Abstract
Objectives
To analyze in detail the fatty acid (FA) composition of follicular fluid (FF) from two-sized follicles at oocyte retrieval and to determine associations of the FAs from large follicles with the woman’s age and the response to ovarian stimulation.
Design
Observational study.
Setting
University and fertility clinic.
Patients
Sixty-four women (age 19–46), consisting of unfertile patients and oocyte donors, undergoing controlled ovarian stimulation.
Interventions
None.
Main outcome measure(s)
FF from small (< 12 mm) and large (≥ 18 mm) follicles was collected at oocyte retrieval. FAs by gas chromatography-flame ionization detection.
Result
Thirty-two FAs with chain lengths ranging from 14 to 25 carbons were identified. There was a readjustment in FA distribution as follicle size increased, raising very long-chain saturated FAs, nervonic (24:1n-9), arachidonic (20:4n-6), and n-3 polyunsaturated FAs (PUFA, P < 0.001), the latter mainly due to an increase in docosahexaenoic acid (22:6n-3, DHA). In large follicles, double bond and peroxidizability indices and total n-3 PUFA, particularly DHA, correlated positively with the woman’s age and negatively with the number of total and mature oocytes, total and top-quality embryos, and fertilization rate.
Conclusions
We have described 32 FAs in ovarian FF, of which 16 changed their distribution with follicle size. The results also indicate that lower n-3 PUFA levels in large follicles, which are associated with younger women, predict a better response to ovarian stimulation based on the recovery of total and mature oocytes, total and top-quality embryos, and fertilization rate per cycle.
Key message
The fatty acid profile of ovarian FF changes as the follicle grows and lower n-3 PUFA levels in large follicles, associated with younger women, predict a better response to ovarian stimulation.
Keywords: Female infertility, Fatty acids, Aging, Gas chromatography, Follicular fluid
Introduction
The woman’s age has a negative impact on fertility and is one of the most common causes of infertility [1]. Assisted reproduction techniques help couples to conceive. A first step in treatment consists basically of controlled ovarian hyperstimulation with gonadotropins, which leads to the growth of multiple follicles. Follicular fluid (FF) surrounds the maturing oocyte prior to fertilization and can therefore influence the outcomes of assisted reproduction, such as fertilization, embryonic development, and pregnancy rate. FF contains fatty acids that are essential for follicular growth and oocyte quality. Fatty acids not only are energy reserve molecules, necessary for cell proliferation during the formation of antral follicles, but also have structural and regulatory functions [2]. They are components of phospholipids and cholesteryl esters in cell membranes, maintaining their fluidity and integrity, and participate in cell signaling and metabolic regulation [3]. Among the different unsaturated fatty acids, the polyunsaturated fatty acids (PUFA) of the omega-6 (n-6) and omega-3 (n-3) series are synthesized from their respective precursors: linoleic acid (n-6) and α-linolenic acid (n-3), which are essential and must therefore be supplied by the diet. The n-3 and n-6 PUFA are necessary in female reproduction [4].
Although some works have described the composition of various fatty acids from the FF of women in the state prior to ovulation [5, 6], little is known about the fatty acid composition of the total lipids during folliculogenesis. The first objective of the present study was to address this issue by comparing the differences that exist between small and large follicles from the same woman. On the other hand, the fatty acid profile of the FF from dominant follicles may be related to the outcomes of ovarian stimulation. The second objective of the work was to establish if there was any correlation between specific fatty acids (or other lipid parameters) with the response to ovarian stimulation, based on the number of total and mature metaphase II oocytes, and total and top-quality embryos that were obtained per cycle, and the fertilization rate. Since the woman’s age is a major factor that negatively affects reproduction, this variable was also considered in the study.
Materials and methods
Study population and sample collection
Sixty-four women were randomly recruited in the assisted reproduction clinic, IVI-RMA in Bilbao (www.ivi.es, Vizcaya, Spain). The women included patients with fertility problems, who entered to the clinic to follow assisted reproduction techniques (n = 43), and fertile women (with at least one living child), who entered to the Egg Donation Program of the clinic (n = 21). Exclusion criteria for participation in the study were (1) vitamin supplementation, (2) cardiovascular medical history, (3) hypertensive disorder, (4) metabolic disease, (5) polycystic ovary syndrome, and (6) endometriosis.
All women underwent gonadotropin treatment according to the standard protocol of the clinic which has been described previously [7]. It consisted in a GnRH agonist protocol. Ovarian stimulation was performed with gonadotropins (recombinant FSH, highly purified human menopausal gonadotropin, or a combination of both) for an average of 9 or 10 days. Ovarian response was assessed by vaginal ultrasound examination and serum estradiol levels every 2 days. Ovulation was induced by single subcutaneous administration of a GnRH agonist (2 mg triptorelin, Ipsen Pharma-Biotech, France) when at least three leading follicles were > 18 mm in diameter. Thirty-six hours later, follicle puncture by transvaginal aspiration was performed. Two contralateral small follicles (diameter < 12 mm) and two contralateral large follicles (diameter ≥ 18 mm) were obtained from each woman. Samples were checked visually for blood contamination, and only uncontaminated samples were used in the study. After centrifugation at 3000g for 10 min, the supernatant was transferred to sterile polypropylene tubes and stored in liquid nitrogen. The tubes were then carried to the University and kept at − 80 °C until analysis. The biochemical analyses were performed by the Free Radicals and Oxidative Stress (FROS) research group of the UPV/EHU (www.ehu.eus/radicaleslibres/).
Due to limitations of the sample volume or contaminations, ten samples from small follicles were discarded, and the corresponding samples from large follicles were unmatched. The FF of a total of 118 follicles (54 small and 64 large) was analyzed.
Sample size
The population size analysis was performed with G*Power 3.1 software [8]. Sample size was calculated as a function of the power level (0.95), the significance level α (0.05), and the effect of the population size to detect a mean difference higher than one standard deviation with probability 0.95 (in this case, medium effect size). According to these parameters, a total sample size of 54 was computed.
Ethical approval
The Ethics Committee of the University UPV/EHU (Ethics Committee for Research involving Human Subjects, CEISH) approved the human subject protocol (CEISH/96/2011/RUIZLARREA), and the study was performed according to the UPV/EHU and IVI-RMA Bilbao agreement, Ref. 2012/01. The project complies with the Spanish Law of Assisted Reproductive Technologies (14/2006). Written informed consent was obtained from all trial subjects for participation in the study.
Fertility variables
Serum 17β-estradiol (E2) was quantified the day of induction of ovulation by radioimmunoassay (Orion Diagnostica, Espoo, Finland) designated for direct quantitative in vitro measurement of unconjugated E2. Antral follicle count (AFC) was the total number of follicles with a diameter between 2 and 10 mm in both ovaries on day 15 before the start of stimulation, as measured by transvaginal ultrasound.
In the IVI-RMA Bilbao clinic, oocytes from large follicles were used for fertilization. Only metaphase II (MII) oocytes were individually identified and included in this study. They were fertilized by ICSI. The fertilization was assessed 16–19 h after microinjection by confirmation of two polar bodies and two pronuclei. Oocytes were evaluated in an inverted microscope at × 400.
The evaluation and grading of embryos were based on a morphological assessment of cell cleavage, cell fragmentation, number of blastomeres, and the presence of multinucleated blastomeres. Embryos were classified into four grades (A–D) based on their implantation potential and in combination with the various mentioned morphological parameters [9]. A top-quality embryo (grade A) was defined as an embryo with four or five blastomeres on day 2 and seven or more on day 3, 10% fragmentation or less on day 3, and absence of multinucleated blastomeres.
Analysis of the acyl composition by gas chromatography/flame ionization detection
The transmethylation of fatty acids from the total lipid fraction of FF was performed following the method of Lepage and Roy [10] adapted to FF. Briefly, tridecanoic acid was added as internal standard to a 50-μl aliquot of FF in a ground glass tube. Two milliliters of methanol/toluene (4/1, v/v) were added and stirred for 1 min in a tube shaker. Transmethylation was carried out in acidic medium by adding 200 μl of acetyl chloride. Samples were incubated at 60 °C overnight. The reaction concluded by adding 3 ml of 10% K2CO3. The methylated fatty acids were extracted from the reaction mixture by adding 500 μl of n-heptane, and the solvent was evaporated in evaporator-concentrator (Savant SpeedVac A290). The extract thus obtained was resuspended in n-heptane, carried to a microvial, and properly encapsulated to proceed to the chromatographic separation.
The methylated fatty acids were separated and quantified on a GC System 7890A with a Series Injector 7683B and equipped with a flame ionization detector (Agilent Technologies Inc., Barcelona, Spain). The capillary column used for the separation had length 30 m, internal diameter 0.25 mm, and film thickness of 0.20 μm (SP 2330, Supelco Company, Bellefonte, PA). The oven temperature was 80 °C during injection and was maintained for 1 min; the temperature was then raised to 150 °C at a rate of 50 °C/min; then, a ramp of 4 °C/min was applied to achieve 190 °C, and the temperature was maintained for 5 min. Finally, the oven was brought to 210 °C at a rate of 4 °C/min and maintained for 10 min at this temperature. The temperatures of the injector and detector were 250 °C. Helium was used as carrier gas with a constant flow rate of 1 ml/min. Nitrogen gas at a flow rate of 40 ml/min was used as adjuvant. The identification of fatty acids was made by comparison with commercial standards (Nu Chek, Elysian, MN, USA). The quantification was carried out by electronic integration and the corresponding implementation of the response factor. Results were expressed as the percentage of total moles of measured fatty acids.
From the fatty acid composition, the following parameters were calculated: average chain length (CL) = [Σ (total14% × 14) + ... + (Σ% totaln × n)]/100 (n = number of carbon atoms), double bond index (DBI) = (Σ % of unsaturated fatty acids × number of double bounds of each unsaturated fatty acid)/100, and peroxidizability index (PI) = [(% monoenoics × 0.025) + (% dienoics × 1) + (% trienoics × 2) + (% tetraenoics × 4) + (% pentaenoics × 6) + (% hexaenoics × 8)]/100.
Statistical analysis
Statistical analysis was performed with Statistics Package for Social Sciences for Windows, version 24 (IBM SPSS, Inc., Chicago, IL). For descriptive statistics, continuous data were expressed as mean ± standard error (SE). The comparisons of a variable between large and small follicles were analyzed by the Student’s t test for paired data and for non-paired data when comparisons were between groups (donors and patients). Normality was examined by the use of the Kolmogorov-Smirnov test. The Wilcoxon rank-sum test (for non-paired data) or the Mann-Whitney U test (for paired data) were used for comparison when data for a particular variable were not normally distributed. Pearson’s correlation was used to determine the relationship between covariates. Stepwise forward multiple linear regression analysis was used for the evaluation of the dependence of total and mature oocytes, the number of antral follicles, serum estradiol, fertilization rate, total and top-quality embryos with the age, and the FAs showing statistically significant associations in the bivariate analysis. All P values were two-sided, and P < 0.05 was considered statistically significant.
Results
Fatty acid composition of FF from small and large follicles
We have identified and quantified 32 fatty acids from total lipids of ovarian FF (Table 1). The chain lengths of the acids varied from 14 to 25 carbon atoms. In large mature follicles, the most abundant fatty acids were, in decreasing order, linoleic, palmitic, and oleic acids, followed by lower levels of stearic and arachidonic acids. The proportion of DHA (about 9-fold lower than linoleic acid) was also noteworthy in FF.
Table 1.
Fatty acid composition of follicular fluid from small and large follicles from women undergoing a controlled ovarian stimulation cycle (n = 54 women)
| Fatty acid | Small follicles | Large follicles | P value |
|---|---|---|---|
| SAT | 36.52 ± 0.34 | 36.32 ± 0.35 | 0.621 |
| Myristic (14:0) | 0.86 ± 0.06 | 0.73 ± 0.05 | 0.029 |
| Pentadecanoic (15:0) | 0.31 ± 0.02 | 0.25 ± 0.01 | 0.001 |
| Palmitic (16:0) | 22.77 ± 0.27 | 22.85 ± 0.27 | 0.798 |
| Heptadecanoic (17:0) | 0.36 ± 0.03 | 0.31 ± 0.01 | 0.134 |
| Stearic (18:0) | 9.45 ± 0.13 | 9.35 ± 0.12 | 0.543 |
| Nonadecanoic (19:0) | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.304 |
| Arachidic (20:0) | 0.34 ± 0.01 | 0.33 ± 0.01 | 0.509 |
| Henicosanoic (21:0) | 0.27 ± 0.02 | 0.32 ± 0.03 | 0.035 |
| Behenic (22:0) | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.984 |
| Tricosanoic (23:0) | 0.32 ± 0.01 | 0.34 ± 0.01 | 0.003 |
| Lignoceric (24:0) | 0.65 ± 0.01 | 0.67 ± 0.02 | 0.046 |
| Pentacosanoic (25:0) | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.298 |
| MUFA | 22.52 ± 0.49 | 22.59 ± 0.74 | 0.931 |
| Myristoleic (14:1n-5) | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.142 |
| Trans-palmitoleic (16:1n-7 trans) | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.019 |
| Palmitoleic (16:1n-7 cis) | 1.18 ± 0.05 | 1.13 ± 0.06 | 0.191 |
| Elaidic (18:1n-9 trans) | 0.14 ± 0.01 | 0.23 ± 0.05 | 0.082 |
| Oleic (18:1n-9 cis) | 17.78 ± 0.45 | 17.80 ± 0.64 | 0.973 |
| Vaccenic (18:1n-7) | 1.58 ± 0.03 | 1.55 ± 0.03 | 0.417 |
| Eicosenoic (20:1n-9) | 0.32 ± 0.01 | 0.34 ± 0.02 | 0.385 |
| Nervonic (24:1n-9) | 1.40 ± 0.04 | 1.47 ± 0.04 | 0.017 |
| PUFA | 40.95 ± 0.50 | 41.08 ± 0.56 | 0.792 |
| n-6 PUFA | 37.00 ± 0.51 | 36.89 ± 0.54 | 0.814 |
| Linoelaidic (18:2n-6 trans) | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.296 |
| Linoleic (18:2n-6 cis) | 26.12 ± 0.43 | 25.59 ± 0.44 | 0.100 |
| γ-Linolenic (18:3n-6) | 0.19 ± 0.01 | 0.18 ± 0.01 | < 0.001 |
| Eicosadienoic (20:2n-6) | 0.39 ± 0.01 | 0.36 ± 0.01 | 0.002 |
| Dihomo-γ-linolenic (20:3n-6) | 1.76 ± 0.06 | 1.88 ± 0.07 | < 0.001 |
| Arachidonic (20:4n-6) | 7.88 ± 0.26 | 8.26 ± 0.25 | 0.012 |
| Docosatetraenoic (22:4n-6) | 0.36 ± 0.01 | 0.31 ± 0.01 | 0.001 |
| Docosapentaenoic (22:5n-6) | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.281 |
| n-3 PUFA | 3.95 ± 0.15 | 4.20 ± 0.17 | < 0.001 |
| α-Linolenic (18:3n-3) | 0.25 ± 0.02 | 0.18 ± 0.01 | < 0.001 |
| Eicosapentaenoic (20:5n-3) | 0.57 ± 0.05 | 0.63 ± 0.06 | 0.038 |
| Docosapentaenoic (22:5n-3) | 0.36 ± 0.01 | 0.38 ± 0.02 | 0.014 |
| DHA (22:6n-3) | 2.77 ± 0.10 | 3.01 ± 0.11 | < 0.001 |
| n-6/n-3 ratio | 10.13 ± 0.42 | 9.60 ± 0.41 | < 0.001 |
| Double bond index | 137.67 ± 1.21 | 139.86 ± 1.15 | 0.011 |
| Chain length | 17.94 ± 0.02 | 17.98 ± 0.02 | < 0.001 |
| Peroxidizability index | 0.91 ± 0.02 | 0.94 ± 0.02 | 0.024 |
| 20:4n-6/18:2n-6 ratio | 0.30 ± 0.01 | 0.33 ± 0.01 | < 0.001 |
Note: Data are expressed as the percentage of total moles of measured fatty acids and are the mean ± standard error of the mean
DHA docosahexaenoic acid, MUFA total monounsaturated fatty acids, PUFA total polyunsaturated fatty acids, SAT total saturated fatty acids
PUFA were the most abundant (41%), followed by saturated (36%) and monounsaturated (22.5%). Among the PUFA, the n-6 series predominated over the n-3 series (almost 9-fold higher).
The comparisons between small and large follicles from the same women (paired data analysis) revealed significant differences for several specific fatty acids. The medium chain saturated myristic (14:0) and pentadecanoic (15:0) and the monunsaturated trans-palmitoleic (16:1) were lower in large follicles, while very long-chain (21 to 24) fatty acids were higher (Table 1). Regarding PUFA, a marked increase of the very long-chain n-3 family, particularly DHA (P < 0.001), and a reduction of its α-linolenic (18:3n-3) precursor are noteworthy. Dihomo-γ-linoleic acid (20:3n-6) and arachidonic acid (20:4n-6) were increased in large follicles, while γ-linolenic (18:3n-6, their common precursor), eicosadienoic (20:2n-6), and docosatetraenoic (22:4n-6) acids decreased with the follicle size. Noteworthy, the 20:4n-6/18:2n-6 ratio, an index reflecting the biosynthetic pathway of arachidonic acid from its precursor, was highly increased in large follicles. The total n-6 PUFA were virtually identical for both follicle sizes. The observed changes in the proportion of n-3 PUFA mainly contributed to a very significant reduction of the n-6/n-3 ratio in large follicles (P < 0.001). The double bond index, the chain length, and the peroxidizability index increased with follicle size.
Characteristics of the study population and fertility parameters
The mean age of the patients was significantly higher than that of the oocyte donors (Table 2). As expected, the counted antral follicles and the number of total and mature oocytes were markedly lower in patients. The fertilization rate also decreased. Despite the lower number of high quality and total embryos obtained from patients, it should be noted that when expressing the mean per oocyte or per fertilized oocyte, the values were similar to those obtained with donors (Table 2).
Table 2.
Characteristics of the studied populations and fertility parameters (n = 64 women with all data)
| Donors | Patients | P value | |
|---|---|---|---|
| Age (years) | 27.2 ± 0.7 | 38.5 ± 0.5 | < 0.001 |
| BMI (kg2/cm) | 23.8 ± 0.6 | 22.9 ± 0.5 | 0.226 |
| Antral follicle count (AFC) | 21.0 ± 1.7 | 13.9 ± 1.1 | < 0.001 |
| aSerum E2 (pg/ml) | 2457 ± 359 | 2039 ± 175 | < 0.001 |
| Total oocytes | 19.0 ± 1.4 | 11.0 ± 0.9 | 0.037 |
| MII oocytes | 15.6 ± 1.2 | 8.9 ± 0.7 | < 0.001 |
| Fertilization rate (%) | 80.4 ± 3.2 | 68.8 ± 3.4 | 0.044 |
| Number of total embryos | 10.8 ± 1.2 | 5.9 ± 0.4 | 0.672 |
| Number of top-quality embryos | 2.89 ± 0.68 | 1.18 ± 0.19 | 0.470 |
| Embryo/oocyte | 0.58 ± 0.05 | 0.55 ± 0.04 | 0.652 |
| Top-quality embryo/oocyte | 0.14 ± 0.03 | 0.12 ± 0.02 | 0.812 |
| Embryo/fertilized oocyte | 0.72 ± 0.05 | 0.74 ± 0.03 | < 0.001 |
| Top-quality embryo/fertilized oocyte | 0.17 ± 0.04 | 0.16 ± 0.03 | 0.243 |
Note: Values are the mean ± standard error of the mean
aSerum E2 at the day of ovulation induction
Association of FF fatty acids from large follicles with age and fertility parameters
A bivariate correlation analysis showed significant negative associations of the woman’s age with the number of total and mature oocytes, the fertilization rate, the total and top-quality embryos, and the AFC (Table 3). Specific fatty acids correlated with age and fertility parameters with opposite Pearson’s coefficients, i.e., positive correlations with age were negative with fertility parameters and vice versa. We highlight a marked (P < 0.001) direct association of 20:5n-3, 22:5n-3, DHA, and total n-3 PUFA with the woman’s age. These fatty acid biomarkers correlated negatively with the number of total and mature oocytes retrieved. The chain length (P < 0.05), the double bond index (P < 0.01), and the peroxidizability index (P < 0.01) also correlated directly with the age. Both indices associated negatively with the number of mature oocytes. The number of total and top-quality embryos was associated negatively with DHA, n-3 PUFA, double bond index, and peroxidizability index. The n-6/n-3 ratio correlated positively with the number of total and mature oocytes and total and top-quality embryos.
Table 3.
Correlation coefficients of fatty acids in follicular fluid from large follicles with the female age and fertility parameters (n = 64 women with all data)
| Fatty acid | Age | P value | Total oocytes | P value | MII oocytes | P value | Fertiliz. rate | P value | Total embryos | P value | Top-quality embryos | P value | AFC | P value | Serum E2 | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | − 0.515 | < 0.001 | − 0.519 | < 0.001 | − 0.357 | 0.005 | − 0.577 | < 0.001 | − 0.419 | < 0.001 | − 0.523 | < 0.001 | ||||
| 16:0 | − 0.364 | 0.003 | − 0.333 | 0.009 | ||||||||||||
| 18:0 | − 0.284 | 0.027 | ||||||||||||||
| 20:0 | 0.303 | 0.015 | − 0.333 | 0.011 | ||||||||||||
| 21:0 | − 0.265 | 0.034 | 0.272 | 0.031 | ||||||||||||
| 23:0 | 0.305 | 0.014 | − 0.278 | 0.032 | − 0.321 | 0.014 | − 0.298 | 0.023 | ||||||||
| SAT | − 0.320 | 0.011 | − 0.370 | 0.003 | ||||||||||||
| 14:1n-5 | − 0.283 | 0.039 | ||||||||||||||
| 16:1n-7cis | − 0.299 | 0.016 | 0.276 | 0.036 | 0.272 | 0.039 | 0.265 | 0.036 | ||||||||
| 18:1n-9trans | − 0.400 | 0.001 | 0.277 | 0.035 | 0.322 | 0.014 | 0.310 | 0.013 | 0.349 | 0.006 | ||||||
| 18:1n-9cis | 0.261 | 0.048 | 0.264 | 0.036 | ||||||||||||
| 20:1n-9 | − 0.311 | 0.012 | 0.264 | 0.035 | 0.312 | 0.017 | 0.407 | 0.001 | 0.343 | 0.007 | ||||||
| 24:1n-9 | 0.363 | 0.003 | ||||||||||||||
| MUFA | 0.267 | 0.043 | 0.263 | 0.037 | ||||||||||||
| 18:2n-6trans | − 0.390 | 0.001 | 0.339 | 0.007 | 0.257 | 0.045 | ||||||||||
| 20:2n-6 | 0.294 | 0.025 | 0.320 | 0.011 | 0.375 | 0.003 | ||||||||||
| 20:4n-6 | − 0.271 | 0.034 | ||||||||||||||
| 18:3n-3 | 0.435 | < 0.001 | ||||||||||||||
| 20:5n-3 | 0.437 | < 0.001 | − 0.308 | 0.013 | − 0.324 | 0.009 | ||||||||||
| 22:5n-3 | 0.361 | 0.003 | − 0.281 | 0.025 | − 0.317 | 0.025 | ||||||||||
| 22:6n-3 | 0.586 | < 0.001 | − 0.432 | < 0.001 | − 0.433 | < 0.001 | − 0.314 | 0.015 | − 0.525 | < 0.001 | − 0.394 | 0.002 | − 0.339 | 0.007 | ||
| n-3 PUFA | 0.575 | < 0.001 | − 0.418 | 0.001 | − 0.425 | < 0.001 | − 0.289 | 0.025 | − 0.525 | < 0.001 | − 0.317 | 0.015 | − 0.310 | 0.013 | ||
| n-6/n-3 | − 0.596 | < 0.001 | 0.439 | < 0.001 | 0.445 | < 0.001 | 0.288 | 0.026 | 0.490 | < 0.001 | 0.277 | 0.036 | 0.305 | 0.015 | ||
| DBI | 0.311 | 0.009 | − 0.316 | 0.011 | − 0.328 | 0.008 | − 0.355 | 0.006 | − 0.321 | 0.014 | ||||||
| CL | 0.315 | 0.011 | − 0.259 | 0.046 | − 0.355 | 0.006 | ||||||||||
| PI | 0.390 | 0.001 | − 0.323 | 0.009 | − 0.279 | 0.031 | − 0.392 | 0.002 | − 0.365 | 0.005 | − 0.276 | 0.029 |
AFC antral follicle count, CL chain length, DBI double bond index, MUFA total monounsaturated fatty acids, PI peroxidizability index, PUFA total polyunsaturated fatty acids, SAT total saturated fatty acids
The fertilization rate correlated inversely with tricosanoic (23:0) and myristoleic (14:1n-5) acids, DHA, n-3 PUFA, the chain length and the peroxidizability index, and positively with the n-6/n-3 ratio.
Saturated FA, particularly 16:0, and n-3 PUFA (notably DHA) correlated negatively with both AFC and serum E2. By contrast, the monounsaturated 20:1n-9 showed a high positive correlation coefficient with both AFC and serum E2.
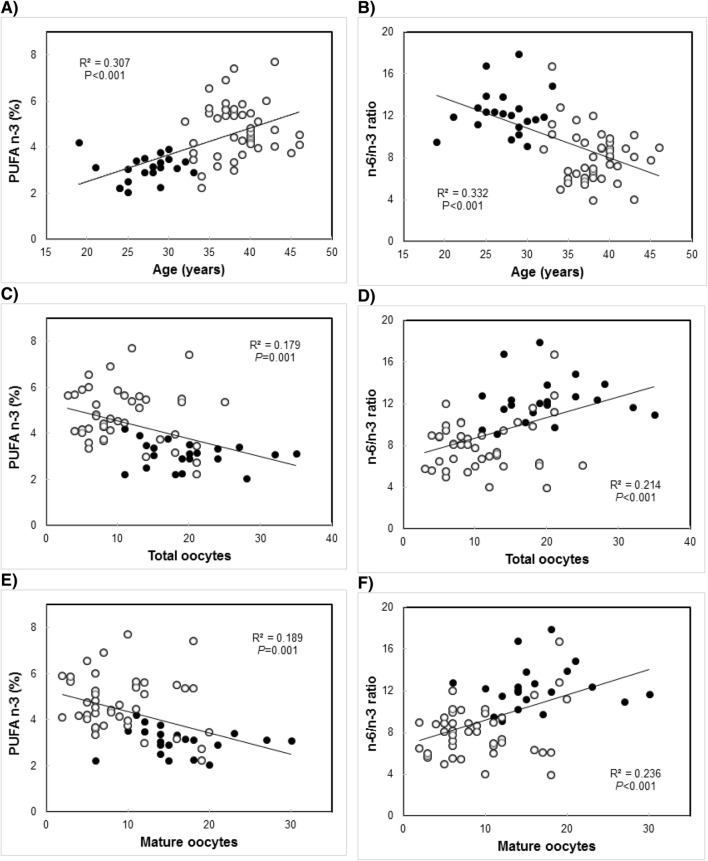
Figure 1 shows the high correlations (P ≤ 0.001) found for total n-3 PUFA and n-6/n-3 ratio with age (a, b) and fertility parameters (c–f). Both biomarkers presented opposite associations between age and the number of total and mature oocytes.
Fig. 1.
Correlation curves for a, c, e PUFA n-3 and b, d, and f n-6/n-3 ratio with a, b age, c, d total oocytes, and e, f mature oocytes. Intrafollicular fatty acids were analyzed in large follicles from oocyte donors (black circles), and infertile patients (open circles); total, 64 women
Multiple linear regression analysis
A multiple linear regression analysis was performed taking as independent variables those that presented statistically significant correlations with fertility parameters (Table 4). The linear regression equations resulting from this analysis show the influence that each predictor variable has on the dependent one, independently of the rest of predictor variables. According to the multiple regression analysis, only the woman’s age remained as a predictor variable for total and mature retrieved oocytes. The age of the woman was a major predictor for the fertilization rate, the number of total and top-quality embryos and AFC. High levels of follicular palmitic acid (16:0) were associated with lower AFC. Similarly, n-3 PUFA was a negative predictor for the number of total embryos. Serum E2 was not dependent on the age, but minor fatty acids, such as 18:1n-9trans (positively) and 18:2n-6trans (negatively), were significant predictors.
Table 4.
Multiple regression models
| aModel 1. Fertilization rate | |||||
| β | SE | β′ | t | P | |
| Constant | 113 | 13.3 | 8.49 | 0.000 | |
| Age | − 1.081 | 0.376 | − 0.342 | − 2.87 | 0.006 |
| 14:1n-5 | − 188 | 85 | − 0.262 | − 2.20 | 0.032 |
| bModel 2. Number of total embryos | |||||
| β | SE | β′ | t | P | |
| Constant | 21.2 | 2.5 | 8.48 | 0.000 | |
| Age | − 0.274 | 0.086 | − 0.410 | − 3.17 | 0.002 |
| n-3 PUFA | − 1.004 | 0.450 | − 0.288 | − 2.22 | 0.030 |
| cModel 3. Number of top-quality embryos | |||||
| β | SE | β′ | t | P | |
| Constant | 8.6 | 1.7 | 4.99 | 0.000 | |
| Age | − 0.115 | 0.039 | − 0.361 | − 2.97 | 0.004 |
| 20:0 | − 8.6 | 4.2 | − 0.249 | − 2.04 | 0.045 |
| dModel 4. Antral follicular count | |||||
| β | SE | β′ | t | P | |
| Constant | 66.1 | 10.1 | 6.49 | 0.000 | |
| Age | − 0.600 | 0.127 | − 0.489 | − 4.73 | 0.000 |
| 16:0 | − 1.249 | 0.417 | − 0.309 | − 2.99 | 0.004 |
| eModel 5. Serum estradiol | |||||
| β | SE | β′ | t | P | |
| Constant | − 1635 | 946 | − 1.72 | 0.090 | |
| 18:3n-3 | 6769 | 2196 | 0.329 | 3.08 | 0.003 |
| 20:2n-6 | 7825 | 2471 | 0.329 | 3.16 | 0.002 |
| 18:1n-9trans | 3359 | 1185 | 0.870 | 2.83 | 0.006 |
| 18:2n-6trans | − 11,395 | 5492 | − 0.635 | − 2.07 | 0.043 |
β regression coefficient, β′ standardized regression coefficient
aIndependent variables included in the model: age, 23:0, 14:1n-5, 22:6n-3, n-3 PUFA, n-6/n-3, chain length, and peroxidizability index. Adjusted R2 = 0.168; standard error of the estimate = 18.563
bIndependent variables included in the model: age, 22:6n-3, n-3 PUFA, n-6/n-3, double bond index, chain length, and peroxidizability index. Adjusted R2 = 0.366; standard error of the estimate = 3.365
cIndependent variables included in the model: age, 20:0, 23:0, 16:1n-7cis, 18:1n-9trans, 18:1n-9cis, 20:1n-9, MUFA, 20:2n-6, 22:6n-3, n-3 PUFA, n-6/n-3, double bond index, and peroxidizability index. Adjusted R2 = 0.206; standard error of the estimate = 1.790
dIndependent variables included in the model: age, 16:0, 21:0, SAT, 18:1n-9trans, 18:1n-9cis, 20:1n-9, MUFA, 18:2n-6trans,20:2n-6, 22:6n-3, n-3 PUFA, n-6/n-3, and peroxidizability index. Adjusted R2 = 0.348; standard error of the estimate = 6.392
eIndependent variables included in the model: 16:0, 18:0, SAT, 16:1n-7cis, 18:1n-9trans, 20:1n-9, 18:2n-6trans, 20:2n-6, 20:4n-6, and 18:3n-3. Adjusted R2 = 0.364; standard error of the estimate = 1054
Discussion
In this work, we have described in detail the fatty acid composition of total lipids in human FF. Thus, 32 fatty acids with chain lengths ranging from 14 to 25 carbons were identified and quantified. Other studies have reported the follicular fatty acid composition of specific lipid classes, such as phospholipids [11] and non-esterified fatty acids [5, 12]. However, the analysis of the acyl composition from a single lipid class provides partial information on the contribution of fatty acids to the numerous functions they have.
The present study shows changes in the fatty acid profile depending on the follicle size. The results suggest that follicular maturation involves the readjustment of fatty acids, increasing the proportion of very long-chain saturated and monounsaturated fatty acids, and long-chain n-3 PUFA, the latter mainly due to DHA. The long-chain n-3 PUFA (docosapentaenoic acid and DHA) are synthesized by the sequential actions of desaturases and elongases from the essential α-linolenic acid, which in our study was decreased in large follicles. The dynamic fatty acid changes taking place in FF could reflect the long-chain fatty acid requirements of the growing oocyte. Recently, Warzych et al. [13] have reported in bovine oocytes that the number of lipid droplets contained in the oocytes enclosed in the primordial follicles correlates positively with the fatty acid composition in FF. These authors have also found that there was a correlation between the content of fatty acids in FF and the expression of several genes related to fatty acid elongation (ELOVL5, fatty acid elongase 5) and desaturation (FADS2, fatty acid desaturase 2, and SCD, and stearoyl-CoA desaturase) in granulosa cells. These data support our findings, which suggest the activation of these enzymes to catalyze reactions that give rise to the observed changes in the acyl profile during follicle growth. In addition, as the follicle grows, there is increased vascularization, and more lipoproteins, mainly HDL, cross the blood-follicle barrier.
We found that the most abundant fatty acids were, in decreasing order, linoleic, palmitic, oleic, stearic, and arachidonic acids. They accounted for more than 80% of all identified fatty acids, and linoleic acid constituted about one-fourth. Similar to our results, Homa and Brown reported that linoleic acid was the most abundant fatty acid in bovine FF, and that its levels were decreased in large follicles [14]. Linoleic acid is an essential fatty acid necessary for the synthesis of all the other n-6 PUFA, and in particular arachidonic acid, precursor of prostaglandins. However, high concentrations of linoleic acid have been reported to impair in vitro oocyte maturation and embryo cleavage in cows [14, 15], as well as to adversely affect oocyte maturation and cumulus cell expansion in sheep [16], and embryo development in murine [17]. Remarkably, we found that arachidonic acid was significantly increased in large follicles, and the ratio of arachidonic acid to 18:2n-6 (an indirect index of the flow through fatty acid desaturase 2 in the biosynthetic pathway of arachidonic acid) was significantly higher in large follicles. These data suggest that linoleic acid could play a role in regulating oocyte development, the lowest linoleic acid concentrations allowing oocytes to mature. The role of arachidonic acid and n-6 PUFA in ovulation and oocyte maturation is unknown. Ciepiela et al. described that high levels of linoleic acid and arachidonic acid derivatives in FF at the time of oocyte retrieval were associated with reduced oocyte competence after ICSI, although they found no correlations with embryo quality or pregnancy rate [18]. Wiener-Megnazi et al. observed a positive correlation between the number of mature oocytes and oxidative stress in FF of infertile women [19], supporting the idea that growth of mature follicles is associated with ROS. Moreover, ovulation is considered an inflammatory process [20], and agents that inhibit acute inflammatory reactions suppress ovulation [21]. Arachidonic acid is a precursor of pro-inflammatory prostaglandins that may mediate both follicular maturation and ovulation processes [3]. These observations, and our results, support the idea that limited physiological oxidative stress may be involved in follicle growth.
As far as we know, this is the first study showing the association of particular fatty acids in the FF with female aging. The long-chain 20:5n-3, 22:5n-3 and DHA correlated positively with the woman’s age and negatively with the number of total and metaphase II oocytes. Bolton-Smith et al. described positive correlations between age and DHA plus eicosapentaenoic acid (20:5n-3) in adipose tissue, regardless of diet [22]. Other works in the literature have also reported age-related increases of n-3 PUFA in lipid fractions from serum and adipose tissue [23, 24]. We cannot rule out that the increase of these n-3 fatty acids in FF is the result of a lower uptake by the oocyte, thus suggesting a role of this lipid class in oocyte development.
An increase in n-3 PUFA is usually accompanied by a decrease in n-6 PUFA, since n-3 and n-6 precursors of both series of PUFA compete for the same desaturases and elongases during the synthesis of longer-chain unsaturated fatty acids [25]. We found that several n-6 fatty acids (18:2n-6trans, 20:2n-6, 22:4n-6) and the n-6/n-3 ratio decreased with age. The n-6 to n-3 PUFA ratio in plasma is a key factor in several diseases [3, 26]. It has been suggested that this index is physiologically more relevant than individual n-3 and n-6 PUFA and to be critical for proper development [27]. In our study, the n-6/n-3 ratio was also positively linked to ovarian response, thus suggesting the importance of this ratio as a marker for achieving mature oocytes in assisted reproduction.
The study of the plasma lipidome as a source of biomarkers related to the aging process is an area of fruitful research. It has been recently reported a lipidomic signature that confers extreme longevity to humans, appearing 16:0 as potential fatty acid biomarker of extreme longevity [28]. Serum palmitic acid levels were substantially lower in young and centenarians than in aged subjects. In our study, the AFC, considered a marker of reproductive aging, is inversely associated with 16:0. In fact, the content of this fatty acid at the follicular level is almost as good predictor as the woman’s age.
There are several studies reporting the direct correlation of serum n-3 PUFA, particularly 20:5n-3 and DHA, with aging in healthy populations [29–32]. As far as we know, there is no data about the evolution of DHA in follicular fluid during aging. In the present study, we describe a progressive increase of n-3 PUFA in the FF with age, in particular 20:5n-3, 22:5n-3, and DHA. These fatty acids are highly unsaturated, a feature that confers high susceptibility to lipoperoxidation by free radical attack. As a result, the obtained data revealed an increase in the peroxidizability index with aging. This microenvironment more prone to oxidative stress may be reflected in the inverse association of age and n-3 PUFA content with the embryos obtained.
One limitation of the study is that all the participants underwent a controlled ovarian stimulation cycle, so that the results related to fatty acid changes that take place during follicle growth cannot be extrapolated to women in a natural cycle. Another question to be resolved is whether the high levels of fatty acids in FF are the result of increased blood entry and/or local synthesis to satisfy the requirements of ovarian cells and embryos, and/or as a result of a lower utilization by follicular cells. Further research is needed on this issue to understand the actual role of fatty acids in reproduction.
Conclusion
This study reports changes in the fatty acid profile of human FF depending on the follicle size. As the follicle grows, the proportion of long-chain fatty acids, n-3 PUFA, particularly DHA, and the double bond and peroxidizability indices increase, while the n-6/n-3 PUFA ratio is reduced in large follicles. Results also indicate that low levels of n-3 PUFA in large follicles, which are associated with younger women, predict a better response to ovarian stimulation in terms of the recovery of total and mature oocytes, total and top-quality embryos, and fertilization rate per cycle. Specific fatty acids are associated with ICSI outcomes, particularly in older women who decide to conceive. Therefore, a more thorough knowledge of the role that lipids play in reproductive processes is necessary before providing nutritional guidelines or dietary supplements to women who undergo assisted reproductive techniques.
Funding
Supported by the “PN de I+D+I” of the Spanish Ministry of Science and Innovation, “ISCIII-Subdirección General de Evaluación y Fomento de la Investigación” and FEDER (ref. FIS/FEDER PI11/02559), University of the Basque Country UPV/EHU (ref. GIU16/62), and the Basque Government (Department of Education, Universities and Research, predoctoral grant to IP, and Department of Development, Economy and Competitiveness, SPRI, ref. IG-2013 0001214).
Ethical approval
The Ethics Committee of the University UPV/EHU (Ethics Committee for Research involving Human Subjects, CEISH) approved the human subject protocol (CEISH/96/2011/RUIZLARREA), and the study was performed according to the UPV/EHU and IVI-RMA Bilbao agreement, Ref. 2012/01. The project complies with the Spanish Law of Assisted Reproductive Technologies (14/2006). Written informed consent was obtained from all trial subjects for participation in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ESHRE Capri Workshop Group A prognosis-based approach to infertility: understanding the role of time. Hum Reprod. 2017;32:1556–1559. doi: 10.1093/humrep/dex071. [DOI] [PubMed] [Google Scholar]
- 2.Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- 5.Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, Moley KH. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95:1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valckx SD, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, Bols PE, Leroy JL. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. 2014;12:13. doi: 10.1186/1477-7827-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares SR, Troncoso C, Bosch E, Serra V, Simón C, Remohí J, Pellicer A. Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J Clin Endocrinol Metab. 2005;90:4399–4404. doi: 10.1210/jc.2004-2252. [DOI] [PubMed] [Google Scholar]
- 8.Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 9.ASEBIR. Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. Cuadernos de Embriología Clínica. 3rd ed. Glóbalo, Agencia Creativa Digital, Madrid; 2015. ISSN: 1888–8011.
- 10.Lepage, Roy CC. Direct transesterification of all classes of lipids in one step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 11.Shaaker M, Rahimipour A, Nouri M, Khanaki K, Darabi M, Farzadi L, Shahnazi V, Mehdizadeh A. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran Biomed J. 2012;16:162–168. doi: 10.6091/ibj.1081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantasri T, Wu LL, Hull ML, Sullivan TR, Barry M, Norman RJ, Robker RL. Distinct localisation of lipids in the ovarian follicular environment. Reprod Fertil Dev. 2015;27:593. doi: 10.1071/RD14321. [DOI] [PubMed] [Google Scholar]
- 13.Warzych E, Pawlak P, Pszczola M, Cieslak A, Madeja ZE, Lechniak D. Interactions of bovine oocytes with follicular elements with respect to lipid metabolism. Anim Sci J. 2017;55:1491–1497. doi: 10.1111/asj.12799. [DOI] [PubMed] [Google Scholar]
- 14.Homa ST, Brown CA. Changes in linoleic acid during follicular development and inhibition of spontaneous breakdown of germinal vesicles in cumulus-free bovine oocytes. J Reprod Fertil. 1992;94:153–160. doi: 10.1530/jrf.0.0940153. [DOI] [PubMed] [Google Scholar]
- 15.Marei WF, Wathes DC, Fouladi-Nashta AA. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction. 2010;139:979–988. doi: 10.1530/REP-09-0503. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffarilaleh V, Fouladi-Nashta A, Paramio MT. Effect of α-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytes. Theriogenology. 2014;82:686–696. doi: 10.1016/j.theriogenology.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Nonogaki T, Noda Y, Goto Y, Kishi J, Mori T. Developmental blockage of mouse embryos caused by fatty acids. J Assist Reprod Genet. 1994;11:482–488. doi: 10.1007/BF02215713. [DOI] [PubMed] [Google Scholar]
- 18.Ciepiela P, Bączkowski T, Drozd A, Kazienko A, Stachowska E, Kurzawa R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization--a prospective analysis of follicular fluid and a matched oocyte in a 'one follicle--one retrieved oocyte--one resulting embryo' investigational setting. PLoS One. 2015;10:e0119087. doi: 10.1371/journal.pone.0119087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82:1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espey LL, Stein VI, Dumitrescu J. Survey of antiinflammatory agents and related drugs as inhibitors of ovulation in the rabbit. Fertil Steril. 1982;38:238–247. doi: 10.1016/S0015-0282(16)46466-6. [DOI] [PubMed] [Google Scholar]
- 22.Bolton-Smith C, Woodward M, Tavendale R. Evidence for age-related differences in the fatty acid composition of human adipose tissue, independent of diet. Eur J Clin Nutr. 1997;51:619–624. doi: 10.1038/sj.ejcn.1600455. [DOI] [PubMed] [Google Scholar]
- 23.Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr. 2008;99:168–174. doi: 10.1017/S000711450779387X. [DOI] [PubMed] [Google Scholar]
- 24.Walker CG, Browning LM, Mander AP, Madden J, West AL, Calder PC, Jebb SA. Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br J Nutr. 2014;111:679–689. doi: 10.1017/S0007114513002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borsonelo EC, Galduróz JCF. The role of polyunsaturated fatty acids (PUFAs) in development, aging and substance abuse disorders: review and propositions. Prostaglandins Leukot Essent Fatty Acids. 2008;78:237–245. doi: 10.1016/j.plefa.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Kang JX, Leaf A. Differential effects of various eicosanoids on the production or prevention of arrhythmias in cultured neonatal rat cardiac myocytes. Prostaglandins. 1997;54:511–530. doi: 10.1016/S0090-6980(97)00122-6. [DOI] [PubMed] [Google Scholar]
- 27.McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev. 2011;24:59–67. doi: 10.1071/RD11907. [DOI] [PubMed] [Google Scholar]
- 28.Jové M, Naudí A, Gambini J, Borras C, Cabré R, Portero-Otín M, et al. A stress-resistant Lipidomic signature confers extreme longevity to humans. J Gerontol A Biol Sci Med Sci. 2017;72:30–7. [DOI] [PubMed]
- 29.Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids. 2013;48:719–727. doi: 10.1007/s11745-013-3763-9. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Secular trend of serum docosahexaenoic acid, eicosapentaenoic acid, and arachidonic acid concentrations among Japanese. A 4- and13-year descriptive epidemiologic study. Prostaglandins Leukot Essent Fatty Acids. 2015;94:35–42. doi: 10.1016/j.plefa.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Rajalahti T, Lin C, Mjøs SA, Kvalheim OM. Changes in serum fatty acid and lipoprotein subclass concentrations from prepuberty to adulthood and during aging. Metabolomics. 2016;12:51. doi: 10.1007/s11306-016-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risé P, Tragni E, Ghezzi S, Agostoni C, Marangoni F, Poli A, Catapano AL, Siani A, Iacoviello L, Galli C. IDEFICS consortium., CHECK group. Different patterns characterize omega 6 and omega 3 long chain polyunsaturated fatty acid levels in blood from Italian infants, children, adults and elderly. Prostaglandins Leukot Essent Fatty Acids. 2013;89:215–220. doi: 10.1016/j.plefa.2013.06.009. [DOI] [PubMed] [Google Scholar]