embryos (mean 6.2). The maternal age-matched control group produced a total of 427 blastocyst-stage embryos (mean 6.9; ns). Embryo aneuploidy rate was calculated as the percent of aneuploid embryos per patient cycle and the existence of an ICE was determined by comparing the aneuploidy rates between the two study groups. Data were analyzed by group Student’s t test and Fisher’s exact test as appropriate with p value of < 0.05 considered to be statistically significant. A post hoc power analysis was performed and there would be a 4.5% difference in aneuploidy needed for it to be significant.
The chromosomes that contained the balanced inversion included 1, 2, 3, 4, 5, 6, 7, 9, 16, 17, and Y. The proportions of the inversions ranged from 6.5% to 73.4% of the chromosome (Table 1). Both paracentric (n = 6 cycles) and pericentric (n = 60 cycles) inversion carriers were represented in this data set.
Table 1.
Inversion patient cytogenetic data
| Karyotype | Inv seg. size (Mbp) | Chr. size (Mbp) | % inverted seg* | n of patients | Cycles |
|---|---|---|---|---|---|
| 46,XY,inv(1)(p22.1p34.1) | 45.2 | 249.25 | 18.1 | 1 | 2 |
| 46,XX,inv(1)(q25q42) | 51.2 | 249.25 | 21.0 | 1 | 2 |
| 46,XX,inv(2)(p11.2q13) | 31.1 | 243.19 | 13.0 | 1 | 1 |
| 46,XY,inv(3)(p21.3p26) | 36.4 | 198.02 | 18.0 | 1 | 2 |
| 46,XY,inv(3)(p11.2q12.1) | 12.8 | 198.02 | 6.5 | 1 | 1 |
| 46,XX,inv(3)(p11.2q12.1) | 12.8 | 198.02 | 6.5 | 1 | 2 |
| 46,XY,inv(3)(p21.31q13.32) | 74.8 | 198.02 | 37.8 | 1 | 1 |
| 46,XY,inv(4)(p15.1q12) | 31.8 | 191.15 | 16.6 | 1 | 1 |
| 46,XX,inv(5)(p13.1q13.3) | 34.4 | 180.91 | 19.0 | 1 | 1 |
| 46,XY,inv(5)(p15.3q14) | 82.5 | 180.91 | 45.6 | 1 | 1 |
| 46,XY,inv(6)(p23q23.3) | 125.6 | 171.11 | 73.4 | 1 | 1 |
| 46,XY,inv(7)(p13q21.12) | 42.8 | 159.14 | 27.0 | 1 | 1 |
| 46,XY,inv(9)(p11q12) | 16.9 | 141.21 | 12.0 | 1 | 1 |
| 46,XX,inv(9)(p11q12) | 16.9 | 141.21 | 12.0 | 1 | 1 |
| 46,XY,inv(9)(p11q13) | 19.1 | 141.21 | 14.0 | 11 | 15 |
| 46,XX,inv(9)(p11q13) | 19.1 | 141.21 | 14.0 | 17 | 22 |
| 46,XY,inv(9)(p12q13) | 25.1 | 141.21 | 18.0 | 3 | 4 |
| 46,XX,inv(9)(p12q13) | 25.1 | 141.21 | 18.0 | 2 | 2 |
| 46,XX,inv(9)(p22q31.2) | 91.4 | 141.21 | 65.0 | 1 | 1 |
| 46,XX,inv(16)(p11.1q11.2) | 12.4 | 90.35 | 13.7 | 1 | 1 |
| 46,XX,inv(16)(q23.1q24.3) | 16.25 | 90.35 | 18.0 | 1 | 1 |
| 46,XY,inv(17)(p13.1q11.2) | 21.1 | 81.19 | 26.0 | 1 | 1 |
| 46,XY,inv(Y)(p11.2q11.22) | 16.8 | 59.37 | 28.3 | 1 | 1 |
Inversion segment size, megabase pair; chromosome size, megabase pair
*Calculations for percentage of chromosome inverted derived using UCSC Genome Browser Feb. 2009 (GRCh37/hg19) assembly
PGT-A and frozen embryo transfer
PGT-A was performed at Reproductive Medicine Associates of New Jersey using an SNP-based microarray analysis on biopsied TE cells as previously described [22]. Briefly, DNA produced using the WGA4 GenomePlex Whole Genome Amplification Kit (Sigma Aldrich) was then evaluated on the NspI GeneChip Mapping 262 K microarray as recommended by the supplier (Affymetrix). Copy number assignments were made using the CNAT 4.0 algorithm (Affymetrix) to assign aneuploidy status to each embryo.
Only blastocysts that were identified as euploid or balanced were subsequently transferred after management of routine endometrial preparation and luteal support. Embryo transfer was performed 3 to 4 h after blastocyst warming under ultrasound guidance using standard techniques. The number of embryos selected for transfer was based on guidelines set forth by the American Society for Reproductive Medicine.
Pregnancy was confirmed at 9 days post embryo transfer by the appropriate bhCG rise and then by ultrasound at 6.5 weeks gestation when a fetal heart rate was observed. Implantation rate was defined as the number of intrauterine gestational sacs with cardiac activity noted on ultrasound examination per number of embryos transferred.
Results
PGT-A data
Inversions defined by the cytogenetic report as polymorphic or non-polymorphic were also identified, however, no difference in ICE was observed in the polymorphic vs. non-polymorphic data set: 48% aneuploidy rate in cycles from polymorphic inversion carriers (n = 51 cycles) versus 52% in the non-polymorphic group (n = 15 cycles) (ns). Additionally, no difference was noted in ICE risk for inv(9)(p11q13) carriers specifically compared to age-matched controls, both with a 48% aneuploidy rate (n = 37 cycles) (ns) (Table 2). The inversion group had an equivalent rate of aneuploidy compared to the maternal age-matched control group when excluding the chromosome with the inversion for the purpose of evaluating for ICE. The aneuploidy rate of the inversion group was 48.8% (± 34.3) and that of the control group was 47.2% (± 28.3) (ns). The aneuploidy rate per inversion varied; however, small sample size limited clinically significant data with the exception of the common inversion 9 (Table 3).
Table 2.
Inversion 9 carrier subgroup
| Inv(9)(p11q13) subtype | Control | |
|---|---|---|
| Maternal age | 34.7 ± 5.6 | 35.9 ± 4.3 |
| Eggs collected | 25.3 ± 12.5 | 21.1 ± 10.4 |
| Fertilized | 14.3 ± 7.5 | 12.3 ± 6.7 |
| Blastocyst biopsied | 6.7 ± 3.8 | 7.1 ± 4.8 |
| Aneuploidy | 48% | 48% |
Data are mean ± standard deviation
Table 3.
IVF cycle and CCS results
| Karyotype | # of IVF cycles | # of patients | # of biopsied embryos | Aneuploid chromosomes detected (quantity if > 1) | Aneuploid rate |
|---|---|---|---|---|---|
| 46,XY,inv(1)(p22.1p34.1) | 2 | 1 | 11 | +7, −10, +11, −12, −14, +14, −16, +19, +20, chaotic(4)* | (11/11) 100% |
| 46,XX,inv(1)(q25q42) | 2 | 1 | 6 | +4, +16, chaotic* | (3/6) 50% |
| 46,XX,inv(2)(p11.2q13) | 1 | 1 | 2 | none | (0/2) 0% |
| 46,XY,inv(3)(p21.3p26) | 2 | 1 | 16 | +16(2), +22, +X(2), −X, chaotic* | (7/16) 44% |
| 46,XY,inv(3)(p11.2q12.1) | 1 | 1 | 6 | −9 | (1/6) 17% |
| 46,XX,inv(3)(p11.2q12.1) | 2 | 1 | 13 | −1, −3, +5, −11, −16, −19(2), −X | (7/13) 54% |
| 46,XY,inv(3)(p21.31q13.32) | 1 | 1 | 2 | none | (0/2) 0% |
| 46,XY,inv(4)(p15.1q12) | 1 | 1 | 8 | +4, +5, +15, −22 | (3/8) 38% |
| 46,XX,inv(5)(p13.1q13.3) | 1 | 1 | 2 | +6, +16 | (2/2) 100% |
| 46,XY,inv(5)(p15.3q14) | 1 | 1 | 14 | −5(2), +5(2), +18 | (5/14) 36% |
| 46,XY,inv(6)(p23q23.3) | 1 | 1 | 3 | −18, −22, chaotic (2)* | (3/3) 100% |
| 46,XY,inv(7)(p13q21.12) | 1 | 1 | 2 | none | (0/2) 0% |
| 46,XY,inv(9)(p11q12) | 1 | 1 | 7 | -X | (1/7) 14% |
| 46,XX,inv(9)(p11q12) | 1 | 1 | 8† | −9, −14, +15, −18 | (3/7†) 43% |
| 46,XY,inv(9)(p11q13) | 15 | 11 | 116† | −1, +1(2), −2, +2, −3, −4, +5, −7, −8, +9(2), −10, +10, −11, −12, −13(2), −14, +14 (3),−15, −16, +16(5), +17, −18(3), +18, −19(2),−20, −21(2), −22(6), +22(2), −X(2), +X, chaotic(4)* | (46/113†) 41% |
| 46,XX,inv(9)(p11q13) | 22 | 17 | 142 | +1(3), −2(2), −4, +4, −5(3), +6, −7, +8, −9(2), +9(3), +unbal inv(9), −10, +10, −11(3), +11, −12(2), +12(2), | |
| −13(2), +13(2), −14(3), +14, −15(4), +15(2), −16(5), +16(11), −17, −18(2), +18(4), −19, +19(2), −20, +20, | |||||
| −21(3), +21, −22(5), +22(10), chaotic(2)* | (73/142) 51% | ||||
| 46,XY,inv(9)(p12q13) | 4 | 3 | 21 | +2, +7(2), −8, +12, −14, −15(3), −16, −18, +19, −21(2), +22, −X | (12/21) 57% |
| 46,XX,inv(9)(p12q13) | 2 | 2 | 12 | −2, +2, −4, −9 | (4/12) 33% |
| 46,XX,inv(9)(p22q31.2) | 1 | 1 | 3 | +14, chaotic* | (2/3) 67% |
| 46,XX,inv(16)(p11.1q11.2) | 1 | 1 | 9 | +3, +13, −14, −15, −16p11.1q11.2(3), −17, −19, chaotic(3)* | (9/9) 100% |
| 46,XX,inv(16)(q23.1q24.3) | 1 | 1 | 2 | −2 | (1/2) 50% |
| 46,XY,inv(17)(p13.1q11.2) | 1 | 1 | 2 | +21 | (1/2) 50% |
| 46,XY,inv(Y)(p11.2q11.22) | 1 | 1 | 3 | none | (0/3) 0% |
(Bold = chromosome associated with inversion)
*Chaotic aneuploidy = > three chromosomes involved in aneuploidy
†Set included inconclusive result(s)
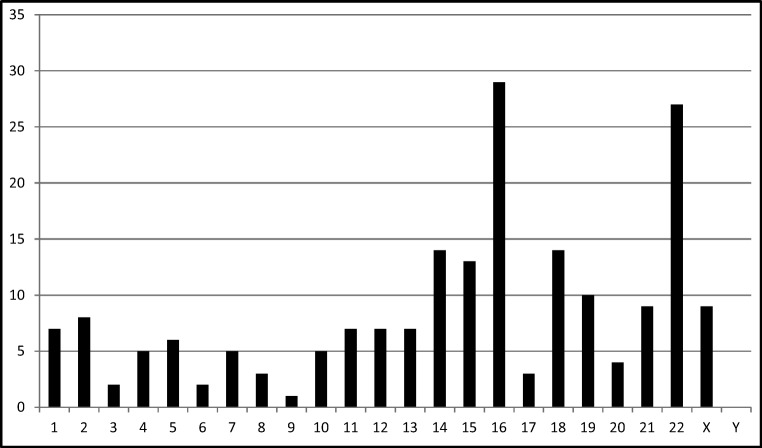
The nature of the chromosome aneuploidies detected in both groups included all autosomes. When excluding for the chromosome with the inversion, and with the exception of chromosome Y, every other chromosome was represented in an aneuploid state at least once in the 66 total cycles (Table 3). The frequency of aneuploidies seen in this data is consistent with common monosomies and trisomies identified in blastocyst embryos. For example, ICE events involving 16 and 22 were observed with the highest frequency as observed in association with advanced maternal age [23] (Fig. 1). It is not always possible to detect recombinant chromosomes due to the variation of coverage among chromosomal locations and the unpredictability of the sites of crossing over events. Incidentally, four recombinant embryos were detected which involved chromosomes 9 and 16. An example of a copy number plot from an unbalanced inversion 9 is included in Fig. 2.
Fig. 1.
ICE events per individual chromosome*
Fig. 2.
PGT copy number plot for an unbalanced inversion (9)(p11q13). Unbalanced inversion 9, balanced, or normal
Within the study group, 28 patients (n = 37 cycles) carried the common polymorphic inv(9)(p11q13). Analysis of this subtype alone compared to the age-matched controls revealed a similar trend seen in the aggregated study group: a similar rate of aneuploidy and no suspected ICE, although not significant (Table 2).
IVF outcome
Following the transfer of euploid blastocyst-stage embryos, inversion carriers experienced excellent implantation, live birth, and miscarriage rates. The implantation rate was 58% from the 54 completed transfers (mean = 1.46 embryos transferred) with a live birth rate of 63% in our study population. None of the patients experienced a miscarriage.
Discussion
This study is the first of its kind to evaluate the incidence of an inter-chromosomal effect in blastocyst embryos due to a parental balanced inversion utilizing a PGT-A platform. Fifty-one heterozygous inversion carrier patients underwent a total of 66 IVF cycles with PGT-A on trophectoderm biopsies. The study group had equal aneuploidy rates for chromosomes unrelated to the inversion compared to age-matched controls (inversion = 48.8% vs. control = 47.2%; ns), suggesting the inversion is not associated with an elevated risk for an ICE. Carriers of balanced inversions experienced excellent ART outcomes following the transfer of euploid/balanced blastocysts as illustrated by a livebirth rate of 63% (34/54 transfers) and no miscarriages (0/34).
It is understood that carriers of inversions are at risk to produce abnormal gametes as a result of malsegregation during meiosis of the chromosome pair affected with the inversion. Initial studies of heterozygous male inversion carriers focused on assessing this risk in mature sperm and concluded that the size and proportion of the inversion within the chromosome were directly associated with the likelihood of an unbalanced recombinant. When such unbalanced spermatozoa fertilize an oocyte, reproductive outcomes are compromised.
Apart from the risk for a genetic imbalance related to the chromosomal pair directly impacted by the inversion, another question remains regarding reproductive risks for inversion carriers: does an inversion have an effect on the segregation of any of the other chromosomes during meiosis? Since aneuploidy is a very common cause of implantation failure, miscarriage and is associated with recognized chromosomal syndromes, understanding a potential increased risk for a full chromosomal aneuploidy is important when developing a patient-specific care plan for heterozygous inversion carrier patients.
Few studies investigating the occurrence of ICE in association with a chromosomal inversion have emerged since the theory was proposed in the 1960s. Therefore, this question remains largely unanswered. Initial studies that investigated a possible impact on ploidy status in inversion carriers were focused only on male inversion carriers. Sperm was analyzed with FISH with conflicting results: some papers detected an ICE with increased aneuploid sperm, whereas other investigations were unable to see an association. These studies were small, only involved sperm, as opposed to embryo samples, and utilized testing platforms that allowed for detection of only a subset of the entire chromosome complement.
Preimplantation genetic testing for aneuploidy has been utilized as a PGT platform for patients with structural chromosome abnormalities such as translocations and inversions for the purpose of detecting unbalanced recombinant chromosomes and to screen for aneuploidy. One study reported on two inversion carrier patients who underwent IVF with PGT-A [20]. Both patients experienced multiple chromosomal errors unrelated to the inverted chromosome. However, no conclusion regarding ICE was made in their investigation.
To our knowledge, this is the first study to evaluate the incidence of an inter-chromosomal effect utilizing PGT-A on blastocyst embryos derived from inversion carriers. Although it is the largest study of its kind, our data set was limited by the quantity and the aggregation of multiple subtypes of inversion carriers for a single analysis. Investigating the risk for ICE separately for polymorphic verses non-polymorphic inversion carriers might yield different results as polymorphic inversions may lead to less meiotic disruption by virtue of their stability and frequency in the population. For this reason, we analyzed the risk for ICE in cycles involving only carriers of the common polymorphic inversion 9 with findings similar to our aggregated group, although the data was not statistically significant (Table 2). We could not investigate possible differences in ICE risk for carriers of pericentric verses paracentric inversions and large verses small inversions due to limited sample sizes. Additionally, it is well-known that the meiotic checkpoints differ in spermatogenesis and oogenesis and therefore could potentially impact gamete outcome for inversion carriers of different sexes. Due to a significant difference in the average maternal age of the male inversion carrier group verses the female inversion carrier group, comparing the risk for an ICE in the male verses female inversion carrier subgroups was not possible with this dataset, as age-related non-disjunction risk could not be controlled.
In summary, our study offers insight into the long-debated question of the existence of an inter-chromosomal effect as it relates to a parental inversion. We found that the inversion itself does not elevate the likelihood of aneuploidy above that of the maternal-age risk. Therefore, for heterozygous inversion carriers who are seeking infertility treatment to achieve a healthy pregnancy, consideration of maternal age and its well-known association with aneuploidy should drive the recommendation for PGT-A to screen for whole chromosome trisomies and monosomies [24]. PGT-A technologies that have the resolution to detect unbalanced recombinants resulting from the specific patient inversion should still be considered for younger patients who do not have an indication for aneuploidy screening but do have a high risk of unbalanced gametes. For most inversion carrier patients, PGT-A remains a singular platform available for both inversion recombinant and age-related aneuploidy screening. This allows for a streamlined PGT approach to select euploid, balanced embryos for transfer, thus improving the likelihood of a viable pregnancy and healthy offspring [21, 25].
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campana M, Serra A, Neri G. Role of chromosome aberrations in recurrent abortion: a study of 269 balanced translocations. Am J Med Genet. 1986;24:341–356. doi: 10.1002/ajmg.1320240214. [DOI] [PubMed] [Google Scholar]
- 2.Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81:171–176. doi: 10.1016/S0301-2115(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 3.Collinson MN, Fisher AM, Walker J, Currie J, Williams L, Roberts P. Inv(10)(p11.2q21.2), a variant chromosome. Hum Genet. 1997;101:175–180. doi: 10.1007/s004390050609. [DOI] [PubMed] [Google Scholar]
- 4.Feuk L. Inversion variants in the human genome: role in disease and genome architecture. Genome Med. 2010;2(2):11. doi: 10.1186/gm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam E, Young EJ, Morris CA, Marshall CR, Loo W, Scherer SW, Mervis CB, Osborne LR. The common inversion of the Williams-Beuren syndrome region at 7q11.23 does not cause clinical symptoms. Am J Med Genet A. 2008;146A:1797–1806. doi: 10.1002/ajmg.a.32360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyce EF, McKim KS. Chromosome axis defects induce a checkpoint-mediated delay and interchromosomal effect on crossing over during Drosophila meiosis. PLoS Genet. 2010;6(8):e1001059. doi: 10.1371/journal.pgen.1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton E, Blanco J, Egozcue J, Vidal F. Sperm studies in heterozygote inversion carriers: a review. Cytogenet Genome Res. 2005;111:297–304. doi: 10.1159/000086903. [DOI] [PubMed] [Google Scholar]
- 8.Anton E, Vidal F, Blanco J. Interchromosomal effect analyses by sperm FISH: incidence and distribution among reorganization carriers. Syst Biol Reprod Med. 2011;57:268–278. doi: 10.3109/19396368.2011.633682. [DOI] [PubMed] [Google Scholar]
- 9.Gardner R, Sutherland G. Chromosome abnormalities and genetic counseling. Oxford: Oxford University Press; 1996.
- 10.Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–337. [PubMed] [Google Scholar]
- 11.Anton E, Vidal F, Egozcue J, Blanco J. Genetic reproductive risk in inversion carriers. Fertil Steril. 2006;85:661–666. doi: 10.1016/j.fertnstert.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Caer E, Perrin A, Douet-Guilbert N, Amice V, De Braekeleer M, Morel F. Differing mechanisms of meiotic segregation in spermatozoa from three carriers of a pericentric inversion of chromosome 8. Fertil Steril. 2008;89:1637–1640. doi: 10.1016/j.fertnstert.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Morel F, Laudier B, Guérif F, Couet ML, Royère D, Roux C, Bresson JL, Amice V, De Braekeller M, Douet-Guilbert N. Meiotic segregation analysis in spermatozoa of pericentric inversion carriers using fluorescence in-situ hybridization. Hum Reprod Oxf Engl. 2007;22:136–141. doi: 10.1093/humrep/del317. [DOI] [PubMed] [Google Scholar]
- 14.Amiel A, Sardos-Albertini F, Fejgin MD, Sharony R, Diukman R, Bartoov B. Interchromosomal effect leading to an increase in aneuploidy in sperm nuclei in a man heterozygous for pericentric inversion (inv 9) and C-heterochromatin. J Hum Genet. 2001;46:245–250. doi: 10.1007/s100380170073. [DOI] [PubMed] [Google Scholar]
- 15.Mikhaail-Philips MM, Ko E, Chernos J, Greene C, Rademaker A, Martin RH. Analysis of chromosome segregation in sperm from a chromosome 2 inversion heterozygote and assessment of an interchromosomal effect. Am J Med Genet A. 2004;127A:139–143. doi: 10.1002/ajmg.a.20693. [DOI] [PubMed] [Google Scholar]
- 16.Mikhaail-Philips MM, McGillivray BC, Hamilton SJ, Ko E, Chernos J, Rademaker A, Martin RH. Unusual segregation products in sperm from a pericentric inversion 17 heterozygote. Hum Genet. 2005;117:357–365. doi: 10.1007/s00439-004-1245-0. [DOI] [PubMed] [Google Scholar]
- 17.Vialard F, Delanete A, Clement P, Simon-Bouy B, Aubriot FX, Selva J. Sperm chromosome analysis in two cases of paracentric inversion. Fertil Steril. 2007;87:418.e1–418.e5. doi: 10.1016/j.fertnstert.2006.05.087. [DOI] [PubMed] [Google Scholar]
- 18.Blanco J, Egozcue J, Vidal F. Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet. 2000;106:500–505. doi: 10.1007/s004390000295. [DOI] [PubMed] [Google Scholar]
- 19.Ferfouri F, Clement P, Gomes DM, Minz M, Amar E, Selva J, Vialard F. Is classic pericentric inversion of chromosome 2 inv(2)(p11q13) associated with an increased risk of unbalanced chromosomes? Fertil Steril. 2009;92:1497.e1–1497.e4. doi: 10.1016/j.fertnstert.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Alfarawati S, Fragouli E, Colls P, Wells D. First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Hum Reprod Oxf Engl. 2011;26:1560–1574. doi: 10.1093/humrep/der068. [DOI] [PubMed] [Google Scholar]
- 21.Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, Scott RT. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96:638–640. doi: 10.1016/j.fertnstert.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Treff NR, Su J, Tao X, Levy B, Scott RT. Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94:2017–2021. doi: 10.1016/j.fertnstert.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 23.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT. Aneuploidy across individual chromosomes at the embryonic level in trophectoderm biopsies: changes with patient age and chromosome structure. J Assist Reprod Genet. 2014;31:1501–1509. doi: 10.1007/s10815-014-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoolcraft WB, Katz-Jaffe MG. Comprehensive chromosome screening of trophectoderm with vitrification facilitates elective single-embryo transfer for infertile women with advanced maternal age. Fertil Steril. 2013;100:615–619. doi: 10.1016/j.fertnstert.2013.07.1972. [DOI] [PubMed] [Google Scholar]
- 25.Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92:157–162. doi: 10.1016/j.fertnstert.2008.05.029. [DOI] [PubMed] [Google Scholar]