Abstract
The effects of Toxoplasma gondii during embryonic development have not been explored despite the predilection of this parasite for neurons and glial cells. Here, we investigated the activation of the purinergic system and proinflammatory responses during congenital infection by T. gondii. Moreover, neuroprotective and neuromodulatory properties of resveratrol (RSV), a polyphenolic natural compound, were studied in infected neuronal progenitor cells (NPCs). For this study, NPCs were isolated from the telencephalon of infected mouse embryos and subjected to neurosphere culture in the presence of EGF and FGF2. ATP hydrolysis and adenosine deamination by adenosine deaminase activity were altered in conditions of T. gondii infection. P2X7 and adenosine A2A receptor expression rates were augmented in infected NPCs together with an increase of proinflammatory (INF-γ and TNF-α) and anti-inflammatory (IL-10) cytokine gene expression. Our results confirm that RSV counteracted T. gondii-promoted effects on enzymes hydrolyzing extracellular nucleotides and nucleosides and also upregulated P2X7 and A2A receptor expression and activity, modulating INF-γ, TNF-α, and IL-10 cytokine production, which plays an integral role in the immune response against T. gondii.
Electronic supplementary material
The online version of this article (10.1007/s11302-018-9634-3) contains supplementary material, which is available to authorized users.
Keywords: NPCs, P2X7 receptor, A2A receptor, Cytokines, Toxoplasmosis
Introduction
Inflammation plays an important role in a diversity of central nervous system diseases including toxoplasmosis disease. Toxoplasma gondii, once having infected the brain, can use different mechanisms in order to subvert the immune response and enable the parasite to persist in the brain throughout the chronic phase of the disease, which can last for many years [1]. However, the emerging role of immunomodulatory mediators and the potential use of their inhibitory effects for pathogen survival and replication in host cells are still a new and poorly understood field.
Several studies have highlighted that T. gondii displays a preference for neurons, in contrast to the low numbers of infected microglial cells during chronic infection [2–4]. Effective control of parasite replication and disruption of the parasitophorous vacuole in host cells depends on the immune response including expression of anti-inflammatory interleukine IL-10 accompanied by the overproduction of proinflammatory cytokines IL-12, IFN-γ, and TNF-α [5–7]. Furthermore, extracellular nucleotides adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and nucleoside adenosine (ADO), as well as their receptors, play an important role in the modulation of a variety of processes, including neuroinflammation [8].
Some purinergic receptors, such as the A2A subtype, promote the suppression of proinflammatory cytokines and stimulate the immune response leading to the production of anti-inflammatory cytokines and protection from oxidative damage in infected cells [9, 10]. P2X7 and A2A receptors are widely expressed in the central nervous system. Several studies have demonstrated that the activation of the P2X7 receptor triggers the elimination of T. gondii [11]. A2A receptor activation may be an important stop signal to prevent excessive stimulation of inflammation, avoiding excessive cellular damage [12]. Some studies conducted by our research group have proposed the influence of the infection by T. gondii on purine levels and adenosine deaminase (ADA) activity in the brain of experimentally infected mice [13]. However, here we determine for the first time, changes in NTPDase, 5′-nucleotidase activities and P2X7 and A2A receptor expression levels in neural progenitor cells (NPCs), which had been experimentally infected with T. gondii. Some researchers have studied the expression of P2X7 receptors in immune cells in mice after T. gondii infection [11].
Given the potential damage, if immune responses were left uncontrolled, it is probable that more than one immunomodulatory pathway has evolved. An understanding of these molecular mechanisms of immune response preconditioning regulation has become relevant. Resveratrol (RSV, 3, 4′, 5-trihydroxy-trans-stilbene), a non-flavonoid polyphenol, naturally present at high concentrations in red wine and grape seeds, have been pharmacologically turned for therapeutic neuroimodulation [14, 15] based on its antioxidant and anti-inflammatory activities [16]. RSV can attenuate activation of immune cells and the subsequent synthesis and release of proinflammatory mediators [17]. Previous studies indicate that RSV confers neuroprotection by inhibiting activation of microglia and reducing the production of proinflammatory factors through cellular cascade signaling pathways [18]. Our research group has proposed that RSV treatment modulates seric cytokine profiles and attenuates the tissue inflammatory process caused by T. gondii infection [19]. In this study, we investigated whether activation of the purinergic system potentiates proinflammatory responses during congenital infection by T. gondii, and whether RSV exerts beneficial effects on the immune response. Thus, we investigated the activity of NTPDase, 5′-nucleotidase and adenosine deaminase, P2X7 and A2A purinergic receptor expression levels and the production of pro- and anti-inflammatory cytokines in infected NPCs. Moreover, we provide evidence for RSV-induced promotion of neuroprotective and neuromodulatory effects in infected NPCs.
Experimental procedures
Animals
All experiments were performed with ten Swiss female mice (age up to 60 days weighing 25 ± 5 g). Animals were kept in boxes with five animals each, under a 12 h light/dark cycle with controlled temperature and humidity (25 °C, 70% respectively), respecting their circadian rhythm, and were fed with commercial feed and water ad libitum. All animals procedures were approved by the Ethics' Committee on Animal Experimentation of the Universidade Federal de Santa Maria under protocol number 9509010915/15.
Parasite infection
For experimental infections, animals were infected orally with T. gondii parasites of the VEG strain (type III, clonal lineage) to mimic a chronic infection (50 parasitic cysts/animal). Twenty days post infection, female mice were put for mating with males. Timed-pregnant animals were obtained by overnight mating, and the efficiency of mating was confirmed by appearance of the vaginal plug.
Isolation and culture of neural progenitor cells (NPCs)
The animals were euthanized using protocols reviewed and approved by the Ethics Committee on Animal Experimentation of the Universidade Federal de Santa Maria (UFSM, 9509010915/15). NPCs were isolated from embryonic day 13 (ED-13) telencephalons of mice embryos according to Hutton and Pevny [20].
Telencephalons were dissected under aseptic conditions and incubated with 0.1% trypsin for 5 min at 37 °C Fetal bovine serum (FBS) was added for inactivation of trypsin. Cells were further mechanically dissociated and then plated at a density of 2 × 105 cells/mL in Dulbecco’s Modified Eagle’s medium (DMEM) F-12 culture medium supplemented with 2% of B-27 (Life Technologies, Carlsbad, CA), 20 ɳg/mL of epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF 2) (both reagents from Sigma-Aldrich, St. Louis, MO), and antibiotics and cultured at 37 °C in a water-saturated atmosphere and 5% of CO2. For the induction of neural differentiation, neurospheres were plated into adherent poly-l-lysine- and laminin-precoated cell-culture grade dishes in 2% B-27 (Life Technologies).
Neurosphere diameters and differentiation characteristics of NPCs derived from infected telencephalon embryos are presented in Supplementary Fig. 1. Obtained results were similar to those of a previous study performed by our research group [21]. Among the main results, we highlight: (1) T. gondii infection altered neurosphere morphology, increased diameters and glial differentiation. (2) T. gondii infection affects proliferation during S and G2/M phases of cell cycle (Suppl. Fig. 1).
Resveratrol
Resveratrol (RSV, C14H12O3; molecular weight 228.25 g/mol; purity > 98%, Sigma Aldrich) was diluted in PBS and added at 10 μM final concentration to NPC cultures during differentiation. This concentration was based on a previous study reported by Kumar et al. [18], where 10 μM RSV showed beneficial effects on survival of NPC cultures. In agreement, a former study by our research group showed that this dose was most effectiveon NPC cultures obtained from T. gondii–infected animals [21].
Flow cytometry analysis
Flow cytometry experiments were performed as described previously [22]. Neurospheres were centrifuged for 5 min at 200×g and dissociated into single cell suspensions. Cells were fixed for 20 min in ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS) and then washed with PBS supplemented with 2% FBS. After 30 min of incubation with rabbit anti-P2X7 purinergic receptor (1:500; Sigma-Aldrich, St. Louis, MO) or mouse anti-A2A adenosine receptor antibodies (1:1000; Sigma-Aldrich, St. Louis, MO). In the same solution, NPCs were washed with PBS and incubated for 1 h at room temperature (RT) with secondary Alexa Fluor 488 or 555 (1:1000; Life Technologies) antibodies. Forty-thousand events were acquired per sample with fluorescence measured in logarithmic scales. Forward and side light-scatter signals were used to exclude dead cells and debris. Data were acquired on a flow cytometer (BD FACSCalibur; BD Biosciences) and analyzed with the FlowJo V10 software (Ashland, OR).
Nucleotide hydrolysis assays
For enzymatic assays, differentiated neurospheres were centrifuged for 5 min at 200×g and dissociated into a single cell suspension with PBS 1X. Twenty microliters of cell suspension (0.9–1.0 mg/mL protein) was added to the reaction mixture of NTPDase or 5′nucleotidase for a final volume of 200 μL and pre-incubated for 10 min at 37 °C according to the method of Lunkes et al. [23]. The reaction was started by the addition of ATP or ADP as substrate at a final concentration of 1.0 mM. E-5′-nucleotidase was determined by the method of Heymann et al. [24]. Phosphate released by ATP, ADP, and AMP hydrolysis was measured using KH2PO4 as standard. Protein levels were measured according to Bradford [25]. The results are reported as ɳmol Pi released/min/mg of protein.
ADA activity levels were estimated in neurospheres spectrophotometrically by the method of Giusti and Galanti [26] as the measurement of ammonia produced when adenosine deaminase acts in excess of adenosine. For the assay, 50 μL of cell suspension reacted for 60 min with 21 mmol/L of adenosine, pH 6.5, at 37 °C. The reaction was stopped by adding a solution of 106.2 mM phenol and 167.8 mM sodium nitroprusside and a hypochlorite solution. The amount of ammonia produced was measured at 620 ɳm, and the results were expressed in units per liter (U/L).
Cytokine gene expression
In this study, qRT-PCR was employed to analyze gene expression modulation of inflammatory cytokines using a similar approach as previously described [27]. In brief, RNA was isolated using TRIzol® reagent (Invitrogen™ Life Technologies, Carlsbad, CA) and quantified at 260 ɳm. Reverse transcription was performed with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA), and RNA was added to a final concentration of 1 μg/μL. The following steps were used for cDNA synthesis: 37 °C for 5 min, heating at 65 °C for 10 min, and cooling for 10 min at 5 °C. Additionally, incubation step at 25 °C for 5 min, 42 °C for 30 min, 85 °C for 5 min, and a final incubation at 5 °C for 60 min were used. Real-time PCR was performed in a volume of 20 μL using 1x QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany) and 1 μL of cDNA in a Rotor-Gene Q (Qiagen) equipment under the following conditions: 95 °C for 3 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s followed by a melting curve of 65 °C to 95 °C. The following forward (5′-3′) and reverse (3′-5′) primer sequences were used: β-Act 5′-CCGTAAAGACCTCTATGCCAAC-3′, 3′-AGGAGCCAGAGCAGTAATCT-5′;IL-1β 5′-GGTACATCAGCACCTCACAA-3′,3′-TTAGAAACAGTCCAGCCCATAC-5′;IL-6 5′-CTTCCATCCAGTTGCCTTCT-3′, 3′-CTCCGACTTGTGAAGTGGTATAG-5′, INF-γ 5′-CTCTTCCTCATGGCTGTTTCT-3′, 3′-TTCTTCCACATCTATGCCACT
T-5′; TNF-α 5′-TTGCTCTGTGAAGGGAATGG-3′, 3′-GCTCTGAGGAGTAGACA
ATAAAG-5′; IL-10 5′-ACAGCCGGGAAGACAATAAC-3′, 3′-CAGCTGGTCCTT
TGTTTGAAAG-5′.
Statistical analysis
Results are expressed as mean ± standard errors of the mean (SEM) for the values obtained from at least three independent experiments. Statistical analysis was performed by two-way ANOVA using Tukey as post hoc test with the Graph Pad Prism (Version 5.0) software. *p < 0.05 was considered statistically significant.
Results
Resveratrol modulates nucleotide hydrolysis of infected NPC
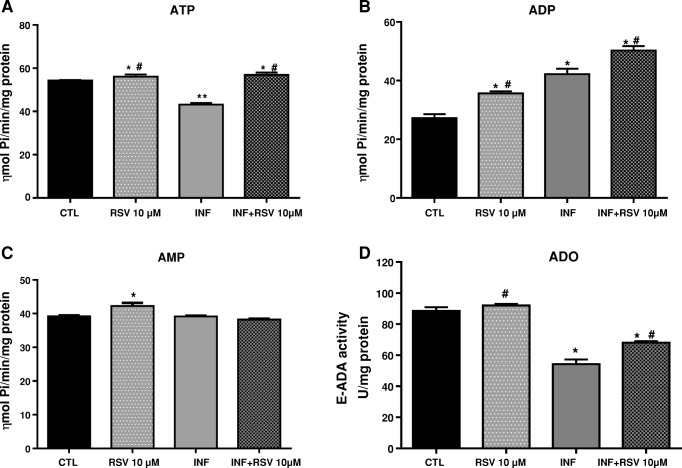
Capacities of uninfected and infected NPCs to hydrolyze extracellular nucleotides and nucleosides were evaluated in this study (Fig. 1a–d). A decrease of ATP hydrolysis (p < 0.05) and an increase of ADP hydrolysis (p < 0.05) was observed in infected NPCs compared to control groups. No significant differences in AMP hydrolysis could be detected in the infected group (Fig. 1c). Infected NPCs decreased adenosine hydrolysis by ADA compared to control (p < 0.01). ATP, ADP and AMP hydrolysis was slightly augmented by RVS as per se effects (p > 0.05). Furthermore, 10 μM RSV restored ATP hydrolysis in infected NPCs. ADA activity was higher in infected and treated NPCs compared to the infected group (Fig. 1d).
Fig. 1.
Effects of T. gondii on nucleotide hydrolysis of control and infected differentiated neurospheres treated with RSV (10 μM). a ATP hydrolysis; b ADP hydrolysis; c AMP hydrolysis; d E-ADA activity. Data represent mean values ± SEM of three independent experiments analyzed by two-way ANOVA with the post hoc Tukey test. *p < 0.05 (*control vs infected groups) (#infected vs control group)
Purine receptor expression is altered following T. gondii infection
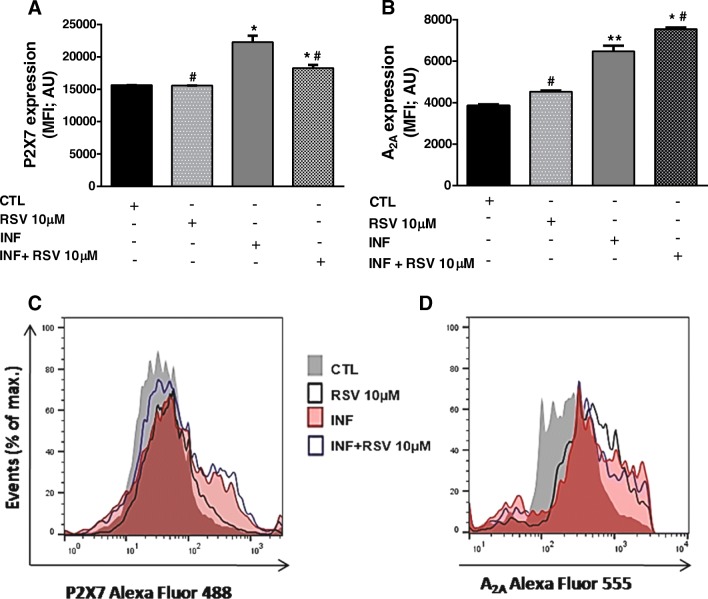
Flow cytometry experiments clearly confirmed augmented P2X7 receptor expression by infected neurospheres (Fig. 2a). Exposure of NPCs to 10 μM RSV did not alter expression rates of P2X7 receptors (p > 0.05). However, RSV attenuated increments in P2X7 receptor protein expression in infected NPCs (Fig. 2a).
Fig. 2.
Effects of RSV on purinergic receptor expression during immune responses induced by T. gondii in differentiated NPCs. a P2X7 receptor expression [(median fluorescence intensity (MFI); arbitrary units (AU)] in control and infected neurospheres. b A2A receptor expression (MFI; AU) in control and infected neurospheres treated with 10 μM RSV. c Representative flow cytometry histogram of P2X7 receptor expression. d representative flow cytometry histogram of A2A receptor expression. The data represent mean values ± SEM of three independent experiments. *p < 0.05, **p < 0.01 (*control vs infected group); (#infected vs experimental group)
Further, an increase of the frequency of A2A receptor-positive cells was shown for the infected group compared to the control group (p < 0.05). RSV (10 μM) did not affect A2A receptor expression (p > 0.05). On the other hand, RSV augmented A2A receptor expression in infected NPCs (Fig. 2b).
Effects of RSV in cytokine gene expression in infected NPC
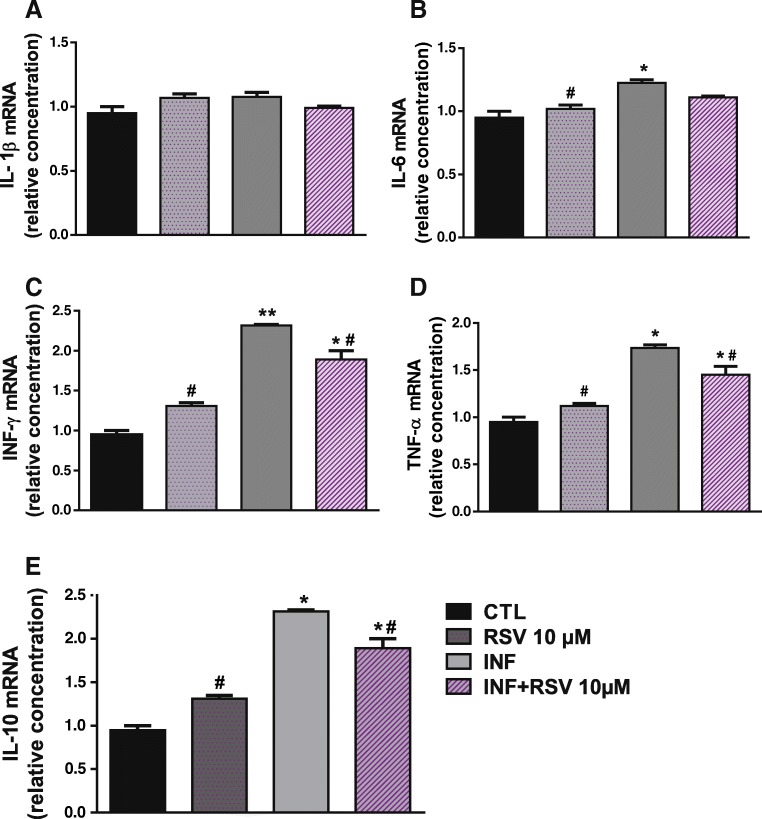
Proinflammatory cytokines play a crucial role in the immune response against T. gondii. In view of that, mRNA transcription levels of IL-1β, IL-6, INF-γ, TNF-α, and anti-inflammatory IL-10 in infected and non-infected NPCs were determined in this study (Fig. 3). A marked increase in transcription levels of mRNA coding for IL-6 (Fig. 3b), INF-γ (Fig. 3c), TNF-α (Fig. 3d), and IL-10 (Fig. 3e) was observed for infected NPCs (p > 0.05) compared to the control group. Further, the treatment with 10 μM RSV reduced INF-γ, TNF-α, and IL-10 gene expression in infected NPCs.
Fig. 3.
Effects of RSV on cytokine gene expression profiles of differentiated NPCs infected with T. gondii. a Anti-inflammatory IL-1β interleukin. b Anti-inflammatory IL-6 interleukin. c Interferon gamma (INF-γ) cytokine. d Tumor necrosis factor alpha (TNF-α) cytokine. e Proinflammatory IL-10 interleukin. **p < 0.01; *p < 0.05 (*control vs infected group); (#infected vs control group)
Discussion
The induction of immunity upon infection by T. gondii is a crucial step in establishing a balanced host-parasite relationship in the central nervous system (CNS). In this study, we investigated the involvement of the purinergic signaling during congenital infection by T. gondii and its relationship with the imunne response. In view of the wide spectrum of biological activities of RSV, our hypothesis was, whether treatment with RSV could modulate inflammatory responses in infected NPCs mediated by purinergic signaling.
After reaching the CNS, the parasite invaded all nucleated cells and initiated activation of neurons and glial cells [3, 28, 29]. Initial signs of glial activation were observed in NPCs from embryos infected through materno-fetal infection by T. gondii. Further, T. gondii induced increased neurosphere size, as we have already shown in a preliminary study by Bottari et al. [21], this supports the notion that extracellular nucleotides also contribute to the control of embryonic neurogenesis.
Signaling pathways employing extracellular nucleotides and adenosine are present during embryonic development of the central nervous system and have a significant role in the activation of neural progenitors after cerebral lesions as induced by T. gondii. The expression of specific subtypes of purinergic receptors or enzymes in neurospheres has been shown by Mishra et al. [30]. In the present study, we evaluated the effects of RSV on nucleotide- and nucleoside-hydrolyzing activity by ectonucleotidases in neurospheres infected by T. gondii.
RSV is a natural polyphenolic compound, that has attracted considerable interest regarding its anti-inflammatory and neuroprotective properties [31, 32]. Beneficial effects of RSV regulating extracellular ATP, ADP, and AMP hydrolysis and ADA activities in infected NPCs (Fig. 1) point at an enhanced break-down of purinergic receptor agonists.
Here, we show that alterations of ectonucleotidase activities in infected NPCs augmented ATP and ADP hydrolysis. Thus, it is possible to suggest that extracellular ATP levels activate the P2X7 receptor on the surfaces of microglial cells (Fig. 1a). The purinergic P2X7 receptor is a bifunctional adenosine triphosphate (ATP)–activated ion channel that contributes to the control of many physiological functions, including cell death, killing infectious organisms, and regulating inflammatory processes [9, 10] as well as survival, mobilization and differentiation of NPCs. Continued activation of P2X7 receptors by ATP during chronic infection has been proposed as a mechanism for the elimination of T. gondii tachyzoites from infected macrophages [11].
Moreover, adenosine A2A receptor expression was increased in infected NPCs (Fig. 2b). These protein G-coupled receptors cause the accumulation of intracellular cAMP, which has strong immunosuppressive effects [33–35]. It is quite possible that this pathway suppresses cell responses upregulating intracellular cAMP levels and downregulates host immune response.
Therefore, ADO in high concentrations can act on A2A receptors, attenuating inflammation and probably tissue damage, concomitantly with the downregulation supposedly provided by the decreased ATP hydrolysis found in our study. It was already reported that the infection by T. gondii causes a significant augmentation in ADO levels in the brain of the mice [13].
These purinergic receptors, when stimulated in T.gondii–activated microglia, possibly induced production of pro-inflammatory cytokines IL-6 (Fig. 3a), INF-γ (Fig. 3b), and TNF-α (Fig. 3c). The activation of microglia cells by T. gondii induced local immune responses as a pathway to control congenital infection by T. gondii [36]. On the other hand, host cells increased anti-inflammatory IL-10 cytokine (Fig. 3e) to prevent neuroinflammation.
The potential inhibitory effects of RSV on cytokine gene expression and their beneficial effects in protecting against neurotoxicity has been reported [37]. Our study suggests that 10 μM RSV reverted elevated INF-γ and TNF-α gene expression (Fig. 3c and Fig. 3d) through the inhibition of P2X7 receptor expression in infected NPCs.
The immune response was regulated by RSV, once 10 μM RSV decreased INF-γ (Fig. 3c) and TNF-α (Fig. 3d) cytokine expression in infected NPCs. Studies have reported that RSV inhibits the production of TNF-α by LPS-activated microglia [17, 38]. It is clear that RSV exerts neuroprotection through inhibiting activation of microglia and the subsequent release of various proinflammatory factors. Furthermore, RSV decreases IL-10 expression (Fig. 3d) in order to restore the immune response against the parasite.
Together, our data point at expression increase of P2X7 and A2A receptors together with enhanced NTPDase activity of infected NPCs. Extracellular ATP contributes to local inflammation and parasite elimination and decreases survival host cell. P2X7 receptors might contribute to T. gondii infection control through the stimulation of the synthesis of pro-inflammatory cytokines. Moreover, this study contributes to elucidate possible therapeutic mechanisms of RSV on purinergic signaling and immune responses during congenital infection by T. gondii.
Conclusion
In summary, our study suggests the importance of purinergic system signaling, possibly via P2X7 and A2A receptors, as components of the inflammatory response against T. gondii. Here, we evaluated the effects of RSV as a neuroprotector and neuromodulator, reporting a positive feedback mechanism mediated by RSV stimulating nucleotide hydrolysis and modulating P2X7 and A2A receptor expression. We conclude that parasite elimination might involve P2X7 receptor signaling, and suggest RSV as a novel therapeutics against T. gondii–based inhibition of immune response by acting on purinergic signalling in infected NPCs.
Electronic supplementary material
(DOCX 940 kb)
Financial support
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX, process number 88887.186030/2018-00). HU is grateful for grant support by the São Paulo State Foundation FAPESP (Project No. 2012/50880-4) and the National Research Council CNPq. MMP is grateful for a post-doctorate fellowship granted by FAPESP (Project No. 2015/19478-3).
Ethics’ committee approval
This experiment was approved by the Ethics’ Committee for Animal Experimentation of the Universidade Federal de Santa Maria (UFSM), under protocol number 9509010915.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nathieli B. Bottari, Phone: 55 49 2049-9560, Email: nathieli_bb@hotmail.com
Micheli M. Pillat, Phone: 55 49 2049-9560, Email: mmpillat@gmail.com
Aleksandro Schafer Da Silva, Phone: 55 49 2049-9560, Email: aleksandro_ss@yahoo.com.br.
References
- 1.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Lüder CGK, Giraldo-Velásquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol. 1999;93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 3.Parlog A, Schlüter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37:159–170. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- 4.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12(2):e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao CC, Hu SX, Gekker G, et al. Effects of cytokines on multiplication of Toxoplasma-gondii in microglial cells. J Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 6.Gaddi PJ, Yap GS. Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol. 2007;85:155–159. doi: 10.1038/sj.icb.7100038. [DOI] [PubMed] [Google Scholar]
- 7.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates Immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho-Silva R, Monteiro da Cruz C, Persechini PM, Ojcius DM. The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signal. 2007;3:83–90. doi: 10.1007/s11302-006-9039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho-Silva R, Ojcius DM. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrêa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5:515–526. doi: 10.1016/S1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 13.Tonin AA, Da Silva AS, Casali EA, et al. Influence of infection by Toxoplasma gondii on purine levels and E-ADA activity in the brain of mice experimentally infected mice. Exp Parasitol. 2014;142:51–58. doi: 10.1016/j.exppara.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 15.Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 17.Meng XL, Yang JY, Chen GL, Wang LH, Zhang LJ, Wang S, Li J, Wu CF. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem Biol Interact. 2008;174:51–59. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS, Yadav S, Khanna VK, Pant AB (2016) Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6. 10.1038/srep28142 [DOI] [PMC free article] [PubMed]
- 19.Bottari NB, Baldissera MD, Tonin AA, Rech VC, Nishihira VSK, Thomé GR, Camillo G, Vogel FF, Duarte MMMF, Schetinger MRC, Morsch VM, Tochetto C, Fighera R, da Silva AS. Effects of sulfamethoxazole-trimethoprim associated to resveratrol on its free form and complexed with 2-hydroxypropyl-β-cyclodextrin on cytokines levels of mice infected by toxoplasma gondii. Microb Pathog. 2015;87:40–44. doi: 10.1016/j.micpath.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Hutton SR, Pevny LH (2008) Isolation, culture, and differentiation of progenitor cells from the central nervous system. Cold Spring Harb Protoc 3. 10.1101/pdb.prot5077 [DOI] [PubMed]
- 21.Bottari NB, Schetinger MR, Pillat MM et al (2018) Resveratrol as a therapy to restore neurogliogenesis of neural progenitor cells infected by Toxoplasma gondii. Mol Neurobiol. 10.1007/s12035-018-1180-z [DOI] [PubMed]
- 22.Pillat MM, Cheffer A, de Andrade CM, Morsch VM, Schetinger MRC, Ulrich H. Bradykinin-induced inhibition of proliferation rate during neurosphere differentiation: consequence or cause of neuronal enrichment? Cytom Part A. 2015;87:929–935. doi: 10.1002/cyto.a.22705. [DOI] [PubMed] [Google Scholar]
- 23.Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, Schetinger MRC. Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res. 2003;109:189–194. doi: 10.1016/S0049-3848(03)00178-6. [DOI] [PubMed] [Google Scholar]
- 24.Heymann D, Reddington M, Kreutzberg GW. Subcellular localization of 5′-nucleotidase in rat brain. J Neurochem. 1984;43:971–978. doi: 10.1111/j.1471-4159.1984.tb12832.x. [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Guist G, Galanti B. Colorimetric method. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1984. pp. 315–323. [Google Scholar]
- 27.Schott KL, Assmann CE, Barbisan F, Azzolin VF, Bonadiman B, Duarte MMMF, Machado AK, da Cruz IBM. Superoxide-hydrogen peroxide genetic imbalance modulates differentially the oxidative metabolism on human peripheral blood mononuclear cells exposed to seleno-L-methionine. Chem Biol Interact. 2017;273:18–27. doi: 10.1016/j.cbi.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Mendez OA, Koshy AA. Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog. 2017;13:e1006351. doi: 10.1371/journal.ppat.1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra SK, Braun N, Shukla V. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Develop. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- 31.Bureau G, Longpré F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 32.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 34.Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem. 2003;3:413–426. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- 35.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari D, Chiozzi P, Falzoni S, Hanau S, di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Wang H, Wu Q, Lu Y, Nie J, Xie X, Shi J. Resveratrol protects cortical neurons against microglia-mediated neuroinflammation. Phyther Res. 2013;27:344–349. doi: 10.1002/ptr.4734. [DOI] [PubMed] [Google Scholar]
- 38.Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 940 kb)