Abstract
Purpose
To compare effects of lipid-soluble statins (simvastatin, lovastatin, atorvastatin) and water-soluble statin (pravastatin) on growth and invasiveness of human endometrial stromal (HES) cells.
Methods
Endometrial biopsies were collected during the proliferative phase from five volunteers. HES cells were isolated and cultured in the absence or in the presence of simvastatin, lovastatin, atorvastatin, and pravastatin. Effects of statins on DNA synthesis, cell viability, activity of caspases 3/7 and invasiveness were evaluated.
Results
The proliferation of HES cells was significantly decreased by simvastatin (by 47–89%), lovastatin (by 46–78%), and atorvastatin (by 21–48%) in a concentration-dependent manner. Activity of executioner caspases 3/7 was significantly increased by simvastatin (by 10–25%), lovastatin (by 19%) and atorvastatin (by 7–10%) in a concentration-dependent manner. The greatest effects were observed in response to simvastatin. Accounting for the effects of statins on cell number, the invasiveness of HES cells was significantly decreased in cells treated with simvastatin (by 49%), lovastatin (by 54%), and atorvastatin (by 53%). Pravastatin had little or no effects on any of the tested endpoints.
Conclusions
Present findings demonstrate that only lipid-soluble among tested statins were effective in inhibition of growth and invasiveness of HES cells. These findings may have clinical relevance in treatment of endometriosis.
Keywords: Statins, Lipophilic, Hydrophilic, Endometrial stroma, Endometriosis
Introduction
Endometriosis is a common and often devastating gynecologic disorder affecting millions of women and associated with dysmenorrhea, dyspareunia, intermenstrual pain and infertility. Its prevalence has been estimated to be approximately 10% among women of reproductive age and the associated healthcare costs of endometriosis, including the costs of productivity loss, may exceed $22 billion/year [1]. Endometrial and endometriotic tissues of women with endometriosis exhibit altered phenotype characterized by increased invasiveness and proliferation facilitating ectopic attachment and growth of endometriotic implants. GWAS identified association of endometriosis with several genes including VEZT (vezatin), FN1(fibronectin) and GREB1 [2]. Interestingly, protein products of two genes, FN1 and VEZT, are involved in cell adhesion, migration, and transmembrane cell junction while GREB1 participates in estrogen-regulating pathway involving estrogen-stimulated cell proliferation [3].
Currently available medical treatments such as GnRH analogs, oral contraceptive pills and progestins are often ineffective or associated with significant side-effects. Surgical treatments of endometriosis may be effective in short-term by reducing pain and possibly improving fertility; however, surgery for endometriosis is technically challenging and is associated with significant intra-operative and long-term risks and complications. Furthermore, surgery often provides only temporary relief followed by return of symptoms and the need for repeat operations; even after a second surgery, 14–20% of patients require a third procedure [4]. In view of these considerations, there is a great need to develop new and effective therapeutic approaches that would either supersede or complement currently available treatments.
Use of statins may offer a potential novel approach for the treatment of endometriosis. In a nude mouse model of experimental endometriosis, we found that simvastatin induced a dose-dependent reduction in the number of implants by up to 85% and a total volume of implants by up to 98% [5]. Statins were also effective in reducing endometriotic lesions in other murine models [6–8] and baboon model [9]. Statins are competitive and reversible inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, a key enzyme in the mevalonate pathway. Statins exhibit anti-inflammatory, antioxidant and immunomodulatory properties, decreasing mediators and markers of inflammation (i.e., C-reactive protein, TNF-α, interleukins, and MCP-1) [10–12]. Statins also inhibit matrix metalloproteinases (MMPs) and increase tissue inhibitor of metalloproteinases (TIMPs), an enzyme system which regulates normal extracellular matrix remodeling, but is dysregulated in endometriosis [13, 14]. We [15–19] and others [7, 20, 21] have shown that statins inhibit growth and invasiveness of human endometrial stromal (HES) cells in vitro. In this study, we compared effects of several statins with different lipophilic/hydrophilic profiles on proliferation, apoptosis and invasiveness of HES cells.
Materials and methods
Acquisition of human tissues
Endometrial biopsy specimens were collected from five participants (aged 21–34 years) during the proliferative phase of their menstrual cycle. Participants had regular menstrual cycles, had no known history of endometriosis, and were free of any hormonal treatment or supplements for at least 3 months prior to their biopsies. Informed consent was obtained from all individual participants included in the study. The University of California, Davis Institutional Review Board approved the collection protocol and the use of human tissues (protocol no. 200715461-3). The tissues were minced and enzymatically digested to purify the endometrial stromal cells. Following the digestion, the cells were passed through a cell strainer and cultured at 37 °C in humidified air with 5% carbon dioxide in DMEM supplemented with 1% antibiotic, 10% charcoal-dextran fetal bovine serum (FBS), and 1 nM estradiol. For individual experiments, the cells were seeded onto 96-well plates (15,000 cells/well) or 24-well tissue culture inserts (50,000 cells/well) as described below. Before the treatment, media were changed for phenol red-free and serum-free DMEM. Each experiment was repeated three times.
Preparation of chemicals
Lipid-soluble statins (simvastatin, lovastatin and atorvastatin) were dissolved in ethanol (EtOH) or dimethyl sulfoxide (DMSO), while water-soluble pravastatin was dissolved directly in cell culture medium. Simvastatin and lovastatin were dissolved in 15% ethanol (v/v) and 0.25% NaOH (w/v), hydrolyzed for 2 h at 50 °C to break the lactone ring, pH-adjusted to 7.4 with 0.1 N HCl, and then filter-sterilized with a 0.2-μ filter. Atorvastatin, generally considered the most potent statin out of the four tested, was dissolved in 100% DMSO [17]. Atorvastatin concentrations in culture differed due to the solubility in the solvent, DMSO, and the solvent’s toxic effects on HES cells. Parallel concentrations of DMSO compared to atorvastatin treatments above 10 μM were found to have negative effects on the control cells. All stock solutions of statins were further diluted in media prior to adding treatments to the cells. Parallel-treated controls of EtOH/NaOH and DMSO were added to all control wells such that final concentrations were identical in all cultures. Chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell proliferation assay and cell viability assay (MTS assay)
Cell proliferation was measured by quantification of DNA synthesis determined using a thymidine incorporation assay. The HES cells were incubated for 24 h in 96-well culture plates with fresh media (phenol red-free and serum-free DMEM), and then cultured without (control) or with simvastatin (3, 10, 30 μM), lovastatin (3, 10, 30 μM), atorvastatin (1, 3, 10 μM), or pravastatin (3, 10, 30 μM) for 48 h. Radiolabeled [3H] thymidine (1 μCi/well) was added to the cells during the last 24 h of culture. At the end of the culture period, the cells were harvested using a multi-well cell harvester (PHD Harvester, Model 290; Cambridge Technology, Inc., Watertown, MA). Radioactivity was measured using a liquid scintillation counter, Wallac 1409 (PerkinElmer, Shelton, CT).
The total number of viable cells was estimated using a CellTiter-Blue Cell Viability Assay (Promega, Madison, WI) 2 h before the end of the culture period. This assay involves the reduction of resazurin to resorufin by metabolically active cells, resulting in the generation of a fluorescent product at the excitation wavelength of 579 nm and the emission wavelength of 584 nm that is proportional to the number of living cells. Fluorescence was determined with the use of a microplate reader (Fluostar Omega, BMG, Durham, NC). Each treatment was carried out in at least eight replicates.
Caspase-3/7 activity assay
Apoptosis of HES cells was evaluated by detection of activity of executioner caspases 3 and 7 using the Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI). The HES cells were incubated for 24 h in 96-well culture plates with fresh media (phenol red-free and serum-free DMEM), and then cultured without (control) or with simvastatin (3, 10, 30 μM), lovastatin (3, 10, 30 μM), atorvastatin (1, 3, 10 μM), or pravastatin (3, 10, 30 μM) for 48 h. The Apo-ONE Caspase-3/7 Reagent contains a substrate that fluoresces after interaction with caspase-3/7. Fluorescence was measured (485Ex/520Em) following a standard 2-h incubation with the reagent in a microplate reader (Fluostar Omega, BMG, Durham, NC). Caspase-3/7 activity was expressed per the number of total viable cells estimated by the cell viability assay. Each treatment was carried out in at least eight replicates.
Invasiveness assay
An invasiveness assay was performed to measure the invasive potential of the cells through an extracellular matrix layer (Matrigel, ECM gel, growth factor reduced, without phenol red from Engelberth-Holm-Swarm mouse sarcoma; Sigma-Aldrich). The protocol for this assay has been described in detail previously [17]. Briefly, the HES cells were seeded in 24-well inserts above a polycarbonate membrane (Transwell Permeable Supports; Corning) coated with Matrigel. Culture media supplemented with 10% serum were added to the lower chamber as a chemical attractant. The HES cells were cultured without (control) or with simvastatin, lovastatin, atorvastatin, or pravastatin at 10 μM for 24 h. Following this treatment, the non-invasive cells were removed from the insert and invading cells were fixed, stained with crystal violet and counted using microscopy. Each treatment was carried out in four replicates.
Statistical analysis
Each assay was repeated a minimum of three times using cells from different participants. Each 96-well plate contained at least 8 replicates per treatment, while each 24-well plate contained 4 replicates. Means were compared by analysis of variance, followed by post hoc comparisons using Dunnett’s test. When appropriate, values were logarithmically transformed before further analysis was performed. The JMP program version 11 (SAS Institute, Cary, NC, USA) was used to carry out all statistical analysis. Results are presented as means ± standard error of the mean (SEM) and expressed as a percentage of control. A value of P < 0.05 was considered statistically significant.
Results
Proliferation assay
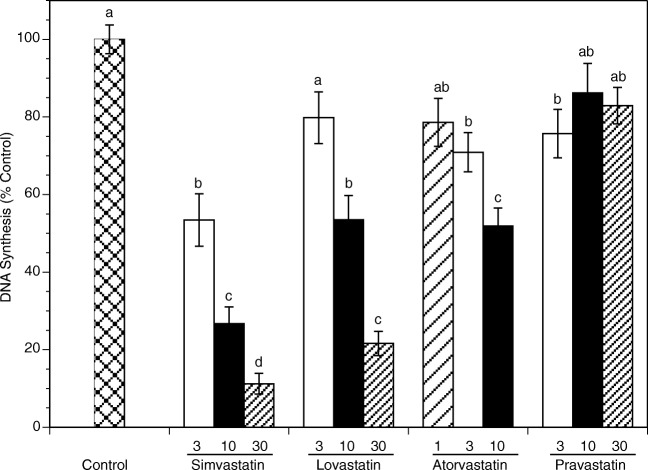
Figure 1 summarizes effects of each statin on proliferation of HES cells. After 48 h, all lipid-soluble statins (simvastatin, lovastatin, and atorvastatin) significantly decreased the number of proliferating cells in a concentration-dependent manner. Simvastatin exerted the greatest effect and decreased proliferation at 3, 10 and 30 μM by 47% (P < 0.0001), 73% (P < 0.0001) and 89% (P < 0.0001), respectively. Lovastatin significantly decreased proliferation at 10 and 30 μM by 46% (P < 0.0001) and 78% (P < 0.0001), respectively. Atorvastatin decreased proliferation at 1, 3 and 10 μM by 21% (P = 0.03), 29% (P = 0.001) and 48% (P < 0.0001), respectively. Pravastatin significantly decreased the number of proliferating cells at 3 μM by 24% (P = 0.01).
Fig. 1.
Effects of statins on DNA synthesis of HES cells cultured for 48 h in the absence (control) or in the presence of simvastatin, lovastatin, pravastatin (3, 10, 30 μM), or atorvastatin (1, 3, 10 μM) as determined by radiolabeled thymidine incorporation assay. Each bar represents the mean ± SEM; means with no letters in common are significantly different (P < 0.05)
Caspase-3/7 activity assay
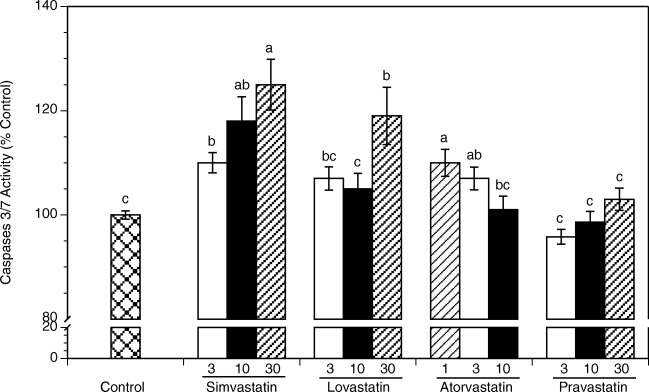
Comparison of effects of individual statins on apoptosis, as measured by the combined activity of executioner caspases 3 and 7 is presented in Fig. 2. At 48 h, cells exposed to simvastatin, lovastatin, and atorvastatin showed a significant increase in caspase-3/7 activity. The greatest and concentration-dependent increases in caspase-3/7 activity were observed in cultures exposed to simvastatin. Simvastatin at 3, 10 and 30 μM increased apoptosis by 10% (P = 0.005), 18% (P < 0.0001) and 25% (P < 0.0001), respectively. Lovastatin had significant effect only at 30 μM increasing caspase-3/7 activity by 19% (P < 0.0001). Atorvastatin at 1 and 3 μM increased activity by 10% (P < 0.0001) and 7% (P = 0.005), respectively. Pravastatin had no significant effect at any of the tested concentrations.
Fig. 2.
Effects of statins on apoptosis of HES cells cultured for 48 h in the absence (control) or in the presence of simvastatin, lovastatin, pravastatin (3, 10, 30 μM), or atorvastatin (1, 3, 10 μM). Each bar represents the mean ± SEM; means with no letters in common are significantly different (P < 0.05)
Cell viability assay (MTS assay)
The net effects of changes in the proliferation and apoptosis of cells result in a change of the number of viable cells, as determined by the MTS assay (Fig. 3). After 48 h, all lipid-soluble statins (simvastatin, lovastatin, and atorvastatin) significantly decreased the number of viable cells. Specifically, the number of viable cells was decreased by simvastatin at 10 μM by 15% (P = 0.04), lovastatin at 30 μM by 16% (P = 0.02) and atorvastatin at 1 and 10 μM by 15% (P = 0.03) and 18% (P = 0.005), respectively. Pravastatin had no statistically significant effect.
Fig. 3.
Effects of statins on the number of viable HES cells cultured for 48 h in the absence (control) or in the presence of simvastatin, lovastatin, pravastatin (3, 10, 30 μM), or atorvastatin (1, 3, 10 μM) as determined by MTS assay. Each bar represents the mean ± SEM; means with no letters in common are significantly different (P < 0.05)
Invasiveness assay
The effects of statins on invasiveness are presented in Fig. 4. The concentration of 10 μM was chosen for the invasiveness assay because it was the lowest concentration that produced a significant effect at 48 h, as seen in the other assays, making it the most applicable concentration. Furthermore, this concentration did not induce significant changes in the number of viable cells at 24 h. Invasiveness decreased significantly for cells treated with simvastatin, lovastatin and atorvastatin at 10 μM by 49% (P < 0.0001), 54% (P < 0.0001) and 53% (P < 0.0001), respectively. In contrast, pravastatin had no significant effect.
Fig. 4.
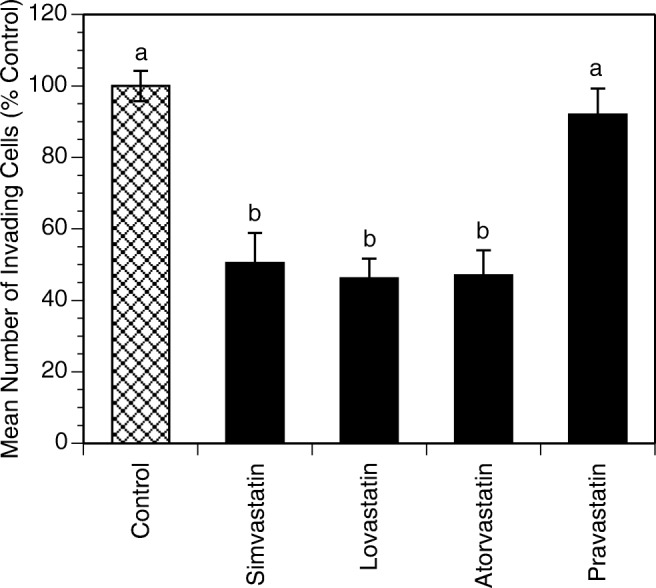
Effects of statins on invasiveness of HES cells cultured for 24 h in the absence (control) or in the presence of simvastatin, lovastatin, pravastatin, or atorvastatin (10 μM). Each bar represents the mean ± SEM; means with no letters in common are significantly different (P < 0.05)
Discussion
We have previously demonstrated that simvastatin affects HES cells proliferation, apoptosis, invasiveness, gene expression in vitro [16–18] and endometriotic lesions in vivo [5]. This novel study demonstrates for the first time that only lipophilic statins, among tested statins, affect endometrial stromal cell growth and invasiveness in vitro. Specifically, we found that simvastatin is the most potent among tested statins in all studied endpoints.
Endometriosis involves the attachment and subsequent growth of endometrial implants outside the uterine cavity. Growth of ectopic endometrial lesions is a net effect of excessive proliferation, decreased apoptosis and increased invasiveness of endometriotic cells. Statins, inhibitors of the rate-limiting enzyme of mevalonate pathway (HMG-CoA reductase), are the most effective and widely prescribed class of drugs to reduce serum cholesterol level and prevent cardiovascular disease. Recent scientific research demonstrated numerous non-cholesterol-lowering properties of statins; these actions are referred to as pleiotropic effects. The pleiotropic effects of statins, broadly studied in various biological systems, including antioxidant [22, 23], anti-inflammatory [24], anti-invasive [25, 26], antiproliferative and pro-apoptotic [27] activities, make them an attractive option as a potential pharmacologic agent to treat endometriosis.
The likely key mechanism responsible for pleiotropic actions of statins is related to inhibition of synthesis of isoprenylation substrates, leading to changes in many important biological pathways. Individual statins, however, have different pharmacokinetic and pharmacodynamic properties as well as different therapeutic dose ranges. These differences are due to their chemical structure, ring attached to the pharmacophore, polar or non-polar substituents, and lipo- or hydrophilicity. Cerivastatin, simvastatin, fluvastatin, lovastatin, atorvastatin and pitavastatin are classified as lipophilic, while pravastatin and rosuvastatin as hydrophilic [28, 29]. Hydrophilic statins tend to be more, but not exclusively [30], hepatoselective, secondary to their tissue-specific active transport system involving organic anion-transporting polypeptide (OATP) [31]. In contrast, lipophilic statins enter cells by passive diffusion through the cell membrane and hence achieve higher concentration in the extrahepatic tissue. These considerations raise the question, which statins are the most effective and appropriate to achieve a given biological effect in specific tissue, in this case for controlling the growth of endometrial implants.
Carrier-mediated uptake makes pravastatin mainly a hepato-specific drug, able to affect cholesterol biosynthesis and other aspects of the mevalonate pathway almost exclusively within the liver [32]. In a study of human smooth muscle cells, both water-soluble and lipid-soluble statins (at 1 μM) affected proliferation, migration and invasion, though pravastatin had the least profound effect [33]. In another study of smooth muscle cells, all statins inhibited proliferation, but the effective doses of lipophilic statins were around 1 μM, while pravastatin was effective at concentrations above 30 μM [34]. In our study, pravastatin had little effect on HES cell proliferation and no effect on apoptotic activity, viable cell number and invasiveness. These limited effects of pravastatin on HES cells were due to the decreased absorption by endometrial stromal cells.
Efficacy of statins depends not only on the presence of tissue-specific active transport system and the ability to diffuse through the cell membrane [30], but also on the dynamics of crossing the cell membrane, metabolism and excretion. While lipophilicity is likely the major factor determining the ability of statins to act on non-hepatic cells, other factors such as absolute hardness, rate of metabolism by specific cytochrome P450 isoenzyme or other pathway(s), ionization energy and buried surface area after binding to the HMG-Co reductase, may explain why not all lipid-soluble statins are equally potent [29]. Indeed, as demonstrated by Turner and co-workers, lipid-soluble statins, in a concentration-dependent manner, inhibited invasion and reduced proliferation in human venous smooth muscle cells in the following order of potency: atorvastatin > simvastatin > lovastatin [35]. Our study has demonstrated that all tested lipid-soluble statins inhibit proliferation, increase apoptosis, decrease the number of viable cells and inhibit invasiveness of HES cells, with simvastatin being the most effective in all studied endpoints. However, it should be emphasized that antiproliferative properties of statins are not universal and are tissue dependent. For example, simvastatin stimulates growth of human osteoblasts and alveolar epithelium [36, 37].
Limitations of our study should be noted. First, this is an in vitro study conducted on a limited number of samples and indicating only a potential superiority of lipophilic statins. Moreover, the study does not address potential differences between healthy women and women with endometriosis with regard to their possibly different responses to statins. To date, there are no published in vivo comparisons of efficacy of various statins in animal models of endometriosis. In vivo studies would also facilitate evaluation of other potential effects of statins on endometriosis, which cannot be reliably tested in endometrial stromal cell cultures, such as inflammation, oxidate stress and especially immune system activation. Another limitation of this study is the use of only one hydrophilic statin. Based on that, the conclusion about class effect of all hydrophilic statins cannot be made with confidence. Consideration should be given to evaluate rosuvastatin, a new-generation hydrophilic statin with high potency, better side-effect profile and different pharmacokinetic profile. It should be emphasized that while lipophilicity of the statins is at least partially responsible for their pleiotropic effects, due to ease of tissue penetration, it also contributes to higher risk of side-effects like myositis and myopathy as well as rhabdomyolysis, impaired insulin secretion and insulin resistance. In addition, different statins also undergo metabolism by different cytochrome P450 isoenzymes, and hence affecting the risk of clinically relevant drug interactions [29].
To date, little is known about effects of the statins on endometriosis in women with this disease. To our knowledge, only one study evaluated effects of a statin on women with endometriosis. In that study, postoperative pain was evaluated in patients who have undergone laparoscopic surgery for endometriosis followed by a 16-week course of simvastatin or GnRH analog. In both groups, there was a significant and comparable reduction of pain at 6 months after surgery. Noted weakness of this study was an absence of a control group evaluating the effect of surgery alone or with placebo treatment [38].
In summary, the present findings demonstrate that only lipid-soluble statins among statins evaluated in our study are effective in inhibiting growth and invasiveness of HES cells in vitro. These findings may be clinically relevant in treatment of endometriosis and suggest that lipid-soluble statins should be preferentially considered for future clinical trials.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54 HD052668.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Sokalska, Email: anna.sokalska@uphs.upenn.edu.
Amanda B. Hawkins, Email: amandablithehawkins@gmail.com
Toshia Yamaguchi, Email: toshia.yamaguchi@ucr.edu.
Antoni J. Duleba, Phone: +1-858-534-8930, Email: aduleba@ucsd.edu
References
- 1.Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20(5):702–716. doi: 10.1093/humupd/dmu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed H, D’Santos C, Serandour AA, Ali HR, Brown GD, Atkins A, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3(2):342–349. doi: 10.1016/j.celrep.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Repetitive conservative surgery for recurrence of endometriosis. Obstet Gynecol. 1991;77(3):421–424. [PubMed] [Google Scholar]
- 5.Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94(7):2489–2494. doi: 10.1210/jc.2008-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oktem M, Esinler I, Eroglu D, Haberal N, Bayraktar N, Zeyneloglu HB. High-dose atorvastatin causes regression of endometriotic implants: a rat model. Human Reprod. 2007;22(5):1474–1480. doi: 10.1093/humrep/del505. [DOI] [PubMed] [Google Scholar]
- 7.Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril. 2010;94(5):1639–46.e1. doi: 10.1016/j.fertnstert.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz B, Ozat M, Kilic S, Gungor T, Aksoy Y, Lordlar N, et al. Atorvastatin causes regression of endometriotic implants in a rat model. Reprod Biomed Online. 2010;20(2):291–299. doi: 10.1016/j.rbmo.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Taylor HS, Alderman Iii M, D’Hooghe TM, Fazleabas AT, Duleba AJ. Effect of simvastatin on baboon endometriosis. Biol Reprod. 2017;97(1):32–38. doi: 10.1093/biolre/iox058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294(3):1043–1046. [PubMed] [Google Scholar]
- 11.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108(4):426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 12.Dje N’Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, et al. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29(3):380–386. doi: 10.1161/ATVBAHA.108.178319. [DOI] [PubMed] [Google Scholar]
- 13.Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006;85(6):1667–1675. doi: 10.1016/j.fertnstert.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 14.Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, Polan ML. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78(4):787–795. doi: 10.1016/S0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ, Seval Y, Cakmak H, Arici A, Duleba AJ. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod. 2006;75:107–111. doi: 10.1095/biolreprod.106.051763. [DOI] [PubMed] [Google Scholar]
- 16.Sokalska A, Wong DH, Cress A, Piotrowski PC, Rzepczynska I, Villanueva J, et al. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab. 2010;95(7):3453–3459. doi: 10.1210/jc.2010-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokalska A, Cress A, Bruner-Tran KL, Osteen KG, Taylor HS, Ortega I, Duleba AJ. Simvastatin decreases invasiveness of human endometrial stromal cells. Biol Reprod. 2012;87:1–6. doi: 10.1095/biolreprod.111.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokalska A, Anderson M, Villanueva J, Ortega I, Bruner-Tran KL, Osteen KG, Duleba AJ. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98:E463–E471. doi: 10.1210/jc.2012-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cakmak H, Basar M, Seval-Celik Y, Osteen KG, Duleba AJ, Taylor HS, et al. Statins inhibit monocyte chemotactic protein 1 expression in endometriosis. Reprod Sci. 2012;19(6):572–579. doi: 10.1177/1933719111430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esfandiari N, Khazaei M, Ai J, Bielecki R, Gotlieb L, Ryan E, Casper RF. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87(2):257–262. doi: 10.1016/j.fertnstert.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril. 2009;92(6):2097–2099. doi: 10.1016/j.fertnstert.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 23.Franzoni F, Quinones-Galvan A, Regoli F, Ferrannini E, Galetta F. A comparative study of the in vitro antioxidant activity of statins. Int J Cardiol. 2003;90(2–3):317–321. doi: 10.1016/S0167-5273(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer M, Mitmaker B, Obrand D, Sheiner N, Abraham C, Dostanic S, Meilleur M, Sugahara T, Chalifour LE. Atorvastatin modulates matrix metalloproteinase expression, activity, and signaling in abdominal aortic aneurysms. Vasc Endovasc Surg. 2010;44(2):116–122. doi: 10.1177/1538574409348352. [DOI] [PubMed] [Google Scholar]
- 26.Porter KE, Naik J, Turner NA, Dickinson T, Thompson MM, London NJ. Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg. 2002;36(1):150–157. doi: 10.1067/mva.2002.122029. [DOI] [PubMed] [Google Scholar]
- 27.Ali OF, Growcott EJ, Butrous GS, Wharton J. Pleiotropic effects of statins in distal human pulmonary artery smooth muscle cells. Respir Res. 2011;12:137. doi: 10.1186/1465-9921-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedi O, Dhawan V, Sharma PL, Kumar P. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedeberg’s Arch Pharmacol. 2016;389(7):695–712. doi: 10.1007/s00210-016-1252-4. [DOI] [PubMed] [Google Scholar]
- 29.Fong CW. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. 2014;85:661–674. doi: 10.1016/j.ejmech.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Abe M, Toyohara T, Ishii A, Suzuki T, Noguchi N, Akiyama Y, Shiwaku HO, Nakagomi-Hagihara R, Zheng G, Shibata E, Souma T, Shindo T, Shima H, Takeuchi Y, Mishima E, Tanemoto M, Terasaki T, Onogawa T, Unno M, Ito S, Takasawa S, Abe T. The HMG-CoA reductase inhibitor pravastatin stimulates insulin secretion through organic anion transporter polypeptides. Drug Metab Pharmacokinet. 2010;25(3):274–282. doi: 10.2133/dmpk.25.274. [DOI] [PubMed] [Google Scholar]
- 31.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19(1):26–37. doi: 10.1016/S0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 32.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 33.Corpataux JM, Naik J, Porter KE, London NJ. The effect of six different statins on the proliferation, migration, and invasion of human smooth muscle cells. J Surg Res. 2005;129(1):52–56. doi: 10.1016/j.jss.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Nègre-Aminou P, van Vliet AK, van Erck M, van Thiel GC, van Leeuwen RE, Cohen LH. Inhibition of proliferation of human smooth muscle cells by various HMG-CoA reductase inhibitors; comparison with other human cell types. Biochim Biophys Acta. 1997;1345(3):259–268. doi: 10.1016/S0005-2760(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 35.Turner NA, Midgley L, O’Regan DJ, Porter KE. Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion. J Cardiovasc Pharmacol. 2007;50(4):458–461. doi: 10.1097/FJC.0b013e318123767f. [DOI] [PubMed] [Google Scholar]
- 36.Chuang SC, Liao HJ, Li CJ, Wang GJ, Chang JK, Ho ML. Simvastatin enhances human osteoblast proliferation involved in mitochondrial energy generation. Eur J Pharmacol. 2013;714(1–3):74–82. doi: 10.1016/j.ejphar.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 37.Xia S, Kang J, Jiang Y, Huang D, Wang S, Pang B. Simvastatin promotes alveolar epithelial cell proliferation and attenuates cigarette smokeinduced emphysema in rats. Mol Med Rep. 2015;12(4):5903–5910. doi: 10.3892/mmr.2015.4172. [DOI] [PubMed] [Google Scholar]
- 38.Almassinokiani F, Mehdizadeh A, Sariri E, Rezaei M, Almasi A, Akbari H, et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:534–539. doi: 10.12659/MSM.883967. [DOI] [PMC free article] [PubMed] [Google Scholar]