Abstract
BACKGROUND:
Liver disease is one of the top causes of death globally. Although liver transplantation is a very effective treatment strategy, the shortage of available donor organs, waiting list mortality, and high costs of surgery remain huge problems. Stem cells are undifferentiated cells that can differentiate into a variety of cell types. Scientists are exploring the possibilities of generating hepatocytes from stem cells as an alternative for the treatment of liver diseases.
METHODS:
In this review, we summarized the updated researches in the field of stem cell-based therapies for liver diseases as well as the current challenges and future expectations for a successful cell-based liver therapy.
RESULTS:
Several cell types have been investigated for liver regeneration, such as embryonic stem cells, induced pluripotent stem cells, liver stem cells, mesenchymal stem cells, and hematopoietic stem cells. In vitro and in vivo studies have demonstrated that stem cells are promising cell sources for the liver regeneration.
CONCLUSION:
Stem cell-based therapy could be a promising therapeutic method for patients with end-stage liver disease, which may alleviate the need for liver transplantation in the future.
Keywords: Stem cells, Liver disease, Stem cell-based therapy, Liver regeneration
Introduction
The liver is essential for maintaining life. It is responsible for bile production, metabolism of nutrients, removal of toxins, blood purification, and immune responses. There are more than 100 different kinds of liver diseases, including fascioliasis, hepatitis, alcoholic liver disease, fatty liver disease, hereditary disease, cirrhosis, etc. These diseases may lead to liver failure and result in an irreversible liver damage. Although the liver can repair itself, when the damage is beyond repair it is dangerous and fatal to patients [1]. According to statistics, the liver disease is among the top 10 leading causes of death in the world [2]. Currently, the most effective treatment option for both chronic and acute liver failure is liver transplant. However the donor organs are limited and the cost of surgery is high [3]. In addition, the recipient immune system may reject the transplanted organ [4]. Therefore, the development of alternative therapeutic strategies for patients with serious liver diseases is an urgent need.
Most of the time, the liver has the ability to heal itself to maintain its important functions. Hepatocytes are the type of cells that make up 70–85% of the liver mass, which will self-renewal after injury. However, this ability will be impacted under the conditions that cause severe liver injury. A possible therapeutic strategy to repair the liver function is to transplant hepatocytes. However, lots of hepatocytes (approximately 5 × 109 cells) are needed for transplantation therapy and these cells are difficult to expand in culture [5]. Recent advances in the cell biology show the therapeutic potential of stem cells [6–8]. This is how stem cells are brought to the attention. Compared with hepatocytes, stem cells are easier to culture and expand in vitro. In addition, they have the ability to differentiate into hepatocytes and other liver cell types. Due to these properties, stem cells are an attractive option for liver tissue regeneration. Lots of studies have shown that a variety of stem cell types are promising candidates for liver cell replacement [9, 10]. This review summarizes current stem cell-based therapies for the treatment of liver diseases and discusses their potentials.
Stem cell sources for liver diseases therapy
Stem cells are incompletely differentiated cells that are able to self-renew and differentiate into a variety of cell types, generating tissues and organs. Based on the development stages, there are two major types of stem cells: embryonic stem cells (ESCs), which are derived from embryos, and adult stem cells (ASCs, also called somatic stem cell), which can be isolated from tissues such as bone marrow and fat. Based on the development potential, stem cells can be divided into five categories: totipotent stem cells, pluripotent stem cells, multipotent, oligopotent, and unipotent stem cells. Totipotent stem cells have the greatest differentiation potential. They can differentiate into all cell types and generate a complete organism. Pluripotent can differentiate into most tissues of the body. Multipotent stem cells are able to differentiate into specialized cells with specific functions. Unipotent stem cells only can divide into a single cell type [11].
It has been reported that many types of stem cells can be used for liver tissue repair [12]. We will summarize the application of each cell type for liver disease treatment and discuss their advantages and limitations.
Embryonic stem cells
ESCs are pluripotent stem cells derived from the early embryonic development stages [13]. The isolation of ESCs dates back to 1981 [14]. Since Evans and Kaufman successfully obtained pluripotent cells from a mouse embryo, the application of ESCs has been studied extensively.
Due to the unlimited differentiation ability, ESCs are a promising cell source in tissue engineering and regenerative medicine [15]. Many studies have reported that ESC-derived hepatocytes display hepatic characteristics. Kuai et al. [16] induced rhesus monkey ESCs towards hepatocyte-like cells (HLCs) by a four-step differentiation process. The differentiated cells displayed morphological features, gene expression patterns and metabolic activities characteristic of hepatocytes. Brolén et al. [17] demonstrated that HLCs derived from human ESCs exhibited liver-like properties including transporter activity and capacity to metabolize drugs.
Currently, the safety and function assessment of human ESC-derived hepatocytes are generally in immune-deficient mice. It has been reported that HLCs derived from human ESCs benefit the recovery of injured liver tissues in mice model, through cell replacement and delivering trophic factors that support liver regeneration [18]. Tolosa et al. [19] evaluated the ability of a European human ESC line (VAL9) to generate hepatocytes after transplantation into mice with acetaminophen-induced acute liver failure, a clinically relevant model. This study revealed an efficient method to induce hepatic differentiation of human ESCs. The differentiated cells were able to repopulate the liver and rescue the liver function in mice with acetaminophen-induced acute toxicity.
It remains a big challenge for clinical applications to generate mature and functional hepatocytes with high efficiency. A study suggested that [20] a polyethyleneimine-modified silica nanoparticles-based sustained delivery system for growth factors was able to induce HLCs from mouse ESCs and produce a high population of well-functional hepatocytes in vitro. When transplanted into liver-injured mice, HLCs that were treated with the delivery system differentiated into hepatocytes in vivo and significantly restored the injured liver.
Induced pluripotent stem cells
A technology pioneered by Shinya Yamanaka’s lab [21] showed that adult cells can be reprogrammed back into a pluripotent state that has an unlimited differentiate ability. These cells are called induced pluripotent stem cells (iPSCs).
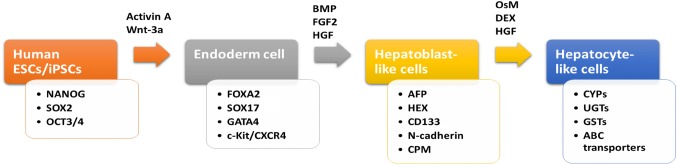
Several studies [22, 23] have shown that iPSCs can be turned into functional hepatocytes that closely resemble normal hepatic cells. Different strategies to generate functional hepatocytes have been carried out. The typical protocol for the differentiation of human ESCs/iPSCs into hepatocyte-like cells is shown in Fig. 1. Cai et al. reported a directed differentiation method [24]. This strategy has been developed very quickly in recent years. However, fully functional hepatocytes are not easy to derive by this method because the whole process involves several steps that would affect the formation of hepatocytes [25].
Fig. 1.
Flow diagram showing typical protocol for the differentiation of human ESCs/iPSCs into hepatocyte-like cells. The protocol consists of three phases and each differentiation phase has specific markers. Representative growth factors, cytokines, and chemicals used in different stages are also summarized. Abbreviations ESCs embryonic stem cells; iPSCs induced pluripotent stem cells; BMP bone morphogenetic protein; FGF2 basic fibroblast growth factor; HGF Hepatocyte growth factor; OsM Oncostatin M; DEX Dexamethasone, SOX2 SRY (sex determining region Y)-box 2; OCT 3/4 octamer-binding transcription factor; FOXA2 forkhead box protein A2; CXCR4 C-X-C chemokine receptor type 4; AFP alpha-fetoprotein; CPM Carboxypeptidase M ; CYPs cytochromes P450; UGTs glucuronosyltransferase; GSTs glutathione s-transferase; ABC transporters ATP-binding cassette transporters
Si-Tayeb et al. [26] reported that mouse iPSCs were induced from somatic cells by forced expression of the reprogramming factors octamer-binding transcription factor (Oct) 3/4 and SRY (sex determining region Y)-box 2 (Sox2) along with either Klf4 or Nanog and Lin28. They demonstrated that mouse iPSCs were induced into hepatocytes and were able to generate intact fetal livers.
Human iPSCs are a potential source for the treatment of end-stage liver disease. Several studies have reported the development process for differentiation of human iPSCs into the hepatocytes. Hannan et al. [27] described a protocol that controlled the differentiation of human ESCs and iPSCs into a near-homogenous population of HLCs by 25 days.
In vitro differentiation of human iPSCs into hepatocytes can be achieved, however it is not clear whether these hepatic cells are capable of treating damaged or diseased liver tissue. Thus, many studies have been carried out. Takebe et al. [28] generated the vascularized and functional human liver from human iPSCs by liver buds transplantation in vitro (iPSC-LBs). Human vasculatures in iPSC-LB transplants were able to function after 48 hours connecting to the host vessels. They also showed that iPSC-LBs mesenteric transplantation rescued the drug-induced lethal liver failure model. Liu et al. [29] showed that hepatic cells at different differentiation stages derived from human iPSCs cloud repopulate the liver tissue in a mouse liver cirrhosis model. Human specific liver proteins were detected in mouse blood. They proved the liver regenerative ability of human iPSC-derived multistage hepatic cells in vivo.
iPSCs hold great promise for regeneration medicine. However, its immunogenicity remains controversial. Zhao et al. [30] reported the abnormal gene expression in some cells derived from iPSCs. The abnormality would subsequently induce the T-cell-dependent immune response in syngeneic recipients. Therefore, they concluded that the immunogenicity of therapeutical cells derived from iPSCs should be evaluated before clinical applications. Another study [31] found little evidence of immunogenicity when iPSCs were differentiated in vitro and transplanted into syngeneic recipients. They supported the idea that the differentiated cells derived from iPSCs could be applied for cell-based therapy without causing immune rejections. Since vector choices for reprogramming may affect immunogenicity [30], the different conclusions may be caused by the different iPSC lines used in these two studies. Although several studies are optimistic about the safety of iPSCs and their progeny, human iPSCs have not been tested for immunogenicity. When considering the therapeutical applications, we have to consider all the potential results and have a balanced view of iPSCs.
Adult stem cells
ASCs are multipotent stem cells with limited cell potential. Examples of ASCs include neural stem cells that give rise to different neural cells, and hematopoietic stem cells that generate all the blood cell types. The major types of ASCs being considered for liver regeneration are mesenchymal stem cells (MSCs) and liver stem cells (LSCs). We will discuss each cell type for the application of cell-based liver therapy and summarize the corresponding updated researches.
Liver stem cells
LSCs (or liver progenitor cells) give rise to a variety of hepatic cell types, including hepatocyte and bile duct epithelial cells. Lots of studies [32–34] have reported that LSCs can generate mature hepatocytes in vitro and could repair and regenerate the liver after transplanted into injured livers. Therefore, LSCs can also be applied as a potential regenerative strategy.
Leucine-rich repeat-containing G-protein coupled receptor (Lgr5) is a biomarker of ASCs in certain tissues such as muscle, spinal cord, and liver. A study by Lu et al. [35] demonstrated that the LSCs were able to expand in vitro and transplant in vivo. The cells can differentiate and reconstitute the liver, leading to an improved liver function and architecture. Huch et al. [36] reported that mouse Lgr5+ LSCs from damaged liver generated organoids in Rspo1-based culture medium. The organoids subsequently differentiated into functional hepatocytes in vitro and in vivo. This study raises the hope that the human counterparts of the mouse LSCs may be induced to differentiate into functional hepatocytes and transplanted to the body. Later the same research group [37] reported that adult bile duct-derived bipotent progenitor cells from human liver were induced to differentiate to functional hepatocytes in vitro and be transplanted in vivo. This study tested human-derived material as a cell source for human liver regeneration, which is one step closer to achieve the goal of realizing cell-based therapy for human liver disease.
Mesenchymal stem cells
It has been reported that the MSCs are able to differentiate into HLCs [38, 39]. They are another cell resource for the treatment of damaged liver tissue. MSCs can be found in a variety of organs and tissues such as the bone marrow, adipose tissue, peripheral blood, synovial membrane, and cartilage [40]. MSCs are the most “multipotent” ASCs in the human body, which means they can differentiate into many cell types [41]. It has been reported that MSCs can give rise to bone, cartilage, muscle, fat, and other tissues [42].
Dowidar and colleagues [43] demonstrated that the transplantation of bone marrow-derived MSCs (BM-MSCs) could treat acetaminophen liver injury by the enhancement of hepatocyte regeneration and inhibition of liver stress and inflammatory signaling in the rat model. Besides BM-MSCs, researchers have found that adipose tissue-derived MSCs (AT-MSCs) also have hepatic differentiation potential in vitro and in vivo [44, 45]. This finding indicates that AT-MSCs are a potential source for the treatment of hepatic injury or dysfunction.
The application of MSCs for liver regeneration is not only studied in experimental animal models but also in human patients with liver diseases. Salama’s group [46] reported that BM-MSCs play a very important role in regenerate liver mass and repair liver functions in human patients. The combination of MSCs and granulocyte colony-stimulating factor (GCSF) is a very effective therapeutic strategy to treat the end-stage liver disease. Wang et al. [47] recruited 10 patients with ursodeoxycholic acid (UDCA)-resistant primary biliary cirrhosis (PBC) to assess the safety and efficacy of allogeneic BM-MSCs transplantation. The efficacy in UDCA-resistant PBC was evaluated throughout a 12-month follow-up. The results showed an improved life quality in patients and no transplant related side effects. Jang et al. [48] studied the safety and antifibrosis effect of MSCs on alcohol-related hepatic fibrosis in human patients. After autologous MSCs injection, they observed histological improvements in 6 of the 11 patients. Although this study has some limitations including lack of control group and sampling errors, it is the first study that describes the effect of BM-MSCs in alcohol-related patients, which indicates BM-MSCs therapy has potential as an anti-fibrotic treatment in cirrhosis.
The MSCs are not only a cell source for liver tissue regeneration, but also able to support the growth and proliferation of liver cells. Hepatocytes transplantation is a therapy option for liver failure; however, maintaining the viability of hepatocytes during culturing process is a challenge. Researchers have found out that MSCs can provide structural support for cells and have anti-apoptotic and immunomodulatory effects. The supportive role of MSCs is very promising in cell transplantation. In order to improve the viability of hepatocytes before transplantation, Gómez-Aristizábal’s group [49] tried to co-culture the hepatocytes with MSCs derived from umbilical cord or fat tissues. The co-culturing showed improved survival and function of hepatocytes. Later, other studies [50–52] also revealed that co-transplantation of hepatocytes and MSCs transplantation is a promising therapeutic strategy for acute liver failure.
Since the MSCs have plasticity (a term describes cells to take on characteristics of other cells in the body), their potential therapeutic application in regeneration field gets a lot of attention. However, researches about MSCs as a successful treatment for the treatment of liver diseases are still needed.
Hematopoietic stem cells
Hematopoietic Stem cells (HSCs) are isolated from bone marrow and umbilical cord blood. They can generate all kinds of blood cell types. It has been demonstrated that HSCs could also be induced to generate hepatocytes for the treatment of damaged liver tissues and the improvement of the liver function [53–56].
Kollet et al. [57] suggested that the interplay between cytokines, chemokines, and proteolytic enzymes promoted the migration of HSCs into the injured liver. And the differentiation of HSCs played an important role in liver repair. It has been reported [58] that granulocyte colony-stimulating factor (G-CSF)–primed HSCs can give rise to hepatocytes, migrate to the injured liver and promote tissue repair. A research studied the beneficial effects of repeated infusion of HSCs in patients with end-stage liver disease [59]. The results showed that the repeated HSC infusion group had a more sustained clinical efficacy, better improvements in liver functions throughout a 12-month follow-up compared with the single HSC infusion group. However, the underlying mechanism of this beneficial effect on liver function is still controversial. Some studies [60, 61] suggest the possible mechanism is the plasticity of stem cells. Other studies [62] indicate the conversion to hepatocytes is because of cell fusion. Although the mechanism is unclear, HSCs still holds great potential in the development of liver tissue regeneration.
Other stem cell sources
Recently more and more studies suggest that ASCs have greater stem cell plasticity than we previously thought. Scientists are investigating the possibility of stem cells from other issue or organs treating liver diseases [63].
Dental pulp is a relatively easy autologous source to access. Among them, a population called dental pulp pluripotent-like stem cells (DPPSCs) expresses pluripotency markers such as OCT3/4 and SOX2. DPPSCs are able to differentiate into tissues of the three embryonic layers and form teratoma-like structures in vitro. They do not have ethical controversy and safety issues that are associated with the application of ESCs or iPSCs. Therefore, DPPSCs are a promising cell source in tissue engineering and regenerative medicine [64]. Gil et al. [65] cultured the cells in a 2D environment supplemented with several growth factors that can recapitulate liver development. DPPSCs were able to give rise to functional HLCs in vitro. B-13 is a rat pancreatic progenitor-like cell line with the capacity to generate unlimited functional HLCs in vitro [66, 67]. Some studies demonstrated [68, 69] that another transdifferentiated rat pancreatic cell line (AR42J-B13) functioned like bona fide hepatocytes.
These studies raise the possibility that other stem cell types could be used to generate hepatocytes for replacing damaged liver tissue.
Considerations of different cell sources
Constantly updated researches keep revealing new information about stem cell-based therapy for liver diseases. Scientists are trying to determine which cell sources have better capabilities and potentials for the therapeutic applications. The studies of using different stem cell types are summarized in Table 1. We will discuss the benefits and limitations of using different cell sources below.
Table 1.
Summary of studies using stem cells for the treatment of liver diseases
| Type of stem cells | HLCs makers | Liver disease models | Outcomes | Reference |
|---|---|---|---|---|
| Rhesus monkey ESCs | AFP, ALB, HNF4, CK8 and CK19 | Rhesus monkey ESCs derived cells displayed hepatic characteristics | Kuai et al. [16] | |
| Human ESCs |
CYP3A4, CYP1A2, CYP2C9, 1-antitrypsin, FP, HNF4, MRP2 and OATP2 |
HLCs generated from human ESCs had liver-like properties | Brolén et al. [17] | |
| Human ESCs | ALB, keratin 18, keratin 7 and Keratin19 | Mice with CCl4 induced acute liver injury | Recovery of injured liver tissues in mice model | Woo et al. [18] |
| human ESC (VAL9) | ALB, CYP3A4, CD81, CLDN1 and ASGR | Mice with acetaminophen-induced acute liver failure | Repopulate the liver and rescue the liver function | Tolosa et al. [19] |
| Mouse ESCs | ALB, AFP, CYP7A1 | Mice with CCl4 induced acute liver injury |
Establish a nanoparticles-based delivery system for growth factors Differentiated HLCs significantly restored the injured liver |
Wang et al. [20] |
| Mouse iPSCs | ALB, AFP | HLCs displayed key liver functions and can integrate into the hepatic parenchyma in vivo | Si‐Tayeb et al. [26] | |
| Human iPSCs | HNF4A, AFP, RBP4, TTR, ALB | Mice with ganciclovir induced liver failure | Vascularized and functional human liver was generated by transplantation of human iPSCs-LBs | Takebe et al. [28] |
| Human LSCs | Tgfb1 and Fgf1 | Mice with CCl4 induced subacute liver injury | hFLMPCs can engraft and repair injured liver | Irudayaswamy et al. [33] |
| HPCs | HNF4α and CYP2D6 | AhCre+Mdm2flox/flox mice | Transplanted HPCs restored liver parenchyma and regenerated hepatocytes and biliary epithelia. | Lu et al. [35] |
| Mouse LSCs | Hnf4-a positive and Krt19 negative | Mice with CCl4 induced liver injury | Lgr5+ stem cells can generate hepatocytes and biliary duct cells during the repair phase | Huch et al. [36] |
| Rat BM-MSCs | ALB, AST, ALP | Acetaminophen liver injury | BM-MSCs enhanced hepatocyte regeneration, and inhibited liver stress and inflammatory signaling | Dowidar [43] |
| Human AT-MSCs | AFP, ALB | Mice with CCl4 induced liver injury | AT-MSCs can relieve the impairment of injured livers | Yin et al. [44] |
| Human BM-MSCs | ALT, AST | Mice With Lethal Fulminant Hepatic Failure | hiPSC-MSCs had therapeutic effect in the mouse AHF |
Salama et al. [46] model |
| Allogeneic BM-MSCs | ALT, AST | Patients with ursodeoxycholic acid resistant primary biliary cirrhosis | Life quality of the patients was improved after BM-MSC transplantation | Wang et al. [47] |
| BM-MSCs | Patients with alcohol-related hepatic fibrosis | BM-MSCs therapy induced a histological and quantitative improvement of hepatic fibrosis |
Jang et al. [48] |
|
| Pancreatic progenitor-like cell | CYP3A4, CPSI and ALB | B-13 progenitor cell can generate hepatocytes with genetic alterations | Fairhall et al. [66] |
ESCs: embryonic stem cells; ASCs: adult stem cells; iPSCs: induced pluripotent stem cells; HPCs: hepatic progenitor cells; MSCs: mesenchymal stem cells; LSCs: liver stem cells; BM-MSCs: bone marrow-derived mesenchymal stem cells; AT-MSCS: adipose tissue-derived mesenchymal stem cells; AFP: α-fetoprotein; ALB: albumin; HNF4: hepatocyte nuclear factor 4; CK8: cytokeratin 8; CK19: cytokeratin 19; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ASGR: asialoglycoprotein receptor; RBP44: retinol binding protein 4; TTR: transthyretin; Tgrb-1: transforming growth factor beta 1, Fgf1: fibroblast growth factor 1; ALP: alkaline phosphatase; CPSI: carbamoyl phosphate synthetase I
ASCs can be isolated from a patient, and then grown into a certain cell type and transplanted back to the same patient. Therefore ASCs have the advantage of avoidance of rejection from patients’ immune system. When using ESCs, patients will need to take immunosuppressing drugs to combat the rejection since the body would consider the new cells as foreigners [70]. In this case, the patients have a high risk of getting infection. The other advantage is that the application of ASCs does not have the ethical problems. ASCs can be isolated from a variety of organs or tissues such as adipose tissue bone marrow, liver, and muscles. However, ESCs are isolated from embryos at an age of 3-5 days. The isolation process will destroy the embryo, which causes a tremendous ethical controversy [71]. This issue hinders the therapeutic application of ESCs.
Although new studies indicate that ASCs may have the ability to differentiate a wider range of cell types than we previously thought [11, 72], ESCs generally have a greater cell differentiation potential. Currently, ESCs still have the advantage to differentiate readily. In addition, ESCs are able to divide without limit and grow very fast in cell culture while ASCs cannot proliferate so readily after isolation from adult organs or tissues. Usually, stem cell transplantation therapies require a large number of cells. Therefore, culturing and harvesting larger numbers of ASCs would be a challenge for their application. Another challenge for the application of ASCs is that they may not proliferate to the same degree. Because the ASCs are not as “young” as ESCs, and they have a greater chance to have abnormalities of DNA mutations which are caused by factors such as the environment or errors during DNA replication [73].
The main functions of ASCs are to maintain and repair the specific tissues or organs where they originate. It is believed for a long time that ASCs are tissue-specific and can only give rise to the cell types corresponding to the tissues where they origin [74]. However, recent researches have indicated that ASCs are capable of generating different cell types under certain conditions [74, 75]. If we have control over this differentiation process, a specific ASC type can be used to treat some serious diseases. In order to put the idea into practice, tremendous challenges should be overcome. Overall, ASCs do not have the ethical controversy as ESCs, but their application still faces huge challenges. Ultimately, ASCs and ESCs are equally valuable to treat liver diseases and prolong human life.
Technologies related to stem cell-based therapies
The application of stem cell-based therapy for liver diseases includes not only cell transplantation, but also culturing technologies.
In vitro culture techniques
A variety of methods have been utilized for stem cells to differentiate into hepatocytes in vitro. Most of them apply growth factors and cytokines such as activin A, Wnt3a, hepatocyte growth factor (HGF), oncostatin M (OsM) to induce liver development in vitro [76, 77]. Some studies use small molecules as an alternative for a more economical in vitro hepatogenic induction. The in vitro culture method is utilizing definitive endoderm (DE) as an interphase during differentiation. It has two steps: the first is to generate DE from stem cells, the second is to induce the differentiation of hepatocytes from DE [78]. This two-step protocol for the in vitro generation of endodermal hepatic cells has proven to be applicable to MSCs and ESCs. Melton et al. [79] identified two small molecules IDE1 and 2 that can direct mouse ESCs differentiation and have a higher efficiency than Activin A.
Another group [80] applied sequential transduction of the transcription factors such as forkhead box protein A2 (FOXA2), forkhead box protein A3 (FOXA3) hepatocyte nuclear factor-4α into pluripotent stem cells to improve the differentiation efficiency. Cai’s group [24] used stepwise titration of growth factors and cytokines to mimic liver developmental signaling pathways in vivo. Studies have shown that hepatocytes differentiated by stem cells are well functioned in vitro and are able to repopulate the liver after transplanting into animal models.
However, there still remain problems. First of all, the cultured stem cells may have genetic instability, which is a safety concern [37]. Secondly, the maturation of differentiated cells still needs improvement compared to their in vivo counterparts. The other issue is related to large-scale production of the cells for therapeutic application. Usually, generating functional HLCs needs almost one month using more than six kinds of cytokines. The long culturing time and continuous consumption of cytokines are big problems for clinical application due to high costs. With the better understanding of signals associated with cell differentiation, scientists are trying to manipulate differentiation process in vitro with small molecular compounds [81]. Du’s group [82] proposed a cost-effective strategy using pure small-molecule cocktails. They showed that activation of Wnt pathway by glycogen synthase kinase (GSK)-3β inhibitor benefited the DE differentiation. The generated hepatocytes by small-molecule-induced approach expressed high levels of hepatocyte-specific markers and displayed the biological functions of liver.
Three-dimensional culture systems
HLCs generated by many protocols will differentiate to several cell types, indicating a purification step is required at the final stage of the differentiation process. Furthermore, it has been reported that the phenotype of HLCs is closer to fetal hepatocytes rather than mature adult hepatocytes [83]. The activity of phase I and II drug metabolism enzymes, and the functions of mature hepatocytes, are significantly decreased in HLCs compared with human primary hepatocytes [84]. Meng et al. [85] reported hepatic canalicular structures are greatly lost in 2D-cultured.
These mismatches between HLCs and primary hepatocytes limit the therapeutic applications of HLCs derived from stem cells. Therefore, the development of further matured HLCs with good hepatic functions is in urgent need.
The Three-dimensional (3D) culture systems have been applied extensively in oncology studies to mimic the in vivo tumor microenvironment [86, 87]. In stem cell-based research area, the 3D hepatic spheroid culture system is one of the most promising methods for HLCs to resemble hepatic functions such as enzyme activity and drug transport. Compared with 2D culture, cells form spheroids in the medium in 3D culture. This culture environment encourages cell–cell and cell–matrix interactions that more closely mimic the native environment found in vivo. Thus the cell morphology more closely represents its natural shape in the body. 3D spheroids consist of cells in different stages, including proliferating, quiescent, and apoptotic cells. The outer layers of a 3D spheroid is exposed to the medium, so outer layer cells are mainly proliferating cells. The inner layers lack of oxygen, growth factors, and nutrients, thus inner cells prefer to stay in a quiescent state. This heterogeneity is more similar to tissues in vivo. In addition, 3D culture models have long-term expression of albumin and high urea secretion projecting on the surface [85]. The main limitation of 3D cultures is the lack of established standards.
Currently, 3D systems can be classified into the following categories (1) scaffold/matrix and (2) scaffold-free 3D formats. In scaffold/matrix based systems, cells are seeded on a 3D matrix or dispersed in a liquid matrix, then solidified or polymerized. Scaffold-free 3D based systems use the forced floating method, the hanging drop method, or agitation-based approaches. There are various choices of matrices or scaffold. The most commonly used matrix/scaffolds are agarose, collagen, fibronectin, gelatin, laminin, and vitronectin.
Lots of studies [88–92] have utilized 3D culture methods to investigate hepatic development, differentiation, and maturation. Kim and colleagues successfully derived HLCs from human ESCs and human iPSCs using 3D spheroid formation with lithium chloride. Recently, one team [93] showed the long-term expansion of adult human liver cells as 3D organoids culture. This 3D culture can produce progenitors with high proliferative capacity, and these progenitors became fully functional hepatocytes that were capable of engraftment in mouse models. Takebe et al. [28] generated in vitro vascularized 3D liver buds to resemble the adult liver tissue by combining endodermal cells derived from iPSCs, human umbilical vein endothelial cells (HUVECs), and MSCs in the same 3D structure. The human liver buds model was able to mimic liver tissue in vitro. And the transplantation of liver buds rescued the drug-induced liver failure in mice.
Bioreactor culture for scale-up
The development of hepatic tissue engineering needs practical methods to manufacture large numbers of functional hepatocytes. Massoud et al. [94] described a scalable stirred-suspension bioreactor culture of HLCs from the human pluripotent stem cells. Rapamycin for “priming” phase and activin A for induction were used to promote the initial differentiation of pluripotent stem cells in a stirred-suspension bioreactor. The cells were further differentiated into HLCs which exhibited multiple features of primary hepatocytes, including the expression of liver-specific markers, albumin secretion, urea production, collagen synthesis, indocyanine green, and LDL uptake. This scale-up method provides a new platform to generate functional HLCs and facilitates medical applications of the stem cell-derived hepatocytes. This scale-up approach opens a new window for pharmaceutical applications.
Cell encapsulation technology
Cell encapsulation technology refers to immobilization of cells within a biocompatible and semi-permeable membrane that permits the diffusion of incoming oxygen and nutrients and outward metabolites and waste products [95]. The benefit of cell encapsulation is the avoidance of transplant rejection. This technology protects the cells from the recipient’s immune system and reduces the usage of immunosuppressive drug. Other advantages of encapsulation include cell expansion, maintenance of viability potency, and controlling cell differentiation toward a desired lineage [96, 97]. Therefore, encapsulated cells are considered as an attractive approach for therapeutic application.
Lots of natural or synthetic polymers are being used for cell encapsulation. Meier et al. microencapsulated human BM-MSCs in novel alginate-poly (ethylene glycol) microspheres [98]. They showed that encapsulated BM-MSCs were able to produce anti-inflammatory IL-1Ra in vitro and significantly delayed the development of bile duct ligation and carbon tetrachloride induced liver fibrosis in the mice model.
In vivo differentiation of stem cells into hepatocytes
In vitro differentiation and culture method have been studied extensively. However, the differentiation of stem cells into hepatocytes can be achieved not only in vitro before their application but also in vivo [99–101].
Choi et al. [102] reported that mouse ESCs differentiated into a mixed population of hepatocytes of varying maturity in vivo. After the injection of ESCs to the spleen of nude mice, histological analysis revealed that some areas of teratomas contained typical hepatocytes arranged in a sinusoidal structure. The hepatic character of these cells was further confirmed by RT-PCR and immunohistochemistry, showing the expression of liver-specific genes and proteins in the differentiated teratoma. In one study, intravenous injection of adult bone marrow cells in the fumarylacetoacetate hydrolase (FAH) knockout mouse, an animal model of tyrosinemia type I, showed in vivo differentiation to HLCs and restored the biochemical liver function [53].
The advances in this field make the application of transplanted stem cells more probable for the treatment of liver diseases. However, signaling pathways controlling cell differentiation, mechanisms, and hepatocyte proliferation conditions should be studied in more depth before considering clinical applications. Hopefully, these new findings can benefit liver failure treatment research.
Clinical trials using stem cell-based therapy for liver disease
The basic researches of stem cell-based therapy for liver disease continue to reveal new findings. Some of the promising results are being translated into clinical trials. But they should be rigorously evaluated and validated before clinical applications. There are over one hundred clinical trials about stem cell-based therapy for liver disease according to ClinicalTrials.gov (https://clinicaltrials.gov/). Among them, most are phase I and phase II studies that aim to evaluate the safety and efficacy of MSCs for liver disease. The MSCs used in clinical trials for liver disease treatment are isolated from the bone marrow, umbilical cord tissue, and adipose tissue. The major administration route is the peripheral blood [103].
Sakai et al. [104] designed a phase I study to evaluate freshly isolated autologous adipose tissue-derived stem (regenerative) cell (ADRC) therapy in liver cirrhotic patients. Serious adverse effects were not observed during the 1-month study period, indicating that ADRCs can be further studied in the future for liver cirrhosis treatment. Shi’s group [105] evaluated the safety and clinical feasibility of umbilical cord-derived mesenchymal stem cell (UC-MSCS) therapy in liver transplant patients with acute graft rejection. During the research, no complications or side effects were observed. They also confirmed that UC-MSC therapy repaired liver damage and improved allograft histology. A phase II Trial [106] evaluated the efficacy of autologous transplantation of BM-MSCs (both undifferentiated and differentiated cells) in cirrhotic patients. The study showed that undifferentiated BM-MSCs and differentiated HLCs transplantation served as a treatment for liver cirrhosis.
In summary, lots of clinical trials are ongoing, and these studies will provide insights into the clinical applications for liver disease. Recent advances on preclinical researches regarding mechanisms of therapeutic effects, safety, and efficacy will also support the clinical applications of stem cell-based therapy in this field.
Advantages and limitations of stem cell-based therapy for liver disease
The goal of stem cell-based therapy for liver disease is to replace unhealthy cells with healthy ones and allow the healthy cells to be functional in the liver. Although the studies about stem cell-based therapy are still ongoing and at experimental stage, scientists have found the huge potential in this field. The plasticity of stem cell has tremendous potential for therapy.
Many research teams around the world are working on the development of stem cell-based therapy for liver diseases. The goals are to identify stem cell types that are suitable for the applications, to transplant them into human body to generate functional hepatocytes, and to develop scale-up methods for the application. To achieve these goals, scientists need to overcome considerable challenges.
Since adult tissues contain many different cell types, the identification and attempting to locate the stem cells are the challenges for scientists. After the cell isolation from the tissues, inducing cells to differentiate into desired cell types is another obstacle. To overcome this problem, we need to have a good knowledge of stem cell regulation. The scientists should confirm the appropriate culture conditions, which is difficult since there are tons of options.
Suppose the problems of cell identification, isolation and controlling of differentiation are all managed, cells may be rejected by patients’ body after implantation, which is another issue. If the patient immune system considers the transplanted cells as ‘foreigners’, the body will trigger immune response and attack the transplanted cells. Therefore, recipients will usually take immunosuppressive drugs to battle the immunological rejection. However, these drugs make the patients vulnerable to the infection.
It has been found that stem cells may benefit tumor growth, indicating that stem cell therapy should be taken carefully. Cancer occurs when cells divide abnormally. It is crucial to find a balance between controlling cell growth and preventing cells from growing excessively and becoming cancerous.
Huge numbers of cells are needed for cell transplant to be effective. However, the method for scale-up production of hepatocytes from stem cells is still difficult to develop. In addition, an effective method for transplanting stem cells or the differentiated cells has not been established. The high blood pressure in the arteries prevents the cells from efficiently entering into the liver. In patients with liver cirrhosis, transplanted cells may direct into the spleen rather than the liver because of a reversal of flow in the portal vein.
The potential of stem cell therapy to alleviate the problem of donor organ shortage motivates scientists to develop new ways for the therapy of liver disease. Although there are many challenges, stem cells still hold great promise for liver diseases treatment.
To overcome the lack of liver donors, the other therapeutic strategy is to activate the LPCs to produce hepatocytes to repair the damaged liver.
In addition to stem cells, the immune system plays an important role in liver repair and regeneration. Another strategy for promoting liver regeneration is controlling immune system via compounds or drug delivery systems. The major challenge is to precisely understand the functions of the immune components and the mechanisms. Another challenge is to develop an efficient delivery system to modulate the immune system for liver repair.
Conclusion
In summary, with recent advances in regenerative medicine, stem cell-based therapy could be a promising therapeutic method for patients with end-stage liver disease, which may alleviate the need for liver transplantation in the future. The road to developing an effective stem cell-based therapy for liver disease is still paved with challenges that will take lots of time to overcome. But good news generated from labs around the world is helpful for the transition of basic research to the clinical applications. Researchers continue to deal with the difficulties in order to utilize the stem cells to regenerate the liver and alleviate the shortage of livers for transplantation.
Acknowledgements
This work was supported by the National Natural Science Fund of the People’s Republic of China (No. 81771304), the National Natural Science Youth Fund of the People’s Republic of China (No. 81601234 and No. 81601073), and the Health Special Fund of Jilin Province, China (SCZSY201616) and the Science and Technology Innovation Development Fund of Jilin (No. 201750246).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Şentürk Ü, Yücedağ I, Polat K. Repetitive neural network (RNN) based blood pressure estimation using PPG and ECG signals. In 2018 2nd International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT) 2018 Oct 19. IEEE.
- 3.Song AT, Avelino-Silva VI, Pecora RA, Pugliese V, D’Albuquerque LA, Abdala E. Liver transplantation: fifty years of experience. World J Gastroenterol. 2014;20:5363–5374. doi: 10.3748/wjg.v20.i18.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltys KA, Setoyama K, Tafaleng EN, Soto Gutiérrez A, Fong J, Fukumitsu K, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66:987–1000. doi: 10.1016/j.jhep.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol. 2015;62:S157–S169. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 7.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol. 2016;18:238–245. doi: 10.1038/ncb3309. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K, et al. A distinct subpopulation of bone marrow mesenchymal stem cells, muse cells, directly commit to the replacement of liver components. Am J Transplant. 2016;16:468–483. doi: 10.1111/ajt.13537. [DOI] [PubMed] [Google Scholar]
- 11.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2014;59:1617–1626. doi: 10.1002/hep.26753. [DOI] [PubMed] [Google Scholar]
- 13.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 14.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 15.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuai XL, Shao N, Lu H, Xiao SD, Zheng Q. Differentiation of nonhuman primate embryonic stem cells into hepatocyte-like cells. J Dig Dis. 2014;15:27–34. doi: 10.1111/1751-2980.12103. [DOI] [PubMed] [Google Scholar]
- 17.Brolén G, Sivertsson L, Björquist P, Eriksson G, Ek M, Semb H, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, et al. Direct and indirect contribution of human embryonic stem cell–derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012;142:602–611. doi: 10.1053/j.gastro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Tolosa L, Caron J, Hannoun Z, Antoni M, López S, Burks D, et al. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res Ther. 2015;6:246. doi: 10.1186/s13287-015-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Yang X, Zhang P, Cai L, Yang X, Chen Y, et al. Sustained delivery growth factors with polyethyleneimine-modified nanoparticles promote embryonic stem cells differentiation and liver regeneration. Adv Sci (Weinh). 2016;3:1500393. doi: 10.1002/advs.201500393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Pettinato G, Wen X, Zhang N. Engineering strategies for the formation of embryoid bodies from human pluripotent stem cells. Stem Cells Dev. 2015;24:1595–1609. doi: 10.1089/scd.2014.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Bujko K, Wojakowski W. Stem cells and clinical practice: new advances and challenges at the time of emerging problems with induced pluripotent stem cell therapies. Pol Arch Med Wewn. 2016;126:879–890. doi: 10.20452/pamw.3644. [DOI] [PubMed] [Google Scholar]
- 24.Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 25.Noto FK, Duncan SA. Generation of hepatocyte-like cells from human pluripotent stem cells. In: Sell S, editor. Stem Cells Handbook. New York: Humana Press; 2013. pp. 139–47. [Google Scholar]
- 26.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannan NR, Segeritz CP, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430–437. doi: 10.1038/nprot.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011; 3:82ra39. [DOI] [PMC free article] [PubMed]
- 30.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 31.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Carraro A, Flaibani M, Cillo U, Michelotto L, Magrofuoco E, Buggio M, et al. A combining method to enhance the in vitro differentiation of hepatic precursor cells. Tissue Eng Part C Methods. 2010;16:1543–1551. doi: 10.1089/ten.TEC.2009.0795. [DOI] [PubMed] [Google Scholar]
- 33.Irudayaswamy A, Muthiah M, Zhou L, Hung H, Jumat NHB, Haque J, et al. Long-term fate of human fetal liver progenitor cells transplanted in injured mouse livers. Stem Cells. 2018;36:103–113. doi: 10.1002/stem.2710. [DOI] [PubMed] [Google Scholar]
- 34.Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2002;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 40.Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2016;99:62–68. doi: 10.1016/j.ymeth.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 42.Porada CD, Zanjani ED, Almeida-Porada G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 43.Dowidar MF, El-Belbasi HI, Ayoub AG, Rashed LA, Elged DW. Biochemical and molecular studies on bone marrow derived stromal stem cells on liver injuries in rats. Zag Vet J. 2017;45:355–365. [Google Scholar]
- 44.Yin L, Zhu Y, Yang J, Ni Y, Zhou Z, Chen Y, et al. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol Med Rep. 2015;11:1722–1732. doi: 10.3892/mmr.2014.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SK, Lee SC, Kim SJ. A novel cell-free strategy for promoting mouse liver regeneration: utilization of a conditioned medium from adipose-derived stem cells. Hepatol Int. 2015;9:310–320. doi: 10.1007/s12072-014-9599-4. [DOI] [PubMed] [Google Scholar]
- 46.Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. doi: 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 48.Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Aristizábal A, Keating A, Davies JE. Mesenchymal stromal cells as supportive cells for hepatocytes. Mol Ther. 2009;17:1504–1508. doi: 10.1038/mt.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebelo SP, Costa R, Silva MM, Marcelino P, Brito C, Alves PM. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2017;11:2034–2045. doi: 10.1002/term.2099. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Zhu Z, Huang Y, Wang P, Luo Y, Gao Y, et al. The subtype CD200-positive, chorionic mesenchymal stem cells from the placenta promote regeneration of human hepatocytes. Biotechnol Lett. 2014;36:1335–1341. doi: 10.1007/s10529-014-1468-7. [DOI] [PubMed] [Google Scholar]
- 52.Fitzpatrick E, Wu Y, Dhadda P, Hughes RD, Mitry RR, Qin H, et al. Coculture with mesenchymal stem cells results in improved viability and function of human hepatocytes. Cell Transplant. 2015;24:73–83. doi: 10.3727/096368913X674080. [DOI] [PubMed] [Google Scholar]
- 53.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 54.Lehwald N, Duhme C, Wildner M, Kuhn S, Fürst G, Forbes SJ, et al. HGF and SDF-1-mediated mobilization of CD 133+ BMSC for hepatic regeneration following extensive liver resection. Liver Int. 2014;34:89–101. doi: 10.1111/liv.12195. [DOI] [PubMed] [Google Scholar]
- 55.Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 56.Wan Z, You S, Rong Y, Zhu B, Zhang A, Zang H, et al. CD34+ hematopoietic stem cells mobilization, paralleled with multiple cytokines elevated in patients with HBV-related acute-on-chronic liver failure. Dig Dis Sci. 2013;58:448–457. doi: 10.1007/s10620-012-2458-z. [DOI] [PubMed] [Google Scholar]
- 57.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, et al. G-CSF–primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108–119. doi: 10.1016/j.exphem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Zekri AR, Salama H, Medhat E, Musa S, Abdel-Haleem H, Ahmed OS, et al. The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Res Ther. 2015;6:118. doi: 10.1186/s13287-015-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 61.Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 62.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 63.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Cell differentiation: hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 64.Martínez Sarrà E. Characterization of dental pulp pluripotent-like stem cells (DPPSC) and their mesodermal differentiation potential. Doctoral dissertation, Universitat Internacional de Catalunya, Barcelona; 2017.
- 65.Gil Recio C. Obtaining hepatocyte-like cells from dental pulppluripotent-like stem cells. Doctoral dissertation, Universitat Internacional de Catalunya, Barcelona; 2015.
- 66.Fairhall EA, Wallace K, White SA, Huang GC, Shaw JA, Wright SC, et al. Adult human exocrine pancreas differentiation to hepatocytes–potential source of a human hepatocyte progenitor for use in toxicology research. Toxicol Res (Camb). 2013;2:80–87. [Google Scholar]
- 67.Probert PM, Chung GW, Cockell SJ, Agius L, Mosesso P, White SA, et al. Utility of B-13 progenitor-derived hepatocytes in hepatotoxicity and genotoxicity studies. Toxicol Sci. 2014;137:350–370. doi: 10.1093/toxsci/kft258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang RY, Shen CN, Lin MH, Tosh D, Shih C. Hepatocyte-like cells transdifferentiated from a pancreatic origin can support replication of hepatitis B virus. J Virol. 2005;79:13116–13128. doi: 10.1128/JVI.79.20.13116-13128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace K, Fairhall EA, Charlton KA, Wright MC. AR42J-B-13 cell: an expandable progenitor to generate an unlimited supply of functional hepatocytes. Toxicology. 2010;278:277–287. doi: 10.1016/j.tox.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hynes RO. US policies on human embryonic stem cells. Nat Rev Mol Cell Biol. 2008;9:993–997. doi: 10.1038/nrm2528. [DOI] [PubMed] [Google Scholar]
- 72.Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol. 2002;197:441–456. doi: 10.1002/path.1176. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Zhang Z, Chi Y, Zhang Q, Xu F, Yang Z, et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4:e950. doi: 10.1038/cddis.2013.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oishi K, Noguchi H, Yukawa H, Hayashi S. Differential ability of somatic stem cells. Cell Transplant. 2009;18:581–589. doi: 10.1177/096368970901805-614. [DOI] [PubMed] [Google Scholar]
- 75.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 77.Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem cell Reports. 2015;4:939–952. doi: 10.1016/j.stemcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, et al. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res. 2013;319:2535–2544. doi: 10.1016/j.yexcr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol Ther. 2012;20:127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou M, Li P, Tan L, Qu S, Ying QL, Song H. Differentiation of mouse embryonic stem cells into hepatocytes induced by a combination of cytokines and sodium butyrate. J Cell Biochem. 2010;109:606–614. doi: 10.1002/jcb.22442. [DOI] [PubMed] [Google Scholar]
- 82.Du C, Feng Y, Qiu D, Xu Y, Pang M, Cai N, et al. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res Ther. 2018;9:58. doi: 10.1186/s13287-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32:504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2010;6:733–746. doi: 10.1517/17425251003674356. [DOI] [PubMed] [Google Scholar]
- 86.Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther. 2016;163:94–108. doi: 10.1016/j.pharmthera.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SE, An SY, Woo DH, Han J, Kim JH, Jang YJ, et al. Engraftment potential of spheroid-forming hepatic endoderm derived from human embryonic stem cells. Stem Cells Dev. 2013;22:1818–1829. doi: 10.1089/scd.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FP. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- 90.Kim JH, Jang YJ, An SY, Son J, Lee J, Lee G, et al. Enhanced metabolizing activity of human ES cell-derived hepatocytes using a 3D culture system with repeated exposures to xenobiotics. Toxicol Sci. 2015;147:190–206. doi: 10.1093/toxsci/kfv121. [DOI] [PubMed] [Google Scholar]
- 91.Ramaiahgari SC, den Braver MW, Herpers B, Terpstra V, Commandeur JN, van de Water B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol. 2014;88:1083–1095. doi: 10.1007/s00204-014-1215-9. [DOI] [PubMed] [Google Scholar]
- 92.Cipriano M, Freyer N, Knöspel F, Oliveira NG, Barcia R, Cruz PE, et al. Self-assembled 3D spheroids and hollow-fibre bioreactors improve MSC-derived hepatocyte-like cell maturation in vitro. Arch Toxicol. 2017;91:1815–1832. doi: 10.1007/s00204-016-1838-0. [DOI] [PubMed] [Google Scholar]
- 93.Garnier D, Li R, Delbos F, Fourrier A, Collet C, Guguen-Guillouzo C, et al. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci Rep. 2018;8:8222. doi: 10.1038/s41598-018-26584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vosough M, Omidinia E, Kadivar M, Shokrgozar MA, Pournasr B, Aghdami N, et al. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013;22:2693–2705. doi: 10.1089/scd.2013.0088. [DOI] [PubMed] [Google Scholar]
- 95.Kang A, Park J, Ju J, Jeong GS, Lee SH. Cell encapsulation via microtechnologies. Biomaterials. 2014;35:2651–2663. doi: 10.1016/j.biomaterials.2013.12.073. [DOI] [PubMed] [Google Scholar]
- 96.Hashemi M, Kalalinia F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015;143:139–146. doi: 10.1016/j.lfs.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Meier RP, Montanari E, Morel P, Pimenta J, Schuurman HJ, Wandrey C, et al. Microencapsulation of hepatocytes and mesenchymal stem cells for therapeutic applications. Methods Mol Biol. 2017;1506:259–271. doi: 10.1007/978-1-4939-6506-9_18. [DOI] [PubMed] [Google Scholar]
- 98.Meier RP, Mahou R, Morel P, Meyer J, Montanari E, Muller YD, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62:634–641. doi: 10.1016/j.jhep.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 99.Hu AB, Cai JY, Zheng QC, He XQ, Shan Y, Pan YL, et al. High-ratio differentiation of embryonic stem cells into hepatocytes in vitro. Liver Int. 2004;24:237–245. doi: 10.1111/j.1478-3231.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 100.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 101.Kuai XL, Bian YH, Cong XQ, Li XL, Xiao SD. Differentiation of mouse embryonic stem cells into hepatocytes in vitro and in vivo. J Dig Dis. 2003;4:75–80. [Google Scholar]
- 102.Choi D, Oh HJ, Chang UJ, Koo SK, Jiang JX, Hwang SY, et al. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002;11:359–368. [PubMed] [Google Scholar]
- 103.Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 104.Sakai Y, Takamura M, Seki A, Sunagozaka H, Terashima T, Komura T, et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen Ther. 2017;6:52–64. doi: 10.1016/j.reth.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]