Abstract
The process of storage and transportation of the red blood cells (RBCs) out of the standard temperature range lead to some biochemical reactions. Infusing inappropriately stored RBCs may cause severe complications. The main objective of this study was to investigate the RBC bags’ temperature during the transfusion chain including storage, transportation, and transfusion. A cross-sectional study was performed on 100 RBC bags that were sent from the blood bank to the cardiac surgical intensive care unit (CSICU) and the operating room (OR). To record the temperature of RBCs, a temperature monitoring device was attached to each bag of RBCs that were transported from the blood bank to the CSICU and the OR. The stored temperature samples in the devices related to different stages were separated. Finally, the normal and non-normal samples of each phase were segregated based on the current guidelines. The results indicated that 10% of 121,262 recorded temperatures samples (per 2 min) were out of the standard range. Of these, 65, 17, 13, and 5% of samples were related to the blood bank, the OR, transportation, and the CSICU, respectively. The minimum and maximum temperatures were 0 °C and 19.5 °C that were below and above the standard, respectively. In the light of findings of the present study, different stages of blood transportation and storage suffer a number of shortcomings, which are more evident in the blood bank. Thus, it is recommended to better manage blood transfusion chain from the blood bank to hospital wards so as to avoid the inadvertent and undesirable consequences of blood transfusion. Because various judgments made by the personnel about the status of blood bags are subjective, a temperature monitoring device can be employed to better monitor the blood transfusion process and compensate for the errors unnoticed by the personnel.
Keywords: Transfusion, Red blood cell, Blood quality, Inappropriate temperature
Introduction
Blood cold chain is an organized process for blood storage and carriage in a secured manner from the time blood is received from the donor till the time it is transfused to the patient. Because of the fact that blood is a biological material, it is vital to keep it in a cold environment to preserve the viability feature and improve the hematologic condition of the patient [1]. The process of storage and carriage of the red blood cells (RBCs) out of the standard temperature range leads to some biochemical reactions. These reactions cause the blood to lose the ability to carry oxygen and carbon dioxide to/from tissues while transfusing the blood [2]. One of the main reasons of preserving blood in the standard temperature is to minimize the bacterial growth. If blood is kept beyond the higher defined limit, even for a short period of time, the bacteria that are infiltrated into blood from the donor will quickly grow and propagate [3]. On the other hand, keeping blood in a temperature less than the defined lower limit might damage the membrane with resultant hemolysis that will increase mortality and morbidity [4]. Thus, it is absolutely necessary to monitor the temperature changes of blood products, characterize the potent failure locations, investigate the influential reasons and modify them. This can prevent adverse events including death, development of organ failure, infection, and long hospitalization.
Based on major European and American guidelines, the temperature of RBC bags must be between 1 and 6 °C while storing and between 1 and 10 °C while carrying [5–7]. Therefore, various researches were conducted to examine the temperature monitoring instruments and their application in order to investigate the storage and transportation condition of blood products [8–11]. It has been demonstrated that these instruments lead to better decision making in comparison to time-based rules and therefore prevent the unnecessary and expensive process of discarding the RBC bags.
By comprehending the importance of the blood transfusion process and the probable issues in the storage and transportation stages, the objective of this study was to investigate the RBC bags’ temperature during the transfusion chain including storage, transportation, and transfusion.
Materials and Methods
Study Design
In a cross-sectional study, the RBC bags that were to send from the hospital blood bank to the cardiac surgical intensive care unit (CSICU) and/or the operating room (OR) were investigated regarding their temperature in an academic hospital located in Mashhad, Iran, in a thirty-day period in winter 2015. The recipients were examined for possible non-immunological hemolytic reactions during and after the blood transfusion.
Sample Size
The sample size in 90% confidence interval, P = 0.05 and d = 10% was estimated roughly at 100. The parameter P examines the ratio of the problem existence in the population. Here the value of P was considered at the worst condition so that the maximum sample number was obtained:
| 1 |
It is worth mentioning that samples were selected by convenience sampling.
Outcome Measurement
The main purpose of the present research was to investigate the blood transfusion chain in terms of temperature fluctuations, identification of stages prone to errors and planning to improve them. To achieve this objective, the time duration for which the blood bags were stored at an improper temperature in different phases was recorded using a temperature monitoring device. Moreover, the rate of non-immunological hemolytic reactions was analyzed due to the non-standard temperature of blood at the time of transfusion. Thus, in case hemolytic symptoms such as fever, bone pain, pallor and hematuria emerged, hemolysis parameters were examined in the laboratory.
Instruments
A temperature monitoring system was developed by the research group for this study and was used to monitor the temperature of RBCs [10]. As shown in Fig. 1, this device was attached to individual RBC bags that were transported from the blood bank to the CSICU and the OR. The temperature monitoring system was equipped with an accurate sensor and a memory chip to save the recorded temperature in the specified time interval (one per 2 min). One of the main features of this system is that it compares the recorded temperatures with the standard range and declares appropriate warning messages. In this study, the alarm capability of the device was disabled and only the RBC temperature during storage and transportation was recorded on the embedded memory chip. Finally, after blood transfusion or return to the blood bank, the temperature monitoring device data was transferred to a computer system for further analysis.
Fig. 1.
Temperature monitoring device attached to a blood bag
To evaluate the accuracy and precision of the device, a laser-thermometer was used (precision of ± 1 °C) as well as a digital thermometer (precision of ± 1 °C) and a HANNA (model number: HI 98509) digital thermometer (precision of ± 0.2 °C). HANNA digital thermometer enjoys a high precision and was, therefore, used as the evaluation gold standard. To meet this objective, in the first phase of the evaluation, standardized blood bags were filled with water and refrigerated so as to simulate natural conditions for blood storage. Then, in different scenarios, the temperature of water was measured and recorded using our temperature monitoring device and the HANNA digital thermometer. In the second phase of evaluation, the expired blood bags were used to measure blood temperature in different scenarios using our temperature monitoring device and the HANNA digital thermometer. In light of the findings of our evaluations (mean temperature difference between the monitoring device and that of the HANNA digital thermometer), the device was calibrated so as to achieve the least difference between our device and the HANNA digital thermometer.
In order to develop the possibility of reexamination of the data recorded in the temperature monitoring devices, a follow-up form was designed based on the different stages that the RBC bag passed from the blood bank center to the transfusion unit or returning to the blood bank. In this form the demographic data of the patient, the RBC bag data, the data of the device attached to the RBC bag, the time of entrance and exit of each bag going through various stages of storage and carriage and also the consequences of transfusion were recorded. After the data entry was completed, the form was attached to the bag.
Implementation Process
Investigating the RBC Bags’ Temperatures
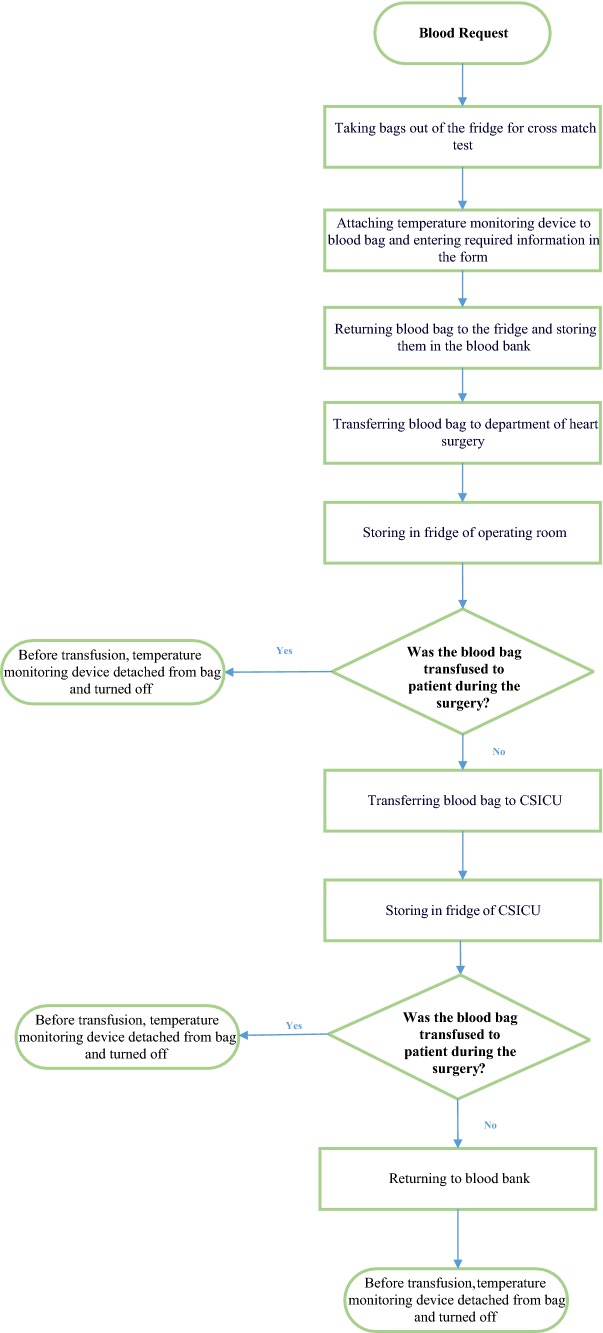
There were six stages of transportation and storage for each blood bag: storage in the blood bank, transportation from the blood bank to the OR, storage in the OR, transportation to the CSICU if not used in the OR, storage in the CSICU, and finally transportation to the blood bank if not used (Fig. 2).
After receipt of request for RBCs by the open heart surgery department, the blood bags with compatible blood groups were selected from the refrigerator. The cords were separated from the blood bag and the cross match was initiated. After cross match, the device was attached to the RBC bag and was turned on. The bag’s unit number and the device number were recorded in the follow-up form attached to the blood bag. The blood bags were returned to the refrigerator in the blood bank.
The next day when delivering blood bags to the operating room, the exit time of the bag from the blood bank was recorded in the follow-up.
While delivering the blood bag to the operating room, the exact time was recorded in the attached form.
Before transfusing the blood bag to the patient, the device was turned off and the transfusion time was also recorded.
Concerning the non-transfused bags that were leaving the operating room, the exit time from the operating room and the delivery time to the CSICU were recorded.
If the bag was transfused in the CSICU, the device would be turned off and the transfusion time would be recorded.
In the case that the blood bag was not used in the CSICU, it was sent to the blood bank and the sending time was recorded.
In the blood bank, the delivery time of the blood bag was written and the device was turned off.
Fig. 2.
Flowchart of transfusion chain using temperature monitoring device
It should be noted the relevant personnel were unaware of the aim of the study, how and why the device worked. They were only trained how to turn on and off the device and how to complete the form.
Investigation of the Fridge Performance
After the data analysis regarding the temperature of the RBC bags and exploitation of the non-standard temperature and their location, we decided to investigate the temperature of the fridges inside the blood bank, the OR and the CSICU. Thus, the temperature monitoring devices were installed in different levels of fridges for a week to monitor the temperature continuously. It should be noted that calibration and qualitative control of all equipment including the fridges was done regularly according to standard operating procedure (SOP). This procedure was done before the study began too.
Data Analysis
Using the follow-up form of each RBC bag and the times recorded, the stored temperature samples in the device related to different stages were categorized and separated. The sum of the samples regarding transferring from the blood bank to the OR, from the OR to the CSICU and from the CSICU to the blood bank was considered as the transferring samples.
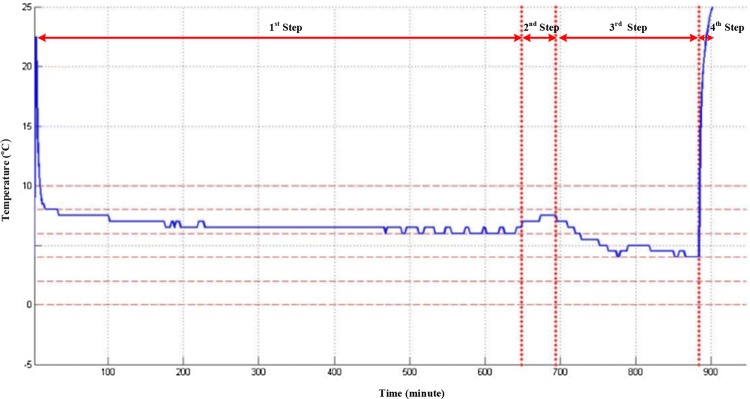
Figure 3 demonstrates a sample of extracted figures from the devices attached to the RBC bags.
Fig. 3.
The temperature recorded by the monitoring device related to a RBC bag. The illustrated steps in the figure are as follows: (1) Attaching the device to the RBC bag and storing in the blood bank fridge. (2) Exiting the RBC bag from the blood bank and transferring to the OR. (3) Storing in the OR fridge till the transfusion time (4) Transfusing the blood and detaching the device from the bag and turning it off
As it is seen in the first step, there was a delay from the time the device was turned on till it was attached to the RBC bag that caused the system to report the room temperature. In order to erase the invalid data, the first 10 samples recorded in the device which were 20 min were deleted. In the final step by using the transfusion time recorded in the follow-up form, the invalid data after it was erased.
Finally based on the guidelines used in the studied center (1–6 °C while storing and 1–10 °C while transferring) the normal and non-normal samples were segregated. Also the non-normal samples were categorized into 3 main parts naming temperature under 1 °C, temperature above 6 °C and temperature above 10 °C.
Results
Investigating the RBC Bags’ Temperatures
The temperature monitoring devices since the turning-on time recorded the temperature of the RBC bags every 2 min continuously. From the 121,262 recorded samples, the temperature of 109,244 samples was in the standard range and 12,018 (10%) samples were out of it (Table 1).
Table 1.
The number of normal and non-normal samples in various stages
| Total | Blood bank | Operating room | CSICU* | Transportation | |
|---|---|---|---|---|---|
| No. all samples | 121,262 | 26,285 (21.6%) | 30,855 (25.5%) | 60,117 (49.6%) | 4005 (3.3%) |
| No. normal samples | 109,244 | 18,504 (17%) | 28,775 (26.4%) | 59,477 (54.4%) | 2488 (2.2%) |
| No. non-normal samples | 12,018 | 7781 (64.8%) | 2080 (17.3%) | 640 (5.3%) | 1517 (12.6%) |
*CSICU Cardiac surgical intensive care unit
Using the non-normal samples in each stage and all the non-normal samples combined, the ratio of the non-normal samples in each stage to all of the non-normal samples was calculated (Fig. 4).
Fig. 4.
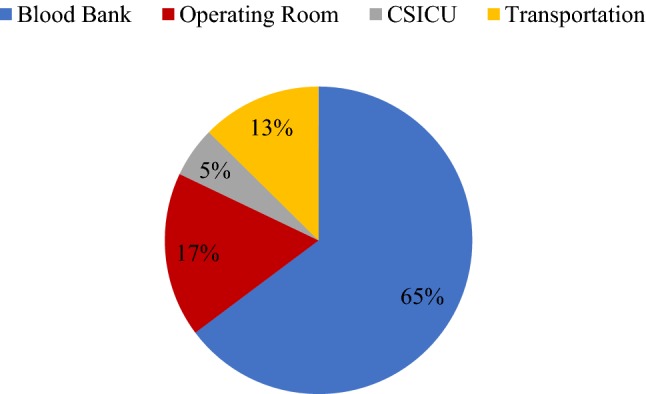
The percentage of the non-normal samples of each stage to the whole non-normal samples
Because of the fact that the time period the RBC bags were in each phase was different for each bag, the critical condition of each phase was established based on the duration of each time period. Therefore the ratio of non-normal data of each phase was calculated relative to the whole data of the respective phase. The percentage of non-normal samples of each stage to the whole samples of the same stage is shown in Fig. 5.
Fig. 5.
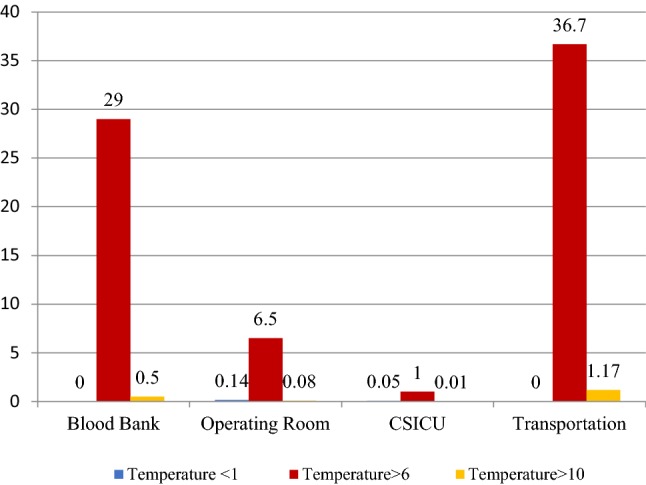
The percentage of non-normal samples of each stage to the whole samples of the same stage
The observation of the recorded samples showed that the minimum temperature 0 °C belonged to the OR and the maximum temperature 19.5 °C belonged to the blood bank (Table 2).
Table 2.
Minimum, maximum and average of blood bag temperature in °C
| Blood bank | Operating room | CSICU* | Transportation | |
|---|---|---|---|---|
| Minimum | 2.5 | 0 | 0.5 | 1 |
| Maximum | 19.5 | 19 | 17 | 16 |
| Average | 6.2 | 5 | 4.1 | 5.4 |
*CSICU Cardiac surgical intensive care unit
Investigation of the Fridge Performance
The observation of the recorded samples showed that not only the fridge temperature was out of the standard range for suitable preservation of the RBC bags, but also suffered from alternative fluctuations that consequently affected the RBC bags. Also the temperature of the bags was different in each level of the fridge. Figure 6 shows the recorded temperature in each level of the OR fridge. Analyzing this figure determined that temperature changes of this fridge had the alternative period of 36 min ranging from − 1.5 °C to 7.5 °C, both of which out of the standard range for safekeeping the RBC bags.
Fig. 6.
Temperature fluctuation in operating room fridge
None of the patients showed any hemolytic reaction symptoms during the study period. So, further laboratory evaluations to determine the hemolysis parameters were not performed.
Discussion
Our study showed that 65% of samples with out of standard temperature were related to the blood bank. According to the ISO15189 standard which is related to the medical laboratory requirements, the blood bank is responsible for keeping the blood product quality stored or sent for transfusion. One of the requirements is supervision of controlling the blood cold chain [12]. Insufficiency of personnel to do the blood compatibility tests and therefore the existence of the RBC bags out of the fridges for a long time, is the most important reason known in this study that made the blood bank to be in top of the list not following the standards in the blood cold chain. As it is mentioned in other studies as well, providing equipment for hospitals’ blood bank centers, skilled personnel and defining standard instructions are among the effective parameters on executing the cold chain [13].The result of Heitmiller et al. showed that 87% of wasted RBCs were caused by inappropriate temperature during storage and transportation. Inadequate knowledge and education of the staff, lack of accountability, inappropriate transportation equipment, and temperature monitoring devices, are declared as the important factors which affected the RBC wastage. The interventions (sigma methodology) conducted by the investigators resulted in a decrease of RBC product wastage from 4.4 to 2% [14]. In another study, the implementation of a feedback-based intervention to increase the awareness of the staff of critical conditions has improved conditions of blood storage and transport [15].
The results also showed that the OR is the second place where the standards for blood preserving temperature are not addressed suitably. The reason why the temperature of the RBC bags has been out of the range in the OR and the CSICU was lack of periodic inspection of blood product storage equipment in these wards. Using non-standard fridges caused alterations in the fridges’ thermostat out of the standard range. The effect of these alterations on the RBC bags kept in the OR and the CSICU fridges had caused the temperature of the RBC bags to drop as low as 0 °C. Temperatures less than the lower end of the standard range could lead to hemolysis and deadly reactions for the patient. As it has been emphasized in other studies, refrigerators should be equipped with a continuous temperature monitoring device with an alarm capability to detect marked fluctuations [16].
Another result worth mentioning is the increase in the RBC bag temperature while transferring. It can be claimed based on the RBC bag temperature figures that there has been increases in temperature while transferring from the blood bank to the OR, from the OR to the CSICU and from the CSICU back to the blood bank. The usage of non-standard cool boxes or the transport of RBCs together with patients between the OR, the CSICU or other wards might cause transportation of RBCs outside the specific defined temperatures. Rising awareness of related staff through providing general information on proper handling of blood products can be effective in reducing blood wastage, as has been noted in other studies [17]. The unit volume, bag width, transferring duration, fluctuation in ambient temperature during transport, the different characteristics and types of cooling boxes including the size and the insulators used in the box production, using coolants like ice packages and the number of RBC bags in the box are suggested among the known factors for controlling the cold chain for carriage [8, 9, 18–22].
However published studies demonstrated that RBC units are associated with a greater degree of hemolysis and septic transfusion reactions when exceeding defined temperature [23–25]. Investigation for the hemolytic consequences of non-standard blood temperature was not performed because of the few number of samples and the limited time for the study. It is expected that by investigating more samples and long follow-ups, the consequences of hemolysis due to keeping the RBC bags in non-standard conditions might be discovered.
In the present study, an uninterrupted temperature monitoring device was used that, compared to available technologies such as temperature and time/temperature tags, possessed more capabilities. For instance, unlike available tags, this device detected and recorded upper and lower bound exit temperatures, simultaneously. Also, uninterrupted reporting of the RBC bag temperature inside the provided storage memory and illustrating it as a diagram provided the capability to characterize temperature alterations. The temperature alterations during blood storage and transportation, even in the standard range, can decrease blood quality. So, as mentioned in other studies, the temperature changes should be monitored and documented using temperature monitoring equipment placed on blood bags [26]. Using temperature monitoring systems with alarm capability as a decision support system in unsuitable temperature conditions can be helpful to detect critical conditions before and after blood corruption and prevent the use of corrupted blood bags. Numerous studies have been conducted on the use of decision support systems for healthcare applications. Decreasing medical errors, increasing preventive care services and improving the tendency towards caring standards are among the benefits provided by clinical decision support systems [27–29].
We demonstrated that a considerable percentage of the RBC bags confront non-standard temperatures at the storage time and carriage time and this requires some modifications in transfusion process. The effect of different interventions on reducing the blood products wastage suggested in several studies can be used to improve the current condition [14, 17, 30, 31]. The effects of interventions such as using critical condition warning systems on the blood bag, replacement or maintenance of old equipment, reporting the errors to the corresponding supervisors and modify them can be investigated in future studies.
Limitations
Our study had several limitations.
It is possible that the blood product from the production time till consumption confronts different scenarios with various temperature conditions. Thus it is vital to monitor the product temperature starting from the production time and being uninterruptedly continued till the consumption time or corruption. In this study because of the limited number of temperature monitoring devices, just the RBC bags that were sent to the open heart surgery ward were investigated.
The temperature monitoring device just like other available instruments is able to check the surface temperature of the bag [32]. However, this issue exists in other instruments including body thermometers as well [33–35].
Turning on the device before attaching it to the RBC bag and turning it off after detaching it from the bag leads to reporting the room temperature. In this study, in order to omit the invalid data, a process called data cleaning was applied manually. In order to solve the problem of reporting invalid data, a method should be used that just after the detachment of the device from the RBC bag it would be stopped.
Conclusion
In the light of findings of the present study, different stages of blood transportation and storage suffer a number of shortcomings, which are more evident in the blood bank. Thus, it is recommended to better manage blood transfusion chain from the blood bank to hospital wards so as to avoid the inadvertent and undesirable consequences of blood transfusion. Because various judgments made by the personnel about the status of blood bags are subjective, a temperature monitoring device can be employed to better monitor the blood transfusion process and compensate for the errors unnoticed by the personnel.
Author’s Contributions
SE, SA, MK, and SA (first author) conceived the study idea and design. SE, HS, and SA (first author) participated in the device development. SA (first author) collected the data. MK, SE and SA interpreted the data. SA (first author) drafted the manuscript. All authors have been involved in critically revising the manuscript. All authors read and approved the final manuscript.
Funding
This research was part of the first author’s MSc thesis which was supported by a grant from Mashhad University of Medical Science Research Council (Khorasan Razavi, Mashhad, Iran; Number: 950116; Date: September 7, 2016).
Conflict of interest
The authoer declare that they have no conflict of interest.
Ethical Approval
Ethical committee approval for the study was obtained from Mashhad University of Medical Sciences on 07 Jun 2015 and numbered 930616.
References
- 1.Mvere D, Bond K. The blood cold chain: guide to the selection and procurement of equipment and accessories. Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1(2):47–51. doi: 10.4103/0973-6247.33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norfolk D. UK blood transfusion services. Handbook of transfusion medicine. 5. London: Stationery Office; 2013. [Google Scholar]
- 4.Groth S. Manual on the management, maintenance and use of blood cold chain equipment. Geneva: World Health Organization; 2005. [Google Scholar]
- 5.Ooley P, Anderson T, Beaton M, Benson K, Cohn C, Domen R. Standards for blood banks and transfusion services. Bethesda: AABB; 2015. [Google Scholar]
- 6.James V, McClelland B. Guidelines for the blood transfusion services in the United Kingdom. London: The Stationery Office; 2005. [Google Scholar]
- 7.Medicines EDftQo. (2015) HealthCare. guide to the preparation, use and quality assurance of blood components. Recommendation No. R (95) 15, Council of Europe
- 8.Nurasyikin Y, Leong C, Fadhlullah T, Hafiz W, Nadiah Z, Atieqah A, et al. Role of blood bag temperature indicators in maintaining patent temperature of the returned unused blood bags in blood bank. Clin Ter. 2011;162(1):19–22. [PubMed] [Google Scholar]
- 9.Reiter U, Wagner T, Kozma N, Reiter G, Lanzer G. Core and surface temperatures in a red-blood-cell unit during storage and transport. Vox Sang. 2011;101(1):10–15. doi: 10.1111/j.1423-0410.2010.01452.x. [DOI] [PubMed] [Google Scholar]
- 10.Aalaei S, Amini S, Keramati M, Shahraki H, Abu-Hanna A, Eslami S. Blood bag temperature monitoring system. Studies Health Technol Inform. 2014;205:730–734. [PubMed] [Google Scholar]
- 11.Sigle JP, Holbro A, Lehmann T, Infanti L, Gerull S, Stern M, et al. Temperature-sensitive indicators for monitoring rbc concentrates out of controlled temperature storage. Am J Clin Pathol. 2015;144(1):145–150. doi: 10.1309/AJCPN7L9RTTPNNRW. [DOI] [PubMed] [Google Scholar]
- 12.Guzel O, Guner EI. ISO 15189 Accreditation: requirements for quality and competence of medical laboratories, experience of a laboratory I. Clin Biochem. 2009;42(4):274–278. doi: 10.1016/j.clinbiochem.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Teimuri Naghadeh H, Karimi G, Rostamian AR, Kiadaliri K, Behzad J, Vafaei Shooshtari M, et al. Assessment of cold chain equipment and the study of effective factors in storage of blood and blood components in Mazandaran and Gilan provinces. Sci J Iran Blood Transfus Organ. 2011;7(4):235–241. [Google Scholar]
- 14.Heitmiller ES, Hill RB, Marshall CE, Parsons BJ, Berkow LC, Barrasso CA, et al. Blood wastage reduction using Lean Sigma methodology. Transfusion. 2010;50(9):1887–1896. doi: 10.1111/j.1537-2995.2010.02679.x. [DOI] [PubMed] [Google Scholar]
- 15.Aalaei S, Amini S, Keramati MR, Tabesh H, Taherzadeh Z, Khoshrounezhad S, et al. Effectiveness of intervention due to feedback on errors arising from inappropriate transportation and storage of blood bags in hospitals: a quasi-experimental study. Electron Physician. 2018;10(5):6764. doi: 10.19082/6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caroline K (2016) Blood Products. In: Yagi K, Holowaychuk M (eds) Manual of veterinary transfusion medicine and blood banking. Wiley & Sons, p 27–42
- 17.Bots M, Grouw E, Rooyen-Schreurs I, Akker G, Sturk A, Klinkspoor J, et al. Strategies to reduce wastage of red blood cell units. Vox Sang. 2016;110(2):143–149. doi: 10.1111/vox.12351. [DOI] [PubMed] [Google Scholar]
- 18.Sharley PH, Williams I, Hague S. Blood transportation for medical retrieval services. Air Med J. 2003;22(6):24–27. doi: 10.1016/j.amj.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Grey D, Fong E, Soares Mendes R, Gilhooley G, Cardey J, Finlayson J. Evaluation of a new transportation container for red cell units in Western Australia. ISBT Sci Ser. 2015;10(2):82–86. doi: 10.1111/voxs.12220. [DOI] [Google Scholar]
- 20.Perry HE, Prasad P, Kirwan S, Huang YQ. Core temperature changes in resuspended red blood cells (RBCs) and pediatric RBCs removed from refrigerated storage. Transfusion. 2010;50(1):174–177. doi: 10.1111/j.1537-2995.2009.02384.x. [DOI] [PubMed] [Google Scholar]
- 21.Reiter U, Reiter G, Wagner T, Kozma N, Roland J, Schöllnast H, et al. Four-dimensional temperature distributions in red blood cells withdrawn from storage and exposed to ambient temperature: a magnetic resonance thermometry study. Transfusion. 2013;53(1):167–173. doi: 10.1111/j.1537-2995.2012.03798.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas S, Wiltshire M, Hancock V, Fletcher S, McDonald C, Cardigan R. Core temperature changes in red blood cells. Transfusion. 2011;51(2):442–443. doi: 10.1111/j.1537-2995.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez-Arcos S, Mastronardi C, Perkins H, Kou Y, Turner T, Mastronardi E, et al. Evaluating the 4-hour and 30-minute rules: effects of room temperature exposure on red blood cell quality and bacterial growth. Transfusion. 2013;53(4):851–859. doi: 10.1111/j.1537-2995.2012.03807.x. [DOI] [PubMed] [Google Scholar]
- 24.Pick P, Fabijanic J. Temperature changes in donor blood under different storage conditions. Transfusion. 1971;11(4):213–215. doi: 10.1111/j.1537-2995.1971.tb04403.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumani D, Goldfinger D, Ziman A. Is the 30-minute rule still applicable in the 21st century? Transfusion. 2013;53(6):1150–1152. doi: 10.1111/trf.12220. [DOI] [PubMed] [Google Scholar]
- 26.Basu D, Kulkarni R. Overview of blood components and their preparation. Indian J Anaesth. 2014;58(5):529. doi: 10.4103/0019-5049.144647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiffman RN, Liaw Y, Brandt CA, Corb GJ. Computer-based guideline implementation systems a systematic review of functionality and effectiveness. J Am Med Inform Assoc. 1999;6(2):104–114. doi: 10.1136/jamia.1999.0060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Arch Intern Med. 2000;160(3):301–308. doi: 10.1001/archinte.160.3.301. [DOI] [PubMed] [Google Scholar]
- 30.Collins RA, Wisniewski MK, Waters JH, Triulzi DJ, Yazer MH. Effectiveness of multiple initiatives to reduce blood component wastage. Am J Clin Pathol. 2015;143(3):329–335. doi: 10.1309/AJCP42WMHSSTPHXI. [DOI] [PubMed] [Google Scholar]
- 31.Whitney GM, Woods MC, France DJ, Austin TM, Deegan RJ, Paroskie A, et al. Reducing intraoperative red blood cell unit wastage in a large academic medical center. Transfusion. 2015;55(11):2752–2758. doi: 10.1111/trf.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson V, Langeberg A, Taye-Makuria A, Sandler SG. Temperature-sensitive labels for containers of RBCs. Am J Clin Pathol. 2006;126(3):406–410. doi: 10.1309/QD8H135A6D72Y22N. [DOI] [PubMed] [Google Scholar]
- 33.Casa DJ, Becker SM, Ganio MS, Brown CM, Yeargin SW, Roti MW, et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42(3):333. [PMC free article] [PubMed] [Google Scholar]
- 34.Mazerolle SM, Ganio MS, Casa DJ, Vingren J, Klau J. Is oral temperature an accurate measurement of deep body temperature? a systematic review. J Athl Train. 2011;46(5):566. doi: 10.4085/1062-6050-46.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran DS, Mendal L. Core temperature measurement. Sports Med. 2002;32(14):879–885. doi: 10.2165/00007256-200232140-00001. [DOI] [PubMed] [Google Scholar]