Abstract
Heat stress is one of the wide varieties of factors which cause oxidative stress in vivo; elevated temperature can lead to oxidative stress of dairy cows that affects milk production. The aim of this study was to determine the capacity of the betaine to act as an antioxidant against oxidative stress induced by heat exposure and apoptosis in mammary epithelial cells (mammary alveolar cells, MAC-T). The MAC-T were divided into four treatment groups: control (37 °C), heat stress (HS, 42 °C), betaine (37 °C), and HS + betaine. MAC-T under heat stress (HS) showed increased ROS accumulation, malondialdehyde (MDA) content, superoxide dismutase (SOD) concentration, and catalase (CAT) activity. During heat stress, betaine decreased the mRNA expression level of HSP70 and HSP27 in MAC-T. Bax/Bcl-2 ratio and caspase-3, the markers of apoptosis, were also elevated in MAC-T under heat stress. The markers of oxidative stress Nrf-2/HO-1 genes were also elevated in MAC-T under heat stress. Pretreatment of betaine reversed the heat-induced depletion in total antioxidant status, ROS accumulation, and SOD and CAT contents in MAC-T. Bax/Bcl-2 ratio and Nrf-2/HO-1 expression of heat-exposed MAC-T were also reduced with betaine supplementation. In conclusion, betaine alleviated oxidative stress and apoptosis of MAC-T by inhibiting ROS accumulation.
Keywords: Heat stress, Reactive oxygen species (ROS), Betaine, Mammary epithelial cells, Apoptosis
Introduction
High temperatures lead to the heat stress of dairy cows, which can have negative effects on milk yield (Hammami et al. 2013; Hill and Wall 2015). This is already costly to the dairy industry in terms of management interventions and lost productivity (St-Pierre et al. 2003). In modern dairy farms, managers will take a variety of approaches to alleviate the effect of heat stress on dairy cows. These approaches usually involve modifying the environment (i.e., cooling cows), modifying ration formulations (i.e., providing some anti-heat stress feed additives), or modifying the genetics of the dairy cows (i.e., breeding to heat-tolerant breeds) during periods of heat stress (Lucy 2002).
Betaine (trimethylglycine, BET) is a well-known methyl donor that participates in many critical biochemical pathways, which provides BET with many of its physiological activities (Jin et al. 2015; Zhang et al. 2016). Betaine is recognized to be the most effective osmoprotectant, and it acts as an osmolyte and confers protection to the cells against environmental stresses (Lang 2007; Zhou et al. 2012). During heat stress, the high ambient temperature may cause water imbalances and osmotic changes in the body cells of animals via dehydration. Betaine may aid in maintaining the cell integrity and hydration under heat stress due to its osmoregulation (Kidd et al. 1997; Mahmoudnia and Madani 2012). Betaine is considered a natural anti-heat stress agent in the livestock and poultry industries. Dietary supplementation of betaine in poultry diets could maintain the avian cellular water and ion balance to improve the avian’s capacity against heat stress (Saeed et al. 2017). A combined supplementation of Se, vitamin E, Cr, and betaine above the National Research Council (NRC)-recommended levels can reduce mobilization of body reserves of lactating sows in summer (Liu et al. 2017). Dietary betaine improved lactation performance of lactating dairy cows (Wang et al. 2010). In recent years, the antioxidant properties of betaine have been widely studied. Betaine exhibits protective effects against oxidative stress inducers, such as thioacetamide and high-fructose diet, in the liver of rats (Giris et al. 2018; Heidari et al. 2018). Dietary betaine supplementation alleviated the negative effects of heat stress on the oxidative status of broilers (Wen et al. 2019). Betaine is considered a promising antioxidant for the alleviation of heat stress and enhancement of milk production in dairy cows (Hall et al. 2016; Zhang et al. 2014). Heat stress is one of the wide varieties of factors which cause oxidative stress in vivo. Oxidative stress is a situation when steady-state ROS concentration is transiently or chronically enhanced, disturbing cellular metabolism and its regulation and damaging cellular constituents (Lushchak 2014). The antioxidant mechanism of betaine in the heat-stressed dairy cow is still unclear.
The potential action sites of betaine were mammalian cells and microbes in the gastrointestinal tract in lactating ruminants (Hall et al. 2016). Thus, we evaluate the role of betaine by using bovine mammary epithelial cells under heat stress condition. We hypothesize that betaine will provide cytoprotection by decreasing reactive oxygen species (ROS) production and cell apoptosis, and improving cell viability in vivo in mammary epithelial cells. Furthermore, this cytoprotection under heat stress would result in greater lactation performance.
Materials and methods
Cell culture and treatments
Bovine mammary epithelial cells (mammary alveolar cells, MAC-T) were a gift from Dr. Youping Sun (Harvard University). The MAC-T were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and 200 U/mL of penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA], and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The medium was changed every 48 h.
To study the protective effect of betaine, MAC-T were divided into control group (37 °C), heat stress (HS, 42 °C for 12 h) group, betaine (25 mM, 37 °C, Sigma, St. Louis, MO, USA) group, and HS (42 °C for 12 h) + betaine (25 mM, 37 °C) group. For heat stress + betaine groups, betaine was added to MAC-T for 12 h prior to treatment with heat stress. Three replicates were used for each treatment.
Cell viability assay
The viability of MAC-T was quantified using a CCK-8 assay kit (Nanjing Jiancheng, China). Cells were seeded in 96-well plates at 1 × 105 cells/well, and after the indicated treatments, the cells were treated with CCK-8 reagent. After 4-h incubation at 37 °C, the absorbance was measured at 450 nm using a microplate reader.
Flow cytometry detection of apoptosis
An Annexin V-FITC/PI Detection Kit (BD Biosciences, San Diego, CA, USA) was used for the determination of cell apoptosis. The cells were treated as described above. Following the manufacturer’s instructions, cells were stained with Annexin V-FITC and PI at room temperature for 25 min. Then, apoptosis was measured with a FACS Calibur (BD Biosciences, Bedford, MA, USA) flow cytometer (FCM). The data analysis was performed using Flowjo software.
Caspase-3 activity measurement
The protein lysates of MAC-T were centrifuged at 18,000 rpm for 15 min at 4 °C, and supernatants were quantified for caspase-3 enzymatic activity using a Caspase Apoptosis Assay Kit (Geno Technology Inc., St. Louis, MO, USA) according to the manufacturer’s instructions.
Total RNA extraction and qRT-PCR
Total RNA was extracted from MAC-T with TRIzol reagent (Invitrogen) and reverse transcribed with PrimeScript RT Master Mix (TaKaRa Biotech, Dalian, China). The purity and concentration of total RNA and cDNA were determined by using a Nanodrop spectrophotometer. The suitable concentration of cDNA products was quantitated with real-time PCR, in which SYBR Premix Ex Taq (TaKaRa Biotech, Dalian, China) and specific primers were used following standard protocols on an ABI StepOne system (Applied Biosystems, Foster City, CA, USA). All the reactions were performed in triplicate with an equal amount of cDNA. β-Actin was used as the housekeeping gene. ΔΔCt method was applied for relative expression analysis. The forward and reverse primer sequences are as follows: HSP27 F-CGGACGCACCCAGACCAGCCAGCAT, R-GGCTGTGGGCCGGATACCAGTCGCG; HSP70 F-CTGAACCCGCAGAACACG, R-CCTTGGTCTCCCCTTTGT; β-actin F -AGTTCGCCATGGATGATGA, R-TGCCGGAGCCGTTGT.
Measurement of reactive oxygen species
The level of ROS was measured using a ROS assay kit (Nanjing Jiancheng, China) following the manufacturer’s instructions and visualized under a fluorescence microscope (Olympus, Tokyo, Japan). The fluorescence was measured using a microplate reader at excitation and emission wavelengths of 488 and 525 nm as previously described (Pulskamp et al. 2007).
MDA, SOD, and CAT assays
BMECs (5 × 103 cells/well in 96-well plates) were treated as described above. Then, the cellular MDA, SOD, and CAT were measured with corresponding assay kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. The optical density of MDA, SOD, and CAT was measured at absorption wavelengths of 450 nm, 450 nm, and 405 nm, respectively, with a microplate reader. Levels of MDA, SOD, and CAT were calculated and normalized to the normal control.
Western blot analysis
Cells were treated as described above, washed with PBS twice, lysed, harvested, and pelleted by centrifugation at 15,000 rpm for 15 min at 4 °C and supernatants were quantified for total protein using a BCA protein assay kit (Beyotime, China) according to the manufacturer’s instructions. Proteins were separated by SDS-PAGE and transferred to the PVDF membrane. The membranes were blocked in TBST containing 5% skim milk for 1 h and incubated with specific primary antibodies against Bax (1:1000; Proteintech, Chicago), Bcl-2 (1:1000; Proteintech), HO-1 (1:500, Abcam), Nrf2 (1:1000; Proteintech), and β-actin (1:2000; Proteintech) at 4 °C overnight. The blots were incubated and HRP-conjugated secondary antibodies and signals were detected by enhanced chemiluminescence (ECL) Western blot detection reagents (Pierce, Rockford, IL, USA). Immunoblots were scanned and densitometry was performed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as mean ± SEM unless indicated otherwise. Statistical analysis was performed using analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test. The data were analyzed with GraphPad Prism version 5.0 (GraphPad Sofware, San Diego, CA, USA). P values less than 0.05 were considered statistically significant (labeled with one star), values less than 0.01 were considered highly significant (labeled with two stars), and values more than 0.05 were considered not significant (labeled with ns).
Results
Effects of betaine treatment on the mRNA expression of HSP27 and HSP70 in response to heat stress
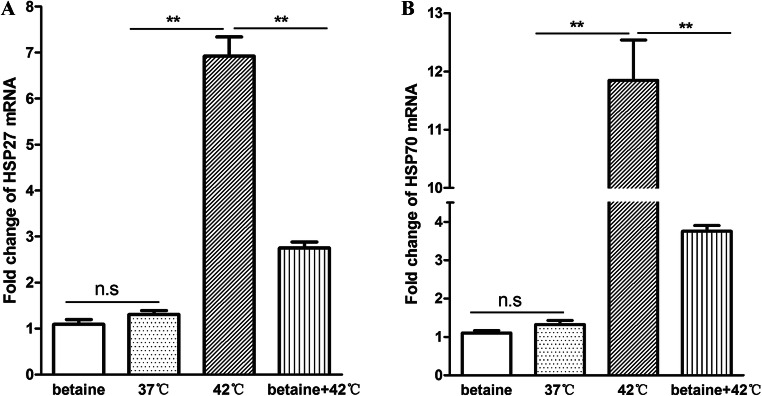
The heat stress responses of cells are adaptive mechanisms to cope with hyperthermia and are essential to the survival of a cell under heat stress. The production of heat shock proteins (HSPs) is the most recognized cellular responses during heat stress. Gene expression of HSP27 and HSP70 in mRNA level was higher in the 42 °C group compared with that of the 37 °C group, while the expression of HSP27 and HSP70 was decreased in the betaine pretreatment group during heat stress (P < 0.01, Fig. 1).
Fig. 1.
a HSP27 and b HSP70 mRNA levels: The mRNA expression level of HSP27 and HSP70 was determined by qRT-PCR. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant
Effects of betaine treatment on the expression of Nrf-2/HO-1 in response to heat stress
Nrf2 is a regulator of the antioxidant system in the cells, as it modulates the expression of a set of antioxidant enzymes like HO-1. HO-1 is also a corresponding oxidative stress marker. Protein expression of both Nrf2 and HO-1 was higher in heat-treated cells compared with that of other groups (P < 0.01, Fig. 2a). Pretreatment of betaine reversed the heat-induced Nrf2/HO-1 expression (P < 0.01, Fig. 2b).
Fig. 2.
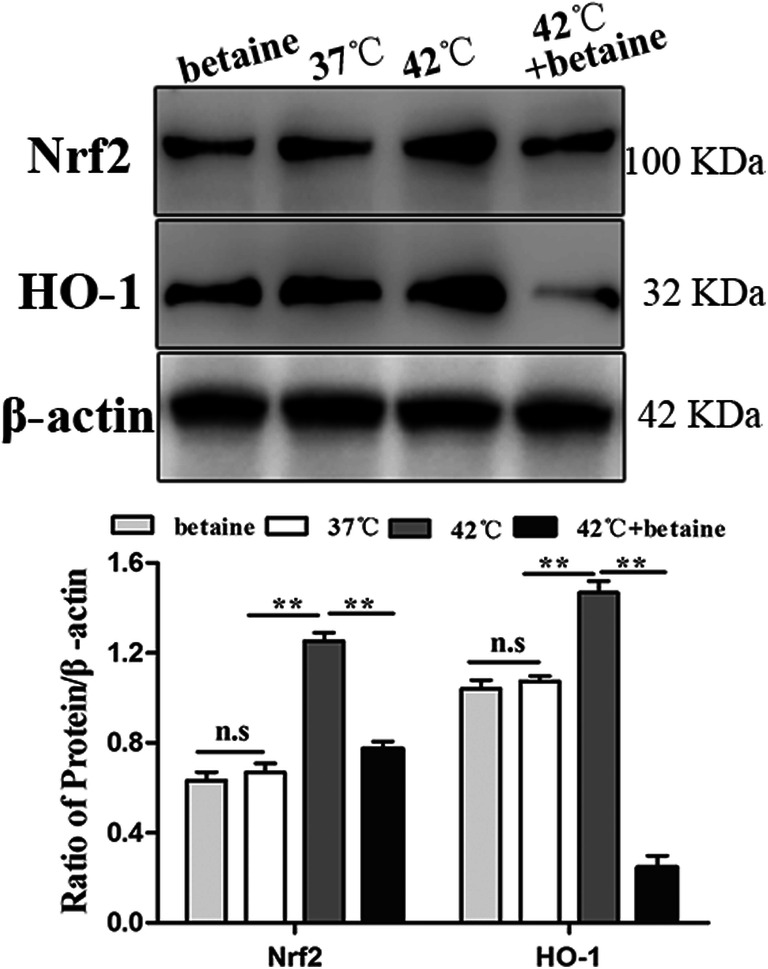
Nrf2 and HO-1 protein levels. a Nrf2 and HO-1 protein expression level was determined by Western blotting, as shown in the figure. Expression level of Nrf2 and HO-1 get increased by heat treatment, and pretreatment of betaine could reverse the increased Nrf2 and HO-1 protein expression. b Densitometric analysis of Nrf2 and HO-1 protein bands of blot (a). Data represent mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant
Effects of betaine treatment on antioxidant enzyme of MAC-T during heat stress
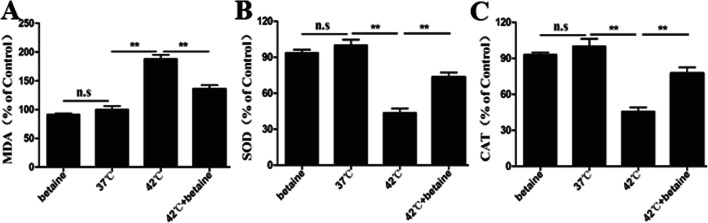
Heat stress is associated with decreased contents of MDA and increased activities of SOD and CAT, which leads to oxidative damage. Our results found that the heat stress–associated oxidative damage is declined by betaine treatment, as shown by enhanced activities of SOD and CAT (P < 0.01) (Fig. 3). These data suggest that the cytoprotective effect of betaine is due to induction of antioxidant enzymes.
Fig. 3.
The activity of antioxidant enzyme in MAC-T. a MDA content. b SOD activity. c CAT activity. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant
Effects of betaine treatment on apoptosis of MAC-T during heat stress
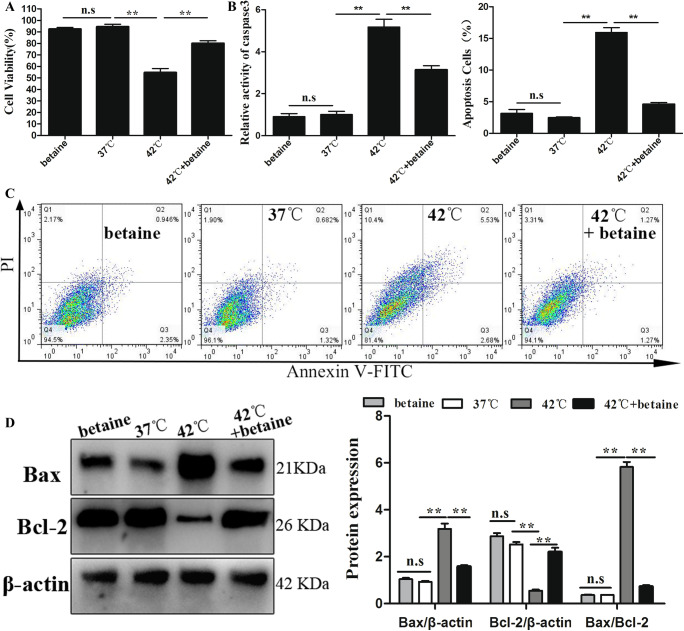
Cells were stained using an Annexin V-FITC/PI detection kit for the determination of cell apoptosis. Early apoptotic cells were stained only with Annexin V (Q3, Annexin V positive); for late apoptotic cells, the cell membrane integrity is lost allowing penetration of PI (Q2, Annexin V and PI positive), and necrotic cells were stained with PI only (Q1, PI positive). Moreover, cell viability assay and the activity of caspases-3 were also analyzed to investigate the effects of betaine treatment on apoptosis of MAC-T during heat stress. The results indicate that the cell viability increased markedly when cells were subjected to pretreatment with betaine compared to heat stress condition (Fig. 4a); furthermore, in the presence of betaine under heat stress condition, the caspase-3 activities in MAC-T decreased markedly compared to heat stress condition (P < 0.01, Fig. 4b). The flow cytometry analysis (Fig. 4c) showed that the percentage of apoptotic MAC-T was elevated after heat stress compared to thermoneutral conditions (P < 0.01, Fig. 4d). Betaine treatment decreased the apoptosis rate of heat-shocked MAC-T (P < 0.01; Fig. 4d), indicating that betaine may protect cells in heat stress through antiapoptotic effects. Figure 4e further confirms that pretreatment with betaine resulted in a significant reduction in the ratio of Bax/Bcl-2 (P < 0.01) compared to the heat stress group.
Fig. 4.
Effect of betaine on MAC-T exposed to heat stress for 12 h: a cellular viability; b caspase-3 activity; c apoptotic rate; d apoptosis events were determined by FACS analysis after PI/Annexin V staining in the treated MAC-T; and e the protein expression of Bax and Bcl-2. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant
Betaine alleviating ROS accumulation during heat stress
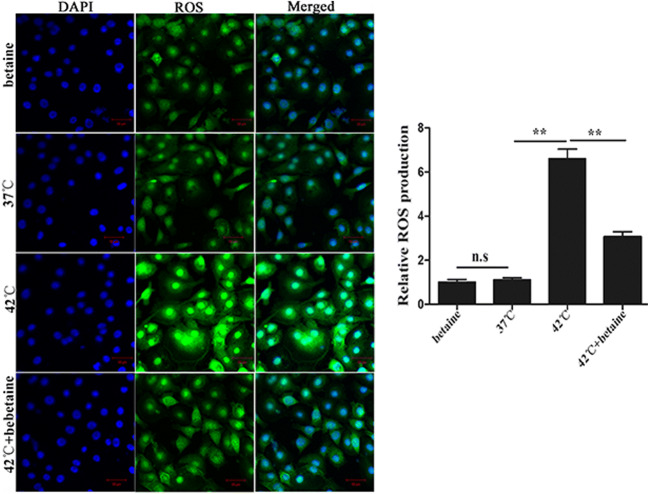
ROS production is a major factor in oxidative damage of cells. To study the direct effect of betaine on heat-induced oxidative stress, we determined the ROS level in MAC-T. We found that pretreatment with betaine alleviated heat stress–induced ROS formation in MAC-T (P < 0.01, Fig. 5).
Fig. 5.
ROS formation after different treatments: ROS production was significantly increased by heat stress, the increased ROS production was significantly alleviated by pretreatment with betaine. The experiment was replicated thrice. Significance of differences for ROS assay: *P < 0.05; **P < 0.01; ns, not significant
Discussion
Heat stress is a major contributing factor to the low performance of lactating dairy cows in hot environments. In modern dairy farms, managers will provide some feed additives of anti-stress to alleviate the heat stress of lactating dairy cows. Heat stress is also one of the wide varieties of factors which cause oxidative stress in vivo. Oxidative stress is a situation when steady-state ROS concentration is transiently or chronically enhanced, disturbing cellular metabolism and its regulation and damaging cellular constituents (Lushchak 2014). Betaine is a well-known methyl donor and a natural anti-stress agent, which plays an important role in regulating nutrient metabolism, osmotic balance, and antioxidant capacity in animals (Eklund et al. 2005). In recent years, the antioxidant properties of betaine have been widely studied. Betaine is also considered a promising antioxidant for the alleviation of heat stress and enhancement of milk production in dairy cows (Hall et al. 2016; Zhang et al. 2014), but the protective effect of betaine for bovine mammary epithelial cells was not yet investigated to the best of our knowledge. This study was carried out to examine the potential effects of betaine supplementation on heat-induced bovine mammary epithelial cells. In order to find out the exact underlying mechanisms of the protective action of betaine, the mRNA expression of HSPs (HSP27 and HSP70), antioxidant activity, and antiapoptotic effect were determined.
The heat stress responses of cells are adaptive mechanisms to cope with hyperthermia and are essential to the survival of a cell under heat stress. The production of HSPs is the most recognized cellular response during heat stress (Li et al. 2015). Our results showed that the betaine pretreatment improved thermotolerance of MAC-T subjected to HS as measured by HSP27 and HSP70 gene decreased expression. Betaine-treated BMEC exhibiting a higher capacity for HSP70 and HSP27 mRNA expression in heat stress was observed (Xiao et al. 2017). Our results are different from those of the previous study. The reason may be that MAC-T and BMEC were probably not at the same stage of development. This might have contributed to their different responses to betaine treatment because signaling pathways have distinct effects on mammary glands at different developmental stages (Macias and Hinck 2012).
Heat stress was suggested to be an environmental factor responsible for stimulating ROS production (Slimen et al. 2014) and an imbalance between ROS production and the available antioxidant defense against them can induce oxidative stress. MDA is the last product of lipid peroxidation, and therefore changes of MDA concentrations can be used as a biomarker of oxidative stress (Bernabucci et al. 2002). In the findings of the present study, MDA levels increased in HS groups. Thereafter, it decreased in betaine pretreatment groups during heat stress. Some authors reported treatment with betaine in rats significantly decreased the MDA level (Sehirli et al. 2016). Betaine could reduce the oxidative stress by suppressing MDA and enhancing SOD in rat brain (Nie et al. 2016). Superoxide dismutase (SOD) and catalase (CAT) constitute the primary enzymatic antioxidant defense against ROS-induced oxidative damage. Heat stress can inhibit SOD expression and activities (Yang and Lin 2002) and this inhibition can be attributed to thermal/oxidative inactivation of the enzyme (Lushchak 2014). In the present study, significant increases in the activity of SOD and CAT in MAC-T were evident in the HS + betaine group compared to the heat stress group. SOD averts oxidative stress by catalyzing superoxide radicals to molecular oxygen and peroxide, and CAT further reduces the hydrogen peroxide decomposition to harmless compounds such as water and oxygen (Sreedhar et al. 2002), which maintains cellular homeostasis by neutralizing excessive ROS levels. Our results indicate that pretreatment of betaine reversed the heat-induced depletion in total antioxidant status by restoring SOD and CAT activities and depressing MDA content.
We further investigated the increased level of ROS in the MAC-T during heat treatment: the result suggests that heat induces oxidative stress in bovine mammary epithelial cells. A majority of the studies dealing with the effects of heat stress on oxidative stress biomarker response have been conducted in circumstances that sustained moderate to severe heat stress under strictly defined experimental conditions (environmentally controlled chambers) on selected high-yielding and intensively managed dairy cattle (Hady et al. 2018). HO-1 is a corresponding oxidative stress marker and plays important roles in iron homeostasis, antioxidant defense, and antiapoptosis of cells (Loboda et al. 2016). Nrf-2 signaling is considered as an important defensive transduction pathway for the body to resist the oxidative and chemical stimuli of the internal environment, which regulates the expression levels of HO-1. To elucidate whether the accumulation of ROS is associated with Nrf2/HO-1 signaling pathway, the expression of Nrf2 and HO-1 protein were examined in MAC-T exposed to heat. Interestingly, the protein expression of Nrf-2 and HO-1 were higher in heat-stressed MAC-T. This is in line with the result of low oxygen levels in bovine embryo in Nrf2-mediated oxidative stress response genes (Amin et al. 2014). Pretreatment with betaine alleviates the expression of Nrf-2 and HO-1. The results showed that heat-induced oxidative stress through Nrf2/HO-1 signaling pathway and betaine could provide protection by the regulation of oxidative stress responses in MAC-T.
In addition, the production of ROS and cell apoptosis are closely related. Our results also showed that the percentage of apoptotic cells was increased significantly with heat treatment, which may be closely correlated with the overproduction of ROS. Heat stress–mediated apoptosis and the involvement of mitochondrial pathways were reported in bovine mammary epithelial cells exposed to higher temperatures in vitro (Du et al. 2008). Therefore, Bax and Bcl-2 are pro-apoptotic and antiapoptotic proteins, respectively, serving as critical control points in the mitochondrial apoptotic pathway (Gu et al. 2015). In the present study, compared with control, MAC-T incubated at 42 °C increased caspase-3 protein level and the ratio of Bax/Bcl-2 significantly; meanwhile, pretreatment of betaine reversed the heat-induced apoptosis. These results suggest that betaine provides protection via inhibition of the mitochondrial apoptotic pathway in heat-associated apoptosis of bovine mammary epithelial cells.
Conclusion
In summary, treatment of MAC-T with betaine effectively inhibits heat exposure–induced oxidative stress and apoptosis via regulation of ROS production. Betaine acts as an antioxidative agent via restoring SOD and CAT activities, and depressing MDA content in heat-insulted MAC-T. Pretreatment with betaine decreases the ratio of Bax/Bcl-2 and caspase-3 activity. The expression levels of Nrf2 and HO-1 protein and heat-associated cell apoptosis were inhibited (Fig. 6). These data emphasize the importance of oxidative stress and caspase signaling cascade in heat-induced apoptosis and suggest that betaine pretreatment reduces severity of oxidative stress probably via antioxidant action and suppression of apoptosis. Altogether, betaine reversed heat-induced apoptosis effectively and predominantly through Nrf2-mediated oxidative stress, which provided the useful evidence that betaine can serve as a novel natural antioxidant in animal feed.
Fig. 6.
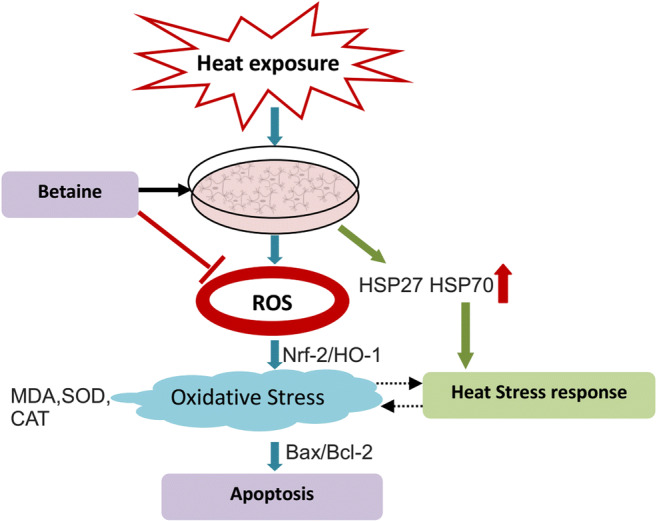
Betaine reversed heat-induced apoptosis effectively and predominantly through Nrf2-mediated oxidative stress
Funding information
This research was supported by the National Key Research and Development Program of China (2018YFD0501600).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amin A, Gad A, Salilew-Wondim D, Prastowo S, Held E, Hoelker M, Rings F, Tholen E, Neuhoff C, Looft C, Schellander K, Tesfaye D. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol Reprod Dev. 2014;81:497–513. doi: 10.1002/mrd.22316. [DOI] [PubMed] [Google Scholar]
- Bernabucci U, Ronchi B, Lacetera N, Nardone A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci. 2002;85:2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3. [DOI] [PubMed] [Google Scholar]
- Du J, Di H-S, Guo L, Li Z-H, Wang G-L. Hyperthermia causes bovine mammary epithelial cell death by a mitochondrial-induced pathway. J Therm Biol. 2008;33:37–47. doi: 10.1016/j.jtherbio.2007.06.002. [DOI] [Google Scholar]
- Eklund M, Bauer E, Wamatu J, Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- Giris M, Dogru-Abbasoglu S, Soluk-Tekkesin M, Olgac V, Uysal M. Effect of betaine treatment on the regression of existing hepatic triglyceride accumulation and oxidative stress in rats fed on high fructose diet. Gen Physiol Biophys. 2018;37:563–570. doi: 10.4149/gpb_2018005x. [DOI] [PubMed] [Google Scholar]
- Gu ZT, Li L, Wu F, Zhao P, Yang H, Liu YS, Geng Y, Zhao M, Su L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca(2+) dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci Rep. 2015;5:11497. doi: 10.1038/srep11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hady MM, Melegy TM, Anwar SR. Impact of the Egyptian summer season on oxidative stress biomarkers and some physiological parameters in crossbred cows and Egyptian buffaloes. Vet World. 2018;11:771–778. doi: 10.14202/vetworld.2018.771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LW, Dunshea FR, Allen JD, Rungruang S, Collier JL, Long NM, Collier RJ. Evaluation of dietary betaine in lactating Holstein cows subjected to heat stress. J Dairy Sci. 2016;99:9745–9753. doi: 10.3168/jds.2015-10514. [DOI] [PubMed] [Google Scholar]
- Hammami H, Bormann J, M’hamdi N, Montaldo HH, Gengler N (2013) Evaluation of heat stress effects on production traits and somatic cell score of holsteins in a temperate environment. J Dairy Sci 96:1844–1855 [DOI] [PubMed]
- Heidari R, Niknahad H, Sadeghi A, Mohammadi H, Ghanbarinejad V, Ommati MM, Hosseini A, Azarpira N, Khodaei F, Farshad O, Rashidi E, Siavashpour A, Najibi A, Ahmadi A, Jamshidzadeh A. Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed Pharmacother. 2018;103:75–86. doi: 10.1016/j.biopha.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Hill DL, Wall E. Dairy cattle in a temperate climate: the effects of weather on milk yield and composition depend on management. Animal. 2015;9:138–149. doi: 10.1017/S1751731114002456. [DOI] [PubMed] [Google Scholar]
- Jin P, Zhang Y, Shan T, Huang Y, Xu J, Zheng Y. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J Agric Food Chem. 2015;63:3654–3659. doi: 10.1021/acs.jafc.5b00605. [DOI] [PubMed] [Google Scholar]
- Kidd M, Ferket P, Garlich J. Nutritional and osmoregulatory functions of betaine. Worlds Poult Sci J. 1997;53:125–139. doi: 10.1079/WPS19970013. [DOI] [Google Scholar]
- Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613s–623s. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- Li L, Sun Y, Wu J, Li X, Luo M, Wang G. The global effect of heat on gene expression in cultured bovine mammary epithelial cells. Cell Stress Chaperones. 2015;20:381–389. doi: 10.1007/s12192-014-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Cottrell JJ, Collins CL, Henman DJ, O’Halloran KSB, Dunshea FR. Supplementation of selenium, vitamin E, chromium and betaine above recommended levels improves lactating performance of sows over summer. Trop Anim Health Prod. 2017;49:1461–1469. doi: 10.1007/s11250-017-1348-y. [DOI] [PubMed] [Google Scholar]
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy MC (2002) Reproductive loss in farm animals during heat stress. In: Proceedings 15th conference on biometeorology and aerobiology (Citeseer), pp 50–53
- Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1:533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudnia N, Madani Y. Effect of betaine on performance and carcass composition of broiler chicken in warm weather-a review. IJAS. 2012;2:675–683. [Google Scholar]
- Nie C, Nie H, Zhao Y, Wu J, Zhang X. Betaine reverses the memory impairments in a chronic cerebral hypoperfusion rat model. Neurosci Lett. 2016;615:9–14. doi: 10.1016/j.neulet.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Saeed M, Babazadeh D, Naveed M, Arain MA, Hassan FU, Chao S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop Anim Health Prod. 2017;49:1329–1338. doi: 10.1007/s11250-017-1355-z. [DOI] [PubMed] [Google Scholar]
- Sehirli AO, Satilmis B, Tetik S, Cetinel S, Yegen B, Aykac A, Sener G. Protective effect of betaine against burn-induced pulmonary injury in rats. Ulus Travma Acil Cerrahi Derg. 2016;22:417–422. doi: 10.5505/tjtes.2015.60137. [DOI] [PubMed] [Google Scholar]
- Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperth. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- Sreedhar A, Pardhasaradhi B, Khar A, Srinivas UK. A cross talk between cellular signalling and cellular redox state during heat-induced apoptosis in a rat histiocytoma. Free Radic Biol Med. 2002;32:221–227. doi: 10.1016/S0891-5849(01)00796-1. [DOI] [PubMed] [Google Scholar]
- St-Pierre N, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci. 2003;86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5. [DOI] [Google Scholar]
- Wang C, Liu Q, Yang W, Wu J, Zhang W, Zhang P, Dong K, Huang Y. Effects of betaine supplementation on rumen fermentation, lactation performance, feed digestibilities and plasma characteristics in dairy cows. J Agric Sci. 2010;148:487–495. doi: 10.1017/S0021859610000328. [DOI] [Google Scholar]
- Wen C, Chen Y, Leng Z, Ding L, Wang T, Zhou Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J Sci Food Agric. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Rungruang S, Hall LW, Collier JL, Dunshea FR, Collier RJ. Effects of niacin and betaine on bovine mammary and uterine cells exposed to thermal shock in vitro. J Dairy Sci. 2017;100:4025–4037. doi: 10.3168/jds.2016-11876. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Lin M-T. Oxidative stress in rats with heatstroke-induced cerebral ischemia. Stroke. 2002;33:790–794. doi: 10.1161/hs0102.100208. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ying SJ, An WJ, Lian H, Zhou GB, Han ZY. Effects of dietary betaine supplementation subjected to heat stress on milk performances and physiology indices in dairy cow. Genet Mol Res. 2014;13:7577–7586. doi: 10.4238/2014.September.12.25. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wu X, Lai F, Zhang X, Wu H, Min T. Betaine inhibits hepatitis B virus with an advantage of decreasing resistance to lamivudine and interferon alpha. J Agric Food Chem. 2016;64:4068–4077. doi: 10.1021/acs.jafc.6b01180. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Holmseth S, Hua R, Lehre AC, Olofsson AM, Poblete-Naredo I, Kempson SA, Danbolt NC. The betaine-GABA transporter (BGT1, slc6a12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am J Physiol Renal Physiol. 2012;302:F316–F328. doi: 10.1152/ajprenal.00464.2011. [DOI] [PubMed] [Google Scholar]